Abstract
Transient receptor potential (TRP) channels often play a role in sensory transduction, including chemosensory transduction. TRP channels, a common downstream target of phosphoinositide (PI) signaling, can be modulated by exogenous phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2], phosphatidylinositol 3,4,5-trisphosphate [PI(3,4,5)P3] and/or diacylglycerol (DAG). Lobster olfactory receptor neurons (ORNs) express a TRP-related, non-selective, calcium/magnesium-permeable, sodium/calcium-gated cation (SGC) channel. Here we report that PIs regulate the function of the calcium-activated form of the lobster channel. Sequestering of endogenous PI(4,5)P2, either with an anti-PI(4,5)P2 antibody or by electrostatic screening with polyvalent cations, blocks the channel. Exogenous PI(3,4,5)P3 activates the channel independently of intracellular sodium and/or calcium. Exogenous non-hydrolysable DAG analogs fail to change the gating parameters of the channel, suggesting the channel is insensitive to DAG. Electrophysiological recording from lobster ORNs in situ using a panel of pharmacological tools targeting the key components of both PI and DAG metabolism (phospholipase C, phosphoinositide 4-kinase and DAG kinase) extend these findings to the intact ORN. PI(4,5)P2 depletion suppresses both the odorant-evoked discharge and whole-cell current of the cells, and does so possibly independently of DAG production. Collectively, our results argue that PIs can regulate output in lobster ORNs, at least in part through their action on the lobster SGC channel.
Keywords: invertebrates, ion channel, olfaction, phosphoinositides, sensory transduction
INTRODUCTION
Phosphoinositides (PIs) are well known to play important and complex roles in the regulation of ion-transporting proteins, ranging from the maintenance of the structural integrity of signaling complexes to the control and fine tuning of the activity of a variety of ion exchangers/channels (for reviews, see Hilgemann, 2003; Raghu, 2006; Logothetis and Nilius, 2007; Nilius et al., 2008; Rohacs and Nilius, 2007; Pochynyuk et al., 2008; Suh and Hille, 2008; Gamper and Shapiro, 2007; Hardie, 2007; Rohacs, 2009). One point that emerges from this understanding is that PIs, specifically phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] and phosphatidylinositol 3,4,5-trisphosphate [PI(3,4,5)P3], are important in regulating ion channels involved in sensory transduction, including olfactory and visual cyclic nucleotide-gated (CNG) channels (Zhainazarov et al., 2004; Brady et al., 2006; Bright et al., 2007) and transient receptor potential (TRP) channels in invertebrate photoreceptors (Raghu et al., 2000) (reviewed in Raghu, 2006; Hardie, 2007; Raghu and Hardie, 2009) and vertebrate hair cells (Hirono et al., 2004). PI-dependent regulation of TRP channels is also increasingly appreciated to be involved in chemosensory transduction. For example, hydrolysis of PI(4,5)P2 leads to desensitization of TRPM5, an essential component of the transduction cascade mediating the taste modalities of bitter, sweet and amino acids (Liu and Liman, 2003). A similar interaction of PI(4,5)P2 has been reported for TRPM4 (Zhang et al., 2005), and sensory transduction in vomeronasal sensory cells appears to involve direct gating of the TRPC2 channel by diacylglycerol (DAG) (Lucas et al., 2003). Data on the extent and mechanisms by which PI signaling modulates the activity of chemosensory transduction channels will likely lead to a better understanding of sensory transduction, and chemosensory transduction in particular.
Lobster ORNs express a TRP-related, sodium-gated non-selective cation (SGC) channel. In inside-out patches excised from cultured ORNs, the channels can be activated by an increase of intracellular sodium and/or calcium (Bobkov and Ache, 2003; Bobkov and Ache, 2005). The calcium-sensitive form predominates in patch clamp recordings obtained from ORN outer dendrite membrane vesicles, suggesting that the calcium-activated form of the channel in particular is involved in olfactory transduction (Bobkov and Ache, 2003). Exogenous PIs modulate the channel function (Zhainazarov and Ache, 1999; Zhainazarov et al., 2001), but it is unclear whether and how the lobster SGC channel is normally regulated by PI signaling in ORNs.
Here we report that PIs can regulate the calcium-sensitive form of the lobster SGC channel. Reducing the endogenous levels of PI(4,5)P2 using either an anti-PI(4,5)P2 antibody or electrostatic screening with polyvalent cations inhibits activity of the channel. In contrast, exogenous non-hydrolysable DAG analogs fail to change the gating parameters of the channel, suggesting the channel is insensitive to DAG. Electrophysiological recording from lobster ORNs in situ using a panel of pharmacological tools targeting the key components of both PI and DAG metabolism [phospholipase C (PLC), phosphoinositide 4-kinase (PI4K) and DAG kinase] extend these findings to the intact ORN. Treatment of the ORNs in ways that would be expected to deplete PI(4,5)P2 suppresses both the odorant-evoked discharge and the odorant-evoked whole-cell current of the cells, and does so possibly independently of DAG production. Collectively, our results argue that PIs can regulate the odor-evoked output of lobster ORNs, at least in part through their action on the lobster SGC channel.
MATERIALS AND METHODS
Cell preparations
Spiny lobsters, Panulirus argus Latrielle 1804, were collected in the Florida Keys, maintained in the laboratory tanks with constant natural sea water flow, and fed shrimps. The experiments were conducted using cultured ORNs and an in situ preparation of the lobster olfactory organ. Primary cultures of lobster ORNs were prepared as described previously (Fadool et al., 1991). Briefly, clusters of ORNs were treated with trypsin (1 mg ml−1, Sigma, St Louis, MO, USA) for 10–40 min, mechanically dissociated, and plated on 35 mm Petri dishes. The cultured ORNs were kept at 21°C. Membrane patches were excised from the soma of cells cultured from 1 to 4 days. The cells were studied in situ using a modification of a preparation developed earlier (Doolin et al., 2002). Separate perfusion contours washed the ORN somata with Panulirus saline and the outer dendrites in the olfactory sensilla with Panulirus saline either alone or containing an odorant or drug. Solution switching was controlled using a multi-channel rapid solution changer (RSC-160, Bio-Logic, Claix, France) or a fast-step SF-77B perfusion system (Warner Instruments Inc., Hamden, CT, USA).
Electrophysiology and data analysis
Currents were measured with Axopatch 200A or 200B patch-clamp amplifiers (Molecular Devices, Sunnyvale, CA, USA) through a digital interface (Digidata 1320A, Molecular Devices), low-pass filtered at 5 kHz, sampled at 5–20 kHz and digitally filtered at 1–1.4 kHz. Data were collected and analyzed with pCLAMP 9.2 software (Molecular Devices) and SigmaPlot 10 (Systat Software, Inc., San Jose, CA, USA). Channel activity was investigated in steady-state conditions at a holding potential of −60 to −70 mV unless otherwise noted. The polarity of the currents is presented relative to the intracellular membrane surface. Patch pipettes were fabricated from borosilicate capillary glass (BF150-86-10, Sutter Instrument Company, Novato, CA, USA) using a Flaming-Brown micropipette puller (P-87, Sutter Instrument Company). Extracellular in situ recordings were conducted using a standard glass electrode filled with Panulirus saline. Odorant-evoked activity was examined after 1–3 min incubation with the solution(s)/drug(s) of interest. In multi-cell extracellular recordings the discharge rates of individual cells were estimated using the threshold or template search procedure provided by pCLAMP 9.2 software. The experimental data were fitted using two variations of the Hill equation: (1) F(x)=Fmax×xh/(x1/2h+xh) for activation, and (2) F(x)=1–Fmax×x1/2h/(xh +xh) for inhibition, where F is the open probability, normalized current or frequency of action potentials, x is the agonist/antagonist concentration, x1/2 is the half-effective agonist/antagonist concentration, and h is the Hill coefficient. An additional parameter reflecting the basal level of F (Fb) was incorporated when necessary. The data are presented as the mean ± s.e. of n observations. Comparisons between data sets were evaluated using Student's t test. All recordings were performed at room temperature (~21–23°C).
Lipid extraction and detection
The olfactory sensilla were removed from two lobster olfactory organs for each sample and transferred to ice-cold Panulirus saline solution. The preparations were centrifuged at 800 g for 5 min at 4°C to pellet the solid debris, and the supernatants containing the outer dendritic membranes were then treated with PLC activator and/or quercetin. Reactions were stopped by the addition of ice-cold 0.5 mol l−1 trichloroacetic acid (TCA). PIP2 was extracted and detected using a protein lipid overlay assay PI(4,5)P2 Mass Strip kit from Echelon Biosciences (Salt Lake City, UT, USA) following the manufacturer's protocol. Blots were incubated with ECL detection reagent (Millipore, Billerica, MA, USA), and the signal captured with a CCD camera (Fluor-S, Bio-Rad Laboratories, Hercules, CA, USA). The signal strength correlates with PI levels in lipid extracts and was determined using MultiAnalyst software (BioRad).
Solutions
Panulirus saline contained (mmol l−1): 486 NaCl, 5–13.4 KCl, 13.6 CaCl2, 9.8 MgCl2 and 10 Hepes, pH 7.8–7.9. Low-calcium sodium solution (210 mmol l−1 NaCl + 10 nmol l−1 Ca2+) contained (mmol l−1): 210 NaCl, 1 EGTA, 0.1 CaCl2, 696 glucose and 10 Hepes, pH 7.8–7.9. Low-calcium lithium solution (210 mmol l−1 LiCl + 10 nmol l−1 Ca2+) consisted of (mmol l−1): 210 LiCl, 1 EGTA, 0.1 CaCl2, 696 glucose and 10 Hepes, pH 7.8. The estimated free calcium concentration in low-calcium sodium/lithium solutions was <10 nmol l−1. Solutions containing 100 μmol l−1 Ca2+ (210 mmol l−1 NaCl + 100 μmol l−1 Ca2+) were prepared without chelating agents. The odorant was an aqueous extract of TetraMarin (TET, Tetra Werke, Melle, Germany), a commercially available fish food. The maximum concentration used in all experiments was 0.5 mg of the dried powder per ml of Panulirus saline. All inorganic salts were purchased from Fisher Scientific (Pittsburgh, PA, USA). All organic compounds were obtained from Sigma except for spermidine trihydrochloride, spermine tetrahydrochloride, m-3M3FBS and d-myo-inositol 1,3,4,5-tetrakisphosphate, octapotassium salt (IP4) which were obtained from Calbiochem. PI(4,5)P2-specific monoclonal antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). The phosphatidylinositol phosphates (phosphoinositides, PIs) were obtained from Cayman Chemical (Ann Arbor, MI, USA) as 1,2-dioctanoyl analogs or, in some cases, from Matreya LLC (Pleasant Gap, PA, USA) as 1,2-dipalmitoyl analogs of natural PIs. Net charges of polyvalent cations were estimated based on pKa values of respective compounds. The predicted pKa values were obtained using the ACD/I-Lab Web service (Advanced Chemistry Development Inc., Toronto, ON, Canada).
RESULTS
The calcium-sensitive form of the lobster SGC channel can be regulated by PIs
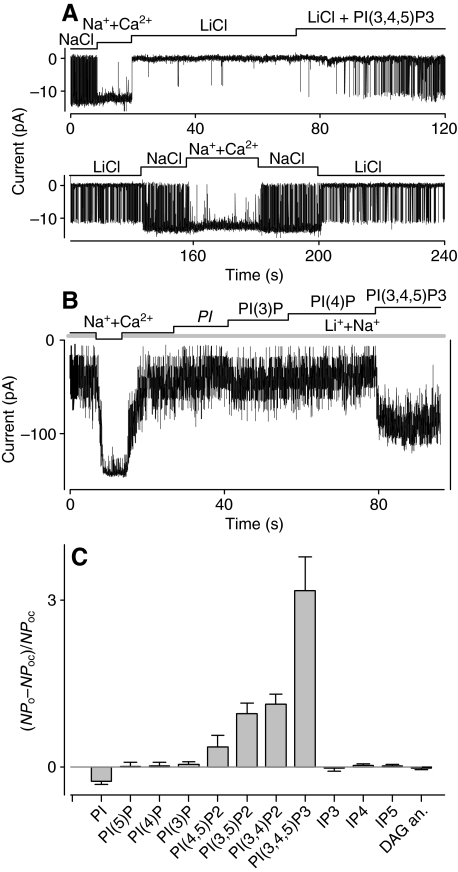
As the calcium-sensitive form of the SGC channel is thought to be predominantly expressed in the transduction compartment (outer dendrites) of the lobster ORNs (Bobkov and Ache, 2003), we first tested whether the PI-dependent modulation of the calcium-sensitive form of the SGC channel was qualitatively consistent with that reported earlier for the calcium-insensitive form of the channel (Zhainazarov et al., 2001). The channel was activated by PIs (1–20 μmol l−1), especially by PI(3,4,5)P3, applied to the cytosolic side of patches containing the calcium-sensitive form of the channel. In single-channel recordings (Fig. 1A), PI(3,4,5)P3 (16 μmol l−1) increased the open probability (Po) of the channel from near 0 to 0.15 (determined at steady-state conditions) in LiCl, and from 0.76 to 0.94 in NaCl. Overall the relative potencies of the PIs tested, estimated as the ratio between the increment in activity following PI (20 μmol l−1) application and the channel activity in control conditions, were: PI(3,4,5)P3>PI(3,4)P2>PI(3,5)P2>PI(4,5)P2, with PI(3)P, PI(4)P, PI(5)P and phosphatidylinositol (PI) being essentially ineffective (Fig. 1B,C). Thus, despite somewhat different experimental conditions, our data for the calcium-sensitive form of the channel are in good agreement with our earlier findings for the calcium-insensitive form (Zhainazarov et al., 2001). The strong sensitivity of the SGC channel to 3-phosphoinositides – PI(3,4,5)P3 could activate the channel even in the absence of Na+ or Ca2+ (Fig. 1A) – suggests phosphoinositide 3-kinase (PI3K) plays a role in activating the SGC channel, and therefore possibly in lobster olfactory transduction.
Fig. 1.
The lobster sodium-gated cation (SGC) channel can be activated and modulated by exogenous lipids in inside-out membrane patches. (A) Current traces of a channel activated by exogenous phosphatidylinositol 3,4,5-trisphosphate [PI(3,4,5)P3, 16 μmol l−1]. Holding potential, −70 mV. Electrode solution: NaCl (210 mmol l−1) + Ca2+ (10 nmol l−1). Bar above current traces shows time course of solution application. LiCl: LiCl (210 mmol l−1) + Ca2+ (10 nmol l−1). NaCl: NaCl (210 mmol l−1) + Ca2+ (10 nmol l−1). Na++Ca2+: NaCl (210 mmol l−1) + Ca2+ (100 μmol l−1). Note: exogenous PI(3,4,5)P3 (1,2-dipalmitoyl analog was used in the experiment) activates the channel independently of intracellular sodium and calcium. Both current traces are gap-free recordings from the same patch. (B) Multi-channel activity in another patch exposed to Na++Ca2+ and different PIs (20 μmol l−1) in the presence of 180 mmol l−1 Li+ and 30 mmol l−1 Na+ (Li++Na+). Holding potential, −60 mV. Electrode solution: NaCl (210 mmol l−1) + Ca2+ (10 nmol l−1). Bar above current traces shows time course of solution application. Note: PI(3,4,5)P3 greatly increased the channel open probability in the presence of 30 mmol l−1 Na+ whereas phosphatidylinositol (PI), PI(3)P and PI(4)P were ineffective. (C) Bar diagram of the relative efficacy of various PIs, a diacylglycerol (DAG) analog and inositol phosphates (IPs) on the SGC channel in membrane patches excised from cultured lobster olfactory receptor neurons (ORNs). Efficacy was calculated as (NPo–NPoc)/NPoc, where N is the number of channels, Poc is the channel open probability in control conditions (Li++Na+) and Po is the channel open probability in Li++Na+ and 20 μmol l−1 of one of the following: PI (n=5), PI(3)P (n=5), PI(4)P (n=5), PI(5)P (n=4), PI(4,5)P2 (n=4), PI(3,5)P2 (n=4), PI(3,4)P2 (n=3), PI(3,4,5)P3 (n=9) or 1-oleoyl-2-acetyl-sn-glycerol (DAG analog, 10 μmol l−1, n=7), IP3 (3–10 μmol l−1, n=8), IP4 (5–20 μmol l−1, n=15) and IP5 (10 μmol l−1, n=5). Note: PI(3,4,5)P3 is the most effective lipid.
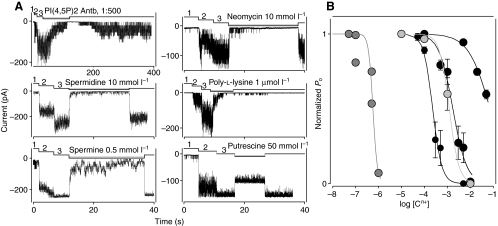
PI(4,5)P2, the precursor of PI(3,4,5)P3 and the most abundant PI lipid in cell membranes, also appears to be necessary to maintain the activity of the channel. Applying a PI(4,5)P2-specific monoclonal antibody to the cytoplasmic side of the membrane patch (1:500 to 1:1000) totally inhibited SGC channel activity (Fig. 2A). This antibody reacts primarily with the head group of PI(4,5)P2 and demonstrates low cross-reactivity with other phospholipids (Thomas et al., 1999). The antibody decreased the open probability of the channel, but did not change the single channel amplitude (e.g. Fig. 2A, 16.6±0.18 pA vs 16.7±0.19 pA, P=0.4, n=8). Applying a PI(3,4,5)P3-specific monoclonal antibody to the cytoplasmic side of the membrane patch (1:500) did not significantly change the gating parameters of the channel, as would be expected given the low levels of PI(3,4,5)P3 in resting cells. The mean NPo estimated 2 min following anti-PI(3,4,5)P3 application and normalized to control was 0.99±0.04 (n=3; 0.92, 0.99, 1.07), further supporting the specificity of the inhibition resulting from application of the PI(4,5)P2-specific monoclonal antibody.
Fig. 2.
Application of organic polyvalent cations (Cn+) and an anti-PI(4,5)P2 antibody blocks the lobster SGC channel in cell-free, inside-out membrane patches. (A) Current traces of channels blocked by Cn+ and an anti-PI(4,5)P2 antibody. Channels were first activated by Na+ (210 mmol l−1) and Ca2+ (100 μmol l−1). Gray lines mark the time courses of an anti-PI(4,5)P2 antibody and Cn+ application. Note, Cn+ reduced the open probability of the channel rather than decreasing the single channel amplitude. The discrete current levels seen on the traces reflect current through different numbers of simultaneously open channels. Note: the PI(4,5)P2 antibody acted considerably slower than the Cn+. Holding potential, −70 mV. Electrode solution: NaCl (210 mmol l−1) + Ca2+ (10 nmol l−1). 1, LiCl (210 mmol l−1) + Ca2+ (10 nmol l−1); 2, NaCl (210 mmol l−1) + Ca2+ (10 nmol l−1); 3, NaCl (210 mmol l−1) + Ca2+ (100 μmol l−1). (B) Plot of the concentration dependence of the effect of Cn+ (in mol l−1) on the SGC channel. Each point is the mean ± s.e.m. of the normalized current from 3–7 patches. Solid lines: Hill plots with the following parameters according to potency: [poly-l-lysine]1/2=0.57 μmol l−1, h=4.5, n=2–6; [spermine]1/2=229 μmol l−1, h=2.2, n=3–7; [neomycin]1/2=1.2 mmol l−1, h=2, n=3–6; [spermidine]1/2 =1.74 −1 mmol l, h=1.4, n=3–6; [putrescine]1/2=73 mmol l−1, h=0.9, n=3–6.
Since polyvalent cations could potentially interact with and sequester anion phospholipids such as PI(4,5)P2 (e.g. Fan and Makielski, 1997; Arbuzova et al., 2000; Suh and Hille, 2007), we also examined the effects of these compounds on channel gating. Organic polyvalent cations, the aminoglycoside antibiotic neomycin, poly-l-lysine and the polyamines spermine, spermidine and putrescine, applied to the cytoplasmic side of inside-out patches containing the calcium-sensitive form of the channel inhibited channel activity in a concentration-dependent manner (Fig. 2). The reversibility of the inhibition varied with the drug (inhibition by poly-l-lysine was only partially reversible even after several minutes of washing, Fig. 2A), the particular patch, and the composition of the bathing solution (divalent cations slowed down recovery). The primary effect of the drugs was to decrease the open probability of the channel (Fig. 2). The relative potencies of the drugs were: [poly-l-lysine]1/2=0.57 μmol l−1, h=4.5, n=2–6; [spermine]1/2=229 μmol l−1, h=2.2, n=3–7; [neomycin]1/2=1.2 mmol l−1, h=2, n=3–6; [spermidine]1/2= 1.74 mmol l−1, h=1.4, n=3–6; and [putrescine]1/2=73 mmol l−1, h=0.9, n=3–6. At their highest concentrations, the drugs also changed the single channel amplitude (Fig. 2A), with the following relative potencies (mean ± s.e.m. of n measurements, P-value of paired t-test): spermine 5 mmol l−1 (16.4±0.1 vs 10.5±1.8 pA, P<0.01, n=5); poly-l-lysine 1 μmol l−1 (16.3±0.2 vs 11.4±0.2 pA, P<0.01, n=6); putrescine 50 mmol l−1 (16.2±0.4 vs 12±0.2 pA, P<0.01, n=5); spermidine 10 mmol l−1 (16.9±0.2 vs 14.4±0.1 pA, P<0.01, n=6); and neomycin 10 mmol l−1 (16.5±0.2 vs 16.3±0.2 pA, P=0.5, n=4). The changes in single channel amplitude suggest that some of the polyvalent cations could act as permeant blocking ions, but detailed analysis of the possible mechanism underlying the observed decrease in channel amplitude was not pursued.
PI(4,5)P2 levels can be manipulated pharmacologically in lobster outer dendrite membranes in vitro
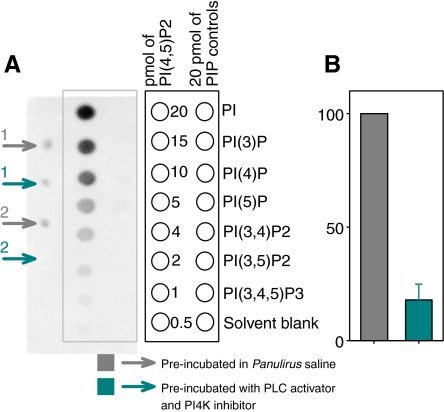
If PI(4,5)P2 plays a role in regulating the SGC channel in situ, it should be possible to show that it occurs in the transduction compartment where the calcium-sensitive form of the channel predominates, and that PI(4,5)P2 levels can be modulated by drugs targeting its metabolism. Lipids were extracted from the outer dendrite membranes of lobster ORNs and PI(4,5)P2 levels measured with a protein–lipid overlay assay that uses the pleckstrin homology domain of the protein PLC-δ1 as a probe for PI(4,5)P2. PI(4,5)P2 could be detected in phospholipids extracted from the outer dendrites of lobster ORNs (Fig. 3A). Treatment with quercetin (40 μmol l−1), a non-specific antagonist of phosphoinositide kinases including PI4K, and the PLC activator m-3M3FBS (20 μmol l−1) reduced the PI(4,5)P2 signal by 82±7% (n=4; Fig. 3B). These drugs, like most, typically are characterized based on their action in vertebrate tissue and these findings also validate their use in subsequent physiological experiments.
Fig. 3.
Treatment with a phospholipase C (PLC) activator and a PI4K inhibitor decreases the level of PI(4,5)P2 in lobster olfactory outer dendritic membranes in vitro. (A) Left: dot blot showing PI(4,5)P2 detected by overlaying the nitrocellulose membrane containing the lipids with a lipid binding protein consisting of a GST-tagged pleckstrin homology domain from PLCδ. Data for two different preparations are shown (1,2). Right (framed): synthetic PI(4,5)P2 and PI controls used in the dot blot as specificity controls (see diagram to the right for description). (B) Bar plot of the magnitude of the PI(4,5)P2 signal before (gray) and after incubating the outer dendrite membranes with a PLC activator (m-3M3FBS, 20 μmol l−1) and a PI4K inhibitor (quercetin, 40 μmol l−1) for 20 min (cyan). Images of the blots were captured with a CCD camera and the grayscale intensity quantified. Signals were normalized to control after the solvent blank was subtracted. Preincubation with the PLC activator and the PI4K inhibitor reduced the PI(4,5)P2 level by 82±7% (n=4).
PIs can regulate the output of lobster ORNs in situ
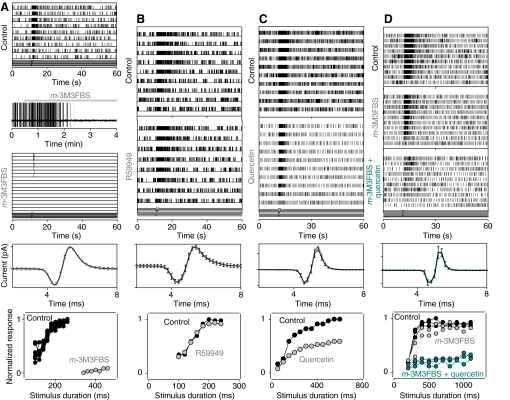
We then used these pharmacological probes to establish whether PI(4,5)P2 pool depletion would change the output of ORNs recorded in situ using standard extracellular loose patch recording. The PLC activator m-3M3FBS (20–40 μmol l−1) applied to the outer dendrites significantly decreased the spontaneous discharge of tonically active ORNs (1.88±0.36 Hz control vs 1.09±0.27 Hz after 3–4 min incubation with the drug, n=28, P<0.005) and significantly reduced odorant-evoked discharge over the range of odorant concentrations tested (Fig. 4A). For the cell shown, for example, the drug almost totally suppressed the evoked response. Overall, application of m-3M3FBS decreased the response 32 of 35 (~90%) ORNs tested by approximately 60% (0.95±0.01 vs 0.38±0.05, average normalized response at saturating stimulus intensities, n=22; Fig. 5 shaded areas and bar plots). Both effects of the drug were reversible.
Fig. 4.
Effect of application of a PLC activator (A,D), a DAG kinase inhibitor (B) and a PI4K inhibitor (C,D) on the discharge of tonically active lobster ORNs in situ. Top panels: raster displays of action potentials from a single ORN in response to odorant pulses of decreasing intensity (from top to bottom), before (control) and in the presence of the drug. Time between successive sweeps, 60 s. (A) Application of m-3M3FBS (40 μmol l−1) completely blocked the spontaneous discharge and strongly reduced the evoked discharge. The gap-free recording between the raster displays shows the time course of the effect. (B) Treatment with the DAG kinase inhibitor R59949 (20 μmol l−1) failed to change either the spontaneous or the evoked activity of another cell. (C) Effect of quercetin (70 μmol l−1) application on spontaneous and odorant-evoked activity. (D) m-3M3FBS (20 μmol l−1) application slightly reduced the evoked discharge of another cell. The output of the cell was reduced further on blocking PI4K with quercetin (70 μmol l−1). The graphs below each set of raster plots show superposition of spikes in control conditions (black) and after incubation with the drugs (gray and cyan). Error bars show standard deviation of the mean (s.d.m.) (n=20–100). The minimal effect on spike shape suggests the drugs had little if any effect on voltage-dependent conductances. The bottom plots show the extent of blockade as a function of odorant intensity, with odorant intensity changed by increasing the duration of the stimulus. The normalized discharge frequency (ordinate) is plotted as a function of stimulus duration (abscissa). Black circles, control; gray and cyan circles, following incubation with the drug indicated on the graph. Data in A–D were obtained from different ORNs.
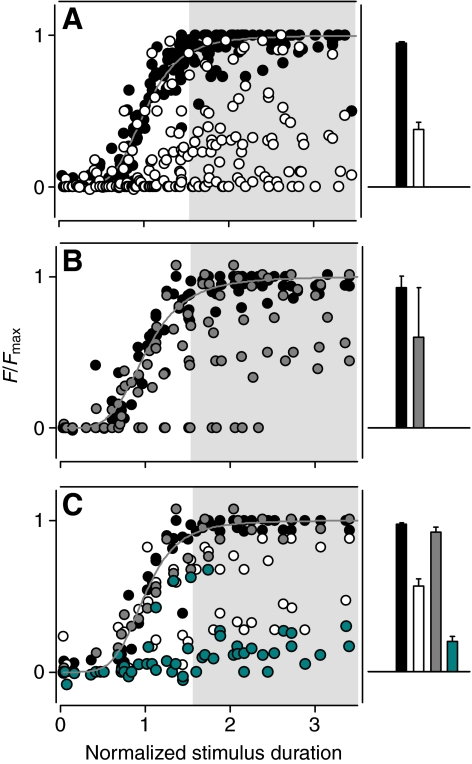
Fig. 5.
The effect of application of m-3M3FBS (A), quercetin (B) and their combination (C) on the odorant-evoked activity of lobster ORNs in situ. Left: the output of all the cells tested (ordinate) plotted as a function of odorant intensity (duration, absissa). The output represents the mean discharge frequency within a 2 s peristimulus interval. The magnitude of the output of each cell was normalized to the Fmax and S1/2 for that cell in control conditions. Solid lines show the fit of the Hill equation, F=Fb+(Fmax–Fb)/[1+(S1/2/S)h] to the data, where Fb is the spontaneous discharge frequency, Fmax is the maximal frequency of response, S1/2 is the duration (concentration) of stimulus required to elicit half-maximal discharge, and h is the Hill coefficient. Right: bar plots of the mean discharge frequency at saturating stimulus intensities (shaded area in plots on the left). Shown are responses in control conditions (black bars) and after at least 10 min incubation with 20 μmol l−1 m-3M3FBS (n=22, white bars), 40–70 μmol l−1 quercetin (n=12, gray bars) or both (n=7, cyan bars). Plots and bar histograms have the same scales (F/Fmax).
As hydrolysis of PI(4,5)P2 by PLC would also be expected to increase inositol trisphosphate (IP3) and DAG, and as DAG has been implicated in chemosensory transduction (Lucas et al., 2003; Chouquet et al., 2009), we tested whether inhibiting the activity of DAG kinase, a key enzyme in DAG metabolism, would mimic the effects of m-3M3FBS or otherwise modulate ORN activity. Applying the DAG kinase antagonist R59949 (20 μmol l−1) to the outer dendrites (5–20 min) failed to change either the spontaneous or odorant-evoked discharge of the cells (Fig. 4B, n=4), arguing that DAG-dependent signal transduction events do not account for the action of the PLC activator.
Preventing PI(4,5)P2 synthesis by suppressing PI kinase activity with quercetin (70 μmol l−1), a non-specific membrane-permeable antagonist of PI kinases shown to greatly reduce PI(4,5)P2 levels for example in hair cells (Hirono et al., 2004), failed to change the spontaneous discharge of lobster ORNs (1.83±0.37 Hz control vs 1.43±0.49 Hz after 3–4 min incubation with the drug, n=12, P=0.4). However, as with m-3M3FBS, application of quercetin significantly reduced the odorant-evoked activity (0.93±0.08 control vs 0.60±0.33 after 2–4 min incubation with the drug, normalized response, n=12, P<0.005, Fig. 4C and Fig. 5B, shaded area). The effect of the drug was reversible in most, but not all, instances.
In some of the cells, treatment with m-3M3FBS had relatively minor effects. For example, for the cell shown in Fig. 4D odorant-evoked activity was reduced only by ~10% after 10 min perfusion with the drug (20 μmol l−1), possibly indicating that in those ORNs PI(4,5)P2 re-synthesis (i.e. the level of PI4K activity) was sufficiently high to compensate for any PLC-dependent hydrolysis. To test this hypothesis we inhibited PI4K while simultaneously activating PLC. Incubation with quercetin (70–140 μmol l−1) together with the PLC activator strongly enhanced the suppression of odorant-evoked ORN activity in 6 of 7 such cells (0.97±0.01 control vs 0.92±0.92 after incubation with quercetin, 0.57±0.04 after incubation with m-3M3FBS, and 0.20±0.03 after simultaneous application of both quercetin and m-3M3FBS, normalized response; Fig. 4D, Fig. 5C). Application of quercetin (70 μmol l−1) and m-3M3FBS (20 μmol l−1) together did not appreciably change the spontaneous activity of the ORNs (1.36±0.37 Hz vs 1.19±0.64 Hz, P=0.8). The remaining cell was insensitive to the drugs.
Except for a minor increase in spike peak amplitude (e.g. Fig. 4C), the absence of any change in spike shapes suggests the drugs had little effect on the conductances associated with action potential generation per se (Fig. 4). However, the finding that pharmacological treatment that potentially blocked the channel decreased the mean frequency of spontaneous discharge suggests, consistent with previous data (e.g. Bobkov and Ache, 2007; Pezier et al., 2009), that the channel might be constitutively active in at least some ORNs and might contribute to setting the resting potential in those ORNs.
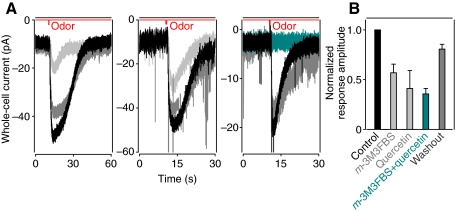
Since extracellular recording does not reveal changes in the underlying receptor current, we also tested the drugs while recording in the whole-cell mode (Fig. 6). Under voltage clamp, odorants generated excitatory inward currents. Application of the PLC activator m-3M3FBS (20 μmol l−1) reduced the odorant-evoked inward current in all of the cells tested (n=5; shown for one cell in Fig. 6A, left) by an average of 43% (0.57±0.09, average normalized response following incubation with the drug, Fig. 6B). Application of quercetin reduced the odorant-evoked inward current (Fig. 6A, middle) by an average of 59% (0.41±0.18, n=4, Fig. 6B). As with the extracellular response, inhibition was even greater if m-3M3FBS and quercetin were applied together (Fig. 6A, right), reducing the odorant-evoked inward current by 65% (0.35±0.06, n=11, Fig. 6B). The effects of the drugs varied from almost total (e.g. Fig. 6A right) to relatively minor (~10–20%) blockade. The inhibition was partially reversible. The resting whole-cell current was basically unchanged by treatment with either drug (Fig. 6A), arguing that the drugs decrease the activity of the channels involved in generating the receptor current rather than, for example, hyperpolarizing the cells.
Fig. 6.
Incubation with m-3M3FBS and quercetin suppresses the odorant-evoked inward current in lobster ORNs in situ. (A) Current traces recorded from three different ORNs evoked by odorants before (black), and after incubation with 20 μmol l−1 m-3M3FBS (light gray, left), 40 μmol l−1 quercetin (light gray, middle) or both (cyan, right). The effects of the drugs were partially reversible on washout (dark gray). Holding potential, −70 mV. Electrode solution (mmol l−1): KCl 180, NaCl 30, EGTA 1, CaCl2 0.1, glucose 696, MgATP 1, Hepes 10. (B) Bar plot of the mean peak inward current obtained in A. Recordings were digitally low-pass filtered at 1 kHz. Current and time scales are different in each case.
DISCUSSION
Collectively, our findings argue that the lobster SGC channel is a target of PI-dependent, and in particular PI(4,5)P2-dependent, regulation in lobster ORNs. PI(4,5)P2 appears to be a critical membrane constituent that maintains the functionality of the SGC channel, as depletion of endogenous PI(4,5)P2 either with an anti-PI(4,5)P2 antibody or by electrostatic screening with polyvalent cations inhibited the channel. Polyvalent organic cations are widely used to sequester anionic phospholipids, including PI(4,5)P2 (Fan and Makielski, 1997; Kozak et al., 2005; Liu and Qin, 2005; Suh and Hille, 2007). The finding that their potency sequence follows the number of positive charges (+nmax/+n7.8, maximum charge/net charge calculated for pH 7.8; IC50): putrescine (+2/+1.5; 73 mmol l−1)<spermidine (+3/+2.48; 1.74 mmol l−1)<spermine (+4/+3.46; 229 μmol l−1)<neomycin (+6/+3.9; 1.2 mmol l−1)<poly-l-lysine (⪢+6; 0.57 μmol l−1)] is consistent with their action in a variety of ion transport systems where PIs are suspected to be important regulatory components (Kozak et al., 2005; Suh and Hille, 2007). However, the relative efficacy of neomycin suggests that net charge of the molecule does not precisely correlate with efficacy and, perhaps, that spatial charge organization is also an important parameter determining potency.
Previous evidence for the involvement of the calcium-sensitive SGC channel in chemosensory transduction in lobster ORNs (Bobkov and Ache, 2005; Bobkov and Ache, 2007), together with the evidence for PI(4,5)P2-dependent regulation of the SGC channel, suggests that the activity of ORNs should be modulated by drugs targeting PIP2 metabolism. Indeed, we found that pharmacological probes potentially accelerating PI(4,5)P2 hydrolysis and those suppressing PI(4,5)P2 synthesis greatly reduced odorant-evoked activity in ORNs recorded in situ. These new findings add to our existing understanding to suggest that PI(4,5)P2 mediates ORN activity in vivo, and that it does so at least in part through the regulation of the SGC channel.
It is important to note that while the effects of these drugs on ORN activity are qualitatively similar they would not necessarily equally change the concentration of PI(4,5)P2 metabolites, suggesting that PI(4,5)P2 itself, and not the metabolites of PI(4,5)P2, regulates ORN activity. That interpretation is further strengthened by the fact that other potential signaling molecules downstream of PI(4,5)P2 hydrolysis, including IP3, IP4, IP5 and the non-hydrolysable DAG analog OAG, failed to influence channel gating (Fig. 1B), and also by the fact that the DAG kinase inhibitor R59949 had no effect on either the spontaneous or the odorant-evoked activity of the ORNs. Thus, PI(4,5)P2-dependent regulation of ORN output would appear to be due to PI(4,5)P2 itself.
PI signaling, of course, involves a complex, dynamic network of inter-converting lipids, each with potential signal function, that are controlled through the combined action of multiple specific phosphatases and kinases. Since exogenous PI(3,4,5)P3 and, although to a considerably lesser extent, PI(3,4)P2 and PI(3,5)P2 activated the channel, these lipids could well be the primary elements gating the channel. PI(3,4,5)P3 in particular might act as such since its action is mainly independent of other factors known to regulate the channel gating (i.e. sodium, calcium – Fig. 1A). This possibility gains credence from evidence that odorants transiently increase levels of PI(3,4,5)P3 in the outer dendritic membranes of lobster ORNs (Corey et al., 2010) and mouse olfactory ciliary-enriched membranes (Klasen et al., 2010). PI(4,5)P2 pool reduction would inevitably reduce the stimulus-dependent increase in PI(3,4,5)P3 and therefore suppress odorant-evoked ORN activity, as we observed. Our data do not exclude other possibilities, however; for example, that olfactory transduction requires both PI(4,5)P2 and PI(3,4,5)P3 as second messengers or that PI(4,5)P2 maintains the structural integrity of the channel and/or its associated signaling complex.
We cannot fully exclude the possibility that PI(4,5)P2 hydrolysis also modulates the activity of other ion transport complexes potentially involved in olfactory transduction. One likely target would be the HCN channel expressed by these cells (Gisselmann et al., 2005). Mammalian HCN channel subtypes can be regulated by PIs; PI(4,5)P2 and PI(3,4,5)P3 shift voltage-dependent channel activation approximately 20 mV toward depolarized potentials (Pian et al., 2006; Zolles et al., 2006; Pian et al., 2007). In the case of the lobster ORN HCN channel, PI(4,5)P2 pool depletion would downregulate the channel activity eventually leading to hyperpolarization of the cell and a reduction in spontaneous discharge and odorant-evoked responses, yet blocking the HCN channel with the specific inhibitor ZD7288 failed to noticeably change the odorant-evoked response of these cells (Gisselmann et al., 2005), and suppression of HCN channel activity would not be expected to reduce the odorant-evoked inward current to the extent seen in our whole-cell recordings (Fig. 6). Thus, we can exclude the possibility that PI(4,5)P2 hydrolysis targets the HCN channel, but other possible targets of PI(4,5)P2 hydrolysis remain to be explored.
In summary, we argue that PIs can regulate the output of lobster ORNs, at least in part by regulating the SGC channel. Sequestering endogenous PI(4,5)P2 inhibited channel activity. Exogenous non-hydrolysable DAG analogs failed to change the gating parameters of the channel, suggesting the channel was insensitive to DAG. Electrophysiological recording from lobster ORNs in situ using a panel of pharmacological tools targeting the key components of both PI and DAG metabolism (PLC, PI4K and DAG kinase) extended these findings to the intact ORN. PI(4,5)P2 depletion suppressed both the odorant-evoked discharge and the odorant-evoked whole-cell current of the cells, and did so possibly independent of DAG production. Although the lobster SGC channel has yet to be identified molecularly, its physiological and pharmacological properties are consistent with TRP channel family assignment. Our findings are consistent with the existing understanding of how TRP channel function is controlled by PI signaling, and extend this understanding to chemosensory receptor neurons. Clearly, further work will be necessary to more completely understand what are likely to be multiple mechanisms of interaction between the lobster channel and the dynamic phosphoinositide environment of the cell, and how such interaction would shape the input and output of the ORN.
ACKNOWLEDGEMENTS
We thank Ms Anna Mistretta-Bradley for her excellent technical assistance. This work was supported by the National Institute on Deafness and Other Communication Disorders through grant DC001655. Deposited in PMC for release after 12 months.
REFERENCES
- Arbuzova A., Martushova K., Hangyás-Mihályné G., Morris A. J., Ozaki S., Prestwich G. D., McLaughlin S. (2000). Fluorescently labeled neomycin as a probe of phosphatidylinositol-4,5-bisphosphate in membranes. Biochim. Biophys. Acta 1464, 35-48 [DOI] [PubMed] [Google Scholar]
- Bobkov Y. V., Ache B. W. (2003). Calcium sensitivity of a sodium-activated nonselective cation channel in lobster olfactory receptor neurons. J. Neurophysiol. 90, 2928-2940 [DOI] [PubMed] [Google Scholar]
- Bobkov Y. V., Ache B. W. (2005). Pharmacological properties and functional role of a TRP-related ion channel in lobster olfactory receptor neurons. J. Neurophysiol. 93, 1372-1380 [DOI] [PubMed] [Google Scholar]
- Bobkov Y. V., Ache B. W. (2007). Block by amiloride derivatives of odor-evoked discharge in lobster olfactory receptor neurons through action on a presumptive TRP channel. Chem. Senses 32, 149-159 [DOI] [PubMed] [Google Scholar]
- Brady J. D., Rich E. D., Martens J. R., Karpen J. W., Varnum M. D., Brown R. L. (2006). Interplay between PIP3 and calmodulin regulation of olfactory cyclic nucleotide-gated channels. Proc. Natl. Acad. Sci. USA 103, 15635-15640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright S. R., Rich E. D., Varnum M. D. (2007). Regulation of human cone cyclic nucleotide-gated channels by endogenous phospholipids and exogenously applied phosphatidylinositol 3,4,5-trisphosphate. Mol. Pharmacol. 71, 176-183 [DOI] [PubMed] [Google Scholar]
- Chouquet B., Debernard S., Bozzolan F., Solvar M., Maobeche-Coisne M., Lucas P. (2009). A TRP channel is expressed in Spodoptera littoralis antennae and is potentially involved in insect olfactory transduction. Insect Mol. Biol. 18, 213-222 [DOI] [PubMed] [Google Scholar]
- Corey E. A., Bobkov Y., Pezier A., Ache B. W. (2010). Phosphoinositide 3-kinase mediated signaling in lobster olfactory receptor neurons. J. Neurochem. 113, 341-350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolin R. E., Ache B. W. (2002). A simple method for focally delivering multiple drugs or ligands to cells. J. Neurosci. Methods 116, 9-14 [DOI] [PubMed] [Google Scholar]
- Fadool D. A., Michel W. C., Ache B. W. (1991). Sustained primary culture of lobster (Panulirus argus) olfactory receptor neurons. Tissue Cell 23, 719-731 [DOI] [PubMed] [Google Scholar]
- Fan Z., Makielski J. C. (1997). Anionic phospholipids activate ATP-sensitive potassium channels. J. Biol. Chem. 272, 5388-5395 [DOI] [PubMed] [Google Scholar]
- Gamper N., Shapiro M. S. (2007). Regulation of ion transport proteins by membrane phosphoinositides. Nat. Rev. Neurosci. 8, 921-934 [DOI] [PubMed] [Google Scholar]
- Gisselmann G., Marx T., Bobkov Y., Wetzel C. H., Neuhaus E. M., Ache B. W., Hatt H. (2005). Molecular and functional characterization of an I-h-channel from lobster olfactory receptor neurons. Eur. J. Neurosci. 21, 1635-1647 [DOI] [PubMed] [Google Scholar]
- Hardie R. C. (2007). TRP channels and lipids: from Drosophila to mammalian physiology. J. Physiol. 578, 9-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgemann D. W. (2003). Getting ready for the decade of the lipids. Annu. Rev. Physiol. 65, 697-700 [DOI] [PubMed] [Google Scholar]
- Hirono M., Denis C. S., Richardson G. P., Gillespie P. G. (2004). Hair cells require phosphatidylinositol 4,5-bisphosphate for mechanical transduction and adaptation. Neuron 44, 309-320 [DOI] [PubMed] [Google Scholar]
- Klasen K., Corey E. A., Kuck F., Wetzel C. H., Hatt H., Ache B. W. (2010). Odorant-stimulated phosphoinositide signaling in mammalian olfactory receptor neurons. Cell Signal. 22, 150-157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak J. A., Matsushita M., Nairn A. C., Cahalan M. D. (2005). Charge screening by internal pH and polyvalent cations as a mechanism for activation, inhibition, and rundown of TRPM7/MIC channels. J. Gen. Physiol. 126, 499-514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B. Y., Qin F. (2005). Functional control of cold- and menthol-sensitive TRPM8 ion channels by phosphatidylinositol 4,5-bisphosphate. J. Neurosci. 25, 1674-1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Liman E. R. (2003). Intracellular Call and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proc. Natl. Acad. Sci. USA 100, 15160-15165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis D. E., Nilius B. (2007). Dynamic changes in phosphoinositide levels control ion channel activity. Pflugers Arch. Eur. J. Physiol. 455, 1-3 [DOI] [PubMed] [Google Scholar]
- Lucas P., Ukhanov K., Leinders-Zufall T., Zufall F. (2003). A diacylglycerolgated cation channel in vomeronasal neuron dendrites is impaired in TRPC2 mutant mice: Mechanism of pheromone transduction. Neuron 40, 551-561 [DOI] [PubMed] [Google Scholar]
- Nilius B., Owsianik G., Voets T. (2008). Transient receptor potential channels meet phosphoinositides. EMBO J. 27, 2809-2816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezier A., Bobkov Y. V., Ache B. W. (2009). The Na+/Ca2+ exchanger inhibitor, KB-R7943, blocks a nonselective cation channel implicated in chemosensory transduction. J. Neurophysiol. 101, 1151-1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pian P., Bucchi A., Robinson R. B., Siegelbaum S. A. (2006). Regulation of Gating and rundown of HCN hyperpolarization-activated channels by exogenous and endogenous PIP2. J. Gen. Physiol. 128, 593-604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pian P., Bucchi A., DeCostanzo A., Robinson R. B., Siegelbaum S. A. (2007). Modulation of cyclic nucleotide-regulated HCN channels by PIP2 and receptors coupled to phospholipase C. Pflugers Arch. Eur. J. Physiol. 455, 125-145 [DOI] [PubMed] [Google Scholar]
- Pochynyuk O., Bugaj V., Stockand J. D. (2008). Physiologic regulation of the epithelial sodium channel by phosphatidylinositides. Curr. Opin. Nephrol. Hypertens. 17, 533-540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghu P. (2006). Regulation of Drosophila TRPC channels by protein and lipid interactions. Semin. Cell Dev. Biol. 17, 646-653 [DOI] [PubMed] [Google Scholar]
- Raghu P., Hardie R. C. (2009). Regulation of Drosophila TRPC channels by lipid messengers. Cell Calcium 45, 566-573 [DOI] [PubMed] [Google Scholar]
- Raghu P., Usher K., Jonas S., Chyb S., Polyanovsky A., Hardie R. C. (2000). Constitutive activity of the light-sensitive channels TRP and TRPL in the Drosophila diacylglycerol kinase mutant, rdgA. Neuron 26, 169-179 [DOI] [PubMed] [Google Scholar]
- Rohacs T. (2009). Phosphoinositide regulation of non-canonical transient receptor potential channels. Cell Calcium 45, 554-565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohacs T., Nilius B. (2007). Regulation of transient receptor potential (TRP) channels by phosphoinositides. Pflugers Arch. Eur. J. Physiol. 455, 157-168 [DOI] [PubMed] [Google Scholar]
- Suh B. C., Hille B. (2007). Electrostatic interaction of internal Mg2+ with membrane PIP2 seen with KCNQ K+ channels. J. Gen. Physiol. 130, 241-256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh B. C., Hille B. (2008). PIP2 is a necessary cofactor for ion channel function: How and why? Annu. Rev. Biophys. 37, 175-195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C. L., Steel J., Prestwich G. D., Schiavo G. (1999). Generation of phosphatidylinositol-specific antibodies and their characterization. Biochem. Soc. Trans. 27, 648-652 [DOI] [PubMed] [Google Scholar]
- Zhainazarov A. B., Ache B. W. (1999). Effects of phosphatidylinositol 4,5-bisphosphate and phosphatidylinositol 4-phosphate on a Na+-gated nonselective cation channel. J. Neurosci. 19, 2929-2937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhainazarov A. B., Doolin R., Herlihy J. D., Ache B. W. (2001). Odor-stimulated phosphatidylinositol 3-kinase in lobster olfactory receptor cells. J. Neurophysiol. 85, 2537-2544 [DOI] [PubMed] [Google Scholar]
- Zhainazarov A. B., Spehr M., Wetzel C. H., Hatt H., Ache B. W. (2004). Modulation of the olfactory CNG channel by Ptdlns(3,4,5)P-3. J. Membr. Biol. 201, 51-57 [DOI] [PubMed] [Google Scholar]
- Zhang Z., Okawa H., Wang Y. Y., Liman E. R. (2005). Phosphatidylinositol 4,5-bisphosphate rescues TRPM4 channels from desensitization. J. Biol. Chem. 280, 39185-39192 [DOI] [PubMed] [Google Scholar]
- Zolles G., Klocker N., Wenzel D., Weisser-Thomas J., Fleischmann B. K., Roeper J., Fakler B. (2006). Pacemaking by HCN channels requires interaction with phosphoinositides. Neuron 52, 1027-1036 [DOI] [PubMed] [Google Scholar]