Abstract
Purpose
Microphthalmia transcription factor (Mitf), which is important in melanocyte development and melanoma growth, was assessed using real-time quantitative reverse transcription-PCR assay to investigate its expression as a marker for circulating melanoma cells in blood and determine the correlation with disease stage and survival in melanoma patients.
Experimental Design
In optimization studies for Mitf, we tested 15 melanoma cell lines, 41 peripheral blood lymphocytes from healthy volunteers, and 21metastatic melanoma tissues. Blood specimens were procured from 90 patients with stage I (n = 20), stage II (n = 20), stage III (n = 28), and stage IV (n = 22) melanoma. Blood specimens were also obtained at four bleed intervals from 58 patients enrolled in a prospective multicenter trial of biochemotherapy before and after surgical treatment of American Joint Committee on Cancer stage III melanoma.
Results
Under the optimized conditions, Mitf was negative in healthy peripheral blood lymphocytes and positive in all melanoma cell lines and 18 (86%) melanoma tissues. In the 90 patients, the rate of Mitf detection was higher with increasing American Joint Committee on Cancer stage (P < 0.0001). In the 58 patients treated with biochemotherapy and surgery, Mitf detection decreased with treatment (P = 0.019). Mitf detection after treatment was associated with a significantly lower relapse-free (P < 0.0001) and overall (P = 0.001) survival and was a significant independent prognostic factor for relapse-free (risk ratio, 5.63; P = 0.0004) and overall (risk ratio, 5.36; P = 0.005) survival.
Conclusions
Mitf detection in blood can indicate subclinical metastatic disease and predict treatment outcome in melanoma patients.
Assessment of circulating tumor cells as a surrogate marker of cancer status is promising because, unlike tumor tissue, blood can be sampled repeatedly with limited invasive procedures (1–4). Recently, real-time quantitative reverse transcription-PCR (RT-PCR) assay has been used to detect circulating tumor cells in blood and only a few studies have shown clinical utility as a predictive surrogate for treatment response (5–8). Because of the heterogeneous expression of tumor genes and variable performance of the assays, the utility of markers in circulating tumor cell detection has not reached consensus. In melanoma, our group introduced the detection of circulating tumor cells using reverse transcription-PCR assay and showed its utility (9, 10). Recently, we showed that a multimarker quantitative RT-PCR assay using melanoma-associated mRNA for MART-1 (melanoma antigen recognized by T cells-1), GalNAc-T (β1→4-N-acetylgalactosaminyltransferase), PAX-3 (paired box homeotic gene transcription factor 3), and MAGE-A3 (melanoma antigen gene-A3 family) was useful for detection of circulating tumor cells in blood and that the detection of markers was associated with melanoma stage and treatment outcome (11, 12).
To assess new potential markers as a surrogate of circulating tumor cells and predictor for disease outcome, we examined a marker that would reflect the biological characteristics of melanoma cells shed into circulation from primary and/or metastatic lesions (1). Recent reports show that key transcriptional factors regulate the phenotype, biology, and growth of melanoma and play an important role in its development and progression (13–15). The phenotype of melanoma cells is associated with its survival and tumor progression (15, 16).
Microphthalmia transcription factor (Mitf) is essential for the development and postnatal survival of melanocytes (17). Recent studies have suggested the correlation of Mitf expression with melanoma cell growth (18–20). Among several isoforms of Mitf, Mitf-M is present in melanoma cells and melanocytes (16, 21). Mitf regulates expression of a variety of genes including melanocyte-differentiating genes, in particular the pigmentation enzymes such as tyrosinase, gp100/pMel17, TRP1, and TRP2 (17, 19, 22, 23). Mitf mutations in humans produce Waardenburg syndrome type 2, a condition characterized by melanocyte deficiency in the skin and inner ear (24, 25). Mitf expression is up-regulated by Wnt signaling via Lef-1/β-catenin complex binding site (26, 27), by α-MSH (α-melanocyte-stimulating hormone) via a cyclic AMP–mediated signal transduction pathway (28, 29), and by c-Kit signaling through mitogen-activated protein kinase phosphorylation (30, 31). Transcriptional factor PAX-3, mutations of which are the cause of Waardenburg syndrome types 1 and 3, and transcriptional factor SOX10, a critical activator of TRP-1 which is correlated with Waardenburg syndrome type 4, also strongly activate Mitf expression in a cooperative manner (32, 33). A recent study shows that Mitf cooperates with Rb1 and can regulate cell cycle progression through activation of p21Cip1 expression (20). Previous studies using immunohistochemistry showed that Mitf has emerged as a potential diagnostic and prognostic marker for melanoma tissue (34, 35); however, its expression has not been well explored as a surrogate marker for circulating melanoma cells in blood.
Our hypothesis is that Mitf expression in blood can be used as a surrogate of circulating tumor cells and predict the subclinical tumor progression and disease outcome in melanoma patients undergoing therapy. To test this hypothesis, we used a quantitative RT-PCR assay for Mitf mRNA to detect circulating tumor cells in blood of melanoma patients. In this study, we showed that Mitf detection in blood was correlated with melanoma stage and survival after neoadjuvant biochemotherapy.
Patients and Methods
Melanoma cell lines
Fifteen melanoma cell lines (MA, MB, MC, MD, ME, MF, MG, MH, MI, MJ, MK, ML, MM, MN, and MO) established and characterized at the John Wayne Cancer Institute were used in the study. Cells were grown in RPMI 1640 containing 10% heat-inactivated FCS and 1% of penicillin/streptomycin (Life Technologies, Inc., Grand Island, NY) in T75-cm2 flask and were used for mRNA analysis when they reached 70% to 80% confluence as previously described (11).
Optimization of the quantitative RT-PCR assay
To optimize the quantitative RT-PCR assay, 15 melanoma cell lines, 41 healthy donor peripheral blood lymphocytes (PBL), and 21 cryopreserved metastatic melanoma tissues were assessed. To determine the detection limit for melanoma cells in blood and assess the clinical feasibility of the assay, we did quantitative RT-PCR on serial diluted melanoma cells mixed with PBLs from healthy donors. Serial dilutions of melanoma cells (103, 102, 10, and 0 cells) that expressed Mitf mRNA were mixed with 107 donor-derived PBLs and assayed for Mitf expression by quantitative RT-PCR. This in vitro assay was done four times to validate its reproducibility and robustness.
Melanoma patients
Two groups of melanoma patients (n = 90 and n = 58; total, 148) were studied to validate the utility of Mitf assay. The first group was composed of 90 patients with American Joint Committee on Cancer (AJCC) stage I (n = 20), stage II (n = 20), stage III (n = 28), and stage IV (n = 22) melanoma. A single blood specimen was obtained from each patient before any treatment at John Wayne Cancer Institute.
The second group of melanoma patients was composed of 58 patients [41 males and 17 females; median age, 41 years (range, 17–76 years); median follow-up, 31.8 months] enrolled in a prospective multicenter phase II clinical trial of adjuvant biochemotherapy before and after surgery for AJCC stage III melanoma between 1999 and 2002. The patients were selected from 92 patients based on the availability of blood specimens and clinical follow-up. All patients received two cycles of biochemotherapy at 3-week intervals before surgery. The biochemotherapy regimen composed of cisplatin, 20 mg/m2 i.v. on days 1 to 4; dacarbazine, 800 mg/m2 i.v. on day 1; vinblastine, 1.6 mg/m2 i.v. on days 1 to 4; interleukin-2, 9 megaunits/m2, continuous i.v. over 24 hours on days 1 to 4, or 36 megaunits/m2 over 4 days via decrescendo schedule (Chiron Corporation, Emeryville, CA); α-IFN, 5 megaunits/m2 s.c. on days 1 to 5 (Schering-Plough, Madison, NJ); and granulocyte colony-stimulating factor, 5 μg/kg s.c. on days 6 to 12 (Amgen, Inc., Thousand Oaks, CA). Patients then underwent therapeutic lymphadenectomy and began two cycles of biochemotherapy within 42 days after surgery; the biochemotherapy regimen and interval were the same as before surgery. Blood samples were obtained immediately before preoperative biochemotherapy (n = 58), before surgery (presurgery, n = 53), after surgery (postsurgery, n = 55), and after postoperative biochemotherapy (n = 55). The interval between each sampling time was ~6 weeks and blood specimens that were not processed within 24 hours after procurement were excluded from the study. All patients were clinically and radiologically evaluated at specified time points during treatment and follow-up.
Both groups of patients signed informed consent forms for the use of their blood specimens. These studies were approved and carried out according to guidelines set forth by the Saint John’s Health Center and John Wayne Cancer Institute Institutional Review Board committee and three independent Institutional Review Board committees for centers participating in the phase II clinical trial (Saint John’s Health Center/John Wayne Cancer Institute, University of Colorado Cancer Center, Aurora, CO; and Hubert H. Humphrey Cancer Center, Robbinsdale, MN). All blood specimens were coded by a computer-generated number and recorded by the database coordinator at John Wayne Cancer Institute independently of investigators and biostatisticians.
Blood processing and quantitative RT-PCR assay
Ten milliliters of blood were collected in sodium citrate–containing tubes from each patient. To eliminate skin-plug contamination of the blood sample from initial venipuncture, the first several milliliters of blood were discarded as previously described (9, 36). Blood cells were prepared using Purescript RBC Lysis Solution (Gentra, Minneapolis, MN) following the instruction of the manufacturer as previously described (11).
Tri-Reagent (Molecular Research Center, Cincinnati, OH) was used to isolate total cellular RNA from blood specimens as previously described (9, 36). RNA was quantified and assessed for purity by UV spectrophotometry. Blood processing, RNA extraction, reverse transcription-PCR assay setup, and post reverse transcription-PCR product analysis were carried out in separate designated rooms to prevent cross-contamination.
Reverse transcription reactions were done using Moloney murine leukemia virus reverse transcriptase (Promega, Madison, WI) with oligo-dT primer (36, 37). A multimarker quantitative RT-PCR assay was done using iCycler iQ Real-Time Thermocycler Detection System (Bio-Rad Laboratories, Hercules, CA) as previously described (11, 37). Primer and probe sequences were designed for the quantitative RT-PCR assay using Oligo Primer Analysis Software, version 5.0 (National Biomedical systems, Plymouth, MN). Primers were designed to span one intron to avoid amplification of contaminating genomic DNA and were synthesized for Mitf-M. Exons, introns, and positions of primers and probe were as follows: exon A (118 bp), intron A (2,644 bp), exon B (74 bp), intron B (4,568 bp), exon C (76 bp), intron C (2,742 bp), and exon D (148 bp); the forward primer spanned intron A, the reverse primer spanned intron C, and the probe were on exon B. Expected amplified product size was 174 bp. Fluorescence resonance energy transfer probe sequences are as follows: Mitf, 5′-FAM-AGAGCACTGGCCAAAGAGAGGCA-BHQ-1-3′; glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 5′-FAM-CAGCAATGCCTCCTGCACCACCAA-BHQ-1-3′. Assay setup involved transferring 5 μL cDNA from 250 ng total RNA to a 96-well PCR plate (Fisher Scientific, Pittsburgh, PA), in which 0.5 μmol/L of each primer, 0.3 μmol/L probe, 1 unit AmpliTaq Gold polymerase (Applied Biosystems, Branchburg, NJ), 200 μmol/L of each deoxynucleotide triphosphate, 4.5 mmol/L MgCl2, and PCR buffer were applied to a final volume of 25 μL. Samples were amplified with a precycling hold at 95°C for 10 minutes, followed by 42 cycles of denaturation at 95°C for 1 minute, annealing at 58°C for 1 minute for Mitf (annealing at 55°C for GAPDH), and extension at 72°C for 1 minute.
Each assay was done at least twice and included marker-positive (melanoma cell line) and marker-negative (PBL with negative for Mitf) controls and reagent with no template controls. GAPDH gene was used as a housekeeping gene to verify integrity of RNA and efficacy of reverse transcription. Any specimen with inadequate GAPDH mRNA was excluded from the study. The mean mRNA copies were used for analysis.
Statistical analysis
In the 90 patients with AJCC stage I–IV melanoma, stage-related differences in Mitf copy number were examined by Kruskal-Wallis test and Mann-Whitney U test; stage-related differences in Mitf positivity were examined by Cochran-Armitage trend test and Fisher’s exact t test.
The study of the 58 patients from the biochemotherapy trial was designed to investigate the changes in circulating tumor cells during treatment course and prognostic effect of circulating tumor burden after overall treatment on disease relapse and survival. Primary outcomes were Mitf detection after all treatments, relapse of the disease, and survival. Wilcoxon signed-rank test was used to compare Mitf copy number during treatment. McNemar’s test was used for assessment of changes in Mitf positivity with treatment. Relapse-free survival after lymphadenectomy and overall survival from the start of biochemotherapy (preoperative biochemotherapy) were used for outcome measurement. Univariate analysis of relapse-free survival and overall survival according to clinicopathologic factors (age, gender, primary site, pT stage, pN stage, and previous therapy) and Mitf detection at each sampling point were done using the log-rank test. Cox proportional hazard model was developed to examine the association of Mitf detection with relapse-free survival and overall survival and used for multivariate analysis. Clinicopathologic variables and Mitf detection at each sampling point were included in the model and a stepwise method was used for prognostic variable selection. Survival curves were generated using the Kaplan-Meier method. The analysis was done using SAS statistical software and all tests were two sided with a significance level of ≤0.05.
Results
Mitf expression in cell lines and tumors
The standard curves generated by using threshold cycles of nine serial dilutions of plasmid templates (108-100 copies) showed the expected linear increase of signal with logarithm of the copy number (data not shown). PCR efficiency evaluated from the slopes of the curves was between 90% and 100%. The correlation coefficient for all standard curves was ≥0.99. We confirmed the product size of Mitf by gel electrophoresis and optimized assay conditions for quantitative RT-PCR (data not shown).
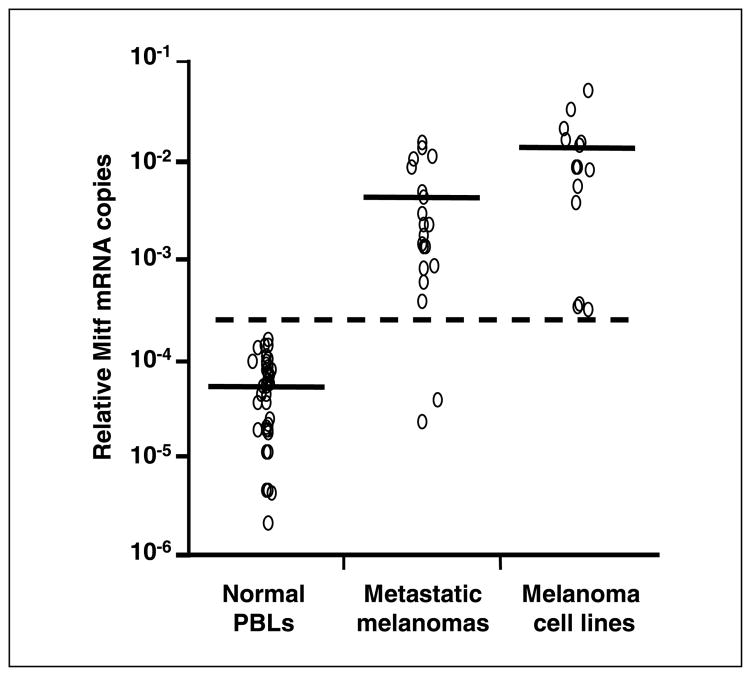
The range of absolute mRNA copies of GAPDH was follows: 3.8 × 105 to 8.4 × 107 (median, 4.0 × 107) per 250 ng total RNA in melanoma cells; 1.2 × 104 to 2.6 × 107 (median, 2.3 × 105) in cryopreserved metastatic melanoma tissues; and 2.5 × 104 to 1.5 × 107 (median, 3.2 × 106) in PBLs from healthy donors. Mitf copies were detected in 15 of 15 (100%) melanoma cell lines, 20 of 21 (95%) metastatic melanoma tissues, and 39 of 41 (95%) PBLs. Based on the variation of mRNA status among samples and compensation for comparison of different assays, relative mRNA copies (absolute mRNA copies of Mitf/absolute mRNA copies of GAPDH) were used to assess the cutoff point and correlation with stage and treatment outcome. Mean relative Mitf copy number (± SD) was 1.3 × 10−2 ± 1.3 × 10−2 (range, 2.8 × 10−4–4.9 × 10−2) in melanoma cell lines, 3.9 × 10−3 ± 4.4 × 10−3 (range, 0–1.4 × 10−2) in metastatic melanoma tissues, and 5.1 × 10−5 ± 4.1 × 10−5 (range, 0–1.4 × 10−4) in PBLs from healthy donors (Fig. 1). Relative Mitf copies were significantly higher in melanoma cell lines and metastatic melanoma tissues than in normal PBLs (P < 0.0001). We set the cutoff for Mitf positivity at 2.8 × 10−4; this was above the Mitf levels of all normal PBLs but below the Mitf levels of all melanoma cell lines and 18 of 21 (86%) metastatic tissues. The cutoff point was above the mean relative Mitf copy number plus 5 SD of healthy donor PBLs. Blood specimens with higher relative Mitf copies than the cutoff point were considered as positive for Mitf.
Fig. 1.
Relative Mitf copies in melanoma cell lines, metastatic melanoma tissues, and PBLs from healthy donors. The cutoff point (dotted line) for Mitf positivity was set at 2.8 × 10−4. The cutoff point was above the mean relative Mitf copy number plus 5 SD of normal PBLs. Horizontal bars, mean mRNA copies.
Sensitivity of quantitative RT-PCR assay for Mitf in melanoma cell dilution study
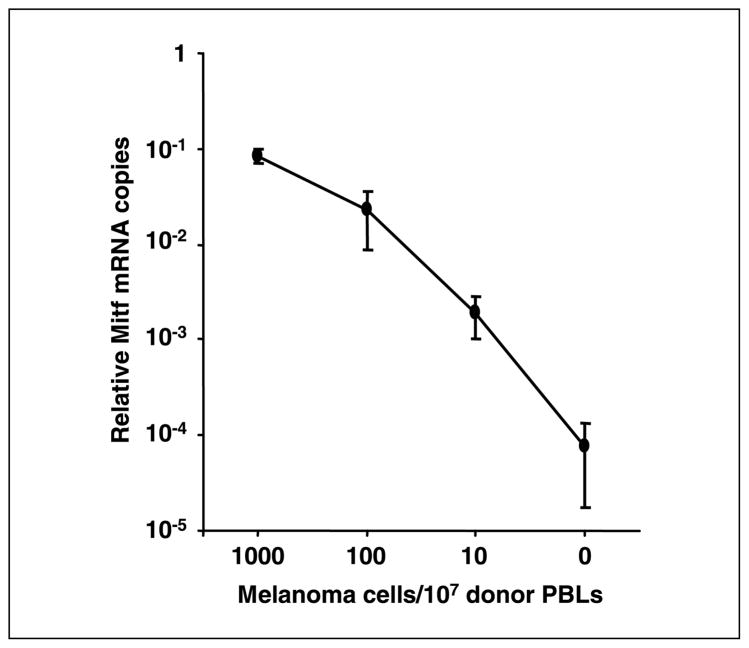
Sensitivity of the optimized quantitative RT-PCR assay for Mitf was assessed. Relative Mitf mRNA copies gradually decreased as the melanoma cells in normal PBLs decreased (Fig. 2). Relative Mitf copies of samples without melanoma cells ranged from 2.5 × 10−5 to 1.0 × 10−4 (mean ± SD, 7.4 × 10−5 ± 5.7 × 10−5). When 10 melanoma cells were mixed in 107 PBLs, relative Mitf copies ranged from 1.0 × 10−3 to 3.0 × 10−3 (mean ± SD, 1.9 × 10−3 ± 8.9 × 10−4). In four separate experiments, all results were higher than the cutoff point of the quantitative RT-PCR assay. We also assessed dilutions of ~1 melanoma cell in 107 healthy donor PBLs. The relative Mitf copies above the cutoff was observed in two of four experiments (data not shown). These studies indicated the feasibility and sensitivity of the quantitative RT-PCR assay to detect occult melanoma cells in blood.
Fig. 2.
Quantitative RT-PCR quantification for melanoma cells mixed with107 PBLs from healthy donor blood. Serial diluted melanoma cells (103,102,10, and 0 cells) were mixed with107 donor-derived PBLs and assayed for Mitf expression by quantitative RT-PCR. The assay was done four times. Points, mean relative Mitf copies given according to serial dilution; bars, SD. Relative Mitf copies of the samples with 10 melanoma cells in 107 PBLs were higher than the cutoff point.
Mitf expression and AJCC stage of disease
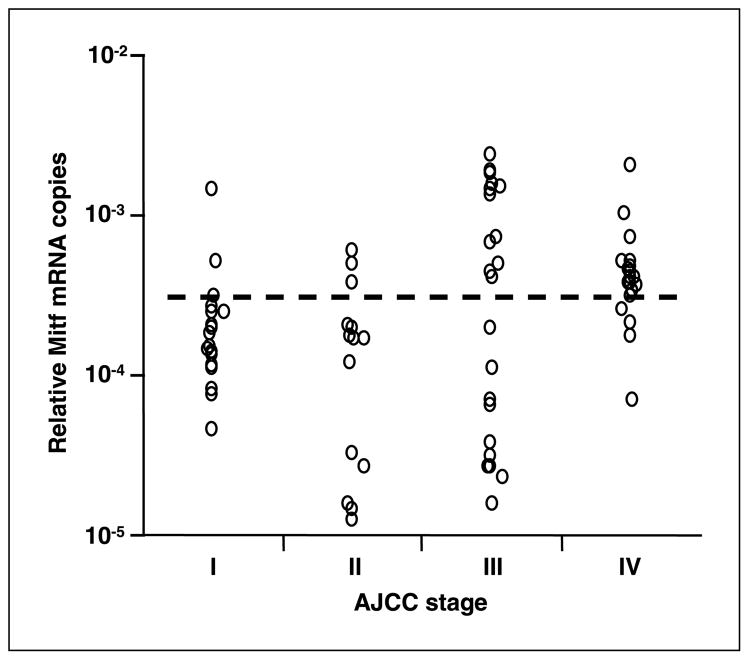
We assessed the Mitf detection in melanoma patients with different AJCC stages. Relative Mitf copies ranged from 0 to 2.4 × 10−3 in blood from the 90 patients with AJCC stage I–IV melanoma (Fig. 3). Stage-related differences in relative Mitf copies were determined significant (P = 0.012, Kruskal-Wallis test) and relative Mitf copies were significantly higher in advanced AJCC stage patients than in earlier stage patients (I versus III, P = 0.04; I versus IV, P = 0.003; II versus III, P = 0.03; II versus IV, P = 0.002; I/II versus III/IV, P = 0.02; Mann-Whitney U test).
Fig. 3.
Correlation between relative Mitf copy number and AJCC stage of melanoma. Dotted line, cutoff level for positive Mitf marker (2.8 × 10−4).
Mitf was positive in blood specimens from 35 of 90 (39%) patients (Table 1). Mitf detection frequency was significantly related to higher AJCC stage (Cochran-Armitage trend test; P < 0.0001). Mitf was positive in only 7 of 40 (18%) stage I/II patients compared with 28 of 50 (56%) stage III/IV patients (P = 0.0002, Fisher’s exact t test).
Table 1.
Mitf detection in different AJCC stages of melanoma
| Mitf | AJCC stage |
P* | ||||
|---|---|---|---|---|---|---|
| I |
II |
III |
IV |
Total |
||
| n = 20 (%) | n = 20 (%) | n = 28 (%) | n = 22 (%) | n = 90 (%) | ||
| Positive | 3 (15) | 4 (20) | 12 (43) | 16 (73) | 35 (39) | <0.0001 |
| Negative | 17 (85) | 16 (80) | 16 (57) | 6 (27) | 55 (61) | |
Comparison between AJCC stage and Mitf detection using Cochran-Armitage trend test.
Correlation of Mitf expression with disease relapse
To further validate the utility of the Mitf quantitative RT-PCR assay, we assessed melanoma patients entered in a prospective phase II multicenter neoadjuvant biochemotherapy trial. In 221 blood specimens from the 58 melanoma patients enrolled in the biochemotherapy trial, relative Mitf copies ranged from 0 to 2.0 × 10−3. Relative Mitf copies decreased with treatment and were significantly lower postoperative biochemotherapy (2.5 × 10−4 ± 2.6 × 10−4) than preoperative biochemotherapy (3.6 × 10−4 ± 3.5 × 10−4; P = 0.029, Wilcoxon signed-rank test).
At a median follow-up time of 31.8 months (range, 4.6–52.4 months), 17 of 58 (29%) patients had relapsed and 41 (71%) were clinically disease-free. The rate of Mitf detection after overall treatment (after postsurgery biochemotherapy) clearly distinguished relapse and relapse-free patient groups: 7% for relapse-free patients versus 53% for relapse patients (P = 0.0005, Fisher’s exact t test; Table 2). In patients who remained relapse-free, the rate of Mitf detection significantly decreased after all treatments (McNemar’s test; P = 0.0002) and after each phase of biochemotherapy (preoperative, P = 0.027; postoperative, P = 0.017). In patients with relapse, there was no significant difference between any two sampling points.
Table 2.
Disease outcome according to Mitf detection in blood during treatment
| Patients (%) |
P* | ||||
|---|---|---|---|---|---|
| Preoperative biochemotherapy | Presurgery | Postsurgery | Postoperative biochemotherapy | ||
| Nonrelapse (n = 41) | 19 (46) | 10 (24) | 12 (29) | 3 (7) | 0.0005 |
| Relapse (n = 17) | 7 (41) | 5 (29) | 2 (12) | 9 (53) | |
Comparison of Mitf positivity at postoperative biochemotherapy with disease outcome using Fisher’s exact t test.
Correlation of Mitf expression with relapse-free survival and overall survival
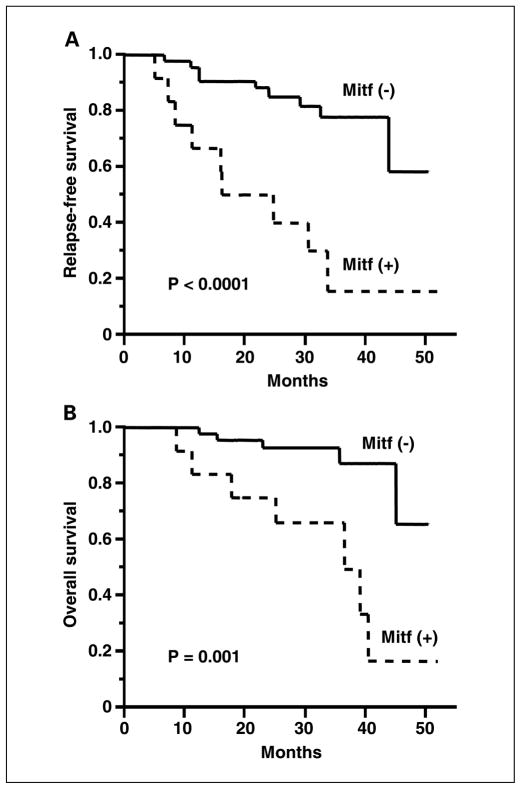
We then evaluated whether Mitf detection related to disease outcome. Before treatment, Mitf detection had no correlation with age, gender, primary site, Breslow thickness, pT stage, pN stage, and previous treatment status. After overall treatment, Mitf detection was inversely correlated with relapse-free survival (P < 0.0001; Fig. 4A). For patients whose specimens did not express Mitf, the estimated relapse-free survival was 92% [95% confidence interval (95% CI), 85–96%] for 12 months and 85% (95% CI, 73–92%) for 24 months. For patients with positive Mitf, the estimated relapse-free survival was 66% (95% CI, 47–81%) for 12 months and 43% (95% CI, 23–67%) for 24 months. Univariate analysis using log-rank test showed that Mitf detection after overall treatment (P < 0.0001) and pN stage (P = 0.04) were significantly correlated with relapse-free survival (Table 3). Multivariate analysis using Cox proportional hazard model showed that Mitf detection after treatment was the only significant predictor for disease relapse (risk ratio, 5.63; 95% CI, 2.16–14.68; P = 0.0004).
Fig. 4.
Kaplan-Meier estimates of relapse-free survival (A) and overall survival (B) based on Mitf detection after all treatment.
Table 3.
Univariate analysis of clinical factors for prediction of relapse-free survival and overall survival
| Factors | Patients | Relapse-free survival |
Overall survival |
||
|---|---|---|---|---|---|
| No. relapse (%) | Log-rank test | No. death (%) | Log-rank test | ||
| Gender | |||||
| Male | 37 | 13 (35) | 0.27 | 8 (22) | 0.91 |
| Female | 21 | 4 (19) | 4 (19) | ||
| Age (y) | |||||
| ≤50 | 45 | 11 (24) | 0.13 | 7 (16) | 0.03 |
| >50 | 13 | 6 (46) | 5 (38) | ||
| Primary site | |||||
| Extremity | 17 | 4 (24) | 0.23 | 4 (24) | 0.38 |
| Head/neck | 8 | 4 (50) | 3 (38) | ||
| Trunk | 25 | 6 (24) | 3 (12) | ||
| pTstage | |||||
| T1/2 | 27 | 6 (22) | 0.16 | 3 (11) | 0.07 |
| T3/4 | 15 | 6 (40) | 5 (33) | ||
| pN stage* | |||||
| N1 | 33 | 5 (15) | 0.04 | 2 (6) | 0.02 |
| N2/3 | 25 | 12 (48) | 10 (40) | ||
| Previous treatment | |||||
| No | 45 | 12 (27) | 0.72 | 9 (20) | 0.49 |
| Yes | 12 | 4 (33) | 2 (17) | ||
| Preoperative biochemotherapy Mitf | |||||
| Negative | 32 | 10 (32) | 0.95 | 4 (13) | 0.08 |
| Positive | 26 | 8 (31) | 8 (31) | ||
| Postoperative biochemotherapy Mitf | |||||
| Negative | 43 | 8 (19) | >0.0001 | 5 (12) | 0.001 |
| Positive | 12 | 9 (75) | 7 (58) | ||
pN stage was determined after two courses of biochemotherapy (at the time of surgery).
After overall treatment, Mitf detection was inversely correlated with overall survival (P = 0.001; Fig. 4B). For patients whose specimens did not express Mitf, the estimated overall survival was 96% (95% CI, 90–98%) for 12 months and 91% (95% CI, 81–96%) for 24 months. For patients with positive Mitf, the estimated overall survival was 78% (95% CI, 61–89%) for 12 months and 61% (95% CI, 39–80%) for 24 months. Univariate analysis showed that Mitf detection after preoperative biochemotherapy (P = 0.04) and Mitf detection after overall treatment (P = 0.001), pN stage (P = 0.02), and age (>50 versus ≤50; P = 0.03) were significantly correlated with overall survival. Multivariate analysis showed that Mitf detection after overall treatment (risk ratio, 5.36; 95% CI, 1.67–17.19; P = 0.005) was a significant independent prognostic factor for overall survival as well as age (risk ratio, 3.82; 95% CI, 1.20–13.32; P = 0.04).
Discussion
Although recent studies have shown the importance of circulating tumor cells in blood (1–3), the clinical utility of molecular detection has had controversy because there is no consistent correlation with disease outcome. Development of sensitive and specific marker assay is needed to evaluate circulating tumor cells as a surrogate marker of disease progression and treatment response. In this study, we showed the utility of a quantitative RT-PCR assay in detecting and measuring Mitf expression in blood directly from patients with different stages of melanoma and in predicting disease progression and treatment outcome.
We evaluated Mitf detection because it is a key transcriptional factor that regulates the melanogenesis pathway. Mitf has been assessed in primary and metastatic melanoma tumors using immunohistochemistry and its utility for diagnosis of melanoma has been shown (34, 35). However, there were no major studies that have assessed Mitf as a surrogate marker of circulating tumor cells and disease outcome (38) and, to our knowledge, none has used a quantitative RT-PCR assay. Recent studies showed that Mitf regulates expression of other genes, such as Bcl-2 and CDK2, and might be a potential drug target in melanoma treatment (18, 19). This regulation by Mitf may contribute to proliferation and/or growth of melanoma (18, 19), which suggests that higher levels of Mitf confer an aggressive phenotype. Although previous studies indicated that Mitf was not expressed in all melanoma cell lines (16, 39), our quantitative RT-PCR assay detected its expression in all melanoma cell lines assessed and in 20 of 21 metastatic melanoma tissues. Whereas expression of many melanocytic markers is often down-regulated in metastatic melanoma, Mitf expression is usually maintained and frequently found in primary and metastatic tumors (34, 40–42); our findings from assessment of metastatic melanoma tissues were consistent with previous reports. The dilution study of melanoma cells in PBLs of normal donors supported a determined cutoff point for Mitf positivity and the quantitative RT-PCR assay for Mitf was sensitive and reliable for detection of circulating tumor cells in blood of melanoma patients.
In the 90 patients with AJCC stage I–IV melanoma, Mitf detection in blood significantly increased according to disease stage. These findings suggested that circulating tumor cells are more frequently found in patients with AJCC stage III/IV (metastatic) diseases. The study suggests that Mitf expression in blood may be a prognostic marker for assessing potential systemic spread of melanoma. The low detection rate in AJCC stage I/II (localized disease) patients likely resulted from the intermittent or low level release of tumor cells into blood and may be related to the low metastatic potential in patients.
In the 58 patients from the biochemotherapy trial, a significant correlation between Mitf detection after overall treatment and lower survival indicated the clinical utility of Mitf as a predictive marker for disease progression and overall survival. The change in Mitf detection was clearly different between relapse-free and relapse groups, and both preoperative and postoperative biochemotherapy significantly reduced Mitf positivity only in relapse-free patients. These results also suggested the potential utility of Mitf detection as a surrogate marker of treatment outcome. After overall treatment, Mitf was detected in more than half of patients with relapse, consistent with the high incidence of disease recurrence associated with AJCC stage III melanoma. These findings indicate the potential value of Mitf detection as a monitoring tool that can detect systemic disease before clinical recurrence (4). Most studies of circulating tumor cells in melanoma patients have used specimens obtained at only one time point. Few studies have reported serial analysis of blood before, during, and after treatment. This is a novel study examining circulating tumor cell levels in a multimodality treatment protocol. Serial assessment can detect intermittent release of tumor cells (4) and assess the responses to different phases of treatment (12). This is essential to assess treatment response or disease progression during treatment. There was no correlation between Mitf detection and surgery in both relapse-free and relapse patients, suggesting that regional lymphadenectomy did not affect circulating tumor cells significantly in stage III melanoma patients. This also suggested that subclinical systemic disease had already been established earlier in the patients who developed relapse of disease. These findings suggest that there may be limited influence of regional nodal surgery for improving overall prognosis in all melanoma patients. This is a subject that is often debated and, presently, multicenter trials are attempting to address this issue.
Development of predictive markers to identify high-risk patients and to monitor the response to adjuvant therapy in patients who are clinically disease-free could contribute significantly to the advancement in the management of melanoma patients. As treatment regimens become multimodal and multiphasic, there will be an urgent need for clinically relevant surrogate markers for treatment response in specimens that can serially be obtained to evaluate different treatment phase. To date, assessment of predictive markers for neoadjuvant therapy of solid tumors has focused on primary and metastatic tumor specimens. However, these are static points that do not indicate whether tumor cells are being shed or whether a specific treatment is reducing subclinical metastasis and the potential for distant clinical metastasis. Serial assessment of molecular markers in blood can indicate the kinetics of tumor cell shedding in response to treatment. Our findings indicate that Mitf is a valuable single marker and may improve detection of circulating tumor cells in combination with other markers. We are currently investigating a panel of mRNA markers including Mitf using a quantitative RT-PCR that will validate the clinical utility of blood assessment as a predictive surrogate of outcome in a randomized multicenter study.
Acknowledgments
Grant support: NIH, National Cancer Institute P01 grants CA 29605 and CA 12528 (D. Hoon), Martin H. Weil Fund (D. Hoon), and Chiron Corporation research grant 05-977 (S. O’Day and R. Gonzalez).
References
- 1.Pantel K, Cote RJ, Fodstad O. Detection and clinical importance of micrometastatic disease. J Natl Cancer Inst. 1999;91:1113–24. doi: 10.1093/jnci/91.13.1113. [DOI] [PubMed] [Google Scholar]
- 2.Hoon DS, Bostick P, Kuo C, et al. Molecular markers in blood as surrogate prognostic indicators of melanoma recurrence. Cancer Res. 2000;60:2253–7. [PubMed] [Google Scholar]
- 3.Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–91. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 4.Voit C, Kron M, Rademaker J, et al. Molecular staging in stage II and III melanoma patients and its effect on long-term survival. J Clin Oncol. 2005;23:1218–27. doi: 10.1200/JCO.2005.04.098. [DOI] [PubMed] [Google Scholar]
- 5.Stathopoulou A, Gizi A, Perraki M, et al. Real-time quantification of CK-19 mRNA-positive cells in peripheral blood of breast cancer patients using the lightcycler system. Clin Cancer Res. 2003;9:5145–51. [PubMed] [Google Scholar]
- 6.Schuster R, Max N, Mann B, et al. Quantitative real-time RT-PCR for detection of disseminated tumor cells in peripheral blood of patients with colorectal cancer using different mRNA markers. Int J Cancer. 2004;108:219–27. doi: 10.1002/ijc.11547. [DOI] [PubMed] [Google Scholar]
- 7.Howe JG, Crouch J, Cooper D, Smith BR. Real-time quantitative reverse transcription-PCR for cyclin D1 mRNA in blood, marrow, and tissue specimens for diagnosis of mantle celllymphoma. Clin Chem. 2004;50:80–7. doi: 10.1373/clinchem.2003.024695. [DOI] [PubMed] [Google Scholar]
- 8.Keilholz U, Goldin-Lang P, Bechrakis NE, et al. Quantitative detection of circulating tumor cells in cutaneous and ocular melanoma and quality assessment by real-time reverse transcriptase-polymerase chain reaction. Clin Cancer Res. 2004;10:1605–12. doi: 10.1158/1078-0432.ccr-0610-3. [DOI] [PubMed] [Google Scholar]
- 9.Hoon DS, Wang Y, Dale PS, et al. Detection of occult melanoma cells in blood with a multiple-marker polymerase chain reaction assay. J Clin Oncol. 1995;13:2109–16. doi: 10.1200/JCO.1995.13.8.2109. [DOI] [PubMed] [Google Scholar]
- 10.Sarantou T, Chi DD, Garrison DA, et al. Melanoma-associated antigens as messenger RNA detection markers for melanoma. Cancer Res. 1997;57:1371–6. [PubMed] [Google Scholar]
- 11.Koyanagi K, Kuo C, Nakagawa T, et al. Multimarker quantitative real-time PCR detection of circulating melanoma cells in peripheral blood: relation to disease stage in melanoma patients. Clin Chem. 2005;51:981–8. doi: 10.1373/clinchem.2004.045096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koyanagi K, O’Day SJ, Gonzalez R, et al. Serial monitoring of circulating melanoma cells during neoadjuvant biochemotherapy for stage III melanoma: outcome prediction in a multicenter trial. J Clin Oncol. 2005;23:8057–64. doi: 10.1200/JCO.2005.02.0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poser I, Bosserhoff AK. Transcription factors involved in development and progression of malignant melanoma. Histol Histopathol. 2004;19:173–88. doi: 10.14670/HH-19.173. [DOI] [PubMed] [Google Scholar]
- 14.Vachtenheim J, Borovansky J. Microphthalmia transcription factor: a specific marker for malignant melanoma. Prague Med Rep. 2004;105:318–24. [PubMed] [Google Scholar]
- 15.Vance KW, Goding CR. The transcription network regulating melanocyte development and melanoma. Pigment Cell Res. 2004;17:318–25. doi: 10.1111/j.1600-0749.2004.00164.x. [DOI] [PubMed] [Google Scholar]
- 16.Selzer E, Wacheck V, Lucas T, et al. The melanocyte-specific isoform of the microphthalmia transcription factor affects the phenotype of human melanoma. Cancer Res. 2002;62:2098–103. [PubMed] [Google Scholar]
- 17.Hemesath TJ, Steingrimsson E, McGill G, et al. Microphthalmia, a critical factor in melanocyte development, defines a discrete transcription factor family. Genes Dev. 1994;8:2770–80. doi: 10.1101/gad.8.22.2770. [DOI] [PubMed] [Google Scholar]
- 18.McGill GG, Horstmann M, Widlund HR, et al. Bcl2 regulation by the melanocyte master regulator Mitf modulates lineage survival and melanoma cell viability. Cell. 2002;109:707–18. doi: 10.1016/s0092-8674(02)00762-6. [DOI] [PubMed] [Google Scholar]
- 19.Du J, Widlund HR, Horstmann MA, et al. Critical role of CDK2 for melanoma growth linked to its melanocyte-specific transcriptional regulation by MITF. Cancer Cell. 2004;6:565–76. doi: 10.1016/j.ccr.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 20.Carreira S, Goodall J, Aksan I, et al. Mitf cooperates with Rb1 and activates p21Cip1 expression to regulate cell cycle progression. Nature. 2005;433:764–9. doi: 10.1038/nature03269. [DOI] [PubMed] [Google Scholar]
- 21.Udono T, Yasumoto K, Takeda K, et al. Structural organization of the human microphthalmia-associated transcription factor gene containing four alternative promoters. Biochim Biophys Acta. 2000;1491:205–19. doi: 10.1016/s0167-4781(00)00051-8. [DOI] [PubMed] [Google Scholar]
- 22.Yasumoto K, Yokoyama K, Shibata K, Tomita Y, Shibahara S. Microphthalmia-associated transcription factor as a regulator for melanocyte-specific transcription of the human tyrosinase gene. Mol Cell Biol. 1994;14:8058–70. doi: 10.1128/mcb.14.12.8058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bentley NJ, Eisen T, Goding CR. Melanocyte-specific expression of the human tyrosinase promoter: activation by the microphthalmia gene product and role of the initiator. Mol Cell Biol. 1994;14:7996–8006. doi: 10.1128/mcb.14.12.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tassabehji M, Newton VE, Read AP. Waardenburg syndrome type 2 caused by mutations in the human microphthalmia (MITF) gene. Nat Genet. 1994;8:251–5. doi: 10.1038/ng1194-251. [DOI] [PubMed] [Google Scholar]
- 25.Hughes AE, Newton VE, Liu XZ, Read AP. A gene for Waardenburg syndrome type 2 maps close to the human homologue of the microphthalmia gene at chromosome 3p12–14.1. Nat Genet. 1994;7:509–12. doi: 10.1038/ng0894-509. [DOI] [PubMed] [Google Scholar]
- 26.Dorsky RI, Moon RT, Raible DW. Control of neural crest cell fate by the Wnt signalling pathway. Nature. 1998;396:370–3. doi: 10.1038/24620. [DOI] [PubMed] [Google Scholar]
- 27.Takeda K, Yasumoto K, Takada R, et al. Induction of melanocyte-specific microphthalmia-associated transcription factor by Wnt-3a. J Biol Chem. 2000;275:14013–6. doi: 10.1074/jbc.c000113200. [DOI] [PubMed] [Google Scholar]
- 28.Bertolotto C, Abbe P, Hemesath TJ, et al. Microphthalmia gene product as a signal transducer in cAMP-induced differentiation of melanocytes. J Cell Biol. 1998;142:827–35. doi: 10.1083/jcb.142.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Price ER, Horstmann MA, Wells AG, et al. α-Melanocyte-stimulating hormone signaling regulates expression of microphthalmia, a gene deficient in Waardenburg syndrome. J Biol Chem. 1998;273:33042–7. doi: 10.1074/jbc.273.49.33042. [DOI] [PubMed] [Google Scholar]
- 30.Hemesath TJ, Price ER, Takemoto C, Badalian T, Fisher DE. MAP kinase links the transcription factor microphthalmia to c-Kit signalling in melanocytes. Nature. 1998;391:298–301. doi: 10.1038/34681. [DOI] [PubMed] [Google Scholar]
- 31.Wu M, Hemesath TJ, Takemoto CM, et al. c-Kit triggers dual phosphorylations, which couple activation and degradation of the essential melanocyte factor Mi. Genes Dev. 2000;14:301–12. [PMC free article] [PubMed] [Google Scholar]
- 32.Bondurand N, Pingault V, Goerich DE, et al. Interaction among SOX10, PAX3 and MITF, three genes altered in Waardenburg syndrome. Hum Mol Genet. 2000;9:1907–17. doi: 10.1093/hmg/9.13.1907. [DOI] [PubMed] [Google Scholar]
- 33.Potterf SB, Furumura M, Dunn KJ, Arnheiter H, Pavan WJ. Transcription factor hierarchy in Waardenburg syndrome: regulation of MITF expression by SOX10 and PAX3. Hum Genet. 2000;107:1–6. doi: 10.1007/s004390000328. [DOI] [PubMed] [Google Scholar]
- 34.King R, Weilbaecher KN, McGill G, Cooley E, Mihm M, Fisher DE. Microphthalmia transcription factor. A sensitive and specific melanocyte marker for Melanoma Diagnosis. Am J Pathol. 1999;155:731–8. doi: 10.1016/S0002-9440(10)65172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salti GI, Manougian T, Farolan M, Shilkaitis A, Majumdar D, Das Gupta TK. Micropthalmia transcription factor: a new prognostic marker in intermediate-thickness cutaneous malignant melanoma. Cancer Res. 2000;60:5012–6. [PubMed] [Google Scholar]
- 36.Miyashiro I, Kuo C, Huynh K, et al. Molecular strategy for detecting metastatic cancers with use of multiple tumor-specific MAGE-A genes. Clin Chem. 2001;47:505–12. [PubMed] [Google Scholar]
- 37.Takeuchi H, Kuo C, Morton DL, Wang HJ, Hoon DS. Expression of differentiation melanoma-associated antigen genes is associated with favorable disease outcome in advanced-stage melanomas. Cancer Res. 2003;63:441–8. [PubMed] [Google Scholar]
- 38.Samija I, Lukac J, Maric-Brozic J, Kusic Z. Microphthalmia-associated transcription factor and tyrosinase as markers of melanoma cells in blood of patients with melanoma. Croat Med J. 2004;45:142–8. [PubMed] [Google Scholar]
- 39.Granter SR, Weilbaecher KN, Quigley C, Fletcher CD, Fisher DE. Microphthalmia transcription factor: not a sensitive or specific marker for the diagnosis of desmoplastic melanoma and spindle cell (non-desmoplastic) melanoma. Am J Dermatopathol. 2001;23:185–9. doi: 10.1097/00000372-200106000-00004. [DOI] [PubMed] [Google Scholar]
- 40.King R, Googe PB, Weilbaecher KN, Mihm MC, Jr, Fisher DE. Microphthalmia transcription factor expression in cutaneous benign, malignant melanocytic, and nonmelanocytic tumors. Am J Surg Pathol. 2001;25:51–7. doi: 10.1097/00000478-200101000-00005. [DOI] [PubMed] [Google Scholar]
- 41.Miettinen M, Fernandez M, Franssila K, Gatalica Z, Lasota J, Sarlomo-Rikala M. Microphthalmia transcription factor in the immunohistochemical diagnosis of metastatic melanoma: comparison with four other melanoma markers. Am J Surg Pathol. 2001;25:205–11. doi: 10.1097/00000478-200102000-00008. [DOI] [PubMed] [Google Scholar]
- 42.Miller AJ, Du J, Rowan S, Hershey CL, Widlund HR, Fisher DE. Transcriptional regulation of the melanoma prognostic marker melastatin (TRPM1) by MITF in melanocytes and melanoma. Cancer Res. 2004;64:509–16. doi: 10.1158/0008-5472.can-03-2440. [DOI] [PubMed] [Google Scholar]