Abstract
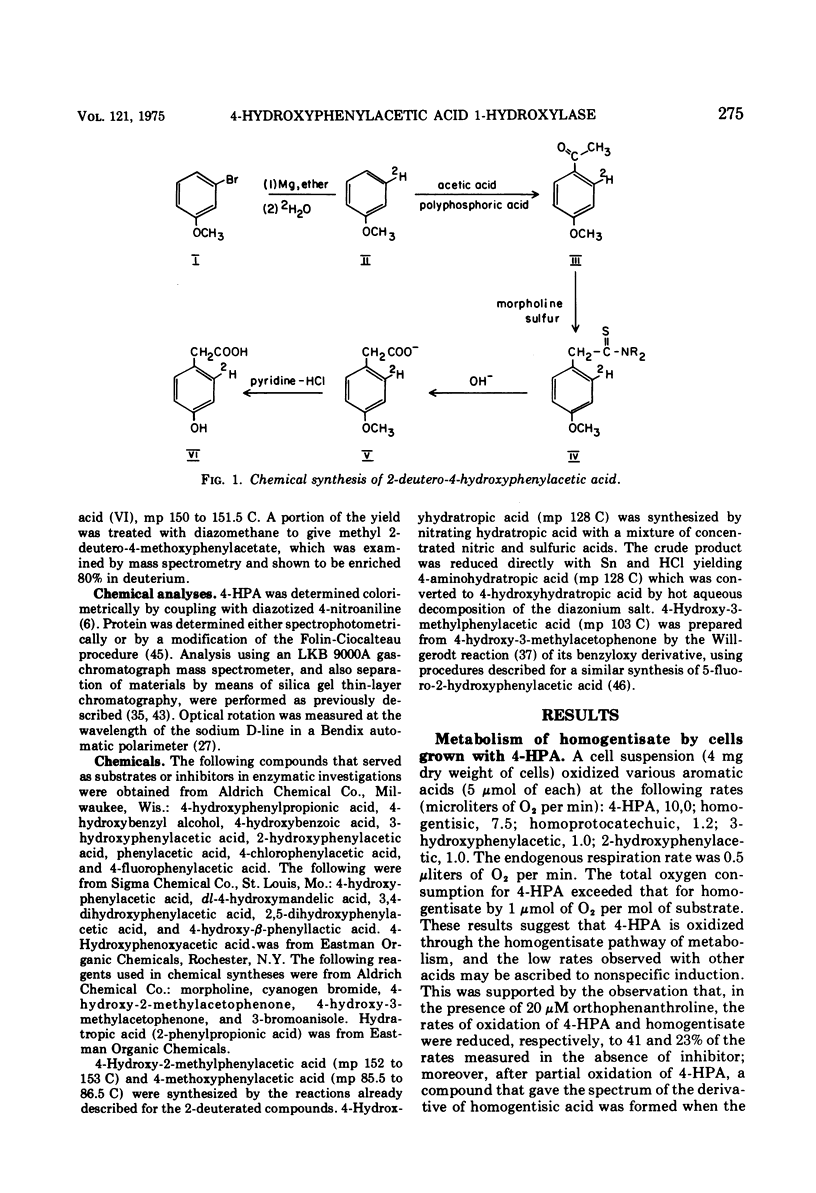
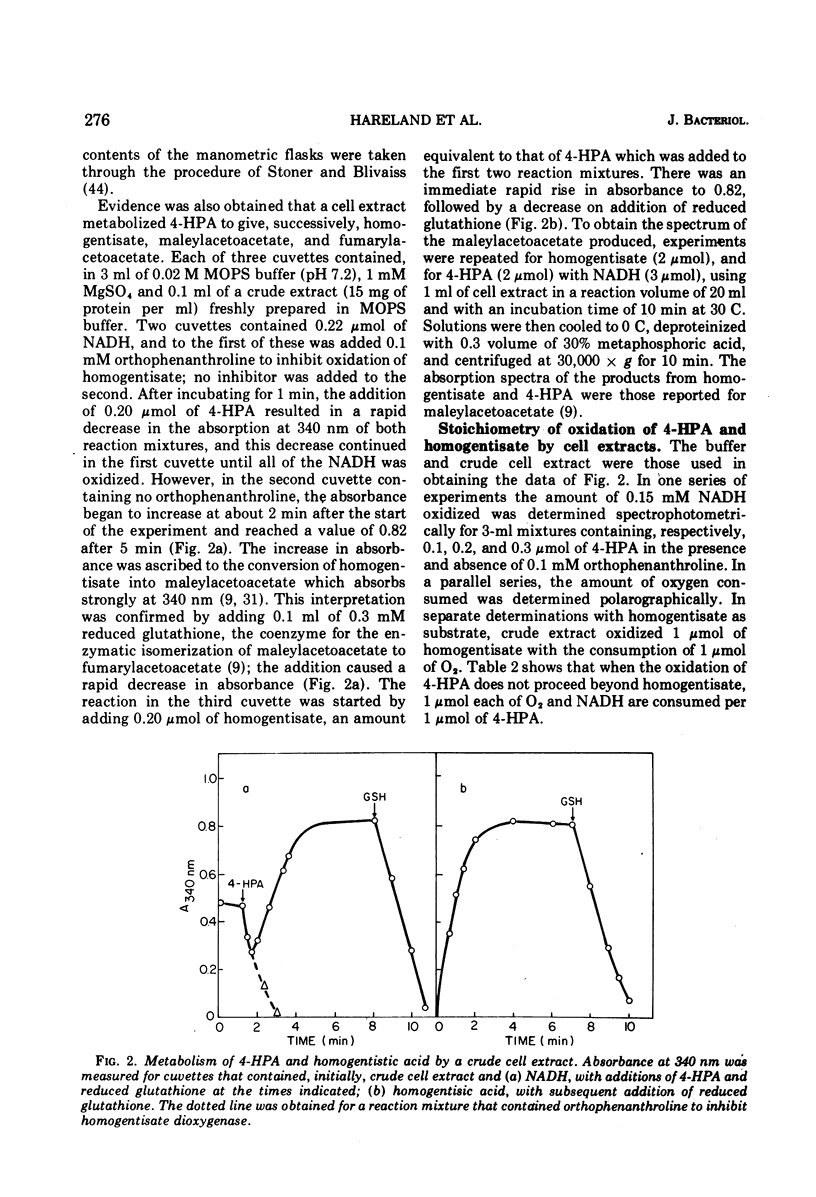
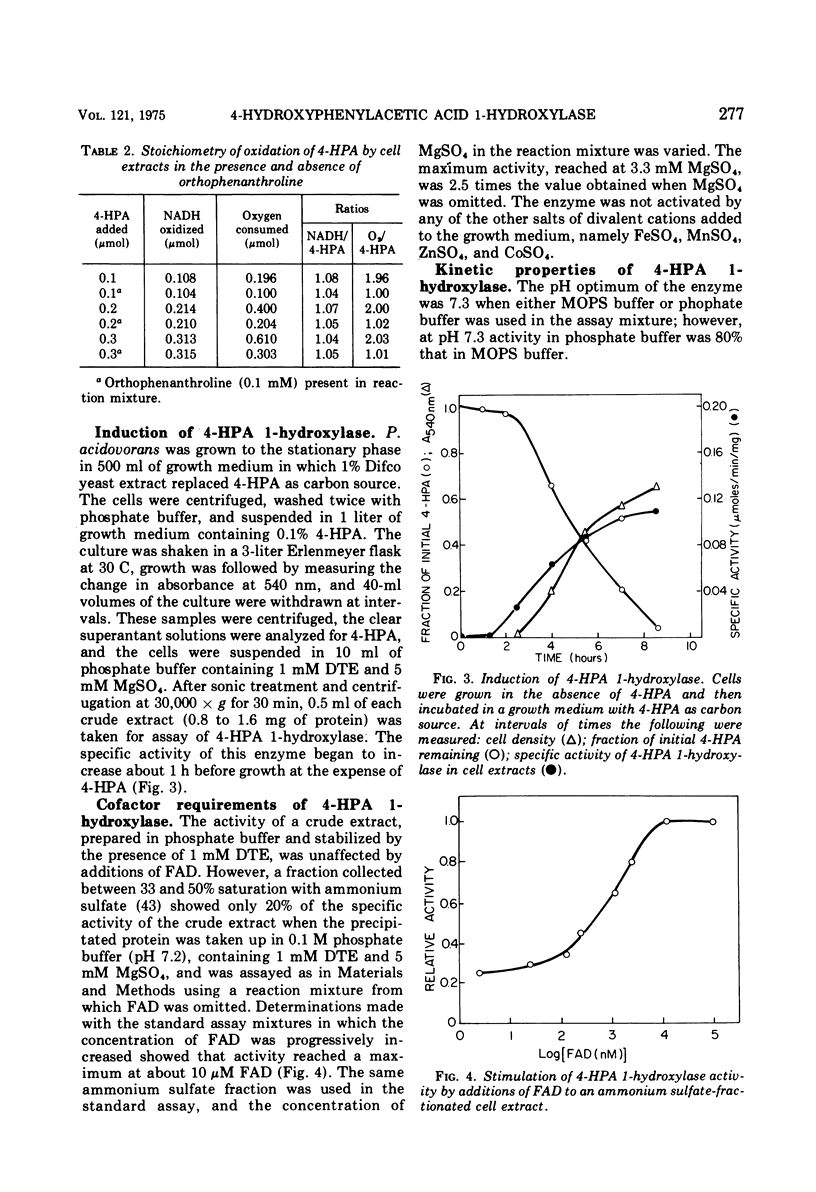
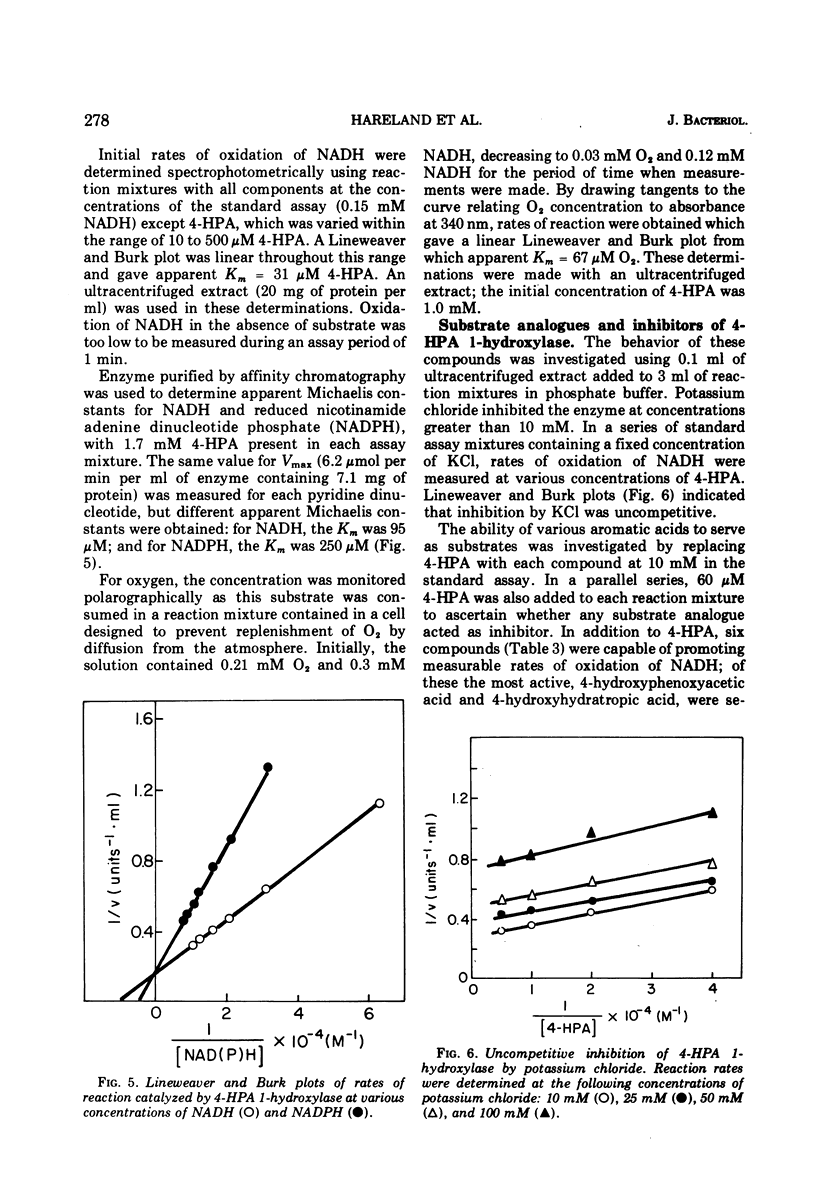
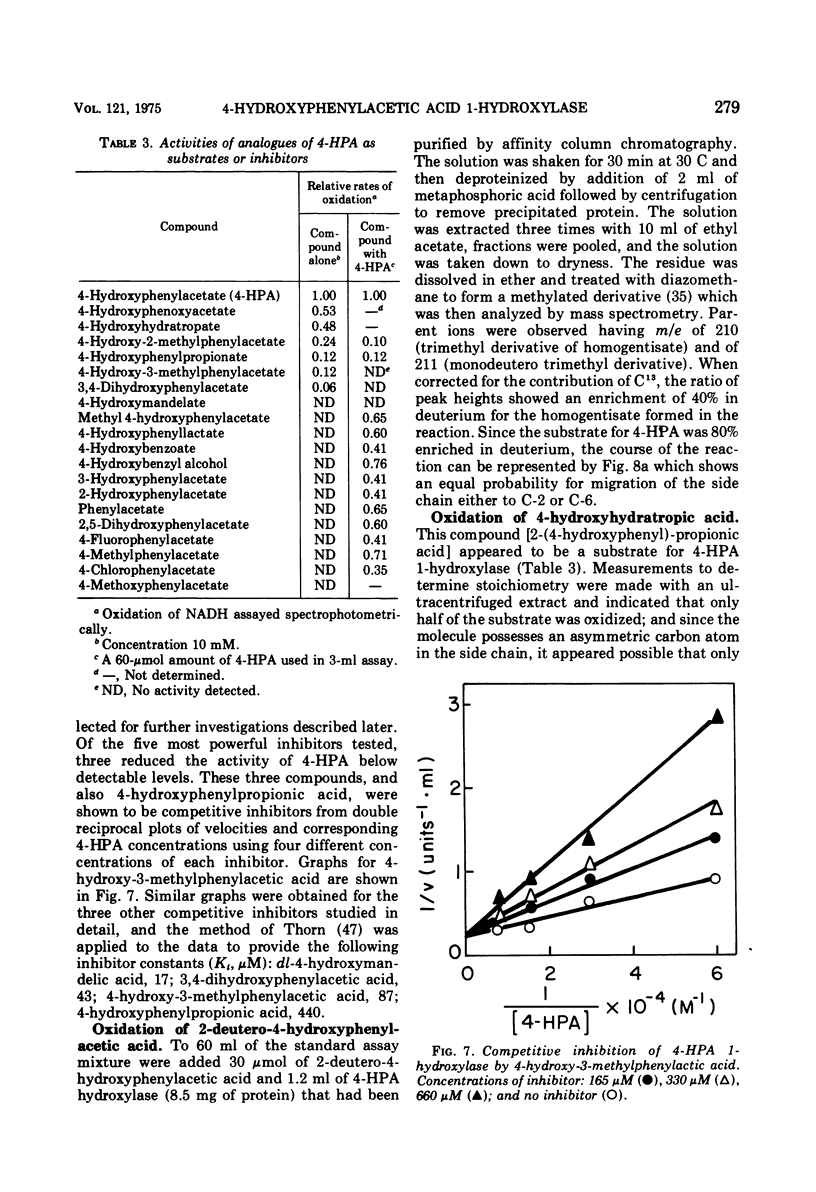
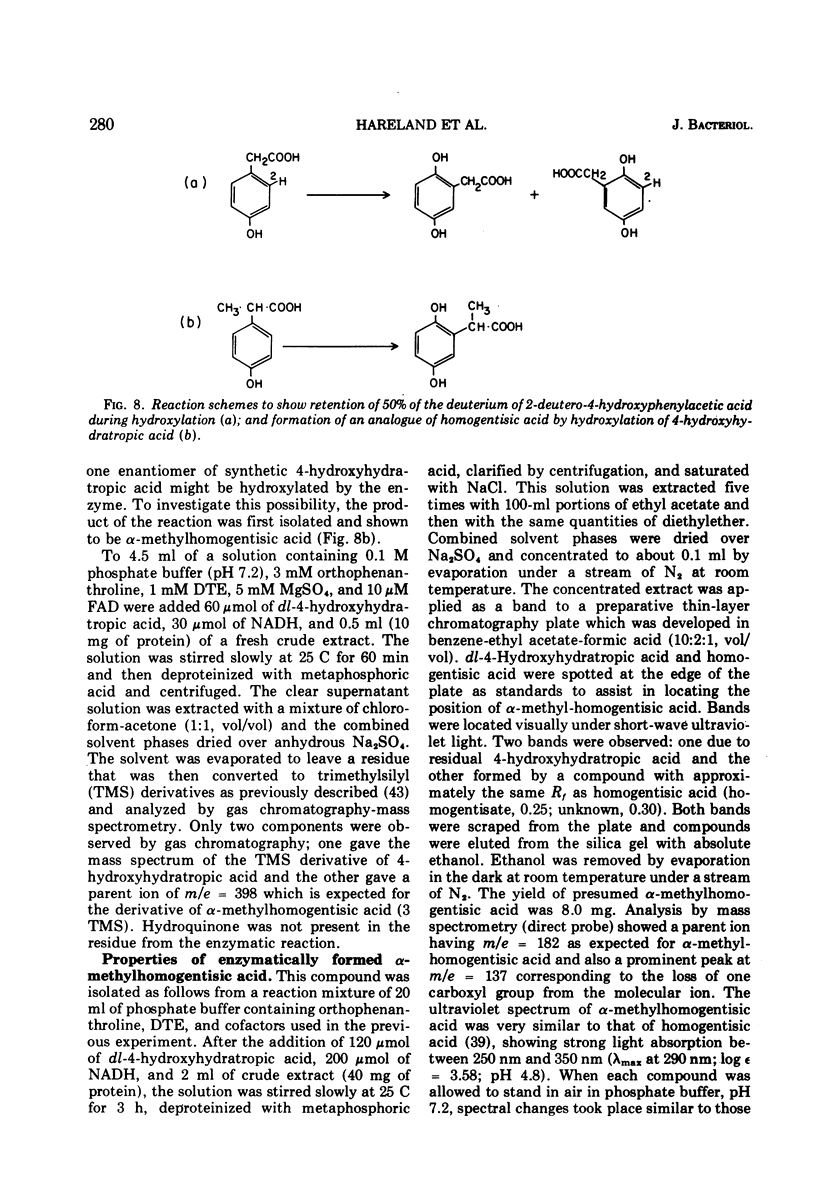
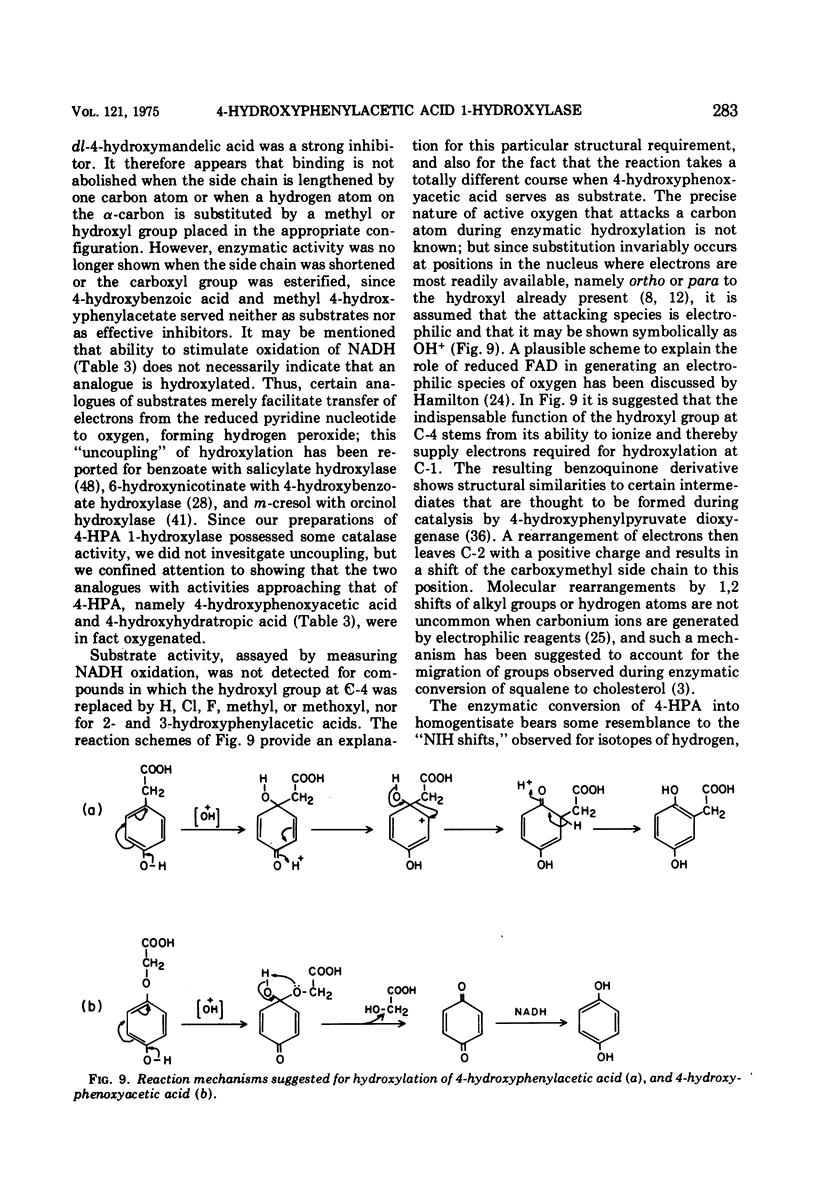
The enzyme 4-hydroxyphenylacetate, NAD(P)H:oxygen oxidoreductase (1-hydroxylating) (EC 1.14.13 ...; 4-hydroxyphenylacetate 1-monooxygenase; referred to here as 4-HPA 1-hydroxylase) was induced in Pseudomonas acidovorans when 4-hydroxyphenylacetate (4-PHA) was utilized as carbon source for growth; homogentisate and maleylacetoacetate were intermediates in the degradation of 4-HPA. A preparation of the hydroxylase that was free from homogentisate dioxygenase and could be stored at 4 C in the presence of dithioerythritol with little loss of activity was obtained by ultracentrifuging cell extracts; but when purified 18-fold by affinity chromatography the enzyme became unstable. Flavin adenine dinucleotide and Mg2+ ions were required for full activity. 4-HPA 1-hydrocylase was inhibited by KCl, which was uncompetitive with 4-HPA. Values of Ki determined for inhibitors competitive with 4-HPA were 17 muM dl-4-hydroxymandelic acid, 43 muM 3,4-dihydroxyphenylacetic acid, 87 muM 4-hydroxy-3-methylphenylacetic acid, and 440 muM 4-hydroxyphenylpropionic acid. Apparent Km values for substrates of 4-HPA 1-hydroxylase were 31 muM 4-HPA, 67 muM oxygen, 95 muM reduced nicotinamide adenine dinucleotide (NADH); AND 250 muM reduced nicotinamide adenine dinucleotide phosphate (NADPH). The same maximum velocity was given by NADH and NADPH. A chemical synthesis is described for 2-deutero-4-hydroxyphenylacetic acid. This compound was enzymatically hydroxylated with retention of half the deuterium in the homogentisic acid formed. Activity as substrate or inhibitor of 4-HPA 1-hydroxylase was shown only by those analogues of 4-HPA that possessed a hydroxyl group substituent at C-4 of the benze nucleus. A mechanism is suggested that accounts for this structural requirement and also for the observation that when 4-hydroxyphenoxyacetic acid was attacked by the enzyme, hydroquinone was formed by release of the side chain, probably as glycolic acid. Only one enantiometer of racemic 4-hydroxyhydratropic acid was attacked by 4-HPA 1-hydroxylase; the product, alpha-methylhomogentisic acid (2-(2,5-dihydroxyphenyl)-propionic acid), exhibited optical activity. This observation suggests that, during its shift from C-1 to C-2 of the nucleus, the side chain of the substrate remains bound to a site on the enzyme while a conformational change of the protein permits the necessary movement of the benzene ring.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADACHI K., TAKEDA Y., SENOH S., KITA H. METABOLISM OF P-HYDROXYPHENYLACETIC ACID IN PSEUDOMONAS OVALIS. Biochim Biophys Acta. 1964 Dec 9;93:483–493. doi: 10.1016/0304-4165(64)90332-0. [DOI] [PubMed] [Google Scholar]
- BACHRACH U. The aerobic breakdown of uric acid by certain pseudomonads. J Gen Microbiol. 1957 Aug;17(1):1–11. doi: 10.1099/00221287-17-1-1. [DOI] [PubMed] [Google Scholar]
- BRAY H. G., THORPE W. V. Analysis of phenolic compounds of interest in metabolism. Methods Biochem Anal. 1954;1:27–52. doi: 10.1002/9780470110171.ch2. [DOI] [PubMed] [Google Scholar]
- Blakley E. R., Kurz W., Halvorson H., Simpson F. J. The metabolism of phenylacetic acid by a Pseudomonas. Can J Microbiol. 1967 Feb;13(2):147–157. doi: 10.1139/m67-021. [DOI] [PubMed] [Google Scholar]
- Blakley E. R. Microbial conversion of p-hydroxyphenylacetic acid to homogentisic acid. Can J Microbiol. 1972 Aug;18(8):1247–1255. doi: 10.1139/m72-193. [DOI] [PubMed] [Google Scholar]
- CHAPMAN P. J., DAGLEY S. Oxidation of homogentistic acid by cell-free extracts of a vibrio. J Gen Microbiol. 1962 Jun;28:251–256. doi: 10.1099/00221287-28-2-251. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. III. Prediction of initial velocity and inhibition patterns by inspection. Biochim Biophys Acta. 1963 Feb 12;67:188–196. doi: 10.1016/0006-3002(63)91816-x. [DOI] [PubMed] [Google Scholar]
- Chamberlain E. M., Dagley S. The metabolism of thymol by a Pseudomonas. Biochem J. 1968 Dec;110(4):755–763. doi: 10.1042/bj1100755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- DAGLEY S., RODGERS A. Estimation of glycollic acid. Biochim Biophys Acta. 1953 Dec;12(4):591–591. doi: 10.1016/0006-3002(53)90196-6. [DOI] [PubMed] [Google Scholar]
- DAGLEY S., TRUDGILL P. W., CALLELY A. G. Synthesis of cell constituents from glycine by a Pseudomonas. Biochem J. 1961 Dec;81:623–631. doi: 10.1042/bj0810623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAGLEY S., TRUDGILL P. W. THE METABOLISM OF GALACTARATE, D-GLUCARATE AND VARIOUS PENTOSES BY SPECIES OF PSEUDOMONAS. Biochem J. 1965 Apr;95:48–58. doi: 10.1042/bj0950048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAGLEY S., TRUDGILL P. W. THE METABOLISM OF TARTARIC ACID BY A PSEUDOMONAS. A NEW PATHWAY. Biochem J. 1963 Oct;89:22–31. doi: 10.1042/bj0890022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagley S. Catabolism of aromatic compounds by micro-organisms. Adv Microb Physiol. 1971;6(0):1–46. doi: 10.1016/s0065-2911(08)60066-1. [DOI] [PubMed] [Google Scholar]
- Daly J. W., Jerina D. M., Witkop B. Arene oxides and the NIH shift: the metabolism, toxicity and carcinogenicity of aromatic compounds. Experientia. 1972 Oct 15;28(10):1129–1149. doi: 10.1007/BF01946135. [DOI] [PubMed] [Google Scholar]
- Daly J., Jerina D., Witkop B. Migration of deuterium during hydroxylation of aromatic substrates by liver microsomes. I. Influence of ring substitutents. Arch Biochem Biophys. 1968 Nov;128(2):517–527. doi: 10.1016/0003-9861(68)90059-3. [DOI] [PubMed] [Google Scholar]
- Gamar Y., Gaunt J. K. Bacterial metabolism of 4-chloro-2-methylphenoxyacetate. Formation of glyoxylate by side-chain cleavage. Biochem J. 1971 May;122(4):527–531. doi: 10.1042/bj1220527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groseclose E. E., Ribbons D. W., Hughes H. 3-Hydroxybenzoate 6-hydroxylase from Pseudomonas aeruginosa. Biochem Biophys Res Commun. 1973 Dec 10;55(3):897–903. doi: 10.1016/0006-291x(73)91228-x. [DOI] [PubMed] [Google Scholar]
- Guroff G., Daly J. W., Jerina D. M., Renson J., Witkop B., Udenfriend S. Hydroxylation-induced migration: the NIH shift. Recent experiments reveal an unexpected and general result of enzymatic hydroxylation of aromatic compounds. Science. 1967 Sep 29;157(3796):1524–1530. doi: 10.1126/science.157.3796.1524. [DOI] [PubMed] [Google Scholar]
- HAGER S. E., GREGERMAN R. I., KNOX W. E. p-Hydroxyphenylpyruvate oxidase of liver. J Biol Chem. 1957 Apr;225(2):935–947. [PubMed] [Google Scholar]
- Hesp B., Calvin M., Hosokawa K. Studies on p-hydroxybenzoate hydroxylase from Pseudomonas putida. J Biol Chem. 1969 Oct 25;244(20):5644–5655. [PubMed] [Google Scholar]
- Hopper D. J., Chapman P. J., Dagley S. Enzymic formation of D-malate. Biochem J. 1968 Dec;110(4):798–800. doi: 10.1042/bj1100798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell L. G., Massey V. A non-substrate effector of p-hydroxybenzoate hydroxylase. Biochem Biophys Res Commun. 1970 Aug 24;40(4):887–893. doi: 10.1016/0006-291x(70)90986-1. [DOI] [PubMed] [Google Scholar]
- Jeffcoat R., Hassall H., Dagley S. Purification and properties of D-4-deoxy-5-oxoglucarate hydro-lyase (decarboxylating). Biochem J. 1969 Dec;115(5):977–983. doi: 10.1042/bj1150977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerina D. M., Daly J. W., Witkop B. The role of arene oxide-oxepin systems in the metabolism of aromatic substrates. II. Synthesis of 3,4-toluene-4-2H oxide and subsequent "NIH shift" to 4-hydroxytoluene-3-2H. J Am Chem Soc. 1968 Nov 6;90(23):6523–6525. doi: 10.1021/ja01025a057. [DOI] [PubMed] [Google Scholar]
- KNOX W. E., EDWARDS S. W. The properties of maleylacetoacetate, the initial product of homogentisate oxidation in liver. J Biol Chem. 1955 Oct;216(2):489–498. [PubMed] [Google Scholar]
- LA DU B. N., ZANNONI V. G. The tyrosine oxidation system of liver. II. Oxidation of p-hydroxyphenylpyruvic acid to homogentisic acid. J Biol Chem. 1955 Dec;217(2):777–787. [PubMed] [Google Scholar]
- LA DU B. N., ZANNONI V. G. The tyrosine oxidation system of liver. III. Further studies on the oxidation of p-hydroxyphenylpyruvic acid. J Biol Chem. 1956 Mar;219(1):273–281. [PubMed] [Google Scholar]
- Leung P. T., Chapman P. J., Dagley S. Purification and properties of 4-hydroxy-2-ketopimelate aldolase from Acinetobacter. J Bacteriol. 1974 Oct;120(1):168–172. doi: 10.1128/jb.120.1.168-172.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindblad B., Lindstedt G., Lindstedt S. The mechanism of enzymic formation of homogentisate from p-hydroxyphenylpyruvate. J Am Chem Soc. 1970 Dec 16;92(25):7446–7449. doi: 10.1021/ja00728a032. [DOI] [PubMed] [Google Scholar]
- MILCH R. A., TITUS E. D., LOO T. L. Atmospheric oxidation of homogentisic acid: spectrophotometric studies. Science. 1957 Aug 2;126(3266):209–210. doi: 10.1126/science.126.3266.209. [DOI] [PubMed] [Google Scholar]
- Otha Y., Ribbons D. W. Crystallization of orcinol hydroxylase from Pseudomonas putida. FEBS Lett. 1970 Dec;11(3):189–192. doi: 10.1016/0014-5793(70)80525-7. [DOI] [PubMed] [Google Scholar]
- Ribbons D. W., Ohta Y. Uncoupling of electron transport from oxygenation in the mono-oxygenase, orcinol hydroxylase. FEBS Lett. 1970 Dec 28;12(2):105–108. doi: 10.1016/0014-5793(70)80574-9. [DOI] [PubMed] [Google Scholar]
- Sparnins V. L., Chapman P. J., Dagley S. Bacterial degradation of 4-hydroxyphenylacetic acid and homoprotocatechuic acid. J Bacteriol. 1974 Oct;120(1):159–167. doi: 10.1128/jb.120.1.159-167.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoner R. E., Blivaiss B. Determination of homogentisic acid in urine. Clin Chem. 1965 Sep;11(9):833–839. [PubMed] [Google Scholar]
- TANIGUCHI K., KAPPE T., ARMSTRONG M. D. FURTHER STUDIES ON PHENYLPYRUVATE OXIDASE. OCCURRENCE OF SIDE CHAIN REARRANGEMENT AND COMPARISON WITH P-HYDROXYPHENYLPYRUVATE OXIDASE. J Biol Chem. 1964 Oct;239:3389–3395. [PubMed] [Google Scholar]
- THORN M. B. Inhibition by malonate of succinic dehydrogenase in heart-muscle preparations. Biochem J. 1953 Jul;54(4):540–547. doi: 10.1042/bj0540540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack B. F., Chapman P. J., Dagley S. Purification and properties of 4-hydroxy-4-methyl-2-oxoglutarate aldolase. J Biol Chem. 1972 Oct 25;247(20):6444–6449. [PubMed] [Google Scholar]
- White-Stevens R. H., Kamin H. Studies of a flavoprotein, salicylate hydroxylase. I. Preparation, properties, and the uncoupling of oxygen reduction from hydroxylation. J Biol Chem. 1972 Apr 25;247(8):2358–2370. [PubMed] [Google Scholar]



