Abstract
Much of the evidence linking the short-latency phasic signaling of midbrain dopaminergic neurons with reward-prediction errors used in learning and habit formation comes from recording the visual responses of monkey dopaminergic neurons. However, the information encoded by dopaminergic neuron activity is constrained by the qualities of the afferent visual signals made available to these cells. Recent evidence from rats and cats indicates the primary source of this visual input originates subcortically, via a direct tectonigral projection. The present anatomical study sought to establish whether a direct tectonigral projection is a significant feature of the primate brain. Injections of anterograde tracers into the superior colliculus of macaque monkeys labelled terminal arbors throughout the substantia nigra, with the densest terminations in the dorsal tier. Labelled boutons were found in close association (possibly indicative of synaptic contact) with ventral midbrain neurons staining positively for the dopaminergic marker tyrosine hydroxylase. Injections of retrograde tracer confined to the macaque substantia nigra retrogradely labelled small to medium sized neurons in the intermediate and deep layers of the superior colliculus. Together, these data indicate that a direct tectonigral projection is also a feature of the monkey brain, and therefore likely to have been conserved throughout mammalian evolution. Insofar as the superior colliculus is configured to detect unpredicted, biologically salient, sensory events, it may be safer to regard the phasic responses of midbrain dopaminergic neurons as ‘sensory prediction errors’ rather than ‘reward prediction errors’, in which case, dopamine-based theories of reinforcement learning will require revision.
Keywords: basal ganglia, oculomotor, dopamine, reward, superior colliculus
Introduction
The short-latency phasic response of dopaminergic (DA) neurons is considered critical for reinforcement learning (Dayan & Balleine, 2002; Schultz, 2002; Montague et al., 2004). Typically, midbrain DA neurons exhibit stereotyped, short latency (<100 ms), short duration (∼100 ms) population responses to unpredicted, biologically salient events (Schultz, 1998). Many believe this signal provides the distributed reinforcement learning mechanism in the basal ganglia and frontal cortex with a ‘reward prediction error’ that is used to adjust subsequent behavioral selections in a manner which maximises future acquisitions of reward (Dayan & Balleine, 2002; Schultz, 2002; Montague et al., 2004).
Despite considerable support for the reward prediction error hypothesis (Schultz, 2006), there are significant observations that conflict with it (Redgrave et al., 1999; Redgrave & Gurney, 2006; Redgrave et al., 2007). First, phasic DA responses are not restricted to reward-related events (Horvitz, 2000; Takikawa et al., 2004). Second, these responses are often remarkably stereotyped across species, stimulus modalities, and experimental paradigms (Schultz, 1998). Third, comparison of the latencies of DA neuron responses (Schultz, 1998) to gaze-shift latencies (Munoz & Guitton, 1986; Jay & Sparks, 1987) indicates that phasic signalling is conducted on the basis of pre-attentive sensory processing occurring before foveation. This is important because the information in the short-latency input to DA neurons must be constrained by the perceptual processing capacity of the sensory systems delivering this information. It is therefore crucial to determine the source(s) of short latency sensory activity in ventral midbrain DA neurons (Kitai et al., 1999; Pan & Hyland, 2005). We have discovered a previously unreported tectonigral pathway in the rat and cat (Comoli et al., 2003; McHaffie et al., 2006), and have demonstrated in the rat that this subcortical projection via the superior colliculus (SC) provides the primary, if not exclusive, source of short latency visual input to midbrain DA neurons (Dommett et al., 2005).
However, much of the evidence linking phasic DA signalling to reward-prediction errors comes from recording visual responses in DA neurons of monkeys (Morris et al., 2004; Takikawa et al., 2004; Bayer & Glimcher, 2005; Schultz, 2006) due to their expected similarity to humans. Thus, it is critical to establish whether the SC is a potential source of short-latency visual input to DA neurons in monkeys. Moreover, basal ganglia systems in primates may be more complex than or exhibit differences from other species (Haber et al., 1995; Damier et al., 1999; Graybiel, 2001; Frankle et al., 2006; Sakai et al., 2002). Furthermore, monkey DA cells are divided into two tiers: the calbindin-positive dorsal tier, which receives input primarily from the ventral striatum, with minor inputs from amygdala and cortex; and the calbindin-negative ventral tier, which receives input primarily from dorsal striatum, but no input from cortex or amygdala (Lynd-Balta & Haber, 1994; Haber & Fudge, 1997). We wished to determine whether the tectonigral projection would also exhibit tier specificity in primates. Consequently, we have employed both anterograde and retrograde tracers to establish the presence and targets of a macaque tectonigral projection (May et al., 2006).
Materials and Methods
All animal protocols used in this study were approved by the appropriate Institutional Animal Care and Use Committees, and were undertaken in compliance with the Guide for the Care and Use of Laboratory Animals at AALAC approved facilities. For the anterograde tracing experiments, a total of 9 adult and young adult macaque monkeys (M. fascicularis and mulatta) of both sexes were used; 5 received injections of biotinylated dextran amine (BDA), and 4 injections of Phaseolus vulgaris leucoagglutinin (PhaL). For the retrograde tracing experiments, wheat germ agglutinin conjugated horseradish peroxidase (WGA-HRP) was used as the tracer. Five relevant cases were selected for analysis from the library of Macaca nemestrina tissue kept in the laboratory of Prof. Suzanne Haber. Consequently, no further animals were used for this part of the investigation.
Anterograde experiments
Tracer injections into the superior colliculus
The animals were subdued with ketamine HCl (10 mg/kg, IM), supplied with an intravenous line and a endotracheal tube, and anesthetized with isoflurane (1.5-3.0 %). Carprophen (3 mg/kg, IM) was given as a presurgical anesthetic. During the surgery, the animals were hydrated with a lactated Ringer's drip, and given dexamethasone (2.5 mg/kg, IM) to control edema and atropine sulfate (0.05 mg/kg, IM) to limit secretions. Core body temperature, heart rate, and exhaled gasses were recorded and maintained within normal parameters. These animals were placed in a stereotaxic head holder and the scalp incised along the midline. Following a craniotomy, the cortex over the midbrain was aspirated to reveal the midbrain surface. A 1.0 μl Hamilton syringe held in a micromanipulator at an angle of 20°, tip up in the parasagittal plane, was used to inject BDA (Moleular Probes, Eugene, OR, USA) into the exposed SC. The 10 % BDA solution (0.1-0.2 μl) was pressure injected at 2-3 sites located 1.5 mm beneath the collicular surface. Alternatively, iontophoresis was used to inject PhaL (Vector Laboratories, Burlingame, CA, USA) into the SC. A positive current (0.7 μA, 10-20 min, 50 % duty cycle, 7 sec each) was used to expel the PhaL (4 % solution in 0.1 M, pH 8.0 phosphate buffer (PB)) from a glass micropipette (tip diameter of 20-30 μm) inserted into the SC. Following these injections, the aspiration defect was filled with damp gelfoam, and the scalp was closed with suture. The incision was infused with Sensorcaine, and the animal was given Buprenex (0.01 mg/kg, IM) as a postoperative analgesic.
Animals injected with BDA survived for ∼ 21 days, and animals injected with PhaL survived for ∼14 days, to allow the tracer to transport. They were then sedated with ketamine HCL (10 mg/kg, IM), and deeply anesthetized with sodium pentobarbital (50-70 mg/kg, IP). Once insensate, they were perfused through the heart with 0.1 M, pH 7.2 phosphate buffered saline (PBS), followed by a fixative containing 1.0 % paraformaldehyde and 1.25 % glutaraldehyde in 0.1 M, pH 7.2 PB. Animals intended for immunohistochemistry were instead perfused with 4.0 % paraformaldyde in 0.1 M, pH 7.2 PB. The brains were postfixed with the same fixative at 4° C for 1-2 hrs (1.0 % / 1.25 %) or overnight (4 %).
Immunohistochemical and histochemical procedures
Brains containing BDA injections were cut into 100 μm sections on a vibratome. Brains with PhaL injections were equilibrated in a 30 % sucrose cryoprotectant, frozen, and cut into 50 μm sections on a sliding microtome. For detailed descriptions of the techniques used see McHaffie and colleagues (McHaffie et al., 2006). Briefly, each ordered series of sections containing BDA was incubated over night at 4° C in a 1:5000 solution of avidin conjugated to horseradish peroxidase (avidin-HRP)(Vector laboratories, Burlingame, CA, USA) in 0.1 M, pH 7.2 PB with 1.0 % Triton-X-100. They were then washed in PB and reacted in a solution containing 0.5 % diaminobenzidine HCl (DAB) and 0.01 % cobalt chloride and nickel ammonium sulphate, to which 0.005 % H2O2 was added to initiate the reaction. To reveal the PhaL, sections were immersed in a solution of 0.3 % Triton-X-100 and 10 % normal goat serum (NGS) in 0.1 M, pH 7.2 PB. They were then incubated overnight in a solution of 1: 200 biotinylated goat anti-PhaL (Vector Laboratories, Burlingame, CA, USA) in 0.1 M, pH 7.2 PB with 1 % NGS. The goat antibody was tagged with HRP by use of a goat ABC kit (Vector Laboratories, Burlingame, CA, USA). The HRP was then visualized with DAB, following the procedure outlined above, except that cobalt chloride was omitted from the reaction mixture. Following the DAB reaction, both sets of sections were rinsed, mounted on gelatinized slides and dried. The slides were then counter-stained with cresyl violet, dehydrated, cleared and cover-slipped.
To reveal the relationship of the PhaL labelled axon terminals to tyrosine-hydroxylase (TH) containing DA neurons, a two step procedure was used. First the PhaL was revealed using the procedures described above. After the sections were washed, they were incubated overnight at 4°C in mouse antibody to TH (1:3,000 dilution in 0.1 M, pH, 7.2 PB with 2 % NGS and 0.3 % Triton-X-100)(Chemicon International, Temecula, CA, USA). Sections were then washed and treated using standard procedures for the mouse ABC kit (Vector Labs, Burlingame, CA, USA). The HRP marking TH-positive (TH+) cells was visualized by use of a VIP staining kit (Vector Labs, Burlingame, CA, USA). These sections were mounted onto gelatinized slides, dehydrated, cleared and cover-slipped.
Analysis
A drawing tube mounted on an Olympus BH-2 microscope was used to plot the location and illustrate the morphology of BDA and PhaL labelled axonal arbors and TH+ neurons. Examples of anterograde labelling within SN were photographed using a Nikon Eclipse-600 microscope equipped with a Nikon DXM 1200 digital colour camera. Metamorph Software (Molecular Devices, Sunnydale, CA, USA) was used to acquire the digital images. Up to 20, 1 μm z-axis slices were combined by use of the stack arithmetic function to make the photomicrographs. The pictures were further adjusted with PhotoshopTM (Adobe, San Jose, CA, USA) software, using the color balance, brightness and contrast functions until the digital images approximated the observed image.
Retrograde experiments
Fudge and Haber (2000; 2001) have previously described both the WGA-HRP injections into the midbrain DA cell containing regions of the 5 monkeys used here, and the patterns of retrograde transport to the amygdala and bed nucleus of the stria terminalis they produced. Further details of the methodology can be found therein.
Tracer injections into the ventral midbrain
Initial sedation via ketamine HCl (10 mg/kg, IM) was followed by inducing a deep surgical level of anesthesia using Na pentobarbital (initial dose 20 mg/kg, IV, with maintenance dosing as needed). WGA-HRP (4.0%, Sigma, St. Louis, MO, USA) was pressure injected (35-40 nl) into discrete regions of the ventral midbrain by use of a 0.5 or l.0 μl Hamilton syringe. The animals were deeply anesthetized with pentobarbital 7-10 days after surgery, and underwent intracardiac perfusion with saline and 4 % paraformaldehyde, as described above.
Immunohistochemical procedures
Once the brains were cryoprotected in sucrose, serial coronal sections (50 μm) were cut on a freezing microtome, and processed to reveal the WGA-HRP by use of immunocytochemical procedures. Sections were first rinsed in 0.1 M, pH 7.4 PBS with 0.3% Triton X-100 (PBS-T), and then preincubated in 10% normal goat serum (NGS) diluted with PBS-T (NGS-PBS-T) for 30 min. Subsequently, they were placed in the primary antisera (rabbit anti-WGA-HRP, 1:2000 (Sigma, St. Louis, MO, USA)), in NGS-PBS-T for approximately 96 h at 4°C. The avidin-biotin reaction (rabbit Vectastain ABC kit, Vector Labs, Burlingame, CA, USA) was used to visualize the tracer. Tissue was incubated for 10-12 min in DAB with cobalt chloride and nickel as described above, to yield a black reaction product. In all cases, the tissue was either counterstained or adjacent sections were stained for Nissl substance. These sections were mounted onto gelatinized slides, dehydrated, cleared and coverslipped.
Analysis
The selected cases had well localised injections in the ventral midbrain that lacked significant necrosis. The locations of retrogradely labelled neurons in the SC were charted using a Nikon Eclipse 800 microscope equipped with brightfield/darkfield illumination, an imaging system, and Neurolucida software (MicroBrightfield, Williston, VT, USA). Photomicrographs were captured digitally, and all image files were processed using Photoshop™ (Adobe, San Jose, CA, USA), Illustrator ™ (Adobe, San Jose, CA, USA), or FreeHand™ (Macromedia, San Jose, CA, USA) software. The images were corrected for contrast, brightness, and color balance. Analysis of the location of retrogradely labelled tectonigral neurons was conducted by plotting the position of each neuron in 3 representative sections selected from the rostral, central and caudal SC onto a drawing of the SC boundaries and layers using the Neurolucida software. Care was taken to select the same 3 levels from each case. Except where specifically mentioned, the number of labelled neurons quoted for each injection site refers to the summed total of labelled cell bodies plotted within the boundaries of the SC on each of the three analysed sections. These raw numbers were not corrected or statistically analyzed as they were solely intended to allow comparison of relative labelling densities with respect to injection site location.
Results
Anterograde Labelling of Tectonigral Axons
Anterogradely labelled axons and terminals were found within the SN in all 9 animals that received collicular injections of BDA or PhaL. Selected cases will be illustrated to convey qualitative descriptions of the anterograde tracing observed within this group.
Anterograde labelling with BDA
An example of a BDA injection site that was confined to the SC, and primarily located within the intermediate gray layer (SGI) of the caudal third of the tectum, is illustrated in figure 1 (insert lower right). [NB: The definition of collicular layers and the nomenclature used to describe them follow the system described in May and Porter (1992)]. In coronally sectioned material, labelled axons from this injection were found throughout the rostrocaudal extent of the ipsilateral SN (Fig. 1A-F), and were located in both pars compacta (SNc) and pars reticulata (SNr). Labelled axons in SNr were generally more numerous in the lateral aspects of the nucleus (Fig. 1B-E). Labelled axons in SNc tended to be denser dorsally, particularly at caudal levels (Fig. 1C-F). Numerous labelled axons were also present in the midbrain reticular formation immediately dorsal to SNc (Fig. 1C-E), as well as in the ventral tegmental area (VTA) medial to SNc (Fig. 1B-E). They were also observed in the subthalamic nucleus (Fig. 1A-D). A small number of BDA labelled axons were found contralaterally in the SNc, SNr and VTA (not illustrated).
Figure 1.
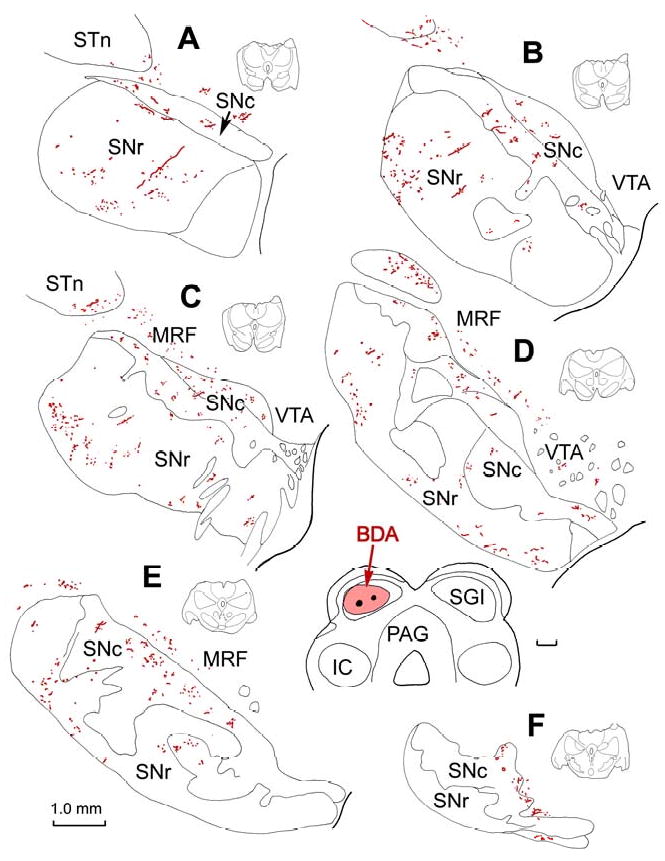
Distribution of labelled tectonigral axons in the substantia nigra. An injection of BDA centered in SGI of the SC (insert, lower right) anterogradely labelled axons throughout the SN, as shown in a rostral to caudal series of frontal sections (A-F). The illustrated levels are indicated by the small schematics to the right of each illustration. Labelled axons are present in both SNc and SNr, as well as in the adjacent midbrain reticular formation, ventral tegmental area, and subthalamic nucleus. Abbreviations used in this in subsequent figures: BC - brachium conjunctivum; BDA - biotinylated dextran amine; IC - inferior colliculus; IIIn - third cranial nerve; MRF - midbrain reticular formation; PAG - periaqueductal gray; PhaL - phaseolus vulgaris leucoagglutinin; SC - superior colliculus; SGI - intermediate gray layer; SGP - deep gray layer; SGS - superficial gray layer; SN - substantia nigra; SNc - substantia nigra pars compacta; SNr - substantia nigra pars reticulata; SO – stratum opticum; STn - subthlamic nucleus; VTA - ventral tegmental area.
Close examination of the anterogradely labelled axons in SN revealed that most were terminal arbors (Fig. 2). The BDA labelled terminal arbors observed in coronal sections through the SNc had relatively fine diameters, and displayed occasional branches (Fig. 2A-D & F). Along their course, they had small enlargements which were at either en passant or terminal locations. While ultrastructural verification is necessary, these presumably represent synaptic boutons. Individual axonal arbors extended among the counter-stained cells in SNc, with boutons displaying close associations (arrowheads) with several different neurons (Fig. 2A-D & F). The BDA labelled arbors usually contributed just a few boutons to any individual neuron in SNc (Fig. 3B), but in some cases multiple boutons were seen in close association (arrowheads) with a single SNc cell (Fig. 3A). These displayed a light brown color due to the presence of neuromelanin granules. BDA labelled terminals were also observed in SNr (Fig. 1A-E), and a portion of these labelled terminal arbors displayed boutons that lay in close association with nigrotectal neurons that had been retrogradely labelled with BDA (Fig. 2G-I). These BDA labelled axons often followed labelled nigrotectal dendrites, contributing a number of close boutonal associations along their course (Fig. 3C). Such close associations are suggestive of synaptic contact between the labelled elements. However, proof of actual synaptic contact requires electron microscopic verification, such as that previously shown between rodent tectonigral terminals and TH+ profiles (Comoli et al., 2003). In other systems it has been noted that more than half of observed close associations represent synaptic contacts (Pilowsky et al., 1992)
Figure 2.
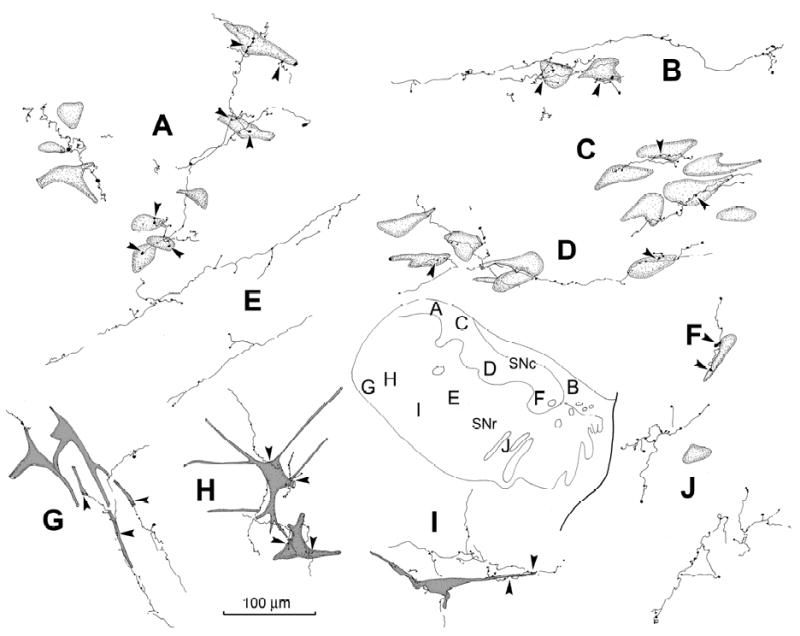
Relationship of BDA labeled tectonigral terminal arbors to components of SN. Individual arbors labelled from the injection illustrated in figure 1 are shown. BDA labelled tectonigral axons were distributed among counter-stained somata in SNc (A-D&F). They were also found in SNr (E&G-J), where they were sometimes associated with retrogradely labelled nigrotectal neurons (G-I). These axons displayed boutonal enlargements that sometimes lay in close association (arrowheads) with cells in SNc and SNr. The location of each illustrated example is shown in the schematic (lower right).
Figure 3.
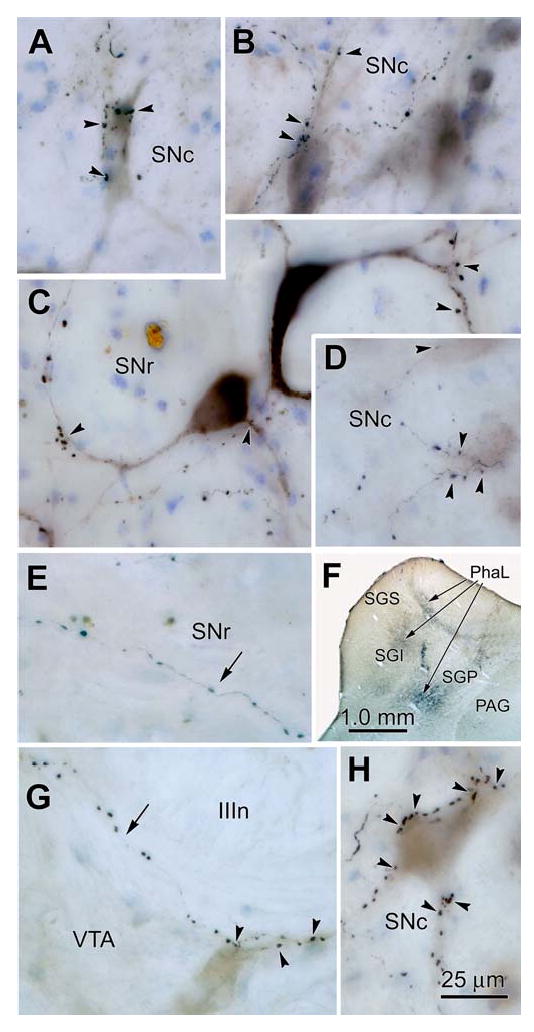
Relationship between BDA (A-C) and PhaL (D,E,G&H) labelled tectonigral boutons and nigral (A-D&H) and VTA (G) neurons. The BDA injection shown in figure 1 produced the labelled tectonigral axons shown in A-C. The boutons of these axons displayed close associations (arrowheads) with ipsilateral SNc neurons (A&B) which contained brown neuromelanin granules. In SNr, anterogradely labelled tectonigral boutons displayed numerous close associations (C) with retrogradely labelled nigrotectal cells, in which dark brown reaction product extended into the dendrites. The labelled axons in E, D, G & H were produced by the PhaL injections shown in F. D is from the contralateral SNc. E shows a PhaL labelled axon in SNr (arrow) that lay parallel to and just above the cerebral peduncle. A labelled axon in the VTA (G, arrow) skirts a fascicle of the oculomotor nerve and shows close associations with a neuron that has numerous neuromelanin granules. The two SNc neurons shown in H are unusual, in that they had numerous labelled boutons in close association with them. Number of z-axis planes: A=11; B=20; C=20; D=10; E=6; F=1; G=7; H=9.
Anterograde labelling with PhaL
Fast spiking GABAergic output neurons of SNr have local axon collaterals that terminate both within SNr and SNc (Grofova et al., 1982; Paladini et al., 1999; Mailly et al., 2003). Consequently, it was possible that the retrogradely transported BDA that labelled nigrotectal neurons in the cases described above might also have labelled their local axon collaterals within SN. The fact that the anterogradely labelled terminals generally appeared to contain denser label than the retrogradely labelled cells argues against this possibility. Nevertheless, to resolve any potential ambiguity associated with BDA tracing, a further 4 subjects received intracollicular injections of the anterograde tracer PhaL. While in some species, PhaL can be occasionally transported in the retrograde direction, this is rarely the case in primates. Combined immunohistochemical procedures were used to reveal the presence of PhaL labelled terminal arbors and TH+ neurons in the same section. Tissue was sectioned in either the coronal or parasagittal planes to further explore the trajectories of tectonigral fibers. In the illustrated example, the injection of PhaL was restricted to a narrow cylindrical area in the caudal SC, crossing all layers (Fig. 4, upper right), and the distribution of labelled fibers is shown in a medial to lateral series of parasagittal sections through the ipsilateral SN (Fig. 4A-F). Although the injection was small and labelled fewer axons than the BDA case illustrated in figure 1, the two tracers provided qualitatively similar results. Thus, most of the labelled axonal arbors were found in SNc (Fig. 4A-E), with the highest concentration in this subject located medially within the dorsal tier (Fig. 4A-C). In this case, only a few labelled axons were found in SNr (Fig. 4C&E), but PhaL labelling in SNr was more evident in other cases (Fig. 3E&F), even in the absence of retrogradely labelled nigrotectal neurons. As was the case for the BDA labelling, PhaL labelled axons were present in the reticular formation dorsal to SNc (Fig. 4A-B), as well as in the ipsilateral VTA (Fig. 3G) and subthalamic nucleus (not illustrated).
Figure 4.
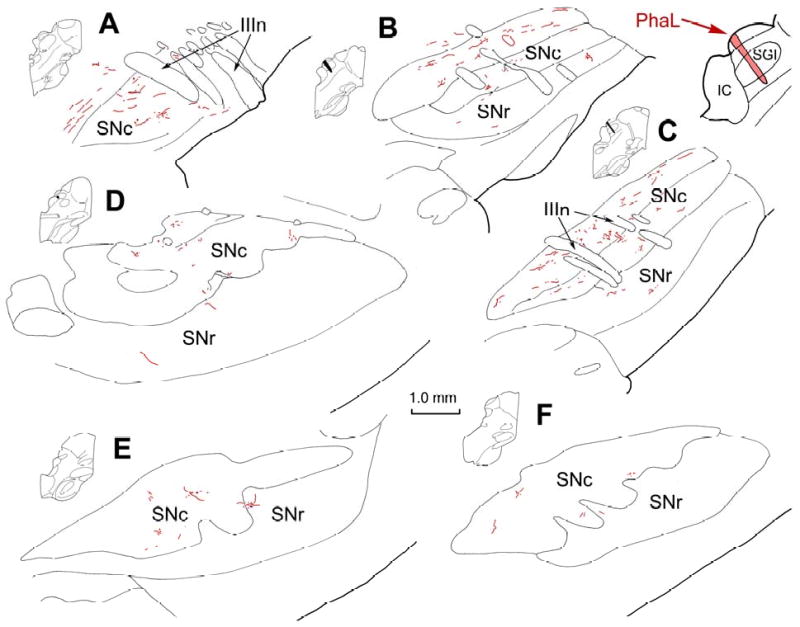
Distribution of labelled tectonigral axons in SN shown in the parasagittal plane. The injection of PhaL, which involved all layers of SC (insert, upper right), anterogradely labelled axons throughout the SN, as shown in a medial to lateral series of parasagittal sections (A-F). The illustrated levels are indicated by the small schematics to the left of each illustration. Labelled axons were present in both SNr and SNc, and were densest in the dorsal tier of SNc.
The terminal arbors labelled in the illustrated case were made up of fine axons that rarely branched, with the exception of short perpendicular extensions (Fig. 5). They were ornamented with many en passant and terminal boutons that formed close associations with TH+ neurons (Fig. 5A-D). The labelled axons could often be followed in the parasagittal plane for considerable distances through the ipsilateral SNc (Fig. 5C). Again, the typical pattern was for an individual axon to have a few boutons associate with each of several TH+ neurons encountered along its course (Fig. 5A-C). In contrast, the labelled axonal arbors seen in ipsilateral SNr were not associated with TH+ somata (Fig. 5E). The photomicrographs in figure 6 show further examples of presumptive contacts formed between anterogradely labelled tectonigral terminals and TH+ neurons in SNc (Fig. 6A,B,E & F) and a terminal arbor in SNr (Fig. 6D). In most cases, only a few boutons were associated with each SNc cell (Fig. 6E), while in others, a cluster of boutons lay adjacent to a TH+ neurons (Fig 6. A&B), or the axon could be followed along a TH+ dendrite (Fig. 6F). Occasionally, neurons with large numbers of associated boutons were present (Fig. 3H). In this case, numerous boutons sit along SNc cells, which were brown in color due to their neuromelanin content. Finally, it was observed that while PhaL labelling in SN was predominantly ipsilateral, a small number of labelled arbors with similar morphology were present contralaterally. These terminal arbors had a similar morphology to those present ipsilaterally, and were mainly distributed amongst neuromelanin containing cells in SNc (Fig. 3D).
Figure 5.
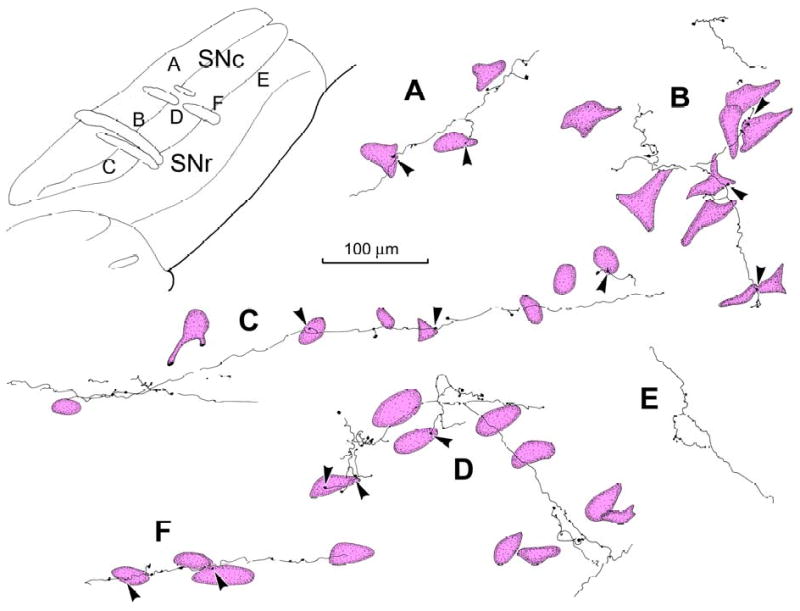
Morphology of PhaL labeled tectonigral terminal arbors and their relationship to TH+, presumed DA, neurons. Individual arbors labeled from the injection illustrated in figure 4 are shown. PhaL labelled tectonigral arbors were distributed among TH+ neurons in SNc (A-D&F), and displayed boutons with close associations (arrowheads) to some cells. They were also found in pars reticulata (E). The location of each illustrated example is shown in the schematic of a parasagittal section (upper right).
Figure 6.
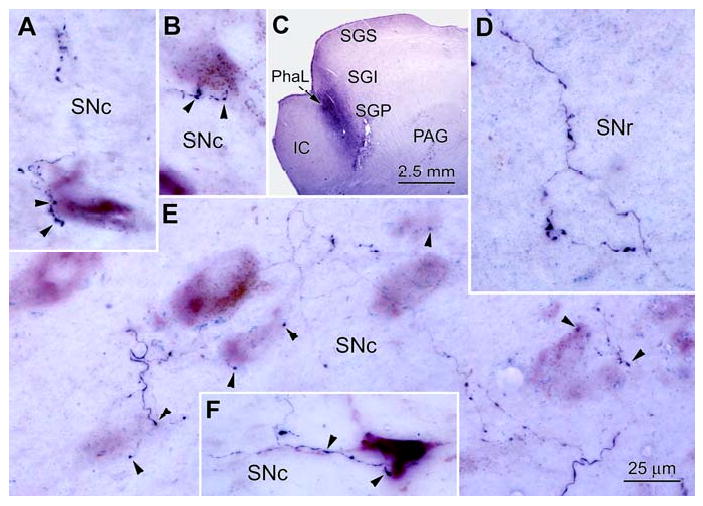
Phal labelled terminal arbors in the SN. PhaL labeled tectonigral axons (black) from the injections shown in Fig. 4 and in C are illustrated in D&E and in A,B&F, respectively. They displayed numerous boutons in SNc (A,B,E&F) and SNr (D). The formert had close associations (arrowheads) with VIP stained (purple) TH+ cells in SNc (A,B,E&F). The morphology of the TH+ cells is accentuated by the presence of brown neuromelanin granules in the cytoplasm. Number of z-axis planes: A=3; B=10; C=1; D=9; E=8; F=11.
In summary, following the use of either of two anatomical tracers, our anterograde tracing data revealed the presence of a direct tectonigral projection in the primate. The tectonigral terminal boutons targeted TH+, presumably DA cell bodies in SNc, ending more densely in the dorsal tier. They also targeted the SNr neuropil, and perhaps even nigrotectal neurons. The next stage of the investigation was to confirm the tectonigral projection using retrograde tracing techniques, and to provide a regional description of the cells of origin of the tectonigral pathway in the primate.
Retrograde Labelling of Tectonigral Cells
Injection sites
Tectonigral cells in the monkey SC were identified within 5 cases in which there were 9 injections of WGA-HRP into the ventral midbrain (Fig. 7). The photomicrograph in figure 7A shows an example of one injection site (Case 91L) where the uptake zone of the tracer was judged to be contained entirely within the SN. The maximum extents of the 9 injections were plotted in relation to the location of ventral midbrain DA neurons defined in adjacent sections (Fig. 7B). Each injection was classified as being either restricted to the SN or the SN and VTA (light grey shading), or predominantly involving these regions, but including dorsal spread into adjacent tissue (dark grey shading).
Figure 7.
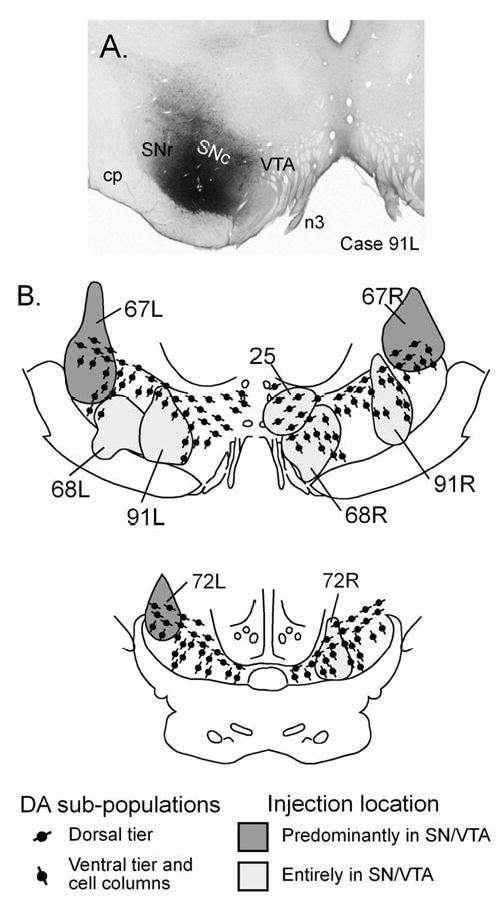
WGA-HRP injection sites in the SN and VTA. An example of an injection confined to the SN is shown in A. B&C are rostral and caudal frontal sections displaying the widest extent of each of the injection sites used in this study. The light gray injection sites were confined to the SN and VTA, while dark gray injection sites extended into the overlying midbrain reticular formation.
Retrogradely labelled tectonigral neurons
In all 5 cases, an overwhelming majority of WGA-HRP labelled neurons (93.6 ± 0.95 %) were located in the intermediate and deep layers of the SC (Fig. 8A). Morphologically, they represent a restricted subset of the different cell types previously described in the primate SC (Ma et al., 1990). The 4 most common classes of retrogradely labelled cells were small neurons that were ovoid, bipolar, or pyramidal in shape, and medium-sized multipolar neurons. All these types were distributed in the intermediate gray and white, and deep gray layers of the SC (SGI, SAI and SGP, respectively). Small ovoid neurons (Fig. 8B) made up about half the labelled cells, and represented the largest class of tectonigral neurons. They had small somata (long axis ∼ 10-20 μm) from which several processes extended abruptly. These ovoid neurons appeared throughout the mediolateral and rostrocaudal extent of the SC. Small bipolar neurons (Fig. 8B) made up approximately 15-20% of the labelled cells. They had elongated cell bodies (long axis ∼ 25-35 μm), and two tapering dendritic processes. The long axes of these cells were more commonly aligned perpendicular to the collicular surface, than parallel to it. These bipolar neurons displayed a tendency for a more lateral distribution. Small pyramidal neurons (Fig. 8C) represented a further 15-20% of labelled cells. They had triangular shaped somata (long axis ∼ 20-35 μm) with dentritic processes emerging from each point. Medium-sized, multipolar neurons (Fig. 8D) represented the remaining significant class of retrogradely labelled tectonigral neurons (∼10-15%). They were a heterogeneous class, whose somata were larger (long axis ∼ 30-50 μm) than those of the other tectonigral populations. Typically, their somata tapered into several dendrites giving them a piriform or stellate appearance. Notably absent from the cell types labelled with WGA-HRP were the classes of large multipolar output neurons (Norita, 1980; Moschovakis et al., 1988; Ma et al., 1990).
Figure 8.
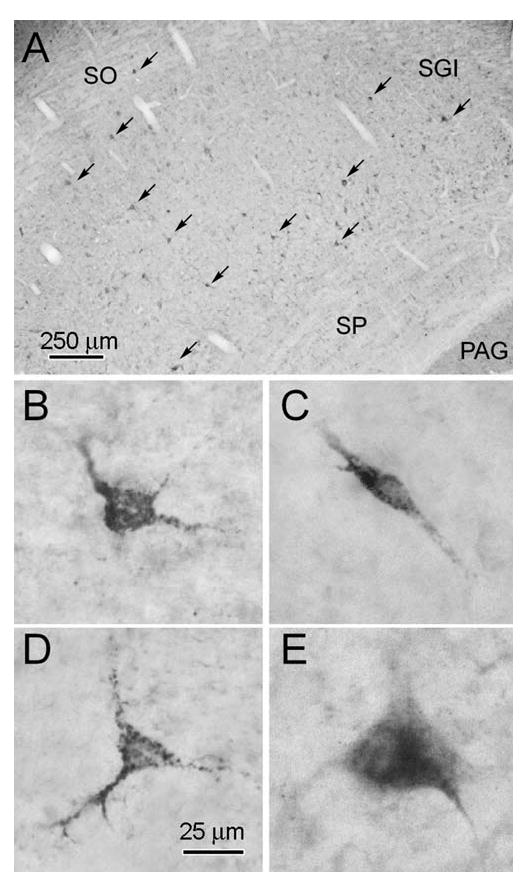
Distribution and morphology of retrogradely labelled tectonigral neurons. The labelled neurons were mainly found in the layers beneath stratum opticum (A). They fell into four morphological classes: small ovoid (B), small fusiform (C), small pyramidal (D) and medium-sized multipolar (E).
Tectonigral projection laterality
Case 25 received a small injection of WGA-HRP located in VTA and the adjacent dorsal tier of medial pars compacta (Fig. 9A). Since this was the only example that received a unilateral injection, our retrograde evidence for the laterality of the projection rests on this case. Nevertheless, in agreement with the anterograde data above, an ipsilateral predominance in the tectonigral projection was clear, as 82% of the retrogradely labelled cells were found in the ipsilateral SC. The distribution of the contralateral labelling in this case represented a weak mirror image of that seen ipsilaterally, with an overwhelming majority of cells on both sides (97 and 96% respectively) confined to the intermediate and deep layers. Assuming comparability between medial and lateral injection sites, these findings suggest that a small portion of the labelled cells observed in the bilateral injection cases described below represents crossed projections.
Figure 9.
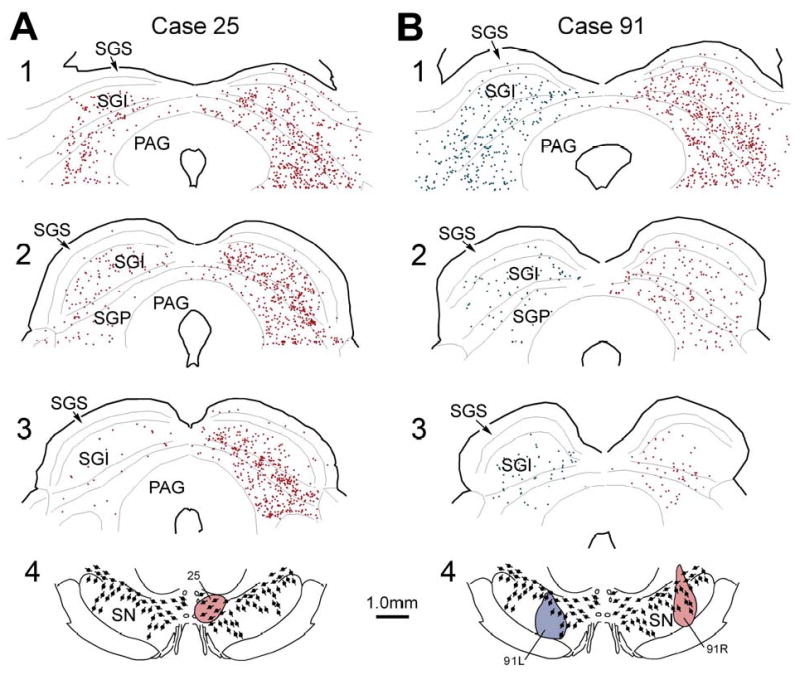
The distribution of retrogradely labelled tectonigral neurons in the SC. A illustrates the location of ipsilateral and contralateral labelled neurons in a rostrocaudal series of frontal sections (A1-3) through the SC following a WGA-HRP injection confined to the VTA and dorsal tier of the SNc (A4). B illustrates the distribution pattern (B1-3) in an animal that received bilateral injections of SN (B4). Cells from 3 adjacent sections were plotted onto each level shown.
Regional distribution of tectonigral cells
In all cases, the overwhelming proportion of retrogradely labelled tectonigral neurons were confined to the intermediate and deep layers of the SC, as depicted in cases 25 and 91 (Fig. 9A and B, respectively). However, in one case (72L, Fig. 7B), an additional population of labelled neurons was found in the ipsilateral superficial layers. A possible reason for this was that the dorsolaterally placed injection site encroached into the lateral accessory nucleus of the optic tract. In 8/9 cases (Case 25 - Fig. 9A – being the exception) the density of retrograde labelling in rostral sections of the SC was clearly stronger than that observed in the caudal ones (see Case 91 – Fig. 9B as a typical example). Across individual layers in the deep SC, and in the mediolateral dimension, the distribution of retrogradely labelled cells in all 9 cases was relatively even (Fig. 9A&B).
Preferential targeting of the dorsal tier
Given that all 9 injection sites had roughly similar dimensions (Fig. 7B), variability in the total numbers of retrogradely labelled cells detected in different cases, as measured in 3 representative sections, may be attributed to the location of an injection site rather than to its size or shape. The dorsomedial injection in Case 25 was the only example in which the injected tracer was largely confined to dorsal tier DA neurons. It is therefore noteworthy that of all the injections confined to SN, it was associated with the greatest number of labelled ipsilateral tectonigral neurons (a total of 1,687 cells). A second case with a more laterally placed injection site that extended from SNr dorsally into an area containing dorsal tier DA neurons also produced large numbers of retrogradely labelled cells in the SC (837 cells, Case 91R - Fig. 9). In contrast, similarly sized injections directed more ventrally, into areas containing concentrations of ventral tier DA neurons and/or the DA cell columns in SNr, were associated with fewer retrogradely labelled neurons in the ipsilateral SC (Case 91L, 395 cells - Fig. 9; and Case 68R, 516 cells, not illustrated). An injection largely confined to SNr was also associated with retrograde labelling in the SC, albeit at similarly reduced levels (Case 68L, 407 cells not illustrated).
Taken together these results indicate, first, that injections of retrograde tracer entirely confined to substantia nigra were associated with significant levels of retrograde labelling of tectonigral neurons. Secondly, the density of retrograde labelling in the SC was greater for injection sites involving dorsal tier DA neurons, in agreement with the anterograde data. However, the anterograde tracers also revealed that many of the afferent fibres innervating more ventral regions of substantia nigra pass through the dorsal tier. Thus, it is possible that the retrogradely labelled population from more dorsally located injections could include cells whose fibers were labelled on their way to more ventral targets. Thirdly, in all cases, retrogradely labelled neurons were mainly small to medium sized and located in the intermediate and deep layers of the SC. Finally, in a majority of cases, rostral sections of the SC were more heavily labelled.
Discussion
This is the first study to demonstrate a direct efferent projection from the superior colliculus to the substantia nigra in primates. The principal findings were: (i) Anterograde tracer injections into the SC labelled fibers and terminals in SNc, and to a lesser extent in SNr and VTA. (ii) Labelled terminal boutons were observed in close association with TH+ neuronal elements, particularly within the dorsal tier of DA neurons. (iii) WGA-HRP injections confined to SN retrogradely labelled 4 distinct classes of cells in the intermediate and deep layers of the SC. The relationship to these findings to those of previous investigations and their functional implications will be discussed below.
Cells of origin of the tectonigral pathway
In both cat (McHaffie et al., 2006) and monkey (Fig. 8), the overwhelming majority of tectonigral neurons belong to a few restricted subcategories of the cells present in the SC (Ma et al., 1990); those with small ovoid, bipolar or pyramidal somata, or medium-sized multipolar somata. The small number of larger neurons observed in the cat (McHaffie et al., 2006) were absent in the monkey. This may reflect a genuine species difference, or that nearby tissue targeted by the ipsilateral descending projections was involved by the cat nigral injections (McHaffie et al., 2006). The diversity of labelled neurons in both species suggests the tectonigral projection provides a heterogeneous input to SN, since the different morphological classes are likely to exhibit different patterns of activity in response to stimuli. Such differences could explain why DA neurons might respond differently to various categories of visual event; e.g., appearance, disappearance, movement (Wurtz & Albano, 1980), and looming (Westby et al., 1990).
It is noteworthy that few tectonigral neurons were found in the exclusively visual, superficial layers of the SC (May, 2006). Moreover, the tectonigral population did not include the large tectoreticulospinal output neurons (Moschovakis et al., 1988; May & Porter, 1992). The former finding suggests that tectonigral cells are not exclusively visual, but are instead probably multisensory neurons tuned to the novelty and saliency presets present in the SC (Stein & Meredith, 1993; Stein & Stanford, 2008). The absence of large, retrogradely labelled, output cells is consistent with the general failure to find motor-related activity in DA neurons (Schultz, 1998), including that associated with shifts of gaze (Wurtz & Albano, 1980; Sparks, 1986). Instead, these cells may include the 40% of SGI units that increase the gain of their activity for reward-related responses, but which do not show motor-related activity (Ikeda & Hikosaka, 2003).
Projections to pars reticulata, a possible feedback pathway
The present study provides evidence for a direct tectal projection to SNr. Specifically, terminals in SNr were labelled with either anterograde tracer, and tectal cells were labelled after injections centered in SNr (Case 68L). Due to deliberately weak TH staining, we were unable to determine whether PhaL labelled terminals in pars reticulata made contact with the dendrites of ventral tier SNc neurons. Thus, it is possible that tectal input to SNr may target both DA and GABAergic elements. Supporting this view are the ultrastructural findings in rat that show tectonigral terminals contacting both DA and non-DA profiles (Comoli et al., 2003). While ultrastructural verification is necessary to prove synaptic contacts, the presence of tectonigral boutons in close association with nigrotectal output neurons (Fig. 3C) raises the possibility that the SC modulates the afferent signals it receives from the basal ganglia. It is well established that the nigrotectal projection provides a pathway whereby the basal ganglia influence the gaze-related activity of the SC (Graybiel, 1978; Chevalier et al., 1981; Hikosaka & Wurtz, 1983; Huerta et al., 1991). Furthermore, this pathway is known to target the cells projecting to brainstem gaze centers via the predorsal bundle (May & Hall, 1984; Bickford & Hall, 1992; Jiang et al., 2003). It is therefore possible that tectonigral input to SNr could modulate the disinhibitory output signals from the basal ganglia that gate SC saccade-related outputs (Chevalier & Deniau, 1990; Hikosaka et al., 2000).
Differential projections to dopaminergic neurons
The demonstration of a tectonigral projection in a primate suggests it is a conserved mammalian feature, and therefore likely also to be present in humans. Furthermore, the results of the anterograde and retrograde tracing experiments suggest that the dorsal tier of DA neurons receives a denser tectonigral projection. It is possible that the level of innervation from the SC is related to the relative density of DA neurons in different sectors of substantia nigra. Thus, the greater number of terminals simply reflects a greater number of targets. Alternatively, it is possible that the dorsal tier of DA is preferentially, although not exclusively, targeted by afferent fibres from the SC. Similar targeting of dorsal tier neurons is a feature of inputs from limbic regions (Lynd-Balta & Haber, 1994; Haber et al., 1995; Fudge & Haber, 2000, 2001). The dorsal tier cells, along with VTA cells, in turn preferentially innervate ventral striatal, and cortical structures (Haber et al., 1995; Haber et al., 2000). Thus, the tectonigral input to these neurons could provide the means whereby biologically salient sensory stimuli might elicit phasic DA modulation in cortico-limbic territories, and through the nigro-striato-nigral system, sequentially influence the dorsal striatum (Belin & Everitt, 2008). This could be of importance for reinforcing cognitive and/or affective representations associated with the unpredicted occurrence of biologically salient sensory events. This possibility has already been included in a recent model (Gruber et al., 2006), in which it was hypothesized that dopamine acts to gate access to working memory in prefrontal corticostriatal loops.
Pre-attentive sensory input to DA neurons
In all species examined (rat, cat and macaque monkey), it is the SC, rather than cortical systems, that provides the most direct visual input to midbrain DA neurons (Comoli et al., 2003; McHaffie et al., 2006). Consequently, subcortical visual processing is the most likely, if not the exclusive, source of the short latency visual drive for phasic DA signalling (Comoli et al., 2003; Dommett et al., 2005). However, as the brain's ‘sentinel’, the SC is activated by biologically salient stimuli irrespective of valence, i.e., it is sensitive both to sensory stimuli that elicit defensive, avoidance responses, as well as stimuli that evoke orienting and appetitive approach responses (Dean et al., 1989). Furthermore, direct stimulation of the SC can elicit either defense- or orienting-like movements (Sahibzada et al., 1986; Sparks, 1986; Cole et al., 2006). In contrast, midbrain DA neurons are sensitive at least to a crude form of valence. They respond at pre-attentive latencies to novel or reward-related stimuli with a positive burst of activity (Horvitz et al., 1997; Schultz, 1998) and to noxious (Ungless et al., 2004; Coizet et al., 2006) or detrimental stimuli (Bayer et al., 2007; Matsumoto & Hikosaka, 2007) with a pause or negative response. An important unresolved question is that of how SC representations of potentially appetitive and aversive stimuli could elicit the appropriate positive/negative response in DA neurons. A possible answer could lie in the discovery of multiple tectonigral cell-types in cat (McHaffie et al., 2006) and monkey (present study), which correlates with the fact that tectonigral terminals form both asymmetric (presumed excitatory) and symmetric (presumed inhibitory) synapses onto both TH+ and TH− neuronal elements in rat SN (Comoli et al., 2003). Thus, the SC could provide both direct and (via SNr) indirect inputs for excitatory and/or inhibitory influences over ventral midbrain DA neurons. Signals relayed via the tectonigral projection may not, however, provide a complete explanation of the bi-directional phasic responses of DA neurons, as the lateral habenula also appears to be involved in phasic suppression of DA neurons (Matsumoto & Hikosaka, 2007).
Role of tectonigral input in reinforcement learning
Here we have demonstrated a tectonigral pathway which appears to terminate in a distributed fashion upon TH+ neurons in SNc. It is likely that this pathway is responsible for the short latency (<100 ms), phasic visual signals expressed by DA neurons, as visual activity is present in the intermediate layer of the superior colliculus at latencies of less than 100 ms (Wurtz & Albano, 1980; Jay & Sparks, 1987). Other prospective sources of visual input where the identity of a visual stimuli are ascertained, such as the P-pathways through the geniculostriate visual system to infra-temporal cortex, could provide such information, but these sources have latencies for activity that are too long to explain the short latency DA activity (Thorpe & Fabre-Thorpe, 2001). This situation would be compounded by the fact foveating eye movements are often necessary for identification of targets. Furthermore, direct contacts between infra-temporal cortex and the ventral midbrain are absent, so appropriate connections would require further relays through pre-frontal cortex (Sesack & Carr, 2002; Frankle et al., 2006) and/or the striatum (Middleton & Strick, 1996). Finally, it has been shown that visual cortex is not necessary for short latency visual activity (Dommett et al., 2005). Instead, the visual response in DA neurons is due to retinal input to SGS, as the effects of the tectonigral projection are dependent upon activity within this layer of the SC (Comoli et al., 2003). Presumably, this visual information is provided to tectonigral neurons in SGI via intralaminar pathways (Hall & Lee, 1997; Isa, 2002).
In this light, the characteristics of the tectonigral visual inputs are particularly relevant. While the physiology of this projection has not been specifically investigated, we do have a general understanding of collicular visual sensory processing (Wurtz & Albano, 1980; Sparks, 1986; Stein & Meredith, 1993; Stein & Stanford, 2008). Within the SC, visual sensory neurons tend to be insensitive to static features, color, and high spatial frequencies, but are exquisitely sensitive to the appearance/disappearance of stimuli and their movement (Wurtz & Albano, 1980). These visual responses often quickly habituate, but there is an enhancement of this activity when the stimulus is used as a target for a rewarded behaviour (Goldberg & Wurtz, 1972). In addition, these responses may be relayed more effectively from superficial to the deep layers of the colliculus when the stimulus is a rewarded target (Isa, 2002). The location of stimuli is encoded by the position of the activated population of collicular neurons within the topographical, retinotopic representation of space. However, this characteristic is probably not transmitted to SNc, as the tectonigral projection appears to be quite diffuse and non-topographic. Thus, if this signal contributes to reinforcement learning mechanisms within the SNc, it would seem to primarily convey information about the occurrence of an event, with relatively little information about its identity or location.
The present anatomical study cannot, in itself, prove or disprove any particular hypothesis of DA signalling, since there are many other sources of non-visual input to DA cells (Kitai et al., 1999; Grace et al., 2007). Nevertheless, the possibility that pre-attentive visual activation of DA neurons occurs via the tectonigral projection raises important questions concerning the role of these cells in reinforcement learning. For example, unlike many of the paradigms commonly used to demonstrate a tight relationship between dopamine signaling and mathematically defined reward prediction errors (Schultz, 2002; Fiorillo et al., 2003; Tobler et al., 2003; Tobler et al., 2005), in real world circumstances, sensory events can be unpredictable in kind, space and time. Under such circumstances, and provided the colliculus is the source of visual input, it is unlikely that the identity (and hence the economic value) of stimuli are known at the time the phasic DA signal (Redgrave & Gurney, 2006). Rather, stimulus identity and economic utility are more likely to be determined by sophisticated cortical perceptual systems after a foveating gaze shift (Thorpe & Fabre-Thorpe, 2001). It is probably safer, therefore, to consider phasic DA signals provided by the colliculus as ‘sensory prediction errors’, rather than ‘reward prediction errors’, as is currently posited (Montague et al., 2004; Schultz, 2006; D'Ardenne et al., 2008). We have argued (Redgrave et al., 2007) that crude valence information available to DA neurons at short latency in the form of sensory prediction errors would be appropriate to reinforce: (i) the discovery of agency; and (ii) the development of novel actions. In the former, the phasic tectonigral input to DA neurons would be used to reinforce the discrimination between externally- and self-generated events. In the latter, the phasic DA response may be used in the process that can identify the specific components in the behavioural output which caused the unpredicted outcome. In this light, instrumental conditioning may resolve into two processes that rely on different mechanisms of reinforcement: (i) discovery of agency and the development of novel actions, which are reinforced by phasic dopamine signals from the superior colliculus (Redgrave & Gurney, 2006); and (ii) reward-based Law of Effect learning, which adjusts the probability of selecting future actions to maximise economic value (Thorndike, 1911). The reinforcement signals for Law of Effect learning are currently unknown, but a good place to start looking would be in perceptual systems that perform detailed discriminations of economic value after foveation (Furuyashiki & Gallagher, 2007; Lee & Seo, 2007; Salzman et al., 2007; Padoa-Schioppa & Assad, 2008).
Acknowledgments
This work was supported by the following grants: EY014263 (PJM), NSF0130954 (PJM), NS35008 (JGM), EY12389 (TS), MH066171 and MH045573 (SNH) & the Wellcome Trust 068012 and 080943 (PR). The authors would also like to acknowledge the technical contributions of Jennifer Cotton, RN and Olga Golanov, MD.
Abbreviations
- BC
brachium conjunctivum
- BDA
biotinylated dextran amine
- DA
dopaminergic
- DAB
diaminobenzidine HCl
- HRP
horseradish peroxidase
- IC
inferior colliculus
- IIIn
third cranial nerve
- MRF
midbrain reticular formation
- NGS
normal goat serum
- PAG
periaqueductal gray
- PB
phosphate buffer
- PBS
phosphate buffered saline
- PBS-T
PBS plus Triton-X 100
- PhaL
Phaseolus vulgaris leucoagglutinin
- SC
superior colliculus
- SAI
intermediate white layer
- SGI
intermediate gray layer
- SGP
deep gray layer
- SGS
superficial gray layer
- SN
substantia nigra
- SNc
substantia nigra pars compacta
- SNr
substantia nigra pars reticulata
- SO
stratum opticum
- STn
subthlamic nucleus
- TH
tyrosine hydroxylase
- VTA
ventral tegmental area
- WGA-HRP
wheat germ agglutinin conjugated HRP
References
- Bayer HM, Glimcher PW. Midbrain dopamine neurons encode a quantitative reward prediction error signal. Neuron. 2005;47:129–141. doi: 10.1016/j.neuron.2005.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer HM, Lau B, Glimcher PW. Statistics of midbrain dopamine neuron spike trains in the awake primate. Journal Of Neurophysiology. 2007;98:1428–1439. doi: 10.1152/jn.01140.2006. [DOI] [PubMed] [Google Scholar]
- Belin D, Everitt BJ. Cocaine seeking habits depend upon doparnine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–441. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Bickford ME, Hall WC. The nigral projection to predorsal bundle cells in the superior colliculus of the rat. J Comp Neurol. 1992;319:11–33. doi: 10.1002/cne.903190105. [DOI] [PubMed] [Google Scholar]
- Chevalier G, Deniau JM. Disinhibition as a basic process in the expression of striatal functions. Trends Neurosci. 1990;13:277–281. doi: 10.1016/0166-2236(90)90109-n. [DOI] [PubMed] [Google Scholar]
- Chevalier G, Deniau JM, Thierry AM, Féger J. The nigrotectal pathway. An electrophysiological reinvestigation in the rat. Brain Res. 1981;213:253–263. doi: 10.1016/0006-8993(81)90232-8. [DOI] [PubMed] [Google Scholar]
- Coizet V, Dommett EJ, Redgrave P, Overton PG. Nociceptive responses of midbrain dopaminergic neurones are modulated by the superior colliculus in the rat. Neuroscience. 2006;139:1479–1493. doi: 10.1016/j.neuroscience.2006.01.030. [DOI] [PubMed] [Google Scholar]
- Cole CE, Gale JT, Gale K, Holmes AL, Malkova L, Zarbalian G. Soc Neurosci Abstr. Atlanta, GA: 2006. GABA receptor manipulation in the primate deep layers of superior colliculus: effects on emotional behaviour. Online: Programme number 815.11. [Google Scholar]
- Comoli E, Coizet V, Boyes J, Bolam JP, Canteras NS, Quirk RH, Overton PG, Redgrave P. A direct projection from superior colliculus to substantia nigra for detecting salient visual events. Nat Neurosci. 2003;6:974–980. doi: 10.1038/nn1113. [DOI] [PubMed] [Google Scholar]
- D'Ardenne K, McClure SM, Nystrom LE, Cohen JD. BOLD responses reflecting dopaminergic signals in the human ventral tegmental area. Science. 2008;319:1264–1267. doi: 10.1126/science.1150605. [DOI] [PubMed] [Google Scholar]
- Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain - I. Nigrosomes and the nigral matrix, a compartmental organization based on calbindin D-28K immunohistochemistry. Brain. 1999;122:1421–1436. doi: 10.1093/brain/122.8.1421. [DOI] [PubMed] [Google Scholar]
- Dayan P, Balleine BW. Reward, motivation, and reinforcement learning. Neuron. 2002;36:285–298. doi: 10.1016/s0896-6273(02)00963-7. [DOI] [PubMed] [Google Scholar]
- Dean P, Redgrave P, Westby GWM. Event or emergency? Two response systems in the mammalian superior colliculus. Trends Neurosci. 1989;12:137–147. doi: 10.1016/0166-2236(89)90052-0. [DOI] [PubMed] [Google Scholar]
- Dommett E, Coizet V, Blaha CD, Martindale J, Lefebvre V, Walton N, Mayhew JE, Overton PG, Redgrave P. How visual stimuli activate dopaminergic neurons at short latency. Science. 2005;307:1476–1479. doi: 10.1126/science.1107026. [DOI] [PubMed] [Google Scholar]
- Fiorillo CD, Tobler PN, Schultz W. Discrete coding of reward probability and uncertainty by dopamine neurons. Science. 2003;299:1898–1902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- Frankle WG, Laruelle M, Haber SN. Prefrontal cortical projections to the midbrain in primates: evidence for a sparse connection. Neuropsychopharmacology. 2006;31:1627–1636. doi: 10.1038/sj.npp.1300990. [DOI] [PubMed] [Google Scholar]
- Fudge JL, Haber SN. The central nucleus of the amygdala projection to dopamine subpopulations in primates. Neuroscience. 2000;97:479–494. doi: 10.1016/s0306-4522(00)00092-0. [DOI] [PubMed] [Google Scholar]
- Fudge JL, Haber SN. Bed nucleus of the stria terminalis and extended amygdale inputs to dopamine subpopulations in primates. Neuroscience. 2001;104:807–827. doi: 10.1016/s0306-4522(01)00112-9. [DOI] [PubMed] [Google Scholar]
- Furuyashiki T, Gallagher M. Neural encoding in the orbitofrontal cortex related to goal-directed behavior. Linking Affect To Action: Critical Contributions Of The Orbitofrontal Cortex. 2007:193–215. doi: 10.1196/annals.1401.037. [DOI] [PubMed] [Google Scholar]
- Goldberg ME, Wurtz RH. Activity of superior colliculus in behaving monkey. II. Effect of attention on neuronal responses. J Neurophysiol. 1972;35:560–574. doi: 10.1152/jn.1972.35.4.560. [DOI] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Organisation of the nigrotectal connection: an experimental tracer study in the cat. Brain Res. 1978;143:339–348. doi: 10.1016/0006-8993(78)90573-5. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Neural networks: Neural systems V: Basal ganglia. American Journal of Psychiatry. 2001;158:21. doi: 10.1176/appi.ajp.158.1.21. [DOI] [PubMed] [Google Scholar]
- Grofova I, Deniau JM, Kitai ST. Morphology of the substantia nigra pars reticulata projection neurons intracellularly labeled with HRP. J Comp Neurol. 1982;208:352–368. doi: 10.1002/cne.902080406. [DOI] [PubMed] [Google Scholar]
- Gruber AJ, Dayan P, Gutkin BS, Solla SA. Dopamine modulation in the basal ganglia locks the gate to working memory. Journal Of Computational Neuroscience. 2006;20:153–166. doi: 10.1007/s10827-005-5705-x. [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL. The primate substantia nigra and VTA: Integrative circuitry and function. Crit Rev Neurobiol. 1997;11:323–342. doi: 10.1615/critrevneurobiol.v11.i4.40. [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. Journal of Neuroscience. 2000;20:2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Ryoo H, Cox C, Lu W. Subsets of midbrain dopaminergic neurons in monkeys are distinguished by different levels of mRNA for the dopamine transporter: comparison with the mRNA for the D2 receptor, tyrosine hydroxylase and calbindin immunoreactivity. J Comp Neurol. 1995;362:400–410. doi: 10.1002/cne.903620308. [DOI] [PubMed] [Google Scholar]
- Hall WC, Lee P. Interlaminar connections of the superior colliculus in the tree shrew. 3. The optic layer. Visual Neuroscience. 1997;14:647–661. doi: 10.1017/s095252380001261x. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Takikawa Y, Kawagoe R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol Rev. 2000;80:953–978. doi: 10.1152/physrev.2000.80.3.953. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Visual and oculomotor function of monkey substantia nigra pars reticulata. IV. Relation of substantia nigra to superior colliculus. J Neurophysiol. 1983;49:1285–1301. doi: 10.1152/jn.1983.49.5.1285. [DOI] [PubMed] [Google Scholar]
- Horvitz JC. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience. 2000;96:651–656. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- Horvitz JC, Stewart T, Jacobs BL. Burst activity of ventral tegmental dopamine neurons is elicited by sensory stimuli in the awake cat. Brain Res. 1997;759:251–258. doi: 10.1016/s0006-8993(97)00265-5. [DOI] [PubMed] [Google Scholar]
- Huerta MF, Van Lieshout DP, Harting JK. Nigrotectal projections in the primate Galago-Crassicaudatus. Exp Brain Res. 1991;87:389–401. doi: 10.1007/BF00231856. [DOI] [PubMed] [Google Scholar]
- Ikeda T, Hikosaka O. Reward-dependent gain and bias of visual responses in primate superior colliculus. Neuron. 2003;39:693–700. doi: 10.1016/s0896-6273(03)00464-1. [DOI] [PubMed] [Google Scholar]
- Isa T. Intrinsic processing in the mammalian superior colliculus. Current Opinion in Neurobiology. 2002;12:668–677. doi: 10.1016/s0959-4388(02)00387-2. [DOI] [PubMed] [Google Scholar]
- Jay MF, Sparks DL. Sensorimotor integration in the primate superior colliculus. I. Motor convergence. J Neurophysiol. 1987;57:22–34. doi: 10.1152/jn.1987.57.1.22. [DOI] [PubMed] [Google Scholar]
- Jiang H, Stein BE, McHaffie JG. Opposing basal ganglia processes shape midbrain visuomotor activity bilaterally. Nature. 2003;423:982–986. doi: 10.1038/nature01698. [DOI] [PubMed] [Google Scholar]
- Kitai ST, Shepard PD, Callaway JC, Scroggs R. Afferent modulation of dopamine neuron firing patterns. Current Opinion in Neurobiology. 1999;9:690–697. doi: 10.1016/s0959-4388(99)00040-9. [DOI] [PubMed] [Google Scholar]
- Lee DY, Seo HJ. Mechanisms of reinforcement learning and decision making in the primate dorsolateral prefrontal cortex. Reward And Decision Making In Corticobasal Ganglia Networks. 2007:108–122. doi: 10.1196/annals.1390.007. [DOI] [PubMed] [Google Scholar]
- Lynd-Balta E, Haber SN. The organization of midbrain projections to the striatum in the primate. Neuroscience. 1994;59:609–623. doi: 10.1016/0306-4522(94)90181-3. [DOI] [PubMed] [Google Scholar]
- Ma PT, Cheng HW, Czech JA, Rafols JA. Intermediate and deep layers of the Macaque superior colliculus: a golgi study. J Comp Neurol. 1990;295:92–110. doi: 10.1002/cne.902950109. [DOI] [PubMed] [Google Scholar]
- Mailly P, Charpier S, Mentrey A, Deniau JM. Three-dimensional organization of the recurrent axon collateral network of the Substantia Nigra Pars Reticulata neurons in the rat. Journal of Neuroscience. 2003;23:5247–5257. doi: 10.1523/JNEUROSCI.23-12-05247.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- May PJ. The mammalian superior colliculus: laminar structure and connections. In: Buttner-Ennever JA, editor. Neuroanatomy of the Oculomotor System. 2006. pp. 321–378. [DOI] [PubMed] [Google Scholar]
- May PJ, Hall WC. Relationships between the nigrotectal pathway and the cells of origin of the predorsal bundle. J Comp Neurol. 1984;226:357–376. doi: 10.1002/cne.902260306. [DOI] [PubMed] [Google Scholar]
- May PJ, McHaffie JG, Jiang H, Stanford TR, Costello MG, Haber SN, Coizet V, Redgrave P. Soc Neurosci Abstr. Atlanta GA: 2006. Projections from the superior colliculus to stubstantia nigra pars compacta in a primate. Online: Programme number 450.2. [DOI] [PubMed] [Google Scholar]
- May PJ, Porter JD. The laminar distribution of macaque tectobulbar and tectospinal neurons. Visual Neurosci. 1992;8:257–276. doi: 10.1017/s0952523800002911. [DOI] [PubMed] [Google Scholar]
- McHaffie JG, Jiang H, May PJ, Coizet V, Overton PG, Stein BE, Redgrave P. A direct projection from superior colliculus to substantia nigra pars compacta in the cat. Neuroscience. 2006;138:221–234. doi: 10.1016/j.neuroscience.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. The temporal lobe is a target of output from the basal ganglia. Proc Natl Acad Sci USA. 1996;93:8683–8687. doi: 10.1073/pnas.93.16.8683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague PR, Hyman SE, Cohen JD. Computational roles for dopamine in behavioural control. Nature. 2004;431:760–767. doi: 10.1038/nature03015. [DOI] [PubMed] [Google Scholar]
- Morris G, Arkadir D, Nevet A, Vaadia E, Bergman H. Coincident but distinct messages of midbrain dopamine and striatal tonically active neurons. Neuron. 2004;43:133–143. doi: 10.1016/j.neuron.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Moschovakis AK, Karabelas AB, Highstein SM. Structure-function relationships in the primate superior colliculus: I Morphological classification of efferent neurons. J Neurophysiol. 1988;60:232–262. doi: 10.1152/jn.1988.60.1.232. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Guitton D. Presaccadic burst discharges of tecto-reticulo-spinal neurons in the alert head-free and -fixed cat. Brain Res. 1986;398:185–190. doi: 10.1016/0006-8993(86)91267-9. [DOI] [PubMed] [Google Scholar]
- Norita M. Neurons and synaptic patterns in the deep layers of the superior colliculus of the cat. A golgi and electron microscopic study. J Comp Neurol. 1980;190:29–48. doi: 10.1002/cne.901900104. [DOI] [PubMed] [Google Scholar]
- Padoa-Schioppa C, Assad JA. The representation of economic value in the orbitofrontal cortex is invariant for changes of menu. Nature Neuroscience. 2008;11:95–102. doi: 10.1038/nn2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paladini CA, Celada P, Tepper JM. Striatal, pallidal, and pars reticulata evoked inhibition of nigrostriatal dopaminergic neurons is mediated by GABA(A) receptors in vivo. Neuroscience. 1999;89:799–812. doi: 10.1016/s0306-4522(98)00355-8. [DOI] [PubMed] [Google Scholar]
- Pan WX, Hyland BI. Pedunculopontine tegmental nucleus controls conditioned responses of midbrain dopamine neurons in behaving rats. J Neurosci. 2005;25:4725–4732. doi: 10.1523/JNEUROSCI.0277-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilowsky P, Llewellyn-Smith IJ, Lipski J, Chalmers J. Substance P immunoreactive boutons form synapses with feline sympathetic preganglionic neurons. J Comp Neurol. 1992;320:121–135. doi: 10.1002/cne.903200109. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Gurney K. The short-latency dopamine signal: a role in discovering novel actions? Nat Rev Neurosci. 2006;7:967–975. doi: 10.1038/nrn2022. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Gurney K, Reynolds J. What is reinforced by phasic dopamine signals? Brain Res Rev. 2007 doi: 10.1016/j.brainresrev.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Prescott TJ, Gurney K. Is the short latency dopamine response too short to signal reward error? Trends Neurosci. 1999;22:146–151. doi: 10.1016/s0166-2236(98)01373-3. [DOI] [PubMed] [Google Scholar]
- Sahibzada N, Dean P, Redgrave P. Movements resembling orientation or avoidance elicited by electrical stimulation of the superior colliculus in rats. J Neurosci. 1986;6:723–733. doi: 10.1523/JNEUROSCI.06-03-00723.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai ST, Inase M, Tanji J. The relationship between MI and SMA afferents and cerebellar and pallidal efferents in the macaque monkey. Somatosens Mot Res. 2002;19:139–48. doi: 10.1080/08990220220131533. [DOI] [PubMed] [Google Scholar]
- Salzman CD, Paton JJ, Belova MA, Morrison SE. Flexible neural representations of value in the primate brain. Linking Affect To Action: Critical Contributions Of The Orbitofrontal Cortex. 2007:336–354. doi: 10.1196/annals.1401.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral theories and the neurophysiology of reward. Annu Rev Psychol. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Carr DB. Selective prefrontal cortex inputs to dopamine cells: implications for schizophrenia. Physiology & Behavior. 2002;77:513–517. doi: 10.1016/s0031-9384(02)00931-9. [DOI] [PubMed] [Google Scholar]
- Sparks DL. Translation of sensory signals into commands for control of saccadic eye movements: role of the primate superior colliculus. Physiol Rev. 1986;66:118–171. doi: 10.1152/physrev.1986.66.1.118. [DOI] [PubMed] [Google Scholar]
- Stein BE, Meredith MA. The merging of the senses. The MIT Press; Cambridge, MA: 1993. [Google Scholar]
- Stein BE, Stanford TR. Multisensory integration: current issues from the perspective of the single neuron. Nat Rev Neurosci. 2008;9:255–266. doi: 10.1038/nrn2331. [DOI] [PubMed] [Google Scholar]
- Takikawa Y, Kawagoe R, Hikosaka O. A possible role of midbrain dopamine neurons in short- and long-term adaptation of saccades to position-reward mapping. J Neurophysiol. 2004;92:2520–2529. doi: 10.1152/jn.00238.2004. [DOI] [PubMed] [Google Scholar]
- Thorndike EL. Animal intelligence. Macmillan; New York: 1911. [Google Scholar]
- Thorpe SJ, Fabre-Thorpe M. Seeking categories in the brain. Science. 2001;291:260–263. doi: 10.1126/science.1058249. [DOI] [PubMed] [Google Scholar]
- Tobler PN, Dickinson A, Schultz W. Coding of predicted reward omission by dopamine neurons in a conditioned inhibition paradigm. Journal of Neuroscience. 2003;23:10402–10410. doi: 10.1523/JNEUROSCI.23-32-10402.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler PN, Fiorillo CD, Schultz W. Adaptive coding of reward value by dopamine neurons. Science. 2005;307:1642–1645. doi: 10.1126/science.1105370. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Magill PJ, Bolam JP. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science. 2004;303:2040–2042. doi: 10.1126/science.1093360. [DOI] [PubMed] [Google Scholar]
- Westby GWM, Keay KA, Redgrave P, Dean P, Bannister M. Output pathways from the rat superior colliculus mediating approach and avoidance have different sensory properties. Exp Brain Res. 1990;81:626–638. doi: 10.1007/BF02423513. [DOI] [PubMed] [Google Scholar]
- Wurtz RH, Albano JE. Visual-motor function of the primate superior colliculus. Ann Rev Neurosci. 1980;3:189–226. doi: 10.1146/annurev.ne.03.030180.001201. [DOI] [PubMed] [Google Scholar]


