Abstract
Objective
Depression and stress promote proinflammatory cytokine production. Dietary intakes of omega-3 (n-3) and omega-6 (n-6) polyunsaturated fatty acids (PUFAs) also influence inflammation; high n-6:n-3 ratios enhance proinflammatory cytokine production, while n-3 has anti-inflammatory properties. This study addressed how interactions between PUFA levels and depressive symptoms were related to proinflammatory cytokine synthesis.
Methods
Blood samples from 43 older adults (mean age = 66.67, SD=10.09) provided data on PUFAs and TNF-α, IL-6, and sIL-6r. Depressive symptoms were assessed by the CES-D.
Results
Depressive symptoms and n-6:n-3 ratios worked together to enhance proinflammatory cytokines beyond the contribution provided by either variable alone, with substantial variance explained by their interaction: 13% for IL-6 and 31% for TNF-α, whereas full models accounted for 18% and 40%, respectively. Although predicted cytokine levels were fairly consistent across n-6:n-3 ratios with low depressive symptoms, higher n-6:n-3 ratios were associated with progressively elevated TNF-α and IL-6 levels as depressive symptoms increased. Higher levels of sIL-6r were associated with higher n-6:n-3 ratios. Six individuals who met criteria for major depressive disorder had higher n-6:n-3 ratios and TNF-α, IL-6, and sIL-6r levels than those who did not meet criteria; excluding these 6 individuals reduced the variance explained by the depressive symptoms and n-6:n-3 ratio interaction.
Conclusions
Diets with high n-6:n-3 PUFA ratios may enhance risk for both depression and inflammatory diseases.
Keywords: depression, proinflammatory cytokines, omega-3, psychoneuroimmunology
Depression is the most common psychiatric illness, and both major depression and subthreshold depressive symptoms carry substantial health risks (1, 2). A number of well-controlled prospective studies have linked depressive symptoms with coronary heart disease (CHD), the leading cause of death in the United States (3); moreover, the nearly 60-fold variance in the annual prevalence of major depression across countries is very similar to the pattern for cardiovascular disease, which shows a strong comorbidity for depression (4, 5).
Epidemiological studies have demonstrated significant inverse relationships between annual fish consumption and prevalence of major depression (4). Fish oil is the prime source for two key omega-3 (n-3) polyunsaturated fatty acids (PUFAs), eicosapentaenoic acid (EPA) and docosahexanoic acid (DHA). Several laboratories have provided evidence that depressed patients have, on average, lower plasma levels of n-3 PUFAs than nondepressed controls; furthermore, there are relationships within these populations between severity of depressive symptoms and lower plasma levels of the n-3 PUFAs (3–6). What is more, all but one of four randomized controlled trials reported significant improvement in the treatment of depression following n-3 PUFA supplementation compared to nonsupplemented controls (6). Significant relationships between lower n-3 PUFA plasma levels and greater negative mood have also been documented in nonpsychiatric populations (7). Omega-6 (n-6) PUFAs are also implicated in depression, with higher n-6:n-3 ratios observed in depressed patients compared to nondepressed controls (3–6).
Arachidonic acid (AA) derived n-6 eicosanoids (primarily from refined vegetable oils such as corn, sunflower, and safflower) increase the production of proinflammatory cytokines, operating as precursors of the proinflammatory eicosanoids of the prostaglandin (PG)2-series (5). In contrast, the n-3 PUFAs, found most abundantly in fish, fish oil, walnuts, wheat germ, and flaxseed can curb the production of AA-derived eicosanoids (5, 8). Thus it is not surprising that both higher levels of n-3 PUFAs as well as lower n-6:n-3 ratios are associated with lower proinflammatory cytokine production (9).
The fatty acid composition of the modern Western diet has changed dramatically during the last century, and these changes are thought to be related to increases in inflammatory-related diseases, including depression and cardiovascular disease (6, 10). For example, the early hunter-gatherer diet had an n-6:n-3 ratio of 2:1 to 3:1 (11). However, during the last century, the typical Western diet underwent fundamental alterations with the enormous growth in refined vegetable oil use, a central n-6 source that replaced n-3 PUFAs from fish, wild game, nuts, seeds, and green leafy vegetables (5, 11); in the contemporary North American diet, the n-6:n-3 ratio is 15:1 to 17:1 (12, 13). In fact, the heightened n-6:n-3 ratio in the Western diet since 1913 has been suggested as a central stimulus for the sharply increased incidence of major depression (4, 5).
The n-6:n-3 ratio is related to serotonergic as well as catecholaminergic neurotransmission (14), providing mechanistic pathways that help explain the relationships with negative mood. Alterations in serotonin (5-HT) receptor numbers and function provoked by changes in PUFAs have been used to link fatty acids with contemporary theories of depression (5, 15). Moreover, immune activation may interact with 5-HT functioning to promote depression (5), because PUFAs influence the synthesis of proinflammatory cytokines.
Furthermore, both syndromal depression and depressive symptoms can enhance production of proinflammatory cytokines, including interleukins 1 and 6 (IL-1 and IL-6) and tumor necrosis factor alpha (TNF-α) (2, 16–21). Brief laboratory stressors can also provoke transient increases in proinflammatory cytokines (1, 22, 23). Moreover, stress and depression may effectively “prime” inflammatory responses, promoting larger cytokine increases in reaction to subsequent stressors and/or pathogens (19, 24–27). Thus, chronic or longer-term stress and depression can elevate both acute and chronic proinflammatory cytokine production (16, 20, 23, 28). Importantly, these are bi-directional relationships; cytokines have substantial effects on the central nervous system (CNS), producing and enhancing negative moods, as well as physical symptoms such as lethargy and fatigue (18). Indeed, there is evidence that cytokines play a role in the neuroendocrine and behavioral features of depressive disorders (18).
In a provocative study relevant to the present investigation, students who had higher n-6:n-3 ratios (above the mean) before exams demonstrated greater TNF-α production by lipopolysaccharide (LPS) and mitogen-stimulated peripheral blood leukocytes (PBLs) during exams than those with lower ratios (29). These data suggest that the n-6:n-3 ratio may influence the proinflammatory response to stressors; because TNF-α and IL-6 are produced by a variety of types of cells, serum cytokine levels may better reflect the overall inflammatory profile than stimulated PBLs or ex vivo production (8). Indeed, the aging and depression literatures have focused on proinflammatory cytokine levels assessed in serum (2, 30).
Accordingly, we were interested in how depressive symptoms and the n-6:n-3 ratio would be related to serum TNF-α, IL-6, and the IL-6 soluble receptor (sIL-6r). We studied an older population because proinflammatory cytokine production is elevated following menopause or andropause even in the absence of infection, trauma, or stress (31). Both higher n-6:n-3 ratios and higher levels of depressive symptoms have been associated with greater production of proinflammatory cytokines; however, in accord with the student examination study (29), we expected that higher n-6:n-3 ratios and higher levels of depressive symptoms would predict higher levels of TNF-α, IL-6, and sIL-6r than either alone.
Methods
Subjects
The subjects were part of a larger project on stress and health in older adults. Subjects were recruited via notices placed in community and university newspapers, senior citizen centers, a collaborating neurologist, as well as through the Alzheimer's Disease Association; thus, the 43 participants included 18 spouses who were currently providing at least 5 hours of week of care for a spouse with Alzheimer's disease or another progressive dementia, and 25 noncaregivers who were demographically indistinguishable from caregivers but had no similar responsibilities. Inclusion of dementia spousal caregivers in this study was desirable because caregivers have, on average, higher levels of syndromal depression as well as depressive symptoms than noncaregivers (28). Subjects with immunologically-related health problems such as cancer or recent surgeries were excluded during recruitment, as well as those taking any medications with broad immunological consequences. These data were collected between September, 2004 and September, 2005.
For this study we used all individuals in the current cohort who were not taking statins, steroids, or medications for diabetes. Of the 23 subjects excluded for these medications, 10 were caregivers and 13 were noncaregivers; 9 of the 28 caregivers who were part of the larger cohort met current criteria for MDD, compared to 4/38 noncaregivers, χ2(1) = 4.32, P=.03. However, when we eliminated subjects based on our medication exclusions, we ended up with 3 caregivers and 3 noncaregivers and a nonsignificant group difference in terms of current MDD, χ2(1) = 0.19, P=.66. Thus, our medication exclusions substantially reduced the number of strained caregivers, and may also have attenuated group-related differences in cytokines (16, 28).
The average age of the 18 men and 25 women in this sample was 66.67 (SD=10.09; the range was 40–86 with one subject under 50, 12 who were 50–59, 13 who were 60–69, 12 who were 70–79, and 5 who were 80 or older); the median education was partial college; three were African American; all were married. The Ohio State University Biomedical Research Review Committee approved the project; all subjects gave written informed consent prior to participation.
Assessment of Mental Health, Depressive Symptoms, and Health-Related Behaviors
The Diagnostic Interview for Genetic Studies (DIGS) (32), developed for the differential diagnosis of major mood and psychotic disorders, provided data on both current and lifetime disorders using DSM-IV criteria. Interviews were administered by well-trained graduate psychology or nursing students who were supervised by a psychologist with extensive experience with structured interviews.
The Center for Epidemiological Studies Depression Scale (CES-D) assessed the severity of depressive symptoms (33, 34). Studies have shown acceptable test-retest reliability and excellent construct validity (34). Widely used, the CES-D has distinguished depressed from non-depressed participants in community and clinical samples (34).
We collected health-related data to assess the possibility that relationships among depressive symptoms, inflammatory markers, and PUFAs might simply reflect the contribution of other variables. Health questions from the Older Adults Resources Survey (OARS) (35) assessed underlying diseases. Several studies have found excellent agreement between self-reports and hospital or physician records for specific conditions of interest, including myocardial infarction, stroke, and diabetes (36, 37). Assessment of health-related behaviors included body mass index (BMI), smoking, medications, and alcohol intake (38). Two questions assessed exercise (39).
The Pittsburgh Sleep Quality Index (PSQI) (40), a self-rated questionnaire, provides data on sleep quality and disturbances over a one-month interval. The PSQI has good diagnostic sensitivity and specificity in distinguishing good and poor sleepers (40). We also asked about the amount of sleep the prior night.
Immunological Assays
All blood samples were drawn between 8 AM and 10 AM to control for diurnal variation. TNF-α, IL-6, and sIL-6r levels were assayed using Quantikine High Sensitivity Immunoassay kits (R&D), per kit instructions, as described elsewhere (19, 28). Serum samples were frozen 45 min - 1 hr after collection. Serum samples were run undiluted in duplicate.
Fatty Acid Analyses
Lipids were extracted from plasma using chloroform: methanol (2:1, v/v) with 0.2 vol. 0.88% KCl (41). Fatty acid methyl esters of the fractions were prepared by incubating the fractions with tetramethylguanidine at 100°C (42) and analyzed by gas chromatography (Hewlett Packard) using a 30-m Omegawax 320 (Supelco-Sigma) capillary column. The helium flow rate was 30 ml/min and oven temperature ramped beginning at 175°C and held for 4 min then increased to 220°C at a rate of 3°C/min as described previously (43). Retention times were compared to authentic standards for fatty acid methyl esters (Supelco-Sigma, St. Louis, MO and Matreya, Inc, Pleasant Gap, PA). Blood samples were not drawn in a fasting state.
Statistical Analysis
Linear regression models were fit to each cytokine, with the n-6:n-3 ratio and the CES-D score as the independent variables. The interaction between the n-6:n-3 ratio and CES-D was also included in each model. Several sociodemographic variables, including age and gender, as well as health behavior and medication usage variables were assessed as potential confounders or effect modifiers. A variable was considered a confounder if inclusion in the model resulted in a change of more than 15% in one of the model coefficients. A variable was considered an effect modifier if there was a significant interaction between that variable and a variable in the primary model. To correct for skewness in the dependent variables, base 10 log-transformed data were used. Statistical significance was evaluated using 2-sided tests at the .05 level of significance.
Fatty acid and cytokine levels were compared for subjects with and without current major depression using analysis of variance (ANOVA) when the data were approximately normally distributed with homogeneous variance. Wilcoxon’s rank sum test was used when the data were not normally distributed.
Results
Table 1 shows the percentages of the key fatty acids. Two subjects with extreme n-6:n-3 ratios of 49.35 and 168.18 (> 9 SDs from the mean) were excluded from analyses using PUFA data as well as from the group means shown in the table. AA/EPA and AA/DHA ratios allow comparisons with recent reports linking these ratios with depression (3, 7).
Table 1.
Relative fatty acid composition of plasma phospholipids
| Fatty Acid | Means (% fatty acid) | SD |
|---|---|---|
| n-3 Series | ||
| Alpha Linolenic 18:3n-3 | 0.64 | 0.22 |
| Stearidonic 18:4n-3 | 0.00 | 0.00 |
| Eicosatetraenoic 20:4n-3 | 0.33 | 0.16 |
| Eicosapentataenoic 20:5n-3 | 0.73 | 0.57 |
| Docosapentaenoic (n-3) 22:5n-3 | 0.19 | 0.13 |
| Docosahexaenoic 22:6n-3 | 0.82 | 0.25 |
| Total EPA and DHA | 1.55 | 0.72 |
| n-3 Total | 2.71 | 0.84 |
| n-6 Series | ||
| Linoleic 18:2n-6 | 25.7 | 5.01 |
| Gamma-linoleic 18:3n-6 | 0.02 | 0.03 |
| Dihomogammalinolenic 20:3n-6 | 1.08 | 2.72 |
| Arachidonic 20:4n-6 | 10.21 | 5.82 |
| Docosadienoic 22:2n-6 | 0.28 | 0.16 |
| n-6 total | 38.53 | 5.98 |
| Ratios | ||
| n-6:n-3 ratio | 14.13 | 3.42 |
| AA/EPA ratio | 19.14 | 27.41 |
| AA/DHA ratio | 12.27 | 10.38 |
Table 2 provides data on the depressive symptoms and cytokines. The correlation between the n-6:n-3 ratio and CES-D scores was r(39)=.24, P=.12.
Table 2.
Means and SDs for Depressive Symptoms and Cytokines
| Measure | Means | SD |
|---|---|---|
| CES-D (depressive symptoms) | 7.41 | 5.86 |
| IL-6, pg/ml (raw) | 1.48 | 1.61 |
| TNF- α, pg/ml (raw) | 1.76 | 1.61 |
| sIL-6r, pg/ml (raw) | 48643.90 | 20364.04 |
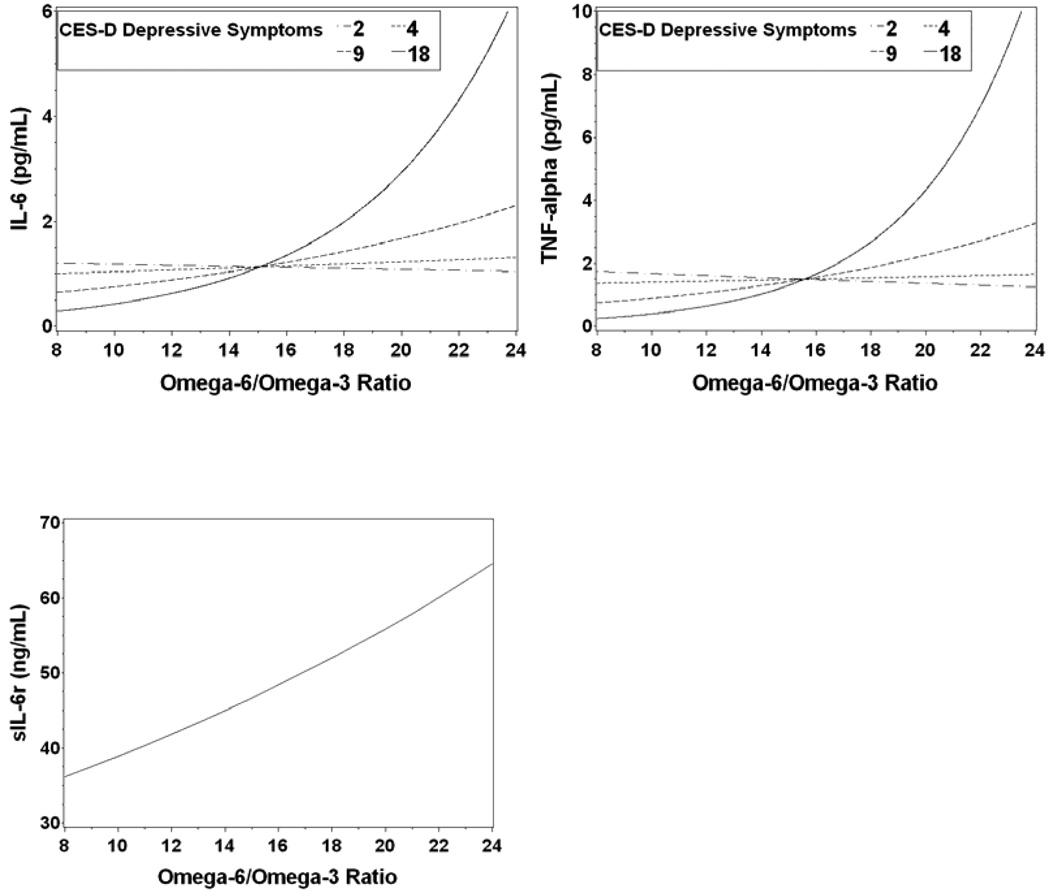
Table 3 shows the significant interaction between the n-6:n-3 ratio and depressive symptoms (CES-D) in the model for IL-6 (F1,37 = 5.76, P = .02) as well as in the model for TNF-α, (F1,37 = 19.55, P < .001). For both the IL-6 and TNF-α models the relationship between cytokine levels and the n-6:n-3 ratio was modified by depressive symptoms. Figures 1a and 1b show that the cytokine levels were relatively consistent at lower depressive symptom levels, while at higher levels there was a marked increase in cytokine levels as the n-6:n-3 ratio increased. The interactions explained substantial amounts of the variance beyond that explained by the individual contributions of depressive symptoms and the n-6:n-3 ratio. The full model explained 18% of the total variance in IL-6, an increase of 13% over the model without the interaction. The full model explained 40% of the total variance in TNF-α, an increase of 31% over the model without the interaction.
Table 3.
Parameter estimates and standard errors for IL-6 and TNF- α*
| Cytokine | Regression Parameter Estimates (Standard Errors) |
Interaction p-value |
|||
|---|---|---|---|---|---|
| Intercept |
n-6:n-3 Ratio |
Depressive Symptoms |
Interaction | ||
| IL-6 (log10) | .275 (.267) | −.015 (.019) | −.083 (.034) | .005 (.002) | .022 |
| TNF-α (log10) | .535 (.187) | −.023 (.013) | −.110 (.024) | .007 (.002) | <.001 |
The parameter estimates can be interpreted in terms of the slope of the regression of the cytokine on the n-6:n-3 ratio dependent on the level of depressive symptoms. For example, the slope for the regression of log-transformed IL-6 on n-6:n-3 ratio is (−).015 + .005·depressive symptoms. Therefore the slope is zero when depressive symptoms are equal to 3.0 and increases by .005 for each unit increase in depressive symptoms.
Figure 1.
a–c. IL-6 (1a) and TNF-α (1b) plots showing the predicted cytokine response by n-6:n-3 ratio separately for different CES-D depressive symptom levels. Although the analyses used continuous variables, predicted cytokine values are plotted for the midpoint of each depressive symptom quartile (2, 4, 9, 18) to illustrate the effect of depression on the cytokine n-6:n-3 ratio relationship. The predicted values for IL-6 and TNF-α are non-linear because the model was on the log scale and then the predicted values were transformed back to the original scale.
In the IL-6sr model (1c) the n-6:n-3 ratio main effect was significant, and the ratio did not differ significantly by depression. Therefore only one response curve is on the plot, at the mean depression level of 7.4. Responses at other depression levels would yield parallel curves.
To assess the estimation of cytokine levels by different fatty acid measures, we compared the results obtained using the n-6:n-3 ratio – the relative balance of n-6 and n-3 – with models that used either n-6 or n-3 individually. Each of these models contained one of either the n-6:n-3 ratio, n-6 alone, or n-3 alone as one independent variable; models also contained CES-D as the second independent variable and the interaction between the two independent variables. For IL-6, the model using the n-6:n-3 ratio accounted for 18% of the total variance, while the model with n-3 alone accounted for 12% of the total variance, and the model with n-6 alone accounted for less than 1%. For TNF-α, the n-6:n-3 ratio model accounted for 40%, the n-3 model 27%, and the n-6 model 3%. Thus, in estimating IL-6 and TNF-α levels, the n-6:n-3 ratio explained more of the total variation than either n-3 or n-6 alone.
Another multiple regression model was fit with sIL-6r as the dependent variable. The n-6:n-3 ratio and CES-D were the independent variables. The n-6:n-3 ratio was significantly associated with sIL-6r (F1,38= 4.17, P = .05). The interaction between the n-6:n-3 ratio and CES-D was not significant and the interaction term was dropped from the model. Model coefficients were 4.46 (SE = .11) for the intercept, .016 (.008) for the n-6:n-3 ratio, and −.004 (.005) for CES-D.
Major Depression
Six participants met criteria for current major depression; 5 of these were taking antidepressants. The n-6:n-3 ratio was significantly higher in those with current major depression than among those without (F1,39 = 10.72, P = .002; 17.92 ± 3.25 vs. 13.49 ± 3.04), while n-3 (P = .12) and n-6 (P = .17) differences were nonsignificant.
Participants with current major depression also had significantly higher cytokine production than those who were not clinically depressed. TNF-α was higher in the former than the latter (Wilcoxon P = .05; 3.16 ± 3.63 vs. 1.49 ± 0.84), as were IL-6 (F1,41 = 4.20, P = .05; 2.86 ± 3.55 vs. 1.22 ± 0.89), and sIL-6r (F1,41 = 4.05, P = .05; 63.93 ± 28.81 vs. 45.74 ± 17.35 ng/mL). Cytokine means and standard deviations are reported on the raw scale, while log-transformed values were used in the analyses.
Exclusion of all participants who met criteria for major depression from the analysis predictably affected the results, reducing the variance explained by the interaction for IL-6 to a nonsignificant increment; however, for TNF-α the full model explained 26% of the variance, with the interaction accounting for 10% of that variance even in this reduced sample.
As described earlier, the relationship between depressive symptoms and cytokines is bi-directional, and thus we calculated how much variance in depressive symptoms was explained by the measured cytokine levels together with n-6:n-3. For IL-6, the interaction was not significant at the 0.05 level (P=0.109). However, the model with the interaction did explain substantially more of the variance in depressive symptoms than the model without the interaction, increasing from 6% to 13%. The interaction between TNF-α and n-6:n-3 was significant (P=0.046) in predicting depressive symptoms. The model with the interaction explained 18% of the variance in depressive symptoms, compared to 8% explained by the model without the interaction.
Depressive Symptoms, Cytokines, Fatty Acids, Sociodemographic Data, and Health Behaviors
Sociodemographic and health behavior variables that were significantly correlated with cytokine levels, fatty acid levels, or depressive symptoms were evaluated as potential confounders or effect modifiers by adding these variables to the models for IL-6, TNF-α, and sIL-6r. None of age, level of education, BMI, hours of sleep the prior night, or exercise (hours of vigorous physical activity per week) were significantly correlated with cytokine levels, fatty acid levels, or depressive symptoms, all Ps>.18. Caregivers and controls did not differ significantly on depressive symptoms, P=.24, the n-6:n-3 ratio, P=.74, IL-6r, P=.82, IL-6, P=.51, or TNF-α, P=.25. Two subjects were smokers. Typical weekly alcohol intake was negatively associated with IL-6, rs=−.47, P=.002; the median number of drinks reported was 1, and only 2 subjects reported more than 7 alcoholic drinks in an average week, (12 and 14 drinks per week, respectively). In addition, sleep over the last month as measured by the PSQI (full scale) was significantly correlated with depressive symptoms, r(41)=.51, P=.001. Including the PSQI and alcohol intake in the models for IL-6, TNF-α, and sIL-6r did not change the significant coefficients by more than 15% for any of the models. Thus, neither of these variables confounded the association between cytokine levels, the n-6:n-3 ratio, and depressive symptoms.
Medication use and chronic health problems were also considered as potential confounders. The most common medications taken by subjects were angiotensin (10 subjects), antidepressants (9), NSAIDs (9), calcium channel blockers (8), anticoagulants (6), thyroid supplements (6), osteoporosis medications (6), antacids (5), diuretics (5), beta blockers (4), and estrogen supplements (4). Variables for the use of each of these medications were added individually to the models for IL-6, TNF-α, and sIL-6r. A variable indicating subjects not taking any medications (11 subjects) was also considered to evaluate the effect of chronic health problems. The CES-D by n-6:n-3 ratio interaction coefficient did not change by more than 15% for any of the medications in the IL-6 or TNF-α models. In the sIL-6r models, antidepressant use had a slight confounding effect on the association between sIL-6r and the n-6:n-3 ratio, decreasing the coefficient for the n-6:n-3 ratio by 16%. However, there was no significant interaction between antidepressant use and the n-6:n-3 ratio, so antidepressant use was not an effect modifier and the relationship between sIL-6r and the n-6:n-3 ratio was consistent among those taking and those not taking antidepressants.
Potential confounders for IL-6, TNF-α, and sIL-6r models were assessed to determine if the significant relationships among cytokines, fatty acids, and depressive symptoms might be a function of differences in sociodemographic variables, health behaviors, or medication use. We found no evidence that the significant interactions between the n-6:n-3 ratio and CES-D in the models for IL-6 and TNF-α or the significant association between the n-6:n-3 ratio and sIL-6r were simply a function of chronic health problems, medications, or health habits.
Discussion
Higher levels of depressive symptoms as well as higher n-6:n-3 ratios worked together to markedly enhance proinflammatory cytokines beyond the contribution provided by either variable alone. Indeed, the amount of variance explained by the interactions alone was substantial,13% for IL-6 and 31% for TNF-α; the full models accounted for 18% and 40%, respectively. These data support and extend the results reported for students taking examinations (29): we found the same pattern they reported for TNF-α with both IL-6 and TNF-α. We also found that individuals who met criteria for syndromal depression had significantly higher n-6:n-3 ratios as well as higher TNF-α, IL-6, and sIL-6r levels than those who did not meet criteria. Moreover, because we were able to show the relationships in serum cytokine levels rather than with levels of cytokines produced by stimulated PBLs, the data provide an important bridge with the aging and depression literatures. Thus, a diet with a high ratio of n-6 to n-3 ratio may enhance risk for both depression as well as inflammatory-related diseases.
Indeed, proinflammatory cytokines influence the onset and course of a spectrum of conditions associated with aging, including CHD, osteoporosis, arthritis, type 2 diabetes, about 15% of cancers, Alzheimer’s disease, and periodontal disease (31, 44, 45). In fact, more globally, chronic inflammation has been suggested as one key biological mechanism that may fuel declines in physical function leading to frailty, disability, and, ultimately, death (30, 46, 47).
The fact that key inflammatory pathways are influenced by both stress and diet provides one obvious mechanism for our findings. Transcription factor nuclear factor kappa B (NF-κB) activation upregulates proinflammatory cytokine production (48–50). Psychological stress promotes NF-κB activation (49, 50), providing a mechanism for translating psychological stress into mononuclear cell activation (49). For example, patients with major depression demonstrated significantly greater stress-induced plasma IL-6 levels and mononuclear cell NF-κB activation than non-depressed controls, and the magnitude of the increase following a laboratory stressor was correlated with depressive symptoms (51).
In contrast, two key n-3 PUFAs, EPA and DHA, can substantially decrease lipopolysaccharide-induced TNF-α expression by blocking NF-κB activation (48). Indeed, recent work suggests that even modest supplementation with n-3 PUFAs reduces plasma norepinephrine, an important link to stress responses via NF-κB (52, 53).
In contrast to IL-6 and TNF-α, sIL-6r was significantly related to the n-6:n-3 ratio, but not to depressive symptoms, or to the interaction between the two. However, patients who met criteria for major depression had higher sIL-6r levels than those who did not meet criteria. Mechanistically, sIL-6R forms a ligand-receptor complex, and thus can regulate the impact of IL-6 on inflammation (54). In effect, the sIL-6R expands the cell types that can respond to IL-6, and thus it is not surprising that higher sIL-6R levels have been associated with inflammatory disorders in addition to depression, including arthritis, asthma, and inflammatory bowel disease (54).
Proinflammatory cytokine secretion increases with age (31), and stress and depression can further enhance age-related production (16–19, 23, 28). In this context it is interesting to note that older subjects show larger immunological changes in n-3 supplementation studies, and also respond with greater increases in plasma EPA and DHA and larger decreases in AA than younger participants (55). The ties between age-associated diseases and proinflammatory cytokine production (31) highlight the potential importance of these changes.
Analyses of fatty acids in plasma reflect the past few weeks or months of dietary intake; longitudinal studies suggest that reliability is good over periods from six months to two to three years (56, 57), and thus it is not surprising that PUFA levels do not appear to change significantly in response to stressors (29). We did not have food frequency questionnaire data which would have been a useful adjunct to evaluate factors such as differences in dietary intake that could be related to depressive symptoms. However, although food frequency questionnaires are well validated and widely used, fatty acid exposure depends not only on intake, but also absorption and metabolism (9), and thus plasma PUFA levels provide a key endpoint. Our n-6:n-3 ratio of 14:1 closely approximates the contemporary North American diet; other studies have reported n-6:n-3 ratios of 15:1 to 17:1 (12, 13).
Some of the strongest data linking PUFAs and serum proinflammatory cytokine levels comes from a large cross-sectional study in which researchers assessed serum fatty acids, as well as key inflammatory markers (9). Higher levels of n-3 PUFAs were associated with lower IL-6 and TNF-α (9). Importantly, in both cases there was an evident dose-response relationship between the n-3 PUFAs and the cytokines (9).
This study is limited by its cross-sectional nature and small sample size; indeed, when individuals who met criteria for major depression were eliminated from analyses, the consequent restricted ranges for both depressive symptoms and n-6:n-3 ratios attenuated the variance explained for IL-6 and TNF-α (although the TNF-α interaction remained significant). We do not see the attenuated variance as a reflection of the unique contributions of clinical depression, because an exam stress study clearly demonstrated that the joint contributions of stressors and higher n-6:n-3 ratios enhance proinflammatory cytokine production independent of syndromal depression (29).
Indeed, although Maes et al. (29) excluded any student who had a current or past Axis I psychiatric disorder, and any student who reported a major negative life event in the prior year, they found substantially enhanced TNF-α production during exams in students with higher n-6:n-3 ratios (> 400%) compared to those with lower ratios (~50%), despite their small sample, n=27. Importantly, their “high” group’s n-6:n-3 ratio was 13.38 (SD=3.29), compared to our sample’s n-6:n-3 mean of 14.13 (SD=3.42), while their “low” group had a mean of 6.15 (SD=1.20); only 4 of our subjects would have fallen into their low group. Thus, together their data and ours suggest that the joint contributions of higher n-6:n-3 ratios and depressive symptoms or stress can markedly enhance proinflammatory cytokine production; a randomized controlled supplementation trial would provide the optimal cause-and-effect demonstration.
Epidemiological studies have clearly linked elevated proinflammatory cytokines with depressive symptoms (2), as well as elevated n-6:n-3 ratios (9). However, other researchers have not always found depression-cytokine relationships or have reported mixed results for different cytokines (18). Our data suggest that a lower n-6:n-3 ratio offers some protection, particularly as depressive symptoms increase; accordingly, it is possible that differences in participants' dietary n-3 intakes may have contributed to the variability in depression-cytokine relationships observed among other studies (18).
Furthermore, increased inflammatory activity has been associated with treatment-resistant depression in several studies (18), and preliminary evidence suggests that n-3 supplementation is beneficial for treatment-resistant depression (58, 59). If the combination of a high n-6:n-3 ratio concomitant with high depressive symptoms promotes inflammation, then our data suggest that one obvious potential benefit of n-3 supplementation would be decrements in cytokine production; as noted earlier, cytokines have substantial CNS effects, including the production and enhancement of negative moods, and thus supplementation may be beneficial because it interrupts a maladaptive feedback loop (18).
In summary, our findings highlight ways in which diet may enhance or inhibit depression-related inflammation among older adults. These behavior-dietary-immune interactions have important implications for both mental and physical health.
Acknowledgments
The study was supported in part by NIH grant AG025732, by General Clinical Research Center Grant MO1-RR-0034, by Ohio State University Comprehensive Cancer Center Core Grant CA16058, and by funds from the Carol S. Kennedy endowment.
We appreciate the helpful assistance of Mary Dodge, Gayle Shrode, Bryon Laskowski, and Monica Litsky with the study, as well as the Central Ohio Alzheimer's Association.
Acronyms
- AA
arachidonic acid
- BMI
body mass index
- CES-D
Center for Epidemiological Studies Depression Scale
- DHA
docosahexanoic acid
- DIGS
Diagnostic Interview for Genetic Studies
- EPA
eicosapentaenoic acid
- IL-6
interleukin 6
- sIL-6r
IL-6 soluble receptor
- n-3
omega-3
- n-6
omega-6
- NF-κB
nuclear factor kappa B
- PSQI
Pittsburgh Sleep Quality Index
- PUFA
polyunsaturated fatty acid
- TNF-α
tumor necrosis factor alpha
References
- 1.Miller GE, Rohleder N, Stetler C, Kirschbaum C. Clinical depression and regulation of the inflammatory response during acute stress. Psychosom Med. 2005;67:679–687. doi: 10.1097/01.psy.0000174172.82428.ce. [DOI] [PubMed] [Google Scholar]
- 2.Penninx BWJH, Kritchevsky SB, Yaffe K, Newman AB, Simonsick EM, Rubin S, Ferrucci L, Harris T, Pahor M. Inflammatory markers and depressed mood in older persons: Results from the health, aging, and body composition study. Biol Psychiatry. 2003;54:566–572. doi: 10.1016/s0006-3223(02)01811-5. [DOI] [PubMed] [Google Scholar]
- 3.Frasure-Smith N, Lesperance F, Julien P. Major depression is associated with lower omega-3 fatty acid levels in patients with recent acute coronary syndromes. Biol Psychiatry. 2004;55:891–896. doi: 10.1016/j.biopsych.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 4.Hibbeln JR. Fish consumption and major depression. Lancet. 1998;351:1213. doi: 10.1016/S0140-6736(05)79168-6. [DOI] [PubMed] [Google Scholar]
- 5.Maes M, Smith RS. Fatty acids, cytokines, and major depression. Biol Psychiatry. 1998;43:313–314. doi: 10.1016/s0006-3223(97)00401-0. [DOI] [PubMed] [Google Scholar]
- 6.Hallahan B, Garland MR. Essential fatty acids and mental health. Br J Psychiatry. 2005;186:275–277. doi: 10.1192/bjp.186.4.275. [DOI] [PubMed] [Google Scholar]
- 7.Tiemeier H, van Tuijl HR, Hofman A, Kiliaan AJ, Breteler MMB. Plasma fatty acid composition and depression are associated in the elderly: The Rotterdam study. Am J Clin Nutr. 2003;78:40–46. doi: 10.1093/ajcn/78.1.40. [DOI] [PubMed] [Google Scholar]
- 8.Pischon T, Hankinson SE, Hotamisligil GS, Rifai N, Willett WC, Rimm EB. Habitual dietary intake of n-3 and n-6 fatty acids in relation to inflammatory markers among US men and women. Circulation. 2003;108:155–160. doi: 10.1161/01.CIR.0000079224.46084.C2. [DOI] [PubMed] [Google Scholar]
- 9.Ferrucci L, Cherubini A, Bandinelli S, Bartali B, Corsi A, Lauretani F, Martin A, Andres-Lacueva C, Senin U, Guralnik JM. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J Clin Endocrinol Metab. 2006;91:439–446. doi: 10.1210/jc.2005-1303. [DOI] [PubMed] [Google Scholar]
- 10.Weber PC, Leaf A. Cardiovascular effects of omega 3 fatty acids: Atherosclerosis risk factor modification by omega 3 fatty acids. World Rev Nutr Diet. 1991;66:218–232. [PubMed] [Google Scholar]
- 11.Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, O'Keefe JH, Brand-Miller J. Origins and evolution of the Western diet: Health implications for the 21st century. Am J Clin Nutr. 2005;81:341–354. doi: 10.1093/ajcn.81.2.341. [DOI] [PubMed] [Google Scholar]
- 12.Hibbeln JR, Umhau JC, George DT, Salem NJ. Do plasma polyunsaturates predict hostility and depression? World Rev Nutr Diet. 1997;82:175–186. doi: 10.1159/000059633. [DOI] [PubMed] [Google Scholar]
- 13.Simopoulos AP. Omega-3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr. 2002;21:495–505. doi: 10.1080/07315724.2002.10719248. [DOI] [PubMed] [Google Scholar]
- 14.Haag M. Essential fatty acids and the brain. Can J Psychiatry. 2003;48:195–203. doi: 10.1177/070674370304800308. [DOI] [PubMed] [Google Scholar]
- 15.Maes M, Christophe A, Delanghe J, Altamura C, Neels H, Meltzer HY. Lowered omega-3 polyunsaturated fatty acids in serum phospholipids and cholesteryl esters of depressed patients. Psychiatry Res. 1999;85:275–291. doi: 10.1016/s0165-1781(99)00014-1. [DOI] [PubMed] [Google Scholar]
- 16.Lutgendorf SK, Garand L, Buckwalter KC, Reimer TT, Hong S, Lubaroff DM. Life stress, mood disturbance, and elevated interleukin-6 in healthy older women. J Gerontol A Biol Sci Med Sci. 1999;54:M434–M439. doi: 10.1093/gerona/54.9.m434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Irwin M. Psychoneuroimmunology of depression: Clinical implications. Brain Behav Immun. 2002;16:1–16. doi: 10.1006/brbi.2001.0654. [DOI] [PubMed] [Google Scholar]
- 18.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glaser R, Robles T, Sheridan J, Malarkey WB, Kiecolt-Glaser JK. Mild depressive symptoms are associated with amplified and prolonged inflammatory responses following influenza vaccination in older adults. Arch Gen Psychiatry. 2003;60:1009–1014. doi: 10.1001/archpsyc.60.10.1009. [DOI] [PubMed] [Google Scholar]
- 20.Maes M, Bosmans E, De Jongh R, Kenis G, Vandoolaeghe E, Neels H. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine. 1997;9:853–858. doi: 10.1006/cyto.1997.0238. [DOI] [PubMed] [Google Scholar]
- 21.Suarez EC, Krishnan RR, Lewis JG. The relation of severity of depressive symptoms to monocyte-associated proinflammatory cytokines and chemokines in apparently healthy men. Psychosom Med. 2003;65:362–368. doi: 10.1097/01.psy.0000035719.79068.2b. [DOI] [PubMed] [Google Scholar]
- 22.Goebel MU, Mills PJ, Irwin MR, Ziegler MG. Interleukin-6 and tumor necrosis factor-alpha production after acute psychological stress, exercise, and infused isoproterenol: Differential effects and pathways. Psychosom Med. 2000;62:591–598. doi: 10.1097/00006842-200007000-00019. [DOI] [PubMed] [Google Scholar]
- 23.Kiecolt-Glaser JK, Loving TJ, Stowell JR, Malarkey WB, Lemeshow S, Dickinson SL, Glaser R. Hostile marital interactions, proinflammatory cytokine production, and wound healing. Arch Gen Psychiatry. 2005;62:1377–1384. doi: 10.1001/archpsyc.62.12.1377. [DOI] [PubMed] [Google Scholar]
- 24.Johnson JD, O'Connor KA, Deak T, Stark M, Watkins LR, Maier SF. Prior stressor exposure sensitizes LPS-induced cytokine production. Brain Behav Immun. 2002;16:461–476. doi: 10.1006/brbi.2001.0638. [DOI] [PubMed] [Google Scholar]
- 25.Johnson JD, O'Connor KA, Deak T, Spencer RL, Watkins LR, Maier SF. Prior stressor exposure primes the HPA axis. Psychoneuroendocrinology. 2002;27:353–365. doi: 10.1016/s0306-4530(01)00057-9. [DOI] [PubMed] [Google Scholar]
- 26.Dantzer R, Wollman E, Vitkovic L, Yirmiya R. Cytokines and depression: Fortuitous or causative association? Mol Psychiatry. 1999;4:328–332. doi: 10.1038/sj.mp.4000572. [DOI] [PubMed] [Google Scholar]
- 27.Maes M, Ombelet W, De Jongh R, Kenis G, Bosmans E. The inflammatory response following delivery is amplified in women who previously suffered from major depression, suggesting that major depression is accompanied by a sensitization of the inflammatory response system. J Affect Disord. 2001;63:85–92. doi: 10.1016/s0165-0327(00)00156-7. [DOI] [PubMed] [Google Scholar]
- 28.Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci U S A. 2003;100:9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maes M, Christophe A, Bosmans E, Lin AH, Neels H. In humans, serum polyunsaturated fatty acid levels predict the response of proinflammatory cytokines to psychologic stress. Biol Psychiatry. 2000;47:910–920. doi: 10.1016/s0006-3223(99)00268-1. [DOI] [PubMed] [Google Scholar]
- 30.Taaffe DR, Harris TB, Ferrucci L, Rowe J, Seeman TE. Cross-sectional and prospective relationships of interleukin-6 and C-reactive protein with physical performance in elderly persons: MacArthur Studies of Successful Aging. J Gerontol A Biol Sci Med Sci. 2000;55:M709–M715. doi: 10.1093/gerona/55.12.m709. [DOI] [PubMed] [Google Scholar]
- 31.Ershler W, Keller E. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000;51:245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- 32.Nurnberger JI, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T. Diagnostic interview for genetic studies. Arch Gen Psychiatry. 1994;51:849–859. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- 33.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 34.Basco MR, Krebaum SR, Rush AJ. Outcome measures of depression. In: Strupp HH, Horowitz LM, Lambert MJ, editors. Measuring patient changes in mood, anxiety, and personality disorders. Washington D. C: American Psychological Association; 1997. pp. 207–245. [Google Scholar]
- 35.Fillenbaum GG, Smyer MA. The development, validity, and reliability of the OARS Multidimensional Functional Assessment Questionnaire. J Gerontol. 1981;36:428–434. doi: 10.1093/geronj/36.4.428. [DOI] [PubMed] [Google Scholar]
- 36.Bush TL, Miller SR, Golden AL, Halle WE. Self-reports and medical report agreement of selected medical conditions in the elderly. Am J Public Health. 1989;79:1554–1556. doi: 10.2105/ajph.79.11.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kehoe R, Wu SY, Leske MC, Chylack LT. Comparing self-reported and physician reported medical history. Am J Epidemiol. 1994;139:813–818. doi: 10.1093/oxfordjournals.aje.a117078. [DOI] [PubMed] [Google Scholar]
- 38.Kiecolt-Glaser JK, Glaser R. Methodological issues in behavioral immunology research with humans. Brain Behav Immun. 1988;2:67–78. doi: 10.1016/0889-1591(88)90007-4. [DOI] [PubMed] [Google Scholar]
- 39.Washburn RA, Adams LL, Hale GT. Physical activity assessment for epidemiologic research: The utility of two simplified approaches. Prev Med. 1987;16:626–646. doi: 10.1016/0091-7435(87)90047-8. [DOI] [PubMed] [Google Scholar]
- 40.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 41.Bligh WJ, Dyer EG. A rapid method of total lipid extraction and purification. Canadian Journal of Biochemical Physiology. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 42.Shantha NC, Decker EA, Hennig B. Comparison of methylation methods for the quantitation of conjugated linoleic acid Isomers. J AOAC Int. 1993;76:644–649. [Google Scholar]
- 43.Belury MA, Kempa-Steczko A. Conjugated linoleic acid modulates hepatic lipid composition in mice. Lipids. 1997;32:199–204. doi: 10.1007/s11745-997-0025-0. [DOI] [PubMed] [Google Scholar]
- 44.Kop WJ, Gottdiener JS, Tangen CM, Fried LP, McBurnie MA, Walston J, Newman A, Hirsch C, Tracy RP. Inflammation and coagulation factors in persons>65 years of age with symptoms of depression but without evidence of myocardial ischemia. Am J Cardiol. 2002;89:419–424. doi: 10.1016/s0002-9149(01)02264-0. [DOI] [PubMed] [Google Scholar]
- 45.Marx J. Inflammation and cancer: The link grows stronger. Science. 2004;306:966–968. doi: 10.1126/science.306.5698.966. [DOI] [PubMed] [Google Scholar]
- 46.Black PH. The inflammatory response is an integral part of the stress response: Implications for atherosclerosis, insulin resistance, type II diabetes and metabolic syndrome X. Brain Behav Immun. 2003;17:350–364. doi: 10.1016/s0889-1591(03)00048-5. [DOI] [PubMed] [Google Scholar]
- 47.Colbert LH, Visser M, Simonsick EM, Tracy RP, Newman AB, Kritchevsky SB, Pahor M, Taaffe DR, Brach J, Rubin S, Harris TB. Physical activity, exercise, and inflammatory markers in older adults: Findings from the health, aging and body composition study. J Am Geriatr Soc. 2004;52:1098–1104. doi: 10.1111/j.1532-5415.2004.52307.x. [DOI] [PubMed] [Google Scholar]
- 48.Zhao Y, Joshi-Barve S, Barve S, Chen LH. Eicosapentaenoic acid prevents LPS-induced TNF-alpha expression by preventing NF-kappaB activation. J Am Coll Nutr. 2004;23:71–78. doi: 10.1080/07315724.2004.10719345. [DOI] [PubMed] [Google Scholar]
- 49.Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, Ferstl R, von Eynatten M, Wendt T, Rudofsky G, Joswig M, Morcos M, Schwaninger M, McEwen B, Kirschbaum C, Nawroth PP. A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci U S A. 2003;100:1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nagabhushan M, Mathews HL, Witek-Janusek L. Aberrant nuclear expression of AP-1 and NFkappaB in lymphocytes of women stressed by the experience of breast biopsy. Brain Behav Immun. 2001;15:78–84. doi: 10.1006/brbi.2000.0589. [DOI] [PubMed] [Google Scholar]
- 51.Pace TWW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, Heim CM. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163:1630–1632. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- 52.Hamazaki K, Itomura M, Huan M, Nishizawa H, Sawazaki S, Tanouchi M, Watanabe S, Hamazaki T, Terasawa K, Yazawa K. Effect of omega-3 fatty acid-containing phospholipids on blood catecholamine concentrations in healthy volunteers: a randomized, placebo-controlled, double-blind trial. Nutrition. 2005;21:705–710. doi: 10.1016/j.nut.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 53.Sawazaki S, Hamazaki T, Yazawa K, Kobayashi M. The effect of docosahexaenoic acid on plasma catecholamine concentrations and glucose tolerance during long-lasting psychological stress: a double-blind placebo-controlled study. J Nutr Sci Vitaminol (Tokyo) 1999;45:655–665. doi: 10.3177/jnsv.45.655. [DOI] [PubMed] [Google Scholar]
- 54.Jones SA, Horiuchi S, Topley N, Yamamoto N, Fuller GM. The soluble interleukin 6 receptor: Mechanisms of production and implications in disease. FASEB J. 2001;15:43–58. doi: 10.1096/fj.99-1003rev. [DOI] [PubMed] [Google Scholar]
- 55.Wu D, Meydani SN. n-3 polyunsaturated fatty acids and immune function. Proc Nutr Soc. 1998;57:503–509. doi: 10.1079/pns19980074. [DOI] [PubMed] [Google Scholar]
- 56.Zeleniuch-Jacquotte A, Chajes V, Van Kappel AL, Riboli E, Toniolo P. Reliability of fatty acid composition in human serum phospholipids. Eur J Clin Nutr. 2000;54:367–372. doi: 10.1038/sj.ejcn.1600964. [DOI] [PubMed] [Google Scholar]
- 57.Kobayashi M, Sasaki S, Kawabata T, Hasegawa K, Akabane M, Tsugane S. Single measurement of serum phospholipid fatty acid as a biomarker of specific fatty acid intake in middle-aged Japanese men. Eur J Clin Nutr. 2001;55:643–650. doi: 10.1038/sj.ejcn.1601194. [DOI] [PubMed] [Google Scholar]
- 58.Nemets B, Osher Y, Belmaker RH. Omega-3 fatty acids and augmentation strategies in treating resistant depression. Essential Psychopharmacology. 2004;6:59–64. [PubMed] [Google Scholar]
- 59.Peet M, Horrobin DF. A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs. Arch Gen Psychiatry. 2002;59:913–919. doi: 10.1001/archpsyc.59.10.913. [DOI] [PubMed] [Google Scholar]