Abstract
Objective
To investigate adrenal steroid regulation in PCOS
Design
5-hour oral glucose tolerance test (OGTT) and frequently sampled-intravenous GTT
Setting
University research center
Patients
Thirty patients
Intervention
None
Main outcome measures
Anthropometrics, leptin, cortisol, DHEAS, glucose, insulin
Results
Morning cortisol correlated with sensitivity index (SI, r=0.540, p=0.0109), DHEAS correlated inversely with age (r=−0.6359), body mass index (BMI, r=−0.6199), fat mass (r=−0.630) and leptin (r=−0.5676) (p< 0.002 for all). Between the 2nd and 4th hour of OGTT, cortisol changes (Δ) exhibited 3 patterns: I. Responders (n=9, Δ:10.7±1.0μg/dL), II. Non-responders (n=10, Δ:−3.5±0.6μg/dL), III. Intermediates (n=11, Δ:4.3±1.0μg/dL). Compared to non-responders, responders were more obese (BMI: 37.0±1.6 vs. 31.7±1.8kg/m2, p< 0.05); had higher leptin (28.9±1.7 vs. 24.1±1.1ng/mL, p<0.03) and lower DHEAS (133±12 vs. 236±32ng/mL, p<0.01), higher glucose at 1h of OGTT (195±13 vs. 131±12mg/dL, p< 0.05), higher AUCGlucose (332±20 vs. 265±17mg/dL, p=0.0208), higher AUCInsulin (244±50 vs. 125±30μU/mL, p=0.05) and lower nadir glucose (61±2 vs. 70±2mg/dL, p=0.0002).
Conclusion
Obesity and insulin resistance are associated with lower morning cortisol and DHEAS but increased cortisol and DHEA responses after glucose ingestion. Morning steroid levels may not reflect the day-long exposure.
Keywords: Adrenal steroids, PCOS, obesity, insulin resistance, leptin
In polycystic ovary syndrome (PCOS), the adrenals contribute to the androgen excess by producing excess amounts of dehydroepiandrostenedione (DHEA) and androstenodione (1, 2). Although obesity and insulin resistance, that are common in PCOS, influence adrenal function, their role is not straightforward. The entire literature is based on the adrenal steroid measurements obtained in the morning. Our recent research demonstrated that PCOS patients secreted cortisol, DHEA and androstenedione after drinking glucose for oral glucose tolerance testing (OGTT) (3). Those who developed even mild postprandial hypoglycemia had a brisk increase in adrenal steroid secretion. Although hypoglycemia is a known stimulator of adrenal steroids, the standard insulin-induced hypoglycemia test used for this purpose aims to serum glucose below 50 mg/dL. Whereas our PCOS patients secreted the adrenal steroids with serum glucose ≤ 69 mg/dL. Similarly, Spyer et al. (4) had reported increased counter-regulatory hormone secretion with plasma glucose concentrations < 67 mg/dL in well controlled diabetic patients and Solter et al. had demonstrated increased counter regulatory hormones during asymptomatic hypoglycemia in obese subjects (5, 6). Thus, it appeared that factors common to PCOS, obesity and type2 diabetes may influence adrenal response. To investigate this possibility, we determined the relationships between the adrenal steroid response and body weight, body composition, insulin secretion and insulin sensitivity in a new group of PCOS patients.
Materials and Methods
Subjects
Thirty patients (23 White, 3 Hispanic, 2 African American and 2 Asian) with PCOS aged 20–45 y and with a body mass index (BMI) of 22–50 kg/m2 were recruited after signing the informed consents approved by the Institutional Review Board of University of California, Davis. The investigators did not have any conflict of interest. All participants were examined by the principal investigator (SEK-K) who is the director of the PCOS program at the Medical Center of the University of California, Davis. The participants fulfilled the NIH criteria for PCOS (7) by having ovarian dysfunction, as evidenced by amenorrhea (no periods for >6 mo) or oligomenorrhea (<6 periods/y), clinical (hirsutism) or laboratory evidence for hyperandrogenemia (total testosterone >54 ng/dL or free testosterone >9.2 pg/mL), along with the absence of any confounding clinical pathology (i.e. Cushing’s disease, 21 hydroxylase deficiency or prolactinoma). Patients were excluded if they used oral contraceptives, antiandrogenic medications, insulin sensitizers, d-chiro inositol, or any other medications or supplements that affect weight or insulin sensitivity during the preceding two months; have impaired glucose tolerance, diabetes mellitus, untreated hypothyroidism, and any other systemic illness such as renal, hepatic, and gastrointestinal disease; smoke; or drink > 2 alcoholic drinks per week.
Study design
Data collection
The OGTT and FS-IVGTT tests were performed at the Clinical and Translational Science Center Clinical Research Center of the University of California, Davis. The subjects were following their habitual diets (1873±104 kcal/d, 34% fat, 50% carbohydrate, 16% protein). They consumed 237±16 g/d carbohydrate prior to testing and they were weight stable.
Anthropometric data
Subjects were seen after an overnight fast. Weight was determined in light clothing using the Tanita BWB800-P Digital Medical Scale. Height without shoes was measured using an Ayrton Model S100 stadiometer. Body composition was determined using bioelectrical impedance (Biostat, British Isles) (8). Because the fluctuations occurring in body-water during menstrual cycles can affect bioelectrical impedance, menstruating women were studied during the first ten days of their cycles.
5-h OGTT
Participants were tested between 0600 and 0900, after an overnight fast. Water intake was permitted. An intravenous catheter was placed into the forearm and kept open with saline. At time point zero, participants drank 75 g of glucose (Glucola ™). Blood samples were obtained at baseline (time point -10 min) and then, after glucose ingestion, at 30-minute intervals for 5 hours. Subjects remained supine throughout the procedure to avoid the confounding effects of physical activity on blood glucose levels. Samples for glucose measurement were collected in Na fluoride containing tubes. Other samples were collected on either serum separation tubes or on tubes containing EDTA or heparin.
3-h FS-IVGTT
Participants were tested between 0600 and 0900, after an overnight fast. An intravenous catheter was placed in their forearm and kept open with normal saline. Heating pads were used in order to maximize blood flow. Three blood samples were obtained at times -15, -10 and -5 minutes. Glucose (0.3u/kg as 25% dextrose) was given intravenously at time 0 min. Intravenous insulin 0.03 u/kg (Humulin Regular: Eli Lilly) was given at time 20 min after the glucose administration. Blood samples were obtained at times 0, 2, 3, 4, 5, 6, 8,10, 12, 14, 16, 19, 22, 23, 24, 25, 27, 30, 40, 50, 60, 70, 90, 100, 120, 140, 160 and 180 min. Blood samples for tests on glucose, insulin, and other parameters were collected as described with the OGTT. Acute insulin response to glucose (AIRGlucose), β-cell function, sensitivity index (SI) and disposition index (DI) was calculated using MiniMod Millennium software (Dr. Bergman, Los Angeles, CA).
Clinical symptoms
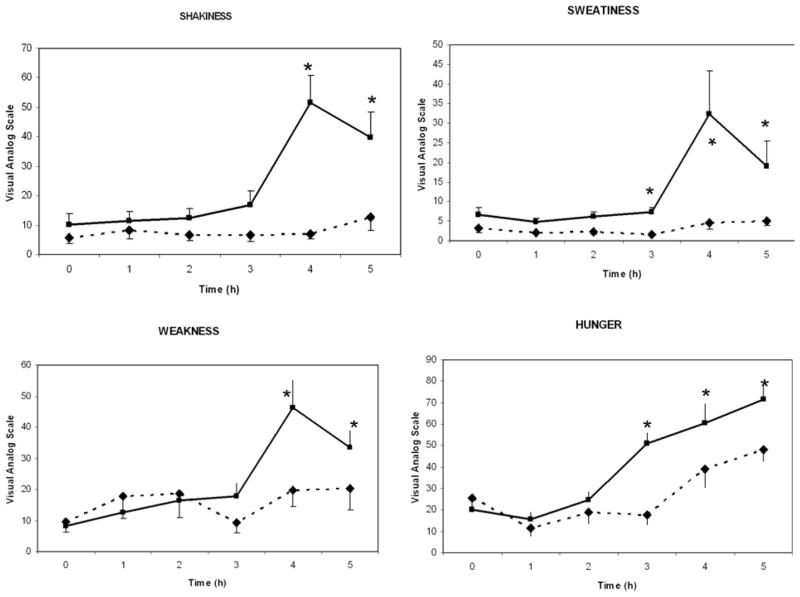
Our recent study indicated a relationship between adrenal steroid secretion and postprandial hypoglycemia. To determine the symptomatology that relates to adrenal steroid secretion, we used the Hypoglycemia Symptoms Logs (HSL) program developed for hand-held computer in collaboration with William Horn, and Nancy Keim, PhD. The key symptoms related to the autonomic response, neuroglycopenia and malaise (sweating, shaking, hunger, weakness, confusion, drowsiness, behavior, speech difficulty, incoordination, nausea and headache) were recorded hourly on a 0–100 scale. The data were transferred from the hand held computer to an Excel™ spreadsheet for analysis, and were plotted against time as shown in Figure 2.
Figure 2.
Clinical symptoms in responders (n = 9, solid line) and non-responders (n = 10, broken line) during oral glucose tolerance test (Mean±SEM, *; p < 0.05 when responders and non-responders are compared to each other).
Biochemical measurements
Plasma and serum samples were obtained after an overnight fast. Glucose was measured using YSI 2300 STAT Plus Glucose & Lactate Analyzer (YSI Life Sciences, Yellow Springs, OH. The coefficient of variation (cv) for glucose was1%. Insulin was measured using RIA kits (Linco Research Inc, St. Charles, MO) with a cv of 8.2%. The homeostatic model assessment (HOMA), a surrogate measure of insulin sensitivity, was calculated using the formula: [fasting insulin (μU/mL) × fasting glucose (mM)]/22.5. Total testosterone, sex hormone binding globulin (SHBG), cortisol and DHEAS were measured by RIA (Diagnostic Systems Laboratories, Webster, TX). The cvs were: 8.3% for testosterone, 4.4% for SHBG, 5.3% for cortisol, 9.6% for DHEAS and 4.9% for DHEA. The reference range for these hormones, measured in a group of lean, healthy women with normal ovarian function (n = 19; age, 40±1 yr; BMI 23.9±1.5 kg/m2) were: testosterone: 0.27±0.029 ng/mL; SHBG, 68.5±6.6 nmol/L, and DHEAS: 116±24 ng/mL.
Statistical Analysis
Statistical analysis was performed using SAS statistical software, version 9.1 (SAS Institute Inc, Cary, NC). Descriptive statistics (mean or adjusted mean, standard error (SE), 95% confidence interval) were calculated for each measurement by the response group (categorized based on change in cortisol) and time point. Pearson’s correlation co-efficients and corresponding p values were calculated for the baseline values of the entire group.
Trajectory of 5-hour change in response levels was estimated by a repeated measures analysis of variance. Individual trajectories of glucose, insulin cortisol, DHEA changes over 11 time points, measured every 30 minutes, were estimated from linear random-effects models. Each response level was entered as the dependent variable (Y). The response group, time (min), and response group × time interaction term were entered as independent variables. To account for between subject heterogeneity in the changes of response levels, intercept and time were modeled as random effects.
Analysis of variance (ANOVA) was performed to assess 1) whether each response level was associated with the response group (overall); and 2) there was significant mean difference between responders and non-responders. A two-sided p-value of 0.05 was considered significant.
Results
Definition of the groups based on cortisol response
The adrenal response was defined based on cortisol changes during OGTT. Those subjects who had a minimum increase of 7.2 μg/dl (200 nM) in cortisol were defines as responders--similar to the interpretation of positive cortrysin stimulation test (9). Those who had no change or a decrease in cortisol from the baseline were defined as non-responders. The responders (n= 9) had 10.7±1.0 μg/dL increase in cortisol between the 2nd and 4th hour of OGTT; the non responders (n=10) had 3.5±0.6 μg/dL decrease; the remaining 11 subjects had intermediate responses (Δ = 4.3±1.0 μg/dL). Cortisol responses differed significantly among these 3 groups (p=0.0001).
The changes in DHEA concentrations followed a similar pattern between the 2nd and 4th hour: Δ = 14.4±1.7 ng/mL in the responders, Δ = 0.4±0.9 ng/mL in the non-responders, and Δ = 3.6±1.2 ng/mL in the intermediates. These differences were also significant (p=0.0003).
Baseline characteristics of responders vs. non-responders (Table 1)
Table 1.
Baseline variables of the PCOS patient who demonstrated increased cortisol response during oral glucose tolerance test (responders) and those who did not have an increase (non-responders).
| Responder (n = 9) | Non-responder (n = 10) | p | |
|---|---|---|---|
| Anthropometric | |||
| Weight (kg) | 105.5 ± 4.9 | 87.5 ± 5.4 | 0.0245 |
| BMI (kg/m2) | 37.0 ± 1.6 | 31.7 ± 1.8 | 0.0458 |
| Fat-mass (kg) | 49.1 ± 3.1 | 37.2 ± 4.9 | 0.0616 |
| Lean-mass (kg) | 57.0 ± 2.9 | 49.8 ± 1.3 | 0.0307 |
| Leptin (ng/mL) | 28.9 ± 1.7 | 24.1±1.1 | 0.0270 |
| Insulin resistance | |||
| Fasting | |||
| Glucose (mg/dL) | 99.7 ± 5.7 | 104.4 ± 5.4 | 0.5689 |
| Insulin (μU/mL) | 18.4 ± 3.0 | 14.0 ± 2.9 | 0.2662 |
| HOMA | 4.01 ± 1.1 | 3.22 ± 1.2 | 0.3241 |
| OGTT | |||
| AUCGlucose | 332 ± 20 | 265 ± 17 | 0.0208 |
| AUCInsulin | 244 ± 50 | 125 ± 30 | 0.0500 |
| GlucoseNadir (mg/dL) | 61.4 ± 2.2 | 70.2 ±2.3 | 0.0002 |
| FS-IVGTT | |||
| AIRGlucose | 843 ± 270 | 504 ± 175 | 0.2972 |
| β-cell function | 287.4 ± 33.0 | 207.6 ± 32.8 | 0.1057 |
| SI | 2.72 ± 0.62 | 4.91 ± 1.38 | 0.1827 |
| DI | 1927 ± 510 | 1584 ± 272 | 0.5486 |
| Steroid hormones | |||
| Cortisol (μg/dL) | 10.9 ± 2.1 | 10.7± 2.0 | 0.7384 |
| DHEA (ng/mL) | 9.9 ± 2.8 | 16.3 ± 2.6 | 0.101 |
| DHEAS (ng/mL) | 133 ± 12 | 236 ±32 | 0.0099 |
| Testosterone (ng/mL) | 0.75±0.05 | 0.80±0.08 | 0.7529 |
| SHBG (nmol/L) | 33.9±3.1 | 58.6±6.7 | 0.0220 |
Age was did not differ significantly between the responders vs. non-responders (Mean±SEM: 34±2 vs. 32±2 years).
Anthropometric variables
Responders were more obese (BMI: 37.0±1.6 vs. 31.7±1.8 kg/m2, p<0.05), had larger lean-mass and a borderline increase in fat-mass. Since body compartment measurements by bioelectrical impedance relate to each other (8), an independent indicator of fat mass was sought. It is well established that serum leptin directly correlates with fat-mass (10). Thus, serum leptin was also measured. Responders had higher serum leptin than non-responders (28.9 vs. 24.1 ng/mL, p=0.0270).
Insulin resistance parameters
Fasting glucose, insulin and HOMA did not differ.
Steroid hormones
Morning DHEAS was lower in responders than in the non-responders (133±12 vs. 236±32 ng/mL, p=0.0099). DHEA tended to be lower also in responders (9.9±2.8 vs. 16.3±2.6 ng/mL, p=0.101). Cortisol and testosterone concentrations were not different. Responders had lower serum SHBG (33.9±3.1 vs. 58.6±6.7 nmol/L, p=0.0220).
Relationships between morning levels of adrenal steroids and anthropometric parameters, insulin resistance, pancreatic β-cell function
Morning cortisol correlated directly with SI (r=0.5405, p=0.002), DI (r=0.3509, p=0.0573), and inversely with β-cell function (r=−0.3969, p=0.0299) indicating that insulin sensitivity was associated with higher serum cortisol in the morning. Cortisol showed a weak inverse correlation with leptin (r=−0.3310, p=0.0740).
Morning DHEAS correlated inversely with age (r=−0.6359, p=0.0002), weight (r=−0.5663, p=0.0011), BMI (r = −0.6199, p = 0.0003), fat-mass (r=−0.06295, p=0.0002), leptin (r=−0.5676, p=0.0011) and fasting insulin (r=−0.4017, p=0.0278). DHEAS correlated directly with SI (r=0.3664, p=0.0464).
Morning DHEA correlated inversely with obesity indicators such as weight, BMI, fat-mass, leptin, fasting glucose and fasting insulin, similarly to DHEAS. DHEA correlated inversely with β-cell function (r=−0.4128, p=0.0234) and directly with SI (r=0.5283, p=0.0027).
Other noteworthy correlations were observed between testosterone and DHEAS and DHEA; between cortisol and DHEAS and DHEA (Table 2). Serum SHBG correlated inversely with leptin (r=−0.4257, p=0.0190) and weight (r=−0.3593, p=0.0512), but directly with SI (r = 0.3535, p=0.0553) and adiponectin (r=0.3369, p=0.0687). Serum leptin correlated with weight (r=0.6727, p<0.0001), BMI (r=0.7814, p<0.0001) and fat mass (r=0.7784, p<0.0001).
Table 2.
Correlations between morning cortisol, DHEAS, DHEA concentrations and anthropometric and insulin resistance variables (n = 30).
| Cortisol | pCortisol | DHEAS | pDHEAS | DHEA | PDHEA | |
|---|---|---|---|---|---|---|
| Age | −0.6359 | 0.0002 | ||||
| Anthropometric | ||||||
| Weight | −0.5663 | 0.0011 | −0.4579 | 0.0109 | ||
| BMI | −0.6199 | 0.0003 | −0.3525 | 0.0560 | ||
| Fat-mass | −0.3851 | 0.0356 | ||||
| Leptin | −0.3310 | 0.0740 | −0.5676 | 0.0011 | −0.5297 | 0.0026 |
| Insulin resistance | ||||||
| Fasting | ||||||
| Glucose | −0.3442 | 0.0625 | ||||
| Insulin | −0.4017 | 0.0278 | −0.3581 | 0.0520 | ||
| FS-IVGTT | ||||||
| β-cell funct. | −0.3969 | 0.0299 | −0.4128 | 0.0234 | ||
| SI | 0.5405 | 0.0020 | 0.3664 | 0.0465 | 0.5283 | 0.0027 |
| DI | 0.3509 | 0.0573 | ||||
| Other | ||||||
| Cortisol | 0.4523 | 0.0121 | 0.6101 | 0.0003 | ||
| DHEAS | 0.7008 | <0.0001 | ||||
| Testosterone | 0.4762 | 0.0078 | 0.523 | 0.0030 | ||
Glucose and insulin changes during OGTT in responders vs. non-responders (Figure 1)
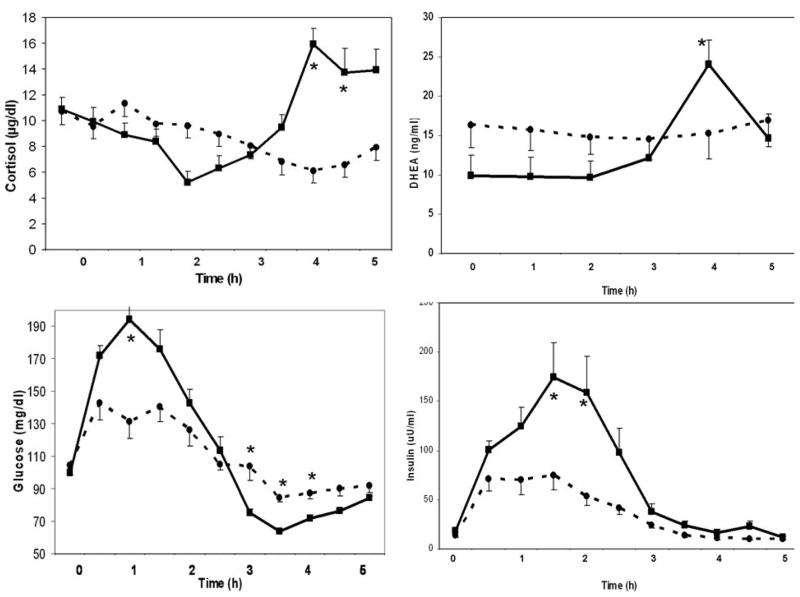
Figure 1.
Changes in cortisol, DHEA, glucose and insulin in responders (n = 9, solid line) and non-responders (n = 10, broken line) during oral glucose tolerance test (Mean±SEM, *; p < 0.05 when responders and non-responders are compared to each other).
Responders had higher glucose at 1h (194±13 vs. 131±12 mg/dL, p< 0.05), and higher area under the curve (AUCGlucose) during the first 2h (332±20 vs. 265±17 mg/dL, p< 0.03). In contrast, responders had lower nadir glucose (61.4±2.2 vs. 70.2±2.3 mg/dL, p=0.0002), 3h-glucose (75.10±8.93 vs. 104.04±8.47 mg/dL, p<0.03) and 4h-glucose (72±4 vs. 87±4 mg/dL, p< 0.02). Responders had also higher AUCInsulin during the first 2h (244±50 vs. 125±30 mU/mL, p=0.05) and higher serum insulin at 2h (159±31 vs. 54±29 mU/mL, p<0.05).
DHEAS and cortisol changes during OGTT in responders vs. non-responders (Figure 1)
Although baseline serum cortisol levels were similar in responders vs. non-responders, they started to diverge during 3.5 h of OGTT. By the 4th hour, cortisol increased from 10.9±2.1 to 16.0±1.7 ng/mL in responders, but had decreased from 10.7±2.0 to 6.2±1.6 ng/mL) in non-responders (p<0.001).
Baseline DHEA concentrations tended to be lower in responders (9.9±2.8 vs. 16.3±2.6 ng/mL, p=0.101). In responders, DHEA increased from 9.9±2.8 to 24.1±3.2 ng/mL, whereas in non-responders, it did not change (from 16.3±2.6 to 16.9±3.1 ng/mL).
Clinical symptoms in responders vs. non-responders (Figure 2)
Starting at the 3rd h, the clinical symptoms diverged: Responders had higher scores in shakiness, sweatiness, weakness and hunger.
Discussion
This study indicated that adrenal steroids are regulated differently in the morning vs. after nutrient intake.
We found that the morning concentrations of the adrenal steroids correlated inversely with age, obesity and insulin resistance. Serum DHEAS and DHEA concentrations related to anthropometric variables much more strongly than serum cortisol. These results are consistent with the observations of Moran at al. who noted that younger and thinner PCOS patients had higher DHEAS levels (11). Age correlated inversely with DHEAS levels in both White and African American women (2). This inverse relationship between DHEAS and obesity and insulin resistance is somewhat unexpected for several reasons. First, it has been hypothesized that a single pathology, namely an overactive serine kinase, may cause both insulin resistance and increased androgens because serine phosphorylation of insulin receptorβ inhibits insulin signaling while serine phosphorylation of P450c17, 17,20-lyase, stimulates DHEA production (12). In this case, serum DHEAS should have correlated with insulin resistance parameters.; our findings showed the opposite. Second, insulin sensitizers lower androgen levels (13–16). However, a closer inspection of the data indicates that this has not been a consistent finding: In one report, metformin treatment did not decrease, but in fact tended to increase morning cortisol levels (p=0.07) and did not affect DHEAS (17). In another, DHEAS and cortisol levels were not reported (16). In yet in another, only serum testosterone decreased, DHEAS did not change, and cortisol levels were not included (13). In addition, these studies showed that the effects of metformin may differ from those of the thiozalidinediones (TZD) (18). In general, TZD caused larger reductions in androgens (13, 15–17, 19, 20). An intriguing question is whether the differential effects of insulin sensitizers relate to their effects on weight; whether TZD lower androgens more than metformin because TZD promote weight gain whereas metformin promotes weight loss (21, 22). Unfortunately, not all studies using insulin sensitizers reported changes in weight.
Recent evidence indicates that the effect of obesity on steroid synthesis may be mediated by leptin. Leptin is an adipose tissue protein. Serum leptin levels correlate directly with fat-mass (23, 24). In humans, serum leptin correlated inversely with cortisol (25). In leptin deficient mice, leptin administration reduced corticosterone levels (26). In our study, serum leptin correlated directly with weight, BMI and fat mass, and inversely with DHEAS and DHEA. There was also a weak inverse correlation between leptin and cortisol (p = 0.0740). It appears that leptin inhibits steroid synthesis by down regulating the rate limiting step–the cholesterol side chain cleavage enzyme (P450scc) (27, 28). It is conceivable that changes in leptin may account for the differential effects of insulin sensitizers on androgens: TZD treatment, which suppresses androgen levels, increases leptin, whereas metformin, which does not affect androgen levels, has no effect on leptin (29, 30).
We investigated dynamic responses of adrenal steroids by measuring cortisol and DHEA during OGTT. We did not measure DHEAS because it has a long half life and does not change promptly during testing. We did not measure androstenodione either because we had demonstrated previously that androstenodione exhibits smaller increases than DHEA during OGTT (3). Similarly, Farah-Eways et al. reported that after ACTH stimulation serum DHEA increased by 222% while androstenodione increased by 31% in PCOS patients, and DHEA increased by 266% while androstenodione increased by 68% in healthy control women (31).
We observed that those individuals with increased cortisol and DHEA responses were more obese and hyperinsulinemic than non-responders. The responders had higher glucose and insulin levels during the first two hours of OGTT, but lower glucose levels afterwards. These findings are consistent with the report of Gambineri et al. (32) that showed that PCOS patients with hyperglycemia and hyperinsulinemia during OGTT exhibited exaggerated cortisol and androstenedione responses to ACTH stimulation. It appears that early hyperinsulinemia led to postprandial hypoglycemia and triggered the hypothalamic-pituitary-adrenal axis. It is possible that obesity augments the adrenal response through two different but complementary mechanisms: First obesity causes hyperinsulinemia and postprandial hypoglycemia, and thus triggers the hypothalamic-pituitary component of the axis. In addition, obesity increases steroid synthesis in the adrenals directly. Several studies that bypassed the hypothalamus and the pituitary and tested only the adrenals by injecting ACTH found increased steroid response in obesity (33–37). Although the underlying mechanism is not yet clear, it is known that rapid steroid response to provocative stimuli is mediated by the steroidogenic acute regulatory protein (StAR) which facilitates the movement of cholesterol from the outer to the inner mitochondrial membrane (38). Recent research indicates that adipose tissue produces signaling molecules that induce the transcription of the StAR promoter and this may account for the effects of obesity (39).
Finally, we wanted to relate the clinical symptomatology to adrenal steroid secretion. It has been recognized that a significant number of PCOS patients complain of symptoms suggestive of hypoglycemia after consuming sweets and simple sugars (3, 40). Our findings indicate that symptoms like shakiness, sweating, weakness and excessive hunger are associated with increased adrenal steroid response, and emphasize the importance of questioning patients specifically for these symptoms.
An important consideration is long-term consequences of repeated stimulation of the hypothalamic-pituitary-adrenal axis by nutrients. It is conceivable that ingestion of simple sugars can cause postprandial hypoglycemia and stimulate secretion of adrenal steroids, which in turn causes further weight gain and insulin resistance, thus creating a vicious cycle. Consistent with this concept we recently demonstrated that PCOS patients who consumed a simple-sugar supplement lost less weight than those who consumed a protein supplement (41). In addition, an earlier study demonstrated that PCOS patients have lower day-long glucose levels as compared to control women (42). Since hypoglycemia stimulates growth hormone secretion, this study monitored growth hormone and found that PCOS patients had higher growth hormone levels throughout the day as well. Recently, Mai et al. reported that infusion of lipid and heparin increased serum levels of free fatty acids and androgens in healthy control women (43). Taken altogether, these studies support that nutrients influence adrenal function. Hormone levels obtained in the morning may not reflect the day-long exposure. Clinical significance of the nutrient-regulated hormone secretion needs to be further investigated.
Acknowledgments
Sources of support: This study was supported by grants from the National Center for Complementary and Alternative Medicine (R21 AT002280) and the ALSAM Foundation to Dr. Kasim-Karakas. The clinical studies were partially supported by the UC Davis Clinical and Translational Science Center Grant (RR 024146).
Footnotes
Conflict of interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chang WY, Knochenhauer ES, Bartolucci AA, Azziz R. Phenotypic spectrum of polycystic ovary syndrome: clinical and biochemical characterization of the three major clinical subgroups. Fertil Steril. 2005;83:1717–23. doi: 10.1016/j.fertnstert.2005.01.096. [DOI] [PubMed] [Google Scholar]
- 2.Kumar A, Woods KS, Bartolucci AA, Azziz R. Prevalence of adrenal androgen excess in patients with the polycystic ovary syndrome (PCOS) Clin Endocrinol (Oxf) 2005;62:644–9. doi: 10.1111/j.1365-2265.2005.02256.x. [DOI] [PubMed] [Google Scholar]
- 3.Kasim-Karakas SE, Cunningham WM, Tsodikov A. Relation of nutrients and hormones in polycystic ovary syndrome. The American journal of clinical nutrition. 2007;85:688–94. doi: 10.1093/ajcn/85.3.688. [DOI] [PubMed] [Google Scholar]
- 4.Spyer G, Hattersley AT, MacDonald IA, Amiel S, MacLeod KM. Hypoglycaemic counter-regulation at normal blood glucose concentrations in patients with well controlled type-2 diabetes. Lancet. 2000;356:1970–4. doi: 10.1016/s0140-6736(00)03322-5. [DOI] [PubMed] [Google Scholar]
- 5.Solter M, Sekso M. Increased posthyperglycemic insulin secretion area in obese subjects with asymptomatic reactive hypoglycemia. Horm Metab Res. 1979;11:252–3. doi: 10.1055/s-0028-1095772. [DOI] [PubMed] [Google Scholar]
- 6.Solter M, Sekso M. Secretion of growth hormone and cortisol in obese subjects with asymptomatic reactive hypoglycemia. Endokrinologie. 1980;75:216–24. [PubMed] [Google Scholar]
- 7.Azziz R. Diagnostic criteria for polycystic ovary syndrome: a reappraisal. Fertility and sterility. 2005;83:1343–6. doi: 10.1016/j.fertnstert.2005.01.085. [DOI] [PubMed] [Google Scholar]
- 8.Pierson RN, Jr, Wang J, Thornton JC. Measurement of body composition: applications in hormone research. Hormone research. 1997;48(Suppl 1):56–62. doi: 10.1159/000191271. [DOI] [PubMed] [Google Scholar]
- 9.Broide J, Soferman R, Kivity S, Golander A, Dickstein G, Spirer Z, et al. Low-dose adrenocorticotropin test reveals impaired adrenal function in patients taking inhaled corticosteroids. The Journal of clinical endocrinology and metabolism. 1995;80:1243–6. doi: 10.1210/jcem.80.4.7714095. [DOI] [PubMed] [Google Scholar]
- 10.Havel PJ, Kasim-Karakas S, Mueller W, Johnson PR, Gingerich RL, Stern JS. Relationship of plasma leptin to plasma insulin and adiposity in normal weight and overweight women: effects of dietary fat content and sustained weight loss. The Journal of clinical endocrinology and metabolism. 1996;81:4406–13. doi: 10.1210/jcem.81.12.8954050. [DOI] [PubMed] [Google Scholar]
- 11.Moran C, Knochenhauer E, Boots LR, Azziz R. Adrenal androgen excess in hyperandrogenism: relation to age and body mass. Fertility and sterility. 1999;71:671–4. doi: 10.1016/s0015-0282(98)00536-6. [DOI] [PubMed] [Google Scholar]
- 12.Bremer AA, Miller WL. The serine phosphorylation hypothesis of polycystic ovary syndrome: a unifying mechanism for hyperandrogenemia and insulin resistance. Fertility and sterility. 2008;89:1039–48. doi: 10.1016/j.fertnstert.2008.02.091. [DOI] [PubMed] [Google Scholar]
- 13.Arslanian SA, Lewy V, Danadian K, Saad R. Metformin therapy in obese adolescents with polycystic ovary syndrome and impaired glucose tolerance: amelioration of exaggerated adrenal response to adrenocorticotropin with reduction of insulinemia/insulin resistance. The Journal of clinical endocrinology and metabolism. 2002;87:1555–9. doi: 10.1210/jcem.87.4.8398. [DOI] [PubMed] [Google Scholar]
- 14.Azziz R, Ehrmann DA, Legro RS, Fereshetian AG, O’Keefe M, Ghazzi MN. Troglitazone decreases adrenal androgen levels in women with polycystic ovary syndrome. Fertility and sterility. 2003;79:932–7. doi: 10.1016/s0015-0282(02)04914-2. [DOI] [PubMed] [Google Scholar]
- 15.Guido M, Romualdi D, Suriano R, Giuliani M, Costantini B, Apa R, et al. Effect of pioglitazone treatment on the adrenal androgen response to corticotrophin in obese patients with polycystic ovary syndrome. Human reproduction (Oxford, England) 2004;19:534–9. doi: 10.1093/humrep/deh145. [DOI] [PubMed] [Google Scholar]
- 16.la Marca A, Egbe TO, Morgante G, Paglia T, Cianci A, De Leo V. Metformin treatment reduces ovarian cytochrome P-450c17alpha response to human chorionic gonadotrophin in women with insulin resistance-related polycystic ovary syndrome. Human reproduction (Oxford, England) 2000;15:21–3. doi: 10.1093/humrep/15.1.21. [DOI] [PubMed] [Google Scholar]
- 17.Vrbikova J, Hill M, Starka L, Cibula D, Bendlova B, Vondra K, et al. The effects of long-term metformin treatment on adrenal and ovarian steroidogenesis in women with polycystic ovary syndrome. European journal of endocrinology/European Federation of Endocrine Societies. 2001;144:619–28. doi: 10.1530/eje.0.1440619. [DOI] [PubMed] [Google Scholar]
- 18.Yilmaz M, Biri A, Karakoc A, Toruner F, Bingol B, Cakir N, et al. The effects of rosiglitazone and metformin on insulin resistance and serum androgen levels in obese and lean patients with polycystic ovary syndrome. Journal of endocrinological investigation. 2005;28:1003–8. doi: 10.1007/BF03345339. [DOI] [PubMed] [Google Scholar]
- 19.Arlt W, Auchus RJ, Miller WL. Thiazolidinediones but not metformin directly inhibit the steroidogenic enzymes P450c17 and 3beta -hydroxysteroid dehydrogenase. The Journal of biological chemistry. 2001;276:16767–71. doi: 10.1074/jbc.M100040200. [DOI] [PubMed] [Google Scholar]
- 20.Azziz R, Ehrmann D, Legro RS, Whitcomb RW, Hanley R, Fereshetian AG, et al. Troglitazone improves ovulation and hirsutism in the polycystic ovary syndrome: a multicenter, double blind, placebo-controlled trial. The Journal of clinical endocrinology and metabolism. 2001;86:1626–32. doi: 10.1210/jcem.86.4.7375. [DOI] [PubMed] [Google Scholar]
- 21.Glueck CJ, Aregawi D, Agloria M, Winiarska M, Sieve L, Wang P. Sustainability of 8% weight loss, reduction of insulin resistance, and amelioration of atherogenic-metabolic risk factors over 4 years by metformin-diet in women with polycystic ovary syndrome. Metabolism: clinical and experimental. 2006;55:1582–9. doi: 10.1016/j.metabol.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Larsen TM, Toubro S, Astrup A. PPARgamma agonists in the treatment of type II diabetes: is increased fatness commensurate with long-term efficacy? Int J Obes Relat Metab Disord. 2003;27:147–61. doi: 10.1038/sj.ijo.802223. [DOI] [PubMed] [Google Scholar]
- 23.Havel PJ, Kasim-Karakas S, Dubuc GR, Mueller W, Phinney SD. Gender differences in plasma leptin concentrations. Nature medicine. 1996;2:949–50. doi: 10.1038/nm0996-949b. [DOI] [PubMed] [Google Scholar]
- 24.Ruhl CE, Harris TB, Ding J, Goodpaster BH, Kanaya AM, Kritchevsky SB, et al. Body mass index and serum leptin concentration independently estimate percentage body fat in older adults. The American journal of clinical nutrition. 2007;85:1121–6. doi: 10.1093/ajcn/85.4.1121. [DOI] [PubMed] [Google Scholar]
- 25.Korbonits M, Trainer PJ, Little JA, Edwards R, Kopelman PG, Besser GM, et al. Leptin levels do not change acutely with food administration in normal or obese subjects, but are negatively correlated with pituitary-adrenal activity. Clinical endocrinology. 1997;46:751–7. doi: 10.1046/j.1365-2265.1997.1820979.x. [DOI] [PubMed] [Google Scholar]
- 26.Stephens TW, Basinski M, Bristow PK, Bue-Valleskey JM, Burgett SG, Craft L, et al. The role of neuropeptide Y in the antiobesity action of the obese gene product. Nature. 1995;377:530–2. doi: 10.1038/377530a0. [DOI] [PubMed] [Google Scholar]
- 27.Herrid M, Xia Y, O’Shea T, McFarlane JR. Leptin inhibits basal but not gonadotrophin-stimulated testosterone production in the immature mouse and sheep testis. Reproduction, fertility, and development. 2008;20:519–28. doi: 10.1071/rd07062. [DOI] [PubMed] [Google Scholar]
- 28.Hsu HT, Chang YC, Chiu YN, Liu CL, Chang KJ, Guo IC. Leptin interferes with adrenocorticotropin/3′,5′-cyclic adenosine monophosphate (cAMP) signaling, possibly through a Janus kinase 2-phosphatidylinositol 3-kinase/Akt-phosphodiesterase 3-cAMP pathway, to down-regulate cholesterol side-chain cleavage cytochrome P450 enzyme in human adrenocortical NCI-H295 cell line. The Journal of clinical endocrinology and metabolism. 2006;91:2761–9. doi: 10.1210/jc.2005-2383. [DOI] [PubMed] [Google Scholar]
- 29.Kim HJ, Kim SK, Shim WS, Lee JH, Hur KY, Kang ES, et al. Rosiglitazone improves insulin sensitivity with increased serum leptin levels in patients with type 2 diabetes mellitus. Diabetes research and clinical practice. 2008;81:42–9. doi: 10.1016/j.diabres.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Romualdi D, Campagna G, Selvaggi L, Jr, Cento R, Proto C, Lanzone A, et al. Metformin treatment does not affect total leptin levels and free leptin index in obese patients with polycystic ovary syndrome. Fertility and sterility. 2008;89:1273–6. doi: 10.1016/j.fertnstert.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Farah-Eways L, Reyna R, Knochenhauer ES, Bartolucci AA, Azziz R. Glucose action and adrenocortical biosynthesis in women with polycystic ovary syndrome. Fertil Steril. 2004;81:120–5. doi: 10.1016/j.fertnstert.2003.05.008. [DOI] [PubMed] [Google Scholar]
- 32.Gambineri A, Pelusi C, Manicardi E, Vicennati V, Cacciari M, Morselli-Labate AM, et al. Glucose intolerance in a large cohort of mediterranean women with polycystic ovary syndrome: phenotype and associated factors. Diabetes. 2004;53:2353–8. doi: 10.2337/diabetes.53.9.2353. [DOI] [PubMed] [Google Scholar]
- 33.Azziz R, Zacur HA, Parker CR, Jr, Bradley EL, Jr, Boots LR. Effect of obesity on the response to acute adrenocorticotropin stimulation in eumenorrheic women. Fertility and sterility. 1991;56:427–33. [PubMed] [Google Scholar]
- 34.Brody S, Carlstrom K, Lagrelius A, Lunell NO, Mollerstrom G. Adrenal steroids in post-menopausal women: relation to obesity and to bone mineral content. Maturitas. 1987;9:25–32. doi: 10.1016/0378-5122(87)90048-x. [DOI] [PubMed] [Google Scholar]
- 35.Komindr S, Kurtz BR, Stevens MD, Karas JG, Bittle JB, Givens JR. Relative sensitivity and responsivity of serum cortisol and two adrenal androgens to alpha-adrenocorticotropin-(1–24) in normal and obese, nonhirsute, eumenorrheic women. The Journal of clinical endocrinology and metabolism. 1986;63:860–4. doi: 10.1210/jcem-63-4-860. [DOI] [PubMed] [Google Scholar]
- 36.Rittmaster RS, Deshwal N, Lehman L. The role of adrenal hyperandrogenism, insulin resistance, and obesity in the pathogenesis of polycystic ovarian syndrome. The Journal of clinical endocrinology and metabolism. 1993;76:1295–300. doi: 10.1210/jcem.76.5.8388405. [DOI] [PubMed] [Google Scholar]
- 37.Vicennati V, Calzoni F, Gambineri A, Gagliardi L, Morselli Labate AM, Casimirri F, et al. Secretion of major adrenal androgens following ACTH administration in obese women with different body fat distribution. Hormone and metabolic research Hormon- und Stoffwechselforschung. 1998;30:133–6. doi: 10.1055/s-2007-978851. [DOI] [PubMed] [Google Scholar]
- 38.Miller WL. StAR search--what we know about how the steroidogenic acute regulatory protein mediates mitochondrial cholesterol import. Molecular endocrinology (Baltimore, Md. 2007;21:589–601. doi: 10.1210/me.2006-0303. [DOI] [PubMed] [Google Scholar]
- 39.Schinner S, Willenberg HS, Krause D, Schott M, Lamounier-Zepter V, Krug AW, et al. Adipocyte-derived products induce the transcription of the StAR promoter and stimulate aldosterone and cortisol secretion from adrenocortical cells through the Wnt-signaling pathway. International journal of obesity (2005) 2007;31:864–70. doi: 10.1038/sj.ijo.0803508. [DOI] [PubMed] [Google Scholar]
- 40.Farshchi H, Rane A, Love A, Kennedy RL. Diet and nutrition in polycystic ovary syndrome (PCOS): pointers for nutritional management. J Obstet Gynaecol. 2007;27:762–73. doi: 10.1080/01443610701667338. [DOI] [PubMed] [Google Scholar]
- 41.Kasim-Karakas SE, Almario RU, Cunningham W. Effects of protein versus simple sugar intake on weight loss in polycystic ovary syndrome (according to the National Institutes of Health criteria) Fertility and sterility. 2008 doi: 10.1016/j.fertnstert.2008.05.065. [DOI] [PubMed] [Google Scholar]
- 42.Prelevic GM, Wurzburger MI, Balint-Peric L, Ginsburg J. Twenty-four-hour serum growth hormone, insulin, C-peptide and blood glucose profiles and serum insulin-like growth factor-I concentrations in women with polycystic ovaries. Hormone research. 1992;37:125–31. doi: 10.1159/000182296. [DOI] [PubMed] [Google Scholar]
- 43.Mai K, Bobbert T, Reinecke F, Andres J, Maser-Gluth C, Wudy SA, et al. Intravenous lipid and heparin infusion-induced elevation in free fatty acids and triglycerides modifies circulating androgen levels in women: a randomized, controlled trial. The Journal of clinical endocrinology and metabolism. 2008 doi: 10.1210/jc.2008-0714. [DOI] [PubMed] [Google Scholar]