Abstract
Formation of the human brain during embryonic and postnatal development is an extraordinarily complex process resulting at maturity in billions of neurons with trillions of specialized connections called synapses. These synapses, composed of a varicosity or bouton from a presynaptic neuron that communicates with a dendritic spine of the postsynaptic neuron, comprise the neural network that is essential for complex behavioral phenomena and cognition. Inappropriate synapse formation or structure is thought to underlie several developmental neuropathologies. Even in the mature CNS, alterations in synapse structure and function continues to be a very dynamic process that is foundational to learning and memory as well as other adaptive abilities of the brain. This synaptic plasticity in mature neurons, which is often triggered by certain patterns of neural activity, is again multifaceted and involves post-translational modifications (e.g. phosphorylation) and subcellular relocalization or trafficking (endocytosis/exocytosis) of existing synaptic proteins, initiation of protein synthesis from existing mRNAs localized in dendrites or spines, and triggering of new gene transcription in the nucleus. These various cellular processes support varying temporal components of synaptic plasticity that begin within 1–2 min but can persist for hours to days. This review will give a critical assessment of activity-dependent molecular modulations of synapses reported over the past couple years. Owing to space limitations, it will focus on mammalian excitatory (i.e. glutamatergic) synapses and will not consider several activity-independent signaling pathways (e.g. ephrinB receptor) that also modulate spine and synapse formation [1,2].
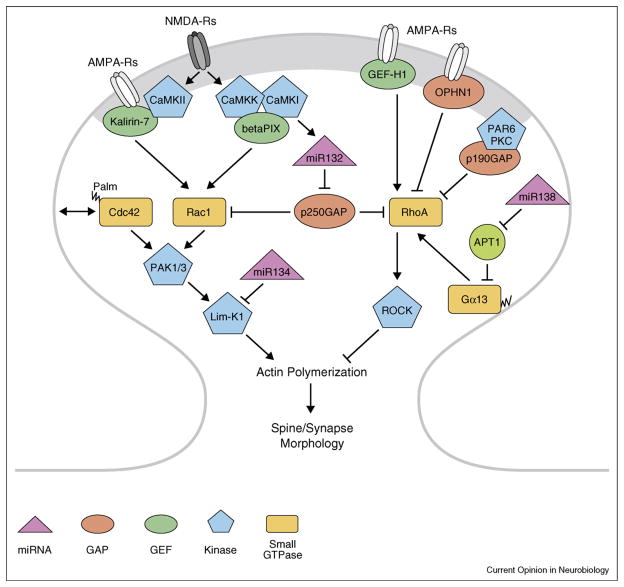
Regulation of spine and synapse formation by small GTPases (see Figure 1)
Figure 1.
Schematic outline of small GTPase regulation of spine morphology via remodeling of the actin cytoskeleton. See text for details. Palm, palmitoylation; miRNA, microRNA.
Mature mushroom-shaped spines are unique micro-compartments that autonomously regulate the electrical and biochemical responses to synaptic activity. It is widely accepted that spine morphology and synapse function, via anchoring of key PSD proteins, is modulated by the actin cytoskeleton that is regulated largely by small GTPases (reviewed in [3]). The family of small GTPases (RhoA, Rac1, and Cdc42) cycle between an active GTP-bound form, promoted by guanine nucleotide exchange factors (GEFs), and an inactive GDP-bound form generated by GTPase-activating proteins (GAPs) that hydrolyze the GTP. GEFs and GAPs are major convergence points of upstream signaling pathways triggered by neuronal activity and growth factors that are often mediated by protein kinases. Major downstream effectors of these small GTPases that regulate the actin cytoskeleton include the p21-activated kinases PAK1 and PAK 3.
Rac-1
Developing neurons exhibit spontaneous calcium spikes triggered by neurotransmitter-evoked activity [4] or growth factors such as brain-derived neurotrophic factor (BDNF) [5]. In mature neurons, Ca2+ entry through NMDA-type glutamate receptors (NMDARs) is crucial for synaptic plasticity. Elevated Ca2+ levels activate calmodulin (CaM) sensitive molecules including CaM-dependent protein kinases (CaMKs) [6]. CaMKs have been implicated in spine structural and synaptic plasticity, but the mechanisms were largely unknown until recently. CaMKII phosphorylates the RacGEF Kalirin-7 at Thr95, and they form a signaling complex with PSD95 and AMPA-type glutamate receptors (AMPARs) [7•], the major transducer of rapid excitatory synaptic transmission in the mammalian CNS. In cultured neurons the general CaMK inhibitor, KN-62, suppressed NMDA-stimulated GEF activity of Kalirin-7, trafficking of AMPARs and spine maturation in cortical neurons. Mutant mice lacking Kalirin-7 show decreased spine density in CA1 pyramidal neurons, deficits in long-term potentiation (LTP), and impaired contextual fear learning [8], supporting the significance of Karilin-7 in synaptic function in vivo. The RacGEF Tiam1 that associates with the NMDAR is also regulated by CaMKII, and dominant-negatives or RNAi inhibition of Tiam1 causes loss of NMDAR-dependent spine formation [9].
Since KN-62 and KN-93 are general CaMK inhibitors, physiological roles previously ascribed to CaMKII based on inhibitory effects of these reagents have recently been re-evaluated. Indeed, other members of the CaMK family, CaMKI and its upstream activator CaMKK, have been demonstrated to play crucial roles in multiple aspects of neuronal development including axon formation [10] and elongation [11], and activity-dependent dendritic arborization [12] and spine/synapse formation [13•]. Enhanced dendritogenesis is mediated via CaMKI-mediated activation of MEK/Erk [14] with resultant CREB-dependent transcription of Wnt-2 [12], which enhances dendrite formation, and microRNA132 levels. MicroRNA132 suppresses translation of p250GAP, resulting in Rac1 activation [15•,16] (see below) and/or inhibition of Rho A [17]. CaMKK/CaMKI form a signaling complex with the GEF betaPIX and its scaffold protein GIT1 that also binds PAK1 [13•]. Activated CaMKI phosphorylates betaPIX at Ser516 and stimulates its Rac-GEF activity. Most of the above studies on CaMKI were performed in cultured hippocampal neurons but have also been replicated in cultured hippocampal slices. Different isoforms of CaMKI appear to specifically mediate these developmental effects as determined by RNAi knockdown.
RhoA
In contrast to Rac and Cdc42, RhoA inhibits spine formation and maturation [18] and should be inactivated during synaptogenesis. It has been reported that the RhoA pathway is regulated through the polarity complex PAR6/atypicalPKC [19]. Overexpression of PAR6, but not the PAR6 mutants lacking binding sites for PKC and PDZ, promotes spine density whereas RNAi suppression of PAR6 reduces spine density and increases RhoA activity. This spine phenotype is rescued by pharmacological inhibition of the RhoA effector kinase, ROCK. One of the RhoA GAPs, P190GAP, is a possible link between PAR6/aPKC and RhoA activity. Indeed, P190GAP co-immunoprecipitates with the PAR6/aPKC complex, and PAR6-induced RhoA inactivation is blocked by P190GAP knockdown. However, the molecular details of how PAR6/aPKC regulate P190GAP remain to be clarified.
Trafficking and activity of AMPARs, which are crucial for maintenance of spines and synaptic plasticity (reviewed in [20,21]), are dynamic processes. What effectors modulate AMPAR actions in spine morphology? The RhoA-specific GEF, GEF-H1, is an AMPAR-interacting protein [22]. A dominant-negative construct and RNAi-mediated knockdown of GEF-H1 increases spine density and length, perhaps due to the ability of RhoA to inhibit Rac1 [23]. Pharmacological inhibition of AMPARs activates RhoA, inactivates Rac1, and decreases spine density—these changes are eliminated by GEF-H1 knockdown. These data suggest that GEF-H1 is a key molecule linking AMPAR signaling and spine morphology. Although GEF-H1 translocates into dendritic spines in response to glutamate in a Ca2+-dependent manner [24], the regulations of GEF-H1-mediated RhoA activation/Rac1 inactivation in response to neuronal activity in spines warrants further investigation. The small GTPases Ras and Rap also appear to govern the synaptic insertion and removal of AMPARs, respectively. The trafficking of AMPARs via Ras is dependent upon its level of activation—low levels of Ras activity lead to incorporation of GluR2 via the MEK-ERK pathway whereas high levels of Ras activity induce GluR1 incorporation via PI3 kinase-PKB/AKT pathway [25]. It should be noted that active Ras is not limited to individual spines but can also spread to adjacent spines where it is thought to reduce the threshold of activation for LTP [26].
The Rho-GTPase signaling pathways have been linked to several forms of mental retardation [27]. A recent study provides a mechanism by which a genetic deficit in the Rho-GAP, oligophrenin-1 (OPHN1) causes impaired synaptic development and glutamatergic dysfunction [28•]. Spontaneous neuronal activity is required for OPHN1 localization in spines where OPHN1 couples to AMPARs, and in developing neurons knockdown of OPHN1 reduces mEPSC frequency. NMDAR-induced endocytosis of AMPAR is suppressed by overexpression of OPHN1 or inhibition of the Rho/ROCK pathway with a ROCK inhibitor, indicating that OPHN1 signaling controls synaptic functions by regulating AMPAR stabilization in the synapse through suppressing the RhoA pathway.
Cdc42
Although multiple functions for RhoA and Rac in regulation of synaptogenesis have been described, roles of Cdc42 are less characterized. For example, expression of constitutively active Cdc42 (V12 mutant) does not appear to affect spine morphology or density [27]. However, a recent study identified Cdc42 as a synaptic palmitoylated protein that is essential for synaptogenesis [29•]. A brain-specific splice variant of Cdc42 is palmitoylated, and glutamate stimulation of cultured neurons causes a rapid depalmitoylation of Cdc42 within 5 min, leading to loss of Cdc42 from dendritic spines. Mechanisms by which neuronal activity induces depalmitoylation of Cdc42 and the role of this pathway in synapse function need further exploration.
MicroRNAs modulate spine formation and morphology
It is well established that localized protein synthesis, often initiated by activity-dependent regulation of translation factors, from selected mRNAs that are transported into dendrites and spines are important in modulating synaptic plasticity [30]. Recently, another mode of activity-modulated translational regulation in neurons via microRNAs (miRs) has been identified. MiRs are non-coding transcripts of approximately 19–24 nucleotides that regulate protein synthesis, either by destabilizing specific mRNAs or suppressing their translation [31]. MiRs have recently been implicated in several neuronal functions including apoptosis, neural patterning, and development of axons and dendrites [32]. Several miRs, together with their processing enzymes, such as Dicer, have been localized in dendritic spines, suggesting they may also play a role in spine/synapse formation and function. Dicer is present in the PSD in an inactive form where its RNAase III activity can be triggered in a Ca2+-dependent manner via calpain cleavage, thereby converting pre-miRs into mature miRs [33]. Transgenic mice lacking forebrain Dicer in neurons lack several miRs, have a 50% decrease in cortical mass due to enhanced apoptosis in early development, and exhibit abnormal hippocampal patterning, decreased dendritic arborization and increased spine length in apical dendrites [34].
Studies in cultured neurons have identified some specific miRs and elucidated their targets that modulate dendrite and spine morphology (Figure 1). A miR that enhances dendritic development in cortical and hippocampal neurons is miR132 [15•,16]. Enhanced neuronal activity via the NMDA receptor and Ca2+-dependent activation of MEK/Erk by CaMKK/CaMKI stimulates CREB-dependent transcription of miR132 to suppress translation of the Rho family GTPase-activating protein p250GAP. Since p250GAP inhibits Rac1, activity-dependent decreases in p250GAP promote dendritic arborization, presumably by enhancing actin polymerization. However, it has been reported that RNAi suppression of p250GAP also activates RhoA and enhances spine size—this phenotype is rescued by dominant-negative RhoA [17]. Since RhoA is normally inhibitory to actin polymerization, the mechanism responsible for spine enlargement needs further clarification. Another miR, miR134, inhibits translation of LIM-kinase 1 that regulates the actin cytoskeleton [35]. Thus, basal miR134 tonically suppresses the size of spines, but this is reversed upon synaptic stimulation through release of BDNF that stimulates synthesis of LIM-kinase 1, perhaps through the mTOR pathway. Mechanisms by which BDNF inactivate the miR134 complex remain to be elucidated. A miR that regulates spine morphology without effect on spine density or dendritic arborization is miR138 [36]. MiR138 decreases spine volume through local suppression of acyl protein thioesterase 1 (APT1). APT1 catalyzes depalmitoylation of proteins, thereby modulating their membrane association. One of the relevant targets of APT1 is the small G protein subunit Gα13 whose membrane association is involved in Rho-dependent signaling.
It is clear that neurons contain numerous miRs, and an increasing number of targets are being identified [37]. Regulation of neuronal development as well as plasticity in mature neurons by miRs and their roles in neurode-generative diseases is an emerging topic that promises to yield rich dividends.
LTP induces spine expansion and AMPAR trafficking
Several forms of synaptic plasticity result in morphological alterations of synapses, both pre- and postsynaptically. Mechanisms regulating trafficking of AMARs during homeostatic synaptic scaling have recently been reviewed [38] and won’t be dealt with here. It is known that LTP-inducing stimuli result in an initial robust and transient expansion of dendritic spines followed by a smaller but sustained increase in spine volume [39]. To date, few studies have examined the molecular mechanisms that underlie LTP-associated spine growth. However, several groups have now shown that pharmacological inhibition of CaMKs blocks the persistent but not the initial spine expansion associated with LTP [40,41••]. Additionally, the persistent increase in spine volume following LTP appears to require the kinase activity of CaMKIIα [42]. A likely target of CaMKIIα, Ser73 of PSD-95, a major synaptic protein, may regulate the termination of activity-dependent spine expansion [41••]. In hippo-campal slice cultures, phosphorylation of PSD95 at Ser73 by CaMKII triggers the displacement of PSD95 and SHANK2 proteins from previously activated spines. SHANK proteins are thought to act as PSD scaffold proteins, linking many PSD proteins such as PSD95, GKAP, and Homer to both ionotropic and metabotrobic glutamate receptors [43]. Overexpression of SHANK leads to enlarged spines [44], so translocation of this scaffold out of the spine may terminate spine growth. Indeed, expression of a phosphomimick mutant of PSD95(S73D) inhibits the increases in both synaptic strength and spine volume associated with LTP, suggesting that phosphorylation of PSD95S73 may contribute to activity-dependent changes in spine growth and synaptic strength [41••].
Recent data indicate that recycling endosomes bring AMPARs as well as the additional membrane necessary for activity-dependent spine growth and remodeling into spines during LTP [45,46]. It should be noted that a mobile pool of AMPARs has been shown to reside at sites adjacent to the PSD and probably play a contributing role in supplying AMPARs to the synapse via means of lateral diffusion [47,48]. Two groups examining the exocytic delivery of AMPARs to the membrane have independently implicated Rab 11 in this process [49,50•]. Rab proteins are crucial regulators of the endosomal membrane system and control membrane trafficking within the exocytic, endocytic and recycling pathways [51]. Using independent techniques, both groups demonstrate that trafficking of GluR1 into spines during LTP requires association with Rab11 containing vesicles. Work by Esteban’s group has also indicated a role for Rab8 in the synaptic delivery of GluR1-containing AMPARs during LTP [52]. Not only are numbers of AMPARs modulated by synaptic activity, but their subunit composition and therefore biophysical properties may also be regulated. The AMPARs at the CA3/CA1 synapse in hippocampus is normally comprised of GluR1/GluR2 subunits, but certain LTP paradigms may promote synaptic incorporation of GluR2-lacking AMPARs ([53,54] but see [55,56]). This possibility is of particular interest as GluR2-lacking AMPARs have higher unity conductances, are permeable to Ca2+ and have been implicated in several neuropathologies (reviewed in [57,58]). Synaptic insertion of GluR1, in addition to increasing synaptic strength, may provide a stable platform to promote spine expansion [59].
Although there is a consensus for the role of Rab proteins in endosome-mediated trafficking of AMPARs during LTP, it is not clear which actin-based myosin motors contribute to the trafficking of these vesicles. Candidate motors include myosin Va [49], Vb [50•] and myosin VI [60]. In the first report, GST-pull downs demonstrated that Rab11 and GluR1, but not a truncated form of GluR1 lacking the last 30 amino acids, binds to the globular tail of Myosin Va. Furthermore, using dominant-negative constructs they show that inhibition of Myosin Va, but not Vb or VI significantly reduces AMPAR-mediated evoked responses, and Myosin Va RNAi impairs pairing-induced LTP. In an independent report, vesicular trafficking of metalloproteinase 9, which has been shown to contribute to persistent spine expansion and synaptic potentiation following LTP [61], into spines was also found to be associated with Myosin Va [62]. It should be noted, however, that synaptic transmission and LTP are not altered in mice that express a functional null mutation in the myosin Va gene [63].
By contrast, Wang et al. [50•] show that endogenous myosin Vb may be the relevant motor protein induced by LTP. Using live-cell imaging, these authors demonstrate that a population of myosin Vb colocalizes with recycling endosomes following NMDAR activation. Binding of MyoVb to Rab11-FIP2 was also shown to be Ca2+ dependent, providing a mechanistic link between NMDAR activation and association of myosin Vb to Rab11-FIP2. Furthermore, acute inhibition of MyoVb by a nonhydrolyzable ADP analog that suppresses the mobility of a MyoVb mutant along F actin, impaired LTP.
If indeed myosin V motors are involved in mediating the enduring synaptic and structural changes associated with LTP, it will be important to learn the mechanism(s) by which these motors are activated. Both groups assume that the trigger leading to myosin activity is increased intracellular Ca2+. While this assumption is in line with the necessity for calcium in LTP, work in the myosin field has shown that Ca2+ binding to the calmodulin light chains actually impairs myosin motility [64]. Therefore, how could calcium influx, necessary for LTP, also be triggering myosin-dependent vesicular movement? According to Sellers et al., myosin motility is most probably being regulated by cargo receptor proteins, where cargo binding to the globular tail domain increases its enzymatic as well as mechanical activity through steric or allosteric regulation [65]. Which cargo-receptor proteins and whether disruption of these proteins interferes with LTP will be of future interest.
LTD and morphological plasticity of spines?
The accepted model to date has been that bidirectional alterations of synaptic strength occur in parallel with corresponding changes in spine geometry. This concept is supported by studies on LTP (see above), and previous studies indicated that LTD is accompanied by a shrinkage in dendritic spines [66,67]. Whether changes in structural plasticity are necessary to adjust synaptic weights or vice versa, however, still remains an open question. Recently, two independent groups challenged this model by testing whether physiological and morphological changes associated with LTD are linked to either a common signaling mechanism or physical process. The first report [68••], using combined live-cell electrophysiology and two-photon microscopy, determined that LTD at identified parallel fiber–Purkinje cell synapses was not associated with structural changes in dendritic spines. These data were not simply the result of an inability to detect changes in spine volume since these same authors were able to detect reductions in spine size associated with depolarization-induced spine retraction. It should be noted, however, that the later manipulation failed to induce changes in evoked synaptic responses, further illustrating a clear disconnect between LTD and spine morphological plasticity.
A separate study [69] using acute hippocampal slices found that when neurons were internally loaded with a phospho-cofilin peptide, to prevent actin depolymerization, LTD was no longer associated with a reduction in spine size, while synaptic depression was still observed. Additionally, application of insulin, which has been shown to result in synaptic rundown and rapid clatherin-mediated internalization of synaptic AMPARs failed to elicit changes in spine volume despite reducing synaptic responses. Alternatively, inhibiting endocytosis with D15 peptide, to disrupt interaction of dynamin with amphiphysin, enhanced synaptic responses but without effects on spine size. Therefore, the lack of association found between LTD and spine morphology as seen in these studies suggests that reductions in synaptic strength may not necessarily correlate with changes in spine morphology as previously thought.
There is now growing evidence that morphological plasticity associated with LTD may be more prominent in presynapitc boutons. Using combined two-photon time lapse microscopy and electrophysiology to monitor labeled presynaptic boutons in hippocampal slice cultures, LTD was found to induce a significant reduction in the size of presynaptic boutons [70•,71]. Therefore, LTD induction can elicit structural remodifications on both sides of the synapse leading to a separation between post and presynaptic contacts. In addition, Becker et al. [71] observed that a major contribution to the loss of synaptic contacts was due to the loss of the partnered presynaptic bouton. Furthermore, they found that while the loss of dendritic spines led to reductions in the size of paired boutons, spine volumes did not change following the gain or loss of partnered boutons. What is the relationship between the loss of synaptic contacts and LTD? Bastrikova et al. [70•] found that the greater the magnitude of synaptic depression they observed following LTD, the greater the reduction in synaptic contacts. These findings suggest that under certain conditions activity-dependent structural changes associated with LTD may predominate in the presynaptic bouton. Whether LTD expression is predominantly due to post-synaptic or presynaptic mechanisms appears to depend on several factors such as brain area, mode of induction, and genetic background (e.g. state of FMR1 gene; reviewed in [72,73]). Future mechanistic studies regarding the signaling involved in determining how an existing synaptic connection is eliminated will be needed. Furthermore, it will be important to understand the contributions or circumstances leading to presynaptic terminal withdrawl, reduced spine size, and AMPAR properties (i.e. internalization, phosphorylation) as a result of LTD stimuli.
Future directions
It is clear that signaling pathways that regulate the actin cytoskeleton via the small GTPases are major players in dictating spine morphology. In fact, several neuropathologies are associated with mutations in these proteins that lead to abnormal spine/synapse maturation. As described in this review, these signaling pathways act on multiple GEFs and GAPs to fine-tune the balance between opposing roles of Rac1 and RhoA. Furthermore, miRs have recently been found to be important regulators of these GTPases. As more cellular targets of various miRs become identified, their roles in spine/synapse maturation will expand. Although numerous regulators of RhoA and Rac1 have emerged, the molecular details of how these regulatory proteins act in concert to promote normal spine/synapse maturation is still lacking. Application of new technologies in live-cell imaging of signaling molecules should further define these intricate cross-talk mechanisms.
Activity-dependent changes in spine/synaptic structure continue to be an area of intense research given that perturbations in signaling molecules associated with activity-dependent structural plasticity lead to cognitive deficits. While the molecular mechanisms contributing to activity-dependent spine expansion/retraction are now starting to emerge, they have also lead to more questions. For example, recent data have implicated a role for myosin-based motors in the establishment of LTP; however, the mechanisms linking LTP and myosin mobility are unclear. Since Ca2+ does not appear to directly enhance myosin mobility, it will be interesting to determine whether any of the cargo-receptor proteins, which most probably effect myosin mobility, are regulated by shifts in intracellular Ca2+. Finally, the association between activity-dependent synaptic plasticity and spine morphology appears to be more complex than previously thought. While LTP remains associated spine enlargement and AMPAR recruitment, structural modification of spines as a result of LTD-inducing stimuli is more complex. Current research has now shown that some forms of synaptic depression are not associated with alterations in spine size, questioning whether postsynaptic morphological changes are necessary for LTD. Future studies examining modulation of presynaptic structures will probably reveal novel mechanisms associated with activity-dependent synaptic pruning.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Essmann CL, Martinez E, Geiger JC, Zimmer M, Traut MH, Stein V, Klein R, Acker-Palmer A. Serine phosphorylation of ephrinB2 regulates trafficking of synaptic AMPA receptors. Nat Neurosci. 2008;11:1035–1043. doi: 10.1038/nn.2171. [DOI] [PubMed] [Google Scholar]
- 2.Tolias KF, Bikoff JB, Kane CG, Tolias CS, Hu L, Greenberg ME. The Rac1 guanine nucleotide exchange factor Tiam1 mediates EphB receptor-dependent dendritic spine development. Proc Natl Acad Sci USA. 2007;104:7265–7270. doi: 10.1073/pnas.0702044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cingolani LA, Goda Y. Actin in action: the interplay between the actin cytoskeleton and synaptic efficacy. Nat Rev Neurosci. 2008;9:344–356. doi: 10.1038/nrn2373. [DOI] [PubMed] [Google Scholar]
- 4.Spitzer NC. Electrical activity in early neuronal development. Nature. 2006;444:707–712. doi: 10.1038/nature05300. [DOI] [PubMed] [Google Scholar]
- 5.Lang SB, Stein V, Bonhoeffer T, Lohmann C. Endogenous brain-derived neurotrophic factor triggers fast calcium transients at synapses in developing dendrites. J Neurosci. 2007;27:1097–1105. doi: 10.1523/JNEUROSCI.3590-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wayman GA, Lee YS, Tokumitsu H, Silva A, Soderling TR. Calmodulin-kinases: modulators of neuronal development and plasticity. Neuron. 2008;59:914–931. doi: 10.1016/j.neuron.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7•.Xie Z, Srivastava DP, Photowala H, Kai L, Cahill ME, Woolfrey KM, Shum CY, Surmeier DJ, Penzes P. Kalirin-7 controls activity-dependent structural and functional plasticity of dendritic spines. Neuron. 2007;56:640–656. doi: 10.1016/j.neuron.2007.10.005. The authors identify a signaling complex of CaMKII, Kalirin-7, PSD95 and AMPARs in neurons. CaMKII can phosphorylate kalirin-7 and appears to regulate its GEF activity, spine morphology and AMPAR trafficking. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma XM, Kiraly DD, Gaier ED, Wang Y, Kim EJ, Levine ES, Eipper BA, Mains RE. Kalirin-7 is required for synaptic structure and function. J Neurosci. 2008;28:12368–12382. doi: 10.1523/JNEUROSCI.4269-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tolias KF, Bikoff JB, Burette A, Paradis S, Harrar D, Tavazoie S, Weinberg RJ, Greenberg ME. The Rac1-GEF Tiam1 couples the NMDA receptor to the activity-dependent development of dendritic arbors and spines. Neuron. 2005;45:525–538. doi: 10.1016/j.neuron.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 10.Davare MA, Fortin DA, Saneyoshi T, Nygaard S, Kaech S, Banker G, Soderling TR, Wayman GA. Transient receptor potential canonical 5 channels activate Ca2+/calmodulin kinase Igamma to promote axon formation in hippocampal neurons. J Neurosci. 2009;29:9794–9808. doi: 10.1523/JNEUROSCI.1544-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wayman GA, Kaech S, Grant WF, Davare M, Impey S, Tokumitsu H, Nozaki N, Banker G, Soderling TR. Regulation of axonal extension and growth cone motility by calmodulin-dependent protein kinase I. J Neurosci. 2004;24:3786–3794. doi: 10.1523/JNEUROSCI.3294-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wayman GA, Impey S, Marks D, Saneyoshi T, Grant WF, Derkach V, Soderling TR. Activity-dependent dendritic arborization mediated by CaM-kinase I activation and enhanced CREB-dependent transcription of Wnt-2. Neuron. 2006;50:897–909. doi: 10.1016/j.neuron.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 13•.Saneyoshi T, Wayman G, Fortin D, Davare M, Hoshi N, Nozaki N, Natsume T, Soderling TR. Activity-dependent synaptogenesis: regulation by a CaM- kinase kinase/CaM-kinase I/betaPIX signaling complex. Neuron. 2008;57:94–107. doi: 10.1016/j.neuron.2007.11.016. This study demonstrates that phosphorylation of the RacGEF betaPIX by CaMKK/CaMKI in a signaling complex with GIT1 regulates Rac1 and activity-dependent synaptogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmitt JM, Guire ES, Saneyoshi T, Soderling TR. Calmodulin-dependent kinase kinase/calmodulin kinase I activity gates extracellular-regulated kinase-dependent long-term potentiation. J Neurosci. 2005;25:1281–1290. doi: 10.1523/JNEUROSCI.4086-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15•.Vo N, Klein ME, Varlamova O, Keller DM, Yamamoto T, Goodman RH, Impey S. A cAMP-response element binding protein-induced microRNA regulates neuronal morphogenesis. Proc Natl Acad Sci USA. 2005;102:16426–16431. doi: 10.1073/pnas.0508448102. This was the first study to provide a mechanism by which microRNAs can regulate neuronal development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wayman GA, Davare M, Ando H, Fortin D, Varlamova O, Cheng HY, Marks D, Obrietan K, Soderling TR, Goodman RH, et al. An activity-regulated microRNA controls dendritic plasticity by down-regulating p250GAP. Proc Natl Acad Sci USA. 2008;105:9093–9098. doi: 10.1073/pnas.0803072105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakazawa T, Kuriu T, Tezuka T, Umemori H, Okabe S, Yamamoto T. Regulation of dendritic spine morphology by an NMDA receptor-associated Rho GTPase-activating protein, p250GAP. J Neurochem. 2008;105:1384–1393. doi: 10.1111/j.1471-4159.2008.05335.x. [DOI] [PubMed] [Google Scholar]
- 18.Govek EE, Newey SE, Van Aelst L. The role of the Rho GTPases in neuronal development. Genes Dev. 2005;19:1–49. doi: 10.1101/gad.1256405. [DOI] [PubMed] [Google Scholar]
- 19.Zhang H, Macara IG. The PAR-6 polarity protein regulates dendritic spine morphogenesis through p190 RhoGAP and the Rho GTPase. Dev Cell. 2008;14:216–226. doi: 10.1016/j.devcel.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Derkach VA, Oh MC, Guire ES, Soderling TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci. 2007;8:101–113. doi: 10.1038/nrn2055. [DOI] [PubMed] [Google Scholar]
- 21.Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- 22.Kang MG, Guo Y, Huganir RL. AMPA receptor and GEF-H1/Lfc complex regulates dendritic spine development through RhoA signaling cascade. Proc Natl Acad Sci USA. 2009;106:3549–3554. doi: 10.1073/pnas.0812861106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Z, Aizenman CD, Cline HT. Regulation of rho GTPases by crosstalk and neuronal activity in vivo. Neuron. 2002;33:741–750. doi: 10.1016/s0896-6273(02)00621-9. [DOI] [PubMed] [Google Scholar]
- 24.Ryan XP, Alldritt J, Svenningsson P, Allen PB, Wu GY, Nairn AC, Greengard P. The Rho-specific GEF Lfc interacts with neurabin and spinophilin to regulate dendritic spine morphology. Neuron. 2005;47:85–100. doi: 10.1016/j.neuron.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 25.Qin Y, Zhu Y, Baumgart JP, Stornetta RL, Seidenman K, Mack V, van Aelst L, Zhu JJ. State-dependent Ras signaling and AMPA receptor trafficking. Genes Dev. 2005;19:2000–2015. doi: 10.1101/gad.342205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harvey CD, Yasuda R, Zhong H, Svoboda K. The spread of Ras activity triggered by activation of a single dendritic spine. Science. 2008;321:136–140. doi: 10.1126/science.1159675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Govek EE, Newey SE, Akerman CJ, Cross JR, Van der Veken L, Van Aelst L. The X-linked mental retardation protein oligophrenin-1 is required for dendritic spine morphogenesis. Nat Neurosci. 2004;7:364–372. doi: 10.1038/nn1210. [DOI] [PubMed] [Google Scholar]
- 28•.Nadif Kasri N, Nakano-Kobayashi A, Malinow R, Li B, Van Aelst L. The Rho-Rho-linked mental retardation protein oligophrenin-1 controls synapse maturation and plasticity by stabilizing AMPA receptors. Genes Dev. 2009;23:1289–1302. doi: 10.1101/gad.1783809. This study is the first to provide a mechanism, Rho regulation of AMPAR dynamics, by which genetic deficits in oligophrenin-1 are linked to abnormal synaptic development and glutamatergic dysfunction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29•.Kang R, Wan J, Arstikaitis P, Takahashi H, Huang K, Bailey AO, Thompson JX, Roth AF, Drisdel RC, Mastro R, et al. Neural palmitoyl-proteomics reveals dynamic synaptic palmitoylation. Nature. 2008;456:904–909. doi: 10.1038/nature07605. The authors present a novel method to isolate neural palmitoylated proteins and describe the mechanism by which Cdc42 regulates synaptogenesis through palmitoyl modification. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Costa-Mattioli M, Sossin WS, Klann E, Sonenberg N. Translational control of long-lasting synaptic plasticity and memory. Neuron. 2009;61:10–26. doi: 10.1016/j.neuron.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klein ME, Impey S, Goodman RH. Role reversal: the regulation of neuronal gene expression by microRNAs. Curr Opin Neurobiol. 2005;15:507–513. doi: 10.1016/j.conb.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 32.Kosik KS. The neuronal microRNA system. Nat Rev Neurosci. 2006;7:911–920. doi: 10.1038/nrn2037. [DOI] [PubMed] [Google Scholar]
- 33.Smalheiser NR, Lugli G. microRNA regulation of synaptic plasticity. Neuromol Med. 2009 doi: 10.1007/s12017-009-8065-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davis TH, Cuellar TL, Koch SM, Barker AJ, Harfe BD, McManus MT, Ullian EM. Conditional loss of Dicer disrupts cellular and tissue morphogenesis in the cortex and hippocampus. J Neurosci. 2008;28:4322–4330. doi: 10.1523/JNEUROSCI.4815-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schratt GM, Tuebing F, Nigh EA, Kane CG, Sabatini ME, Kiebler M, Greenberg ME. A brain-specific microRNA regulates dendritic spine development. Nature. 2006;439:283–289. doi: 10.1038/nature04367. [DOI] [PubMed] [Google Scholar]
- 36.Siegel G, Obernosterer G, Fiore R, Oehmen M, Bicker S, Christensen M, Khudayberdiev S, Leuschner PF, Busch CJ, Kane C, et al. A functional screen implicates microRNA-138-dependent regulation of the depalmitoylation enzyme APT1 in dendritic spine morphogenesis. Nat Cell Biol. 2009;11:705–716. doi: 10.1038/ncb1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schratt G. Fine-tuning neural gene expression with microRNAs. Curr Opin Neurobiol. 2009;19:213–219. doi: 10.1016/j.conb.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 38.Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Segal M. Dendritic spines and long-term plasticity. Nat Rev Neurosci. 2005;6:277–284. doi: 10.1038/nrn1649. [DOI] [PubMed] [Google Scholar]
- 40.Lee SJ, Escobedo-Lozoya Y, Szatmari EM, Yasuda R. Activation of CaMKII in single dendritic spines during long-term potentiation. Nature. 2009;458:299–304. doi: 10.1038/nature07842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41••.Steiner P, Higley MJ, Xu W, Czervionke BL, Malenka RC, Sabatini BL. Destabilization of the postsynaptic density by PSD-95 serine 73 phosphorylation inhibits spine growth and synaptic plasticity. Neuron. 2008;60:788–802. doi: 10.1016/j.neuron.2008.10.014. Demonstrates CaMKII phosphorylation of PSD95 at S73 triggers the termination of spine expansion by displacing PSD95 and SHANK2 from previously activated spines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamagata Y, Kobayashi S, Umeda T, Inoue A, Sakagami H, Fukaya M, Watanabe M, Hatanaka N, Totsuka M, Yagi T, et al. Kinase-dead knock-in mouse reveals an essential role of kinase activity of Ca2+/calmodulin-dependent protein kinase IIalpha in dendritic spine enlargement, long-term potentiation, and learning. J Neurosci. 2009;29:7607–7618. doi: 10.1523/JNEUROSCI.0707-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hayashi MK, Tang C, Verpelli C, Narayanan R, Stearns MH, Xu RM, Li H, Sala C, Hayashi Y. The postsynaptic density proteins Homer and Shank form a polymeric network structure. Cell. 2009;137:159–171. doi: 10.1016/j.cell.2009.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sala C, Piech V, Wilson NR, Passafaro M, Liu G, Sheng M. Regulation of dendritic spine morphology and synaptic function by Shank and Homer. Neuron. 2001;31:115–130. doi: 10.1016/s0896-6273(01)00339-7. [DOI] [PubMed] [Google Scholar]
- 45.Park M, Salgado JM, Ostroff L, Helton TD, Robinson CG, Harris KM, Ehlers MD. Plasticity-induced growth of dendritic spines by exocytic trafficking from recycling endosomes. Neuron. 2006;52:817–830. doi: 10.1016/j.neuron.2006.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park M, Penick EC, Edwards JG, Kauer JA, Ehlers MD. Recycling endosomes supply AMPA receptors for LTP. Science. 2004;305:1972–1975. doi: 10.1126/science.1102026. [DOI] [PubMed] [Google Scholar]
- 47.Petrini EM, Lu J, Cognet L, Lounis B, Ehlers MD, Choquet D. Endocytic trafficking and recycling maintain a pool of mobile surface AMPA receptors required for synaptic potentiation. Neuron. 2009;63:92–105. doi: 10.1016/j.neuron.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heine M, Groc L, Frischknecht R, Beique JC, Lounis B, Rumbaugh G, Huganir RL, Cognet L, Choquet D. Surface mobility of postsynaptic AMPARs tunes synaptic transmission. Science. 2008;320:201–205. doi: 10.1126/science.1152089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Correia SS, Bassani S, Brown TC, Lise MF, Backos DS, El-Husseini A, Passafaro M, Esteban JA. Motor protein-dependent transport of AMPA receptors into spines during long-term potentiation. Nat Neurosci. 2008;11:457–466. doi: 10.1038/nn2063. [DOI] [PubMed] [Google Scholar]
- 50•.Wang Z, Edwards JG, Riley N, Provance DW, Jr, Karcher R, Li XD, Davison IG, Ikebe M, Mercer JA, Kauer JA, et al. Myosin Vb mobilizes recycling endosomes and AMPA receptors for postsynaptic plasticity. Cell. 2008;135:535–548. doi: 10.1016/j.cell.2008.09.057. Demonstrates that NMDA-dependent plasticity induces Myosin Vb association with recycling endosomes and subsequent trafficking of GluR1 into spines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gabe Lee MT, Mishra A, Lambright DG. Structuralmechanisms for regulation of membrane traffic by Rab GTPases. Traffic. 2009 doi: 10.1111/j.1600-0854.2009.00942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brown TC, Correia SS, Petrok CN, Esteban JA. Functional compartmentalization of endosomal trafficking for the synaptic delivery of AMPA receptors during long-term potentiation. J Neurosci. 2007;27:13311–13315. doi: 10.1523/JNEUROSCI.4258-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Plant K, Pelkey KA, Bortolotto ZA, Morita D, Terashima A, McBain CJ, Collingridge GL, Isaac JT. Transient incorporation of native GluR2-lacking AMPA receptors during hippocampal long-term potentiation. Nat Neurosci. 2006;9:602–604. doi: 10.1038/nn1678. [DOI] [PubMed] [Google Scholar]
- 54.Guire ES, Oh MC, Soderling TR, Derkach VA. Recruitment of calcium-permeable AMPA receptors during synaptic potentiation is regulated by CaM-kinase I. J Neurosci. 2008;28:6000–6009. doi: 10.1523/JNEUROSCI.0384-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Adesnik H, Nicoll RA. Conservation of glutamate receptor 2-containing AMPA receptors during long-term potentiation. J Neurosci. 2007;27:4598–4602. doi: 10.1523/JNEUROSCI.0325-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gray EE, Fink AE, Sarinana J, Vissel B, O’Dell TJ. Long-term potentiation in the hippocampal CA1 region does not require insertion and activation of GluR2-lacking AMPA receptors. J Neurophysiol. 2007;98:2488–2492. doi: 10.1152/jn.00473.2007. [DOI] [PubMed] [Google Scholar]
- 57.Kwak S, Weiss JH. Calcium-permeable AMPA channels in neurodegenerative disease and ischemia. Curr Opin Neurobiol. 2006;16:281–287. doi: 10.1016/j.conb.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 58.Liu SJ, Zukin RS. Ca2+-permeable AMPA receptors in synaptic plasticity and neuronal death. Trends Neurosci. 2007;30:126–134. doi: 10.1016/j.tins.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 59.Kopec CD, Real E, Kessels HW, Malinow R. GluR1 links structural and functional plasticity at excitatory synapses. J Neurosci. 2007;27:13706–13718. doi: 10.1523/JNEUROSCI.3503-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Osterweil E, Wells DG, Mooseker MS. A role for myosin VI in postsynaptic structure and glutamate receptor endocytosis. J Cell Biol. 2005;168:329–338. doi: 10.1083/jcb.200410091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang XB, Bozdagi O, Nikitczuk JS, Zhai ZW, Zhou Q, Huntley GW. Extracellular proteolysis by matrix metalloproteinase-9 drives dendritic spine enlargement and long-term potentiation coordinately. Proc Natl Acad Sci USA. 2008;105:19520–19525. doi: 10.1073/pnas.0807248105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sbai O, Ferhat L, Bernard A, Gueye Y, Ould-Yahoui A, Thiolloy S, Charrat E, Charton G, Tremblay E, Risso JJ, et al. Vesicular trafficking and secretion of matrix metalloproteinases-2, -9 and tissue inhibitor of metalloproteinases-1 in neuronal cells. Mol Cell Neurosci. 2008;39:549–568. doi: 10.1016/j.mcn.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 63.Schnell E, Nicoll RA. Hippocampal synaptic transmission and plasticity are preserved in myosin Va mutant mice. J Neurophysiol. 2001;85:1498–1501. doi: 10.1152/jn.2001.85.4.1498. [DOI] [PubMed] [Google Scholar]
- 64.Sellers JR, Thirumurugan K, Sakamoto T, Hammer JA, 3rd, Knight PJ. Calcium and cargoes as regulators of myosin 5a activity. Biochem Biophys Res Commun. 2008;369:176–181. doi: 10.1016/j.bbrc.2007.11.109. [DOI] [PubMed] [Google Scholar]
- 65.Wu X, Sakamoto T, Zhang F, Sellers JR, Hammer JA., 3rd In vitro reconstitution of a transport complex containing Rab27a, melanophilin and myosin Va. FEBS Lett. 2006;580:5863–5868. doi: 10.1016/j.febslet.2006.09.047. [DOI] [PubMed] [Google Scholar]
- 66.Nagerl UV, Eberhorn N, Cambridge SB, Bonhoeffer T. Bidirectional activity-dependent morphological plasticity in hippocampal neurons. Neuron. 2004;44:759–767. doi: 10.1016/j.neuron.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 67.Zhou Q, Homma KJ, Poo MM. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron. 2004;44:749–757. doi: 10.1016/j.neuron.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 68••.Sdrulla AD, Linden DJ. Double dissociation between long-term depression and dendritic spine morphology in cerebellar Purkinje cells. Nat Neurosci. 2007;10:546–548. doi: 10.1038/nn1889. Demonstrates that electrical or chemical LTD of identified parallel fiber–Purkinje cell synapses is not associated with structural changes in dendritic spines. [DOI] [PubMed] [Google Scholar]
- 69.Wang XB, Yang Y, Zhou Q. Independent expression of synaptic and morphological plasticity associated with long-term depression. J Neurosci. 2007;27:12419–12429. doi: 10.1523/JNEUROSCI.2015-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70•.Bastrikova N, Gardner GA, Reece JM, Jeromin A, Dudek SM. Synapse elimination accompanies functional plasticity in hippocampal neurons. Proc Natl Acad Sci USA. 2008;105:3123–3127. doi: 10.1073/pnas.0800027105. Used virally infected CA1 and CA3 neurons to visualize coupled pre- and postsynaptic structures and found that LTD induced presynaptic terminal withdrawal from coupled dendritic spines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Becker N, Wierenga CJ, Fonseca R, Bonhoeffer T, Nagerl UV. LTD induction causes morphological changes of presynaptic boutons and reduces their contacts with spines. Neuron. 2008;60:590–597. doi: 10.1016/j.neuron.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 72.Richter JD, Klann E. Making synaptic plasticity and memory last: mechanisms of translational regulation. Genes Dev. 2009;23:1–11. doi: 10.1101/gad.1735809. [DOI] [PubMed] [Google Scholar]
- 73.Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]