Abstract
Acyclovir suppressive therapy (400 mg twice daily) reduces herpes simplex virus type 2 (HSV-2) associated genital ulcer disease (GUD) and lesional HSV shedding. In an international trial of acyclovir for HSV-2 suppression to prevent HIV acquisition (HPTN 039), acyclovir had a smaller effect on the frequency of GUD as well as the frequency and quantity of lesional HSV DNA in African women and Peruvian men compared with men in the United States. The observed regional variation in the clinical and virologic efficacy of acyclovir for HSV suppression warrants further evaluation of determinants of responses to acyclovir.
Keywords: herpes simplex virus type 2, acyclovir, suppression, genital ulcer disease, viral shedding, genital herpes
Introduction
Herpes Simplex Virus 2 (HSV-2) is the most common etiology of genital ulcer disease (GUD) worldwide and has been associated with a two to four-fold increase in HIV acquisition [1]. It is hypothesized that increased HIV acquisition risk is conferred by breaches in genital epithelium as well as genital inflammation during HSV reactivation. Herpetic ulcerations compromise the integrity of genital epithelium and mucosa and recruit activated CD4+ and CD8+ T lymphocytes and dendritic cells that may facilitate HIV attachment and infection during sexual intercourse [2].
We recently completed a randomized placebo controlled trial (HPTN 039) to evaluate whether 400 mg twice daily acyclovir could reduce HIV acquisition among 3172 HSV-2 seropositive men who have sex with men (MSM) in the US and Peru and women in sub-Saharan Africa by suppressing HSV [3]. This trial failed to demonstrate a protective effect of this regimen on HIV incidence, confirming results from another trial testing the same intervention in Tanzanian women [4]. As a secondary objective, HPTN 039 evaluated the impact of acyclovir on symptomatic genital ulcers, as well as on virologic endpoints, defined by frequency and amount of HSV detected in GUD observed on examination. The efficacy of acyclovir has been extensively characterized in developed countries; however, there is a paucity of data on acyclovir efficacy in resource-poor countries. While acyclovir has now been added to the WHO essential drug list, many STD clinics in Africa still do not have access to the drug, and the cost of the medication limits its use. In addition, evaluation of acyclovir in this setting has focused on treatment of genital ulcer disease and not on prevention of HSV recurrences. Thus, our trial provides novel information about the clinical and virologic efficacy of suppressive acyclovir in resource-poor settings.
Subjects and Methods
A total of 3127 HIV negative, HSV-2 antibody positive participants were enrolled into HPTN 039 and evaluable. MSM were enrolled at sites in the US (Seattle, San Francisco and New York) and Peru (Lima, Iquitos, and Pucallpa) and women were enrolled at sites in Harare, Zimbabwe; Lusaka, Zambia; and Johannesburg, South Africa. Informed consent was obtained from study participants and Institutional Review Boards approved the protocol at each participating trial site. Eligibility criteria and screening procedures are further detailed elsewhere [3].
Participants were randomized to receive acyclovir 400mg twice daily or a matching placebo and seen monthly for 12–18 months. During monthly visits, participants were asked about symptoms of genital herpes in the previous 7 days. Clinicians performed a genital exam at all quarterly study visits and at any monthly or interim study visit if symptoms of genital ulcers were reported. The study staff obtained swabs from lesions clinically consistent with a herpes recurrence; swabs were placed into polymerase chain reaction (PCR) media, frozen and shipped to the University of Washington Virology laboratory. HSV DNA PCR assay was performed according to validated, previously published procedures [5,6]. Samples were analyzed using a real time fluorescent probe-based PCR assay (TaqMan; Applied Biosystems) to quantitate HSV and were considered positive for HSV-2 if more than 3 copies per reaction, or 150 copies per mL of fluid were detected [7]. Mean, median and standard deviations of HSV-2 DNA copy number from genital ulcers were calculated by study arm, and the distribution for log10 HSV PCR titer was plotted by study arm and region. Monthly and quarterly study drug adherence was computed based on monthly pill counts from returned study drug bottles and self-report. Since each participant provided samples at up to six different study visits, we employed generalized estimated equations to analyze numbers of GUDs, HSV-2 positivity from lesional swabs, and mean log10 HSV copies. Models for mean reduction in HSV were adjusted for age and report of genital ulcers in the three months prior to enrollment. To explore whether reductions in HSV shedding were affected by adherence to study drug, analyses were stratified by adherence level measured by monthly pill counts averaged for the prior quarter(<90%, >90%). For counts (i.e. numbers of GUDs) and binary results (HSV-2 positivity), a log link and negative binomial distribution was used. For continuous outcomes (i.e. log10 HSV copies), an identity link and normal distribution was used. An independence working correlation and robust covariance estimate were used in all analyses.
Results
A total of 459 MSM participants were enrolled at US sites, 1355 MSM at Peruvian sites, and 1358 women from African sites. At baseline, 29% of US and 17% of Peruvian MSM reported anogenital herpes symptoms over the prior 3 months; 3–4% had GUD clinically diagnosed at enrollment. In contrast, 33% of women reported symptoms over this time period and 17% had GUD diagnosed on exam. During the 18 month study follow-up, 915 (29%) participants had GUD on exam for a total of 1664 episodes detected. The GUD incidence varied by study arm and population. The overall rate of GUD on exam was 55 per 100 person-years (p-yrs) in the placebo group compared with 30 per 100 p-yrs in the acyclovir group (p<0.0001). For the US MSM, the rate of GUD was 53 per 100 p-yrs in the placebo compared with 15 per 100 p-yrs in the acyclovir group; for the Peruvian MSM, 34 per 100 p-yrs in the placebo compared with 16 per 100 p-yrs in the acyclovir group; and in the African women, 75 per 100 p-yrs in the placebo compared with 46 per 100 p-yrs in the acyclovir group. Overall, acyclovir was associated with a 47% [RR 0.53 (95% CI 0.46, 0.62)] reduction in GUD; 71% [RR 0.29 (95% CI 0.18, 0.47) in US MSM, but only 53% [0.47 (95% CI 0.36, 0.62) in the MSM from Peru and 39% [0.61 (95% CI 0.51, 0.74)] in African women (p=0.0003 for difference in treatment effect by region). Thus, we observed statistically significant regional variation in the proportional reduction in GUD with acyclovir suppression.
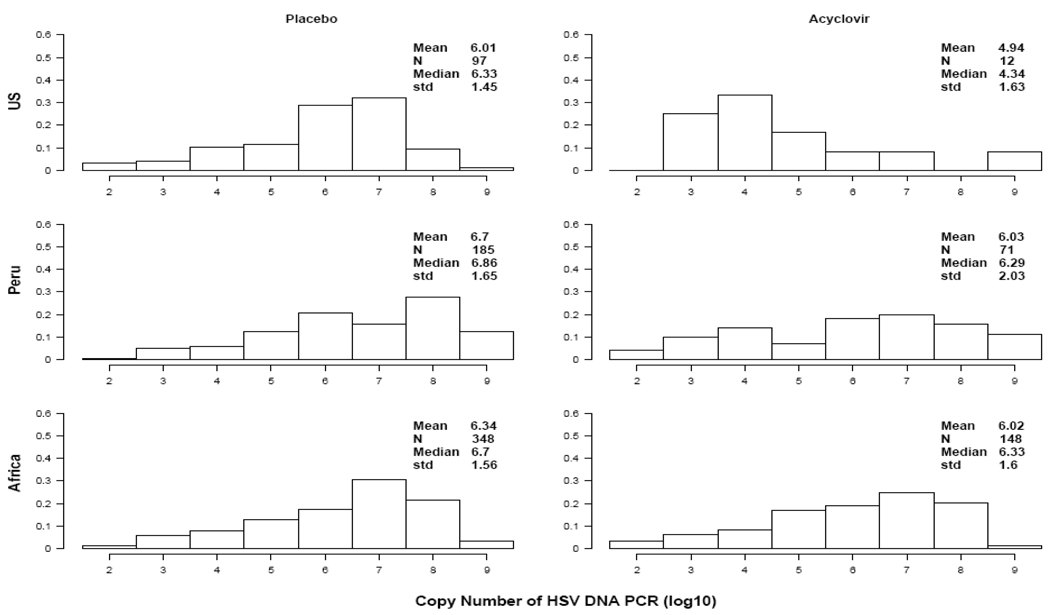
To explore these differences, we examined virologic data obtained from 1468 swabs collected from participants with GUD. Overall, 861(59%) were positive for HSV-2 (630 out of 962 in the placebo arm compared with 231 out of 506 in the acyclovir arm, p<0.0001). This represented a 63% reduction in the rate of ulcers with detectable HSV-2, although regional differences persisted. We found an 88% reduction in HSV-2 positive breakthrough genital ulcers among enrolled US MSM [97 out of 137 in the placebo arm compared with 12 out of 43 in the acyclovir arm, RR 0.12 (95% CI, 0.05–0.29)], but only 61% in MSM from Peru [185 out of 261 in the placebo arm compared with 71 out of 121 in the acyclovir arm, RR 0.39 (95% CI, 0.28–0.56)], and 57% in African women [348 out of 564 in the placebo arm compared with 148 out of 342 in the acyclovir arm, RR 0.43 (95% CI, 0.34–0.56)] (p< 0.0001 for difference in RR between regions). Of the samples with detectable HSV, the mean HSV-2 copy number detected in lesions was reduced by 0.43 log10. However, as shown in Figure 1, the reduction was highest among US MSM with an observed 1.07 log10 reduction with acyclovir (95% CI 0.33–1.80 log10 copies, p=0.0043), a 0.68 log reduction (95% CI 0.04–1.32 log10 copies, p=0.04) among Peruvian MSM, and only a 0.32 log10 reduction (95% CI 0.01–0.63 log10 copies, p=0.05) in the African women (p= 0.13 for difference between regions). As seen in Table 1, these estimates were minimally affected when adjusted for age and reported history of GUD in the 3 months prior to enrollment. In an unadjusted model restricting the analysis to HSV-2 detected in swabs collected from participants reporting >90% adherence to treatment by average drug adherence in the prior quarter (n=627 swabs), the mean reduction in log10 HSV DNA copies increased marginally in all groups, however, regional differences persisted (1.31, 0.89, and 0.47 log10 HSV DNA copy reduction in the US MSM, Peruvian MSM and African women, respectively; p =0.30 for difference between regions).
Figure 1. Distribution of log 10 HSV PCR copy number isolated from GUD, by Arm and Group.
Comparison of HSV DNA copy number from genital ulcers by study arm and region from 861 swabs with detectable HSV. Number of swabs (N), mean, median and standard deviation of the HSV DNA log10 copy number is provided for participants randomized to placebo (left column) and acyclovir (right column).
Table 1.
Acyclovir associated mean reductions in log10 Herpes Simplex Virus (HSV) DNA copies isolated from HSV positive genital ulcer disease (GUD), by region
| Mean Reduction in log10 HSV copies compared to placebo | |||||
|---|---|---|---|---|---|
| Analysis | No. swabs |
Total | US MSM | Peruvian MSM | African women |
| Unadjusted | 861 | 0.43 (95% CI 0.15, 0.71) p=0.003 |
1.07 (95% CI, 0.33, 1.80) | 0.68 (95% CI 0.04, 1.32) | 0.32 (95% CI 0.01, 0.63) |
| Adjusted* | 861 | 0.46 (95% CI 0.18, 0.75) p=0.0014 |
1.12 (95% CI, 0.40, 1.84) | 0.70 (95% CI 0.05, 1.34) | 0.32 (95% CI 0.01,0.64) |
| >90% Adherence¥ |
627 | 0.57 (95% CI 0.25, 0.89) p=0.0005 |
1.31 (95% CI, 0.20, 2.42) | 0.89 (95% CI 0.15, 1.63) | 0.47 (95% CI 0.11,0.83) |
GEE multivariate analysis adjusted for age and baseline report of GUD 3 months prior to enrollment.
Unadjusted analysis restricted to swabs collected from men who have sex with men (MSM) and female participants with >90% average quarterly adherence to study drug measured by monthly pill count
Discussion
In this large multi-site, international trial of suppressive HSV-2 therapy, the standard dose of twice daily acyclovir reduced GUD recurrence overall by half, frequency of ulcers with detectable HSV-2 by 63%, and amount of HSV DNA recovered from those ulcers by 0.43 log10 copies. However, we observed significant regional variation in the incidence of GUD on examination as well as the frequency and amount of HSV detected from ulcers in US and Peruvian MSM and African women. These differences by region in the quantity of HSV-2 detected in swabs from genital herpes lesions persisted after adjusting for level of adherence to antiviral therapy in the prior quarter, and thus are unlikely to be explained by inadequate drug intake. Other possible explanations for the lower efficacy of acyclovir on GUD-associated HSV-2 shedding in populations outside the US, particularly in Africa, include strain variation resulting in inherent acyclovir resistance among HSV strains from Africa, or unappreciated differences in acyclovir absorption or pharmacokinetics. Detailed studies using frequent assessment of genital shedding combined with drug level evaluation may elucidate the mechanism underlying our observations.
The substantial body of evidence establishing the efficacy of acyclovir suppression on clinical and subclinical HSV shedding comes from studies of US and European cohorts [8,9]. For example, a recent report by Gupta and colleagues [10] enrolling men and women at research clinics from the Northwest US observed a 1.2–1.6 log10 reduction in copy number by PCR comparing acyclovir and valacyclovir to placebo which mirrors our estimates from US participants. Despite increased use of acyclovir for syndromic management of HSV-2 worldwide, data are limited on the efficacy of suppressive HSV therapy in resource poor settings. However, over the past several years, studies in Peru [11] and Africa [12] evaluating HSV-2 suppression with both drugs in HIV-infected men and women to reduce HIV shedding and disease progression have also shown efficacy in reducing HSV-2 viral shedding based on molecular diagnostic methods similar to those used in this study. The authors of the Tanzanian trial [4] suggest that suboptimal adherence to 400mg twice daily acyclovir may account for the lack of efficacy in reducing HIV acquisition among the HIV negative women enrolled in their study, as evidenced by a lower than anticipated reduction in HSV shedding from cervicovaginal lavage specimens. However, in our study, adherence rates, measured primarily by pill count, were greater than 90% on average [3]. Moreover, our GUD analysis showed only marginal improvements in the reduction of HSV shedding across all groups when restricted to those with the highest level of adherence.
A limitation of this analysis is that we did not obtain HSV cultures at the time of GUD swabbing which would have permitted us to phenotype viral isolates for acyclovir sensitivity, nor were genotypic assays performed to determine whether non-US populations had a greater prevalence of acyclovir resistance, although future studies are planned Given that acyclovir resistance is infrequent in immunocompetent persons from settings with high levels of background acyclovir use [13], it is unlikely that resistance from this mechanism would be observed in Peru and Africa where acyclovir availability remains very limited.
Regional differences in HSV-2 shedding despite suppressive therapy were unexpected and may have several implications. Potential biologic explanations require further exploration to assess differential effects of acyclovir by population. Thus far, liquid chromatography performed on study drug stored in the field has confirmed expected drug potency [3], and studies are currently underway to assess pharmacokinetics and GUD healing among African women treated with standard episodic acyclovir dosing. In addition, HSV-2 genotypic strain variation has been reported (14), and while an association with lower susceptibility to acyclovir has not been observed, further study of viral polymorphisms as a potential explanation for the regional differences in acyclovir response is warranted. Of note, while our study relied on pill count and self report to measure adherence to study product, as is the standard for most biomedical prevention trials, these methods may overestimate adherence [15]. Finally, efforts to identify a safe and effective HSV-2 vaccine must continue in order to reduce the burden of new HSV-2 infections globally, and potentially reduce HIV transmission risk in those regions most affected by the epidemic.
Acknowledgements
The authors would like to thank Scott Rose and Sam Griffith of Family Health International for study implementation support. We wish to acknowledge Dr. Susan Buchbinder and Theresa Wagner, MPH, for their input on the manuscript; and the extraordinary commitment of the HPTN 039 trial sites, volunteers, and participating community advisory boards.
Footnotes
Potential conflicts of interest
CC has received research grant support from GlaxoSmithKline and has served on an advisory board to GlaxoSmithKline. JS has received grant support from GlaxoSmithKline. FC has received research grant support from GlaxoSmithKline. The University of Washington Virology Division has received grant funding from GlaxoSmithKline and Novartis to undertake HSV serological assays and PCR assays for studies funded by these companies. LC directs these laboratories; he receives no salary support from these grants. AW has received grant support from GlaxoSmithKline, Antigenics, and Astellas. All other authors declare no conflict of interest
Financial support: National Institute of Allergy and Infectious Diseases, National Institutes of Health (grants U01 AI052054 and AI30731) and by the HIV Prevention Trials Network (HPTN) under Cooperative Agreement U01 AI46749 sponsored by the National Institute of Allergy and Infectious Diseases, National Institute of Child Health and Human Development, National Institute of Drug Abuse, National Institute of Mental Health, and Office of AIDS Research. Dr. Wald is also supported by K24 AI07113. Study drug was purchased with a grant provided by GlaxoSmithKline.
Preliminary data presented at the 15th Conference of Retroviruses and Opportunistic Infections, Boston, MA, 03–06 February 2008 (abstract 32).
The study is registered with Clinicaltrials.gov, number NCT00076232
References
- 1.Freeman EE, Weiss HA, Glynn JR, et al. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20:73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 2.Zhu J, Hladlik F, Woodward A, et al. Persistence of HIV-1 receptor-positive cells after HSV-2 reactivation is a potential mechanism for increased HIV-1 acquisition. Nature Medicine. 2009 Aug;15(8):886–892. doi: 10.1038/nm.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Celum C, Wald A, Hughes J, et al. Effect of aciclovir on HIV acquisition in herpes simplex virus 2 seropositive women and men who have sex with men: a randomized, double-blind, placebo controlled trial. Lancet. 2008;371:2109–2119. doi: 10.1016/S0140-6736(08)60920-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watson-Jones D, Weiss HA, Rusizoka M, et al. Effect of herpes simplex suppression on incidence of HIV among women in Tanzania. N Engl J Med. 2008;358:1560–1571. doi: 10.1056/NEJMoa0800260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jerome K, Huang M, Wald A, et al. Quantitative stability of DNA after extended storage of clinical specimens as determined by real-time PCR. J Clinc Microbiol. 2002;40:2609–2611. doi: 10.1128/JCM.40.7.2609-2611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corey L, Huang ML, Selke S, Wald A. Differentiation of herpes simplex virus 1 and 2 in clinical samples by a real-time taqman PCR assay. J Med Virol. 2005;76:350–355. doi: 10.1002/jmv.20365. [DOI] [PubMed] [Google Scholar]
- 7.Magaret A, Wald A, Huang ML, et al. Optimizing PCR positivity criterion for Detection of Herpes Simplex Virus DNA on Skin and Mucosa. J Clin Micro. 2007;45:1618–1620. doi: 10.1128/JCM.01405-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Douglas JM, Critchlow C, Benedetti J, et al. A double blind study of oral acyclovir for suppression of recurrences of genital herpes simplex virus infection. N Engl J Med. 1984;310:1551–1556. doi: 10.1056/NEJM198406143102402. [DOI] [PubMed] [Google Scholar]
- 9.Wald A, Zeh J, Barnum G, Davis LG, Corey L. Suppression of subclinical shedding of herpes simplex virus type 2 with acyclovir. Ann Intern Med. 1996;124:8–15. doi: 10.7326/0003-4819-124-1_part_1-199601010-00002. [DOI] [PubMed] [Google Scholar]
- 10.Gupta R, Wald A, Krantz E, et al. Valacyclovir and acyclovir for suppression of shedding of herpes simplex virus in the genital tract. J Infect Dis. 2004;190:1374–1381. doi: 10.1086/424519. [DOI] [PubMed] [Google Scholar]
- 11.Zuckerman RA, Lucchetti A, Whittington WL, et al. Herpes simplex virus (HSV) suppression with valacyclovir reduces rectal and blood plasma HIV-1 levels in HIV-1/HSV-2-seropositive men: a randomized, double-blind, placebo-controlled crossover trial. J Infect Dis. 2007;196:1500–1508. doi: 10.1086/522523. [DOI] [PubMed] [Google Scholar]
- 12.Cowan FM, Pascoe SJ, Barlow KL, et al. A randomised placebo-controlled trial to explore the effect of suppressive therapy with acyclovir on genital shedding of HIV-1 and herpes simplex virus type 2 among Zimbabwean sex workers. Sex Transm Infect. 2008;84:548–553. doi: 10.1136/sti.2008.031153. [DOI] [PubMed] [Google Scholar]
- 13.Bacon TH, Levin MJ, Leary JJ, Sarisky RT, Sutton D. Herpes simplex virus resistance to acyclovir and penciclovir after two decades of antiviral therapy. Clin Microbiol Rev. 2003;16:114–128. doi: 10.1128/CMR.16.1.114-128.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Norberg P, Kasubi MJ, Haarr L, et al. Divergence and recombination of clinical herpes simplex virus type 2 isolates. J Virol. 2007 Dec;81(23):13158–13167. doi: 10.1128/JVI.01310-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berg K, Arnsten J. Practical and conceptual challenges in measuring antiretroviral adherence. J Acquir Immune Defic Syndr. 2006;43:S79–S87. doi: 10.1097/01.qai.0000248337.97814.66. [DOI] [PMC free article] [PubMed] [Google Scholar]