Abstract
The role of estrogen receptor α (ER-α) in melanoma is unknown. ER-α expression may be regulated in melanoma via hypermethylation of promoter CpG islands. We assessed ER-α hypermethylation in primary and metastatic melanomas and sera as a potential tumor progression marker. ER-α methylation status in tumor (n = 107) and sera (n = 109) from American Joint Committee on Cancer (AJCC) stage I to IV melanoma patients was examined by methylation-specific PCR. The clinical significance of serum methylated ER-α was assessed among AJCC stage IV melanoma patients receiving biochemotherapy with tamoxifen. Rates of ER-α methylation in AJCC stage I, II, and III primary melanomas were 36% (4 of 11), 26% (5 of 19), and 35% (8 of 23), respectively. Methylated ER-α was detected in 42% (8 of 19) of stage III and 86% (30 of 35) of stage IV metastatic melanomas. ER-α was methylated more frequently in metastatic than primary melanomas (P = 0.0003). Of 109 melanoma patients’ sera in AJCC stage I, II, III, and IV, methylated ER-α was detected in 10% (2 of 20), 15% (3 of 20), 26% (5 of 19), and 32% (16 of 50), respectively. Serum methylated ER-α was detected more frequently in advanced than localized melanomas (P = 0.03) and was the only factor predicting progression-free [risk ratio (RR), 2.64; 95% confidence interval (95% CI), 1.36–5.13; P = 0.004] and overall survival (RR, 2.31; 95% CI, 1.41–5.58; P = 0.003) in biochemotherapy patients. Hypermethylated ER-α is a significant factor in melanoma progression. Serum methylated ER-α is an unfavorable prognostic factor.
Introduction
Because it is difficult to predict which primary tumors will progress to regional or distant metastases, cutaneous melanoma remains a challenging disease to manage (1). New strategies for the identification of epigenetic biomarkers may improve the clinical management of melanoma by facilitating earlier disease diagnosis and providing more accurate prognostic information. No major study has examined the epigenetic alterations of hormone receptors in the progression from primary to metastatic melanoma in a large series of patients.
Hypermethylation of gene promoter CpG islands plays a significant role in the development and progression of various cancers, including melanoma (2–6). The identification of hypermethylated genes in tumors has become an accepted approach to assess tumor-related gene inactivation (6–9). We previously reported tumor-related gene hypermethylation in primary and metastatic melanomas (10). Thereafter, we showed the hypermethylation of multiple tumor-related and tumor suppressor genes during progression from primary to metastatic lesions (11). Several genes methylated in primary and metastatic melanomas were also detected in serum as methylated circulating DNA (11). The observation that tumor-related DNA could be detected in circulating serum provided a method of disease surveillance independent of the availability of gross tumor tissue (12–17).
Estrogen receptor α (ER-α) belongs to a superfamily of transcription activators (18, 19) involved in many physiologic processes, including tumor progression (20–22). Loss of ER-α expression has been associated with aberrant CpG island hypermethylation in breast cancer cell lines and tumors (23–27) and shown to modulate breast cancer progression (5). Several studies have reported the presence of ER in melanoma cell lines but analysis of human melanomas has shown variable ER-α expression (28–31). Several in vitro experiments established that tamoxifen is an effective growth inhibitor of melanoma cells (32, 33). Based on the variable presence of ER-α in melanoma cells, as well as anecdotal reports of clinical responses to antiestrogen therapy, several studies of hormonal and chemohormonal treatments were coordinated. Initial trials were encouraging, with improved response rates and median overall survival in patients receiving tamoxifen, particularly women (34, 35). Subsequent trials, however, failed to show significant differences in response rates or overall survival when tamoxifen was used alone or in combination with systemic therapies (36–42). Reasons for the discrepancies in response to antiestrogen therapy between these trials are unknown.
Mechanisms regulating the expression of ER-α in melanoma are poorly defined; to date, no mutation or other gross structural alteration of the ER-α gene has been reported in melanoma. We hypothesized that ER-α gene silencing via gene promoter hypermethylation in primary and metastatic melanoma plays an important role in melanoma progression and may be used as a prognostic molecular biomarker.
Materials and Methods
Melanoma cell line and tumor DNA isolation
DNA was extracted from 11 melanoma cell lines established from metastatic tumors at John Wayne Cancer Institute and one breast cancer cell line (MCF-7) from American Type Culture Collection (Manassas, VA) as previously described (14). Institutional Review Board approval for the use of human tissues was obtained from Saint John’s Health Center and John Wayne Cancer Institute before beginning the study. Patients who underwent surgery for American Joint Committee on Cancer (AJCC) stages I, II, III, and IV melanoma (11 stage I primary tumors; 19 stage II primary tumors; 23 stage III primary tumors; 19 stage III metastatic tumors; and 35 stage IV metastatic tumors) were selected consecutively by the database coordinator from our institutional melanoma patient and specimen database (Table 1). Paraffin-embedded tumor specimens from these patients were obtained from the Division of Surgical Pathology at Saint John’s Health Center.
Table 1.
Clinical characteristics of melanoma patients
| Patient characteristics | n (%) |
|---|---|
| Total patients (tissue) | 107 |
| Sex | |
| Male | 58 (54%) |
| Female | 49 (46%) |
| Age (median), y | |
| <50 | 25 (23%) |
| ≥60 | 50 (47%) |
| Stage | |
| I | 11 (10%) |
| II | 19 (18%) |
| III (primary) | 23 (21%) |
| III (metastasis) | 19 (18%) |
| IV (metastasis) | 35 (33%) |
| Total patients (serum) | 109 |
| Sex | |
| Male | 73 (67%) |
| Female | 34 (31%) |
| Unknown | 2 (2%) |
| Age (median 45), y | |
| <50 | 43 (41%) |
| ≥60 | 51 (48%) |
| Stage | |
| I | 20 (19%) |
| II | 20 (19%) |
| III | 19 (18%) |
| IV | 50 (48%) |
Several 8-µm sections were cut from formalin-fixed, paraffin-embedded blocks as previously described (43). One section from each tumor block was deparaffinized, mounted on a glass slide, and stained with H&E for microscopic analysis. Light microscopy was used to confirm tumor location and assess tissue homogeneity. Additional sections from the tumor block were mounted on glass slides and microdissected under light microscopy. Dissected tissues were digested with 50 µL of proteinase K–containing lysis buffer at 50°C for 12 hours, followed by heat deactivation of proteinase K at 95°C for 10 minutes (5). DNA was extracted as previously described (10).
Serum DNA isolation
AJCC stage I (n = 20), stage II (n = 20), stage III (n = 19), and stage IV patients (n = 50) diagnosed with melanoma were assessed for this study (Table 1). Stage I, II, and III patients received no additional adjuvant therapy but stage IV patients received a systemic concurrent biochemotherapy regimen of dacarbazine (DTIC) or temazolamide, cisplatin, vinblastine, IFN-α2b, interleukin-2, and tamoxifen in the setting of one of several phase II trials, as previously reported (40–42).
AJCC stage IV patients (Table 2) were selected and coded by the clinical study coordinator and assessed in laboratory and statistical analyses in a blinded fashion. The selection of stage IV patients was based on patient response or nonresponse to biochemotherapy, availability of clinical follow-up data, completion of the biochemotherapy trial, and specimen availability. Patients were categorized as responders or nonresponders to biochemotherapy based on clinical response criteria (42). Those showing a complete response (n = 13) or partial response (n = 10) were included in the responder group (n = 23) whereas patients showing progressive disease (n = 24) were deemed nonresponders. Patients exhibiting stable disease (n = 3) were considered neither responders nor nonresponders. One patient in the responder group was lost to follow-up and excluded from the survival analysis. Serum drawn from healthy donors (n = 40) served as normal controls.
Table 2.
Clinical demographics of stage IV melanoma patients receiving biochemotherapy
| Patient characteristics (serum donors) | n (%) |
|---|---|
| Total patients | 50 |
| Sex | |
| Male | 38 (76%) |
| Female | 12 (24%) |
| Age (median 45), y | |
| <50 | 34 (68%) |
| ≥60 | 16 (32%) |
| ECOG | |
| 0 | 14 (28%) |
| 1 | 12 (24%) |
| 2 | 24 (48%) |
| Biochemotherapy response Responder |
|
| CR | 13 (26%) |
| PR | 10 (20%) |
| Nonresponder | |
| PD | 24 (48%) |
Abbreviations: CR, complete response; PR, partial response; PD, progressive disease.
Stage IV patients’ blood was drawn for serum before administration of biochemotherapy. Ten milliliters of blood were collected in serum separator tubes, centrifuged, run through a 13-mm serum filter (Fisher Scientific, Pittsburgh, PA), aliquoted, and cryopreserved at −30°C. DNA was extracted and processed from serum as previously described (6). DNA quantification was done on all serum specimens using the PicoGreen quantification assay (Molecular Probes, Eugene, OR; ref. 44).
Cell line and tissue DNA sodium bisulfite modification
Extracted DNA from cell lines and paraffin-embedded melanoma tumors was subjected to sodium bisulfite modification (11). Briefly, 2 µg DNA was denatured in 0.3 mol/L NaOH for 3 minutes at 95°C and then 550 µL of a 2.5 mol/L sodium bisulfite/125 mmol/L hydroquinone solution were added. Samples were incubated under mineral oil in the dark for 3 hours at 60°C. Salts were removed using the Wizard DNA Clean-Up System (Promega, Madison, WI) and desulfonated in 0.3 mol/L NaOH at 37°C for 15 minutes. Modified DNA was precipitated with ethanol using Pellet Paint NF (Novagen, Madison, WI) as a carrier and resuspended in molecular grade H2O. DNA samples were cryopreserved at −30°C until methylation-specific PCR was done.
Serum DNA sodium bisulfite modification
Extracted DNA from serum was subjected to sodium bisulfite modification (44). Briefly, DNA from 500 µL of serum was supplemented with 1 µg salmon sperm DNA (Sigma Chemical Co., St. Louis, MO) and denatured in 0.3 mol/L NaOH for 3 minutes at 95°C. Overall, 550 µL of a 2.5 mol/L sodium bisulfite/125 mmol/L hydroquinone solution were added. Samples were incubated under mineral oil in the dark for 3 hours at 60°C. Salts were removed using the Wizard DNA Clean-Up System (Promega) and desulfonated in 0.3 mol/L NaOH at 37°C for 15 minutes. Modified serum DNA was prepared and stored identically to tissue samples.
Detection of methylated ER-α
ER-α methylation status was assessed using two sets of fluorescent labeled primers specifically designed to amplify methylated or unmethylated DNA sequences of the ER-α promoter region. Primer sequences are provided as methylated sense and antisense followed by unmethylated sense and antisense sequences, with annealing temperatures and PCR product size: ER-α methylated-specific forward, 5′-TAAATAGAGATATATCGGAGTTTGGTACG-3′, and reverse, 5′-AACTTAAAATAAACGCGAAAAACGA-3′ (61°C, 96 bp); unmethylated-specific forward, 5′-TAAATAGAGATATATTGGAGTTTGGTATGG-3′, and reverse, 5′-AACTTAAAATAAACACAAAAAACAAA-3′ (58°C, 96 bp). Bisulfite-modified DNA was subjected to PCR amplification in a final reaction volume of 20 µL containing PCR buffer, 2.5 mmol/L MgCl2, deoxynucleotide triphosphates (dNTP), 0.3 µmol/L primers, and 0.5 units of AmpliTaq Gold DNA polymerase (Applied Biosystems, Foster City, CA). PCR was done with an initial incubation at 95°C for 10 minutes, followed by 40 cycles of denaturation at 95°C for 30 seconds, annealing for 30 seconds, extension at 72°C for 30 seconds, and final hold at 72°C for 7 minutes. DNA from the ER-α-positive breast cancer cell line MCF-7 was used as a control to verify the presence of ER-α; DNA from the ER-α-negative melanoma cell line MCA was used as a control to verify the absence of ER-α. A universal unmethylated control was synthesized from normal DNA by φ-29 DNA polymerase and served as a positive unmethylated control (45). Unmodified lymphocyte DNA was used as a negative control for methylated and unmethylated reactions. Lymphocyte DNA treated with SssI methylase (New England BioLabs, Beverly, MA) was used as a positive methylated control. PCR products were visualized using capillary array electrophoresis (CEQ 8000 XL, Beckman Coulter, Inc., Fullerton, CA) in a 96-well microplate format (6). Methylated and unmethylated PCR products from each sample were assessed simultaneously using forward primers labeled with Beckman Coulter WellRED dye-labeled phosphoramidites (Genset oligos, Boulder, CO). Forward methylated-specific primers were labeled with D4pa dye and forward unmethylated-specific primers were labeled with D2a dye. One microliter of methylated PCR product and 1 µL of unmethyated PCR product were mixed with 40-µL loading buffer and a 0.5-µL dye-labeled size standard (Beckman Coulter). Each marker was optimized with methylated and unmethylated controls. Samples showing a peak at the base pair size marker for unmethylated DNA were considered unmethylated whereas those showing a peak at the base pair size marker for methylated DNA were considered methylated.
Melanoma cell line 5-aza-2-deoxycytidine and trichostatin a treatment
To confirm down-regulation of ER-α expression by hypermethylation of the ER-α promoter region, we treated cell lines with the DNA-demethylating agent 5-aza-2-deoxycytidine (5-aza-CdR) and the histone deacetylase inhibitor trichostatin A (TSA). In combination with 5-aza-CdR treatment, TSA can up-regulate the mRNA expression of genes silenced due to hypermethylation (26, 27). The MCF-7 cell line was used as a ER-α-positive control and the MCA cell line was used as a ER-α-negative control. Cell lines were maintained in RPMI 1640 supplemented with heat-inactivated 10% fetal bovine serum, penicillin G, and streptomycin (100 units/mL). Cells were treated with 1,000 nmol/L TSA for 24 hours (Wako Biochemicals, Osaka, Japan) and 1,000 nmol/L 5-aza-CdR for 5 days (Sigma). After treatment with 5-aza-CdR and TSA, melanoma cells were washed with PBS and harvested with 0.25% trypsin-0.53 mmol/L EDTA (Life Technologies, Inc., Auckland, NJ). The mRNA expression level of ER-α was assessed by reverse transcriptase PCR (RT-PCR) before and after 5-aza-CdR and TSA treatment.
mRNA analysis
Total cellular RNA from melanoma cell lines was extracted using Tri-Reagent (Molecular Research Center, Inc., Cincinnati, OH) as previously described (6). The RNA was quantified and assessed for purity using UV spectrophotometry and the RIBOGreen detection assay (Molecular Probes). The expression of mRNA for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), an internal reference housekeeping gene, was assessed by RT-PCR on all RNA samples to verify the integrity of RNA and to indicate equal loading of PCR products for gel electrophoresis.
All reverse transcription reactions were done using Moloney murine leukemia virus reverse-transcriptase (Promega) with oligo-dT (GeneLink, Hawthorne, NY) priming as previously described (6). cDNA from 250 ng of total RNA was used for each reaction (46). The RT-PCR reaction mixture consisted of 1 µmol/L of each primer, 1 units of AmpliTaq Gold polymerase (Applied Biosystems), 200 µmol/L of each dNTP, 4.5 mmol/L MgCl2, and AmpliTaq buffer to a final volume of 25 µL. The primer sequences used were as follows: ER-α, 5′-AGACATGAGAGCTGCCAACC-3′ (forward) and 5′-GCCAGGCACATTCTAGAAGG-3′ (reverse); GAPDH, 5′-GGGTGTGAACCATGAGAAGT-3′ (forward) and 5′-GACTGTGGTCATGAGTCCT-3′ (reverse). Samples were amplified with 30 cycles of denaturation at 95°C for 30 seconds, annealing at 58°C for 30 seconds, and extension at 72°C for 30 seconds for ER-α and GAPDH, respectively.
ER-α-positive (MCF-7 cell line) and ER-α-negative (MCA melanoma cell line) controls and reagent controls for RT-PCR assays were included as previously described (46). All PCR products were separated on 1.5% Tris-borate EDTA (TBE) agarose gels for ER-α and 2% TBE agarose gels for GAPDH and stained with SYBR Gold (Invitrogen Detection Techonologies, Eugene, OR). Each assay was repeated in triplicate.
Biostatistical analysis
The correlation between ER-α methylation status of primary and metastatic melanomas with AJCC stage was assessed using the χ2 method. Similarly, the correlation between ER-α methylation status of circulating serum DNA with known clinical prognostic factors and biochemotherapy response was assessed by the χ2 method. Additionally, a multivariate logistic regression model was developed to correlate clinical prognostic factors and serum circulating ER-α methylation status with response to biochemotherapy.
Survival length was determined from the first day of biochemotherapy treatment to death or the date of last clinical follow-up. Survival curves were derived using the Kaplan-Meier method and the differences between curves were analyzed using the log-rank test. Cox’s proportional hazards regression model was used for multivariate analyses. Age, gender, Eastern Cooperative Oncology Group (ECOG) status, lactate dehydrogenase (LDH) level, number of metastasis sites, and ER-α methylation status were included in the multivariate model using a stepwise method for variable selection.
Results
Detection of methylated ER-α DNA in cell lines
Initially, we assessed ER-α in established metastatic melanoma cell lines. The frequency of hypermethylated ER-α in metastatic melanoma cell lines was 91% (10 of 11). Among these lines, six had only a methylated-specific peak whereas four cell lines showed both methylated- and unmethylated-specific peaks. These experiments optimized our methylation-specific PCR assay for ER-α and showed the high frequency of hypermethylated ER-α in metastatic melanoma cells cultured in vitro.
ER-α reexpression with 5-aza-CdR and TSA treatment
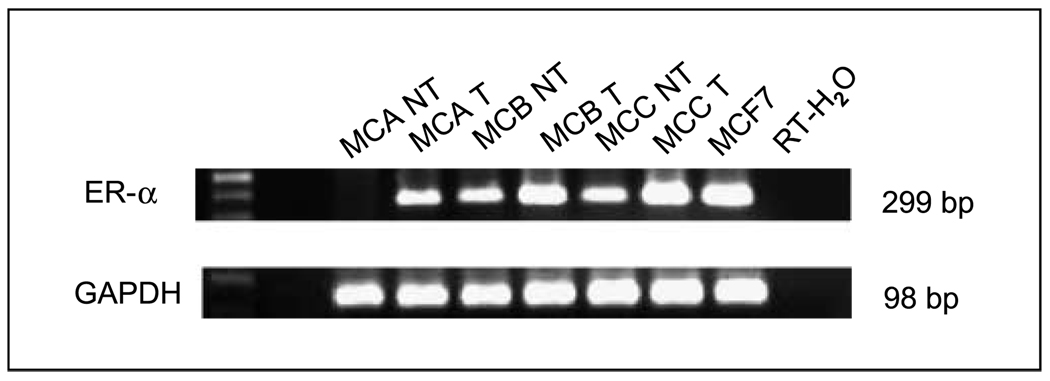
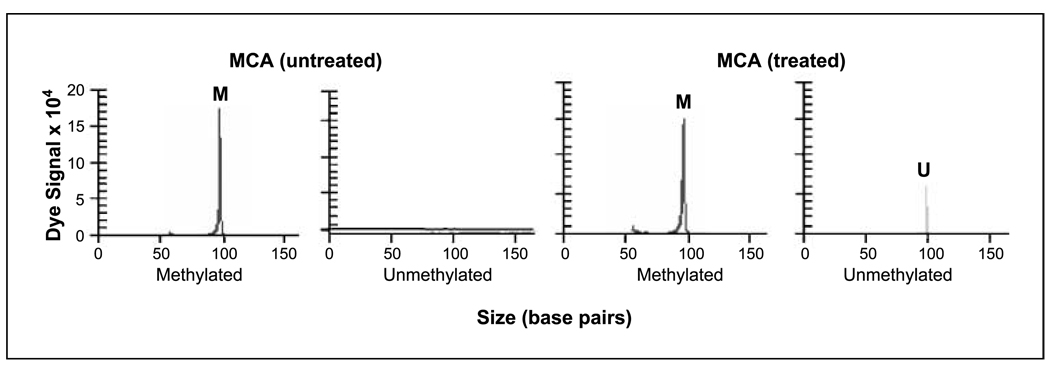
To determine if cells with hypermethylated ER-α can be induced to reexpress ER-α mRNA, we treated cell lines with 5-aza-CdR and TSA. In untreated cell lines, ER-α mRNA was detected in MCB and MCC, but not in MCA (Fig. 1). ER-α mRNA expression was restored to a detectable level in MCA after 5-aza-CdR and TSA treatment (Fig. 1). After treatment with 5-aza-CdR for 5 days followed by treatment with TSA for 24 hours, the MCA showed an unmethylated-specific DNA peak when assessed by methylation-specific PCR (Fig. 2). To further verify hypermethylation of the ER-α gene promoter region in melanoma, purified PCR products after sodium bisulfite modification were directly sequenced using a CEQ DYE Terminator Cycle Sequencing Kit (Beckman Coulter). Promoter region CpG islands were fully methylated in the MCA cell line, which does not express ER-α, whereas MCC, a cell line that expresses ER-α, showed no evidence of promoter region CpG island hypermethylation. With an optimized assay for the detection of methylated ER-α and the demonstration that reversal of methylation leads to reexpression of ER-α mRNA, we then approached the detection of methylated ER-α in paraffin-embedded melanoma specimens.
Figure 1.
Representative expression and reexpression of ER-α in three melanoma lines (MCA, MCB, and MCC) treated with 5-aza-CdR and TSA. mRNA expression was analyzed by RT-PCR. The housekeeping gene GAPDH was included as a RT-PCR control. NT, cell line not treated with 5-aza-CdR and TSA; T, cell line treated with 5-aza-CdR and TSA.
Figure 2.
Representative methylation-specific PCR results of melanoma cell line (MCA) with and without 5-aza-CdR plus TSA treatment. M, methylated-specific product; U, unmethylated-specific product. Only a methylated peak was initially observed (untreated). An unmethylated peak appeared after treatment with 5-aza-CdR plus TSA (treated).
Detection of methylated ER-α in melanomas
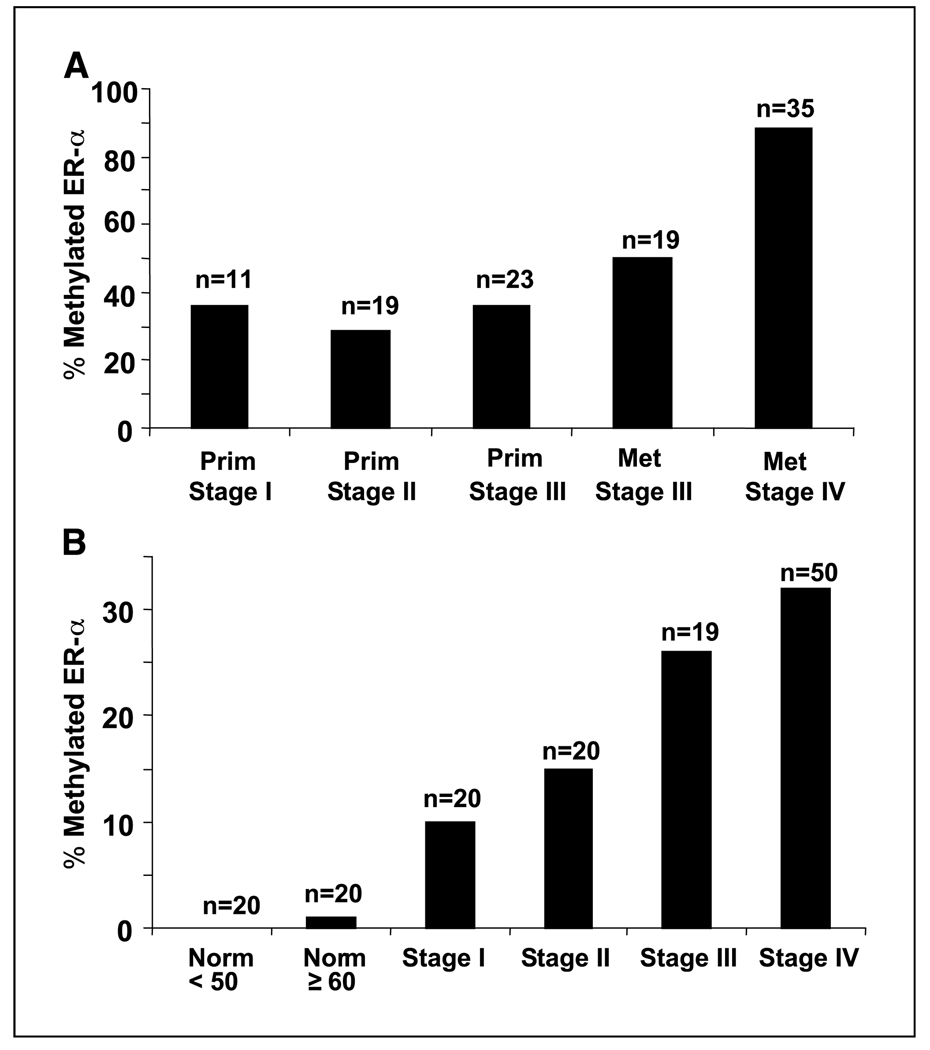
We evaluated 53 paraffin-embedded primary melanomas (stage I, n = 11; stage II, n = 19; stage III, n = 23) using methylation-specific PCR. Overall, the frequency of methylated ER-α in primary melanomas was 32% (17 of 53). Similar rates of methylated ER-α were detected in primary tumors among the patients assessed regardless of stage. The frequency of ER-α methylation in AJCC stage I, II, and III primary melanoma tumors was 36% (4 of 11), 26% (5 of 19), and 35% (8 of 23), respectively (Fig. 3A).
Figure 3.
A, frequency of methylated ER-α DNA in melanoma tumors according to AJCC stage. Prim, primary melanoma tumor; Met, metastatic melanoma tumor. B, frequency of methylated ER-α DNA in melanoma patients’ sera according to AJCC stage. Norm <50, normal healthy volunteers younger than 50 years. Norm ≥60, normal healthy volunteers ages 60 years or older.
Additionally, 54 paraffin-embedded metastatic melanomas were assessed, including stage III lymph node metastases (n = 19) and stage IV distant metastases (n = 35; 14 s.c., 9 lymph nodes, 6 lung, 5 colorectal, and 1 liver). Methylated ER-α was detected in 42% (8 of 19) of stage III and 86% (30 of 35) of IV metastatic melanomas (Fig. 3A). The frequency of methylated ER-α detected in stage IV metastatic tumors was significantly higher than in stage III metastatic tumors (P = 0.0003). Overall, ER-α was methylated in 70% (38 of 54) of metastatic tumors, a >2-fold increase in frequency compared with primary melanomas.
ER-α methylation status was also determined for 10 paraffin-embedded normal tissues from various organ sites (pancreas, n = 2; liver, n = 2; thymus, n = 2; lung, n = 2; and skin, n = 2). Methylated ER-α was detected in 90% (9 of 10) of normal tissues, indicating that ER-α is usually methylated and silenced in normal tissue.
Because we frequently detected methylated ER-α in paraffin-embedded primary and metastatic melanomas, we assessed the detection of methylated ER-α in the serum of AJCC stage Ito IV melanoma patients to evaluate its role as a blood marker for disease detection.
Detection of circulating methylated ER-α DNA in serum
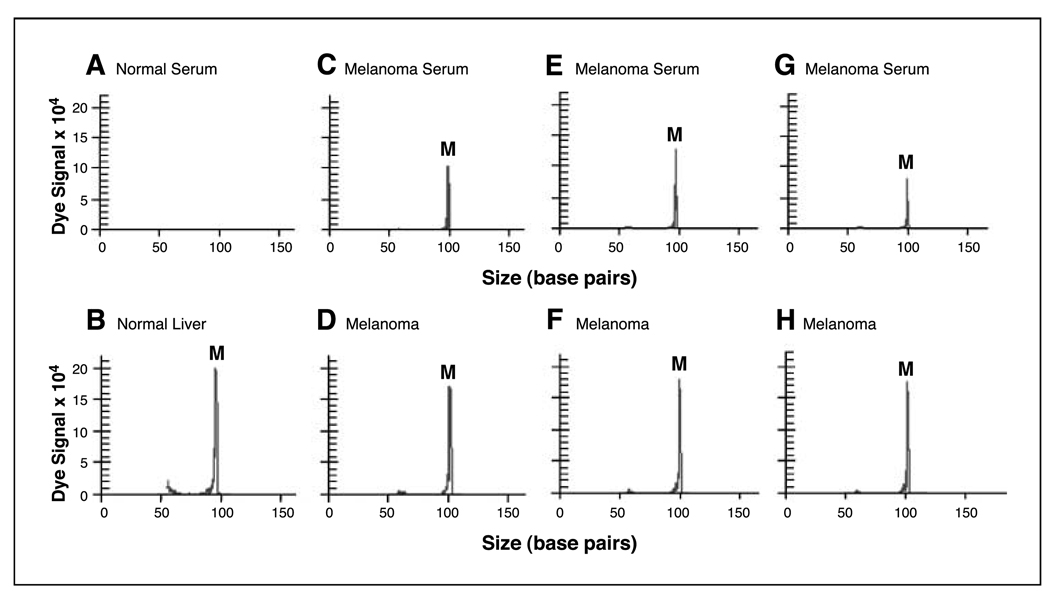
Previously, we showed that circulating methylated DNA markers can be valuable surrogates of tumor progression (11, 44). Hence, we developed an optimized assay to detect the presence of free circulating methylated ER-α DNA in serum. The frequency with which methylated ER-α was detected in serum increased with tumor progression and according to AJCC stage. In the analysis of 109 melanoma patients’ sera, the frequency of circulating methylated ER-α in AJCC stage I, II, III, and IV sera was 10% (2 of 20), 15% (3 of 20), 26% (5 of 19), and 32% (16 of 50), respectively (Fig. 3B). The frequency of serum methylated ER-α was increased in patients with more advanced disease; methylated ER-α was detected in stage III/IV more frequently than in stage I/II (P = 0.034). Methylated ER-α was detected in the sera of only 1 of 40 healthy normal donors, an 82-year-old female. Representative methylation peaks from normal donor sera, normal liver tissue, melanoma patient sera, and melanoma tumors are provided in Fig. 4. Healthy normal donors ranged in age from 20 to 84 years (mean, 56 years); the gender distribution of normal volunteers was comparable to that of melanoma patients assessed. Having established that methylated ER-α can be reliably detected in the sera of melanoma patients but not in normal volunteers and is a marker of disease progression, we focused our attention on assessing the clinical utility of methylated ER-α as a predictor of disease outcome.
Figure 4.
Representative methylation-specific PCR results of sera and tissue specimens. No methylation peak appeared in serum of healthy donor (A). A methylation peak appeared in normal liver tissue (B). A single methylation peak was detected in sera and paraffin-embedded specimens from stage IV melanoma patients (C–H). E and F, paired specimens from the same patient.
Clinical utility of circulating methylated ER-α
Before receiving systemic concurrent biochemotherapy, blood from AJCC stage IV melanoma patients was obtained and retrospectively assayed for the detection of circulating methylated ER-α DNA. Serum ER-α methylation from stage IV patients was assessed to predict the patients most likely to respond to biochemotherapy. The median time of clinical follow-up after the initial blood draw was 12.5 months. The frequency of circulating methylated ER-α for responders (4 of 23, 17%) was significantly lower (P = 0.018) than nonresponders (12 of 24, 50%). In a multivariate logistic regression model that included known clinical prognostic factors for melanoma, the presence of circulating serum methylated ER-α DNA was the only factor that significantly correlated with response to biochemotherapy [risk ratio (RR), 0.21; 95% confidence interval (95% CI), 0.06–0.81; P = 0.023]. Patients categorized as biochemotherapy responders had significantly better overall survival compared with patients deemed biochemotherapy nonresponders (log-rank, P < 0.0001).
Regardless of response to biochemotherapy, patients with serum methylated ER-α had significantly worse progression-free survival compared with patients in whom methylated ER-α was not detected (log-rank, P = 0.002). Serum methylated ER-α, LDH >190 IU/L, and age <50 years were significantly correlated with progression-free survival in a univariate analysis (log-rank; methylated ER-α, P = 0.002; LDH >190 IU/L, P = 0.013; age <50 years, P = 0.028).
Similarly, patients with circulating methylated ER-α had significantly worse overall survival compared with patients in whom methylated ER-α was not detected (log-rank, P = 0.002). Circulating methylated ER-α and serum LDH >190 IU/L significantly correlated with overall survival (log-rank; methylated ER-α, P = 0.002; LDH >190 IU/L, P = 0.015). Other prognostic factors (gender, age, ECOG, and number of metastatis sites) were not significant.
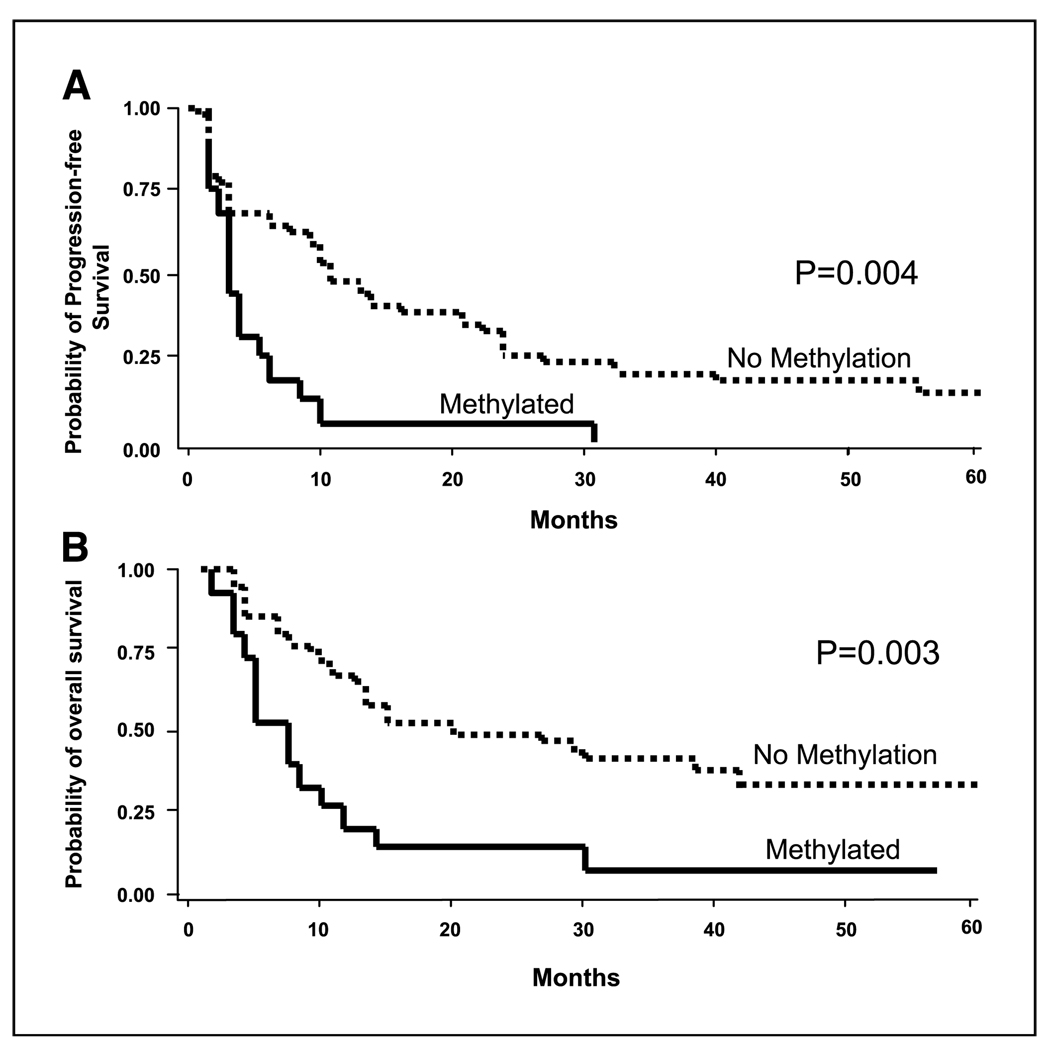
A multivariate Cox’s proportional hazard regression model was developed to correlate clinical factors and ER-α methylation status with progression-free and overall survival. Age, gender, ECOG status, LDH level, number of metastasis sites, and ER-α methylation status were included in the model using a stepwise method for variable selection. Serum methylated ER-α was the only independent factor predicting progression-free (Fig. 5A; RR, 2.64; 95% CI, 1.36–5.13; P = 0.004) and overall survival (Fig. 5B; RR, 2.31; 95% CI, 1.41–5.58; P = 0.003).
Figure 5.
A, Kaplan-Meier curves showing the correlation of pre-biochemotherapy serum ER-α methylation status with progression-free survival (Cox proportional hazard, P = 0.004). Methylated, patients with serum methylated ER-α DNA. No methylation, patients with no detectable serum methylated ER-α. B, Kaplan-Meier curves showing the correlation of pre-biochemotherapy serum ER-α methylation status with overall survival (Cox proportional hazard, P = 0.003).
Methylated ER-α: gender and age
Because ER-α hypermethylation is influenced by both age and gender in other cancers, we assessed the relation of these factors to methylated ER-α status in primary and metastatic melanomas and serum. There was no significant difference in the frequency of methylated ER-α in paraffin-embedded tumors or sera between male and female patients, nor was there any significant difference in the frequency of methylated ER-α in tumors between patients ≥60 years old and patients <50 years old.
Discussion
Methylated ER-α has been detected in neoplasia of the colorectum, lung, and breast (21, 22, 24, 26, 27). The reported expression level of ER-α in melanoma has been variable, with several studies failing to show the presence of ER-α using monoclonal antibodies (28–31). Tamoxifen has been used in chemotherapy and biochemotherapy regimens for over a decade (36–39). Although improved response rates have been reported with its use, tamoxifen has not been shown to significantly improve overall survival in advanced melanoma (40, 41). This is the first study reporting a potential mechanism for the failure of tamoxifen in the treatment of melanoma. We have shown that the variable down-regulation of ER in melanoma is due to epigenetic control of its expression via gene promoter region hypermethylation.
Our studies show that methylated ER-α can be detected in melanoma cell lines and ER-α mRNA expression can be reestablished after demethylation with 5-aza-CdR and TSA. Additionally, methylated ER-α can be detected in paraffin-embedded primary and metastatic melanoma tumors, showing its value as a biomarker of tumor progression. Methylated ER-α DNA was detected in the serum of melanoma patients with AJCC stage Ito IV disease and was a biomarker of disease progression. Furthermore, serum circulating hypermethylated ER-α in AJCC stage IV melanoma patients predicted response to biochemotherapy, progression-free survival, and overall survival.
Our in vitro experiments showed that all but one of the 11 metastatic melanoma cell lines assayed had methylated ER-α. This suggests that in vitro culturing may promote the epigenetic silencing of ER-α or select for a subpopulation of cells with methylated ER-α. 5-aza-CdR alone did not significantly increase ER-α mRNA expression (data not shown); the histone deacetylase inhibitor TSA was necessary to significantly increase expression above pretreatment levels. A histone deacetylase inhibitor, such as TSA, modulates chromatin histones and, together with 5-aza-CdR, can effectively activate gene expression. That TSA treatment was a necessary step for ER-α mRNA reexpression suggests that histone acetylation also plays an important regulatory role in ER-α expression (26, 27). Similar epigenetic regulation in breast cancer, ovarian cancer, prostate cancer, and hepatocellular cancer has been reported (3, 23).
The frequency of ER-α methylation served as a marker of progression from primary to metastatic disease and from regional nodal metastasis to distant visceral metastasis. As with breast cancer, the expression of ER-α mRNA as regulated by ER-α methylation is directly or indirectly related to the development of metastasis.
Because we were able to detect methylated ER-α in primary and metastatic melanomas, we assessed whether or not methylated ER-α could function as a blood-based biomarker for diagnosis and disease surveillance. In the current study, methylated ER-α was detected in the serum of AJCC stage Ito IV melanoma patients in a pattern related to disease progression. In a subset of matched melanoma tumor and serum sample pairs, all patients with methylated DNA detected in serum had primary or metastatic tumors with methylated ER-α as well (data not shown).
Knowing that we could detect methylated ER-α in serum, we assessed the predictive utility of this marker in a select population of stage IV melanoma patients enrolled in a concurrent biochemotherapy trial. We attempted to predict the response to therapy based on the methylation status of circulating ER-α. Response rates for systemic therapies in advanced metastatic melanoma are alarmingly low. Biochemotherapy, the use of chemotherapy in conjunction with immune modulators, has produced better response rates (40–42) but outcomes differ greatly between responders and nonresponders. It has been difficult to predict tumor response before or in the early phases of biochemotherapy. Identifying molecular predictors of therapeutic response may permit physicians to treat those patients most likely to respond to therapy while sparing nonresponsive patients unnecessary treatment and its associated morbidity. Methylated ER-α was more commonly detected in the serum of patients who failed to respond to biochemotherapy and was the only factor predictive of response to biochemotherapy. Serum methylated ER-α was the only independent predictor of progression-free and overall survival in a multivariate analysis, surpassing even known clinical prognostic factors.
There are several possible explanations for these findings. First, tamoxifen, a member of the selective ER modulator family, was used in the biochemotherapy regimen of 44 of 50 patients. Patients without serum methylated ER-α, who therefore express ER-α, may be more likely to respond to the antitumor effects of tamoxifen. Conversely, the failure of patients to respond to biochemotherapy may be partially explained by the inability of tamoxifen to exert its antitumor effects when ER-α expression is silenced due to promoter region hypermethylation. This is akin to the clinical situation seen in breast cancer in which tumors not expressing ER-α do not respond to hormone therapy and carry a poorer prognosis (23). ER-α methylation could also reflect a pathophysiologic event that includes a more global hypermethylation of tumor-related genes, thereby providing tumor cells with a growth advantage (8).
Methylated ER-α is present in normal cells of different histology (47, 48). In our serum analysis, however, ER-α was not detected in the serum from normal healthy donors. Normal cells containing methylated ER-α would be expected to release this DNA into the bloodstream. Why, then, was methylated ER-α not detected in normal healthy donors? We believe that methylated ER-α from tumors is cleared less efficiently than methylated ER-α from normal cells. The destruction of normal cells is primarily through apoptosis-related events, resulting in the release of small, characteristic enzyme-degraded fragments of DNA. As a result, the DNA released from normal cells is cleared rapidly and not readily detected in blood. On the contrary, tumor cells disrupted by physical trauma or cell necrosis release intact large fragments of DNA (49). Melanoma patients release both free DNA and tumor cells into the blood stream. Circulating tumor cells may release large fragments of DNA due to nonapoptotic death mechanisms.5 The detection of methylated ER-α in melanoma patients strongly suggests that the circulating DNA is tumor related.
Age-dependent methylation of ER-α has previously been implicated in other studies (50). In this study, we did not find age differences in ER-α methylation. Among 40 healthy volunteers, methylated ER-α was detected only in one 82-year-old donor, which may be due to factors unrelated to aging, including subclinical cancer. Further detailed studies will validate the presence and significance of ER-α methylation in healthy elderly volunteers.
This is the first study showing the detection of methylated ER-α in both melanoma patients’ tumor tissues and sera. The detection of methylated ER-α in tumors or sera correlates with tumor progression and is therefore prognostically important. Our findings indicate that detection of methylated ER-α in serum may identify a population of patients with poor melanoma outcome and poor response to systemic therapy in whom alternative treatment management should be considered. Furthermore, our data support the initiation of a prospective biochemotherapy trial for stage IV melanoma based on serum ER-α methylation status. Such a trial would provide valuable information about the clinical value of tamoxifen in the treatment of melanoma and further test the ability of the ER-α methylation assay to predict response to biochemotherapy.
Acknowledgments
Grant support: NIH, National Cancer Institute Project II P0 CA029605, CA012582, and R33-CA100314.
Footnotes
Unpublished results.
References
- 1.Balch CM, Soong SJ, Atkins MB, et al. An evidence-based staging system for cutaneous melanoma. CA Cancer J Clin. 2004;54:131–149. doi: 10.3322/canjclin.54.3.131. [DOI] [PubMed] [Google Scholar]
- 2.Dulaimi E, Hillinck J, Ibanez de Caceres I, Al-Saleem T, Cairns P. Tumor suppressor gene promoter hyper-methylation in serum of breast cancer patients. Clin Cancer Res. 2004;10:6189–6193. doi: 10.1158/1078-0432.CCR-04-0597. [DOI] [PubMed] [Google Scholar]
- 3.Jeronimo C, Henrique R, Hoque MO, et al. A quantitative promoter methylation profile of prostate cancer. Clin Cancer Res. 2004;10:8472–8478. doi: 10.1158/1078-0432.CCR-04-0894. [DOI] [PubMed] [Google Scholar]
- 4.Lapidus RG, Ferguson AT, Ottaviano YL, et al. Methylation of estrogen and progesterone receptor gene 5′ CpG islands correlates with lack of estrogen and progesterone receptor gene expression in breast tumors. Clin Cancer Res. 1996;2:805–810. [PubMed] [Google Scholar]
- 5.Shinozaki M, Hoon DS, Giuliano AE, et al. Distinct hypermethylation profile of primary breast cancer is associated with sentinel lymph node metastasis. Clin Cancer Res. 2005;11:2156–2162. doi: 10.1158/1078-0432.CCR-04-1810. [DOI] [PubMed] [Google Scholar]
- 6.Umetani N, Mori T, Koyanagi K, et al. Aberrant hypermethylation of ID4 gene promoter region increases risk of lymph node metastasis in T1 breast cancer. Oncogene. 2005;24:4721–4727. doi: 10.1038/sj.onc.1208538. [DOI] [PubMed] [Google Scholar]
- 7.Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003;349:2042–2054. doi: 10.1056/NEJMra023075. [DOI] [PubMed] [Google Scholar]
- 8.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3:415–428. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 9.Sidransky D. Emerging molecular markers of cancer. Nat Rev Cancer. 2002;2:210–219. doi: 10.1038/nrc755. [DOI] [PubMed] [Google Scholar]
- 10.Spugnardi M, Tommasi S, Dammann R, Pfeifer GP, Hoon DS. Epigenetic inactivation of RAS association domain family protein 1 (RASSF1A) in malignant cutaneous melanoma. Cancer Res. 2003;63:1639–1643. [PubMed] [Google Scholar]
- 11.Hoon DS, Spugnardi M, Kuo C, Huang SK, Morton DL, Taback B. Profiling epigenetic inactivation of tumor suppressor genes in tumors and plasma from cutaneous melanoma patients. Oncogene. 2004;23:4014–4022. doi: 10.1038/sj.onc.1207505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujimoto A, O’Day SJ, Taback B, Elashoff D, Hoon DS. Allelic imbalance on 12q22-23 in serum circulating DNA of melanoma patients predicts disease outcome. Cancer Res. 2004;64:4085–4088. doi: 10.1158/0008-5472.CAN-04-0957. [DOI] [PubMed] [Google Scholar]
- 13.Fujiwara Y, Chi DD, Wang H, et al. Plasma DNA microsatellites as tumor-specific markers and indicators of tumor progression in melanoma patients. Cancer Res. 1999;59:1567–1571. [PubMed] [Google Scholar]
- 14.Hoon DS, Bostick P, Kuo C, et al. Molecular markers in blood as surrogate prognostic indicators of melanoma recurrence. Cancer Res. 2000;60:2253–2257. [PubMed] [Google Scholar]
- 15.Nakayama T, Taback B, Nguyen DH, et al. Clinical significance of circulating DNA microsatellite markers in plasma of melanoma patients. Ann N Y Acad Sci. 2000;906:87–98. doi: 10.1111/j.1749-6632.2000.tb06596.x. [DOI] [PubMed] [Google Scholar]
- 16.Taback B, Hoon DS. Circulating nucleic acids and proteomics of plasma/serum: clinical utility. Ann N Y Acad Sci. 2004;1022:1–8. doi: 10.1196/annals.1318.002. [DOI] [PubMed] [Google Scholar]
- 17.Taback B, O’Day SJ, Boasberg PD, et al. Circulating DNA microsatellites: molecular determinants of response to biochemotherapy in patients with metastatic melanoma. J Natl Cancer Inst. 2004;96:152–156. doi: 10.1093/jnci/djh011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beato M, Herrlich P, Schutz G. Steroid hormone receptors: many actors in search of a plot. Cell. 1995;83:851–857. doi: 10.1016/0092-8674(95)90201-5. [DOI] [PubMed] [Google Scholar]
- 19.Mangelsdorf DJ, Thummel C, Beato M, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonkhoff H, Fixemer T, Hunsicker I, Remberger K. Estrogen receptor expression in prostate cancer and premalignant prostatic lesions. Am J Pathol. 1999;155:641–647. doi: 10.1016/S0002-9440(10)65160-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujii S, Tominaga K, Kitajima K, et al. Methylation of the oestrogen receptor gene in non-neoplastic epithelium as a marker of colorectal neoplasia risk in longstanding and extensive ulcerative colitis. Gut. 2005;54:1287–1292. doi: 10.1136/gut.2004.062059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jensen EV, Cheng G, Palmieri C, et al. Estrogen receptors and proliferation markers in primary and recurrent breast cancer. Proc Natl Acad Sci U S A. 2001;98:197–202. doi: 10.1073/pnas.211556298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lapidus RG, Nass SJ, Davidson NE. The loss of estrogen and progesterone receptor gene expression in human breast cancer. J Mammary Gland Biol Neoplasia. 1998;3:85–94. doi: 10.1023/a:1018778403001. [DOI] [PubMed] [Google Scholar]
- 24.Leu YW, Yan PS, Fan M, et al. Loss of estrogen receptor signaling triggers epigenetic silencing of downstream targets in breast cancer. Cancer Res. 2004;64:8184–8192. doi: 10.1158/0008-5472.CAN-04-2045. [DOI] [PubMed] [Google Scholar]
- 25.O’Neill PA, Davies MP, Shaaban AM, et al. Wild-type oestrogen receptor β (ERβ1) mRNA and protein expression in Tamoxifen-treated post-menopausal breast cancers. Br J Cancer. 2004;91:1694–1702. doi: 10.1038/sj.bjc.6602183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang X, Ferguson AT, Nass SJ, et al. Transcriptional activation of estrogen receptor α in human breast cancer cells by histone deacetylase inhibition. Cancer Res. 2000;60:6890–6894. [PubMed] [Google Scholar]
- 27.Yang X, Phillips DL, Ferguson AT, Nelson WG, Herman JG, Davidson NE. Synergistic activation of functional estrogen receptor (ER)-α by DNA methyl-transferase and histone deacetylase inhibition in human ER-α-negative breast cancer cells. Cancer Res. 2001;61:7025–7029. [PubMed] [Google Scholar]
- 28.Cohen C, DeRose PB, Campbell WG, Schlosnagle DC, Sgoutas D. Estrogen receptor status in malignant melanoma. Am J Dermatopathol. 1990;12:562–564. doi: 10.1097/00000372-199012000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Fisher RI, Neifeld JP, Lippman ME. Oestrogen receptors in human malignant melanoma. Lancet. 1976;2:337–339. doi: 10.1016/s0140-6736(76)92592-7. [DOI] [PubMed] [Google Scholar]
- 30.Flowers JL, Seigler HF, McCarty KS, Sr, Konrath J, McCarty KS., Jr Absence of estrogen receptor in human melanoma as evaluated by a monoclonal antiestrogen receptor antibody. Arch Dermatol. 1987;123:764–765. [PubMed] [Google Scholar]
- 31.Walker MJ, Beattie CW, Patel MK, Ronan SM, Das Gupta TK. Estrogen receptor in malignant melanoma. J Clin Oncol. 1987;5:1256–1261. doi: 10.1200/JCO.1987.5.8.1256. [DOI] [PubMed] [Google Scholar]
- 32.Gill PG, De Young NJ, Thompson A, Keightley DD, Horsfall DJ. The effect of tamoxifen on the growth of human malignant melanoma in vitro. Eur J Cancer Clin Oncol. 1984;20:807–815. doi: 10.1016/0277-5379(84)90220-7. [DOI] [PubMed] [Google Scholar]
- 33.Kanter-Lewensohn L, Girnita L, Girnita A, et al. Tamoxifen-induced cell death in malignant melanoma cells: possible involvement of the insulin-like growth factor-1 (IGF-1) pathway. Mol Cell Endocrinol. 2000;165:131–137. doi: 10.1016/s0303-7207(00)00253-7. [DOI] [PubMed] [Google Scholar]
- 34.Cocconi G, Bella M, Calabresi F, et al. Treatment of metastatic malignant melanoma with dacarbazine plus tamoxifen. N Engl J Med. 1992;327:516–523. doi: 10.1056/NEJM199208203270803. [DOI] [PubMed] [Google Scholar]
- 35.Flaherty LE, Liu PY, Mitchell MS, et al. The addition of tamoxifen to dacarbazine and cisplatin in metastatic malignant melanoma. A phase II trial of the Southwest Oncology Group (SWOG-8921) Am J Clin Oncol. 1996;19:108–113. doi: 10.1097/00000421-199604000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Rumke P, Kleeberg UR, MacKie RM, et al. Tamoxifen as a single agent for advanced melanoma in postmenopausal women. A phase II study of the EORTC Malignant Melanoma Cooperative Group. Melanoma Res. 1992;2:153–156. doi: 10.1097/00008390-199209000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Falkson CI, Ibrahim J, Kirkwood JM, Coates AS, Atkins MB, Blum RH. Phase III trial of dacarbazine versus dacarbazine with IFN α-2b versus dacarbazine with tamoxifen versus dacarbazine with IFN α-2b and tamoxifen in patients with metastatic malignant melanoma: an Eastern Cooperative Oncology Group study. J Clin Oncol. 1998;16:1743–1751. doi: 10.1200/JCO.1998.16.5.1743. [DOI] [PubMed] [Google Scholar]
- 38.Agarwala SS, Ferri W, Gooding W, Kirkwood JM. A phase III randomized trial of dacarbazine and carboplatin with and without tamoxifen in the treatment of patients with metastatic melanoma. Cancer. 1999;85:1979–1984. [PubMed] [Google Scholar]
- 39.Chiarion Sileni V, Nortilli R, Aversa SM, et al. Phase II randomized study of dacarbazine, carmustine, cisplatin and tamoxifen versus dacarbazine alone in advanced melanoma patients. Melanoma Res. 2001;11:189–196. doi: 10.1097/00008390-200104000-00015. [DOI] [PubMed] [Google Scholar]
- 40.O’Day SJ, Gammon G, Boasberg PD, et al. Advantages of concurrent biochemotherapy modified by decrescendo interleukin-2, granulocyte colony-stimulating factor, and tamoxifen for patients with metastatic melanoma. J Clin Oncol. 1999;17:2752–2761. doi: 10.1200/JCO.1999.17.9.2752. [DOI] [PubMed] [Google Scholar]
- 41.O’Day SJ, Boasberg PD, Piro L, et al. Maintenance biotherapy for metastatic melanoma with interleukin-2 and granulocyte macrophage-colony stimulating factor improves survival for patients responding to induction concurrent biochemotherapy. Clin Cancer Res. 2002;8:2775–2781. [PubMed] [Google Scholar]
- 42.O’Day SJ, Atkins MB, Weber J. A phase II multi-center trial of maintenance biotherapy (MBT) after induction concurrent biochemotherapy (BCT) for patients (Pts) with metastatic melanoma; Proc ASCO; 2005. p. 710s. [DOI] [PubMed] [Google Scholar]
- 43.Takeuchi H, Morton DL, Kuo C, et al. Prognostic significance of molecular upstaging of paraffin-embedded sentinel lymph nodes in melanoma patients. J Clin Oncol. 2004;22:2671–2680. doi: 10.1200/JCO.2004.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mori T, O’Day SJ, Umetani N, et al. Predictive utility of circulating methylated DNA in serum of melanoma patients receiving biochemotherapy. J Clin Oncol. 2005;23:9351–9358. doi: 10.1200/JCO.2005.02.9876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Umetani N, de Maat MF, Mori T, Takeuchi H, Hoon DS. Synthesis of universal unmethylated control DNA by nested whole genome amplification with phi29 DNA polymerase. Biochem Biophys Res Commun. 2005;329:219–223. doi: 10.1016/j.bbrc.2005.01.088. [DOI] [PubMed] [Google Scholar]
- 46.Koyanagi K, O’Day SJ, Gonzalez R, et al. Serial monitoring of circulating melanoma cells during neo-adjuvant biochemotherapy for stage III melanoma: outcome prediction in a multicenter trial. J Clin Oncol. 2005;23:8057–8064. doi: 10.1200/JCO.2005.02.0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu L, Zhang J, Bates S, et al. A methylation profile of in vitro immortalized human cell lines. Int J Oncol. 2005;26:275–285. [PubMed] [Google Scholar]
- 48.Zhao C, Lam EW, Sunters A, et al. Expression of estrogen receptor β isoforms in normal breast epithelial cells and breast cancer: regulation by methylation. Oncogene. 2003;22:7600–7606. doi: 10.1038/sj.onc.1207100. [DOI] [PubMed] [Google Scholar]
- 49.Hiramatsu S, Umetani N, Amersi F, Martino S, Giuliano AE, Hoon DS. Prediction of breast metastasis by integrity of free circulating DNA in serum. Clin Chem. 2005;51:25. doi: 10.1200/JCO.2006.05.9493. [DOI] [PubMed] [Google Scholar]
- 50.Li LC, Shiina H, Deguchi M, et al. Age-dependent methylation of ESR1 gene in prostate cancer. Biochem Biophys Res Commun. 2004;321:455–461. doi: 10.1016/j.bbrc.2004.06.164. [DOI] [PubMed] [Google Scholar]