Abstract
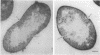
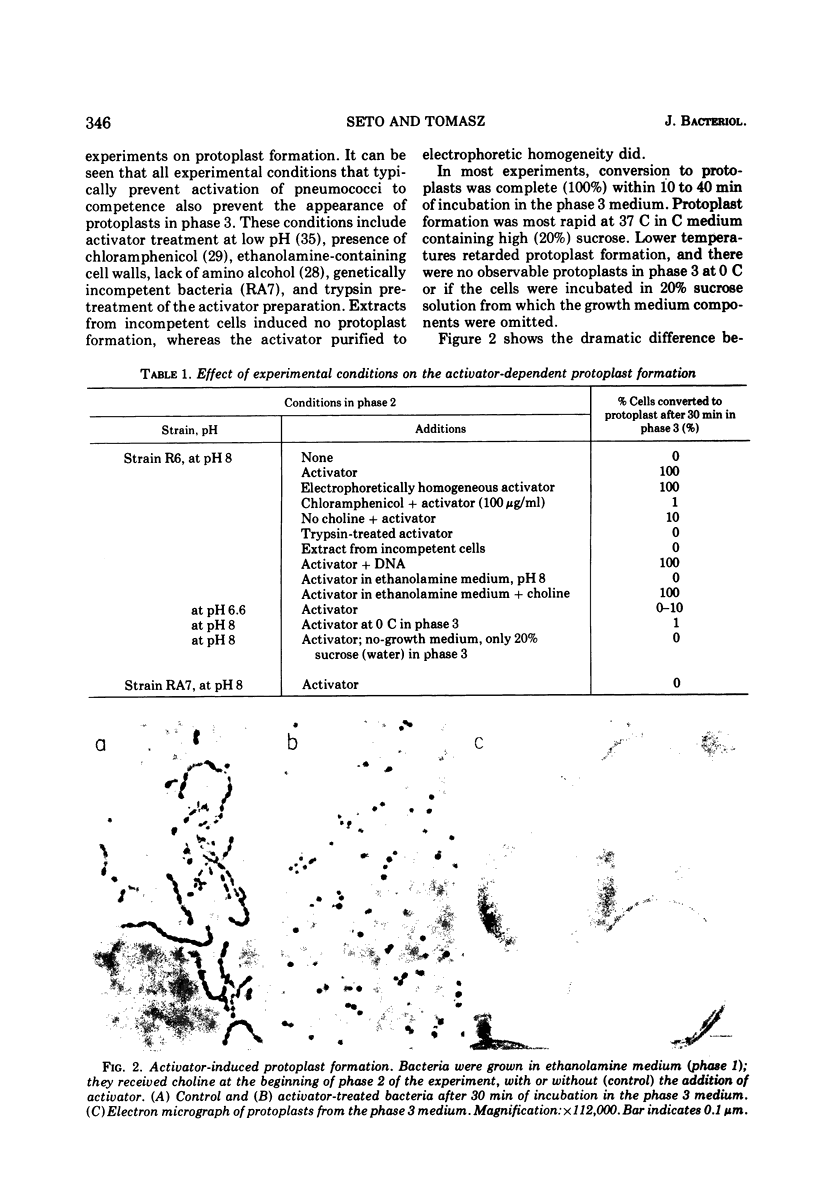
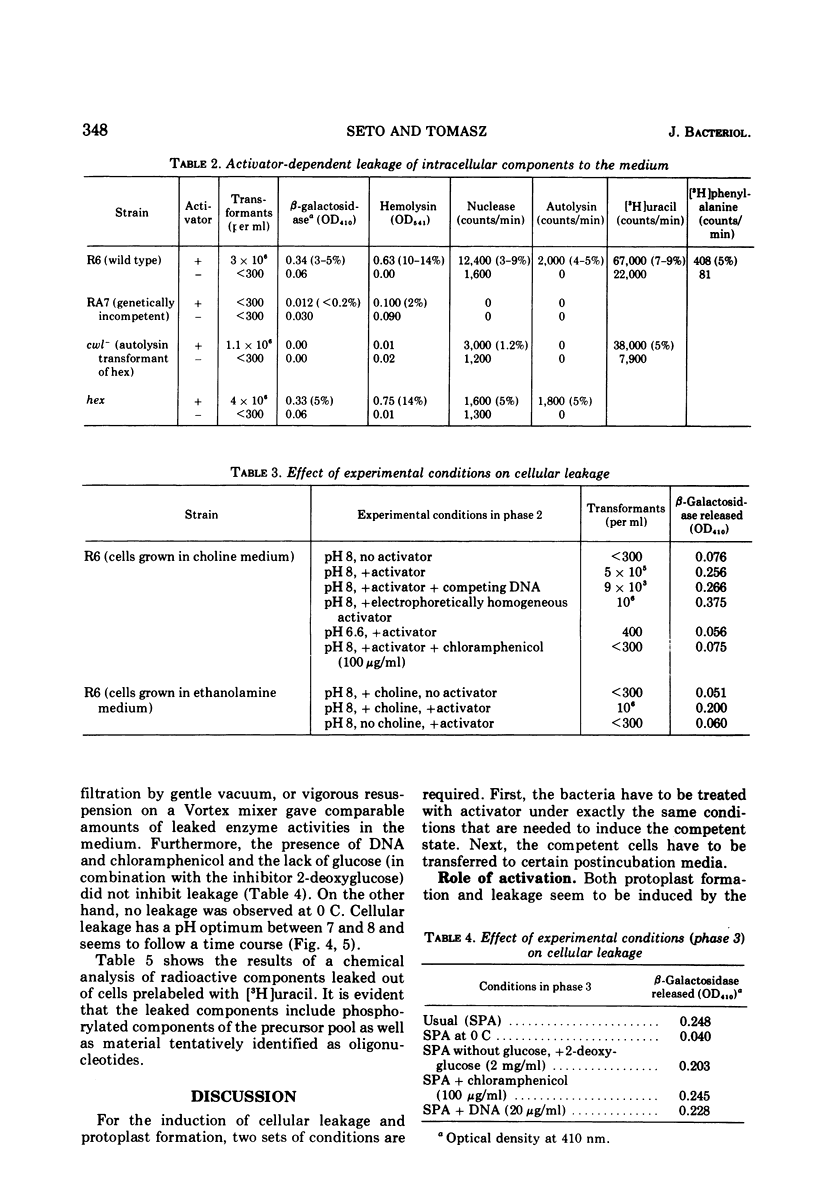
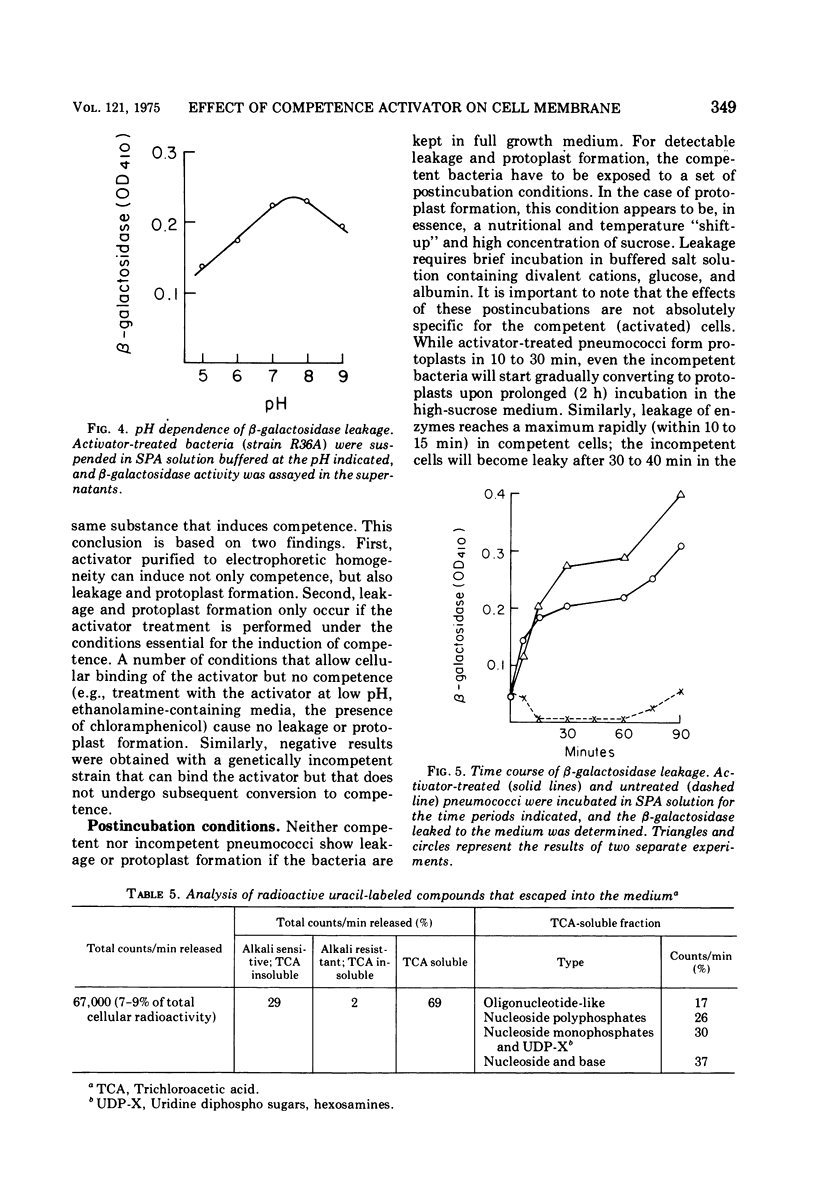
Treatment of pneumococci with activator (a protein that induces bacterial "competence" to absorb deoxyribonucleic acid molecules and undergo genetic transformation) can cause either protoplast formation or leakage of intracellular components to the medium depending on postincubation conditions. The leaked intracellular components include nucleoside phosphates, beta-galactosidase, deoxyribonuclease, autolysin, and hemolysin. Leakage and protoplast formation are induced by the electrophoretically pure activator, and these phenomena require the same conditions as induction of competence for genetic transformation, namely, genetic capacity for competence, protein synthesis, incorporation of choline, and the optimal pH for activation. It is suggested that the activator protein accelerates a normal process of transport (leakage) of autolysin molecules into the periplasmic space. The activity of these autolysin molecules from within would then unmask deoxyribonucleic acid binding sites located on the plasma membrane.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akrigg A., Ayad S. R., Barker G. R. The nature of a competence-inducing factor in Bacillus subtilis. Biochem Biophys Res Commun. 1967 Sep 27;28(6):1062–1067. doi: 10.1016/0006-291x(67)90090-3. [DOI] [PubMed] [Google Scholar]
- Akrigg A., Ayad S. R. Studies on the competence-inducing factor of Bacillus subtilis. Biochem J. 1970 Apr;117(2):397–403. doi: 10.1042/bj1170397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briles E. B., Tomasz A. Radioautographic evidence for equatorial wall growth in a gram-positive bacterium. Segregation of choline-3H-labeled teichoic acid. J Cell Biol. 1970 Dec;47(3):786–790. doi: 10.1083/jcb.47.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D., Cirigliano C. Fate of transforming DNA following uptake by competent Bacillus subtilis. Formation and properties of products isolated from transformed cells which are derived entirely from donor DNA. J Mol Biol. 1972 Feb 28;64(1):9–29. doi: 10.1016/0022-2836(72)90318-x. [DOI] [PubMed] [Google Scholar]
- Ephrussi-Taylor H., Freed B. A. Incorporation of thymidine and amino acids into deoxyribonucleic acid and acid-insoluble cell structures in pneumococcal cultures synchronized for competence to transform. J Bacteriol. 1964 May;87(5):1211–1215. doi: 10.1128/jb.87.5.1211-1215.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERSHEY A. D., DIXON J., CHASE M. Nucleic acid economy in bacteria infected with bacteriophage T2. I. Purine and pyrimidine composition. J Gen Physiol. 1953 Jul;36(6):777–789. doi: 10.1085/jgp.36.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback H. R. Transport. Annu Rev Biochem. 1970;39:561–598. doi: 10.1146/annurev.bi.39.070170.003021. [DOI] [PubMed] [Google Scholar]
- Kohoutová M., Braná H., Holubová I. Isolation and purification of a substance inducing competence and inactivating transforming DNA in Pneumococcus. Biochem Biophys Res Commun. 1968 Jan 25;30(2):124–129. doi: 10.1016/0006-291x(68)90458-0. [DOI] [PubMed] [Google Scholar]
- Lacks S., Greenberg B. Competence for deoxyribonucleic acid uptake and deoxyribonuclease action external to cells in the genetic transformation of Diplococcus pneumoniae. J Bacteriol. 1973 Apr;114(1):152–163. doi: 10.1128/jb.114.1.152-163.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S. Mutants of Diplococcus pneumoniae that lack deoxyribonucleases and other activities possibly pertinent to genetic transformation. J Bacteriol. 1970 Feb;101(2):373–383. doi: 10.1128/jb.101.2.373-383.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy C., Nester E. W. Macromolecular synthesis in newly transformed cells of Bacillus subtilis. J Bacteriol. 1967 Jul;94(1):131–140. doi: 10.1128/jb.94.1.131-140.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. A., Guild W. R. Structure of deoxyribonucleic acid on the cell surface during uptake by pneumococcus. J Bacteriol. 1973 Sep;115(3):1055–1062. doi: 10.1128/jb.115.3.1055-1062.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser J. L., Tomasz A. Choline-containing teichoic acid as a structural component of pneumococcal cell wall and its role in sensitivity to lysis by an autolytic enzyme. J Biol Chem. 1970 Jan 25;245(2):287–298. [PubMed] [Google Scholar]
- NESTER E. W., STOCKER B. A. BIOSYNTHETIC LATENCY IN EARLY STAGES OF DEOXYRIBONUCLEIC ACIDTRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1963 Oct;86:785–796. doi: 10.1128/jb.86.4.785-796.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OTTOLENGHI E., HOTCHKISS R. D. Release of genetic transforming agent from pneumococcal cultures during growth and disintegration. J Exp Med. 1962 Oct 1;116:491–519. doi: 10.1084/jem.116.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PUCK T. T., LEE H. H. Mechanism of cell wall penetration by viruses. II. Demonstration of cyclic permeability change accompanying virus infection of Escherichia coli B cells. J Exp Med. 1955 Feb 1;101(2):151–175. doi: 10.1084/jem.101.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakula R., Hauschild A. H. The effect of "competase" on DNA uptake in provoked transformation of a streptococcus. Can J Microbiol. 1965 Oct;11(5):823–827. doi: 10.1139/m65-111. [DOI] [PubMed] [Google Scholar]
- Ranhand J. M., Leonard C. G., Cole R. M. Autolytic activity associated with competent group H streptococci. J Bacteriol. 1971 Apr;106(1):257–268. doi: 10.1128/jb.106.1.257-268.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J. Bacterial growth and the cell envelope. Bacteriol Rev. 1970 Jun;34(2):194–214. doi: 10.1128/br.34.2.194-214.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel R., Slade H. D. Properties of a Streptococcus sanguis (group H) bacteriocin and its separation from the competence factor of transformation. J Bacteriol. 1973 Aug;115(2):655–661. doi: 10.1128/jb.115.2.655-661.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Daneo-Moore L., Higgins M. L. Problems of cell wall and membrane growth, enlargement, and division. Ann N Y Acad Sci. 1974 May 10;235(0):161–197. doi: 10.1111/j.1749-6632.1974.tb43265.x. [DOI] [PubMed] [Google Scholar]
- Shockman G. D., Thompson J. S., Conover M. J. Replacement of Lysine by Hydroxylysine and Its Effects on Cell Lysis in Streptococcus faecalis. J Bacteriol. 1965 Sep;90(3):575–588. doi: 10.1128/jb.90.3.575-588.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMAS R. Recherches sur la cinétique des transformations bactériennes. Biochim Biophys Acta. 1955 Dec;18(4):467–481. doi: 10.1016/0006-3002(55)90137-2. [DOI] [PubMed] [Google Scholar]
- TOMASZ A., HOTCHKISS R. D. REGULATION OF THE TRANSFORMABILITY OF PHEUMOCOCCAL CULTURES BY MACROMOLECULAR CELL PRODUCTS. Proc Natl Acad Sci U S A. 1964 Mar;51:480–487. doi: 10.1073/pnas.51.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tichy P., Landman O. E. Transformation in quasi spheroplasts of Bacillus subtilis. J Bacteriol. 1969 Jan;97(1):42–51. doi: 10.1128/jb.97.1.42-51.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A. Biological consequences of the replacement of choline by ethanolamine in the cell wall of Pneumococcus: chanin formation, loss of transformability, and loss of autolysis. Proc Natl Acad Sci U S A. 1968 Jan;59(1):86–93. doi: 10.1073/pnas.59.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A. Cellular metabolism in genetic transformation of pneumococci: requirement for protein synthesis during induction of competence. J Bacteriol. 1970 Mar;101(3):860–871. doi: 10.1128/jb.101.3.860-871.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A., Mosser J. L. On the nature of the pneumococcal activator substance. Proc Natl Acad Sci U S A. 1966 Jan;55(1):58–66. doi: 10.1073/pnas.55.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A. The role of autolysins in cell death. Ann N Y Acad Sci. 1974 May 10;235(0):439–447. doi: 10.1111/j.1749-6632.1974.tb43282.x. [DOI] [PubMed] [Google Scholar]
- Tomasz A., Zanati E., Ziegler R. DNA uptake during genetic transformation and the growing zone of the cell envelope. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1848–1852. doi: 10.1073/pnas.68.8.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDEL W., PELZER H. BAGSHAPED MACROMOLECULES--A NEW OUTLOOK ON BACTERIAL CELL WALLS. Adv Enzymol Relat Areas Mol Biol. 1964;26:193–232. doi: 10.1002/9780470122716.ch5. [DOI] [PubMed] [Google Scholar]
- Ziegler R., Tomasz A. Binding of the competence factor to receptors in the spheroplast membrane of pneumococci. Biochem Biophys Res Commun. 1970 Dec 9;41(5):1342–1349. doi: 10.1016/0006-291x(70)90236-6. [DOI] [PubMed] [Google Scholar]