Abstract
Auditory spatial acuity was measured in mice using prepulse inhibition (PPI) of the acoustic startle reflex (ASR) as the indicator response for stimulus detection. The prepulse was a “speaker swap” (SSwap), shifting a noise between two speakers located along the azimuth. Their angular separation, and the spectral composition and sound level of the noise were varied, as was the interstimulus interval (ISI) between SSwap and ASR elicitation. In Experiment 1 a 180° SSwap of wide band noise (WBN) was compared with WBN Onset and Offset. SSwap and WBN Onset had near equal effects, but less than Offset. In Experiment 2 WBN SSwap was measured with speaker separations of 15°, 22.5°, 45°, and 90°. Asymptotic level and the growth rate of PPI increased with increased separation from 15° to 90°, but even the 15° SSwap provided significant PPI for the mean performance of the group. SSwap in Experiment 3 used octave band noise (2–4, 4–8, 8–16, or 16–32 kHz) and separations of 7.5° to 180°. SSwap was most effective for the highest frequencies, with no significant PPI for SSwap below 8–16 kHz, or for separations of 7.5°. In Experiment 4 SSwap had WBN sound levels from 40 to 78 dB SPL, and separations of 22.5°, 45°, 90° and 180°: PPI increased with level, this effect varying with ISI and angular separation. These experiments extend the prior findings on sound localization in mice, and the dependence of PPI on ISI adds a reaction-time-like dimension to this behavioral analysis.
Keywords: Spatial processing, processing time, minimum audible angle, startle inhibition
Introduction
The neurobiological explanation of how mammals locate sound objects in space has historically advanced through multidisciplinary research projects that have combined anatomical and/or electrophysiological methods with behavioral psychophysics (for example, the seminal works of Neff et al., 1956; Galambos et al., 1959; Masterton et al., 1975). These experiments, and the many that have succeeded them, have most often studied sound localization in large laboratory animals, e.g., cats, dogs, or monkeys, which have sufficiently large heads to provide both major cues for sound localization along the azimuth: the interaural timing difference cues (ITD) for low frequency signals; and interaural level difference cues (ILD) for high frequency signals. In addition, these animals also make use of monaural mid- and high-frequency spectral cues that are created by head and pinna position relative to the sound source for additional azimuthal guidance as well as for elevation location (e.g. in the cat, Rice et al., 1992). Further benefits of using these particular animals for sensory behavioral studies are that they can be readily motivated and trained in auditory discrimination tasks, and that the data they provide can rival the psychophysical results obtained from human observers (as shown, for example, by Huang & May, 1996; Populin & Yin, 1998). In total, these research programs have produced a systems level description of the neural mechanisms by which monaural spectral and binaural timing and level cues are first separated and then re-integrated in the auditory brainstem, and at more rostral sites, thus providing the neural basis for sensory-behavioral spatial localization as described, for example, by Yin (2002) and Schofield (2005).
In contrast to this preference for large laboratory animals in the study of central auditory processing, hearing science in recent years has increasingly focused on the mouse as being most useful for understanding the biological composition and biophysical operation of the peripheral auditory system. This trend is due to advances in molecular biology and is most obvious in the growing application of mouse genetic engineering to the study of peripheral hearing loss and its genetic and cellular bases (Brown et al., 2008).
Historically, the mouse has not been considered an appropriate animal for the study of sound localization, mainly because in contrast to larger mammals it is reasonably understood to make little if any use of ITD cues, relying primarily on ILD cues to locate sounds. The diameter of the mouse head is ~16 mm (Chen et al., 1995), which provides a maximal left vs. right ITD of only about 100 µsec. Li and Borg (1991) among others have shown that it has very poor sensitivity to low frequency stimuli below about 1.5 kHz, known to provide useful ITD information in mammals (e.g., Mills 1958). In contrast, the maximal left-right ILD varies over a useful range of 10 to about 60 dB with increasing audio-frequency from 10 kHz to 80 kHz (Chen et al., 1995). Consequently, there is only a scant behavioral literature devoted to the study of sound localization in the mouse.
Ehret & Dreyer (1984) trained water-deprived outbred NMRI mice (successfully in 8 of a beginning group of 10) with a water reward that the mice received for approaching distant sounds placed around the perimeter of a small arena. They reported minimum audible angles for threshold discrimination (MAA) of 7° for broadband noise and 9.5–15° for tonal stimuli, but their mice required continuous presentation of the stimuli during the trials to perform this task. Heffner & Heffner (1988a) used a conditioned avoidance procedure and a 50% criterion for threshold detection to show that water deprived predator grasshopper mice (n = 3) had an MAA of about 19°, while Heffner et al. (2001) later tested C57/BL6 mice (n = 3) with the same procedure to find an MAA of 33° at 2 months of age that increased to 46° at 7 months of age. This strain is well known for its age-related progressive hearing loss beginning with the highest audio-frequencies (shown by Mikaelian, et al., 1974, in behavioral tests, and more recently with ABR thresholds by Li & Borg, 1991, and many others). The increase in the MAA of older C57 mice was attributed by Heffner et al. (2001) to its high frequency hearing loss and the consequent loss of ILD cues. Heffner at al. (2001) also used different upper cut-offs in low-pass noise to show that their young C57 mice performed less well when the stimulus band was restricted to 20 kHz and below, this supporting their explanation of the poorer performance of the older mice. Their data in young mice (Heffner & Heffner, 1988a; Heffner et al., 2001) contrast with the Ehret & Dreyer (1984) report of a MAA of 12° for 1 kHz tones. This discrepancy between these two experiments is readily ascribed to the unique semi-realistic test arena used by Ehret & Dreyer (1984), as the freely moving animal could possible learn to “home-in” on the target by listening for increases and decreases in sound level as it explored the test chamber.
In addition to poor ITD sensitivity, another impediment to the study of sound localization in mice is that some aspects of the classic behavioral-psychophysical tests based on reinforcement or punishment training schedules, so useful for assessing sensory abilities in other laboratory animals (cf. Fay, 1988), have been difficult to apply to mice, as described, for example, by Heffner & Heffner (2001). As such, these methods are best employed for studying small groups of animals that can be presumed to be representative of the strain or species, or in “life-span” experiments in which the behavioral effects of prolonged initial training can be maintained with repeating testing (e.g., May et al., 2006; Mikalian et al., 1974). The assumption of homogeneity across animals may not hold for genetically-engineered animals and, further, their having a normal lifespan is not assured: beyond these caveats, it is almost certain that testing relatively large groups of mice will be necessary to assure sufficient power for the statistical comparison of differences between groups of normal vs. mutant animals.
Recent in vitro and in vivo electrophysiological investigations of the mouse auditory brainstem that include the study of genetically engineered mice have greatly enlarged our understanding of the neurobiology of brainstem nuclei that process sound location cues (for example, Brew & Forsythe, 1995; Golding et al., 1999; Rothman & Manis, 2003; Brew et al., 2003; Kopp-Scheinpflug et al., 2003; Gittelman & Temple, 2006). However, the present lack of rapid and objective methods for studying auditory processing in awake and behaving mice is an impediment to transporting these same genetic engineering techniques into the study of the molecular basis of sensory and perceptual processes.
With these requirements in mind, in the present experimental series we use a novel application of Reflex Modification Audiometry (RMA, Young & Fechter, 1983) to assess the ability of CBA/CaJ mice to detect a change in the location of a sound source. RMA is a rapid method of determining whether an animal has detected a signal (the prepulse) by observing its indirect effect on the expression of a reflex by an eliciting stimulus (ES) presented shortly afterward.
Ison & Hoffman (1983) reviewed the history of research on reflex modification, which dates back to the initial discoveries of Sechenov (1863/1965), while Koch (1999) and Swerdlow et al. (2001) have reviewed its physiological bases. As described by Hoffman & Ison (1992), RMA provides a sensitive objective test of sensory function in developmental and comparative research that is non-invasive, does not require food or water deprivation, and requires limited or no training prior to testing. These characteristics may make this method very useful for the behavioral study of spatial localization as well as for other examples of complex auditory processing in genetically modified mice.
Reflex modification by preliminary stimuli varies with the time interval by which the prepulse leads the ES (the interstimulus interval, ISI). Reflex effects at ISIs beyond about 20 to 40 ms are sensitive to pharmacological challenges and to cortical ablation, which have no effect at shorter ISIs (for gap detection in rats, Ison et al., 1991; Ison & Bowen, 2000; and Bowen et al., 2003; and for SSwap in mice, Ison et al., 2009). These data suggest the involvement of different neural loci in PPI with increasing ISI. Changes in PPI with latency might reflect a temporal progression of processing along the afferent auditory pathway, though at present this model lacks a detailed neurophysiological basis, in contrast with other methods such as the ABR (for example, Melcher et al. 1996).
The empirical aim of the present investigation was to determine the effects on reflex modification of manipulating the standard stimulus parameters important for sound localization, namely, the angular separation between two sounds, their spectral composition, and their sound level.
Methods
Subjects
The mice (N = 31 in total) were all of the CBA/CaJ inbred strain, bred at the University of Rochester from Jackson Laboratory stock (Bar Harbor, ME). The same group of 3–4 month old mice (10 male and 3 female) was tested in Experiments 1 and 2. A second group of 11–14 month old mice (3 male and 9 female) were tested in Experiment 3, and a third group of 6 month old mice (3 male and 3 female) were tested in Experiment 4. While many strains of mice show early onset age-related hearing loss (Zheng et al., 1999), the CBA/CaJ does not show significant high frequency hearing loss until they approach 2 years of age (Li and Borg, 1991). Thus in contrast to the C57BL/6J mice studied by Heffner et al. (2001), there is no a priori reason to expect age-related performance differences between these three groups of adult mice. All mice were group-housed (2 to 3 mice per cage) in the University of Rochester vivarium in a controlled constant climate and a 12/12 h normal L/D cycle, with testing during the daylight hours. Food and water was freely available except during testing, which lasted for about 1 h per session. The ambient noise level in the colony was 40 dB SPL at 2 kHz and decreased linearly to 25 dB SPL at 24 kHz on a log-frequency scale. The University of Rochester Committee on Animal Resources approved all procedures, which were in accord with USPHS regulations and the Federal Animal Welfare Act.
Apparatus
Experiments were conducted within a sound-attenuating room (IAC, Bronx, NY) with echo-attenuating acoustical foam (Sonex; Illbruck, Minneapolis, MN) lining the walls. One mouse was tested at a time while confined in an aluminum wire cage, 5 cm wide, 7 cm long, and 4 cm high, having free sound penetration. The mouse was further restricted by adjustable wire combs oriented with the long dimension of the cage, which lightly pressed against its sides. Startle stimuli were 15 ms broad-band noise bursts (rectangular-gated, 50 kHz bandwidth, 120 dB SPL) digitally generated using a Tucker-Davis Technology (TDT, Alachua, FL) RP2.1 Real-time Processor. Startle eliciting stimuli (ES) were attenuated using a TDT PA5, then amplified with an Adcom (East Brunswick, NJ) GFA-535 II amplifier and broadcast from 15 cm above the mouse via a Yamaha JA4281B compression tweeter. Carrier stimuli in these experiments were broadcast from one of two matched TDT-ES1 electrostatic speakers located 50 cm from the mouse’s head in the azimuthal plane (but 100 cm in Experiments 3 and 4). The carriers in Experiments 1, 2 and 4 consisted of rectangular-gated (~0 ms rise-fall time) broadband noise (1 – 50 kHz and 70 dB SPL in Experiments 1 and 2, but varying between 40, 50, 60, 70, and 78 dB SPL in Experiment 4). The carrier in Experiment 3 was a 70 dB SPL broadband noise that was then octave-band filtered using a 4th order digital IIR filter implemented onboard the RP2.1, with coincident 2 ms rise-fall time shaping and a 50 dB SPL broadband floor to mask any switching transients. These carriers were digitally generated using a second TDT-RP2 (100 kHz sample rate) in real time. Sound levels were measured with a ¼” microphone (Bruel & Kjær model 4135) connected to a measuring amplifier (Bruel & Kjær model 2610). The two matched carrier speakers were positioned with angular separations of 180, 90, 45, 22.5, or 15° (7.5° instead of 15° in Experiment 3; only 180° in Experiment 1), with each speaker facing the test cage. The testing cage was oriented so that the mouse’s head faced the mid-line between the two speakers and was mounted on a 15-cm long pedestal that was bolted to a suspended acrylic platform to which an accelerometer was attached. The startle speaker and its supports, the pedestal and the acrylic shelf, and the table on which the apparatus was placed were all covered with echo absorbing foam or carpeting. The force of the startle reflex was transduced by the accelerometer and the voltage output sampled at 1 kHz by the first RP2.1. The startle response amplitude was the RMS of this output in the 100 ms period after the delivery of the startle stimulus. The experimental stimuli were controlled and the responses recorded by a PC using a custom LabView (National Instruments) front-end. The mouse was placed into the test cage in a second IAC-sound attenuating holding room, and then the cage was taken next door to the test room and fastened to the pedestal. Test trials began 2–4 minutes later and continued for about 60 minutes.
General Procedures
The prepulse in each experiment was a change in the location of the noise carrier between two matched speakers (SSwap). In Experiment 1 there were two additional conditions of Noise Onset or Noise Offset from a single speaker. The stimuli were presented at different interstimulus intervals (ISI) prior to the ES, in order to determine the time course of their effect as well as its peak. In Experiment 1 there were two test sessions, in Experiment 2 there were four, in Experiment 3 there were five, and in Experiment 4 there were eight. Each session lasted approximately 60 minutes and several rest days separated the sessions. The speakers had a fixed angular spacing in any one test session, while different audio-frequency (Experiment 3) or sound level (Experiment 4) conditions for the noise band carrier were varied within each sessions. All sessions began with the mouse being placed within the testing cage in the startle chamber for a 2 minute period in the presence of continuous background noise presented from one of the carrier speakers (or quiet for the ‘Onset’ session in Experiment 1). There were 11 presentations of each condition, these being block randomized with 16 conditions in each block. These conditions were: presentation of the prepulse with ISI = 1, 2, 5, 10, 20, 30, 40, 50, 60, 100, 150, 200, or 300 ms (10, 50, and 200 ms in Experiments 3 and 4); two no-prepulse baseline startle control trials; and a no-ES control to measure background activity. The intertrial interval averaged 20 s and was randomly selected from the range 15 to 25 s. Stimulus presentation and response recording were both controlled by the computer and the animal’s general behavior (for example, grooming or head orientation) was not monitored by the experimenters during the test period, nor was there any later selection or rejection of the resulting responses.
Data Analysis
The mean of the ASR values for each condition was calculated for each subject, after excluding the first block of trials to avoid possible skewing effects of an initial adaptation to the ES. The mean ASR amplitude measures are given in arbitrary voltage units (aV-units), which are internally consistent across experiments. Prepulse inhibition scores were calculated as a ratio of each subject’s mean response amplitude in the prestimulus condition (ASRp) compared with the no-prepulse control baseline (ASRc), using the formula
Repeated-measures analyses of variance (ANOVA) with ISI duration, speaker angle, and/or noise band spectrum and level as within-subject variables were performed with SPSS v.16 (SPSS Inc, Chicago, IL). The p-values provided by the ANOVA were adjusted via the Hunyh-Feldt method for non-homogeneity of between-cell correlations and effect sizes were determined by SPSS partial-Eta-squared measures (ηp2). Correlations between conditions across subjects are reported as Pearson’s r-statistic. Graphical presentation of the data and supplemental t-tests on specific stimulus conditions within- and between-subjects used GraphPad Prism software (version 4.2), and effect sizes were determined by R2. The effect sizes for individual animals and conditions was provided by Cohen’s d-statistic (Cohen, 1992, p. 157), calculated for each subject as the mean ASR difference within each block between the mean of the two C trials and the mean of selected P condition trials, and the mean of the block-means was divided by the standard deviations of the differences.
Experimental Designs, Rationale, and Results
Experiment 1
Experiments on reflex modification have typically used a simple prepulse such as a burst or an increment in acoustic energy, or less often an offset or energy decrement as the prepulse, or a photic prepulse, but it has been hypothesized that any perturbation in the stimulus environment immediately prior to a reflex eliciting stimulus would affect reflex expression (Hoffman & Ison, 1980). Thus Experiment 1 was designed to determine if PPI would be observed when an ongoing noise was moved over a 180° separation from the right side to the left side of the mouse while maintaining the overall sound level. The SSwap condition was compared with the two separate manipulations of the offset of an ongoing noise, or the onset of noise presented in a quiet background.
In the interpretation of the results of this experimental design it is important to note the startle amplitudes are affected by prevailing background noise as well as by prepulse stimuli (e.g., Hoffman & Searle, 1965). For this reason, it was anticipated that the control reflex amplitudes would differ between the noise offset vs. onset sessions because the control ES was delivered in noise vs. in quiet, and also the PPI values would in part reflect the noise levels prevailing at the time of the prepulse. In principle, of the three prepulse types studied here, the SSwap session provides the best example of a pure prepulse effect, because this prepulse is an exchange of sound location between the two ears that does not alter the overall noise level.
Procedure
The general methods were those described above. There were three conditions, each testing a different type of prestimulus: SSwap, with the speakers separated by 180° right and left; Noise Offset in the right hand speaker, with the ES presented in quiet; and Noise Onset in the left hand speaker with the ES presented in a noise background. The prestimuli were followed by the ES at ISI of 1, 2, 5, 10, 20, 30, 40, 50, 60, 100, 150, 200, and 300 ms, in order to track both the temporal development and the decay of PPI as well as its maximum strength. The experiment was planned to last for three test days with all animals being tested in each of the three conditions, but ended after two days when the results were already decisive: at this time 9 mice had been tested with SSwap, 11 with Noise Offset, and 6 with Noise Onset.
Results
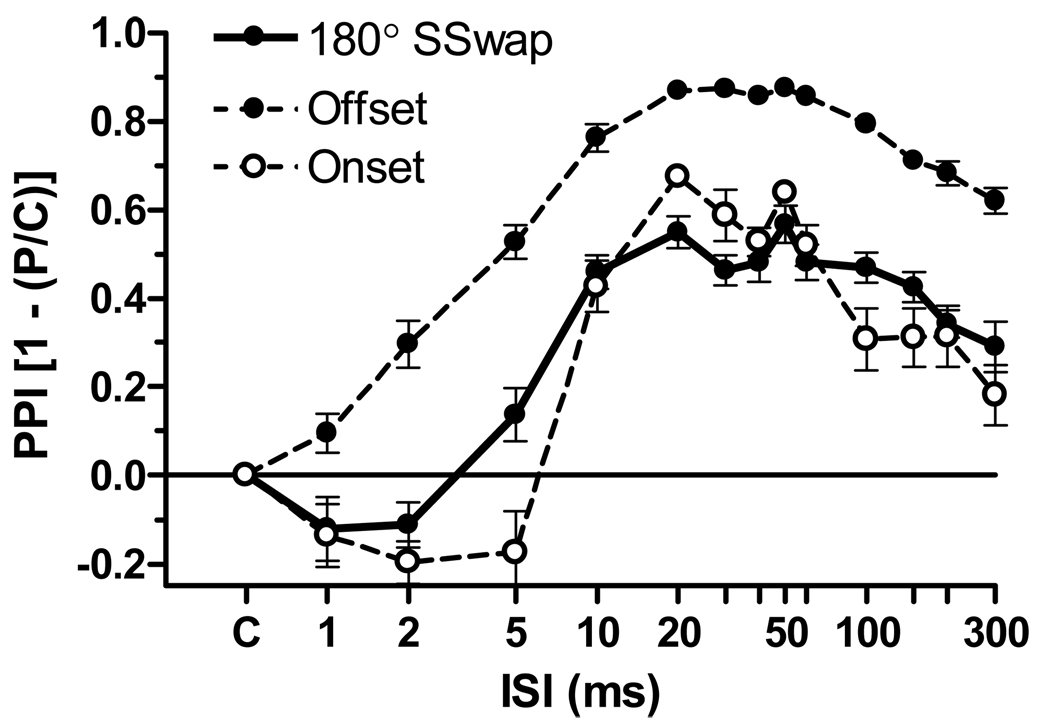
Figure 1 shows the Mean (SEM) PPI values as a function of ISI for each of the three stimulus conditions. All provided a rapidly increasing PPI effect that rose to a broad peak beginning at about 10 to 20 ms, this followed by a more gradual decay after about 60 to 100 ms. The ANOVA of these data proceeded on the conservative assumption that the comparisons across the three stimulus conditions were uncorrelated so that stimulus conditions could be treated as a between-subjects effect, while the comparison across ISI was a within-subjects effect. Over all three conditions the main effect of ISI was significant, F(13/299) = 167.20, p < 0.001, ηp2 = 0.88, as was the stimulus condition, F(2/23) = 85.53, p < .001, ηp2 = 0.36. The contrast of SSwap with Noise Onset was significant only for their interaction with ISI, F (13/169) = 3.57, p < 0.001, ηp2 = 0.21. Of primary importance, the 180° SSwap alone provided very strong PPI, that was significant for the group as early as 5 ms, t(8) = 2.32, p < 0.05, R2 = 0.40, and reached a near asymptotic peak at 10 ms that persisted for about 100 ms before beginning a gradual decay. Seven of the nine mice provided significant PPI (p < 0.01) for SSwap between 10 and 100 ms, the two exceptions showing “near” significant results at p = 0.07 and p = 0.08.
Figure 1.
Mean (SEM) PPI values as a function of the ISI. Three prepulse conditions were compared: 1) a 180° change in the location of a wide band noise (WBN) from right to left; 2) the offset of WBN that had been presented in the right hand speaker, the ES being presented in quiet for prepulse trials and in a background of WBN for control trials; and 3) the onset of the WBN in the left hand speaker, the ES being presented in a background of quiet in control trials and in the presence of the WBN for prepulse trials. (Experiment 1, n = 9, 11, and 6).
Noise Offset provided more PPI at its peak and a more rapid development with increasing ISI than SSwap. An Offset PPI effect that was marginal at the 1 ms ISI, t(10) = 2.19, p = 0.054, R2 = 0.32, was highly significant for every ISI thereafter (p < 0.001). Noise Onset provided a similar level of peak PPI as SSwap but had the longest delay, with significant PPI at 10 ms, t(5) = 7.49, p < 0.001, R2 = 0.91, but also significant levels of PPI for every ISI thereafter (at least p < 0.01, save at 300 ms, p < 0.05). It may be noted in Figure 1 that both SSwap and Noise Onset provided an initial brief period of reflex facilitation but this was significant only for the Noise Onset condition at the 2 ms ISI, t(5) = 4.09, p < 0.001, R2 = 0.77. Startle reflex facilitation by auditory onsets and inhibition by offsets at very brief ISIs are common finding in similar experiments (Ison et al., 1997; Ison et al., 2003).
The effect of the background noise level on the amplitude of the baseline ASR was prominent in Experiment 1: the control baseline ASR mean (SD, n) for the SSwap condition was 4029 (1374, 9) aV-units, which was very similar to the mean for the Noise Offset condition of 4057 (1019, 11). However these contrasted with the mean for the Noise Onset condition for which the control startle was given in quiet, which was just 2516 (608, 6). The ANOVA of these data provided a significant effect of stimulus condition, F(2/25) = 4.53, p = 0.02, ηp2 = 0.28.
These data indicate that the effects of the Noise Onset and Offset prestimuli have two components, one being the transient prepulse effect and the other the ongoing effect of the continuous noise at the time of ASR elicitation. This latter effect adds to the SSwap PPI for the Offset condition but diminishes it for the Onset condition, a difference that possibly accounts for the PPI difference between these two stimulus types apparent in Figure 1. In contrast, the same level of background noise was always present for the SSwap condition, and its PPI effect can be ascribed entirely to the SSwap stimulus. Table 1 provides the Mean (SD, Range) for Cohen’s d effect size statistic for the three prepulse conditions, averaged over ISI values of 10 to 100 ms: this measure complements the average PPI statistics with a index that reflects the degree of trial-to-trial variability between control and inhibited trials within each subject.
Table 1.
Cohen’s d: The Effect Size for SSwap in Each Experiment
| Exp # (n) | Source | Mean | SD | Range |
|---|---|---|---|---|
| EXP 1(13) | ||||
| Onset | +2.57 | 0.95 | +1.70, +4.67 | |
| Offset | +1.50 | 0.81 | +0.80, +3.00 | |
| 180° SSwap | +1.35 | 0.53 | +0.63, +2.18 | |
| EXP 2 (13) | ||||
| 90° SSwap | +0.98 | 0.48 | +0.27, +1.69 | |
| 45° SSwap | +1.30 | 0.51 | +0.63, +2.58 | |
| 22.5° SSwap | +0.97 | 0.95 | +0.01, +3.76 | |
| 15° SSwap | +0.51 | 0.34 | −0.08, +1.00 | |
| EXP 3(12) | ||||
| 16–32 kHz | ||||
| 180° SSwap | +0.68 | 0.66 | −0.44, +1.98 | |
| 90° SSwap | +0.55 | 0.40 | −0.12, +1.34 | |
| 45° SSwap | +0.54 | 0.54 | +0.14, +1.32 | |
| 22.5° SSwap | +0.24 | 0.27 | −0.34, +0.66 | |
| 7.5° SSwap | +0.04 | 0.04 | −0.54, +0.65 | |
| 8–16 kHz | ||||
| 180° SSwap | +0.62 | 0.31 | +0.19, +1.09 | |
| 90° SSwap | +0.30 | 0.427 | −0.46, +1.53 | |
| 4–8 kHz | ||||
| 180° SSwap | +0.09 | 0.51 | −0.36, +0.26 | |
| 2–4 kHz | ||||
| 180° SSwap | +0.03 | 0.43 | −1.09, +0.51 | |
| EXP 4 (6) | ||||
| 40 dB SPL | ||||
| 180° SSwap | +0.61 | 0.25 | +0.24, +0.98 | |
| 90° SSwap | +0.41 | 0.48 | −0.20, +1.44 | |
| 45° SSwap | +0.54 | 0.21 | +0.30, +0.97 | |
| 22.5° SSwap | +0.47 | 0.15 | +0.26, +0.63 | |
| 78 dB SPL | ||||
| 180° SSwap | +1.80 | 0.66 | +1.16, +2.76 | |
| 90° SSwap | +1.02 | 0.26 | +0.71, +1.26 | |
| 45° SSwap | +1.07 | 0.26 | +0.81, +1.35 | |
| 22.5° SSwap | +1.04 | 0.31 | +0.65, +1.54 | |
Experiment 2
Experiment 1 established that the maximal possible shift in sound location for a wide band noise over a separation of 180° provides a very strong PPI effect on the ASR. PPI reached a near asymptotic level within ISIs of about 10 ms and then gradually decayed beyond about 100 ms. These positive findings encouraged this second experiment, designed to determine how PPI would change as the angular separation between the two speakers in the SSwap condition was progressively reduced. As described above, previous work on spatial acuity in the mouse has variously reported thresholds for noise bands between 9° and 33°, this depending on the strain of mouse and the testing method (Ehret & Dreyer, 1984; Heffner et al., 2001). In Experiment 2 we used four angular separations of 90°, 45°, 22.5° and 15°, and again used the same ISI of 1 to 300 ms in order to determine the onset latency and the temporal development and decay of PPI.
Procedure
The general methods were those described above, and the subjects had been previously tested in Experiment 1. There were four experimental sessions, each presenting the SSwap paradigm described in Experiment 1, but with a different angle between the speakers in each session. The first session used a 15° speaker angle as a contrast to the very effective 180° condition given in Experiment 1. Given modest evidence of PPI at 15°, the next session presented used the 90° separation, followed then by 45°, and finally the 22.5° separation. The SSwap prestimuli were followed by the ES as in Experiment 1 with ISI of 1, 2, 5, 10, 20, 30, 40, 50, 60, 100, 150, 200, and 300 ms, there were two ES-alone control trials, and one no-stimulus condition to measure background activity.
Results
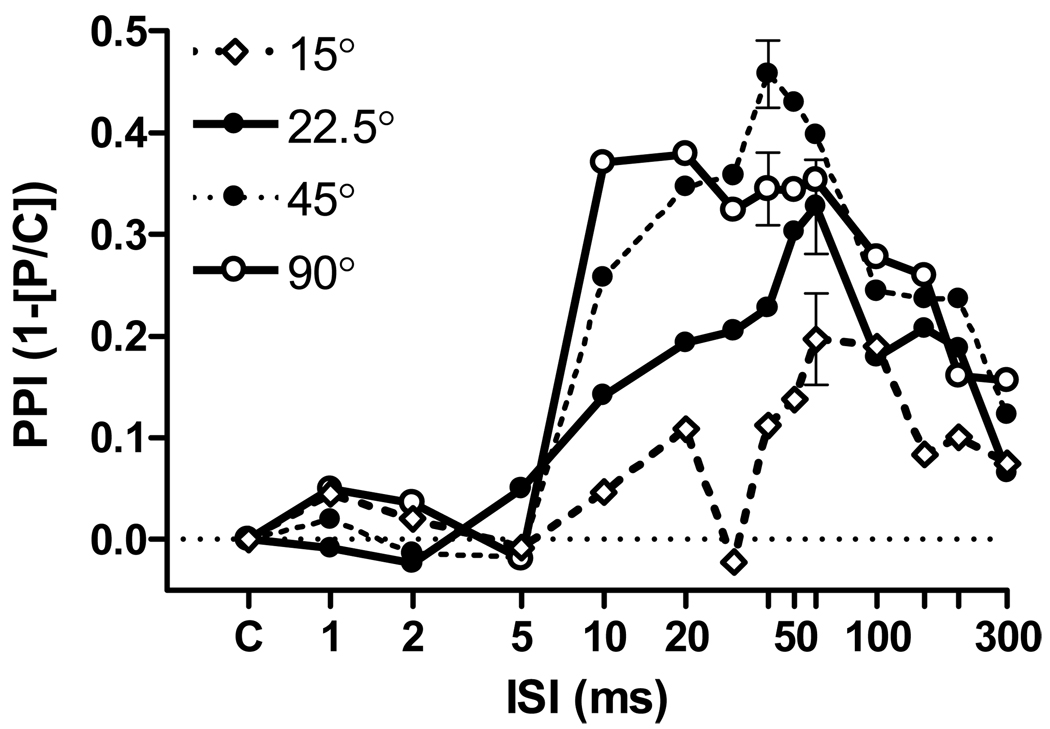
Figure 2 depicts the Mean (SEM) levels of PPI for the four angular separations. The peak level of PPI increased with increasing angular separation from 15° up to 45°, and the peak for 90° was approximately equal to that obtained for the 45° separation. The latency of both PPI onset and its peak varied with increasing separation. In contrast to the 5 ms onset latency obtained for the 180° separation in Experiment 1, here PPI onset was delayed until at least 10 ms, and the peak level of PPI was present at 10 ms for the 90° separation, but later at 40 to 60 ms for the smaller separations. The ANOVA of these data provided significant main effects for Angle, F(3/36) = 5.92, p = 0.002, ηp2 = 0.33; for ISI, F(13/156) = 32.86, p < 0.001, ηp2 = 0.73; and for their interaction, F(39/468) = 3.16, p < 0.01, ηp2 = 0.21. The main effect of ISI was highly significant (p < 0.001) for every angle, indicating that the change in location of even the 15° difference could be detected in the group data. For the 90° separation at ISI of 10 and 20 ms all 13 mice had significant PPI at p < 0.05; for 45° at ISI of 40 and 50 ms, 12 of the 13 mice had significant PPI at p < 0.05, the exception providing p = 0.076; for 22.5° at ISI of 50 and 60 ms, 7 of the 13 mice showed significant PPI; and for 15° for ISI of 60 and 100 ms, just 3 of the 13 mice had significant levels of PPI. Post-hoc analyses of the effect size of SSwap are given in Table 1, with Cohen’s d-statistic for individual mice calculated using the two ISIs that provided the largest group PPI values for each angle as is shown in Figure 2. A repeated measure ANOVA of the d-scores provided a significant effect of Angular Separation, F(3/36) = 3.89, p = 0.023, ηp2 = 0.24.
Figure 2.
Mean (SEM) PPI values as a function of the ISI between the SSwap and the ES, when the prepulse was a 90° , 45°, 22.5°, or 15° change in the angular separation of WBN from the right to the left hand speakers, centered in the frontal plane. To maintain clarity in the figure only representative SEM are presented. (Experiment 2, n = 13).
There were no significant differences in the control ASR scores across the four experimental sessions, (F < 1), which indicates that there was no habituation of the startle response caused by repeated testing. The mean control ASR (SD) ranged from 4324 (1039) at the 15° separation to 4723 (1458) at 45°. The overall correlation of control ASR values for the differences between mice across testing sessions was r = +0.77, p < 0.001, while neither the positive correlation of d-scores (r = +0.47) or of peak PPI values (r = +0.51) achieved statistical significance.
Experiment 3
Experiment 3 was designed to determine which spectral components of broadband noise provide the acoustic cues responsible for the detection of a change in noise location in the prior experiments. Heffner et al. (2001) reported that near asymptotic sound localization in young C57BL/6J mice was maintained as the upper cutoff of low-pass noise dropped from 80 kHz to 40 kHz, but then declined markedly as it was reduced to 20 kHz and further to 10 kHz. Changing the low-pass cut-off of the noise certainly reduced the potential contribution of high frequencies to localization but was also accompanied by an overall decrease in its bandwidth and thus its spread along the basilar membrane: this might have also affected performance in their mice. In Experiment 3 the test stimuli were four adjacent 1-octave noise bands, and while this manipulation also confounds bandwidth with the upper frequency of each band, it may be assumed at least that different octave bands occupy approximately equal areas along the mouse basilar membrane (Ehret, 1978). A secondary objective in Experiment 3 was to determine if these mice could detect a change in sound source location when the speaker separation is just 7.5°, this being the detection threshold obtained in young adult mice by Ehret & Dreyer (1984) when the mice were able to home-in on a succession of noise bursts.
Procedure
The subjects were 12 CBA mice, 9 female and 3 male, on average 12 months of age. Five angular separations were used, one on each of five test days: 180°, 90°, 45°, 22.5° and 7.5°, presented in counterbalanced order across mice. On each test day the SSwap stimuli consisted of four different octave bands of 2–4, 4–8, 8–16, and 16–32 kHz. In addition, a continuous 50 kHz wide band noise presented at 50 dB SPL was played from both speakers, which was intended to mask any spectral transients remaining with the 2 ms cosine-squared gating of the stimuli. For each octave band there were four different conditions; the no-prepulse control, and the SSwap prepulse conditions presented at 10 ms, 50 ms, and 200 ms ISI. A previous report has shown that all of these octave bands are audible to CBA/CaJ mice and yield significant PPI when presented as brief pulses in quiet (Ison et al., 2005).
Results
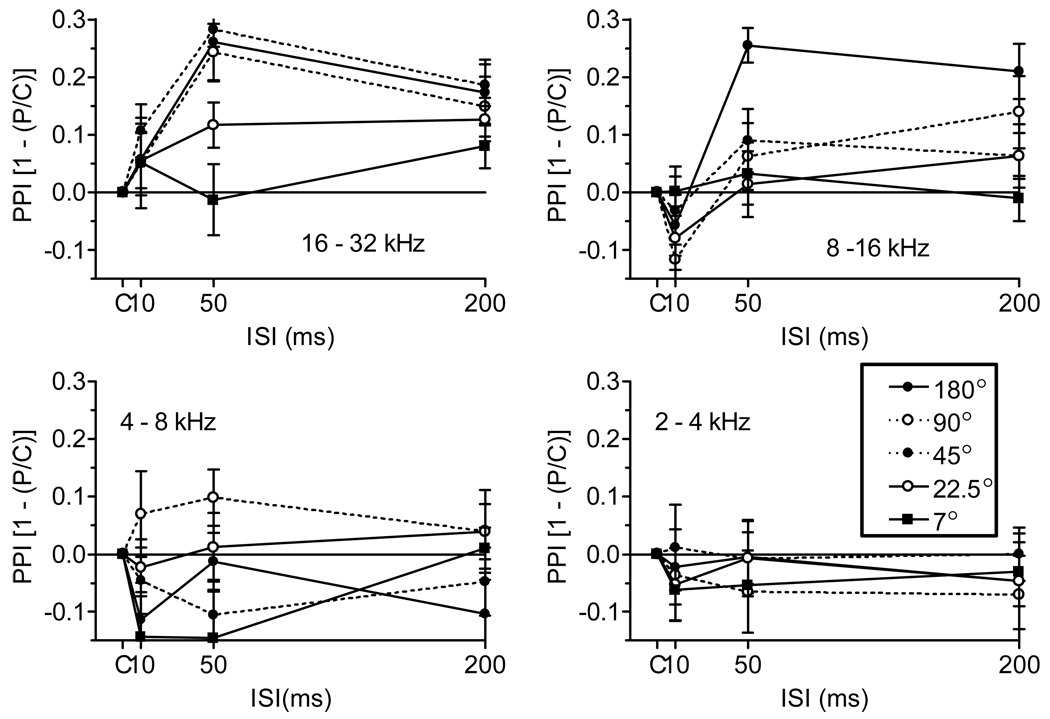
Figure 3 presents the Mean (SEM) PPI vs. ISI for the four different spectral bands, with the five different angular separations presented for each band. Inspection of this figure reveals that for the highest frequency band of 16–32 kHz the 180°, 90°, and 45° angles were equally effective; that for the band of 8–16 kHz only the 180° angular separation had a substantial effect at the 50 ms ISI as well as the 200 ms ISI; and the 4–8 kHz and the 2–4 kHz octave bands had minimal effects for any separation. The overall ANOVA of these data provided significant main effects for Band, F(3/33) = 8.78, p < 0.001, ηp2 = 0.44, and for ISI, F(3/33) = 17.56, p < 0.001, ηp2 = 0.62; and for the interactions of Angle and ISI, F(12/132) = 2.13, p = 0.02, ηp2 = 0.16, and Band and ISI, F(9/99) = 6.32, p < 0.001, ηp2 = 0.36. There were no differences in baseline ASR amplitudes associated with either the different frequency bands or the angular separations (all p > 0.2).
Figure 3.
Mean (SEM) PPI values as a function of the ISI between the SSwap and the ES, when the prepulse was a 180°, 90° , 45°, 22.5°, or 7° change in the angular separation of one-octave band noise, of 2–4, 4–8, 8–16, and 16– 32 kHz, from the right to the left hand speakers, centered in the frontal plane. (Experiment 3, n = 12).
Separate post-hoc ANOVA revealed that neither of the lower two bands, 2–4 and 4–8 kHz, provided significant PPI at any ISI or speaker angle (p > 0.10) while both the higher bands, 8–16 kHz and 16–32 kHz, provided significant interactions for Angle and ISI (p < 0.01). Post-hoc analyses of the Cohen’s d effect size statistic for SSwap at 50 ms are shown in Table 1, presented for all of the statistically significant conditions, and also for the largest separation at each frequency band that failed to provide significant inhibition. Analyses of these data provided significant effects (p < 0.01) at an ISI of 50 ms for the 16 to 32 kHz band at all angular separations between 180° and 22.5°, but not 7.5 °; and also for the 8 to 16 kHz band at 180°, but not 90 °; and there were no significant effects at any angle for the two lowest octave bands. The correlation of the peak d-statistics among mice across bandwidths within a day was always positive but never statistically significant (+0.33 < r < +0.56), but there were stable individual differences in ASR control levels across all conditions (r = +0.58, p = 0.01), while correlations across different octave bands within a single day all exceeded r = 0.96 (p < 0.01).
Experiment 4
Experiment 4 was designed to investigate the effect of sound intensity on the PPI effect of SSwap at the peak level for 50 ms and also its early onset at 10 ms and its later decay at 200 ms. We have not found in the literature any reference to the dependence of spatial acuity on sound level in mice, but there are relevant psychophysical studies in human observers beginning with Altschuler & Comalli (1975) and including more recently Su & Recanzone (2001) and Sabin et al. (2005). Generally, localization of sounds and sensitivity to a relative change their position improves with increasing intensity above the absolute threshold for the carrier. Recanzone & Beckerman (2004) reported similar behavioral effects in monkeys to those reported for humans, and Woods et al. (2006), in an electrophysiological study conducted in awake and behaving monkeys, found that the numbers of spatially responsive cortical neurons and their rate of firing to stimulus onsets and offsets increased over the entire range of stimulus intensities.
Procedure
The general methods were those described above. The subjects were 6-month old CBA/CaJ mice (3 M, 3F). There were eight test days and on each the angular separation for SSwap was held constant at 22.5°, 45°, 90°, or 180°. The testing order was counterbalanced among the mice and each angle tested twice. Two stimulus dimensions were varied on each test day, the Noise Level of the WBN (40, 50, 60, 70, and 78 dB SPL) and the ISI (10, 50, and 200 ms), plus there were baseline conditions of a single ES alone in each noise level.
Results
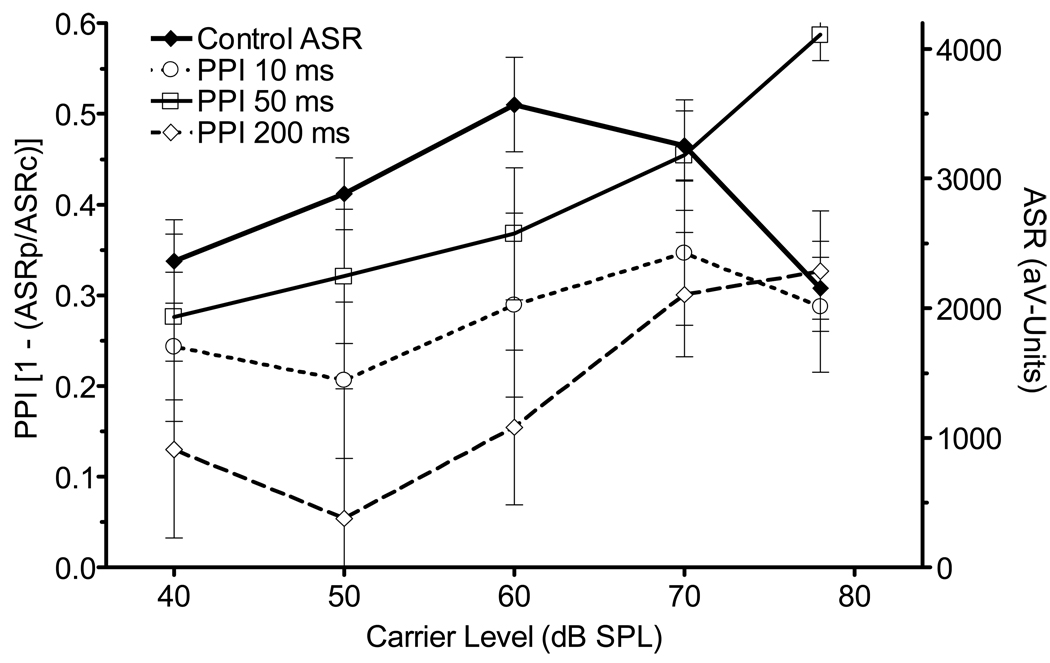
Figure 4 shows the effect of carrier level on both the control startle reflex (ASR) and PPI at 10, 50, and 200 ms ISI, both dimensions averaged across angle. As anticipated the inhibitory effect of SSwap was greatest at the 50 ms ISI, next with the 10 ms interval, and least at 200 ms. The effect of level on the ASR, here peaking at 60 dB SPL, is similar to that previously reported in rats (for example, Ison & Hammond, 1971): the initial increase in the ASR is thought to reflect noise-related arousal, while the subsequent downturn possibly represents some form of sensory masking (Davis, 1974). In contrast, the effect of level on PPI was a monotonic increasing function overall, this most apparent for the 50 ms ISI, least apparent for the 10 ms ISI. The ANOVA of the ASR data provided a main effect for Level, F(4/20) = 13.60, p < 0.01, ηp2 = 0.74, with significant quadratic and cubic trends (p < 0.01) attesting to the non-monotonic first increasing then decreasing effect. The ANOVA of the level effect on PPI in the entire set of data that included all three ISIs and all four angular separations provided a main effect of Level that was significant, F(4/20) = 4.23, p = 0.017, ηp2 = 0.47, with a linear increasing trend, F(1/5) = 20.66, p < 0.01, ηp2 = 0.80; a significant main effect for ISI , F(3/15) = 7.73, p = 0.002, ηp2 = 0.61, and also a significant interaction between Level and ISI, F(9/45) = 10.94, p < 0.001, ηp2 = 0.69: this interaction appears to capture the greater relative effect of high carrier levels on PPI at the 50 and 200 ms compared to the 10 ms ISI. There was also an expected significant effect for Angular Separation, F(3/15) = 153.24, p < 0.001, ηp2 = 0.97, and a relatively small but significant 3-way interaction between Angle, Level and ISI, F (36/180) = 1.82, p = 0.035, ηp2 = 0.28. This latter 3-way interaction (not depicted in Fig. 4 in order to maintain the clarity of the larger effects) may reflect the effect of increased noise level being most evident at longer ISIs and at greater angles, and least evident at 10 ms for the 22.5 ° angle. The post-hoc analysis of Cohen’s d-statistic at the 50 ms ISI showed these effects in the performance of individual mice, and are presented in Table 1 for the extreme levels, comparing 40 vs. 78 dB SPL. The average d-statistic was higher for 78 dB compared to 40 dB for each mouse, t(5) = 9.05, p < 0.001, R2 = 0.94.
Figure 4.
On the left axis, mean (SEM) of control ASR amplitudes and on the right axis, mean (SEM) PPI for Sswap at the 10, 50, and 100 ms ISI with increasing level of the WBN, both summed over angular separation to maintain clarity. (Experiment 4, n = 6).
Discussion
The results of these experiments reinforce and extend the limited data previously available on the ability of mice to detect a change in sound source location, and underscore the value of the RMA methods for studying this aspect of complex auditory processing in mice.
The data obtained in Experiment 1 show that a 180° shift in sound from right to left in the frontal plane is a very effective stimulus for producing PPI, with its effect being nearly equal to that obtained with separate noise onset and noise offset conditions. It is particularly interesting that these simple onsets and offsets had significant effects at ISIs of just 2 ms between the prepulse and the startle stimulus, with reflex facilitation provided by noise onset and reflex inhibition by noise offset. The most plausible explanation of these effects is that at very brief intervals the prepulse combines with the startle stimulus at or near the spinal root nucleus, which is the putative origin of the startle reflex in rodents (Nodal & Lopez, 2003). The small initial hint of reflex facilitation provided by SSwap at 2 ms followed by significant PPI at 5 ms suggests that the coincident onsets and offsets processed by the most caudal monaural pathways may have independent and competing effects on startle reflex expression.
The data obtained in Experiment 2 show that the strength of PPI was reduced with smaller angular separations of the two speakers, but that even the smallest separation of 15° produced a statistically significant effect for the group of mice. However just 3 of the 13 mice showed significant PPI (p < 0.05) at 15°, while 7 mice had significant PPI at 22.5°, and all 12 mice provided significant PPI at 45° and 90°. With a less stringent criterion for threshold performance (for example, a 1-tail test) more mice would be counted as having detected the 15° SSwap (in fact, 8 of 13). We note that the experimental paradigm could be made more powerful for each subject by increasing the numbers of presentation of each condition in each session, and by duplicating sessions on different testing days, and such statistical and experimental design features can be tailored to the nature of the hypothesis.
An important feature of the Experiment 2 data is that the growth of PPI with increasing ISI was slower as the angular separation between the speakers decreased. This is another indication that the wider angular separations provided the mouse with a more salient stimulus. The graded series of relatively slow onsets of PPI in the second experiment is also consistent with the hypothesis that smaller angular separations for SSwap demand more neural processing than the more rapidly appearing effects of noise onsets and offsets. Here it is of interest that a phenomenon of “binaural sluggishness” has been observed in human psychoacoustic experiments, describing the delayed detection of a binaural masked stimulus (Grantham & Wightman, 1978; Kollmeier & Gilkey, 1990) or binaural gap detection (Summerfield &Akeroyd, 2000). Alternatively, monaural processing of intensity changes at the two ears might also provide sufficient cues for detection of the SSwap prepulse. The relative contributions of monaural and binaural cues for detection are not easily determined in free-field behavioral experiments with intact normal animals. However, as described above, there are pharmacological and physiological manipulations that target long latency PPI but which do not affect short latency PPI (Ison et al., 1991; Ison & Bowen, 2000; Bowen et al., 2003, and Ison et al., 2008). Further experiments of this kind would help to resolve any present ambiguities concerning the neural bases of PPI for SSwap.
The results of Experiment 3 are consistent with the understanding that very high audio-frequencies are necessary for the mouse to distinguish one sound location from another (Heffner et al., 2001). At the highest octave band of 16 to 32 kHz the group of mice showed significant PPI for all shifts in location between 180° and 22.5 °, while for the 8 to 16 kHz octave band only the 180° SSwap was detected, and SSwap for the lower frequency bands of 4 to 8 kHz and 2 to 4 kHz did not provide significant PPI for any degree of separation. Ison et al., (2005) has shown that the simple onset of both of these lower frequency octave bands presented in quiet inhibits the ASR, so their failure here to generate PPI cannot be attributed to these stimuli being inaudible. Rather, given their long wave-length in comparison to the diameter of the mouse head, that they are likely equally present at the two ears of the mouse regardless of their location.
In principle, the essential cues contained in the high frequency octave bands that did produce PPI could be both the location-specific monaural spectral cues resulting from the filtering effect of the pinna as well as the binaural cues of interaural level and possibly onset-time differences. The effectiveness of monaural cues was obvious to the human experimenters when listening to SSwap with one ear blocked, as the change in the location of the broadband noise was readily detected. We ascribe this effect to head and ear-shaped spectral cues because we could not detect the change in the sound when the output of the two speakers was transmitted through a tube that was inserted into the ear canal. For mice, both monaural and binaural cues are liely involved in SSwap. Karcz et al. (2008) showed that monauralized mice showed significant PPI for a 90° SSwap, this revealing an effect of monaural cues, but a reduced level of PPI compared to that obtained when the same animal was tested under binaural conditions. The binaural advantage revealed the significant contribution of binaural processing to SSwap performance in these animals.
As shown in Experiment 4, the increasing SSwap PPI with increasing noise level is consistent with the psychophysical data on localization provided by human listeners (for example, Altschuler & Comalli, 1975; Su & Recanzone, 2001; and Sabin et al., 2005) and monkeys (Recanzone & Beckerman, 2004), save that most earlier reports suggest a performance benefit only over a small range of increased levels above the absolute threshold. Instead, the steady increase in PPI with an increase in level from 40 dB up to 78 dB at the 50 ms ISI shown in Figure 4 agrees more with the steady increase in onset- and offset- evoked neural activity in monkey auditory cortex with increasing levels of spatially distinct stimuli reported by Woods et al., (2006). It should also be noted that the present experiment was primarily designed to test the effects of suprathreshold stimulus levels on performance, and PPI would certainly have declined sharply as the absolute threshold was approached with levels 10 to 30 dB below those used in this experiment. The data of Experiment 4 demonstrate that SSwap PPI in the mouse does depend on carrier intensity. As such, future experiments must consider the potential effects of peripheral hearing loss on the MAA in the mouse. Equally though, it is important to note that mice can be expected to detect changes in sound location over small angular separations even at moderate sound levels.
The four experiments reported here are concerned with the ability of mice to detect a change in the location of the sound, following the path taken by most animal behavioral studies that was first developed by Mills (1958) in an experiment with human observers. Experiments of this type yield psychophysical functions describing the relationship between angular separation and behavioral measures (for example, Heffner & Heffner, 1988a, 1988b, or the behavioral functions presented here for PPI), or a single derived measure of spatial acuity, the MAA, for example, Mills, (1958) in which the MAA was defined as 50% of the angular separation between the two angles that provided 25% and 75% correct detection. Heffner & Heffner (1988a) described four ways to calculate the MAA threshold for three grasshopper mice trained with a conditioned suppression procedure. The means of these four different calculations varied only slightly, from 17.5° to 19.2°. In our data here, the group mean PPI at 15° for the CBA/CaJ mice tested in Experiment 2 was significantly different from the control condition, while the 7.5° condition in Experiment 3 never had significant PPI. Our RMA paradigm appears to have produced an estimate of the MAA that approximates the threshold value for mice of Heffner and Heffner (1988a), despite the very different testing conditions.
We also note that both RMA and the psychophysical measures that rely on a trained indicator response provide similar outcomes for gerbils as well as mice. Heffner and Heffner (1988b) reported an significant effect (p < 0.01) for gerbil’s ability to distinguish between noise bursts presented with 12° angular separation. Similarly, we have reported for a group of young gerbils that a WBN SSwap produced significant PPI (p < 0.05) at a 15° degree separation for every ISI from 50 to 200 ms, and even for a 7.5° separation for ISIs between 150 and 300 ms. These similarities between thresholds obtained by RMA and via behavioral training methods should encourage its further use for measuring the spatial abilities of groups of mice and other rodents.
In these experiments we did not fix the head position of the mouse. Initial observations indicated that tight constraint of the mouse caused agitation and increased variability in the ASR. In a previous investigation using mildly tranquilized mice (Ison et al., 1998) we were able to successfully fix the mouse head by temporarily gluing a horizontal rod between the pinna. However, it was very difficult to titrate the drug level for each mouse in each test period in order to calm the mouse but not eliminate its startle response. We ruled out this procedure for the present experiments since it is incompatible with high-throughput testing. The preliminary observation of the normal non-tranquilized mouse in the test cage was that its trunk remained oriented towards the speakers because of the restriction provided by the wire combs, but the head tended to rove from side to side within an approximate 30 degrees arc. These mice also engaged in bouts of head and ventral body grooming during which their heads moved vigorously both from side to side and up and down.
The freedom from restraint of our mice here contrasts with the typical procedures used in many free-field auditory localization studies, in which the heads of human participants have been fixed more or less rigidly within a fixed frame or otherwise held in place by bite-bars. Likewise. awake animal preparations often use a rigid post surgically attached to the skull in order to restrain the head. Head restraint is necessary in humans when variation in the MAA is investigated as a function of eccentricity (Mills, 1958) or the accuracy of sound localization is to be coordinated with initial eye-position (Lewald & Getzmann, 2006; Razavi et al., 2007). It is also essential in awake animals in experiments intended to correlate sound localization behaviors with central neural activity (in monkeys, Recanzone et al., 2000; or cats, Tollin et al., 2005), this being necessary for accurate recording. Behavioral experiments on spatial localization have generally made an effort to stabilize head position in order to better define the stimulus conditions, for example, by having the subject close a circuit between the floor and a drinking spout in order to initiate a trial (in rats and mice, for example, Kelly & Kavanagh, 1986; Heffner & Heffner, 1988a).
The defense for holding the head in a stable position from trial to trial (as recommended by Heffner & Heffner, 2001, in their discussion of sensory-behavioral testing in mice) is that this is necessary for the experimenter to maintain control over the stimulus conditions. The interaural time and level differences and the spectral cues that vary with the spatial location of the sound source must vary also with the shifting orientation of the subject’s head and ears, unless this movement is somehow constrained.
In the present experiments the SSwap test stimulus is presented not when the animal is quiet with its head in some designated orientation, but at some quasi-randomly computer-designated time regardless of the animal’s behavior. Further, during the average 20 s period that begins each trial the location of the sound is stable, but the critical binaural and monaural acoustic cues at the ears must constantly fluctuate over some unknown range with every movement of the head and pinna of the mouse. It seems then reasonable to argue, a priori, that this internally produced flux would obscure the reversal of the acoustics at the two ears provided by the external SSwap stimulus, and thus reduce or even eliminate PPI for this stimulus.
The empirical question, however, is whether this movement-associated fluctuation in the acoustic stimulus actually impacts these results. Our findings demonstrate that the sensitivity to differences in spatial location obtained with SSwap at least approximates the values obtained previously in experiments that attempt to stabilize the position of the head of the mouse. This challenges the assumption that fixing the head is necessary in order to provide a valid measure of the MAA. Other reports have shown that head restraint is not beneficial for localization acuity, with absolute spatial localization better in both monkeys (Populin, 2006) and cats (Tollin et al., 2004) when their heads are unrestrained. Additionally, it has been shown that even in human listeners the accuracy of sound localization is little affected by their changing head or eye position just prior to signal presentation (Goossen & van Opstal, 1999; Populin, 2008). These several examples indicate that the auditory system can disambiguate the acoustic fluctuations that result from moving the head from those that result from an external shift in sound location.
Shore and Zhou (2006) recently reviewed the somatosensory influence on auditory encoding. The central auditory system is not isolated from the other senses, but has multiple somatosensory and proprioceptive inputs generated by movements of the head, neck, and pinna that feed into its different levels from the most caudal divisions of the cochlear nucleus up to the auditory cortex. The associated physiological data show that the inputs into the auditory system from movements of the pinna can variously inhibit or can enhance neural activity evoked in the auditory system by acoustic input, for example, in the dorsal cochlear nucleus, as documented by Kanold & Young (2001). We speculate that similar mechanisms exist in the neural pathways mediating PPI, such that startle is modulated for external changes in auditory cues, and not by those generated by the animal’s own movement.
The RMA-based technique introduced here requires little or no adaptation to the test stimuli or the test environment and it may find application in situations where training the animal is impractical or impossible – e.g. in early development, or for genetically modified animals with brief life spans (e.g., Brew et al., 2005). The validity of prepulse inhibition and reflex modification audiometry to study monaural stimulus detection is well established (Yerkes, 1905; Young & Fechter, 1983; Simmons, 1988; Hoffman & Ison, 1992; Ison, 2001), with PPI values having strong correlates to both mouse auditory neurophysiology and human psychoacoustics (Barsz et al., 2001). It is only the use of RMA to study monaural/binaural stimulus discrimination based on location cues that is novel in the present work. That this and other reflex-based methods offer attractions for sensory work in animals is seen also in the report of Bala & Takahashi (2000), and their recent use of dishabituation of the pupillary dilation response in owls to test their ability to discriminate sound location and frequency change. Further, these investigators went on to show that the dishabituation phenomenon correlated well with other behavioral and neurophysiological measures of detection and encoding in the owl. As described above, the present results correspond well with previous measurements of MAA in the mouse and the validity of this RMA procedure may be strengthened by subsequent neurophysiological measurements of the encoding of these stimuli in the mouse brain.
A more general limitation of the mouse model for understanding the molecular basis of binaural sound localization in mammals is suggested by the traditional view that mice depend solely on LSO processing of the ILD cues provided by high audio-frequencies. As such they cannot contribute to our understanding the way in which the MSO system functions, or the role of the ITD cues that are more important for large mammals, including humans. However. the brainstem of the mouse does include an MSO, albeit it is relatively small compared to that of large animals (Heffner & Masterton, 1990). There are to our knowledge no published data yet available for the mouse MSO, but data obtained in the similarly sized MSO of the bat by Grothe & Neuweiler (2000) have shown that neurons in this nucleus are sensitive to the same range of ITDs that is found in the MSO cells of large animals. These are ITD values that could not arise from a single sound source presented at any degree of eccentricity in the bat or in the mouse, and while some of these large values do provide realistic cues for localization in the ITD sensitive cells of the MSO of larger animals, some do not. McFadden (1973) was the first to question the evolutionary relevance of these cues, suggesting that they would be normally encountered as near-coincident multiple copies of the same sound, for example, as echoes of a single acoustic event in a reverberant listening environment. Grothe & Neuweiler (2000) came to a similar conclusion, and suggested that providing an echo suppression mechanism that processes the location of only the first of multiple inputs (“the precedence effect”) may have been the original function of the MSO in the primordial small mammal, and that has been adapted for a related purpose in larger mammals. The temporal binding or separation of spectrally-defined similar events must be important for small and large animals alike and both the MSO and LSO of modern mammals may have preserved this function. Thus we conclude that the study of spatial hearing in the mouse arguably may elucidate not only the task of locating high audio-frequency sound objects in humans and other large mammals, but may also illuminate certain aspects of a more general use for ITD cues to represent acoustic space.
In conclusion, prepulse inhibition of the acoustic startle response can be used to measure the ability of mice to detect change in sound source location. Using this method, the minimum audible angle of the 3 month old CBA/CaJ mouse appears to be between 7.5° and 15°. An ISI delay of 60 to 100 ms between the prepulse and the ASR is required to achieve this degree of resolution, while an angular separation of 180° reaches a peak with only a 10 ms delay. Detection of the change in location is mediated by frequencies above 8 kHz, and particularly by those above 16 kHz, and the effectiveness of the SSwap stimuli in producing PPI increases with carrier intensity from 40 dB SPL at least up to 78 dB SPL. In addition the salience of different SSwap conditions is evident in the kinetics of PPI, in its rate of development with an increase in the ISI. This technique permits a rapid behavioral assessment of auditory spatial discrimination in mice that will prove useful in determining the behavioral consequences of genetic and pharmacological manipulations.
Acknowledgements
This research was supported by USPHS NIH-NIA P01 Grant AG09524, NIH-NIDCD P30 Grant DC005409, and by the Schmitt Foundation for Integrative Brain Research. We thank two anonymous reviewers for helpful comments on the manuscript.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
References
- Altshuler MW, Comalli PE. Effects of stimulus intensity and frequency on median horizontal plane sound localization. Journal of Auditory Research. 1975;5:262–265. [Google Scholar]
- Bala ADS, Takahashi TT. Pupillary dilation response as an indicator of auditory discrimination in the barn owl. Journal of Comparative Physiology A. 2000;186:425–434. doi: 10.1007/s003590050442. [DOI] [PubMed] [Google Scholar]
- Barsz K, Ison JR, Snell KB, Walton JP. Behavioral and neural measures of auditory temporal acuity in aging humans and mice. Neurobiology of Aging. 2002;23:565–578. doi: 10.1016/s0197-4580(02)00008-8. [DOI] [PubMed] [Google Scholar]
- Bowen GP, Taylor MK, Lin D, Ison JR. Auditory cortex lesions impair both temporal acuity and intensity discrimination in the rat, suggesting a common mechanism for sensory processing. Cerebral Cortex. 2003;13:815–822. doi: 10.1093/cercor/13.8.815. [DOI] [PubMed] [Google Scholar]
- Brew HM, Allen PD, Moore JT, Rivoli PJ, Tempel BL, Ison JR. Complex auditory processing matures rapidly in mice aged 11 to 18 days. Society of Neuroscience Abstracts. 2005;19:#44. [Google Scholar]
- Brew HM, Forsythe ID. Two voltage-dependent K+ conductances with complementary functions in postsynaptic integration at a central auditory synapse. Journal of Neuroscience. 1995;15:8011–8022. doi: 10.1523/JNEUROSCI.15-12-08011.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew HM, Hallows J, Tempel BL. Hyperexcitability and reduced low threshold potassium currents in auditory neurons of mice lacking the channel subunit Kv1.1. Journal of Physiology. 2003;548:1–20. doi: 10.1113/jphysiol.2002.035568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SD, Hardisty-Hughes RE, Mburu P. Quiet as a mouse: dissecting the molecular and genetic basis of hearing. Nature Reviews Genetics. 2008;9:277–290. doi: 10.1038/nrg2309. [DOI] [PubMed] [Google Scholar]
- Chen Q-C, Cain D, Jen PH-S. Sound pressure transformation at the pinna of mus domesticus. Journal of Experimental Biology. 1995;198:2007–2023. doi: 10.1242/jeb.198.9.2007. [DOI] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychological Bulletin. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Davis M. Signal-to-noise ratio as a predictor of startle amplitude and habituation in the rat. Journal of Comparative & Physiological Psychology. 1974;86:812–825. doi: 10.1037/h0036417. [DOI] [PubMed] [Google Scholar]
- Ehret G. Stiffness gradient along the basilar membrane as a basis for spatial frequency analysis within the cochlea. Journal of the Acoustical Society of America. 1978;64:1723–1726. doi: 10.1121/1.382153. [DOI] [PubMed] [Google Scholar]
- Ehret G, Dreyer A. Localization of tones and noise in the horizontal plane by unrestrained house mice (Mus musculus) Journal of Experimental Biology. 1984;109:163–174. doi: 10.1242/jeb.109.1.163. [DOI] [PubMed] [Google Scholar]
- Fay RR. Hearing in Vertebrates: A psychophysics database. Winnetka IL: Hill-Fay Associates; 1988. [Google Scholar]
- Finlayson PG, Caspary DM. Low-frequency neurons in the lateral superior olive exhibit phase-sensitive binaural inhibition. Journal of Neurophysiology. 1991;65:598–605. doi: 10.1152/jn.1991.65.3.598. [DOI] [PubMed] [Google Scholar]
- Galambos R, Schartzkopff J, Rupert A. Microelectrode study of superior olivary nuclei. American Journal of Physiology. 1959;197:527–536. doi: 10.1152/ajplegacy.1959.197.3.527. [DOI] [PubMed] [Google Scholar]
- Gittelman JX, Tempel BL. Kv1.1-containing channels are critical for temporal precision during spike initiation. Journal of Neurophysiology. 2006;96:1203–1214. doi: 10.1152/jn.00092.2005. [DOI] [PubMed] [Google Scholar]
- Golding NL, Ferragamo MJ, Oertel D. Role of intrinsic conductances underlying responses to transients in octopus cells of the cochlear nucleus. Journal of Neuroscience. 1999;19:2897–2905. doi: 10.1523/JNEUROSCI.19-08-02897.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham DW, Wightman FL. Detectability of varying interaural temporal difference. Journal of the Acoustical Society of America. 1978;63:511–523. doi: 10.1121/1.381751. [DOI] [PubMed] [Google Scholar]
- Goossens HHLM, van Opstal AJ. Influence of head position on the spatial representation of acoustic targets. Journal of Neurophysiology. 1999;81:2720–2736. doi: 10.1152/jn.1999.81.6.2720. [DOI] [PubMed] [Google Scholar]
- Grothe B, Neuweiler G. The function of the medial superior olive in small mammals: temporal receptive fields in auditory analysis. Journal of Comparative Physiology A-Sensory Neural & Behavioral Physiology. 2000;185:413–423. doi: 10.1007/s003590050441. [DOI] [PubMed] [Google Scholar]
- Heffner RS, Heffner HE. Sound localization in a predatory rodent, the northern grasshopper mouse (Onychomys leucogaster) Journal of Comparative Psycholology. 1988a;102:66–71. doi: 10.1037/0735-7036.102.1.66. [DOI] [PubMed] [Google Scholar]
- Heffner RS, Heffner HE. Sound localization and use of binaural cues by the gerbil (Meriones unguiculatus) Behavioral Neuroscience. 1988b;102:422–428. doi: 10.1037//0735-7044.102.3.422. [DOI] [PubMed] [Google Scholar]
- Heffner HE, Heffner RS. Behavioral assessment of hearing in mice. In: Willott JF, editor. Handbook of mouse auditory research: From behavior to molecular biology. Boca Raton, FL: CRC press; 2001. pp. 19–29. [Google Scholar]
- Heffner RS, Koay G, Heffner HE. Sound-localization acuity changes with age in C57/BL6J mice. In: Willott JF, editor. Handbook of mouse auditory research: From behavior to molecular biology. Boca Raton, FL: CRC Press; 2001. pp. 31–35. [Google Scholar]
- Heffner RS, Masterton RB. Ch. 9: Sound Localization in Mammals: Brain Stem Mechanisms. In: Berkley MA, Stebbins WC, editors. Comparative Perception. Vol. 1. New York: John Wiley & Sons; 1990. pp. 285–314. [Google Scholar]
- Hoffman HS, Ison JR. Reflex modification in the domain of startle: I. Some empirical findings and their implications for how the nervous system processes sensory input. Psychological Review. 1980;87:175–189. [PubMed] [Google Scholar]
- Hoffman HS, Ison JR. Reflex modification and the analysis of sensory processing in developmental and comparative research. In: Campbell BA, Hayne H, Richardson R, editors. Attention and information processing in infants and adults: perspectives from human and animal research. Hillsdale, NJ: Erlbaum; 1992. pp. 83–111. [Google Scholar]
- Hoffman HS, Searle J. Acoustic variables in the modification of startle reaction in the Rat. Journal of Comparative & Physiological Psychology. 1965;60:53–58. doi: 10.1037/h0022325. [DOI] [PubMed] [Google Scholar]
- Huang AY, May BJ. Spectral cues for sound localization in cats: effects of frequency domain on minimum audible angles in the median and horizontal planes. Journal of the Acoustical Society of America. 1996;100:2341–2348. doi: 10.1121/1.417943. [DOI] [PubMed] [Google Scholar]
- Ison JR. The acoustic startle response in the mouse: reflex elicitation and reflex modification by preliminary stimuli. In: Willott JF, editor. Handbook of mouse auditory research: From behavior to molecular biology. Boca Raton, FL: CRC Press; 2001. pp. 59–82. [Google Scholar]
- Ison JR, Agrawal P. The effect of spatial separation of signal and noise on masking in the free field as a function of signal frequency and age in the mouse. Journal of the Acoustical Society of America. 1998;104:1689–1695. doi: 10.1121/1.424381. [DOI] [PubMed] [Google Scholar]
- Ison JR, Allen PD. A diminished rate of "physiological decay" at noise offset contributes to age-related changes in temporal acuity in the CBA mouse model of presbycusis. Journal of the Acoustical Society of America. 2003;114:522–528. doi: 10.1121/1.1577553. [DOI] [PubMed] [Google Scholar]
- Ison JR, Allen PD, Rivoli PJ, Moore JT. The behavioral response of mice to gaps in noise depends on its spectral components and its bandwidth. Journal of the Acoustical Society of America. 2005;117:3944–3951. doi: 10.1121/1.1904387. [DOI] [PubMed] [Google Scholar]
- Ison JR, Bowen GP. Scopolamine reduces sensitivity to auditory gaps in the rat, suggesting a cholinergic contribution to temporal acuity. Hearing Research. 2000;145:169–176. doi: 10.1016/s0378-5955(00)00088-5. [DOI] [PubMed] [Google Scholar]
- Ison JR, Hammond GR. Modification of the startle reflex in the rat by changes in the auditory and visual environments. Journal of Comparative & Physiological Psychology. 1971;75:435–452. doi: 10.1037/h0030934. [DOI] [PubMed] [Google Scholar]
- Ison JR, Hoffman HS. Reflex modification in the domain of startle: II. The anomalous history of a robust and ubiquitous phenomenon. Psychological Bulletin. 1983;94:3–17. [PubMed] [Google Scholar]
- Ison JR, Housel N, Allen PD, Kopp-Scheinpflug C, Forsythe ID. Antagonists of neural nitric oxide synthase affect auditory behaviors in mice: A study of the acoustic startle reflex (ASR) and its inhibition by gaps in noise and a change in sound source location. Association of Research in Otolaryngology Abstracts. 2009;35:#429. [Google Scholar]
- Ison JR, O'Connor K, Bowen GP, Bocirnea A. Temporal resolution of gaps in noise by the rat is lost with functional decortication. Behavioral Neuroscience. 1991;105:33–40. doi: 10.1037//0735-7044.105.1.33. [DOI] [PubMed] [Google Scholar]
- Ison JR, Taylor MK, Bowen GP, Schwarzkopf SB. Facilitation and inhibition of the acoustic startle reflex in the rat after a momentary increase in background noise level. Behavioral Neuroscience. 1997;111:1335–1352. doi: 10.1037//0735-7044.111.6.1335. [DOI] [PubMed] [Google Scholar]
- Joris PX, Yin TC. Envelope coding in the lateral superior olive. I. Sensitivity to interaural time differences. Journal of Neurophysiology. 1995;73:1043–1062. doi: 10.1152/jn.1995.73.3.1043. [DOI] [PubMed] [Google Scholar]
- Kanold PO, Young ED. Proprioceptive information from the pinna provides somatosensory input to cat dorsal cochlear nucleus. Journal of Neuroscience. 2001;21:7848–7858. doi: 10.1523/JNEUROSCI.21-19-07848.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karcz A, Allen P, Kopp-Scheinpflug C, Ison J. Kcna1 null mutant mice, with known temporal processing deficits in the auditory brainstem, show spatial-location behavioral deficits under both monaural and binaural conditions. Association for Research in Otolaryngology Abstracts. 2008;30:#877. [Google Scholar]
- Koch M. The neurobiology of startle. Progress in Neurobiology. 1999;59:107–128. doi: 10.1016/s0301-0082(98)00098-7. [DOI] [PubMed] [Google Scholar]
- Kollmeier B, Gilkey RH. Binaural forward and backward masking: evidence for sluggishness in binaural detection. Journal of the Acoustical Society of America. 1990;87:1709–1719. doi: 10.1121/1.399419. [DOI] [PubMed] [Google Scholar]
- Kopp-Scheinpflug C, Fuchs K, Lippe WR, Tempel BL, Rubsamen R. Decreased temporal precision of auditory signaling in Kcna1-null mice: An electrophysiological study in vivo. Journal of Neuroscience. 2003;23:9199–9207. doi: 10.1523/JNEUROSCI.23-27-09199.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewald J, Getzmann S. Horizontal and vertical effects of eye-position on sound localization. Hearing Research. 2006;213:99–106. doi: 10.1016/j.heares.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Li H-S, Borg E. Age-related loss of auditory sensitivity in two mouse phenotypes. Acta Oto-Laryngologica. 1991;111:827–834. doi: 10.3109/00016489109138418. [DOI] [PubMed] [Google Scholar]
- Masterton B, Thompson GC, Bechtold JK, RoBards MJ. Neuroanatomical basis of binaural phase-difference analysis for sound localization: a comparative study. Journal of Comparative & Physiological Psychology. 1975;89:379–386. doi: 10.1037/h0077034. [DOI] [PubMed] [Google Scholar]
- May BJ. Role of the dorsal cochlear nucleus in the sound localization behavior of cats. Hearing Research. 2000;148:74–87. doi: 10.1016/s0378-5955(00)00142-8. [DOI] [PubMed] [Google Scholar]
- May BJ, Kimar S, Prosen CA. Auditory filter shapes of CBA/CaJ mice: Behavioral assessments. Journal of the Acoustical Society of America. 2006;120:321–330. doi: 10.1121/1.2203593. [DOI] [PubMed] [Google Scholar]
- McFadden D. Precedence effects and auditory cells with long characteristic delays. Journal of the Acoustical Society of America. 1973;54:528–530. doi: 10.1121/1.1913611. [DOI] [PubMed] [Google Scholar]
- Melcher JR, Knudson IM, Fullerton BC, Guinan JJ, Jr, Norris BE, Kiang NY. Generators of the brainstem auditory evoked potential in cat. I. An experimental approach to their identification. Hearing Research. 1996;93:1–27. doi: 10.1016/0378-5955(95)00178-6. [DOI] [PubMed] [Google Scholar]
- Middlebrooks JC, Green DM. Directional dependence of interaural envelope delays. Journal of the Acoustical Society of America. 1990;87:2149–2162. doi: 10.1121/1.399183. [DOI] [PubMed] [Google Scholar]
- Mikaelian DO, Warfield D, Norris BA. Genetic progressive hearing loss in the C57/b16 mouse: Relation of behavioral responses to cochlear pathology. Acta Oto-Laryngologica. 1974;77:327–334. doi: 10.3109/00016487409124632. [DOI] [PubMed] [Google Scholar]
- Mills AW. On the minimum audible angle. Journal of the Acoustical Society of America. 1958;30:237–246. [Google Scholar]
- Neff WD, Fisher JF, Diamond IT, Yela M. Role of auditory cortex in discrimination requiring localization of sound in space. Journal of Neurophysiology. 1956;19:500–512. doi: 10.1152/jn.1956.19.6.500. [DOI] [PubMed] [Google Scholar]
- Nodal FR, Lopez DE. Direct input from cochlear root neurons to pontine reticulospinal neurons in albino rat. Journal of Comparative Neurology. 2003;460:80–93. doi: 10.1002/cne.10656. [DOI] [PubMed] [Google Scholar]
- Populin LC. Monkey sound localization: head-restrained versus head-unrestrained orienting. Journal of Neuroscience. 2006;26:9820–9832. doi: 10.1523/JNEUROSCI.3061-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Populin LC. Human sound localization: measurements in untrained, head-unrestrained subjects using gaze as a pointer. Experimental Brain Research. 2008;190:11–30. doi: 10.1007/s00221-008-1445-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Populin LC, Yin TC. Behavioral studies of sound localization in the cat. Journal of Neuroscience. 1990;18:2147–2160. doi: 10.1523/JNEUROSCI.18-06-02147.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razavi B, O'Neill WE, Paige GD. Auditory spatial perception dynamically realigns with changing eye position. Journal of Neuroscience. 2007;27:10249–10258. doi: 10.1523/JNEUROSCI.0938-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recanzone GH, Beckerman NS. Effects of intensity and location on sound location discrimination in macaque monkeys. Hearing Research. 2004;198:116–124. doi: 10.1016/j.heares.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Rice JJ, May BJ, Spirou GA, Young ED. Pinna-based spectral cues for sound localization in cat. Hearing Research. 1992;58:132–152. doi: 10.1016/0378-5955(92)90123-5. [DOI] [PubMed] [Google Scholar]
- Rothman JS, Manis PB. The roles potassium currents play in regulating the electrical activity of ventral cochlear nucleus neurons. Journal of Neurophysiology. 2003;89:3097–3113. doi: 10.1152/jn.00127.2002. [DOI] [PubMed] [Google Scholar]
- Sabin AT, Macpherson EA, Middlebrooks JC. Human sound localization at near-threshold levels. Hearing Research. 2005;199:124–134. doi: 10.1016/j.heares.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Schofield BR. Superior olivary complex and lateral lemniscal connections of the auditory midbrain. In: Winer JA, Schreiner CE, editors. The Inferior Colliculus. New York: Springer; 2005. pp. 132–154. [Google Scholar]
- Sechenov I. In: Reflexes of the brain. Belsky S, translator. Cambridge MA: MIT Press; 1965. Originally published 1863. [Google Scholar]
- Shore SE, Zhou J. Somatosensory influence on the cochlear nucleus and beyond. Hearing Research. 2006;216–217:90–99. doi: 10.1016/j.heares.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Simmons AM. Masking patterns in the bullfrog (Rana catesbeiana). I: Behavioral effects. Journal of the Acoustical Society of America. 1988;83:1087–1092. doi: 10.1121/1.396053. [DOI] [PubMed] [Google Scholar]
- Su TI, Recanzone GH. Differential effect of near-threshold stimulus intensities on sound localization performance in azimuth and elevation in normal human subjects. Journal of the Association for Research in Otolaryngology. 2001;2:246–256. doi: 10.1007/s101620010073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield MA, Akeroyd AQ. A binaural analog of gap detection. Journal of the Acoustical Society of America. 1999;105:2807–2820. doi: 10.1121/1.426897. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology. 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Tollin DJ, Populin LC, Moore JM, Ruhland JL, Yin TCT. Sound localization performance in the cat: the effect of restraining the head. Journal of Neurophysiology. 2005;93:1223–1234. doi: 10.1152/jn.00747.2004. [DOI] [PubMed] [Google Scholar]
- Woods TM, Lopez SE, Long JH, Rahman JE, Recanzone GH. Effects of stimulus azimuth and intensity on the single-neuron activity in the auditory cortex of the alert macaque monkey. Journal of Neurophysiology. 2006;96:3323–3337. doi: 10.1152/jn.00392.2006. [DOI] [PubMed] [Google Scholar]
- Wu SH, Kelly JB. Binaural interaction in the lateral superior olive: time difference sensitivity studied in mouse brain slice. Journal of Neurophysiology. 1992;68:1151–1159. doi: 10.1152/jn.1992.68.4.1151. [DOI] [PubMed] [Google Scholar]
- Yerkes RM. The sense of hearing in frogs. Journal of Comparative Neurology and Psychology. 1905;15:279–304. [Google Scholar]
- Yin TCT. Neural mechanisms of encoding binaural localization cues. In: Oertel D, Fay RR, Popper AN, editors. Integrative Functions in the Mammalian Auditory Pathway. NY: Springer-Verlag; 2002. pp. 99–159. [Google Scholar]
- Young JS, Fechter LD. Reflex inhibition procedures for animal audiometry: A technique for assessing ototoxicity. Journal of the Acoustical Society of America. 1983;73:1686–1693. doi: 10.1121/1.389391. [DOI] [PubMed] [Google Scholar]
- Zheng QY, Johnson KR, Erway LC. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hearing Research. 1999;130:94–107. doi: 10.1016/s0378-5955(99)00003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]