Abstract
To provide evidence of large numbers of missed opportunities for early HIV diagnosis we designed a retrospective cohort study linking surveillance data from the South Carolina HIV/AIDS Reporting System to a statewide all payer health care database. We determined visits and diagnoses occurring before the date of the first positive HIV test and medical encounters were categorized to distinguish visits that were likely versus unlikely to have prompted an HIV test. Of the 4117 HIV-positive individuals newly diagnosed between 2001 and 2005, 3021 (73.4%) visited a South Carolina health care facility one or more times prior to testing HIV positive. Of these 3021, 1311 (43.4%) were late testers, and 1425 (47.2%) were early testers. Females were less likely than males to be late testers (odds ratio [OR] 0.55, 95% confidence interval [CI] 0.45–0.68), blacks were more likely than whites to be late testers (OR 1.37, 95% CI 1.10–1.71), and persons 50 years of age and older more likely to be late testers (OR 7.16, 95% CI 3.84–13.37). A total of 78.8% of the 13,448 health care visits for both late and early testers were for health care diagnoses unlikely to prompt an HIV test. These findings underscore the need for more routine HIV testing of adults and adolescents visiting health care facilities in order to facilitate early diagnosis.
Introduction
Since 1998, the state of South Carolina has consistently ranked among the top 10 for annual AIDS case rates in the United States. In 2005, South Carolina ranked ninth with 15.7 AIDS cases per 100,000 population,1 even though the state lacks large metropolitan areas and 39.5% of the population is considered rural. Unlike large urban centers, the state of South Carolina is not perceived as an AIDS epicenter. However, South Carolina ranked sixth among states and territories in reported new AIDS cases between 2000 to 2004.2 Both the high AIDS case rates and trends in new AIDS cases suggest that opportunities for early HIV diagnosis are being missed.
Most health care institutions in South Carolina have relied on a risk-based HIV testing strategy, which has been the standard of care for many years. However, providers and patients often incorrectly perceive HIV risk as low, resulting in missed opportunities for early HIV diagnosis. This is especially true for black women who are disproportionately impacted by the HIV epidemic (2005; black/white female relative risk, 20.5) and who may be at risk through their own or their partners' practices.3,4 A recent analysis of HIV screening of asymptomatic male and nonpregnant female patients by U.S. physicians showed that only 28.4% self-reported screening for HIV infection.5 To redirect local health jurisdictions in taking a broader approach to HIV testing in their communities, the Centers for Disease Control and Prevention (CDC) published revised recommendations for HIV testing in health care settings in September 2006.6
Recently published data from the South Carolina Department of Health and Environmental Control (DHEC) HIV/AIDS Reporting System (HARS) suggests that risk-based testing has not been effective for detecting HIV infection early because many individuals are first diagnosed when they present with advanced disease.7 Among South Carolina patients newly diagnosed with HIV infection from May 2004 to April 2005, 34% had a CD4+ T cell count 200 or fewer cells per mm3 and 56% had a CD4+ T cell count 350 or fewer cells per mm3. Given the natural history of HIV infection, these data suggest long durations of undiagnosed HIV infection. The existing testing strategy in South Carolina institutions may have contributed to the high prevalence of HIV disease because undiagnosed persons remained unaware for years of their HIV status and may not benefit from risk reduction counseling that may have facilitated ongoing HIV transmission.
To explore the frequency of missed opportunities for early HIV diagnosis, a population-based retrospective cohort study was devised that linked HIV case surveillance data to a statewide health care database. Linkage of the two databases allowed the investigators to determine: how often HIV-infected individuals accessed medical services before being tested for HIV; what factors are associated with late versus early HIV testing; and whether diagnostic codes from earlier health care visits were likely to have prompted an earlier HIV test. This investigation expands our previous report8 on late testers (those diagnosed with AIDS within 1 year of their initial HIV diagnosis) to include information on individuals with HIV-only (not AIDS) and reports on laboratory markers obtained at the time of HIV diagnosis.
Methods
The data for this study were obtained by merging variables from two sources: the health care database of the South Carolina Office of Research and Statistics (ORS) of the State Budget and Control Board and the South Carolina HIV/AIDS Reporting System (HARS). The South Carolina DHEC Institutional Review Board and the ORS Data Oversight Committee approved the study.
South Carolina ORS data
In accordance with South Carolina state law, the ORS has received health care data from emergency departments, hospital inpatient facilities, hospital ambulatory care facilities, and outpatient surgery facilities within South Carolina since 1996. These South Carolina health care facilities report greater than 98% of their data to the ORS within 1 year from date of collection (ORS, unpublished data, 2007). In addition, the ORS has a contractual relationship granting access to data from several free medical clinics (FMC). Each individual who has accessed health care services from these facilities is assigned a Unique Patient Identifier Number (UPIN) that has been validated internally by the ORS.
For the purposes of this study, health care data included International Classification of Diseases (ICD) codes, admission dates at health care facilities, source of payment at last visit, physician specialty code, location of the care facility, bed size of the facility, the classification of the health care facility (private, public), and patient zip code. Health care data for this report were supplied by 60 emergency departments, 62 inpatient facilities, 63 ambulatory facilities or outpatient surgery facilities, and 19 free medical clinics around the state for visits that occurred from January 1, 1997 through December 31, 2005. ICD codes from all health care visits made before the first positive HIV test date (see HARS section below) were grouped by the authors into two categories: (1) diagnoses not suggestive of HIV infection and unlikely to have prompted an HIV test (e.g., hypertension, diabetes, constipation); and (2) diagnoses that should have prompted an HIV test: those suggestive of HIV risk, e.g., sexually transmitted diseases, intravenous drug use; diseases possibly related to HIV, e.g., symptoms suggestive of acute retroviral syndrome,9 thrombocytopenia, peripheral neuropathy, pneumonia; and diseases probably related to HIV infection, e.g., cerebral toxoplasmosis, Pneumocystis jiroveci pneumonia, thrush.10
South Carolina HARS data
HIV infection has been reportable by name to the South Carolina Department of Health and Environmental Control (DHEC) since 1986. Data quality from the South Carolina HARS exceeds CDC minimum standards on reporting timeliness (95% within 6 months of a diagnosis) and completeness of reporting (98%, based on a comparison with other data sources) (South Carolina DHEC, unpublished data, 2005). Beginning January 1, 2004, state law required all CD4+ T cell counts and HIV viral load values to be reported to South Carolina DHEC, and recorded in the HARS database.
South Carolina HARS data on individuals reporting a first HIV diagnosis were included for diagnosis dates between January 1, 2001 and December 31, 2005. AIDS diagnosis reports on these individuals were included through December 31, 2006. Follow-up for inclusion of AIDS diagnoses was needed to permit a proper classification of individuals into early and late testers. The HARS data file had duplicate records removed and included only persons 18 years of age and older who were residents of South Carolina at the time of HIV diagnosis. The resulting data file contained records on 4117 persons and included the date of first positive HIV test, date of AIDS diagnosis, source of report, mode of exposure, patient name, birth date, gender, race/ethnicity, social security number (if available), HARS number, county of residence, initial CD4+ T cell counts and HIV viral load values.
Data linkage
The HARS and the ORS health care data files were linked using patient name, birth date, gender, race/ethnicity, social security number (if available), and county of residence. The date of the first positive HIV test recorded in HARS was used to identify encounters from the ORS health care database that occurred before that date. Authorized persons trained in HARS security guidelines and Health Insurance Portability and Accountability Act (HIPAA) confidentiality procedures linked the data in a secured location. Deidentified data with all identifiers (names, addresses, and social security numbers) removed were then provided to the investigators.
Late and early testers
We compared data from individuals who had made prior health care visits and developed AIDS within 1 year of testing HIV positive (late testers)13 with data from HIV-positive individuals who were not diagnosed with AIDS within 1 year of testing HIV positive, nor within subsequent follow-up until December 31, 2006 (early testers). For analyses, we limited the group of early testers to those individuals with health care visits no more than 3 years before the first positive HIV test date. This limited the time interval for the early testers' missed opportunities to be within the estimated duration of infection for individuals with a median of 500 CD4+ cells at diagnosis.11 Clinical illness and subsequent CD4 values reported to HARS after the HIV diagnosis date were used to determine AIDS status. 285 persons who received an AIDS diagnosis between 1 year post-HIV diagnosis and the end of follow-up were excluded from the analysis. These individuals were intermediate between the early and late testers in terms of CD4+ cell counts at diagnosis.
Statistical analysis
We performed descriptive analyses on data from the 3021 HIV-infected individuals with health care visits in the years preceding their first positive HIV test date (health care visit dates included the period from January 1, 1997 to December 31, 2005). We analyzed late versus early testers on factors potentially associated with late versus early testing, as well as numbers, percentages and ICD codes of health care visits. We used adjusted odds ratios (OR) and 95% confidence intervals (CI) from a logistic regression to assess the association of gender, race/ethnicity, transmission category, and number of health care visits for persons with late compared to early testing status. Categorical variables were summarized with percentages, and continuous variables were summarized using median and range; CD4 and viral load contrasts used Kruskal-Wallis two-sample nonparametric medians tests. The first CD4+ T cell count obtained at diagnosis was used to determine disease stage at the time of the HIV diagnosis. Significance testing was not done on health care visit data because there were multiple health care visits per individual, i.e., an individual may appear in more than one time period or may have diagnostic codes that fall into more than one category. At a specific visit, any diagnosis recorded that is likely to prompt an HIV test counts that visit once in the “likely to prompt” category, for example. However, if that visit had two diagnoses likely to prompt a test (e.g., syphilis and aseptic meningitis), that visit was counted both in the sexually transmitted disease (STD) category and the Diagnoses Possibly Related to HIV category. Plasma viral load results reported as undetectable had values changed to 200 copies per milliliter to allow for statistical analysis.12 Values over 750,000 copies per milliliter were considered to be out of range, and values were changed to 750,000 copies per milliliter for analysis. Statistical analysis was performed using SAS version 8.2 (SAS Institute Inc., Cary, NC).
Results
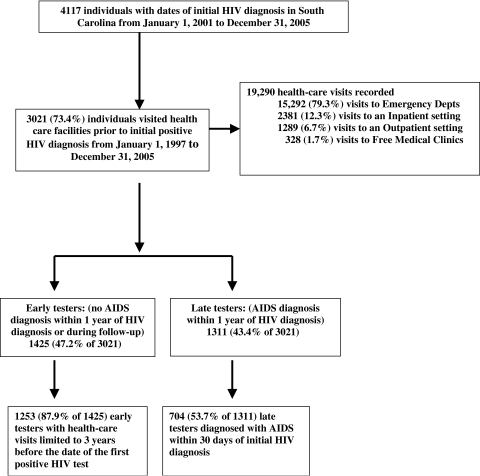
Of the 4117 persons newly diagnosed with HIV infection between 2001 and 2005, 3021 (73.4%) visited a South Carolina health care facility one or more times prior to testing HIV positive (Fig. 1). Of the 3021 individuals with previous visits, 1311 (43.4%) developed AIDS within 1 year of testing (late testers), and 1425 (47.2%) had HIV infection only within 1 year of HIV testing, with additional follow-up until December 31, 2006 (early testers). Of the late testers, 704 (53.7%) had AIDS diagnosed at or within 30 days of their initial HIV diagnoses. Of the early testers, 1253 (87.9%) had health care encounters in the 3-year period prior to the first positive HIV test date (early testers) and were used in the comparisons of late versus early testers.
FIG. 1.
Missed opportunities for earlier diagnosis of HIV infection.
For the 1311 late and 1253 early testers, 2229 (86.9%) had an initial CD4+ T cell count and 1946 (75.9%) had an initial HIV plasma viral load documented in HARS. Compared to the early testers' median initial CD4+ T cell count of 513 cells/mm3 (range, 211–1677 cells/mm3), the late testers' median CD4+ T cell count of 90 cells/mm3 (range, 0–1188cells/mm3) was significantly lower (p < 0.0001).
Compared to the early testers' median initial HIV plasma viral load of 12,228 copies/ml (range: 200 to >750,000 copies per milliliter), the late testers' median HIV plasma viral load of 81,891 copies per milliliter (200 to >750,000 copies per milliliter) was significantly higher (p < 0.0001).
Of the 19,290 health care visits recorded for the 3021 individuals who had a documented previous visit to a South Carolina health care facility since January 1, 1997, 15,292 (79.3%) visits were to emergency departments, 2381 (12.3%) visits were to inpatient settings, 1289 (6.7%) visits were to outpatient facilities, and 328 (1.7%) visits were to free clinics (Fig. 1). Total visits ranged from 1 to 133 per patient and the median number of visits was four per individual. Visits occurring 6 months or less before HIV diagnosis accounted for 2811 (14.6%) of the 19,290 visits; 1959 (10.2%) of the visits were made more than 6 months to 1 year before, 3413 (17.7%) were more than 1 to 2 years before, 3188 (16.5%) were more than 2 to 3 years before, and 7919 (41.1%) were more than 3 years before HIV diagnosis.
In the adjusted analysis using logistic regression (Table 1), women were less likely than men to be late testers (OR 0.56, 95% CI 0.45–0.68), and blacks were more likely than whites to be late testers (OR 1.37, 95% CI 1.10–1.71). Adults age 50 years and older were more likely than younger individuals to be late testers (OR 7.20, 95% CI 3.86–13.45), and the odds of late testing rose linearly with increasing year of age (p value for test of trend, p < 0.0001). There was a statistically significant higher average number of visits to health-care facilities made by late versus early testers (p < 0.0001). Mode of HIV transmission was not found to be a significant predictor of late versus early testing, except for the risk not specified individuals, who were less likely than men who have sex with men (MSM) to have been late testers (OR 0.70, 95% CI 0.55–0.90).
Table 1.
Adjusted Odds of Being a Late Tester Among 2564 Individuals Diagnosed with HIV between 2001 and 2005 and Seen in a South Carolina Health Care Facility before the Date of Diagnosis
| |
HIV diagnostic status |
|
|
|
|
|||
|---|---|---|---|---|---|---|---|---|
| |
Early testersa(no AIDS within 1 year of HIV diagnosis or during follow-up) |
Late testersb(AIDS within 1 year of HIV diagnosis) |
Crude odds ratio |
Adjusted odds ratio |
||||
| Characteristic | No. | (%) | No. | (%) | OR | 95% CI | OR | 95% CI |
| Gender | ||||||||
| Female | 548 | 43.7 | 422 | 32.2 | 0.61 | 0.52, 0.72 | 0.56 | 0.45, 0.68 |
| Male | 705 | 56.3 | 889 | 67.8 | 1.00 | — | 1.00 | — |
| Race/ethnicityc | ||||||||
| Black | 983 | 78.5 | 1,061 | 80.9 | 1.19 | 0.97, 1.46 | 1.37 | 1.10, 1.71 |
| Hispanic | 21 | 1.7 | 21 | 1.6 | 1.10 | 0.59, 2.08 | 1.53 | 0.78, 3.01 |
| White | 243 | 19.4 | 220 | 16.8 | 1.00 | — | 1.00 | — |
| Age (yrs) | ||||||||
| 18–19 | 52 | 4.2 | 16 | 1.2 | 1.00 | — | 1.00 | — |
| 20–24 | 201 | 16.0 | 80 | 6.1 | 1.29 | 0.70, 2.40 | 1.25 | 0.66, 2.35 |
| 25–29 | 192 | 15.3 | 129 | 9.9 | 2.18 | 1.19, 3.99 | 2.28 | 1.23, 4.24 |
| 30–39 | 379 | 30.3 | 429 | 32.7 | 3.68 | 2.07, 6.55 | 4.17 | 2.30, 7.59 |
| 40–49 | 307 | 24.5 | 413 | 31.5 | 4.37 | 2.45, 7.80 | 4.86 | 2.67, 8.85 |
| ≥50 | 122 | 9.7 | 244 | 18.6 | 6.50 | 3.56, 11.86 | 7.20 | 3.86, 13.45 |
| Transmission categoryd | ||||||||
| Heterosexual | 517 | 41.3 | 491 | 37.5 | 0.87 | 0.72, 1.06 | 0.81 | 0.63, 1.04 |
| IDU | 50 | 4.0 | 82 | 6.3 | 1.51 | 1.03, 2.21 | 0.94 | 0.62, 1.43 |
| MSM/IDU | 13 | 1.0 | 16 | 1.2 | 1.13 | 0.54, 2.39 | 0.82 | 0.37, 1.79 |
| Risk not specified | 358 | 28.6 | 376 | 28.7 | 0.96 | 0.78, 1.19 | 0.70 | 0.55, 0.90 |
| MSM | 314 | 25.1 | 342 | 26.1 | 1.00 | — | 1.00 | — |
| Previous visits | ||||||||
| 1 Visit | 371 | 29.6 | 281 | 21.4 | 1.00 | — | 1.00 | — |
| 2–5 Visits | 592 | 47.2 | 568 | 43.3 | 1.27 | 1.04, 1.54 | 1.31 | 1.07, 1.61 |
| 6–10 Visits | 195 | 15.6 | 263 | 20.1 | 1.78 | 1.40, 2.27 | 2.12 | 1.64, 2.75 |
| >10 Visits | 95 | 7.6 | 199 | 15.2 | 2.77 | 2.07, 3.69 | 3.63 | 2.66, 4.97 |
n = 1253. Reported to HARS in South Carolina during 2001–2005. No AIDS within 1 year of HIV diagnosis or during subsequent follow-up to December 31, 2006. Restricted to individuals whose health care visits occurred within 3 years of the date of the first positive HIV diagnosis.
n = 1311. Reported to HARS in South Carolina during 2001–2005. AIDS within 1 year of HIV diagnosis.
Asians/Pacific Islanders, American Indians/Alaska Natives, and persons of multiple races were excluded because numbers were too small for meaningful analysis.
Transfusion recipients and persons with hemophilia were excluded because numbers were too small for meaningful analysis.
OR, odds ratio; CI, confidence interval; IDU, injection drug user; MSM, men who have set with men; HARS, HIV/AIDS Reporting System.
The analysis of health care visits and diagnoses for the 2564 early and late testers is presented in Table 2. The majority of the 5326 health care visits for early testers (78.8%) were for diagnoses that would not have been likely to prompt an HIV test. Similarly, the majority of the 8122 health care visits for late testers (78.8%) were for diagnoses that would not have been likely to prompt an HIV test. Therefore, for both early and late testers about 80% of their health care visits would not have prompted an HIV test, thus representing encounters that would not have provided an opportunity for HIV testing under a risk-based testing paradigm. From Table 1 we have that 377 (30.1%) early testers and 440 (33.6%) late testers were identified as either injection drug users or MSM at visits prior to their HIV diagnosis. These are persons with high-risk practices that should have prompted HIV screening if risk histories had been elicited during the health care visits.
Table 2.
Number and Percentage of Health Care Visits by Early Testersa and Late Testersb who had Visited a Heath Care Facility before the Date of HIV Diagnosis, by Health Care Visit Diagnosis—South Carolina, 1997–2005
| |
HIV diagnostic status |
|||
|---|---|---|---|---|
| |
Early testers (no AIDS within 1 year of HIV diagnosis or during follow-up) |
Late testers (AIDS within 1 year of HIV diagnosis) |
||
| Health care visit diagnosis | No. | (%) | No. | (%) |
| Visits with diagnoses likely to prompt an HIV testc | 1131 | 21.2 | 1726 | 21.2 |
| Sexually transmitted diseases and related diagnoses | 179 | 166 | ||
| Symptoms suggestive of acute retroviral syndromed | 805 | 1,201 | ||
| Diseases possibly related to HIVe | 212 | 495 | ||
| Diseases probably related to HIVf | 11 | 91 | ||
| Intravenous drug use and related behaviors | 115 | 83 | ||
| Visits with diagnoses not likely to prompt an HIV test | 4195 | 78.8 | 6396 | 78.8 |
| Total visits | 5326 | (100) | 8122 | (100) |
n = 1253. Reported to HARS in South Carolina during 2001–2005. No AIDS within 1 year of HIV diagnosis or during subsequent follow-up to December 31, 2006. Restricted to individuals whose health care visits occurred within 3 years of the date of the first positive HIV diagnosis.
n = 1311. Reported to HARS in South Carolina during 2001–2005. AIDS within 1 year of HIV diagnosis.
The five categories of diagnoses can occur more than once in a single visit; thus the total diagnoses exceed the number of visits.
Including fever, lymphadenopathy, and rash.
Including peripheral neuropathy, pneumonia, and thrombocytopenia.
Including cerebral toxoplasmosis, pulmonary tuberculosis, and thrush.
HARS, HIV/AIDS Reporting System.
Discussion
The findings in this report demonstrate that current HIV testing practices in South Carolina health care facilities have failed to diagnose many individuals early in the course of their HIV infection despite documented prior encounters with the medical care system at times when these persons were probably HIV infected. This population–based study found that approximately three fourths of individuals testing HIV positive from 2001 through 2005 had visited a South Carolina health care facility prior to the date of their first positive HIV test. Furthermore, of the 43% of persons diagnosed between 2001–2005 who had a late diagnosis, more than half progressed to AIDS within 30 days of their initial diagnosis. Approximately 80% of the health care visits before HIV diagnosis for both late and early testers were for conditions not likely to prompt HIV testing in a nonroutine testing environment. These findings strongly suggest that routine HIV screening in health care settings of all adults and adolescents might have resulted in earlier diagnoses in South Carolina.
These data also do not support the practice of targeted testing of risk groups as a sufficient HIV screening strategy. Mode of exposure has been cited as a significant variable among those who test late in other studies,13 but this was not confirmed in this statewide study. Our adjusted analysis did not show that transmission category was significant in differentiating late from early testers. Race and age, two factors that were associated with late versus early testing, are not behavioral or clinical risk characteristics that would have met the criteria for targeted testing as defined in 2001.14 A separate analysis of statewide surveillance data in South Carolina found that the majority of South Carolina HIV/AIDS cases were not due to MSM exposure: 34% of HIV-infected individuals in South Carolina reported heterosexual contact as their mode of exposure and 26% reported no identifiable risk.7 The high proportion of diagnoses at health care encounters that would not have prompted an HIV test under a risk-based testing strategy suggests that a clinical risk-based testing strategy, even if implemented successfully in all these facilities would still have missed an earlier diagnosis the majority of the time.
Certain characteristics and trends were notable findings in this analysis. First, women were more likely to be early as opposed to late testers. Routine offering of HIV testing during prenatal services might explain why women were diagnosed earlier in infection; prenatal testing is routine screening and delayed diagnosis in men may support the use of this practice outside the prenatal setting. Blacks were more likely than whites to be late testers. This suggests that a change to a routine screening paradigm in South Carolina would greatly benefit the black population if done comprehensively. Adults age 50 years and older compared to those aged 18 to 19 were much more likely to be late testers. This suggests either transmission at older ages or prolonged periods of unidentified infection in older adults who may not be perceived by themselves or health care workers to be at risk for HIV infection because of their age.14–16 Finally, the odds of testing late for HIV infection increased with the frequency of prior health care encounters. This finding suggests a role for illness in accounting for this association.
The most common diagnostic codes among early and late testers were unlikely to prompt HIV testing using a risk-based testing strategy. Because individuals with HIV infection do become ill with diseases that affect the general population (such as hypertension and diabetes), continuing the practice of relying on targeted HIV testing in these health care facilities will delay the diagnosis of many HIV-infected persons. This delay in diagnosis provides opportunities for spread of HIV disease by individuals unaware of their serostatus. The CDC has estimated that the approximately 25% of HIV-infected persons who are unaware of their infection account for 54% of new infection transmissions.17 It is estimated that knowledge of HIV serostatus in unaware persons could reduce new infections by greater than 30%. A recent meta-analysis estimated that unprotected anal or vaginal intercourse with HIV-seronegative partners was reduced by 68% among HIV-infected persons who knew of their positive serostatus compared with those who were unaware.18 In South Carolina, expanded routine opt-out HIV screening in health care settings should reduce the number of persons who are unaware of their HIV-infected status.
Institution of routine HIV screening, in addition to reducing the burden of late HIV diagnosis, improve treatment coverage and so reduce mortality and morbidity, and has been shown to reduce stigma and generate higher HIV test acceptance rates.20–22 Normalizing HIV testing in the minds of the general public is important. Freely available materials in the proper language and educational level should accompany a general consent for care that encompasses HIV testing. Because some newly diagnosed individuals may not have previously considered the possibility that they might be HIV infected and are unprepared to cope with an HIV diagnosis, providers need to be trained on how to effectively intervene to prevent or moderate potential psychological and social harms.19
An increased number of diagnoses also will increase demands for public health services, especially partner notification. In this regard, important steps for clinicians and case managers include: communicating with health department partner services staff to become familiar with the services and how to access them; asking at the patient's initial visit about sex and drug injection partners and whether they have been informed of their risk; screening patients for behavioral risks and sexually transmitted infections that may indicate a need for further discussion about partners; and referring patients to the health department for assistance with partner notification and other prevention services.
However, a key challenge to implementation of routine screening remains costs. Three recently published papers concerning the cost-effectiveness of HIV screening concluded that even when the prevalence of HIV infection in specific populations is substantially lower than 1%, screening for HIV is cost effective relative to other established screening programs.20–22 And while the public health benefits are clear—individuals on therapy have more productive lives, avert opportunistic infections, as well as secondary transmissions—the costs associated with routine screening are not trivial. Although most practitioners would agree that providing accessible testing benefits patients, the capacity of treatment and preventive services will need to be increased if routine HIV testing is to translate into earlier treatment. In addition, a need for targeted counseling and testing services will remain in specific settings and may be preferred to opt-out testing because it may diagnose more HIV infections and do so at a lower gross cost per infection averted.23 In settings with a documented HIV seroprevalence of less than 0.3%, for instance, routine screening may not be warranted. Such prevalence data, however, should be prospectively generated and not estimated.
The findings in this report are not without limitations. First, some of the late and early testers might not have been HIV-infected at the time of some of their health care encounters. Some of the late testers' health care encounters occurred up to 8 years before AIDS was diagnosed, and some of the early testers' health care encounters occurred up to 3 years before their HIV diagnosis; therefore, some of these instances might not have been missed opportunities. However, given the long average latent period of approximately 10 years before the onset of AIDS,23 most persons who had AIDS within a year after a 2001–2005 diagnosis would already have been HIV-infected during most of their health care visits beginning in 1997. The CD4+ cell count of early testers at HIV diagnosis suggests most would have been HIV infected during their health care visits up to 3 years before that diagnosis. Second, although several variables were available for linking records between the two datasets, matching might not have been successful in all cases. Third, HIV testing might have been recommended but rejected by certain patients during earlier visits. Fourth, a referral for HIV testing might have occurred during some of the health care encounters before HIV was diagnosed, making these visits not truly missed opportunities. Finally, although HARS and ORS data are considered comprehensive, certain HIV/AIDS diagnoses and health-care visits may not have been reported.
In summary, a majority of persons with newly diagnosed HIV infection were found to have had many prior health care visits that could have provided an opportunity for earlier HIV diagnosis. As most of the diagnostic codes on these health care visits would not have prompted an HIV test, these data underscore the need for routine HIV screening of adults and adolescents visiting health care facilities. The benefits of earlier diagnosis and linkage to care, access to medications and prevention services for HIV infected persons should result in improved health outcomes and reduced HIV transmission.
Acknowledgments
We thank Bernard Branson, M.D. and Lytt Gardner, Ph.D. for their role in mentorship, study design, and critical revision of the manuscript. The persons listed in this section received no compensation for their work.
Presented in part as poster at the 14th Conference on Retroviruses and Opportunistic Infections, February 25–28, 2007, Los Angeles, California.
Support for this study was provided through a USC CSTA Planning Grant (NIH 1P20RR023476-01 [W.D., H.A.]).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Centers for Disease Control and Prevention. Cases of HIV and AIDS in the United States. HIV/AIDS Surveillance Report. 2005. www.cdc.gov/hiv/topics/surveillance/resources/slides/epidemiology/slides/EPI-AIDS_11.ppt. [Feb 23;2009 ]. www.cdc.gov/hiv/topics/surveillance/resources/slides/epidemiology/slides/EPI-AIDS_11.ppt
- 2.Qian HZ. Taylor RD. Fawal HJ. Vermund SH. Increasing AIDS case reports in the South: U.S. trends from 1981–2004. AIDS Care. 2006;18(Suppl 1):S6–S9. doi: 10.1080/09540120600839074. [DOI] [PubMed] [Google Scholar]
- 3.Millett G. Malebranche D. Mason B. Spikes P. Focusing “down low”: Bisexual black men, HIV risk and heterosexual transmission. J Natl Med Assoc. 2005;97(7 Suppl):52S–9S. [PMC free article] [PubMed] [Google Scholar]
- 4.Racial/ethnic disparities in diagnoses of HIV/AIDS—33 states, 2001–2005. MMWR Morb Mortal Wkly Rep. 2007;56:189–193. [PubMed] [Google Scholar]
- 5.Bernstein KT. Begier E. Burke R. Karpati A. Hogben M. HIV screening among U.S. physicians, 1999–2000. AIDS Patient Care STDs. 2008;22:649–656. doi: 10.1089/apc.2007.0261. [DOI] [PubMed] [Google Scholar]
- 6.Branson BM. Handsfield HH. Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55:1–17. quiz CE1–4. [PubMed] [Google Scholar]
- 7.Ogbuanu IU. Torres ME. Kettinger L. Albrecht H. Duffus WA. Epidemiologic Characterization of individuals with newly reported HIV-infection in South Carolina, May 2004 to April 2005. Am J Public Health. 2009;99(Suppl 1):S111–S1117. doi: 10.2105/AJPH.2006.104323. epub 2007 Nov 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Missed opportunities for earlier diagnosis of HIV infection—South Carolina, 1997–2005. MMWR Morb Mortal Wkly Rep. 2006;55:1269–1272. [PubMed] [Google Scholar]
- 9.Kahn JO. Walker BD. Acute human immunodeficiency virus type 1 infection. N Engl J Med. 1998;339:33–39. doi: 10.1056/NEJM199807023390107. [DOI] [PubMed] [Google Scholar]
- 10.Greenwald JL. Rich CA. Bessega S. Posner MA. Maeda JL. Skolnik PR. Evaluation of the Centers for Disease Control and Prevention's recommendations regarding routine testing for human immunodeficiency virus by an inpatient service: Who are we missing? Mayo Clin Proc. 2006;81:452–458. doi: 10.4065/81.4.452. [DOI] [PubMed] [Google Scholar]
- 11.Longini IM., Jr. Clark WS. Gardner LI. Brundage JF. The dynamics of CD4+ T-lymphocyte decline in HIV-infected individuals: A Markov modeling approach. J Acquir Immune Defic Syndr. 1991;4:1141–1147. [PubMed] [Google Scholar]
- 12.Giordano TP. Gifford AL. White AC, Jr., et al. Retention in care: A challenge to survival with HIV infection. Clin Infect Dis. 2007;44:1493–1499. doi: 10.1086/516778. [DOI] [PubMed] [Google Scholar]
- 13.Late versus early testing of HIV—16 Sites, United States, 2000–2003. MMWR Morb Mortal Wkly Rep. 2003;52:581–586. [PubMed] [Google Scholar]
- 14.Akers A. Bernstein L. Henderson S. Doyle J. Corbie-Smith G. Factors associated with lack of interest in HIV testing in older at-risk women. J Womens Health (Larchmt) 2007;16:842–858. doi: 10.1089/jwh.2006.0028. [DOI] [PubMed] [Google Scholar]
- 15.Luther VP. Wilkin AM. HIV infection in older adults. Clin Geriatr Med. 2007;23:567–583. doi: 10.1016/j.cger.2007.02.004. vii. [DOI] [PubMed] [Google Scholar]
- 16.Mugavero MJ. Castellano C. Edelman D. Hicks C. Late diagnosis of HIV infection: The role of age and sex. Am J Med. 2007;120:370–373. doi: 10.1016/j.amjmed.2006.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marks G. Crepaz N. Janssen RS. Estimating sexual transmission of HIV from persons aware and unaware that they are infected with the virus in the USA. AIDS. 2006;20:1447–1450. doi: 10.1097/01.aids.0000233579.79714.8d. [DOI] [PubMed] [Google Scholar]
- 18.Marks G. Crepaz N. Senterfitt JW. Janssen RS. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: Implications for HIV prevention programs. J Acquir Immune Defic Syndr. 2005;39:446–453. doi: 10.1097/01.qai.0000151079.33935.79. [DOI] [PubMed] [Google Scholar]
- 19.Galletly CL. Pinkerton SD. Petroll AE. CDC recommendations for opt-out testing and reactions to unanticipated HIV diagnoses. AIDS Patient Care STDs. 2008;22:189–193. doi: 10.1089/apc.2007.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bozzette SA. Routine screening for HIV infection—Timely and cost-effective. N Engl J Med. 2005;352:620–621. doi: 10.1056/NEJMe048347. [DOI] [PubMed] [Google Scholar]
- 21.Paltiel AD. Weinstein MC. Kimmel AD, et al. Expanded screening for HIV in the United States--an analysis of cost-effectiveness. N Engl J Med. 2005;352:586–595. doi: 10.1056/NEJMsa042088. [DOI] [PubMed] [Google Scholar]
- 22.Sanders GD. Bayoumi AM. Sundaram V, et al. Cost-effectiveness of screening for HIV in the era of highly active antiretroviral therapy. N Engl J Med. 2005;352:570–585. doi: 10.1056/NEJMsa042657. [DOI] [PubMed] [Google Scholar]
- 23.Holtgrave DR. Costs and consequences of the US Centers for Disease Control and Prevention's recommendations for opt-out HIV testing. PLoS Med. 2007;4:e194. doi: 10.1371/journal.pmed.0040194. [DOI] [PMC free article] [PubMed] [Google Scholar]