Abstract
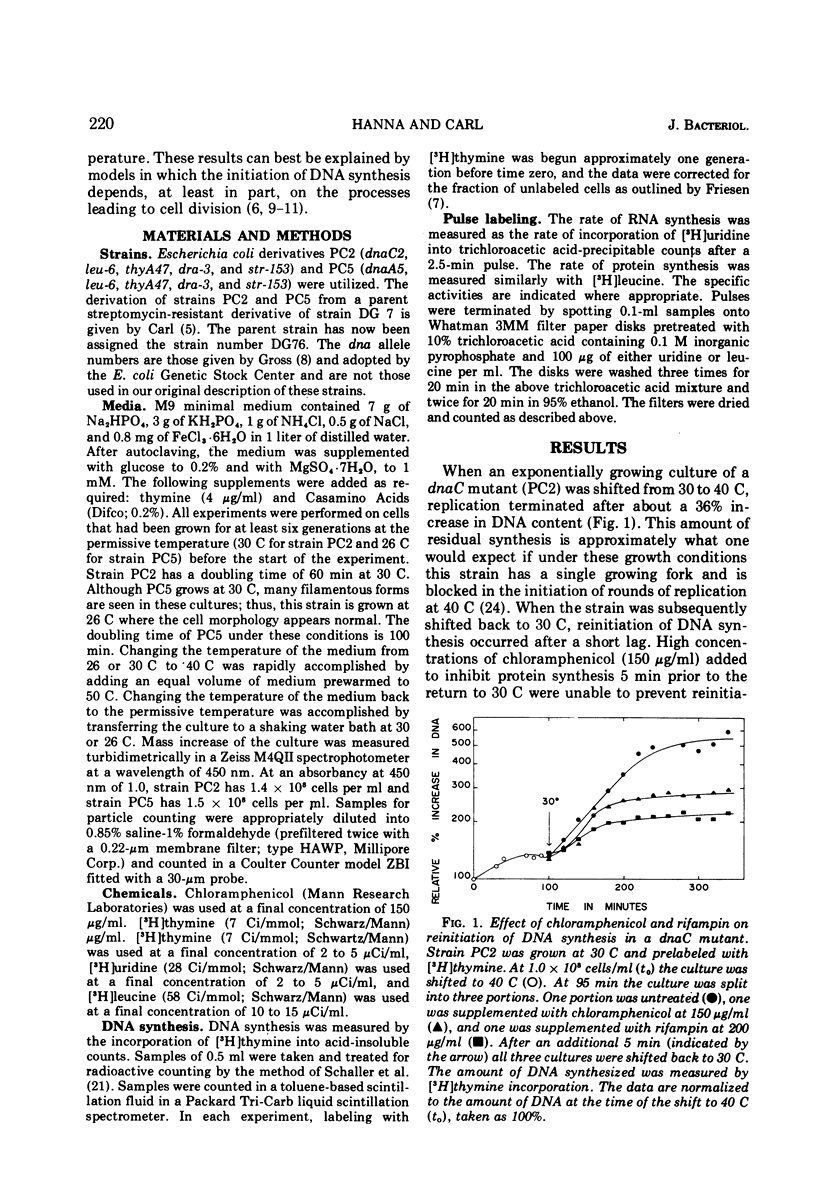
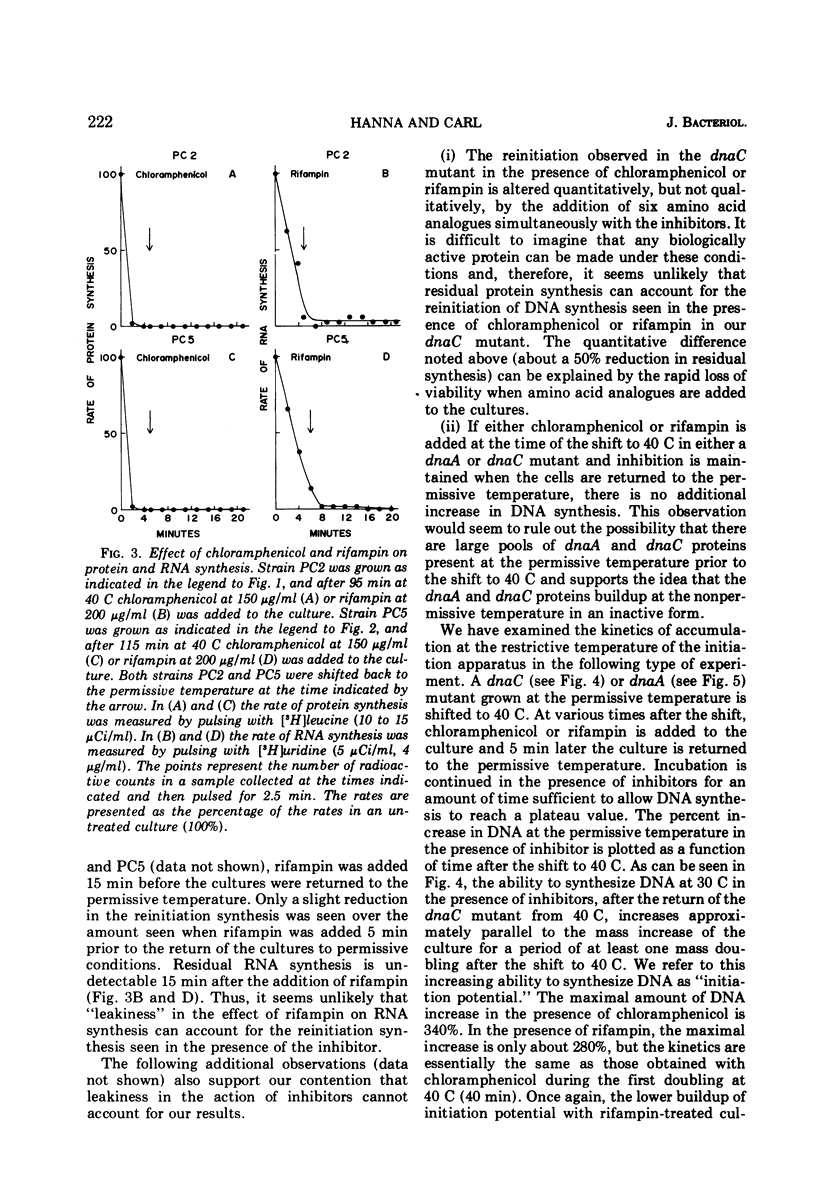
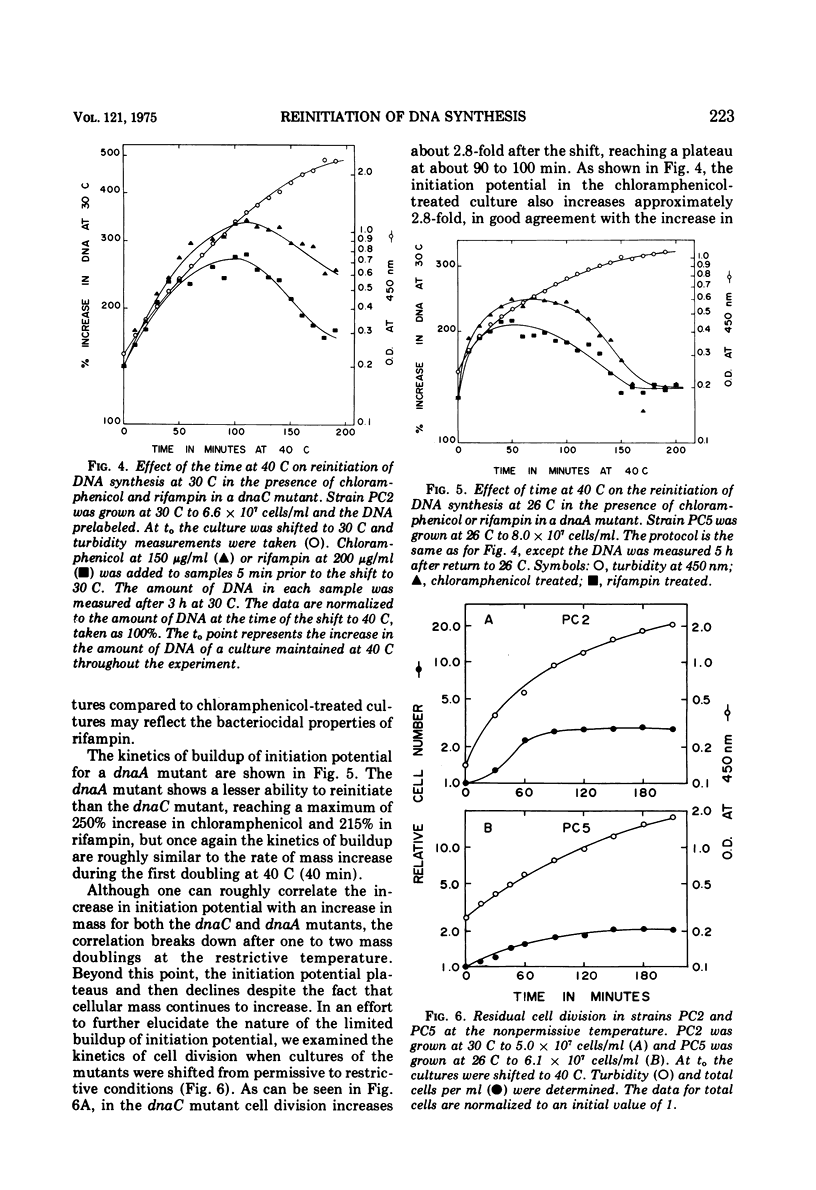
The dnaA and dnaC genes are thought to code for two proteins required for the initiation of chromosomal deoxyribonucleic acid replication in Escherichia coli. When a strain carrying a mutation in either of these genes is shifted from a permissive to a restrictive temperature, chromosome replication ceases after a period of residual synthesis. When the strains are reincubated at the permissive temperature, replication again resumes after a short lag. This reinitiation does not require either protein synthesis (as measured by resistance to chloramphenicol) or ribonucleic acid synthesis (as measured by resistance to rifampin). Thus, if there is a requirement for the synthesis of a specific ribonucleic acid to initiate deoxyribonucleic acid replication, this ribonucleic acid can be synthesized prior to the time of initiation and is relatively stable. Furthermore, the synthesis of this hypothetical ribonucleic acid does not require either the dnaA of dnaC gene products. The buildup at the restrictive temperature of the potential to reinitiate deoxyribonucleic acid synthesis at the permissive temperature shows rather complex kinetics the buildup roughly parallels the rate of mass increase of the culture for at least the first mass doubling at the restrictive temperature. At later times there appears to be a gradual loss of initiation potential despite a continued increase in mass. Under optimal conditions the increase in initiation potential can equal, but not exceed, the increase in cell division at the restrictive temperature. These results are most easily interpreted according to models that postulate a relationship between the initiation of deoxyribonucleic acid synthesis and the processes leading to cell division.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe M., Tomizawa J. Chromosome replication in Escherichia coli K12 mutant affected in the process of DNA initiation. Genetics. 1971 Sep;69(1):1–15. doi: 10.1093/genetics/69.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyersmann D., Schlicht M., Schuster H. Temperature-sensitive initiation of DNA replication in a mutant of Escherichia coli K12. Mol Gen Genet. 1971;111(2):145–158. doi: 10.1007/BF00267789. [DOI] [PubMed] [Google Scholar]
- Blair D. G., Sherratt D. J., Clewell D. B., Helinski D. R. Isolation of supercoiled colicinogenic factor E 1 DNA sensitive to ribonuclease and alkali. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2518–2522. doi: 10.1073/pnas.69.9.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brutlag D., Schekman R., Kornberg A. A possible role for RNA polymerase in the initiation of M13 DNA synthesis. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2826–2829. doi: 10.1073/pnas.68.11.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carl P. L. Escherichia coli mutants with temperature-sensitive synthesis of DNA. Mol Gen Genet. 1970;109(2):107–122. doi: 10.1007/BF00269647. [DOI] [PubMed] [Google Scholar]
- Gross J. D. DNA replication in bacteria. Curr Top Microbiol Immunol. 1972;57:39–74. doi: 10.1007/978-3-642-65297-4_2. [DOI] [PubMed] [Google Scholar]
- Gudas L. J., Pardee A. B. Deoxyribonucleic acid synthesis during the division cycle of Escherichia coli: a comparison of strains B-r, K-12, 15, and 15T- under conditions of slow growth. J Bacteriol. 1974 Mar;117(3):1216–1223. doi: 10.1128/jb.117.3.1216-1223.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstetter C. E. Initiation of chromosome replication in Escherichia coli. I. Requirements for RNA and protein synthesis at different growth rates. J Mol Biol. 1974 Mar 25;84(1):1–19. doi: 10.1016/0022-2836(74)90209-5. [DOI] [PubMed] [Google Scholar]
- Helmstetter C. E. Initiation of chromosome replication in Escherichia coli. II. Analysis of the control mechanism. J Mol Biol. 1974 Mar 25;84(1):21–36. doi: 10.1016/0022-2836(74)90210-1. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Mordoh J., Jacob F. On the process of cellular division in Escherichia coli. 3. Thermosensitive mutants of Escherichia coli altered in the process of DNA initiation. J Mol Biol. 1970 Nov 14;53(3):369–387. doi: 10.1016/0022-2836(70)90072-0. [DOI] [PubMed] [Google Scholar]
- Ikemura T., Dahlberg J. E. Small ribonucleic acids of Escherichia coli. I. Characterization by polyacrylamide gel electrophoresis and fingerprint analysis. J Biol Chem. 1973 Jul 25;248(14):5024–5032. [PubMed] [Google Scholar]
- Kuempel P. L. Temperature-sensitive initiation of chromosome replication in a mutant of Escherichia coli. J Bacteriol. 1969 Dec;100(3):1302–1310. doi: 10.1128/jb.100.3.1302-1310.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARK K. G., REPKO T., HOFFMAN E. J. THE EFFECT OF AMINO ACID DEPRIVATION ON SUBSEQUENT DEOXYRIBONUCLEIC ACID REPLICATION. Biochim Biophys Acta. 1963 Sep 17;76:9–24. [PubMed] [Google Scholar]
- Lark K. G. Evidence for the direct involvement of RNA in the initiation of DNA replication in Escherichia coli 15T. J Mol Biol. 1972 Feb 28;64(1):47–60. doi: 10.1016/0022-2836(72)90320-8. [DOI] [PubMed] [Google Scholar]
- Laurent S. J. Initiation of deoxyribonucleic acid replication in a temperature-sensitive mutant of B. subtilis: evidence for a transcriptional step. J Bacteriol. 1973 Oct;116(1):141–145. doi: 10.1128/jb.116.1.141-145.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAALOE O., HANAWALT P. C. Thymine deficiency and the normal DNA replication cycle. I. J Mol Biol. 1961 Apr;3:144–155. doi: 10.1016/s0022-2836(61)80041-7. [DOI] [PubMed] [Google Scholar]
- Messer W. Initiation of deoxyribonucleic acid replication in Escherichia coli B-r: chronology of events and transcriptional control of initiation. J Bacteriol. 1972 Oct;112(1):7–12. doi: 10.1128/jb.112.1.7-12.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pato M. L. Regulation of chromosome replication and the bacterial cell cycle. Annu Rev Microbiol. 1972;26:347–368. doi: 10.1146/annurev.mi.26.100172.002023. [DOI] [PubMed] [Google Scholar]
- Schaller H., Otto B., Nüsslein V., Huf J., Herrmann R., Bonhoeffer F. Deoxyribonucleic acid replication in vitro. J Mol Biol. 1972 Jan 28;63(2):183–200. doi: 10.1016/0022-2836(72)90369-5. [DOI] [PubMed] [Google Scholar]
- Schekman R., Wickner W., Westergaard O., Brutlag D., Geider K., Bertsch L. L., Kornberg A. Initiation of DNA synthesis: synthesis of phiX174 replicative form requires RNA synthesis resistant to rifampicin. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2691–2695. doi: 10.1073/pnas.69.9.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubach W. H., Whitmer J. D., Davern C. I. Genetic control of DNA initiation in Escherichia coli. J Mol Biol. 1973 Feb 25;74(2):205–221. doi: 10.1016/0022-2836(73)90107-1. [DOI] [PubMed] [Google Scholar]
- Sueoka N., Yoshikawa H. The chromosome of Bacillus subtilis. I. Theory of marker frequency analysis. Genetics. 1965 Oct;52(4):747–757. doi: 10.1093/genetics/52.4.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler J. A., Gross J. D. Escherichia coli mutants temperature-sensitive for DNA synthesis. Mol Gen Genet. 1971;113(3):273–284. doi: 10.1007/BF00339547. [DOI] [PubMed] [Google Scholar]
- Westergaard O., Brutlag D., Kornberg A. Initiation of deoxyribonucleic acid synthesis. IV. Incorporation of the ribonucleic acid primer into the phage replicative form. J Biol Chem. 1973 Feb 25;248(4):1361–1364. [PubMed] [Google Scholar]
- Wickner W., Brutlag D., Schekman R., Kornberg A. RNA synthesis initiates in vitro conversion of M13 DNA to its replicative form. Proc Natl Acad Sci U S A. 1972 Apr;69(4):965–969. doi: 10.1073/pnas.69.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams H., Boyer H. W., Helsinki D. R. Size and base composition of RNA in supercoiled plasmid DNA. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3744–3748. doi: 10.1073/pnas.70.12.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf B. The characteristics and genetic map location of a temperature sensitive DNA mutant of E. coli K12. Genetics. 1972 Dec;72(4):569–593. doi: 10.1093/genetics/72.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]


