Abstract
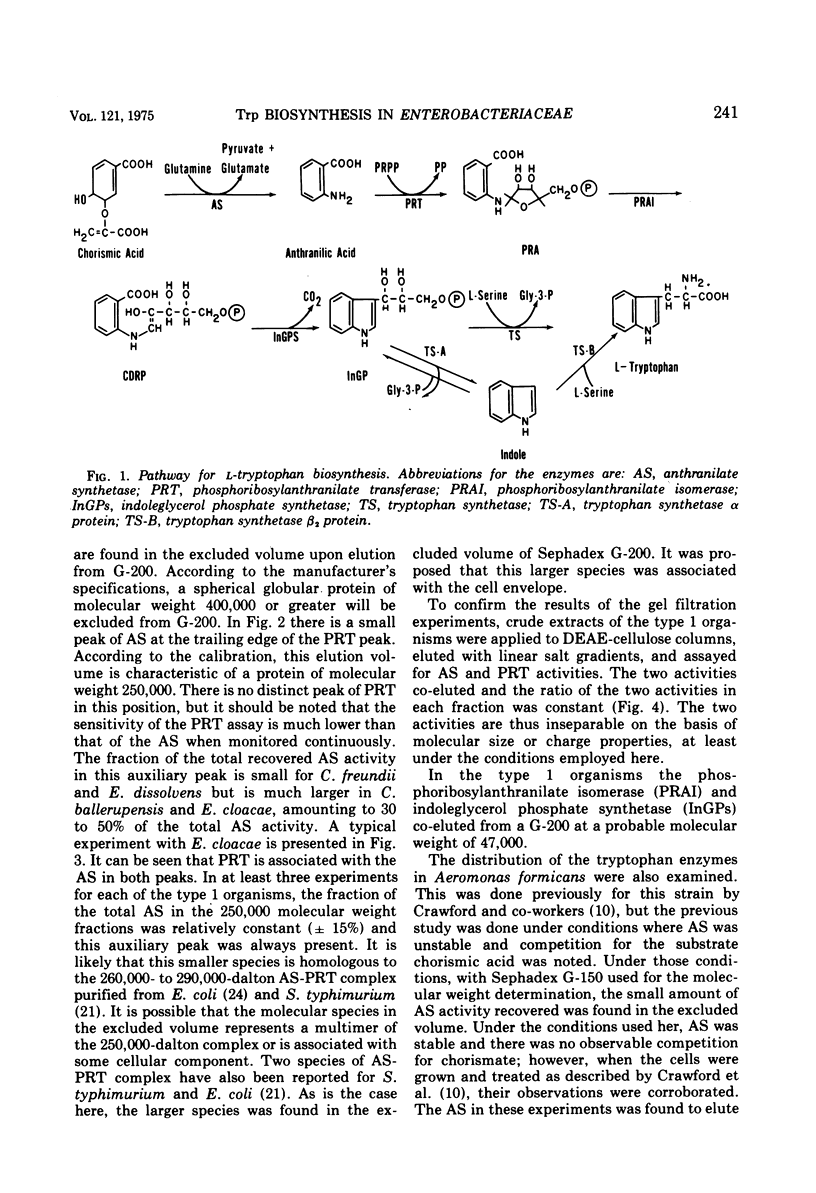
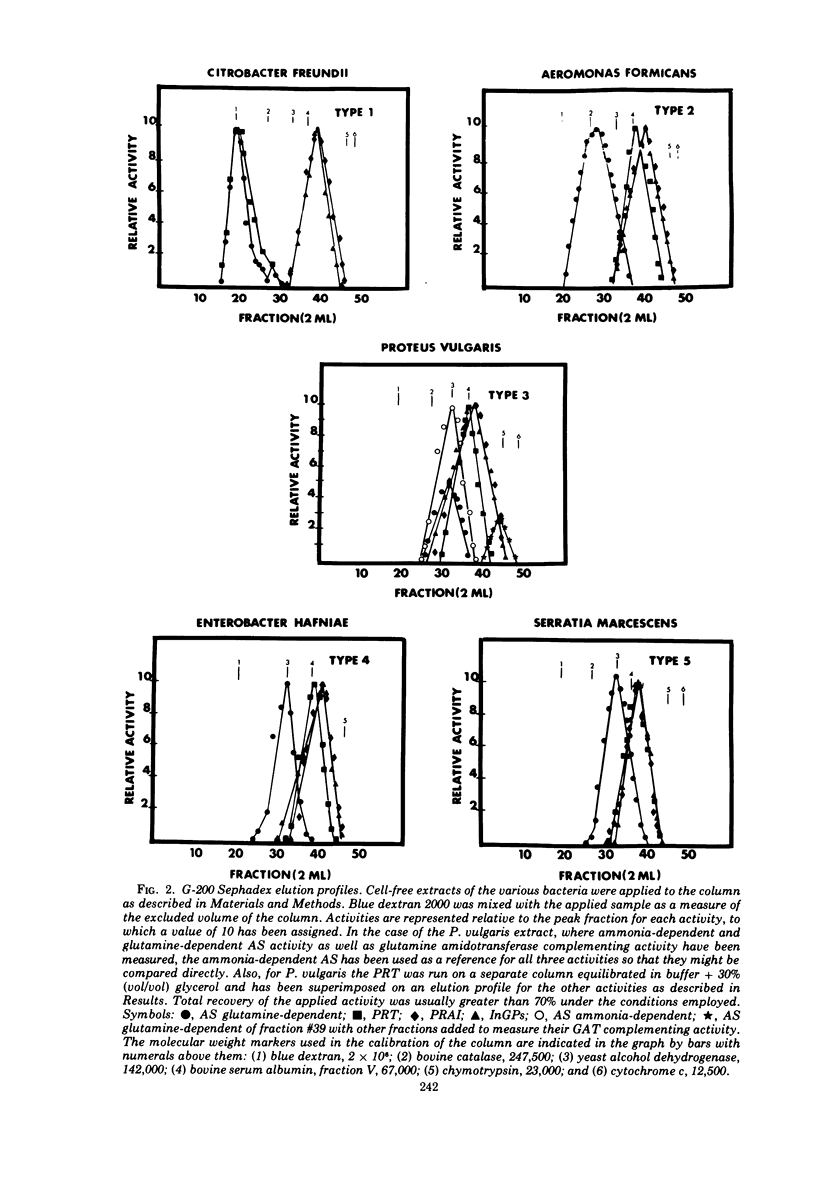
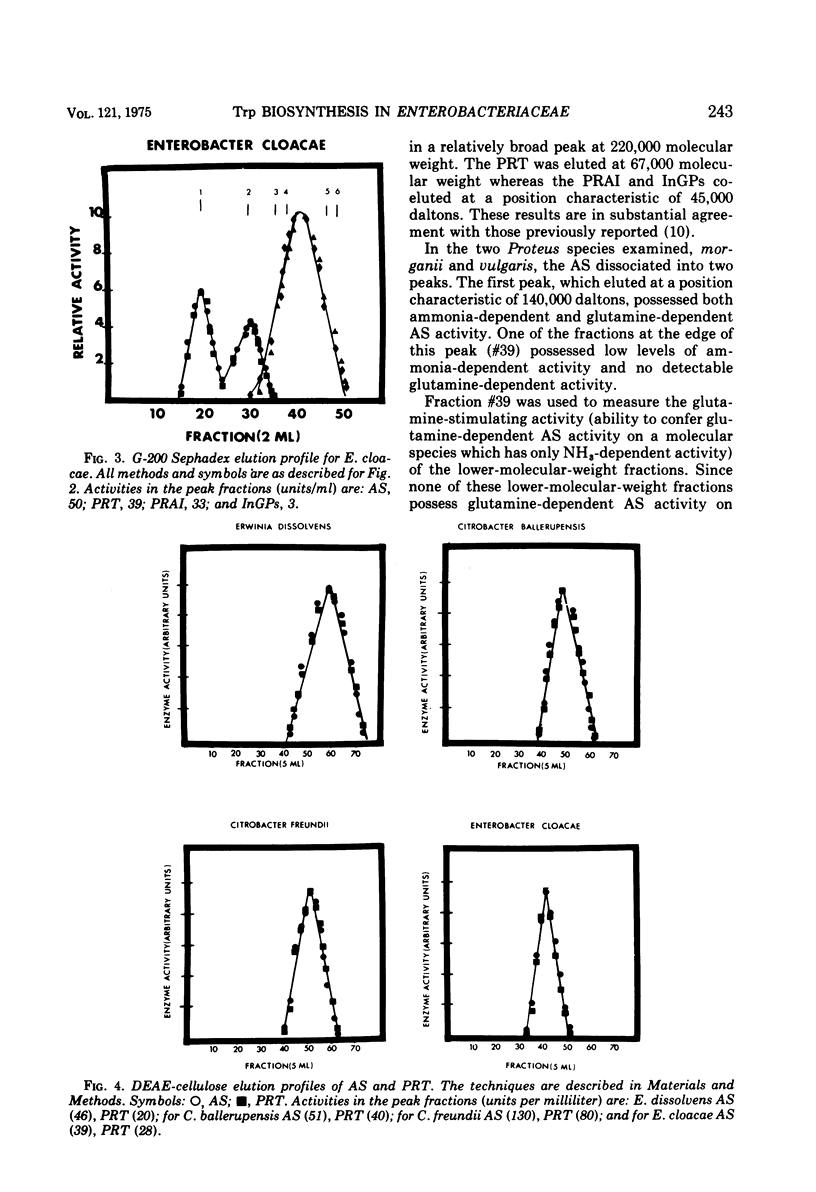
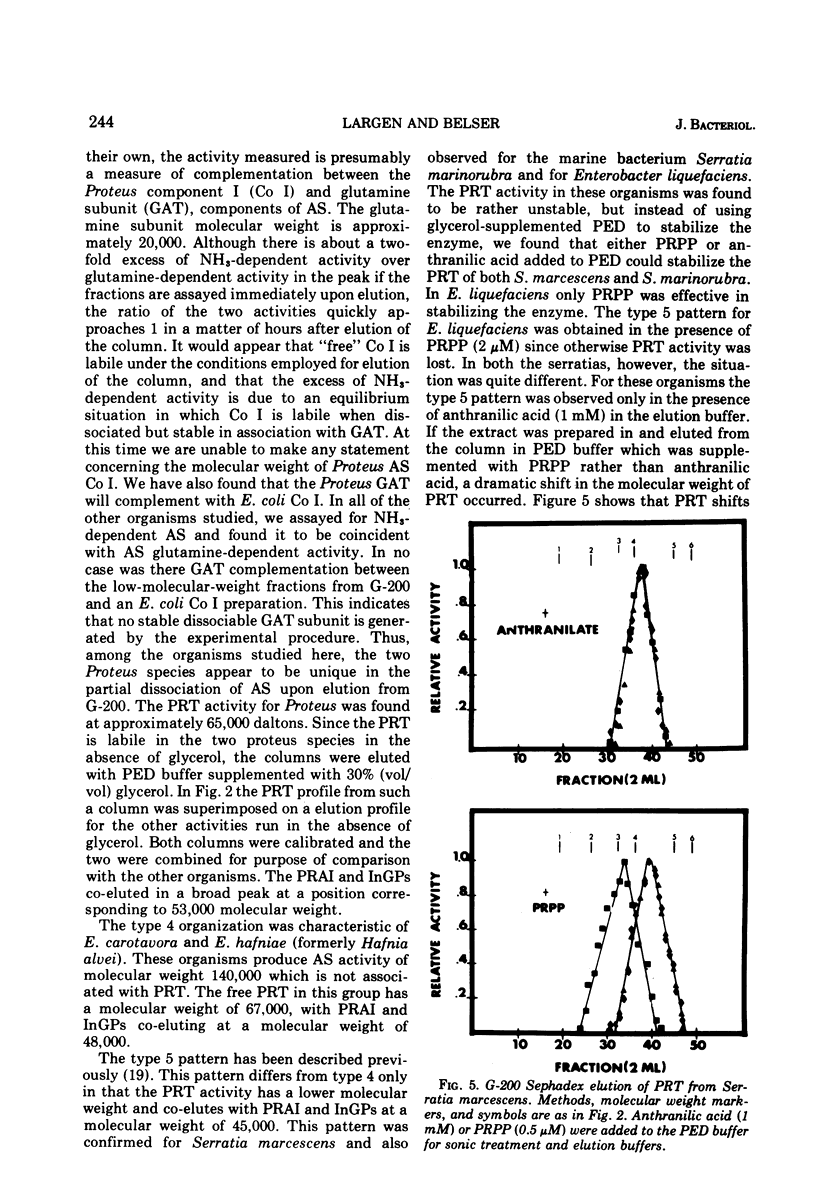
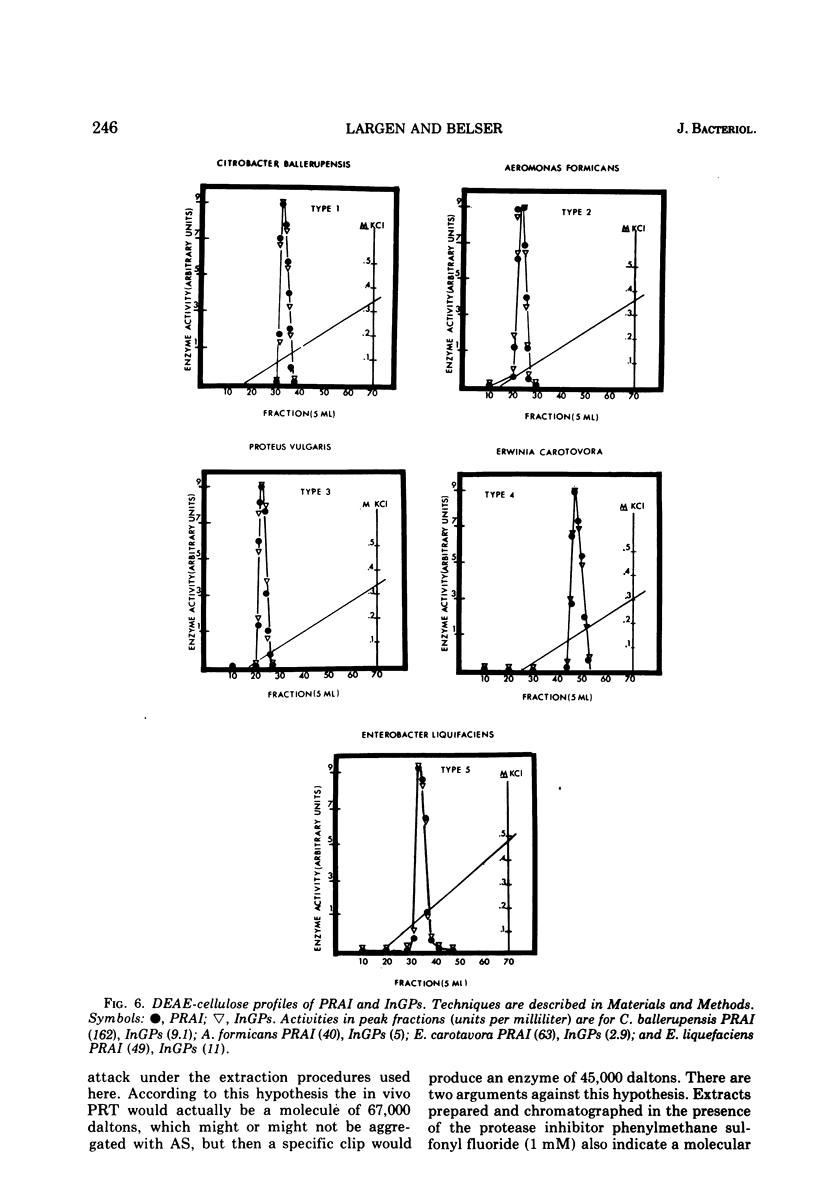
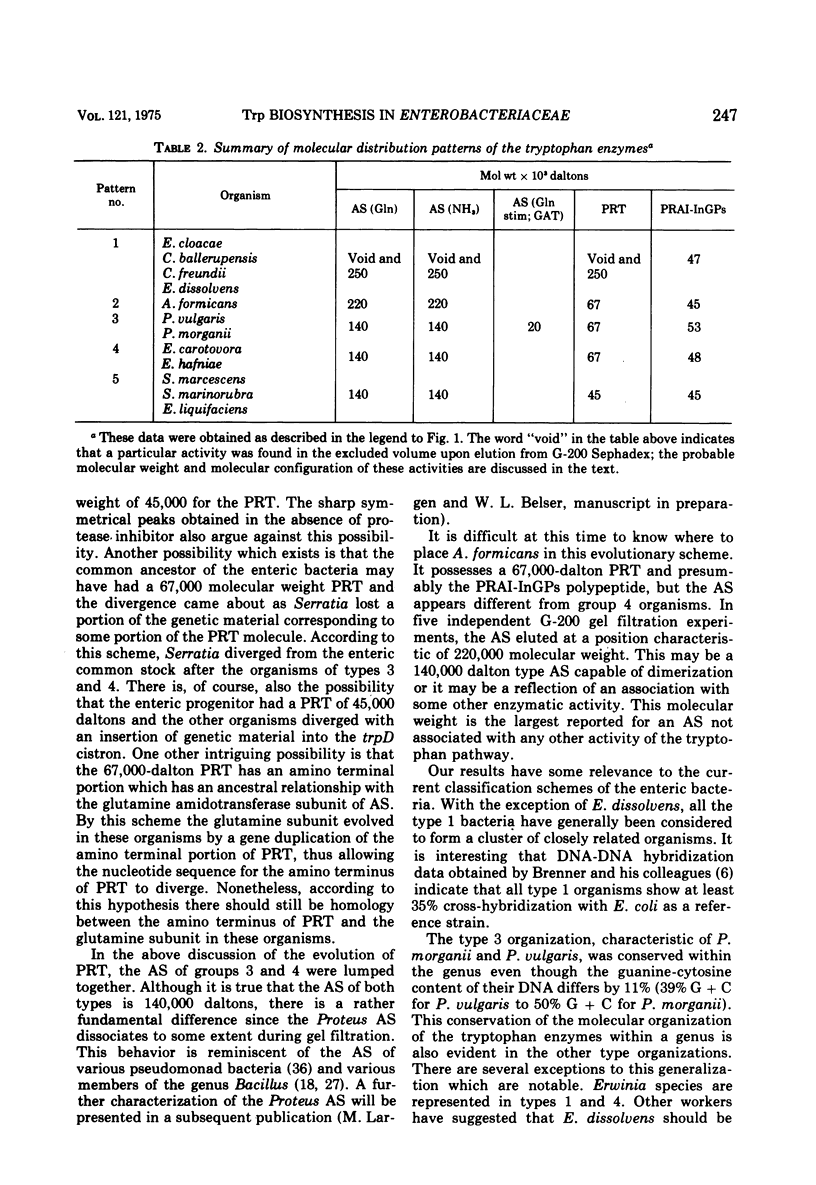
Several physical properties of the first four enzymatic activities of the tryptophan pathway were examined using gel filtration and ion exchange chromatography. Five different patterns were noted. Differences in the anthranilate synthetase (AS) and phosphoribosylanthranilate transferase (PRT) defined these patterns. In all the organisms studied phosphoribosylanthranilate isomerase and indoleglycerol phosphate synthetase co-eluted from both diethylaminoethyl-cellulose and G-200 and thus probably are contained in a single polypeptide of 50,000 daltons. An AS-PRT complex was found in Citrobacter species, Enterobacter cloacae, and Erwinia dissolvens. In all the other bacteria examined AS and PTR were separate molecules. In Serratia marcescens, S. marinorubra, and Enterobacter liquefaciens, AS was 140,000 daltons and PRT was 45,000 daltons. In Erwinia carotavora and Enterobacter hafniae the AS was the same size as the Serratia species but the PRT was larger at 67,000 daltons. Two Proteus species had an AS and PRT of the same size as E. carotavora and E. halfniae but the Proteus AS was different in that it partially dissociated upon gel filtration. Aeromonas formicans was unique in its possession of an AS with a molecular weight of 220,000. The PRT of A. formicans was found to elute at 67,000 daltons. Possible paths of evolution of the tryptophan enzymes are discussed in terms of the results of this study. The results presented here are also considered with respect to existing taxonomic schemes of the enteric bacteria.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Garry B. COORDINATE REPRESSION OF THE SYNTHESIS OF FOUR HISTIDINE BIOSYNTHETIC ENZYMES BY HISTIDINE. Proc Natl Acad Sci U S A. 1959 Oct;45(10):1453–1461. doi: 10.1073/pnas.45.10.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bascomb S., Lapage S. P., Willcox W. R., Curtis M. A. Numerical classification of the tribe Klebsielleae. J Gen Microbiol. 1971 Jun;66(3):279–295. doi: 10.1099/00221287-66-3-279. [DOI] [PubMed] [Google Scholar]
- Benson A., Tomoda K., Chang J., Matsueda G., Lode E. T., Coon M. J., Yasunobu K. T. Evolutionary and phylogenetic relationships of rubredoxin-containing microbes. Biochem Biophys Res Commun. 1971 Feb 19;42(4):640–646. doi: 10.1016/0006-291x(71)90536-5. [DOI] [PubMed] [Google Scholar]
- Brenner D. J., Falkow S. Genetics of the Enterobacteriaceae. C. Molecular relationships among members of the Enterobacteriaceae. Adv Genet. 1971;16:81–118. doi: 10.1016/s0065-2660(08)60355-7. [DOI] [PubMed] [Google Scholar]
- Brenner D. J., Fanning G. R., Steigerwalt A. G. Deoxyribonucleic acid relatedness among species of Erwinia and between Erwinia species and other enterobacteria. J Bacteriol. 1972 Apr;110(1):12–17. doi: 10.1128/jb.110.1.12-17.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee J. N. Genetics of the Proteus group. Annu Rev Microbiol. 1972;26:23–54. doi: 10.1146/annurev.mi.26.100172.000323. [DOI] [PubMed] [Google Scholar]
- Cohen G. N., Stanier R. Y., Le Bras G. Regulation of the biosynthesis of amino acids of the aspartate family in Coliform bacteria and Pseudomonads. J Bacteriol. 1969 Sep;99(3):791–801. doi: 10.1128/jb.99.3.791-801.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford I. P., Sikes S., Melhorn D. K. The natural relationships of Aeromonas formicans. Arch Mikrobiol. 1967;59(1):72–81. doi: 10.1007/BF00406318. [DOI] [PubMed] [Google Scholar]
- Crawford I. P., Yanofsky C. Pseudomonas putida tryptophan synthetase: partial sequence of the subunit. J Bacteriol. 1971 Oct;108(1):248–253. doi: 10.1128/jb.108.1.248-253.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson F., Pittard J., Reich E. Ammonium ions as the source of nitrogen for tryptophan biosynthesis in whole cells of Escherichia coli. Biochim Biophys Acta. 1967 Apr 25;136(3):573–576. doi: 10.1016/0304-4165(67)90020-7. [DOI] [PubMed] [Google Scholar]
- Hamon Y., Le Minor L., Peron Y. Les bactériocines d'Enterobacter liquefaciens. Intérêt taxonomique de leur étude. C R Acad Sci Hebd Seances Acad Sci D. 1970 Feb 9;270(6):886–889. [PubMed] [Google Scholar]
- Hoch S. O., Crawford I. P. Enzymes of the tryptophan pathway in three Bacillus species. J Bacteriol. 1973 Nov;116(2):685–693. doi: 10.1128/jb.116.2.685-693.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson M. A., Belser W. L. Enzymes of tryptophan biosynthesis in Serratia marcescens. J Bacteriol. 1969 Apr;98(1):109–115. doi: 10.1128/jb.98.1.109-115.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang L. H., Zalkin H. Multiple forms of anthranilate synthetase-anthranilate 5-phosphoribosylpyrophosphate phosphoribosyltransferase from Salmonella typhimurium. J Biol Chem. 1971 Apr 25;246(8):2338–2345. [PubMed] [Google Scholar]
- Hütter R., DeMoss J. A. Organization of the tryptophan pathway: a phylogenetic study of the fungi. J Bacteriol. 1967 Dec;94(6):1896–1907. doi: 10.1128/jb.94.6.1896-1907.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J., Cox E. C., Yanofsky C. Anthranilate synthetase, an enzyme specified by the tryptophan operon of Escherichia coli: purification and characterization of component I. J Bacteriol. 1969 Feb;97(2):725–733. doi: 10.1128/jb.97.2.725-733.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J. Hybrid anthranilate synthetase molecules from enterobacterial sources. Nature. 1969 Jul 5;223(5201):57–59. doi: 10.1038/223057a0. [DOI] [PubMed] [Google Scholar]
- Ito J., Yanofsky C. Anthranilate synthetase, an enzyme specified by the tryptophan operon of Escherichia coli: Comparative studies on the complex and the subunits. J Bacteriol. 1969 Feb;97(2):734–742. doi: 10.1128/jb.97.2.734-742.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. A., Nasser D. S., Nester E. W. Comparative control of a branch-point enzyme in microorganisms. J Bacteriol. 1967 Nov;94(5):1582–1593. doi: 10.1128/jb.94.5.1582-1593.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D., Sneath P. H. Genetic transfer and bacterial taxonomy. Bacteriol Rev. 1970 Mar;34(1):40–81. doi: 10.1128/br.34.1.40-81.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Largen M., Belser W. The apparent conservation of the internal low efficiency promoter of the tryptophan operons of several species of Enterobacteriaceae. Genetics. 1973 Sep;75(1):19–22. doi: 10.1093/genetics/75.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. L., Hanlon J., Yanofsky C. Structural homology of the glutamine amidotransferase subunits of the anthranilate synthetases of Escherichia coli, Salmonella typhimurium and Serratia marcescens. Nature. 1974 Mar 1;248(5443):48–50. doi: 10.1038/248048a0. [DOI] [PubMed] [Google Scholar]
- Li S. L., Yanofsky C. Amino acid sequences of fifty residues from the amino termini of the tryptophan synthetase chains of several enterobacteria. J Biol Chem. 1972 Feb 25;247(4):1031–1037. [PubMed] [Google Scholar]
- McQuade J. F., 3rd, Creighton T. E. Purification and comparison of the N-(5'-phosphoribosyl)anthranilic acid isomerase-indole-3-glycerol phosphate synthetase of tryptophan biosynthesis from three species of Enterobacteriaceae. Eur J Biochem. 1970 Oct;16(2):199–207. doi: 10.1111/j.1432-1033.1970.tb01072.x. [DOI] [PubMed] [Google Scholar]
- Murphy T. M., Mills S. E. Immunochemical and enzymatic comparisons of the tryptophan synthase alpha subunits from five species of Enterobacteriaceae. J Bacteriol. 1969 Mar;97(3):1310–1320. doi: 10.1128/jb.97.3.1310-1320.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts J. M., Drapeau G. R. Partial characterization of phosphoribosyl transferase, phosphoribosyl anthranilate isomerase, and indole glycerol phosphate synthase from Serratia marcescens. J Bacteriol. 1972 Aug;111(2):334–339. doi: 10.1128/jb.111.2.334-339.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queener S. F., Gunsalus I. C. Anthranilate synthase enzyme system and complementation in Pseudomonas species. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1225–1232. doi: 10.1073/pnas.67.3.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb F., Belser W. L. Anthranilate synthetase. In vitro complementation between Serratia marcescens and Escherichia coli subunits. Biochim Biophys Acta. 1972 Nov 28;285(1):243–252. [PubMed] [Google Scholar]
- Robb F., Hutchinson M. A., Belser W. L. Anthranilate synthetase. Some physical and kinetic properties of the enzyme from Serratia marcescens. J Biol Chem. 1971 Nov 25;246(22):6908–6912. [PubMed] [Google Scholar]
- Rocha V., Crawford I. P., Mills S. E. Comparative immunological and enzymatic study of the tryptophan synthetase beta 2 subunit in the Enterobacteriaceae. J Bacteriol. 1972 Jul;111(1):163–168. doi: 10.1128/jb.111.1.163-168.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr M. P., Mandel M. DNA base composition and taxonomy of phyopathogenic and other enterobacteria. J Gen Microbiol. 1969 Apr;56(1):113–123. doi: 10.1099/00221287-56-1-113. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]


