Abstract
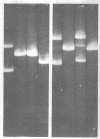
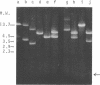
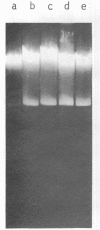
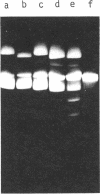
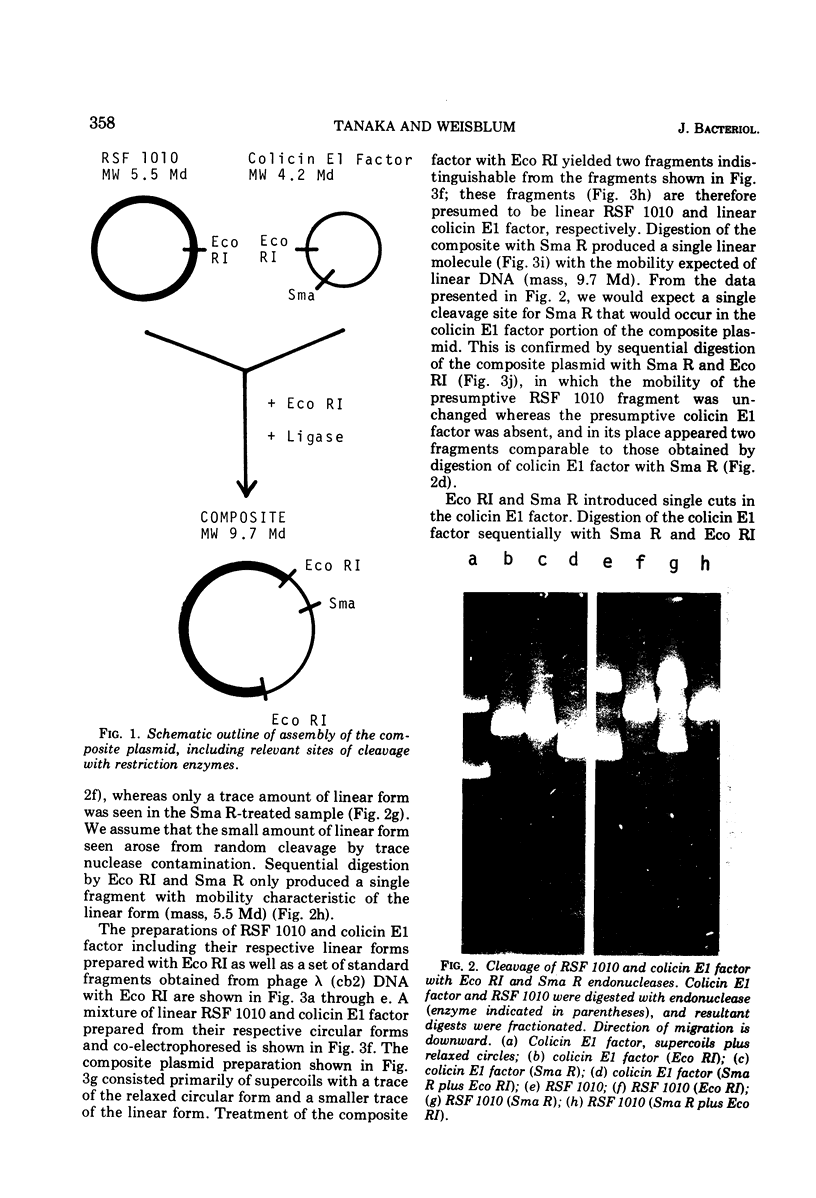
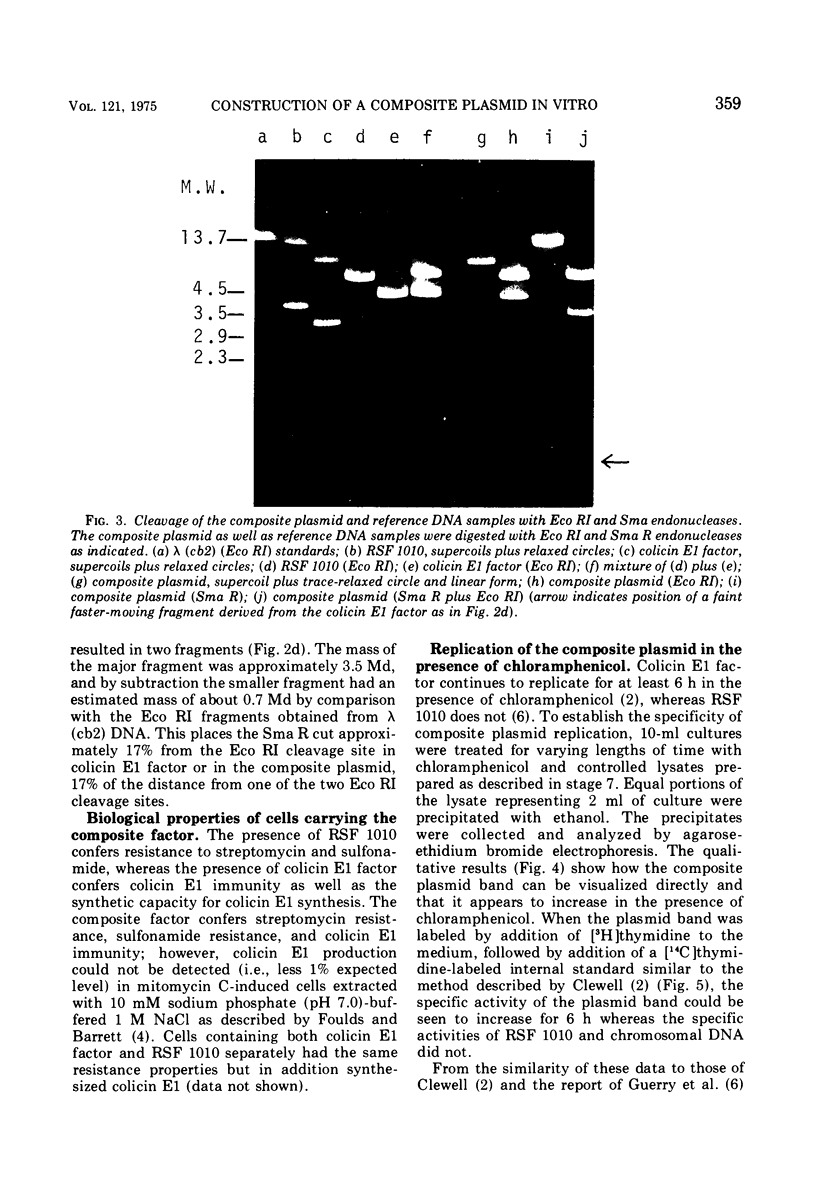
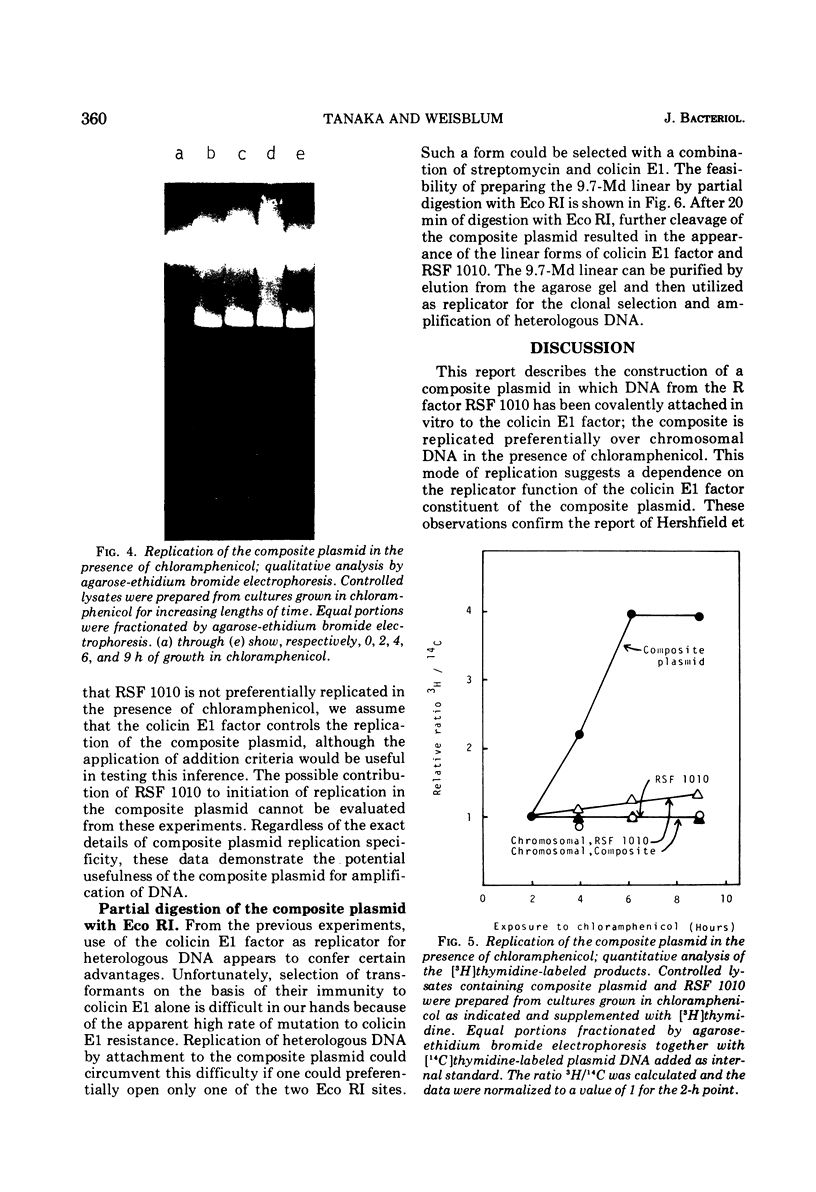
A composite plasmid has been constructed in vitro from colicin E1 factor (mass of 4.2 megadaltons [Md]) and nontransmissible resistance factor RSF 1010 (mass, 5.5. Md) deoxyribonucleic acids (DNAs) by the sequential action of Escherichia coli endonuclease (RI (Eco RI) and T4 phage DNA ligase on the covalently closed circular forms of the constituents. The composite plasmid was selected and amplified in vivo by sequential transformation of E. coli C600 with the ligated mixture and selection of transformants in medium containing streptomycin plus colicin E1, followed by amplification in the presence of chloramphenicol and purification of the extracted plasmid by dye-buoyant density gradient centrifugation in ethidium bromide-cesium chloride solution. Treatment of the composite plasmid with Eco RI yielded two fragments with mobilities corresponding to the linear forms of the parental plasmids, whereas Serratia marscesens endonuclease R (SmaR), which introduces a single scission in the colicin E1 factor but not in RSF 1010, convErted the composite plasmid to a single linear molecule (mass, 9.7 Md). Sequential degradation of colicin E1 factor with Sma R and Eco RI produced two fragments with masses of 3.5 and 0.7 Md; sequential degradation of RSF 1010 produced only one fragment (due to the cleavage with Eco RI), and sequential degradation of the composite plasmid produced the expected three fragments--an RSF 1010 Eco RI linear and the two expected products from the colicin E1 factor moiety. The composite plasmid conferred on the host cell resistance to streptomycin, sulfonamides, and colicin E1, but colicin E1 itself was not synthesized. In contrast, colicin E1 was synthesized by cells containing simultaneously both colicin E1 factor and RSF 1010 as separate entities. In the presence of chloramphenicol, the composite plasmid continued to replicate for 6 h. whereas replication of RSF 1010 and chromosomal DNA stopped within 2 h. Continued replication in the presence of chloramphenicol suggests that the replicator of the colicin E1 factor is functional in the composite plasmid.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allet B., Jeppesen P. G., Katagiri K. J., Delius H. Mapping the DNA fragments produced by cleavage by lambda DNA with endonuclease RI. Nature. 1973 Jan 12;241(5385):120–123. doi: 10.1038/241120a0. [DOI] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Boyer H. W., Helling R. B. Construction of biologically functional bacterial plasmids in vitro. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3240–3244. doi: 10.1073/pnas.70.11.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds J., Barrett C. Characterization of Escherichia coli mutants tolerant to bacteriocin JF246: two new classes of tolerant mutants. J Bacteriol. 1973 Nov;116(2):885–892. doi: 10.1128/jb.116.2.885-892.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerry P., LeBlanc D. J., Falkow S. General method for the isolation of plasmid deoxyribonucleic acid. J Bacteriol. 1973 Nov;116(2):1064–1066. doi: 10.1128/jb.116.2.1064-1066.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerry P., van Embden J., Falkow S. Molecular nature of two nonconjugative plasmids carrying drug resistance genes. J Bacteriol. 1974 Feb;117(2):619–630. doi: 10.1128/jb.117.2.619-630.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershfield V., Boyer H. W., Yanofsky C., Lovett M. A., Helinski D. R. Plasmid ColEl as a molecular vehicle for cloning and amplification of DNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3455–3459. doi: 10.1073/pnas.71.9.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Mertz J. E., Davis R. W. Cleavage of DNA by R 1 restriction endonuclease generates cohesive ends. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3370–3374. doi: 10.1073/pnas.69.11.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Weiss B., Jacquemin-Sablon A., Live T. R., Fareed G. C., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid. VI. Further purification and properties of polynucleotide ligase from Escherichia coli infected with bacteriophage T4. J Biol Chem. 1968 Sep 10;243(17):4543–4555. [PubMed] [Google Scholar]