Abstract
Activation of viral promoter transcription is a crucial event in the life cycle of several viruses. Hypoxia inducible factor-1 alpha (HIF-1α) is an inducible transcription factor whose activity is dependent on environmental conditions, most notably oxygen levels and cellular stress. HIF-1α has been implicated in the pathogenesis of several viruses, including HIV-1, HHV-8 and RSV. Under hypoxic conditions or oxidative stress, HIF-1α becomes stable and translocates to the nucleus, where it modulates gene transcription. The objective of the present study was to investigate a possible role for HIF-1α in the activation of JCV. Glial cell cultures infected with JCV demonstrated a significant increase in the levels of HIF-1α, in where it is located to the nucleus. Immunohistochemical studies corroborated upregulation of HIF-1α in JCV infected oligodendrocytes and astrocytes in clinical samples of PML compared with normal glial cells from the same samples in which HIF-1α expression is weak. CAT assays performed in co-transfected glial cells demonstrated activation of the JCV early promoter in the presence of HIF-1α. This activation was potentiated in the presence of Smad3 and Smad4. Finally, chromatin immunoprecipitation assays demonstrated the binding of HIF-1α to the JCV control region. These results suggest a role for HIF-1α in the activation of JCV; understanding of this pathway may lead to the development of more effective therapies for PML, thus far an incurable disease.
Keywords: Progressive Multifocal Leukoencephalopathy, JCV, HIF-α, ChIP assay, Smads
Introduction
Progressive Multifocal Leukoencephalopathy (PML) is a fatal demyelinating disease of the central nervous system (CNS) caused by the opportunistic human neurotropic polyomavirus, JCV. Once considered a rare disease, the incidence of PML dramatically increased after the human immunodeficiency virus-1 (HIV-1) pandemic, and is now considered an AIDS-defining condition. It is estimated that more than 4% of all HIV-1-infected patients will develop PML [4]. The histopathology of PML is characterized by the presence of extensive areas of demyelination in the subcortical white matter of the brain, with predominance for the frontal and parieto-occipital lobes [4]. Histological hallmarks of the disease include the presence of enlarged oligodendrocytes with intra-nuclear eosinophilic inclusions and giant bizarre transformed astrocytes within the demyelinated plaques. Multiple foamy macrophages, microglial nodules and perivascular cuffing of lymphocytes can also be observed, as in all types of viral encephalitis [10, 44].
JCV belongs to the family of polyomaviruses, along with SV40 and BK virus. It is a non-enveloped, small (38–40 nm), circular double-stranded DNA (5,130 bp) virus with an icosahedral capsid that shows a predilection for infecting glial cells and is suspected to remain latent in peripheral B-lymphocytes, stromal cells of the tonsils [34, 56] and renal tubular cells [54]. A recent study also shows the presence of viral genomic sequences in normal astrocytes and oligodendrocytes [37]. The JCV genome can be divided into three regions: (1) an early region that encodes the early proteins, large T-Antigen and small t-antigen; (2) a late region, that contains the coding sequences of the capsid proteins, VP1, VP2 and VP3, and the accessory Agnoprotein; and (3) a non-coding regulatory region (control region, CR) where the origin of replication and binding sites for several transcription factors are located [26, 43]. Upon entrance into the cell and viral DNA migration into the nucleus, cellular factors initiate transcription of the JCV early region resulting in expression of T-Antigen. The latter not only facilitates viral DNA replication, but also initiates transcription of the late region resulting in the production of VP1, VP2 and VP3, and Agnoprotein. Finally, virion assembly occurs with the release of newly formed infectious viral particles and cell death [26].
Several cellular pathways can be disrupted by JCV to complete its viral productive infection, including those related to cell cycle regulation and DNA damage repair. In JCV-infected glial cells, cyclins A, E, B1 [41], the DNA repair protein Rad51 [8], and the anti-apoptotic protein Survivin [40] have been shown to be up-regulated and may play a crucial role in the pathogenesis of PML. In addition, results from micro-RNA array analysis suggest that proteins of well-known cellular pathways can be used by JCV as co-factors for viral transcription [41]. For example, Smad proteins, the downstream signal modulators of the transforming growth factor-β (TGF-β) pathway, have been shown to play a role in the activation of the early and late promoters of JCV (JCV-E and JCV-L, respectively) in JCV infected cells and in cases of PML, either alone or in cooperation with the HIV-1 transactivator protein, Tat [13]. These mechanisms may explain, at least in part, why PML is significantly more frequent in patients with AIDS than in any other immuno-suppressive condition.
On the other hand, hypoxia and oxidative stress are important biological events that negatively affect cells in many different aspects including DNA repair, mitochondrial function, necrosis and apoptosis among others. In order to counteract these hazardous stimuli, cells possess different subsets of mechanisms to protect themselves [60]. Under hypoxic conditions, cells specifically respond through a finely regulated mechanism mediated by the hypoxia inducible factor-1 (HIF-1) [21, 51]. HIF-1 is a heterodimeric protein composed of an inducible hypoxia-regulated HIF-1α subunit, and a constitutively expressed HIF-1β subunit (aryl hydrocarbon receptor nuclear translocator, ARNT). Both molecules contain basic helix–loop– helix (bHLH) and PAS domains, which are crucial for the formation of the HIF-1α/HIF-1β heterodimer and for DNA binding, respectively [23, 58]. Under non-hypoxic conditions, HIF-1α is generally detected at low levels in the cytoplasm due to its constant degradation mediated through the ubiquitine/proteasome complex [22, 31]. However, under hypoxic conditions, ubiquitination and degradation of HIF-1α are inhibited, resulting in stable levels of the protein in the cytoplasm, which later translocates into the nucleus [21]. It is in this site where HIF-α dimerizes with HIF-1β, to become an active molecule, and in cooperation with other cofactors (CBP/p300, Sp1) [2, 12, 33] binds to specific DNA sequences, known as HIF-responsive elements (core sequence, 5′-RCGTG-3′), found in several target genes (i.e. erythropoietin, glycolytic enzymes, vascular endothelial growth factor, etc.) to initiate their transcription [50]. These genes are in control of either to prepare the cell to rapidly counteract hypoxia (by initiating glucose uptake, switching metabolism to anaerobic glycolysis, activating chaperones, etc.), or to adapt to chronic hypoxic conditions (through angiogenesis and erythrocytosis). However, if the damage is so severe that even these mechanisms are exceeded, cell survival and apoptotic pathways can also be activated through HIF-α or different mechanisms [1, 17, 29, 46, 52]. In response to hypoxia, other signaling pathways may be activated by HIF-1, for example, the TGF-β pathway downstream effectors, Smad2, Smad3 and Smad4 can cooperate with HIF-1α to increase VEGF expression or regulate erythropoietin levels in order to restore oxygen levels [48, 49, 64].
Interestingly, HIF-1α can also be modulated through hypoxia-independent mechanisms. In addition, several reports have identified HIF-1α as a transcriptional regulator during viral infection and inflammation; most notably HIF-1α has been associated with the Respiratory Syncytial Virus (RSV) [19] and to the activation of the Epstein–Barr virus lytic cycle [24]. Since JCV is capable of promoting cellular stress and several transcription factors have been implicated in the activation of the JCV promoter, in the present study, we aimed to determine if HIF-1α plays a role on JCV activation and in the pathogenesis of PML. To our knowledge, this is the first description of a link between the main regulatory factor of hypoxia, HIF-1α, its interaction with the transcriptional machinery of JCV and the development of PML.
Materials and methods
Clinical samples
A total of 10 autopsy samples of PML were collected from the archives of the Pathology Institute, University of Laussane, Switzerland (4 cases), and from the Manhattan Brain Bank (R24MH59724) at Mount Sinai Medical Center, New York (6 cases). Eight samples were from HIV-1 infected patients and two cases were of non-AIDS-related PML. Normal brain sections from two patients who died of non-neurologic conditions were used as controls. All tissues were obtained under approval from Institutional Review Board (IRB). Table 1 describes the age, gender and associated condition of the PML cases.
Table 1.
Immunohistochemistry for HIF-1α in clinical samples of PML
| No. | Age (years) |
Gender | Diagnosis | Associated | Immunohistochemistry |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Oligodendrocytes |
Astrocytes |
|||||||||
| VP-1 | Agno | HIF-1α | VP-1 | Agno | HIF-1α | |||||
| 1 | 73 | Female | Non-AIDS-PML | Renal Transplant | ++n | ++cy | +++n | ++n/+cy | ++cy | +++n/cy |
| 2 | 46 | Male | Non-AIDS-PML | Lymphocytic Leukemia | +++n | +cy | +++n | ++n | +cy | ++n/cy |
| 3 | 31 | Female | AIDS-PML | AIDS | +++n/++cy | +++cy | ++n | ++n | ++cy | ++cy |
| 4 | 36 | Male | AIDS-PML | AIDS | +++n | ++cy | ++++n | ++n/+cy | ++cy | +cy |
| 5 | 37 | Male | AIDS-PML | AIDS | +++n | +++cy | +++n | +++n | ++cy | ++n/cy |
| 6 | 48 | Female | AIDS-PML | AIDS | +++n | ++cy | +n | ++n/+cy | +++cy | ++n/cy |
| 7 | 44 | Male | AIDS-PML | AIDS | +++n/+cy | +cy | ++n | ++n/+cy | ++cy | ++n/cy |
| 8 | 38 | Male | AIDS-PML | AIDS | ++n | +cy | ++n | ++n | +cy | +cy |
| 9 | 35 | Female | AIDS-PML | AIDS | ++n | ++cy | +++n/+cy | ++n/+cy | +cy | ++n/cy |
| 10 | 52 | Male | AIDS-PML | AIDS | +++n/++cy | +++cy | ++n | +++n/++cy | ++cy | ++cy |
| 11 | 42 | Male | Normal Brain | Myocradial Infarction | − | − | − | − | − | +weak cy |
| 12 | 62 | Female | Normal Brain | Renal Failure | − | − | − | − | − | − |
AIDS Acquired Immuno Deficiency Syndrome, PML Progressive Multifocal Leukoencephalopathy, VP-1 JCV Capsid Protein VP1, Agno JCV Late Product Agnoprotein, n nuclear, cy cytoplasmic, − negative, + weak immunoreactivity, ++ moderate immunoreactivity, +++ robust immunoreactivity
Histological and immunohistochemical evaluation
The paraffin-embedded tissue was sectioned at 4 µm thickness and stained with Hematoxylin and Eosin for routine histological diagnosis. A special staining for myelin (Luxol Fast Blue) was performed to highlight areas of demyelination. Immunohistochemistry was performed using the avidin–biotin–peroxidase complex system according to the manufacturer’s instructions (Vectastain Elite ABC Kit, Vector Laboratories, Burlingame CA). Our modified protocol includes deparaffinization in xylenes, re-hydration through alcohol up to water and non-enzymatic antigen retrieval in 0.01 M citrate buffer (pH 6.0) heated to 95°C for 40 min in a vacuum oven. After a cooling period of 30 min, slides were rinsed in PBS and incubated in MeOH/3% H2O2 for 20 min to quench endogenous peroxidase. Sections were then rinsed with PBS and blocked with 5% normal horse or goat serum for 2 h at room temperature. Primary antibodies were incubated overnight at room temperature in a humidified chamber. The primary antibodies used in this study included a rabbit polyclonal anti-Agnoprotein (1:2000 dilution, provided by Dr. Mahmut Safak, Temple University, Philadelphia, PA); a rabbit polyclonal antibody against the JCV capsid protein VP1 (1:4000 dilution, kindly provided by Dr. Walter Atwood, Brown University, Providence RI); a mouse monoclonal antibody against SV-40 T-Antigen, which cross-reacts with JCV T-Antigen (clone pAb416, 1:100 dilution, Oncogene, Boston, MA), and a mouse monoclonal antibody for the detection of HIF-1α (clone ESEE122, 1:1000 dilution, Novus Biologicals, Littleton CO). For the detection of the TGF-β downstream pathway molecules, Smads, we used a rabbit polyclonal antibody against Smad3 (FL-425, 1:200 dilution, Santa Cruz Biotechnology Inc, Santa Cruz, CA), and a mouse monoclonal antibody against Smad4 (clone B-8, 1:100 dilution, Santa Cruz Biotechnology). For cellular markers, we utilized mouse monoclonal antibodies for Glial fibrillary acidic protein, GFAP (Clone 6F2, 1:100 dilution, DAKO, Carpinteria CA), and Galactocerebroside, GalC (Clone mGalC, 1:200 dilution, Millipore, Billerica MA). After rinsing thoroughly with PBS, biotinylated secondary anti-mouse or anti-rabbit antibodies were incubated for 1 h at room temperature. Finally, sections were incubated with avidin–biotin–peroxidase complexes (ABC kit) for 1 h at room temperature, rinsed with PBS, and developed with Diaminobenzidine (Boehringer Mannheim, Germany). Sections were counterstained with Hematoxylin and mounted with Permount (Fisher Scientific, Pittsburgh, PA).
Double labeling immunofluorescence
For tissue, deparaffination, antigen retrieval, and blocking steps were performed as described above. For cell cultures, U-87 MG cells were plated on poly-l-lysine-coated glass chamber slides, allowed to attach overnight, fixed with ice-cold acetone for 3 min and washed in PBS. The first mouse monoclonal primary antibody was incubated over night. After washing with PBS the FITC-conjugated secondary antibodies were incubated for 1 h. After rinsing thoroughly, the second primary antibodies (rabbit polyclonal) were incubated over night, followed by incubation with anti-rabbit rhodamine-conjugated secondary antibodies. Finally, the slides were washed and mounted with Vecta-Shield mounting medium (Vector Laboratories) and visualized by fluorescence microscopy in a Nikon inverted microscope equipped with Deconvolution software (Slidebook 4.0, Intelligent Imaging, Denver, CO).
Cell culture conditions
Primary human oligodendrocytes were isolated following a previously described and well-established methodology [61]. Briefly, CNS tissue obtained from biopsies of patients undergoing lobectomies for epilepsy were trypsinized, filtered through mesh and centrifuged on a 30% Percoll gradient. The initial mixture of dissociated glial cells was suspended in minimal essential medium (MEM, Life Technologies) with 5% FBS, 50 U/ml penicillin and 50 µg/ml streptomycin, and cultured for 48 h in culture flasks. With this protocol, adherent cells such as astrocytes and microglia were separated from the non-adherent oligodendrocytes. The oligodendrocyte fraction was plated at 105 cells/well onto poly-l-lysine coated wells of 16-well chamber slides, and cultured for 2 weeks in MEM and 5% FBS. The phenotype of the cells and the purity of the cultures were corroborated by immunohistochemistry with specific markers including Galactocerebroside (Gal-C) and GFAP.
U-87 MG cells were maintained with Dulbecco modified Eagle medium supplemented with 10% fetal calf serum, 100 U/ml of penicillin, and 100 mg/ml of streptomycin at 37°C. Supernatant from both cell cultures was examined for the presence of Mycoplasma with a PCR based assay (PCR Mycoplasma Test Kit, MD Biosciences, Saint Paul, Minnesota).
Infections and Western blot analysis
One million primary oligodendrocytes were infected with 100 HA units of the Mad1/SVEΔ strain of JCV, equivalent to a multiplicity of infection (MOI) of 1, in the absence of serum for 3 h at 37°C as previously described [41]. This hybrid JCV contains the sequences for all JCV coding regions and a modified non-coding region in which the distal portion of the second 98-bp repeat sequence has been replaced with an analogous portion of the 72-bp repeat sequence of SV-40, resulting in a more effective viral replication. After infection, cells were washed and re-fed with growth media supplemented with 15% FBS. The efficiency of viral gene expression and viral replication in the infected cells cultures was evaluated by Western blot and immunocytochemistry using anti-Agnoprotein and anti-VP1 antibodies, respectively.
For Western blot analysis, JCV infected and non-infected cells were lysed in TNN buffer (50 mM Tris, pH 7.4, 50 mM NaCl, 5 mM MgCl2, 0.5% NP-40) at 5, 10 and 15 days post-infection. Proteins were separated using an 8% acrylamide gel and transferred to a supported nitrocellulose membrane (Trans-blot® Transfer Medium, Bio-Rad Laboratories, Hercules, CA). 50 µg of protein was loaded for each condition. A mouse monoclonal antibody against HIF-1α (clone 54, BD Biosciences, San Jose, CA) was used at a 1:500 dilution and a horse anti-mouse horseradish peroxidase secondary antibody (Thermo Scientific, Rockford, IL) was used at a 1:5,000 dilution. Visualization of proteins was performed using the Amersham ECL Plus Detection System (GE Healthcare, Buckinghamshire, UK). Experiments were performed in duplicates. Grb-2 was used as a loading control for both, HIF-1α and Agnoprotein.
Chloramphenicol Acetyl Transferase assay (CAT assay)
U-87 MG cells were transiently transfected using the calcium phosphate precipitation method described by Graham and Van der Eb, with some variations [18]. Briefly, cells were plated, grown overnight and re-fed 3 h before transfection. 10 µg of each plasmid were used for transfection. DNA concentrations were kept constant by adding empty vector. The DNA was diluted up to 250 µl of water and mixed with the same volume of 0.5 M CaCl2. The resulting DNA–CaCl2 cocktail was added drop by drop into a tube containing HNP pH 7.1 buffer previously warmed up to 37°C, and after mixing for 15 min added to the cells. After a 5-h incubation period, the medium was removed and glycerol shock was performed (2 ml of 10% glycerol for 1 min). Finally, cells were re-fed with fresh medium. Cells were transfected with reporter constructs containing the JCV promoter from the Mad-1 strain linked to the chloramphenicol acetyltransferase (CAT) gene in the early (JCVE-CAT) and late orientations (JCVL-CAT), plasmids pBLCAT3-Mad1E and pBLCAT3-Mad1L, respectively. The early and late promoters were cloned separately in a unique BamH1 restriction site (position 426). Cells were then co-transfected with a human HIF-1α expressing plasmid (pcDNA3-hHIF-1α: BamHl-XbaI), kindly provided by Dr. Olay Batuman, SUNY Health System, Brooklyn NY; a Smad 3 containing plasmid (pRK5-Smad3Flag: BamHI-SalI into pRK5F), and a Smad 4 containing plasmid (pRK5-Smad4Flag: EcoRI-SalI into pRK5F).
Cells were harvested after 48 h post-transfection into TEN buffer (40 mM Tris–HCl, pH 7.5, 1 mM EDTA, pH 8.0, and 150 mM NaCl) and lysed in 250 mM Tris, pH 7.8, by several freezes in a dry ice ethanol bath and subsequent thaws at 37°C. Equal amounts of extract protein were analyzed in each assay (5–20 µg). These extracts were incubated with 8 pmol of acetyl CoA and 0.1 µCi of 14C-chloramphenicol at 37°C for 1 h. After extraction with ethyl acetate, the samples were spotted onto thin-layer chromatography plates, and various forms migrated in a methanol–chloroform (5:95) mixture. The percentage of conversion of 14C-chloramphenicol was determined with the Molecular Imager FX System (Bio-Rad Laboratories Inc, Hercules, CA). All experiments were performed in triplicates.
Immunoprecipitation, SDS-PAGE and Western blot analyses
U-87 MG cells were harvested 48 h after transient co-transfection with the following plasmids: for HIF-1α, pcDNA3h-HIF-1α myc tagged: BamHI-XbaI (kindly provided by Dr. Olcay Batuman); for Smad 3, pRK5-Smad3 Flag: BamHI-SalI into pRK5F; and for Smad4, pRK5-Smad4 Flag: BamHI-SalI into pRK5F. For immunoprecipitation experiments, cells were lysed in TNN buffer and extracts were subjected to immunoprecipitation with either rabbit anti-Smad3 or mouse anti-Smad4 antibodies (10 µl, respectively) using protein A-Sepharose beads (GE Healthcare, Buckinghamshire, UK). Cell extracts were separated using an 8% acrylamide gel and transferred to a pure nitrocellulose membrane (Trans-blot Transfer Medium, Bio-Rad Laboratories Inc, Hercules, CA). A monoclonal mouse anti-HIF-1α antibody (clone 54, BD Biosciences, San Jose, CA) was incubated overnight at 4°C at a 1:500 dilution, and an anti-mouse horseradish peroxidase-conjugated secondary antibody (Thermo Scientific) was used at a 1:5,000 dilution. Negative controls were carried out by incubating protein extracts without a primary antibody and with a non-specific IgG of the same species as the primary antibody. The anti-HIF-1α antibody utilized is different from the one used for immunohistochemistry since the latter is specifically for detection of HIF-1α in paraffin embedded tissues. Detection of proteins was performed using the Amersham ECL Plus Detection System (GE Healthcare) according to the manufacturer’s instructions. All experiments were performed in duplicates.
Chromatin immunoprecipitation (ChIP) assay
One million U-87 MG cells were grown overnight in 100 mm dishes to 60–70% confluency; cells were then transiently co-transfected with 2 µg of h-HIF-1α plasmid (pcDNA3h-HIF1α myc-tagged: BamHI-XbaI), and with pBJC plasmid that contains the whole JCV genome (cloned into a pBR322 plasmid: EcoRI), using a FuGene 6 transfection reagent (Roche Applied Sciences). The same plasmid lacking the JCV control region was used as a negative control. Plates were returned to the incubator for 48 h. Cells were cross-linked with 1% formaldehyde, harvested and ChIP was performed. The remainder of the procedure followed standard protocols for ChIP analysis, according to the manufacturer’s instructions (Upstate Biotechnology). DNA was sheared by 3–4 cycles Sonication, Amplitude 30, for 10 s. The primary antibody used in the ChIP procedure was a rabitt polyclonal anti-HIF-1α ChIPGrade (ab2185, AbCam, Cambridge, MA) and for negative control a non-specific non-conjugated goat anti-rabbit IgG (Thermo Scientific, Rockford, IL). The following primers spanning the JCV non-coding control region (NCCR) were used for PCR amplification: 5′-tggattcctccctattcagcac-3′ (NCRR1 4993–5004 forward) and 5′-atggccagctggtgacaagc-3′ (NCRR2 258–279 reverse). PCR was 35 cycles (94°C for 45 s, 56°C for 45 s, and 72°C for 45 s), followed by extension at 72°C for 7 min.
Results
Histological and immunohistochemical characterization of PML cases
All PML cases were histologically characterized by the presence of multiple foci of demyelination in the sub-cortical white matter in the sections stained with Hematoxylin and Eosin (H&E). Luxol Fast Blue was used to highlight the absence of myelin in those particular areas (Fig. 1a, b). At higher magnification, multiple pathognomonic bizarre astrocytes as well as enlarged oligodendrocytes harboring intra-nuclear eosinophilic inclusion bodies were found within the demyelinated plaques (Fig. 1c, d). In addition, several foamy macrophages were observed in the demyelinated plaques and perivascular cuffing of lymphocytes were present in the periphery of the lesions. The presence of JC viral proteins was corroborated by immunohistochemistry; cytoplasmic expression of the viral accessory Agnoprotein was observed in the bizarre astrocytes as well as in the cytoplasm of infected oligodendrocytes. Likewise, expression of the JCV capsid protein, VP-1, was detected in the intra-nuclear inclusion bodies of oligodendrocytes and in the cytoplasm and nuclei of bizarre astrocytes (Fig. 1e, f). Expression of T-Antigen was also detected in infected glial cells, astrocytes and oligodendrocytes within demyelinated plaques (data not shown).
Fig. 1.
Histological characterization of Progressive Multifocal Leukoencephalopathy. PML is characterized by multiple foci of myelin loss in the sub-cortical white matter (a Hematoxylin & Eosin). These plaques of demyelination are highlighted with a special stain for myelin (b Luxol Fast Blue). Bizarre astrocytes (c), as well as enlarged oligodendrocytes harboring intra-nuclear eosinophilic inclusions (d) can be found within the demyelinated plaques. Expression of the JCV capsid protein VP1 is detected by immunohistochemistry in the nuclei and cytoplasm of bizarre astrocytes (e), and in the intranuclear inclusion bodies of oligodendrocytes (f). Original magnification a and b ×100. c and e ×400. d and f ×1,000
Upregulation of HIF-1α in clinical samples of PML
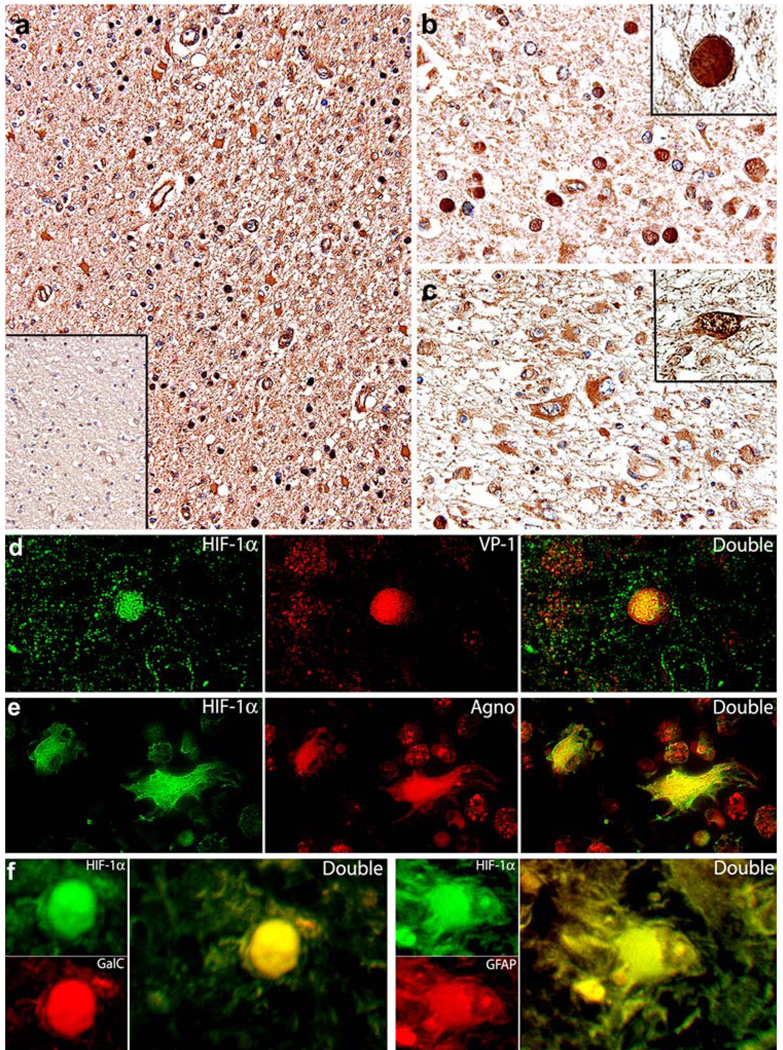
In order to determine the expression of HIF-1α in the context of JCV infection, we performed immunohistochemical analysis in the PML samples and compared the results with HIF-1α expression in non-affected areas of the same samples and with normal brain tissue (Fig. 2a). Robust immunoreactivity for HIF-1α was observed in both phenotypes of JCV-infected cells; in the intra-nuclear inclusion bodies of infected oligodendrocytes (Fig. 2b), and in bizarre astrocytes with a homogeneous cytoplasmic pattern of expression (Fig. 2c), with just a few cells exhibiting a positive reaction in the nucleus (inset). Normal oligodendrocytes, located in the adjacent non-affected areas of the same samples, were consistently negative for HIF-1α, while astrocytes in these adjacent areas were weakly immunoreactive. On the other hand, normal control brain showed no expression of HIF-1α in oligodendrocytes and again a very weak expression in the cytoplasm of normal astrocytes (Fig. 2a, inset). Interestingly, endothelial cells from small capillaries present throughout the brain parenchyma in areas of demyelination also demonstrated up-regulation of HIF-1α. Since endothelial cells are capable of sustaining active viral infection, these results corroborate the observations from oligodendrocytes and bizarre astrocytes.
Fig. 2.
Expression of HIF-1α in clinical samples of PML. Immunohistochemistry revealed the up-regulation of HIF-1α in the demyelinated plaques of PML cases at low magnification (a) compared to a very weak signal in the non-affected adjacent normal brain (inset). At higher magnification, HIF-1α is located into the intra-nuclear inclusion bodies of oligodendrocytes (b), and in the cytoplasm of bizarre astrocytes within plaques of demyelination (c). Double labeling with HIF-1α (fluorescein) and the capsid protein VP-1 (rhodamine) demonstrates the co-localization of both proteins in the nuclei of oligodendrocytes harboring inclusion bodies (d). Double labeling with HIF-1α (fluorescein) and the accessory Agnoprotein (rhodamine) corroborates the presence of HIF-1α in bizarre astrocytes undergoing active productive infection (e). To corroborate the phenotype of cells expressing HIF-1α, we performed double labeling with specific cellular markers Gal-C for oligodendrocytes, and GFAP for astrocytes (f). Original magnifications, a and inset ×200, b and c ×400, Insets and double labeling panels, ×1,000
In order to demonstrate co-expression of HIF-1α and JCV proteins in both types of JCV-infected cells, we performed double immunofluorescence in the same PML samples. Co-localization between HIF-1α and the capsid protein VP-1 was found in the nuclei of oligodendrocytes harboring inclusion bodies (Fig. 2d), whereas HIF-1α and Agnoprotein co-localized in the cytoplasm of bizarre astrocytes (Fig. 2e). Although the morphological characteristics of bizarre astrocytes and oligodendrocytes harboring inclusion bodies are unmistakable, in order to demonstrate their phenotype, we performed double labeling with specific cellular markers. HIF-1α is present in cells expressing Gal-C (oligodendrocytes), and GFAP (astrocytes) (Fig. 2f).
Upregulation of HIF-1α upon JCV infection in vitro
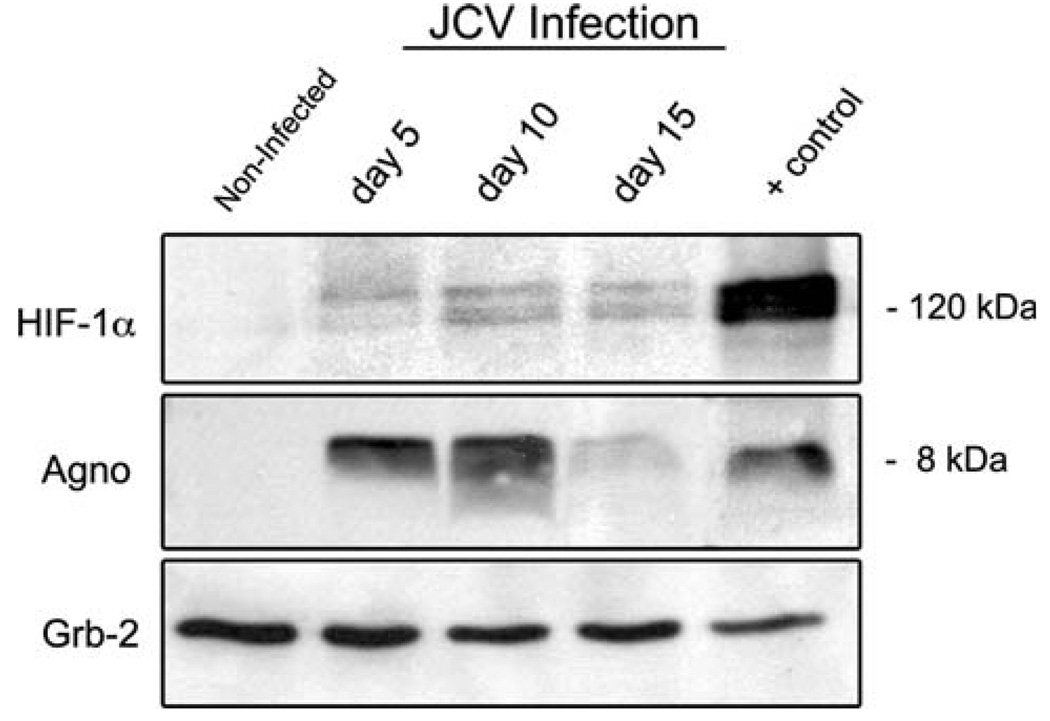
Based on these findings, the next series of experiments was aimed to determine whether the expression of HIF-1α was induced upon JCV infection in vitro. By Western blot analysis, we found that infection with JCV was capable of inducing HIF-1α protein expression in primary oligoden-drocytes, when compared with uninfected cells in which HIF-1α levels remain undetectable (Fig. 3). Expression of HIF-1α seemed to remain constant at different time points of infection (days 5, 10 and 15). Non-infected cells at the same time points (5, 10 and 15 days) also showed undetectable levels of HIF-1α, corroborating that induction of HIF-1α is the result of JCV infection and not of stress of cell culture conditions (data not shown). Western blot analysis for the viral late product, Agnoprotein, was performed with the same extracts to corroborate that the infection of oligodendrocytes with JCV was active and productive. Grb-2 from the same cell extracts is shown as a loading control for HIF-1α.
Fig. 3.
Induction of HIF-1α after JCV-infection in glial cell cultures. Western blot analysis demonstrated HIF-1α up-regulation in whole cell extracts from primary oligodendrocytes (upper panel) at different time points after JCV-infection compare with uninfected cells in which expression of HIF-1α is absent. Detection of the viral accessory product Agnoprotein indicates the presence of productive JCV infection in the same extracts (middle panel). Cell extracts from U-87 MG transiently transfected with a HIF-1α vector were used as positive control; extracts from glial cells infected with JCV were used as positive control for detection of Agnoprotein. Grb-2 from same cell extracts was used as a loading control for HIF-1α
Activation of the JCV promoter by HIF-1α and Smads
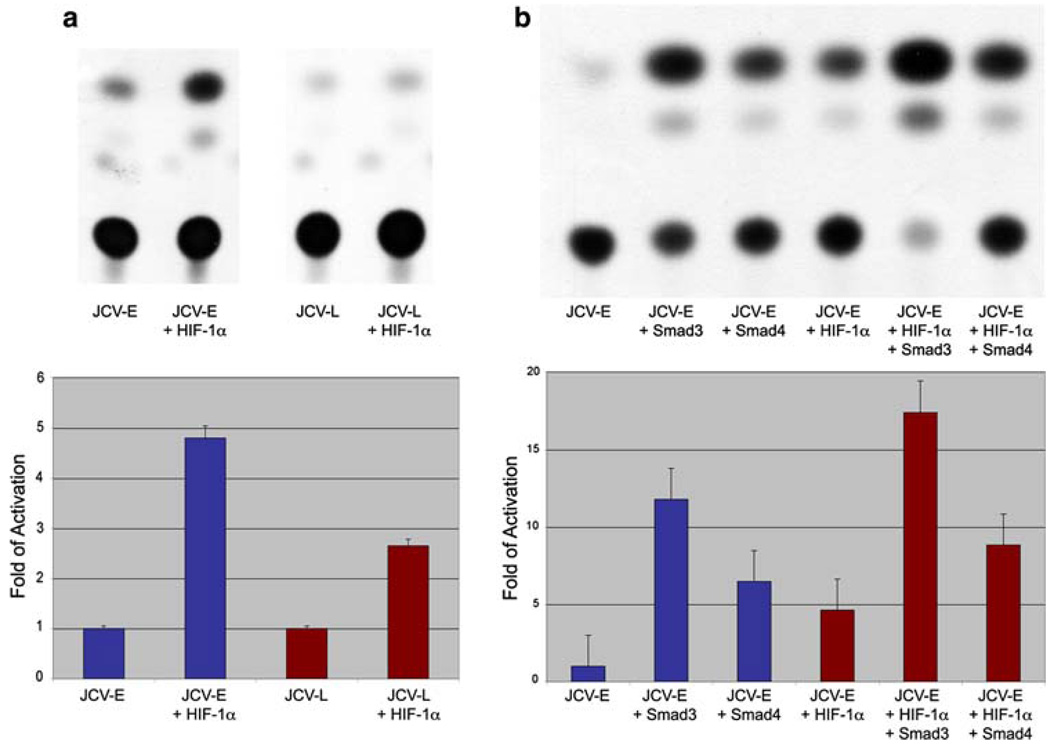
Next, we investigated the up-regulation of HIF-1α and its effect on JCV transcriptional regulation. We performed different sets of CAT assays to determine the involvement of HIF-1α in the regulation of both JCV early and late promoters. Interestingly, we found that both promoters can be activated by HIF-1α. Activation of JCV-E promoter was increased in 4.80-fold, while the JCV-L promoter was activated 2.65-fold (Fig. 4a).
Fig. 4.
HIF-1α activation of the JCV promoter. CAT assays performed on lysates of U-87 MG cell cultures transiently co-transfected with the JCV early (JCV-E) and late (JCV-L) promoters and HIF-1α, demonstrated a significant activation of both JCV promoters in the presence of HIF-1α (a), and the activation of the JCV-E promoter by Smad3 and Smad4. Both signaling molecules showed cooperativity with HIF-1α in the activation of JCV-E promoter (b). All experiments were performed in triplicates
As we have previously reported, the TGF-β pathway downstream molecules, Smad3 and Smad4, can activate both the early and late promoters of JCV in glial cells [13]. Likewise, cooperation between Smad3, Smad4 and HIF-1α in response to hypoxia has also been shown in different cell cultures [48] but not in astrocytic cell lines. Thus, our next set of experiments included the study of the activation and putative cooperation between HIF-1α, Smad3 and Smad4 on the JCV-E promoter. Our findings not only corroborated activation of the JCV-E promoter by Smad3 and Smad4 (11.80 and 6.48-fold, respectively) as expected, but also showed the synergy of these molecules with HIF-1α at the level of transcription. Interestingly, activation of JCV-E promoter by Smad3 and HIF-1α was significantly higher than the activation observed with Smad4 and HIF-1α (17.42 compared to 8.84-fold, respectively) (Fig. 4b).
Co-localization and physical interaction between HIF-1α and Smads in JCV infected cells
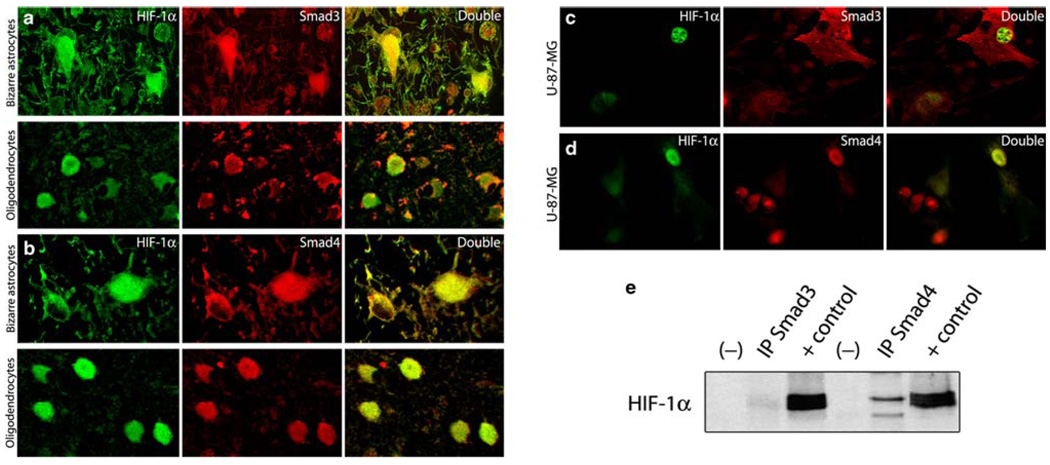
Based on these results, in the next series of experiments we determined if co-localization and physical interaction between HIF-1α and Smads3 and 4 occur in PML samples and in cell cultures. To achieve this, double-labeling immunofluorescence was performed in PML cases as well as in U-87 MG cell cultures. In both, co-localization of HIF-1α (fluorescein) and either Smad3 or Smad4 (rhodamine) was observed (Fig. 5). In PML samples, HIF-1α and Smad3 were found co-localizing in the nuclei and cytoplasm of bizarre astrocytes, and in the nuclei of oligodendrocytes harboring inclusion bodies (Fig. 5a). In contrast, Smad4 was found in co-localization with HIF-1α in the nuclei and cytoplasm of bizarre astrocytes, and in a large number of JCV infected oligodendrocytes (Fig. 5b). In U-87 MG cell cultures, HIF-1α and Smad3 were found in the nuclei (Fig. 5c), and HIF-1α and Smad4 were found co-localizing in the nuclear and cytoplasmic compartments (Fig. 5d). Corroborating these results, immunoprecipitation with Smad3 and Smad4 primary antibodies followed by Western blot for HIF-1α demonstrated not only co-localization, but also a direct interaction between these proteins (Fig. 5e).
Fig. 5.
Co-localization and physical interaction between HIF-1α, Smad3 and Smad4. Double labeling for HIF-1α (fluorescein) and Smad3 (rhodamine) in PML samples showed cytoplasmic and nuclear co-localization of both proteins in bizarre astrocytes and in the nuclei of few oligodendrocytes harboring inclusion bodies (a). HIF-1α and Smad4 were also found co-localizing in the nuclei and cytoplasm of bizarre astrocytes and in the nuclei of JCV-infected oligodendrocytes (b). All panels original magnification 400×. Double labeling performed in transiently co-transfected U-87 MG cells with the HIF-1α (fluorescein) and Smad3 and Smad4 plasmid vectors (rhodamine) demonstrated nuclear co-localization between HIF-1α and Smad3 (c) and nuclear and cytoplasmic co-localization between HIF-1α and Smad4 in transfected cells (d). Co-immunoprecipitation assay in U-87 MG whole cell extracts co-transfected with HIF-1α and Smad3 and Smad4, respectively, demonstrated a direct physical interaction between these proteins. Positive and negative controls are also shown (e)
HIF-1α binds to the control region of JCV
Once the activation of the JCV promoter by HIF-1α was established, chromatin immunoprecipitation (ChIP) assay was performed to determine if HIF-1α is bound in vitro to the control region of JCV (CR). U-87 MG cells were transiently co-transfected with a HIF-1α plasmid and a pBJC plasmid, which contains the whole JCV genome, cross-linked, lysed and sonicated under standardized conditions (see “Material and methods”). Immunoprecipitation was performed with an anti-HIF-1α antibody or a non-specific rabbit IgG. DNA associated with the resulting immunoprecipitates was amplified using primers spanning the JCV CR (see “Material and methods”). A 426 bp PCR product corresponding to the amplified CR was observed when anti-HIF-1α antibody was present (lane 3) but not when using a non-specific IgG (lane 4), indicating that HIF-1α physically binds to the JCV CR. A plasmid lacking the JCV control region was used as an additional negative control, ruling out HIF-1α interactions with plasmid sequences (Fig. 6a). HIF-1α protein expression in non-transfected and transfected cell lysates was demonstrated by Western blot analysis (Fig. 6b).
Fig. 6.
Interaction between HIF-1α and the JCV promoter region. a ChIP assays performed in U-87 MG cells transiently co-transfected with a plasmids containing HIF-1α and the whole JCV genome (pBJC plasmid). The approximate size of the PCR product of the JCV CR is indicated. Lane 1 DNA ladder. Lane 2 1/10 of the input extract of Lane 3. In lane 3, a specific anti-HIF-1α antibody was used. Non-specific IgG was used as a control of specificity (lane 4), a plasmid lacking the JCV control region was used as a negative control (lane 5); no antibody used as another negative control (lane 6). Negative control for PCR is shown in lane 7, and the pBJC plasmid, used as a positive control for the PCR reaction in Lane 8. Protein levels of HIF-1α were confirmed by Western blot (b)
Discussion
Infectious processes, including viral infections, represent important stressful conditions for living organisms. At the same time, appropriate tissue conditions need to be in place for viruses to effectively replicate and complete their life cycles. During the last decade, the activation and replication of different viruses have been linked to the HIF-1 pathway. For example, parvovirus B19, a virus that shows predilection for erythroid progenitor cells, completes virion assembly through a HIF-1α dependent mechanism. The underlying physiologic hypoxia found in the bone marrow increases HIF-1α intracellular levels and apparently, may be a determinant for completing the viral infectious cycle [39]. Another common pathogen, respiratory syncytial virus (RSV), considered the main cause of bronchiolitis in children, can be activated through stabilization of HIF-1α in cultured bronchial epithelial cells [19, 27]. The present study was aimed to determine if an association between HIF-1α and the human neurotropic virus JCV exists. Our first objective was to determine the levels of expression of HIF-1α in PML archival tissue. HIF-1α was found up-regulated in glial cells where JCV replication takes place, bizarre astrocytes and oligodendrocytes. We believe that this increase in the levels of HIF-1α is the response of cellular stress caused by the infection with JCV. Interestingly, while the expression of HIF-1α in oligodendrocytes is predominantly nuclear, in bizarre astrocytes in predominantly cytoplasmic, with some cells exhibiting both, nuclear and cytoplasmic. This could be explained by the different behavior shown by oligodendrocytes and astrocytes in response to JCV infection. While viral replication takes place in the nuclei of infected oligodendrocytes, resulting in their lytic death, in astrocytes viral replication takes place in both cellular compartments and it does not result in their cytolysis but in their bizarre phenotype. Another interesting observation was the up-regulation of HIF-1α in endothelial cells from small capillaries present within areas of demyelination. Previous reports have demonstrated that endothelial cells are capable of sustaining active viral replication by JCV [7], which in association with the enormous stress of these areas caused by the demyelinating process are responsible for the up-regulation of HIF-1α in these cells.
To corroborate the results observed in the clinical samples in an in vitro system, in the next series of experiments, we studied the levels of HIF-1α in primary oligodendroglial cultures and confirmed that JCV infection results in the activation of HIF-1α, compared with uninfected cells in which the levels of HIF-1α remain undetectable.
On the other hand, several transcription factors have the ability to bind to the JCV CR and have been implicated in the activation of the JCV promoter. For example, YB-1 binds in close proximity to the TATA box and enhances late gene transcription [25], NF-κB, important for early and late activation [32, 42, 47], the novel DNA binding protein Pur-α, which is capable to bind to the CR and stimulate transcription of T-Antigen [15, 16], BAG-1 [11], the early growth response protein 1 or Egr-1 [45], NF-1 [30], and Smads, the downstream molecules of TGF-β [13], among others. However, there are still several other proteins that can interact with the JCV control region but remain unknown. Considering that HIF-1α is another prominent transcription factor that has been implicated in the activation of several viruses, in the next series of experiments, we performed transcription activation assays to determine the possible activation of the JCV promoter by HIF-1α and we found activation of both the early and late promoters. Interestingly, the activation was potentiated in the presence of Smads, which suggests cooperation between HIF-1α and other proteins in the stimulation of JCV transcription and replication. Thus, in the next series of experiments, we demonstrated the co-localization of HIF-1α and Smads in the nuclei of JCV affected glial cells in cases of PML and in vitro, as well as their physical interaction by co-immunoprecipitation. Finally, through chromatin immunoprecipitation assay, we demonstrate the binding of HIF-1α to the JCV control region.
We hypothesize that under oxidative stress conditions caused by the presence of HIV, HIF-1α levels increase, leading to the direct and/or indirect activation of the JCV promoter, promoting a more efficient active viral replication. However, future studies are needed to unravel more detailed mechanisms of this activation, including the interaction between different signaling molecules affected by the presence of JCV. The Survivin pathway is, in our opinion of particular interest, since this protein, which is normally absent in fully differentiated tissues, has been demonstrated to be reactivated by JCV infection, resulting in prevention of apoptosis and efficient viral replication [40] and Survivin activation has recently been associated with the presence of HIF-1α [14, 59].
In addition several questions arise. For example, HIF-1α has also been implicated in viral oncogenesis [38]. Perhaps one of the most studied viruses in association to the HIF-1α machinery is the Kaposi’s sarcoma-associated herpes virus (KSHV) [5, 6, 9, 20]. KSHV is able to stabilize HIF-1α through phosphorylation by MAP kinases and PI3/Akt, and to force HIF-1α to bind to HREs found in the viral genome, stimulating the formation of new virions [53]. However, the secondary activation of HIF-1α by HHV-8 also induces the transcription of VEGF and other factors that promote endothelial cell proliferation and may facilitate the progression of Kaposi sarcoma [5, 6, 9, 20, 38]. In a similar way, hepatitis B virus (HBV) [35, 62, 63], hepatitis C virus (HCV) [36], Epstein–Barr virus (EBV) [24, 28, 57] and recently, the human T-cell leukemia virus-1 (HTLV-1) [55] can stabilize HIF-1α and utilize this protein either to complete their lytic cycle or to facilitate tumor progression as well as intra-tumoral neo-angiogenesis. On the other hand, the oncogenic potential of JCV has been extensively demonstrated in vitro and in animal models, and there is mounting evidence suggesting a role for JCV in human cancer. Therefore, new studies are needed to establish if there is any correlation between the expression of HIF-1α and the transforming abilities of T-Antigen, which may represent a contributing factor in the oncogenesis of JCV.
Another interesting topic regarding the association of HIF-1α with JCV infection is viral latency and reactivation. It is well established that after a sub-clinical primary infection, JCV remains in a latent state in the kidney [3], one of the organs that has the capability to detect hypoxia and to stimulate erythropoiesis. It could be hypothesized that hypoxic conditions, with their consequent activation of HIF-1α, may also stimulate the reactivation of JCV. One possible mechanism that may be favorable for viral replication involves glycolysis, which is activated during hypoxic conditions, resulting in the increased synthesis of pyrimidines and purines, required by viruses to achieve replication.
Taken together, these results show for the first time the involvement of HIF-1α in the activation of the JCV promoter, a crucial step for viral transcription and viral replication, which along with other pathways affected by JCV, such as Survivin, will eventually result in the development of PML. Understanding of the molecular mechanisms involved in these events may result in the development of more effective treatments for PML, a thus far fatal disease.
Acknowledgments
We would like to express our gratitude to the members of the Department of Neuroscience who have generously shared their ideas and reagents. We would like to thank Dr. Martyn White in particular for his insightful thoughts and discussion on this manuscript. This work was made possible thanks to funding from NIH grants awarded to KK and LDV (P01 NS030916) and LDV (R01 NS055644).
Contributor Information
Sergio Piña-Oviedo, Department of Neuroscience, Center for Neurovirology and Neuropathology Core, Temple University School of Medicine, 1900 N. 12th Street, Philadelphia, PA 19122, USA.
Kamel Khalili, Department of Neuroscience, Center for Neurovirology, Temple University School of Medicine, 1900 N. 12th Street, Philadelphia, PA 19122, USA.
Luis Del Valle, Department of Neuroscience, Center for Neurovirology and Neuropathology Core, Temple University School of Medicine, 1900 N. 12th Street, Philadelphia, PA 19122, USA, luis.del.valle@temple.edu.
References
- 1.Aminova LR, Chavez JC, Lee J, Ryu H, Kung A, Lamanna JC, Ratan RR. Prosurvival and prodeath effects of hypoxiainducible factor-1alpha stabilization in a murine hippocampal cell line. J Biol Chem. 2005;280:3996–4003. doi: 10.1074/jbc.M409223200. doi:10.1074/jbc.M409223200. [DOI] [PubMed] [Google Scholar]
- 2.Arany Z, Huang LE, Eckner R, Bhattacharya S, Jiang C, Goldberg MA, Bunn HF, Livingston DM. An essential role for p300/CBP in the cellular response to hypoxia. Proc Natl Acad Sci USA. 1996;93:12969–12973. doi: 10.1073/pnas.93.23.12969. doi:10.1073/pnas.93.23.12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beckmann AM, Shah KV. Propagation and primary isolation of JCV and BKV in urinary epithelial cell cultures. Prog Clin Biol Res. 1983;105:3–14. [PubMed] [Google Scholar]
- 4.Berger JR, Concha M. Progressive multifocal leukoencephalopathy: the evolution of a disease once considered rare. J Neurovirol. 1995;1:5–18. doi: 10.3109/13550289509111006. doi:10.3109/13550289509111006. [DOI] [PubMed] [Google Scholar]
- 5.Carroll PA, Kenerson HL, Yeung RS, Lagunoff M. Latent Kaposi’s sarcoma-associated herpesvirus infection of endothelial cells activates hypoxia-induced factors. J Virol. 2006;80:10802–10812. doi: 10.1128/JVI.00673-06. doi:10.1128/JVI.00673-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catrina SB, Botusan IR, Rantanen A, Catrina AI, Pyakurel P, Savu O, Axelson M, Biberfeld P, Poellinger L, Brismar K. Hypoxia-inducible factor-1alpha and hypoxia-inducible factor-2alpha are expressed in Kaposi sarcoma and modulated by insulin-like growth factor-I. Clin Cancer Res. 2006;12:4506–4514. doi: 10.1158/1078-0432.CCR-05-2473. doi:10.1158/1078-0432.CCR-05-2473. [DOI] [PubMed] [Google Scholar]
- 7.Chapagain ML, Verma S, Mercier F, Yanagihara R, Nerurkar VR. Polyomavirus JC infects human brain microvascular endothelial cells independent of serotonin receptor 2A. Virology. 2007;364:55–63. doi: 10.1016/j.virol.2007.02.018. doi:10.1016/j.virol.2007.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darbinyan A, White MK, Akan S, Radhakrishnan S, Del Valle L, Amini S, Khalili K. Alterations of DNA damage repair pathways resulting from JCV infection. Virology. 2007;364:73–86. doi: 10.1016/j.virol.2007.02.015. doi:10.1016/j.virol.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis DA, Rinderknecht AS, Zoeteweij JP, Aoki Y, Read-Connole EL, Tosato G, Blauvelt A, Yarchoan R. Hypoxia induces lytic replication of Kaposi sarcoma-associated herpesvirus. Blood. 2001;97:3244–3250. doi: 10.1182/blood.v97.10.3244. doi:10.1182/blood.V97.10.3244. [DOI] [PubMed] [Google Scholar]
- 10.Del Valle L, Piña-Oviedo S. HIV disorders of the brain: pathology and pathogenesis. Front Biosci. 2006;11:718–732. doi: 10.2741/1830. doi:10.2741/1830. [DOI] [PubMed] [Google Scholar]
- 11.Devireddy LR, Kumar KU, Pater MM, Pater A. BAG-1, a novel Bcl-2-interacting protein, activates expression of human JC virus. J Gen Virol. 2000;81:351–357. doi: 10.1099/0022-1317-81-2-351. [DOI] [PubMed] [Google Scholar]
- 12.Discher DJ, Bishopric NH, Wu X, Peterson CA, Webster KA. Hypoxia regulates beta-enolase and pyruvate kinase-M promoters by modulating Sp1/Sp3 binding to a conserved GC element. J Biol Chem. 1998;273:26087–26093. doi: 10.1074/jbc.273.40.26087. doi:10.1074/jbc.273.40.26087. [DOI] [PubMed] [Google Scholar]
- 13.Enam S, Sweet TM, Amini S, Khalili K, Del Valle L. Evidence for involvement of transforming growth factor beta1 signaling pathway in activation of JC virus in human immunodeficiency virus 1-associated progressive multifocal leukoencephalopathy. Arch Pathol Lab Med. 2004;128:282–291. doi: 10.5858/2004-128-282-EFIOTG. [DOI] [PubMed] [Google Scholar]
- 14.Fan LF, Dong WG, Jiang CQ, Qian Q, Yu QF. Role of Hypoxia-inducible factor-1alpha and Survivin in colorectal carcinoma progression. Int J Colorectal Dis. 2008;23:1057–1064. doi: 10.1007/s00384-008-0511-3. doi:10.1007/s00384-008-0511-3. [DOI] [PubMed] [Google Scholar]
- 15.Gallia GL, Johnson EM, Khalili K. Puralpha: a multifunctional single-stranded DNA- and RNA-binding protein. Nucleic Acids Res. 2000;28:3197–3205. doi: 10.1093/nar/28.17.3197. doi:10.1093/nar/28.17.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallia GL, Safak M, Khalili K. Interaction of the single-stranded DNA-binding protein Puralpha with the human polyomavirus JC virus early protein T-antigen. J Biol Chem. 1998;273:32662–32669. doi: 10.1074/jbc.273.49.32662. doi:10.1074/jbc.273.49.32662. [DOI] [PubMed] [Google Scholar]
- 17.Gardner LB, Li Q, Park MS, Flanagan WM, Semenza GL, Dang CV. Hypoxia inhibits G1/S transition through regulation of p27 expression. J Biol Chem. 2001;276:7919–7926. doi: 10.1074/jbc.M010189200. doi:10.1074/jbc.M010189200. [DOI] [PubMed] [Google Scholar]
- 18.Graham FL, van der Eb AJ. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973;52:456–467. doi: 10.1016/0042-6822(73)90341-3. doi:10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 19.Haeberle HA, Durrstein C, Rosenberger P, Hosakote YM, Kuhlicke J, Kempf VA, Garofalo RP, Eltzschig HK. Oxygen-independent stabilization of hypoxia inducible factor (HIF)-1 during RSV infection. PLoS ONE. 2008;3:e3352. doi: 10.1371/journal.pone.0003352. doi:10.1371/journal.pone.0003352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haque M, Davis DA, Wang V, Widmer I, Yarchoan R. Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) contains hypoxia response elements: relevance to lytic induction by hypoxia. J Virol. 2003;77:6761–6768. doi: 10.1128/JVI.77.12.6761-6768.2003. doi:10.1128/JVI.77.12.6761-6768.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hopfl G, Ogunshola O, Gassmann M. HIFs and tumors— causes and consequences. Am J Physiol Regul Integr Comp Physiol. 2004;286:R608–R623. doi: 10.1152/ajpregu.00538.2003. doi:10.1152/ajpregu.00538.2003. [DOI] [PubMed] [Google Scholar]
- 22.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. doi:10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 23.Jiang BH, Rue E, Wang GL, Roe R, Semenza GL. Dimerization, DNA binding, and transactivation properties of hypoxia-inducible factor 1. J Biol Chem. 1996;271:17771–17778. doi: 10.1074/jbc.271.30.17771. doi:10.1074/jbc.271.30.17771. [DOI] [PubMed] [Google Scholar]
- 24.Jiang JH, Wang N, Li A, Liao WT, Pan ZG, Mai SJ, Li DJ, Zeng MS, Wen JM, Zeng YX. Hypoxia can contribute to the induction of the Epstein–Barr virus (EBV) lytic cycle. J Clin Virol. 2006;37:98–103. doi: 10.1016/j.jcv.2006.06.013. doi:10.1016/j.jcv.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 25.Kerr D, Chang CF, Chen N, Gallia G, Raj G, Schwartz B, Khalili K. Transcription of a human neurotropic virus promoter in glial cells: effect of YB-1 on expression of the JC virus late gene. J Virol. 1994;68:7637–7643. doi: 10.1128/jvi.68.11.7637-7643.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khalili K, White MK. Human demyelinating disease and the polyomavirus JCV. Mult Scler. 2006;12:133–142. doi: 10.1191/135248506ms1264oa. doi:10.1191/135248506ms1264oa. [DOI] [PubMed] [Google Scholar]
- 27.Kilani MM, Mohammed KA, Nasreen N, Tepper RS, Antony VB. RSV causes HIF-1alpha stabilization via NO release in primary bronchial epithelial cells. Inflammation. 2004;28:245–251. doi: 10.1007/s10753-004-6047-y. doi:10.1007/s10753-004-6047-y. [DOI] [PubMed] [Google Scholar]
- 28.Kondo S, Seo SY, Yoshizaki T, Wakisaka N, Furukawa M, Joab I, Jang KL, Pagano JS. EBV latent membrane protein 1 up-regulates hypoxia-inducible factor 1alpha through Siah1-mediated down-regulation of prolyl hydroxylases 1 and 3 in nasopharyngeal epithelial cells. Cancer Res. 2006;66:9870–9877. doi: 10.1158/0008-5472.CAN-06-1679. doi:10.1158/0008-5472.CAN-06-1679. [DOI] [PubMed] [Google Scholar]
- 29.Koshiji M, Kageyama Y, Pete EA, Horikawa I, Barrett JC, Huang LE. HIF-1alpha induces cell cycle arrest by functionally counteracting Myc. EMBO J. 2004;23:1949–1956. doi: 10.1038/sj.emboj.7600196. doi:10.1038/sj.emboj.7600196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar KU, Devireddy LR, Tang SC, Pater A, Pater MM. Human JC virus nuclear factor 1 binding motifs and large tumor antigen region required for transactivation of late promoter. J Neurochem. 1996;67:473–481. doi: 10.1046/j.1471-4159.1996.67020473.x. [DOI] [PubMed] [Google Scholar]
- 31.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxiainducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. doi:10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 32.Mayreddy RP, Safak M, Razmara M, Zoltick P, Khalili K. Transcription of the JC virus archetype late genome: importance of the kappa B and the 23-base-pair motifs in late promoter activity in glial cells. J Virol. 1996;70:2387–2393. doi: 10.1128/jvi.70.4.2387-2393.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miki N, Ikuta M, Matsui T. Hypoxia-induced activation of the retinoic acid receptor-related orphan receptor alpha4 gene by an interaction between hypoxia-inducible factor-1 and Sp1. J Biol Chem. 2004;279:15025–15031. doi: 10.1074/jbc.M313186200. doi:10.1074/jbc.M313186200. [DOI] [PubMed] [Google Scholar]
- 34.Monaco MC, Atwood WJ, Gravell M, Tornatore CS, Major EO. JC virus infection of hematopoietic progenitor cells, primary B lymphocytes, and tonsillar stromal cells: implications for viral latency. J Virol. 1996;70:7004–7012. doi: 10.1128/jvi.70.10.7004-7012.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moon EJ, Jeong CH, Jeong JW, Kim KR, Yu DY, Murakami S, Kim CW, Kim KW. Hepatitis B virus X protein induces angiogenesis by stabilizing hypoxia-inducible factor-1alpha. FASEB J. 2004;18:382–384. doi: 10.1096/fj.03-0153fje. [DOI] [PubMed] [Google Scholar]
- 36.Nasimuzzaman M, Waris G, Mikolon D, Stupack DG, Siddiqui A. Hepatitis C virus stabilizes hypoxia-inducible factor 1alpha and stimulates the synthesis of vascular endothelial growth factor. J Virol. 2007;81:10249–10257. doi: 10.1128/JVI.00763-07. doi:10.1128/JVI.00763-07. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Perez-Liz G, Del Valle L, Gentilella A, Croul S, Khalili K. Detection of JC virus DNA fragments but not proteins in normal brain tissue. Ann Neurol. 2008;64:379–387. doi: 10.1002/ana.21443. doi:10.1002/ana.21443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pillet S, Le Guyader N. Interaction of viruses with cellular response to hypoxia. Med Sci (Paris) 2005;21:517–522. doi: 10.1051/medsci/2005215517. [DOI] [PubMed] [Google Scholar]
- 39.Pillet S, Le Guyader N, Hofer T, NguyenKhac F, Koken M, Aubin JT, Fichelson S, Gassmann M, Morinet F. Hypoxia enhances human B19 erythrovirus gene expression in primary erythroid cells. Virology. 2004;327:1–7. doi: 10.1016/j.virol.2004.06.020. doi:10.1016/j.virol.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 40.Piña-Oviedo S, Urbanska K, Radhakrishnan S, Sweet T, Reiss K, Khalili K, Del Valle L. Effects of JC virus infection on anti-apoptotic protein Survivin in progressive multifocal leukoencephalopathy. Am J Pathol. 2007;170:1291–1304. doi: 10.2353/ajpath.2007.060689. doi:10.2353/ajpath.2007.060689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Radhakrishnan S, Otte J, Enam S, Del Valle L, Khalili K, Gordon J. JC virus-induced changes in cellular gene expression in primary human astrocytes. J Virol. 2003;77:10638–10644. doi: 10.1128/JVI.77.19.10638-10644.2003. doi:10.1128/JVI.77.19.10638-10644.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ranganathan PN, Khalili K. The transcriptional enhancer element, kappa B, regulates promoter activity of the human neurotropic virus, JCV, in cells derived from the CNS. Nucleic Acids Res. 1993;21:1959–1964. doi: 10.1093/nar/21.8.1959. doi:10.1093/nar/21.8.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ravichandran V, Major EO. Viral proteomics: a promising approach for understanding JC virus tropism. Proteomics. 2006;6:5628–5636. doi: 10.1002/pmic.200600261. doi:10.1002/pmic.200600261. [DOI] [PubMed] [Google Scholar]
- 44.Richardson EP., Jr Progressive multifocal leukoencephalopathy. N Engl J Med. 1961;265:815–823. doi: 10.1056/NEJM196110262651701. [DOI] [PubMed] [Google Scholar]
- 45.Romagnoli L, Sariyer IK, Tung J, Feliciano M, Sawaya BE, Del Valle L, Ferrante P, Khalili K, Safak M, White MK. Early growth response-1 protein is induced by JC virus infection and binds and regulates the JC virus promoter. Virology. 2008;375:331–341. doi: 10.1016/j.virol.2008.02.021. doi:10.1016/j.virol.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Royds JA, Dower SK, Qwarnstrom EE, Lewis CE. Response of tumour cells to hypoxia: role of p53 and NFkB. Mol Pathol. 1998;51:55–61. doi: 10.1136/mp.51.2.55. doi:10.1136/mp.51.2.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Safak M, Gallia GL, Khalili K. A 23-bp sequence element from human neurotropic JC virus is responsive to NF-kappa B subunits. Virology. 1999;262:178–189. doi: 10.1006/viro.1999.9886. doi:10.1006/viro.1999.9886. [DOI] [PubMed] [Google Scholar]
- 48.Sanchez-Elsner T, Botella LM, Velasco B, Corbi A, Attisano L, Bernabeu C. Synergistic cooperation between hypoxia and transforming growth factor-beta pathways on human vascular endothelial growth factor gene expression. J Biol Chem. 2001;276:38527–38535. doi: 10.1074/jbc.M104536200. [DOI] [PubMed] [Google Scholar]
- 49.Sanchez-Elsner T, Ramirez JR, Sanz-Rodriguez F, Varela E, Bernabeu C, Botella LM. A cross-talk between hypoxia and TGF-beta orchestrates erythropoietin gene regulation through SP1 and Smads. J Mol Biol. 2004;336:9–24. doi: 10.1016/j.jmb.2003.12.023. doi:10.1016/j.jmb.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 50.Semenza GL. HIF-1 and human disease: one highly involved factor. Genes Dev. 2000;14:1983–1991. [PubMed] [Google Scholar]
- 51.Semenza GL. Surviving ischemia: adaptive responses mediated by hypoxia-inducible factor 1. J Clin Invest. 2000;106:809–812. doi: 10.1172/JCI11223. doi:10.1172/JCI11223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Semenza GL. Hypoxia-inducible factor 1: control of oxygen homeostasis in health and disease. Pediatr Res. 2001;49:614–617. doi: 10.1203/00006450-200105000-00002. doi:10.1203/00006450-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 53.Sodhi A, Montaner S, Patel V, Zohar M, Bais C, Mesri EA, Gutkind JS. The Kaposi’s sarcoma-associated herpes virus G protein-coupled receptor up-regulates vascular endothelial growth factor expression and secretion through mitogen-activated protein kinase and p38 pathways acting on hypoxia-inducible factor 1alpha. Cancer Res. 2000;60:4873–4880. [PubMed] [Google Scholar]
- 54.Tominaga T, Yogo Y, Kitamura T, Aso Y. Persistence of archetypal JC virus DNA in normal renal tissue derived from tumor-bearing patients. Virology. 1992;186:736–741. doi: 10.1016/0042-6822(92)90040-v. doi:10.1016/0042-6822(92)90040-V. [DOI] [PubMed] [Google Scholar]
- 55.Tomita M, Semenza GL, Michiels C, Matsuda T, Uchihara JN, Okudaira T, Tanaka Y, Taira N, Ohshiro K, Mori N. Activation of hypoxia-inducible factor 1 in human T-cell leukaemia virus type 1-infected cell lines and primary adult T-cell leukaemia cells. Biochem J. 2007;406:317–323. doi: 10.1042/BJ20070286. doi:10.1042/BJ20070286. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 56.Tornatore C, Berger JR, Houff SA, Curfman B, Meyers K, Winfield D, Major EO. Detection of JC virus DNA in peripheral lymphocytes from patients with and without progressive multifocal leukoencephalopathy. Ann Neurol. 1992;31:454–462. doi: 10.1002/ana.410310426. doi:10.1002/ana.410310426. [DOI] [PubMed] [Google Scholar]
- 57.Wakisaka N, Kondo S, Yoshizaki T, Murono S, Furukawa M, Pagano JS. Epstein-Barr virus latent membrane protein 1 induces synthesis of hypoxia-inducible factor 1 alpha. Mol Cell Biol. 2004;24:5223–5234. doi: 10.1128/MCB.24.12.5223-5234.2004. doi:10.1128/MCB.24.12.5223-5234.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. doi:10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wei H, Wang C, Chen L. Proliferating cell nuclear antigen, survivin, and CD34 expressions in pancreatic cancer and their correlation with hypoxia-inducible factor 1alpha. Pancreas. 2006;32:159–163. doi: 10.1097/01.mpa.0000202961.71600.9b. doi:10.1097/01.mpa.0000202961.71600.9b. [DOI] [PubMed] [Google Scholar]
- 60.Weir EK, Lopez-Barneo J, Buckler KJ, Archer SL. Acute oxygen-sensing mechanisms. N Engl J Med. 2005;353:2042–2055. doi: 10.1056/NEJMra050002. doi:10.1056/NEJMra050002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yong V, Antel JP. Culture of glial cells from human brain biopsies. In: Federoff S, Richardson A, editors. Protocols for Neural Cell Culture. New York: Humana Press; 1992. pp. 81–96. [Google Scholar]
- 62.Yoo YG, Cho S, Park S, Lee MO. The carboxy-terminus of the hepatitis B virus X protein is necessary and sufficient for the activation of hypoxia-inducible factor-1alpha. FEBS Lett. 2004;577:121–126. doi: 10.1016/j.febslet.2004.10.004. doi:10.1016/j.febslet.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 63.Yoo YG, Oh SH, Park ES, Cho H, Lee N, Park H, Kim DK, Yu DY, Seong JK, Lee MO. Hepatitis B virus X protein enhances transcriptional activity of hypoxia-inducible factor-1alpha through activation of mitogen-activated protein kinase pathway. J Biol Chem. 2003;278:39076–39084. doi: 10.1074/jbc.M305101200. doi:10.1074/jbc.M305101200. [DOI] [PubMed] [Google Scholar]
- 64.Zhang H, Akman HO, Smith EL, Zhao J, Murphy-Ullrich JE, Batuman OA. Cellular response to hypoxia involves signaling via Smad proteins. Blood. 2003;101:2253–2260. doi: 10.1182/blood-2002-02-0629. doi:10.1182/blood-2002-02-0629. [DOI] [PubMed] [Google Scholar]