Abstract
Objective
We used positron emission tomography (PET) to study differences in longitudinal changes in regional cerebral blood flow (rCBF) between APOE ε4 carriers and non-carriers in non-demented older adults from the Baltimore Longitudinal Study of Aging (BLSA). Our main aim was to examine whether there are regionally specific longitudinal changes in rCBF in APOE ε4 carriers that might be related to its well-established role as a genetic risk factor for Alzheimer’s disease (AD).
Methods
Using [15O]water PET and voxel-based analysis, we compared changes in rCBF over an 8-year period between non-demented APOE ε4 carriers (N=29) and non-carriers (N=65) over 55 years of age. Serial neuropsychological data were collected for all participants.
Results
Widespread differences were observed in longitudinal change in rCBF between ε4 carriers and non-carriers. The predominant pattern was greater rCBF decline in ε4 carriers. These differences were observed in the frontal, parietal and temporal cortices. The brain regions affected are those that are especially vulnerable to AD pathology. Both ε4 carriers and non-carriers remained free of clinical diagnoses of dementia or mild cognitive impairment during the course of the study.
Interpretation
Our findings suggest that APOE ε4-mediated risk for AD is associated with widespread decline in rCBF over time that precedes the onset of dementia. Accelerated rates of decline in brain function in APOE ε4 individuals may contribute to their increased risk for AD and lower age-at-onset.
Introduction
Numerous epidemiological studies, as well as recent genome wide association studies, have established that the apolipoprotein E (APOE) ε4 allele is a risk factor for Alzheimer’s disease (AD), increasing the risk by 3–8 fold and lowering the age of onset by 7–15 years in a dose-dependent manner 1–3. The ε4 allele is associated with integral features of AD neuropathology including neuritic plaques and neurofibrillary tangles 4. Moreover, it confers a greater risk of conversion to AD in subjects with mild cognitive impairment (MCI) 5. However, despite overwhelming evidence linking it with both incipient and established AD, the precise mechanism by which APOE ε4 mediates increased risk for AD is poorly understood.
Functional neuroimaging methods have been applied to study changes in brain function in APOE ε4 carriers. Cross sectional studies in healthy young, middle aged and elderly APOE ε4 carriers have reported reductions in both regional cerebral blood flow (rCBF) and regional cerebral glucose metabolism in several brain regions, including those that are especially vulnerable to AD pathology 6. Cross sectional studies, however, are limited in their ability to address the effects of an AD-risk factor over time. Longitudinal functional neuroimaging studies in healthy elderly individuals are especially advantageous as they examine brain function at two or more discrete time points and can thereby address the role, if any, of the ε4 allele in increasing disease risk over time. However, to date, such studies in cognitively normal ε4 carriers have been somewhat inconsistent. These inconsistent findings may be due to the fact that some of these studies have been carried out in middle-aged subjects 7, and in the limited number of longitudinal studies carried out in the elderly, ε4-related effects were observed only in relatively small numbers of subjects with accompanying cognitive decline and/or age-associated memory impairment 8, 9.
Longitudinal neuroimaging studies on the influence of APOE genotype upon changes in brain function in cognitively normal elderly individuals are valuable as differences in brain metabolism or rCBF have the potential to be useful surrogate markers of AD in preclinical studies. Such predictive biomarkers of AD have considerable potential value in clinical practice and also in research where they may help accelerate the development of novel disease-modifying treatments. In both the US and Europe public/private consortia have been formed to conduct trials to discover such antecedent biomarkers 10, 11.
Here we report associations between APOE genotype and longitudinal changes in rCBF in non-demented, older adults in the Baltimore Longitudinal Study of Aging (BLSA). We hypothesized that APOE ε4 carriers will exhibit significant changes in rCBF over time in brain regions vulnerable to AD pathology in comparison to non-carriers.
Methods
Subjects
We used PET data from 94 participants in the neuroimaging substudy 12 of the BLSA13. We excluded individuals with intracranial tumors and clinical strokes. Data from participants who fulfilled consensus criteria (NINCDS-ADRDA) for AD 14 and MCI were excluded from the time of diagnosis. PET and neuropsychological data from these participants were included from baseline through the time point preceding the diagnosis of AD or MCI. A diagnosis of MCI was assigned by consensus conference if a participant had deficits in either a single cognitive domain (usually memory) or had more than one cognitive deficit but did not have functional loss in activities of daily living.
In order to examine the cardiovascular risk profile of participants in the two groups, we calculated the Framingham risk score for each subject at baseline to derive a 10-year risk profile for coronary heart disease (CHD) 15. This composite score of cardiovascular risk was based on the presence of the following specific risk factors: age, total serum cholesterol concentration, hypertension, diabetes mellitus and smoking. A positive family history of dementia was defined as ≥1 first degree relative with a clinical diagnosis of dementia at the time of entry into the study.
This study was approved by the local Institutional Review Board. All participants provided written informed consent prior to each assessment.
APOE genotype analysis was performed on DNA extracted from fresh blood by restriction enzyme isoform genotyping in all participants 16. The two groups were defined as APOE ε4 carriers (both heterozygous; n=22 and homozygous; n=7 i.e. ε4/ε4) and non-carriers (n=65). Annual neuropsychological data were available for all participants. Data from the imaging baseline (year 1) and last available follow-up time points were used in the analysis. The mean (SD) follow-up interval was 7.8 (1.1) years. The demographic characteristics of the participants are detailed in Table 1.
Table 1.
Demographic characteristics of APOE ε4 carriers and non-carriers
| Whole Sample | ε4 non-carriers | ε4 carriers | p-Value | |
|---|---|---|---|---|
| N | 94 | 65 | 29 | |
| Sex (% of males) | 58.5 | 61.5 | 51.72 | 0.37 |
| Baseline age | 69.2 (7.1) | 70.0 (7.7) | 67.4 (5.0) | 0.06 |
| Education | 16.3 (2.8) | 16.2 (3.0) | 16.5 (2.3) | 0.62 |
| MMSE 1st year | 28.9 (1.3) | 28.8 (1.5) | 29.1 (1.0) | 0.19 |
| MMSE last year | 28.9 (1.2) | 28.9 (1.2) | 28.8 (1.3) | 0.61 |
| Interval (years) | 7.8 (1.1) | 7.7 (1.2) | 7.8 (0.7) | 0.72 |
| Framingham risk score for CHD |
13.35 (7.43) | 14.02 (7.40) | 11.86 (7.40) | 0.19 |
| Family history of dementia |
27 (28%) | 19 (29%) | 8 (28%) | 0.8 |
Data are expressed as mean ± (standard deviation)
Neuropsychological Testing
During each neuroimaging visit, participants completed a battery including 12 neuropsychological tests evaluating six cognitive domains. Memory was assessed using the California Verbal Learning Test (CVLT) and Benton Visual Retention Test (BVRT). Word knowledge and verbal ability were measured using Primary Mental Abilities Vocabulary (PMA). Verbal fluency was assessed by Letter (i.e. FAS) and Category fluency tests. Attention and working memory were measured by the Digit Span Test of the Wechsler Adult Intelligence Scale-Revised, and the Trail Making Test. Digits Backward, Trails B, and Verbal Fluency (categories and letters) assessed executive function. The Card Rotations Test assessed visuospatial function. Data from evaluations at baseline and the last follow-up were used to examine differences in change in performance over time between the two groups.
PET Scanning Parameters
Participants underwent PET scans at baseline (year-1) and up to eight annual follow-ups. Each imaging session included a resting scan in which participants were instructed to keep their eyes open and focused on a computer screen covered by a black cloth. Participants also underwent PET scans during verbal and figural recognition memory tasks. Scan order was counter-balanced but remained constant over repeated assessments. For comparability with previous literature, we present analyses of the resting state PET data.
PET measures of rCBF were obtained using [15O] water. For each scan, 75 mCi of [15O] water were injected as a bolus. Scans were performed on a GE 4096+ scanner, which provides 15 slices of 6.5 mm thickness. Images were acquired for 60 seconds from the time the total radioactivity counts in the brain reached threshold level. Attenuation correction was performed using a transmission scan acquired prior to the emission scans.
MRI Scanning Parameters
A 3-D spoiled gradient refocused (SPGR) MRI scan (124 slices, 256×256 matrix, 0.94×0.94mm voxel size, 1.5 mm slice thickness) was obtained on a 1.5 T GE Signa scanner at each imaging visit.
PET Data Analysis
Data from PET scans obtained at baseline and the last available follow-up time points were used in the analyses. The mean interval between baseline and last follow-up PET scans did not differ significantly between ε4 carriers (7.8 years) and non-carriers (7.7 years). The PET scans were realigned and spatially normalized into standard stereotactic space and smoothed to full width at half maximum of 12×12×12 mm in the x, y, and z planes. To control for variability in global flow, rCBF values at each voxel were ratio adjusted to the mean global flow and scaled to 50 ml/100g/min for each image. The image data were analyzed using Statistical Parametric Mapping (SPM2; Wellcome Department of Cognitive Neurology, London, England).
To examine the differences in longitudinal rCBF change between the ε4+ and ε4− groups, voxel-based group × time interactions for rCBF change between baseline and last follow-up scans were performed. Baseline age and sex were included as covariates and significant effects for each contrast were based on the magnitude of activation (p≤0.005) and spatial extent (>50 voxels). For those regions showing differences in longitudinal changes in rCBF between the ε4+ and ε4− groups, a region-of-interest (ROI) analysis was performed to quantify both baseline rCBF as well as the magnitude of change in rCBF over time. The ROI analysis was performed by extracting the adjusted rCBF values at the local maxima for each region using a 4 mm spherical search area.
MRI Volumetric Data Analysis
Additional analyses were performed to control for the effects of potential differences in tissue volume between the two groups that could account for differences in rCBF changes. The MRI scans were segmented into gray matter, white matter and cerebrospinal fluid and spatially normalized into stereotactic space using a high-dimensional elastic warping method and a volume-preserving transformation 17. For each participant, binary maps of the regions showing significant differences in rCBF over time between the ε4+ and ε4− groups were registered with the MRI image. Total volumes of gray + white matter were subsequently calculated for each region. The PET data were then reanalyzed using the total tissue volume (gray + white matter) of these regions as additional covariates. Total gray + white matter volume could not be calculated for one region in the right inferior temporal cortex due to a registration error.
Results
Sample characteristics
APOE ε4 carriers tended to be slightly younger than non-carriers (p=0.06). There were no significant differences in sex distribution between the two groups. There were also no significant differences between the two groups in educational status or mini-mental state examination (MMSE) scores either at baseline or last follow-up (Table 1). There was no significant difference in the cardiovascular risk profile between ε4 carriers and non-carriers as determined by the composite Framingham risk score for each group. There were no significant inter-group differences in the proportion of subjects with a family history of dementia. APOE ε4 carriers tended to perform slightly worse than non-carriers in the Trails-A cognitive task after controlling for age and sex (35.17±1.88 and 30.59±1.26 seconds respectively; p=0.05) There were no significant differences at baseline between the groups on any other cognitive task.
Neuropsychological performance
For each task or cognitive domain, a linear mixed-effects model, adjusting for sex and baseline age, was used to compare changes in performance from the baseline to the last follow-up time points for ε4 carriers versus non-carriers (Table 2). APOE ε4 non-carriers compared with carriers showed significantly greater decline in category fluency over time (p < 0.05). There were no significant inter-group differences in performance over time on any other cognitive task.
Table 2.
Change over time in neuropsychological performance for APOE ε4 carriers and non-carriers after controlling for sex and baseline age.
| Domain | ε4 non- carriers |
ε4 carriers |
Difference | p-value |
Effect size |
|
|---|---|---|---|---|---|---|
| Memory | CVLT | 1.83 (1.39) |
−2.10 (2.03) |
3.93 (2.46) |
0.1137 | 1.60 |
| BVRT errors |
−1.41 * (0.45) |
−0.93 (0.65) |
−0.48 (0.79) |
0.5501 | −0.60 | |
| Verbal Fluency |
Letters | −0.48 (0.41) |
−0.24 (0.61) |
−0.25 (0.73) |
0.7392 | −0.33 |
| Categories | −1.21 * (0.34) |
0.24 (0.50) |
−1.45 (0.61) |
0.0206 | −2.38 | |
| Attention and working memory |
Digits forward |
0.34 (0.24) |
−0.33 (0.36) |
0.67 (0.43) |
0.1250 | 1.55 |
| Digits back | −0.29 (0.33) |
−0.19 (0.49) |
−0.09 (0.59) |
0.8749 | −0.16 | |
| Trails A | 1.77 (1.50) |
−1.22 (2.22) |
2.99 (2.67) |
0.2663 | 1.12 | |
| Trails B | −10.78* (5.15) |
−3.70 (7.80) |
−7.08 (9.35) |
0.4518 | −0.76 | |
| Visuospatial | Card rotation |
2.22 (2.92) |
9.71 * (4.22) |
−7.49 (5.13) |
0.1485 | −1.46 |
Data are expressed as mean ± (standard error)
Indicates significant change within groups (p<0.05)
For each task or cognitive domain, a linear mixed-effect model, adjusting for sex and baseline age, was used to compare changes from the baseline to the last follow-up time points for ε4 carriers versus non-carriers. For BVRT and Trails A and B tasks, positive slopes indicate improvement and negative slopes indicate decreased performance over time respectively.
Effect size was computed as ‘Difference in performance’ divided by the standard error.
Longitudinal changes in rCBF
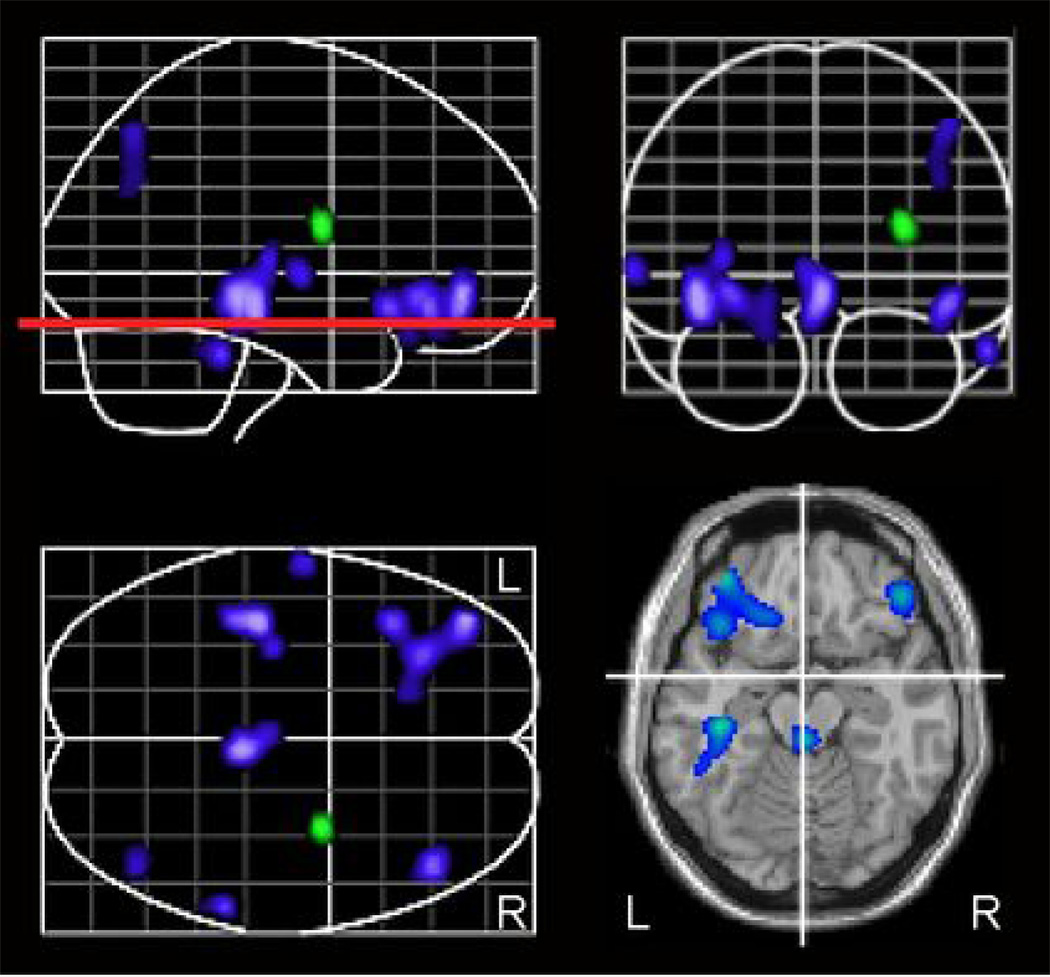
Analysis of group differences in longitudinal change from baseline to last follow-up revealed several regions of rCBF declines that differed significantly between ε4 carriers versus non-carriers (Table 3, Figure 1). The brain regions exhibiting significantly greater rCBF declines over time in ε4 carriers included the right orbitofrontal cortex (Brodmann area; BA 47), left middle (BA 10) and inferior frontal (BA 47) gyri, left middle (BA 20) and superior temporal (BA 22) gyri, right inferior temporal cortex (BA 20) and right superior parietal cortex (BA 7). Significantly greater increase in rCBF was observed in ε4 carriers relative to non-carriers in the right insula. The ROI analysis in these regions showed that the magnitude of the change in rCBF over time in ε4 carriers ranged from two to six percent relative to baseline. We also observed significantly greater baseline rCBF in APOE ε4 carriers in each of these regions (Table 3).
Table 3.
Local maxima within areas of significant differences in the magnitude of longitudinal changes in rCBF between APOE ε4 positive and negative groups. Coordinates are in stereotactic space and Brodmann areas are in parentheses.
| Decreases in Resting rCBF | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Coordinate | ||||||||||
| Region | Side | x | y | z | T-value | puncorrected | Number of voxels | Percentage change from baseline |
Baseline rcbf (ml/100g/min) |
|
| ε4+ve | ε4 −ve | |||||||||
| APOE ε4 POSITIVE > APOE ε4 NEGATIVE | ||||||||||
| Orbitofrontal cortex (47) | R | 46 | 36 | −12 | 3.64 | <0.001 | 162 | −4% | 86.18 (5.2)* | 81.80 (4.6) |
| Middle frontal gyrus (10) | L | −42 | 48 | −8 | 3.90 | <0.001 | 6361 | −6% | 75.96 (4.1)* | 71.42 (4.9) |
| Inferior frontal gyrus (47) | L | −40 | 22 | −12 | 3.56 | <0.001 | 6361 | −6% | 95.75 (5.7)* | 88.06 (7.1) |
| Superior parietal cortex (7) | R | 46 | −66 | 50 | 3.21 | 0.001 | 1362 | −6% | 77.43 (4.6)* | 71.49 (5.5) |
| Angular gyrus (39) | R | 42 | −68 | 34 | 2.90 | 0.002 | 1362 | −3% | 89.03 (5.7)* | 81.39 (5.4) |
| Brainstem | R | 2 | −30 | −8 | 3.90 | <0.001 | 327 | −2% | 80.56 (8.1)† | 76.49 (5.7) |
| Middle temporal gyrus (20) | L | −40 | −24 | −12 | 3.93 | <0.001 | 455 | −2% | 70.49 (7.8)* | 64.68 (4.3) |
| Inferior temporal cortex (20)¶ | R | 60 | −36 | −28 | 3.50 | <0.001 | 62 | −2% | 78.23 (6.2)* | 72.96 (4.6) |
| Superior temporal gyrus (22)§ | L | −64 | −8 | 0 | 3.37 | 0.001 | 57 | −5% | 73.11 (4.4)* | 67.15 (4.6) |
| Increases in Resting rCBF | ||||||||||
| APOE ε4 POSITIVE > APOE ε4 NEGATIVE | ||||||||||
| Insula | R | 32 | −2 | 16 | 3.71 | 0.000 | 56 | +5% | 67.95 (5.8)‡ | 65.15 (5.1) |
Denotes local maxima contained within the same cluster
Denotes local maxima contained within the same cluster
Each voxel represents 2mm x 2mm x 2mm tissue volume
Volume correction was not performed on this region due to registration errors
Differences in rCBF in this region were not statistically significant after correcting for total tissue volume
p<0.001
p<0.007
p=0.02.
Baseline rCBF values in regions showing longitudinal changes are presented as Mean (S.D.)
Figure 1. Differences in longitudinal change in rCBF between APOE ε4 positive and ε4 negative groups.
Areas in blue show significantly greater longitudinal decreases in rCBF in the APOE ε4 carriers and those in green denote areas showing greater longitudinal decrease in rCBF in APOE ε4 non-carriers. The red line illustrates the z level of the representative slice shown.
These longitudinal differences in rCBF between the two groups were largely preserved after correcting for tissue volume in each of the above regions, with the exception of the left superior temporal gyrus. The observed decline in rCBF in this region in ε4 carriers compared to non-carriers was no longer significant after correcting for the corresponding regional tissue volume.
Discussion
Our principal objective in the present study was to examine differences in changes in rCBF over time in ε4 carriers versus non-carriers in non-demented older individuals. Consistent with our hypothesis, longitudinal changes in rCBF differed between ε4 carriers and non-carriers in several brain regions that are especially vulnerable to AD pathology. Carriers of the APOE ε4 allele showed greater longitudinal declines in rCBF in the frontal, parietal and temporal cortices. These results, based on the largest sample of older adults studied to date, demonstrate that there indeed are widespread longitudinal differences in rCBF change between older ε4 carriers and non-carriers. Furthermore, we demonstrate that the majority of these differences in brain function over time were evident even after adjusting for differential loss of brain tissue.
Studies in middle aged individuals have indicated accelerated decline in regional cerebral glucose metabolism (CMRglu) in APOE ε4 carriers 7. For example, Reiman and colleagues used FDG-PET in late-middle aged subjects (10 ε4 heterozygotes and 15 ε4 non-carriers) with at least one first degree relative with probable AD and showed significantly greater decline in rCMRglu over two years in ε4 carriers 7. The regions showing the greatest declines in ε4 carriers were bilateral temporal, occipitotemporal and prefrontal cortices, as well as the right hippocampus and left fusiform gyrus.
However, previous longitudinal PET studies on the effect of APOE genotype on cerebral metabolism in older adults have been inconclusive 8, 9. In the few studies that have demonstrated APOE genotype-related changes in regional cerebral metabolism over time in older people, such effects were only observed in relatively small numbers of subjects with a family history of AD or in those who underwent significant cognitive decline over the follow-up period. Using F-18 fluorodeoxyglucose (FDG)-PET, de Leon and colleagues studied 12 individuals over 70 years of age who underwent significant cognitive decline over a period of three years 8. Using a region of interest (ROI) approach, the authors observed a significantly greater decline in regional CMRglu in the lateral temporal lobe (averaged across hemispheres) in ε4 carriers compared to non-carriers. However, using a similar approach in an older sample of 10 ε4 carriers and 10 ε4 non-carriers with both age-associated memory impairment and a family history of AD, Small and colleagues found no significant group differences in longitudinal change in CMRglu 9.
The predominant pattern of differences in rCBF over time in our study is one of greater decline in ε4 carriers. We also confirmed that ε4-associated changes in rCBF were largely independent of changes in tissue volume between the two groups. Previous PET studies on the effect of APOE-genotype on longitudinal changes in brain function have not corrected for differences in tissue volume in brain regions showing significant changes in ε4 carriers 7–9. These additional volumetric analyses are especially important in the light of previous data showing atrophic changes in several brain regions in cognitively normal ε4 carriers 18–20. Although our study does not address the precise mechanisms underlying the observed APOE-genotype associated changes in rCBF over time, we suggest that the widespread decline in rCBF in ε4 carriers might represent regional patterns of neuronal vulnerability in subjects at risk for AD. It is likely that these changes reflect declining brain function resulting from multiple biological effects mediated by the APOE ε4 allele. These may include impaired brain repair mechanisms in ε4 carriers 21 rendering specific regions differentially vulnerable to the deleterious effect of environmental risk factors. It is interesting that we observed higher baseline rCBF values in APOE ε4 carriers in regions exhibiting significant longitudinal declines in rCBF. It is plausible that these increments in baseline rCBF represent compensatory mechanisms in APOE ε4 carriers. These findings are in partial agreement with earlier studies using [15O] water PET to study rCBF in young adult APOE ε4 carriers 22. The observed longitudinal changes in rCBF in this study may also reflect to a certain extent, early neuropathological changes such as β-amyloid deposition 23 or neurofibrillary degeneration 24 in ε4 carriers. Some ε4-associated changes in rCBF observed in our study are distributed in brain regions that are particularly vulnerable to AD pathology such as temporal cortex and bilateral frontal lobes. Moreover, the patterns of rCBF decline shows some regional overlap with the distribution of β-amyloid deposition measured with [11C]Pittsburgh Compound B (PIB) PET both in normal aging and AD 25.
It is important to note that in this relatively healthy sample excluding individuals with cognitive impairment, we did not detect significant differences in memory decline in ε4 carriers compared to non-carriers. APOE ε4-associated cognitive decline in non-demented subjects is reported to be especially prominent in those with low educational levels 26. As the BLSA is a volunteer sample with an unusually high level of education 27, it is possible that cognitive reserve protects them from objective memory problems until later in the course of a preclinical disease process. Furthermore, participants are rigorously evaluated during study visits to detect both clinical and occult cardiovascular disease 28. Many participants in our study undergo early treatment and lifestyle-based interventions against established vascular risk factors for dementia such as diabetes, hypertension and hyperlipidemia. Taken together, these factors may have contributed to the absence of cognitive decline in ε4 carriers in our study, perhaps by modifying the preclinical course of disease.
In summary, we used [15O] water PET to demonstrate greater longitudinal decline in rCBF in cognitively intact, older subjects carrying the APOE ε4 allele compared to non-carriers. These results suggest that APOE ε4-mediated risk for AD is associated with widespread changes in rCBF over time and that these changes precede the development of cognitive decline. These findings have potential for utility as neuroimaging markers of longitudinal changes in brain function in subjects at risk for AD as well as for monitoring response to novel disease-modifying treatments in clinical trials.
Acknowledgements
This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging and by Research and Development Contract N01-AG-3–2124. Partial support was also through a R&D contract with MedStar Research Institute. We are grateful to the BLSA participants and neuroimaging staff for their dedication to these studies and the staff of the Johns Hopkins PET facility for their assistance.
Footnotes
Conflict of interest: Nothing to disclose
REFERENCES
- 1.Coon KD, Myers AJ, Craig DW, et al. A high-density whole-genome association study reveals that APOE is the major susceptibility gene for sporadic late-onset Alzheimer's disease. J Clin Psychiatry. 2007;68:613–618. doi: 10.4088/jcp.v68n0419. [DOI] [PubMed] [Google Scholar]
- 2.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 3.Saunders AM, Strittmatter WJ, Schmechel D, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer's disease. Neurology. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- 4.Tiraboschi P, Hansen LA, Masliah E, et al. Impact of APOE genotype on neuropathologic and neurochemical markers of Alzheimer disease. Neurology. 2004;62:1977–1983. doi: 10.1212/01.wnl.0000128091.92139.0f. [DOI] [PubMed] [Google Scholar]
- 5.Modrego PJ. Predictors of conversion to dementia of probable Alzheimer type in patients with mild cognitive impairment. Curr Alzheimer Res. 2006;3:161–170. doi: 10.2174/156720506776383103. [DOI] [PubMed] [Google Scholar]
- 6.Scarmeas N, Stern Y. Imaging studies and APOE genotype in persons at risk for Alzheimer's disease. Curr Psychiatry Rep. 2006;8:11–17. doi: 10.1007/s11920-006-0076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reiman EM, Caselli RJ, Chen K, et al. Declining brain activity in cognitively normal apolipoprotein E epsilon 4 heterozygotes: A foundation for using positron emission tomography to efficiently test treatments to prevent Alzheimer's disease. Proc Natl Acad Sci U S A. 2001;98:3334–3339. doi: 10.1073/pnas.061509598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Leon MJ, Convit A, Wolf OT, et al. Prediction of cognitive decline in normal elderly subjects with 2-[(18)F]fluoro-2-deoxy-d-glucose/poitron-emission tomography (FDG/PET) Proc Natl Acad Sci U S A. 2001;98:10966–10971. doi: 10.1073/pnas.191044198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Small GW, Ercoli LM, Silverman DH, et al. Cerebral metabolic and cognitive decline in persons at genetic risk for Alzheimer's disease. Proc Natl Acad Sci U S A. 2000;97:6037–6042. doi: 10.1073/pnas.090106797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lovestone S, Francis P, Strandgaard K. Biomarkers for disease modification trials--the innovative medicines initiative and AddNeuroMed. J Nutr Health Aging. 2007;11:359–361. [PubMed] [Google Scholar]
- 11.Mueller SG, Weiner MW, Thal LJ, et al. Ways toward an early diagnosis in Alzheimer's disease: The Alzheimer's Disease Neuroimaging Initiative (ADNI) Alzheimers Dement. 2005;1:55–66. doi: 10.1016/j.jalz.2005.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Resnick SM, Goldszal AF, Davatzikos C, et al. One-year age changes in MRI brain volumes in older adults. Cereb Cortex. 2000;10:464–472. doi: 10.1093/cercor/10.5.464. [DOI] [PubMed] [Google Scholar]
- 13.Shock NW, Greulich RC, Andres R, et al. Normal human aging: the Baltimore Longitudinal Study of Aging. Washington, D.C: U.S. Government Printing Office; 1984. [Google Scholar]
- 14.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 15.Wilson PW, D'Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 16.Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31:545–548. [PubMed] [Google Scholar]
- 17.Shen D, Davatzikos C. HAMMER: hierarchical attribute matching mechanism for elastic registration. IEEE Trans Med Imaging. 2002;21:1421–1439. doi: 10.1109/TMI.2002.803111. [DOI] [PubMed] [Google Scholar]
- 18.den Heijer T, Oudkerk M, Launer LJ, et al. Hippocampal, amygdalar, and global brain atrophy in different apolipoprotein E genotypes. Neurology. 2002;59:746–748. doi: 10.1212/wnl.59.5.746. [DOI] [PubMed] [Google Scholar]
- 19.Soininen H, Partanen K, Pitkanen A, et al. Decreased hippocampal volume asymmetry on MRIs in nondemented elderly subjects carrying the apolipoprotein E epsilon 4 allele. Neurology. 1995;45:391–392. doi: 10.1212/wnl.45.2.391. [DOI] [PubMed] [Google Scholar]
- 20.Wishart HA, Saykin AJ, McAllister TW, et al. Regional brain atrophy in cognitively intact adults with a single APOE epsilon4 allele. Neurology. 2006;67:1221–1224. doi: 10.1212/01.wnl.0000238079.00472.3a. [DOI] [PubMed] [Google Scholar]
- 21.Arendt T, Schindler C, Bruckner MK, et al. Plastic neuronal remodeling is impaired in patients with Alzheimer's disease carrying apolipoprotein epsilon 4 allele. J Neurosci. 1997;17:516–529. doi: 10.1523/JNEUROSCI.17-02-00516.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scarmeas N, Habeck CG, Stern Y, et al. APOE genotype and cerebral blood flow in healthy young individuals. JAMA. 2003;290(12):1581–1582. doi: 10.1001/jama.290.12.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morishima-Kawashima M, Oshima N, Ogata H, et al. Effect of apolipoprotein E allele epsilon4 on the initial phase of amyloid beta-protein accumulation in the human brain. Am J Pathol. 2000;157:2093–2099. doi: 10.1016/s0002-9440(10)64847-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghebremedhin E, Schultz C, Braak E, Braak H. High frequency of apolipoprotein E epsilon4 allele in young individuals with ver mild Alzheimer's disease-related neurofibrillary changes. Exp Neurol. 1998;153:152–155. doi: 10.1006/exnr.1998.6860. [DOI] [PubMed] [Google Scholar]
- 25.Rowe CC, Ng S, Ackermann U, et al. Imaging beta-amyloid burden in aging and dementia. Neurology. 2007;68:1718–1725. doi: 10.1212/01.wnl.0000261919.22630.ea. [DOI] [PubMed] [Google Scholar]
- 26.Mayeux R, Small SA, Tang M, et al. Memory performance in healthy elderly without Alzheimer's disease: effects of time and apolipoprotein-E. Neurobiol Aging. 2001;22:683–689. doi: 10.1016/s0197-4580(01)00223-8. [DOI] [PubMed] [Google Scholar]
- 27.Grober E, Hall CB, Lipton RB, et al. Memory impairment, executive dysfunction, and intellectual decline in preclinical Alzheimer's disease. J Int Neuropsychol Soc. 2008;14:266–278. doi: 10.1017/S1355617708080302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a "set up" for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]