Abstract
Previous work has demonstrated an important role for adrenergic receptors in memory processes in fear and drug conditioning paradigms. Recent studies have also demonstrated alterations in extinction in these paradigms using drug treatments targeting β- and α2-adrenergic receptors, but little is known about the role of α1-adrenergic receptors in extinction. The current study examined whether antagonism of α1-adrenergic receptors would impair the consolidation of extinction in fear and cocaine conditioned place preference (CPP) paradigms. After contextual fear conditioning, injections of prazosin (1.0 or 3.0 mg/kg) following nonreinforced context exposures slowed the loss of conditioned freezing over the course of five extinction sessions (Experiment 1). After cocaine place conditioning, prazosin had no effect on the rate of extinction over eight nonreinforced test sessions. Following post-extinction reconditioning, however, prazosin-treated mice showed a robust place preference, but vehicle-treated mice did not, suggesting that prazosin reduced the persistent effects of extinction (Experiment 2). These results confirm the involvement of the α1-adrenergic receptor in extinction processes in both appetitive and aversive preparations.
Keywords: extinction, fear conditioning, cocaine, conditioned place preference, memory, noradrenergic receptors
Introduction
Recent studies of the cellular and molecular mechanisms involved in memory have focused on mechanisms that underlie extinction, a process by which conditioned behavior is eliminated as a result of nonreinforced exposure to previously conditioned stimuli. Understanding these mechanisms is important not just for a general understanding of the mechanisms involved in memory formation, but also for clinical applications, in which pharmacotherapies targeting extinction represent an important treatment strategy for conditions such as learned fears, post-traumatic stress disorder (PTSD), and drug addiction (Amaral & Roesler, 2008; Taylor, Olausson, Quinn, & Torregrossa, 2009). The noradrenergic system has received a great deal of attention in animal models of these disorders, due to the involvement of norepinephrine (NE) in a variety of cognitive processes, including attention, arousal, emotion, learning, and memory consolidation (reviewed in McGaugh & Roozendaal, 2009; Sara, 2009).
NE exerts its effects through activation of three families of adrenergic receptors (ARs), β, α1 and α2 (Bylund et al., 1994). The majority of studies examining the role of NE in facilitating plasticity related to learning and memory have focused on the β-AR, demonstrating a requirement for this receptor in memory processes involved in fear (e.g., Ferry & McGaugh, 1999; Liang, Juler, & McGaugh, 1986) and drug conditioning (e.g., Bernardi, Lattal, & Berger, 2006; Bernardi, Ryabinin, Berger, & Lattal, 2009; Fricks-Gleason & Marshall, 2008; Robinson & Franklin, 2007), including the extinction of learned fear (Berlau & McGaugh, 2006; Cain, Blouin, & Barad, 2004; Mueller, Porter, & Quirk, 2008). Recent studies have also begun to elucidate the role of the α2-AR in learning and retrieval processes. This receptor has been shown to be involved in learning, consolidation and recall (Ferry and McGaugh, 2008; Galeotti, Bartolini, & Ghelardini, 2004; Gibbs & Summers, 2003; Samini, Kardan, & Mehr, 2008; Tahsili-Fahadan et al., 2006), as well as extinction (Cain et al., 2004; Kupferschmidt, Tribe, & Erb, 2009; Morris & Bouton, 2007), in fear and drug conditioning preparations.
Little is known about the role of the α1-AR in extinction, despite the fact that this class of adrenergic receptors has been shown to be important for memory consolidation processes that follow initial learning and retrieval in both fear and drug paradigms (Bernardi et al., 2009; Ferry, Roozendaal, & McGaugh, 1999a, 1999b; see also Walker, Rasmussen, Raskin, & Koob, 2008). For example, selective activation of the α1-AR in the basolateral nucleus of the amygdala (BLA), a site that has a well-demonstrated role in memory processing (McGaugh & Roozendaal, 2009), has been shown to enhance memory for an inhibitory avoidance task in rodents, while blockade with intra-BLA administration of the α1-AR antagonist, prazosin, impaired long-term retention. These results are likely due to the influence of the α1-AR on β-AR activity, as prazosin has been shown to impair the enhancement of memory retention caused by the β-AR agonist clenbuterol, but not the synthetic cAMP analog 8-bromo-cAMP (Ferry et al., 1999a), and antagonism at β-AR has been shown to attenuate the effects of α1-AR agonism on memory consolidation (Ferry et al., 1999b). Because many studies have shown that consolidation processes also operate after extinction (e.g., Berlau & McGaugh, 2006), it is likely that α1-ARs may be involved in modulating these processes.
Several recent studies have noted similarities in the circuitry involved in the extinction of both learned fears and conditioned drug seeking behaviors (reviewed in Peters, Kalivas, & Quirk, 2009). For example, the medial prefrontal cortex (mPFC) has been demonstrated to play a significant role in both fear extinction (e.g., Burgos-Robles, Vidal-Gonzalez, & Quirk, 2009; Mueller et al., 2008) and drug seeking behaviors following extinction (Peters, LaLumiere, & Kalivas, 2008; Peters, Vallone, Laurendi, & Kalivas, 2008), likely via projections from the infralimbic cortex (IL) of the mPFC to the BLA and nucleus accumbens (NAcc) (reviewed in Peters et al., 2009). Furthermore, dopamine projections to the PFC, which are commonly studied in reward-related learning (reviewed in Hyman, Malenka, & Nestler, 2006), have been shown to be involved in the extinction of fear (Morrow, Elsworth, Rasmusson, & Roth, 1999; Storozheva, Afanas'ev, Proshin, & Kudrin, 2003). In addition, alterations in glutamatergic signaling can impair or promote extinction of fear or drug-induced CPP, depending on the alteration (e.g., Engblom et al., 2008; Groblewski, Lattal, & Cunningham, 2009; Walker, Ressler, Lu, & Davis, 2002), and drugs that promote or inhibit gene expression can also alter extinction in these different preparations (e.g., Lattal, Barrett, & Wood, 2007; Malvaez, Sanchis-Segura, Vo, Lattal, & Wood, 2009; Santini, Ge, Ren, Pena de Ortiz, & Quirk, 2004). Together, these findings suggest that extinction as a general process may share underlying neurobiological mechanisms that are at least somewhat independent of the unconditioned stimulus that supports the original learning.
In the following experiments, we examined the effects of systemic administration of the α1-AR antagonist prazosin on the extinction of contextual fear conditioning (Experiment 1) and cocaine CPP (Experiment 2) in mice. If the α1-AR is important for post-extinction memory processes, then long-term extinction should be impaired in mice that receive prazosin after the extinction sessions.
Methods
Subjects
Male C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) aged 8–16 weeks served as subjects. Mice were housed four per cage in a temperature-controlled (21 °C) environment maintained on a 12-hr light-dark cycle (lights on at 6 a.m.). Food and water were available ad libitum. Experiments were performed in accordance with the NIH guidelines for the care and use of laboratory animals and the IACUC of Oregon Health & Science University. All behavioral testing was conducted during the light phase between 0700 h and 1600 h.
Drugs
For cocaine CPP, cocaine HCl (Sigma, St. Louis, MO) was dissolved in physiological saline for intraperitoneal (IP) injection and administered at 20 mg/kg for conditioning trials and 5 and 20 mg/kg for reconditioning trials in a volume of 10 ml/kg. Prazosin (Sigma) was dissolved in sterile water and administered at 1.0 or 3.0 mg/kg IP in a volume of 10 ml/kg. These doses were based on previous studies in mice examining the effects of prazosin on memory (Galeotti et al., 2004; Introini-Collison, Saghafi, Novack, & McGaugh, 1992; Knauber & Muller, 2000).
Experiment 1: Fear Conditioning
Apparatus
Four Coulbourn Instruments mouse conditioning chambers (H10-11M-TC) were used. The floor consisted of stainless steel grid rods spaced 6.4 mm apart. On top of the floor was a clear Plexiglas cylinder (15.2 cm in diameter). The chambers were housed in sound- and light-attenuating shells and a fan provided background noise at 70 dB. Scrambled shock (2 s, 0.35 mA) was delivered to the grid floor by a computer-controlled shock generator (Coulbourn H13–15). Mounted 18 cm above the floor of each chamber was an automated infrared activity monitor (Coulbourn H24–61). Experimental events were controlled by Graphic State 3.01 software.
Behavioral Procedures
Mice were handled for 1–2 min/day for 3 days prior to the experiment. On Day 1 (conditioning), mice received a 12-min exposure to the context with four unsignaled shocks, each delivered on average every 180 s (range = 60–300 s). On Days 2–4, mice received a 3-min nonreinforced exposure to the context, followed immediately by an injection of 1.0 mg/kg of prazosin (n=26), 3.0 mg/kg of prazosin (n=15) or vehicle (n=15). On Days 4 and 5, mice received a 12-min nonreinforced exposure to the context followed by prazosin or vehicle injections. Mice received one additional 12-min test on Day 12. Again, activity was recorded by infrared activity monitors mounted on the ceiling of each chamber, and freezing was defined as bouts of inactivity that lasted at least 3 s. Total time meeting this criterion was divided by the total time in the session to calculate percent time freezing (see Lattal, 2007).
Experiment 2: Conditioned Place Preference
Apparatus
Conditioned place preference was assessed using an unbiased design (Cunningham, Gremel, & Groblewski, 2006) in four automated one-compartment place conditioning boxes housed in sound- and light-attenuating melamine shells (McCarthy Manufacturing, Gresham, OR). Each conditioning box consisted of a clear acrylic test cage (30 cm × 15 cm × 15 cm) with removable floors composed of interchangeable halves (left/right) of two distinct floor types. A GRID floor consisted of 2.3-mm stainless steel rods mounted 6.4 mm apart in an acrylic frame. A HOLE floor consisted of perforated 16 gauge stainless steel sheets with 6.4-mm diameter round holes on 9.5 mm staggered centers. Position in the box (left/right side) and general activity were assessed by EthoVision computer software (Noldus, Leesburg, VA) that records and analyzes the position of the mouse within the apparatus via a camera mounted to the ceiling of the melamine shell.
Behavioral Procedures
Place conditioning involved the following phases: habituation, conditioning, postconditioning preference/extinction testing, reconditioning, and postreconditioning preference testing.
Habituation (1 session)
During habituation, mice were injected with saline (IP, 10 ml/kg) and placed in the apparatus without floors for 15 min to reduce the stress associated with injections and exposure to the apparatus.
Conditioning (8 sessions)
Mice in each drug treatment group were randomly assigned to one of two conditioning subgroups (cocaine on GRID floor = GRID+; cocaine on HOLE floor = GRID-) and exposed to a Pavlovian discrimination conditioning procedure (Cunningham et al., 2006). Thus, on alternate days over eight conditioning sessions (four cocaine sessions and four saline sessions), mice in the GRID+ subgroup received cocaine (20 mg/kg IP) immediately prior to 15-min conditioning trials on the GRID floor and saline (10 ml/kg IP) immediately prior to 15-min trials on the HOLE floor. Alternatively, mice in the GRID− subgroup received cocaine (20 mg/kg IP) immediately prior to 15-min conditioning trials on the HOLE floor and saline (10 ml/kg IP) immediately prior to 15-min trials on the GRID floor. The order of treatment exposure was counterbalanced within each GRID+ and GRID− subgroup, such that half of the mice in each subgroup received conditioning to cocaine during the first conditioning trial and half of the mice received saline during the first trial. During conditioning trials, left and right floor types were identical and mice had access to both sides of the apparatus (Cunningham et al., 2006).
Postconditioning preference/extinction testing (8 sessions)
Mice were randomly assigned to one of three groups: vehicle (n = 16; 8 per GRID+/GRID− conditioning subgroups), 1 mg/kg prazosin (n = 15; 7/8 per GRID+/GRID− conditioning subgroups), and 3 mg/kg prazosin (n = 16; 8 per GRID+/GRID− conditioning subgroups). During Test 1, which occurred 72 hr following the end of conditioning trials, mice received a saline injection (1 ml/kg IP) immediately prior to placement into the apparatus with half GRID floor and half HOLE floor for a 12-min preference test designed to demonstrate the acquisition of the cocaine-cue association during the conditioning phase. For the half GRID floor and half HOLE floor combination, the position of the floors (left vs. right) was counterbalanced within each GRID+ and GRID− subgroup. Immediately following this test session, mice received drug treatment injections (vehicle, 1 mg/kg prazosin, or 3 mg/kg prazosin) and were returned to their home cages. Beginning twenty-four hours later, mice received an additional five daily 12-min choice extinction trials (Tests 2–6, with a two-day break between Tests 4 and 5) and two 30-min choice extinction sessions (Tests 7–8), each immediately followed by vehicle or prazosin injections to assess the effects of post-test prazosin on the extinction of a cocaine-induced conditioned place preference. Magnitude of place preference was determined by comparing the amount of time spent on the GRID floor between the GRID+ and GRID− conditioning subgroups, as well as percent time spent on the drug-paired floor (collapsed across conditioning subgroup).
Reconditioning and post-reconditioning preference testing (6 sessions)
Four days following the last extinction session, mice received a single pair of 15-min CS+ (5 mg/kg cocaine)/CS− (vehicle) conditioning trials (as described earlier) and a 15-min preference test conducted over three consecutive days. Seven days later, mice received another pair of 15-min CS+ (20 mg/kg cocaine)/CS− (vehicle) conditioning trials (as described earlier) and a 15-min preference test over three consecutive days. Again, magnitude of place preference was determined by comparing the amount of time spent on the GRID floor between the GRID+ and GRID− conditioning subgroups, as well as percent time spent on the drug-paired floor (collapsed across conditioning subgroup).
Data analysis
Fear conditioning data were analyzed using repeated measures ANOVA and Student’s t test. CPP data were analyzed in two ways. First, to simplify the presentation and analysis during extinction, GRID+ and GRID− groups were collapsed and the effects of the drug treatment on time spent on the cocaine-paired floor was analyzed. Second, differences between GRID+ and GRID− subgroups in time spent on the GRID floor were used to assess preference as a function of counterbalancing assignments (Cunningham, Ferree, & Howard, 2003). Repeated measures ANOVA (drug treatment × extinction session), two-way ANOVA [prazosin dose × conditioning subgroup (GRID+/GRID−) and Student’s t-test were used for these comparisons. Bonferroni-corrected alpha levels are reported for cases of multiple Student’s t tests.
Results
Experiment 1: Fear Conditioning
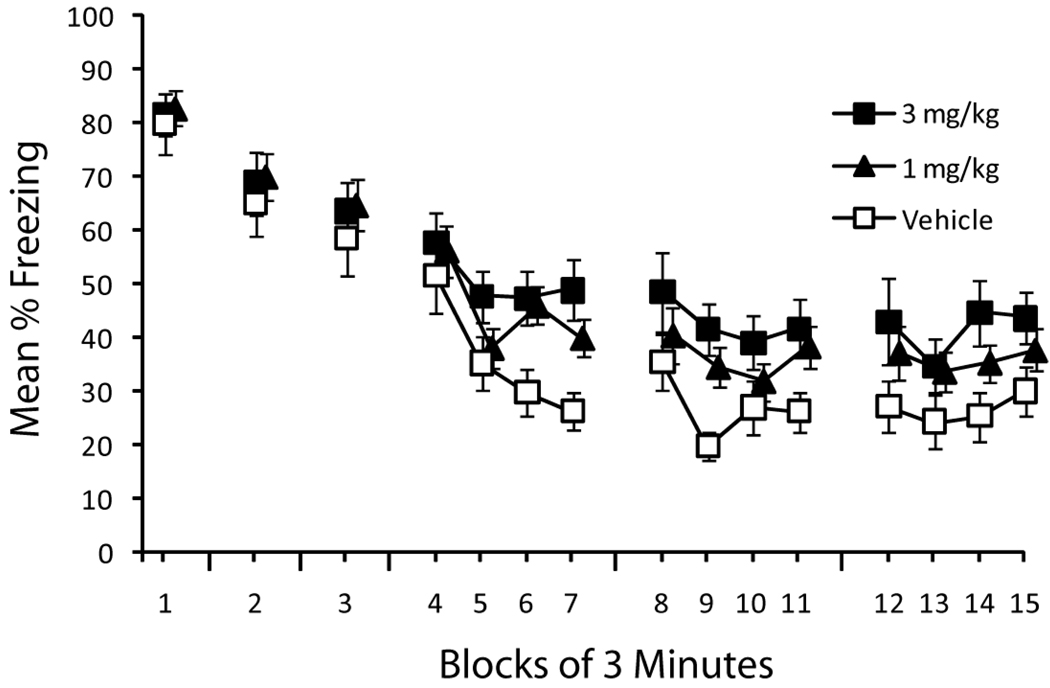
Mice showed high levels of freezing that decreased over the course of the six extinction sessions. Effects of post-session administration of prazosin became evident during the fourth extinction session (the first of the longer extinction sessions; Figure 1). During the first three days of extinction (Figure 1, Blocks 1–3), which consisted of a 3-min exposure to the context with no shock, prazosin did not reliably impair extinction [reliable main effect of session, F(2,106)=36.0, p<0.001; no reliable main effect of dose or interaction, Fs<1]. However, effects of prazosin were detected during the longer extinction sessions conducted on Days 4, 5, and 12. Separate ANOVAs with dose and 3-min time block as factors revealed reliable main effects of dose [Fs(2, 53)>3.2, ps<0.05] and time block [F(3, 159)>5.4, ps<0.001], but no interactions [Fs(6, 159)<1.9, ps>.09] during Days 4 and 5. During Day 12, the main effect of dose was not reliable [F(2, 53)=2.7, p=0.07].
Figure 1.
Effects of post-session prazosin injections on extinction of context-evoked fear. Sessions 1–3 of extinction consisted of a 3-min nonreinforced context exposure; sessions 4–6 consisted of a 12-min exposure. Each of the first five sessions was followed by an IP injection of 1.0 mg/kg prazosin (n=26), 3.0 mg/kg prazosin (n=15), or vehicle (n=15). Error bars represent standard error of the mean.
Analyses of the simple effects during each of Days 4, 5, and 12 revealed reliable differences between mice treated with vehicle and 3.0 mg/kg prazosin [main effect of dose: Fs(1,28)>5.1, ps<.05; no interactions: Fs<1.8, ps>.15], but no reliable differences between mice treated with vehicle and 1.0 mg/kg prazosin [Fs(1,39)>2.8, ps>0.08; no interactions (Fs<2.2, ps>0.08]. Together, these findings show that a higher dose (3.0 mg/kg) of prazosin impaired extinction over multiple days.
Experiment 2: Conditioned Place Preference
Performance during extinction and tests after reconditioning are shown both as percent time on the cocaine-paired floor (Figure 2A) and time (s/min) on the Grid floor (Figures 2B and 2C). Both measures confirmed that prazosin did not affect rate of extinction, but did result in enhanced post-extinction reconditioning.
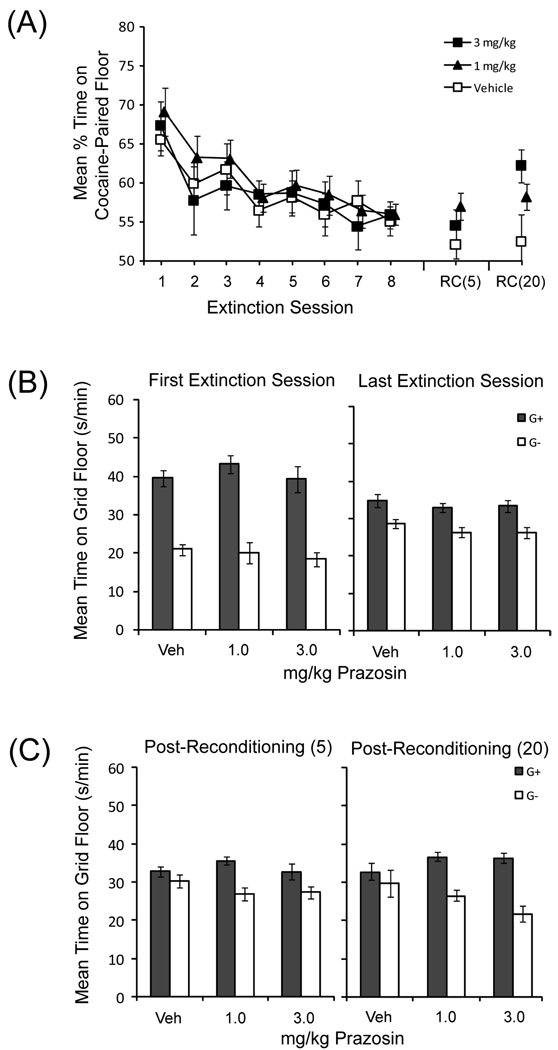
Figure 2.
Effects of post-session prazosin injections on extinction of cocaine-induced conditioned place preference. (A) Percent time spent on the cocaine-paired floor is shown for the eight sessions of extinction and for the two test sessions following post-extinction reconditioning (RC) with 5 mg/kg (RC5) or 20 mg/kg (RC20) of cocaine. Each of the extinction sessions was followed by IP injection of 1.0 mg/kg prazosin (n=15), 3.0 mg/kg prazosin (n=16), or vehicle (n=16). Mean time spent on the GRID floor during (B) the first and last extinction session and (C) the two sessions following reconditioning is shown for mice that received cocaine pairings with the GRID floor (G+) and mice that received pairings with the HOLE floor (G−). Error bars represent standard error of the mean.
Extinction
A repeated measures ANOVA (dose × session) of percent time on the cocaine floor during the eight sessions of extinction (Figure 2A; Extinction 1–8) revealed a significant main effect of session [F(7,308) = 7.2, p < .001], but no interaction or main effect of dose (Fs < 1). Similar statistical results were obtained with the analysis of time spent on the grid floor in the counterbalanced subgroups (Figure 2B). There was no effect of time block within the 30-min extinction sessions [Sessions 7 and 8; Fs(4, 164)=1.1, ps>.35], so the analyses included the mean preference from those sessions.
Mice in each drug subgroup group showed a conditioned place preference during Test 1, prior to drug treatment (Figure 2B, Panel 1; First Extinction Session). A two-way ANOVA revealed a significant main effect of conditioning subgroup [F(1,41) = 112.2, p < .001], indicating reliable preference across drug treatments for the cocaine-paired floor, but no interaction or main effect of dose (Fs < 1). Comparing the first and last extinction session revealed only a reliable interaction between conditioning subgroup and session [F(1,41)=41.8, p<.0001], demonstrating a large loss of preference from the beginning to the end of extinction. During the final extinction session (Figure 2B, Panel 2), there were no effects of prazosin, as indicated by a significant main effect of conditioning subgroup [F(1,41) = 31.3, p < .001], but no interaction (F < 1) or main effect of dose [F(2,41) = 1.2, p = .29].
Reconditioning
Prazosin impaired the persistence of extinction, as indicated by stronger reconditioning compared to vehicle. When post-reconditioning preference data were examined [Figure 2A; RC(5) and RC(20)], ANOVA revealed that following reconditioning with 5 mg/kg cocaine, there was no significant difference between the groups [F(2,44) = 1.7, p = .19], but following reconditioning with 20 mg/kg cocaine, there was a significant main effect of dose [F(2,44) = 3.7, p < .05]. Student’s t-test confirmed that although the difference in percent preference between the vehicle and 1 mg/kg prazosin did not reach significance [t(29) = 1.7, p = .15], there was a reliable difference in percent preference between the vehicle and 3 mg/kg prazosin groups [t(30) = 2.4, p =.023; Bonferroni-corrected α=0.025].
Figure 2C, Panel 1 shows the mean (+SEM) time spent on the GRID floor during the post-reconditioning (5 mg/kg cocaine) preference test for groups treated with vehicle, 1, or 3 mg/kg prazosin during extinction. There was no difference between the groups in the magnitude of preference, as indicated by a significant main effect of conditioning subgroup [F(1,41) = 17.8, p < .001], but no interaction [F(2,41) = 1.8, p = .19] or main effect of dose (F < 1). However, post-hoc tests found a reliable difference between GRID+ and GRID− groups within the 1 mg/kg dose [t(13)=4.2, p<.001], but no difference in the 3 mg/kg group [t(14)=2.1, p=.056] or vehicle [t(14)=1.2, p=.25; Bonferroni-adjusted alpha=.017]. Following reconditioning with 20 mg/kg cocaine, mice that received either dose of prazosin during extinction showed greater reacquisition of cocaine CPP compared to mice that received vehicle during extinction. Figure 2C, Panel 2 shows the mean (+SEM) time spent on the GRID floor during the post-reconditioning (20 mg/kg cocaine) preference test for groups vehicle, 1 and 3 mg/kg prazosin. There was a significant dose × conditioning subgroup interaction [F(2,41) = 17.8, p < .001], suggesting a difference in preference as a function of prazosin treatment, as well as a main effect of conditioning subgroup [F(1,41) = 27.0, p < .001] and no main effect of dose (F < 1). Further analysis with Student’s t-test comparing time spent on the GRID floor for the GRID+ and GRID− subgroups within each drug treatment confirmed that the vehicle group [t(14) = .71, p = .49] failed to show a preference for the cocaine-paired floor following reconditioning, while both the 1 mg/kg [t(13) = 5.4, p < .001; Bonferroni alpha = 0.017] and 3 mg/kg [t(14) = 5.8, p < .001; Bonferroni alpha = 0.017] prazosin groups continued to show reliable preferences, indicative of a prazosin-mediated impairment of the persistence of extinction.
Discussion
This study demonstrates an impairment in extinction of fear and cocaine-induced CPP by systemic administration of the α1-AR antagonist prazosin immediately after the extinction sessions. In both cases, however, the effects on extinction were evident only after multiple extinction sessions. In the case of fear extinction, these effects were most apparent beginning on the fourth session of extinction. In the case of CPP extinction, no effects on rate of extinction were observed, but reconditioning was more pronounced in prazosin-treated mice, consistent with a weaker extinction effect in those mice. In general, these findings are consistent with studies of initial memory consolidation, which have found effects of α1-AR antagonists on the consolidation of inhibitory avoidance (Ferry et al., 1999a, 1999b). Further, these results extend previous demonstrations of the importance of adrenergic signaling in fear extinction, including studies of the α2-AR and β-AR (Berlau & McGaugh, 2006; Cain et al., 2004; Morris & Bouton, 2007; Mueller et al., 2008), to the α1-AR in extinction of fear. The precise relationship of α1-ARs with α2-ARs and β-ARs and the locus of action of α1-AR-mediated impairment of extinction remain to be systemically characterized, but previous studies have reported interactions between α1-ARs and both α2-ARs and β-ARs in the BLA in mediating the memory enhancing effect of NE (Ferry et al., 1999a, 1999b). Furthermore, recent evidence has further implicated the BLA, as well as the IL cortex, in mediating extinction and extinguished behaviors in fear and drug conditioning paradigms, respectively (Bernardi et al., 2009; Peters et al., 2008b; Vidal-Gonzalez, Vidal-Gonzalez, Rauch, & Quirk, 2006).
The most obvious explanation for the failure to observe an effect of prazosin early in extinction is that behavior was at a ceiling. Vehicle-treated animals did not show a large loss of freezing between the initial extinction sessions, which makes observing a drug-induced impairment in extinction even more difficult. Only after behavior began to move to the lower part of the scale in vehicle-treated animals were the effects of prazosin observed. In CPP, vehicle-treated animals moved from an approximate 65% preference to a 55% preference over the course of extinction, which may not have provided enough room in behavior to see effects on rate of extinction. Post-extinction reconditioning was stronger in prazosin-treated animals, however, which is consistent with the idea that extinction was deeper in vehicle-treated animals. Together, these findings demonstrate that multiple extinction and reconditioning sessions may reveal pharmacological effects that were not evident in behavior during early stages of extinction.
These findings also are consistent with some literature on extinction showing that extinction must develop in behavior before pharmacological effects can be observed (Bouton, Vurbic, & Woods, 2008; Weber, Hart, & Richardson, 2007). If little or no extinction occurs, animals may not be sensitive to the extinction contingencies, meaning that there is no learning to be impaired. The gradual loss of freezing over several extinction sessions in our experiments is consistent with this idea, with the effects being most evident when behavior moved to the lower parts of the scale on Days 4–6 in fear.
As with fear paradigms, few studies have examined a role for the α1-AR in memories associated with drug conditioning (e.g., Bernardi et al., 2009). Using CPP, we found here that prazosin did not affect the rate at which extinction developed. However, prazosin did impair the persistence of extinction, as mice treated with both 1 and 3 mg/kg showed a reacquisition of cocaine CPP following a single re-exposure to 20 mg/kg cocaine with the CS+ after extinction. Vehicle-treated animals failed to reacquire a cocaine CPP following reconditioning with 20 mg/kg cocaine. Our reconditioning data with prazosin are an important extension to the study of extinction processes in animal models of drug-seeking behaviors, as the conditions under which drug cues reacquire motivational value following abstinence in humans remain the biggest detriment to developing pharmacotherapies for addiction. Furthermore, previous work has implicated the reconditioning of drug cues as a more important contributor to relapse than reinstatement (Leri & Rizos, 2005). Leri and Rizos (2005) demonstrated using heroin CPP in rats that the persistence of drug-seeking behaviors following reconditioning with heroin after extinction, lasting at least 96 hours, was stronger than that of heroin-primed reinstatement following extinction, which was absent 24 hours later, and thus more relevant to the human condition.
Other studies of reconditioning after extinction of CPP have demonstrated rapid reconditioning in vehicle-treated animals (e.g., ethanol CPP, Groblewski et al., 2009; heroin CPP, Leri & Rizos, 2005). Groblewski et al. (2009), for example, found that the partial NMDA receptor agonist D-cycloserine (DCS) administered during extinction of ethanol CPP led to weaker reconditioning compared to vehicle-treated mice. Groblewski et al. (2009) concluded that DCS did enhance extinction, even though rate of extinction was unaffected (see also Nic Dhonnchadha et al., 2010). These findings, like ours, suggest that post-extinction reconditioning is a useful tool for unmasking extinction effects that may not be evident during the course of CPP extinction itself. At a theoretical level, our findings of enhanced reconditioning in groups that received prazosin during extinction is consistent with the idea that the learning processes that occurred during extinction were not as strong as in those animals that received vehicle.
Describing the general role of noradrenergic receptors in extinction is complicated because studies have shown that in addition to impairing extinction, noradrenergic antagonists can impair the expression of conditioned behavior (Rodriguez-Romaguera, Sotres-Bayon, Mueller, & Quirk, 2009) or enhance behavioral extinction (Bernardi et al., 2009; Debiec & Ledoux, 2004). One notable difference between our experiments and those of Bernardi et al. (2009), who found rapid loss of CPP following post-retrieval injections of prazosin in rats, is that in the Bernardi et al. paper, the difference in loss of preference in vehicle- and prazosin-treated animals was dramatic between Tests 1 and 2, with very little change in vehicle-treated animals. In the current experiments, there was a change in preference in the 1 mg/kg group, as shown in the previous paper, but with a similar change in preference in vehicle-treated mice. Thus, the effects of prazosin may depend on the amount of extinction that occurs in vehicle-treated animals (e.g., Lee, Milton, & Everitt, 2006; Suzuki et al., 2004). Furthermore, because of the more rapid decline in preference in vehicle-treated animals between Tests 1 and 2 in the current study as compared to Bernardi et al. (2009), it is plausible that the cocaine memory was stronger in this prior study, and previous work has suggested a correlation between memory strength and susceptibility to disruption, such that stronger memories may be more susceptible to pharmacological impairment than weaker ones (e.g., Eisenberg, Kobilo, Berman, & Dudai, 2003). Clearly the effects of prazosin during extinction are complex and future studies will need to determine the conditions under which α1-AR blockade can promote or retard extinction.
In an animal model of the hyper-responsiveness and increased startle associated with PTSD in humans (Servatius, Ottenweller, & Natelson, 1995), prazosin was shown to attenuate the stress-induced elevation of the acoustic startle response in rats, suggesting a potential clinical efficacy of this drug in fear- and stress-related paradigms. In humans, prazosin has been studied as a pharmacotherapy for PTSD, and has been demonstrated to reduce the nightmares associated with this illness in a variety of populations (Raskind et al., 2003; Taylor et al., 2008). Though not thought to be due to effects on extinction of fear, but rather the retrieval of fearful memories (Miller, 2008), these studies indicate a role for the α1-AR in memory processes in humans. Further characterizing the effects of prazosin on extinction of fear in clinical populations will be important, because one implication of our findings is that an unintended consequence of prazosin in these populations could potentially be a poorer response to behavioral therapies that use extinction techniques.
In conclusion, our findings implicate the α1-ARs in extinction in two very different behavioral paradigms, and are consistent with the idea that common mechanisms are involved in extinction in fear and drug learning paradigms (Peters et al., 2009). Similar behavioral effects on extinction in these paradigms suggest that targeting the α1-AR may be fruitful in developing pharmacotherapies for disorders involving deficits in extinction.
Acknowledgements
This research was supported by grants from the National Institute of Mental Health to K. Matthew Lattal (R01 MH077111), and National Institute on Drug Abuse to K. Matthew Lattal and Marcelo A. Wood (R01 DA025922) and to Rick E. Bernardi (F31 DA022844). We thank James Stafford for helpful comments and Scott Bolkan and Ellen McCleery for help with data collection.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
References
- Amaral OB, Roesler R. Targeting the NMDA receptor for fear-related disorders. Recent Pat CNS Drug Discov. 2008;3:166–178. doi: 10.2174/157488908786242470. [DOI] [PubMed] [Google Scholar]
- Berlau DJ, McGaugh JL. Enhancement of extinction memory consolidation: the role of the noradrenergic and GABAergic systems within the basolateral amygdala. Neurobiol Learn Mem. 2006;86:123–132. doi: 10.1016/j.nlm.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Bernardi RE, Lattal KM, Berger SP. Postretrieval propranolol disrupts a cocaine conditioned place preference. Neuroreport. 2006;17:1443–1447. doi: 10.1097/01.wnr.0000233098.20655.26. [DOI] [PubMed] [Google Scholar]
- Bernardi RE, Ryabinin AE, Berger SP, Lattal KM. Post-retrieval disruption of a cocaine conditioned place preference by systemic and intra-basolateral amygdala β2- and α1-adrenergic antagonists. Learn Mem. 2009;16:777–789. doi: 10.1101/lm.1648509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Vurbic D, Woods AM. D-cycloserine facilitates context-specific fear extinction learning. Neurobiol Learn Mem. 2008;90:504–510. doi: 10.1016/j.nlm.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ. Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. J Neurosci. 2009;29:8474–8482. doi: 10.1523/JNEUROSCI.0378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylund DB, Eikenberg DC, Hieble JP, Langer SZ, Lefkowitz RJ, Minneman KP, Molinoff PB, Ruffolo RR, Jr, Trendelenburg U. International Union of Pharmacology nomenclature of adrenoceptors. Pharmacol Rev. 1994;46:121–136. [PubMed] [Google Scholar]
- Cain CK, Blouin AM, Barad M. Adrenergic transmission facilitates extinction of conditional fear in mice. Learn Mem. 2004;11:179–187. doi: 10.1101/lm.71504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Ferree NK, Howard MA. Apparatus bias and place conditioning with ethanol in mice. Psychopharmacology (Berl) 2003;170:409–422. doi: 10.1007/s00213-003-1559-y. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA. Drug-induced conditioned place preference and aversion in mice. Nat Protoc. 2006;1:1662–1670. doi: 10.1038/nprot.2006.279. [DOI] [PubMed] [Google Scholar]
- Debiec J, Ledoux JE. Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience. 2004;129:267–272. doi: 10.1016/j.neuroscience.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Eisenberg M, Kobilo T, Berman DE, Dudai Y. Stability of retrieved memory: inverse correlation with trace dominance. Science. 2003;301:1102–1104. doi: 10.1126/science.1086881. [DOI] [PubMed] [Google Scholar]
- Engblom D, Bilbao A, Sanchis-Segura C, Dahan L, Perreau-Lenz S, Balland B, Parkitna JR, Lujan R, Halbout B, Mameli M, Parlato R, Sprengel R, Luscher C, Schutz G, Spanagel R. Glutamate receptors on dopamine neurons control the persistence of cocaine seeking. Neuron. 2008;59:497–508. doi: 10.1016/j.neuron.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Ferry B, McGaugh JL. Clenbuterol administration into the basolateral amygdala post-training enhances retention in an inhibitory avoidance task. Neurobiol Learn Mem. 1999;72:8–12. doi: 10.1006/nlme.1998.3904. [DOI] [PubMed] [Google Scholar]
- Ferry B, McGaugh JL. Involvement of basolateral amygdala alpha2-adrenoceptors in modulating consolidation of inhibitory avoidance memory. Learn Mem. 2008;15:238–243. doi: 10.1101/lm.760908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry B, Roozendaal B, McGaugh JL. Basolateral amygdala noradrenergic influences on memory storage are mediated by an interaction between beta- and alpha1-adrenoceptors. J Neurosci. 1999a;19:5119–5123. doi: 10.1523/JNEUROSCI.19-12-05119.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry B, Roozendaal B, McGaugh JL. Involvement of alpha1-adrenoceptors in the basolateral amygdala in modulation of memory storage. Eur J Pharmacol. 1999b;372:9–16. doi: 10.1016/s0014-2999(99)00169-7. [DOI] [PubMed] [Google Scholar]
- Fricks-Gleason AN, Marshall JF. Post-retrieval {beta}-adrenergic receptor blockade: Effects on extinction and reconsolidation of cocaine-cue memories. Learn Mem. 2008;15:643–648. doi: 10.1101/lm.1054608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeotti N, Bartolini A, Ghelardini C. Alpha-2 agonist-induced memory impairment is mediated by the alpha-2A-adrenoceptor subtype. Behav Brain Res. 2004;153:409–417. doi: 10.1016/j.bbr.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Gibbs ME, Summers RJ. Alpha 2-adrenoceptors in the basal ganglia have a role in memory consolidation and reinforcement. Neuropharmacology. 2003;45:355–367. doi: 10.1016/s0028-3908(03)00172-2. [DOI] [PubMed] [Google Scholar]
- Groblewski PA, Lattal KM, Cunningham CL. Effects of D-cycloserine on extinction and reconditioning of ethanol-seeking behavior in mice. Alcohol Clin Exp Res. 2009;33:772–782. doi: 10.1111/j.1530-0277.2009.00895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Introini-Collison I, Saghafi D, Novack GD, McGaugh JL. Memory-enhancing effects of post-training dipivefrin and epinephrine: involvement of peripheral and central adrenergic receptors. Brain Res. 1992;572:81–86. doi: 10.1016/0006-8993(92)90454-h. [DOI] [PubMed] [Google Scholar]
- Knauber J, Muller WE. Subchronic treatment with prazosin improves passive avoidance learning in aged mice: possible relationships to alpha1-receptor up-regulation. J Neural Transm. 2000;107:1413–1426. doi: 10.1007/s007020070005. [DOI] [PubMed] [Google Scholar]
- Kupferschmidt DA, Tribe E, Erb S. Effects of repeated yohimbine on the extinction and reinstatement of cocaine seeking. Pharmacol Biochem Behav. 2009;91:473–480. doi: 10.1016/j.pbb.2008.08.026. [DOI] [PubMed] [Google Scholar]
- Liang KC, Juler RG, McGaugh JL. Modulating effects of posttraining epinephrine on memory: involvement of the amygdala noradrenergic system. Brain Res. 1986;368:125–133. doi: 10.1016/0006-8993(86)91049-8. [DOI] [PubMed] [Google Scholar]
- Lattal KM. Effects of ethanol on encoding, consolidation, and expression of extinction following contextual fear conditioning. Behav Neurosci. 2007;121:1280–1292. doi: 10.1037/0735-7044.121.6.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattal KM, Barrett RM, Wood MA. Systemic or intrahippocampal delivery of histone deacetylase inhibitors facilitates fear extinction. Behav Neurosci. 2007;121:1125–1131. doi: 10.1037/0735-7044.121.5.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JL, Milton AL, Everitt BJ. Reconsolidation and extinction of conditioned fear: inhibition and potentiation. J Neurosci. 2006;26:10051–10056. doi: 10.1523/JNEUROSCI.2466-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leri F, Rizos Z. Reconditioning of drug-related cues: a potential contributor to relapse after drug reexposure. Pharmacol Biochem Behav. 2005;80:621–630. doi: 10.1016/j.pbb.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Malvaez M, Sanchis-Segura C, Vo D, Lattal KM, Wood MA. Modulation of Chromatin Modification Facilitates Extinction of Cocaine-Induced Conditioned Place Preference. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL, Roozendaal B. Drug enhancement of memory consolidation: historical perspective and neurobiological implications. Psychopharmacology (Berl) 2009;202:3–14. doi: 10.1007/s00213-008-1285-6. [DOI] [PubMed] [Google Scholar]
- Miller LJ. Prazosin for the treatment of posttraumatic stress disorder sleep disturbances. Pharmacotherapy. 2008;28:656–666. doi: 10.1592/phco.28.5.656. [DOI] [PubMed] [Google Scholar]
- Morris RW, Bouton ME. The effect of yohimbine on the extinction of conditioned fear: a role for context. Behav Neurosci. 2007;121:501–514. doi: 10.1037/0735-7044.121.3.501. [DOI] [PubMed] [Google Scholar]
- Morrow BA, Elsworth JD, Rasmusson AM, Roth RH. The role of mesoprefrontal dopamine neurons in the acquisition and expression of conditioned fear in the rat. Neuroscience. 1999;92:553–564. doi: 10.1016/s0306-4522(99)00014-7. [DOI] [PubMed] [Google Scholar]
- Mueller D, Porter JT, Quirk GJ. Noradrenergic signaling in infralimbic cortex increases cell excitability and strengthens memory for fear extinction. J Neurosci. 2008;28:369–375. doi: 10.1523/JNEUROSCI.3248-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nic Dhonnchadha BA, Szalay JJ, Achat-Mendes C, Platt DM, Otto MW, Spealman RD, Kantak KM. D-cycloserine Deters Reacquisition of Cocaine Self-Administration by Augmenting Extinction Learning. Neuropsychopharmacology. 2010;35:357–367. doi: 10.1038/npp.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem. 2009;16:279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci. 2008a;28:6046–6053. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Vallone J, Laurendi K, Kalivas PW. Opposing roles for the ventral prefrontal cortex and the basolateral amygdala on the spontaneous recovery of cocaine-seeking in rats. Psychopharmacology. 2008b;197:319–326. doi: 10.1007/s00213-007-1034-2. [DOI] [PubMed] [Google Scholar]
- Raskind MA, Peskind ER, Kanter ED, Petrie EC, Radant A, Thompson CE, Dobie DJ, Hoff D, Rein RJ, Straits-Troster K, Thomas RG, McFall MM. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003;160:371–373. doi: 10.1176/appi.ajp.160.2.371. [DOI] [PubMed] [Google Scholar]
- Robinson MJ, Franklin KB. Central but not peripheral beta-adrenergic antagonism blocks reconsolidation for a morphine place preference. Behav Brain Res. 2007;182:129–134. doi: 10.1016/j.bbr.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Romaguera J, Sotres-Bayon F, Mueller D, Quirk GJ. Systemic propranolol acts centrally to reduce conditioned fear in rats without impairing extinction. Biol Psychiatry. 2009;65:887–892. doi: 10.1016/j.biopsych.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samini M, Kardan A, Mehr SE. Alpha-2 agonists decrease expression of morphine-induced conditioned place preference. Pharmacol Biochem Behav. 2008;88:403–406. doi: 10.1016/j.pbb.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Santini E, Ge H, Ren K, Pena de Ortiz S, Quirk GJ. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J Neurosci. 2004;24:5704–5710. doi: 10.1523/JNEUROSCI.0786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci. 2009;10:211–223. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- Servatius RJ, Ottenweller JE, Natelson BH. Delayed startle sensitization distinguishes rats exposed to one or three stress sessions: further evidence toward an animal model of PTSD. Biol Psychiatry. 1995;38:539–546. doi: 10.1016/0006-3223(94)00369-E. [DOI] [PubMed] [Google Scholar]
- Storozheva ZI, Afanas’ev II, Proshin AT, Kudrin VS. Dynamics of intracellular dopamine contents in the rat brain during the formation of conditioned contextual fear and extinction of an acoustic startle reaction. Neurosci Behav Physiol. 2003;33:307–312. doi: 10.1023/a:1022831104116. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahsili-Fahadan P, Yahyavi-Firouz-Abadi N, Khoshnoodi MA, Motiei-Langroudi R, Tahaei SA, Ghahremani MH, Dehpour AR. Agmatine potentiates morphine-induced conditioned place preference in mice: modulation by alpha2-adrenoceptors. Neuropsychopharmacology. 2006;31:1722–1732. doi: 10.1038/sj.npp.1300929. [DOI] [PubMed] [Google Scholar]
- Taylor FB, Martin P, Thompson C, Williams J, Mellman TA, Gross C, Peskind ER, Raskind MA. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma posttraumatic stress disorder: a placebo-controlled study. Biol Psychiatry. 2008;63:629–632. doi: 10.1016/j.biopsych.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JR, Olausson P, Quinn JJ, Torregrossa MM. Targeting extinction and reconsolidation mechanisms to combat the impact of drug cues on addiction. Neuropharmacology. 2009;56 Suppl 1:186–195. doi: 10.1016/j.neuropharm.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem. 2006;13:728–733. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Rasmussen DD, Raskind MA, Koob GF. alpha1-noradrenergic receptor antagonism blocks dependence-induced increases in responding for ethanol. Alcohol. 2008;42:91–97. doi: 10.1016/j.alcohol.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J Neurosci. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M, Hart J, Richardson R. Effects of D-cycloserine on extinction of learned fear to an olfactory cue. Neurobiol Learn Mem. 2007;87:476–482. doi: 10.1016/j.nlm.2006.12.010. [DOI] [PubMed] [Google Scholar]