Abstract
Euryhaline teleosts such as Atlantic killifish (Fundulus heteroclitus) are able to acclimate to changing environmental salinity by tightly regulating NaCl absorption and secretion across their gills. Many studies have examined the mechanisms responsible for long-term (days) salinity acclimation; however, much remains unknown about the mechanisms of acute (hours) salinity acclimation. In this study, we tested the hypotheses that phosphorylation of the Na+–K+–Cl− cotransporter (NKCC1) located in the basolateral membrane of the gill plays a role in acute salinity acclimation and that changes in NKCC1 phosphorylation are mediated by a cAMP–protein kinase A (cAMP–PKA) pathway. Using a phospho-specific antibody, we determined the time course of changes in total and phosphorylated NKCC1 protein during acclimation to water of various salinities. Long-term (≥14 days) acclimation of killifish to seawater (SW) and 2× SW resulted in 4- to 6-fold and 5- to 8-fold increases, respectively, in total gill NKCC1 protein relative to fish maintained in freshwater (FW). NKCC1 was found to be between 20% and 70% activated in fish, with lower average activation in fish acclimated to SW and 2× SW compared with FW fish. Increases and decreases in the fractional level of NKCC1 phosphorylation were seen within 1 h of transfer of fish to water of higher and lower salinity, respectively, consistent with a regulatory role of phosphorylation prior to an increase in the biosynthesis of NKCC1; large changes in protein expression of NKCC1 were observed over periods of hours to days. We found that NKCC1 phosphorylation is acutely regulated in the killifish gill in response to changing environmental salinity and that phosphorylation in excised gills increases in response to forskolin stimulation of the cAMP–PKA pathway. The role of phosphorylation is further underscored by the observation that mRNA expression of sterile 20 (Ste20)-related proline–alanine-rich kinase (SPAK) changes with salinity acclimation, being 2.7-fold greater in SW-acclimated killifish relative to FW fish. Overall, these results demonstrate an important role of NKCC1 phosphorylation in the gill of Atlantic killifish during acute salinity acclimation.
Keywords: Na+–K+–Cl− cotransporter, teleost, gills, Atlantic killifish, phosphorylation, mitochondria-rich cell, phospho-specific antibody, SPAK kinase
INTRODUCTION
Euryhaline teleosts such as Atlantic killifish (Fundulus heteroclitus) are capable of rapidly acclimating to changes in environmental salinity through structural and functional alterations in the gill and therefore provide an excellent model for studying ion-transporting epithelia. During acclimation to seawater (SW), fish drink continuously and plasma osmolality rises due to the environmental salt load. Increased plasma osmolality results in cell shrinkage and within minutes initiates an increase in Cl− secretion through mitochondria-rich cells (MRCs) or chloride cells located in the gill (Foskett and Scheffey, 1982; Zadunaisky et al., 1995). Conversely, when fish are exposed to a hypotonic medium plasma osmolality is rapidly decreased and Cl− secretion is inhibited. The mechanism controlling active NaCl secretion in fish resembles that of other secretory epithelia (Boucher and Larsen, 1988; Nauntofte, 1992; Riordan et al., 1994; Melvin et al., 2005) involving three principle ion transporters: Na+,K+-ATPase, Na+–K+–Cl− cotransporter (NKCC1), and an apical Cl− channel (cystic fibrosis transmembrane conductance regulator; CFTR) (Marshall and Bryson, 1998; Evans et al., 2005; Hwang and Lee, 2007). In this model, basolaterally located Na+,K+-ATPase creates the electrical and chemical gradient to drive Na+ and Cl− into the cell via basolateral NKCC1. Na+ and Cl− then leave the cell through a paracellular pathway and CFTR, respectively. Less is known about the model of NaCl uptake in fish. Recent work in tilapia (Oreochromis mossambicus) has proposed ion uptake models that incorporate an apically located Na+–Cl− cotransporter and Na+/H+ exchanger (Hiroi et al., 2008); however, it is likely that ion uptake mechanisms differ across different teleost species.
Early work in killifish demonstrated inhibition of Cl− secretion by bumetanide, providing evidence for a role of NKCC1 in the initial step of rapid regulatory salt acclimation in teleosts (Eriksson et al., 1985; Zadunaisky et al., 1995). This was supported by subsequent work demonstrating the blockage of Cl− secretion in Na+- and K+-free saline (Marshall and Bryson, 1998). Since then, numerous studies have confirmed the presence of NKCC1 in the gills of a wide-variety of teleosts, including killifish, and have examined the regulation of NKCC1 protein expression during long-term salinity acclimation (Hwang and Lee, 2007). Specifically, NKCC1 has been localized to the basolateral membrane of MRCs in the gill and typically co-localizes with Na+,K+-ATPase (Hwang and Lee, 2007). In killifish, both gill mRNA and protein abundance of NKCC1 are up- and down-regulated during SW and FW acclimation, respectively (Scott et al., 2004; Scott et al., 2005). Increased mRNA and protein expression of NKCC1 during long-term salinity acclimation is thought to be mediated by increases in plasma hormones including cortisol and growth hormone (Pelis and McCormick, 2001; Tipsmark et al., 2002).
In many salt-secreting epithelia, NKCC1 activation is mediated by cotransporter phosphorylation (Lytle and Forbush, 1992; Lytle and Forbush, 1996; Kurihara et al., 1999). Specifically, two threonine residues (T184 and T189) at the N-terminus of the NKCC1 sequence have been identified as important phosphorylation sites for cotransporter activation, and this region is highly conserved among shark, teleost and mammalian NKCC1s, as well as between NKCC1 (1a and 1b in teleosts) and NKCC2 (Lytle and Forbush, 1996; Darman and Forbush, 2002). Phosphorylation of NKCC1 is currently thought to be mediated directly by the sterile 20 (Ste20)-related proline–alanine-rich kinase (SPAK) (downstream of WNK kinases) (Delpire and Gagnon, 2008) in response to decreases in cell volume and intracellular Cl− concentration. In secretory epithelia, decreases in cell volume and intracellular Cl− occur when apical Cl− channels (CFTR) are opened by protein kinase A (PKA)-mediated phosphorylation (Berger et al., 1991; Tabcharani et al., 1991). Phosphorylation and thus activation of NKCC1 then allows the influx of Cl− into the cell, restoring cell volume and intracellular Cl− concentration. Eventually, NKCC1 is deactivated by protein phosphatases, including protein phosphatase 1 (PP1) (Darman et al., 2001; Darman and Forbush, 2002).
In teleosts, several studies have demonstrated the presence and regulation of protein kinases and phosphatases in osmoregulatory tissues and have hypothesized that as part of an acute acclimation response to changing environmental salinity, regulation of NKCC1 activity is under rapid control: being turned on and off via phosphorylation in response to cell shrinkage (hypertonic stress) and cell swelling (hypotonic stress), respectively (Hoffmann et al., 2007; Marshall et al., 2007; Katoh et al., 2007). However, despite these studies the direct effect of environmental salinity on the phosphorylation state of NKCC1 and the role of phosphorylation in regulating NKCC1 in the teleost gill during acute salinity acclimation remain unclear.
The major objective of the present study was to determine whether NKCC1 phosphorylation is part of the regulatory process in the killifish gill during salinity acclimation. We hypothesize that NKCC1 plays an important role in the killifish gill during both short- and long-term salinity acclimation – short-term acclimation being achieved by activation of cotransporter through phosphorylation, which increases the activity of existing cotransporter present in MRCs before new protein synthesis can occur, and long-term acclimation being achieved by de novo synthesis of cotransporter. To test this, we exposed killifish to varying environmental salinities and subsequently examined both total NKCC1 protein expression and the fractional level of NKCC1 phosphorylation in the gills, by utilizing a phospho-specific NKCC1 antibody (R5). We also measured gill mRNA expression of SPAK in FW- and SW-acclimated killifish to examine a potential role for this kinase in the regulation of salt secretion. Preliminary reports of part of this work have been presented (Flemmer et al., 1999; Behnke et al., 1999).
MATERIALS AND METHODS
Fish
Atlantic killifish (F. heteroclitus L.) were collected from the estuarine waters of Northeast Creek (Mount Desert Island, ME, USA) and held at the Mount Desert Island Biological Laboratory, MDIBL (Salisbury Cove, ME, USA). Prior to the start of experiments, fish were held in running SW tanks and were maintained at 23°C under natural photoperiod conditions for a period of 2 weeks to 2 months.
Experimental design
Approximately 380 killifish were used in these experiments (6 for each condition and time point), principally in four cohorts investigated over a 2 year period; experiments were conducted between August and November. Fish ranged in size from 2.5 to 9 g, with more than 80% in the 3–6g range, and fish of different sizes were distributed equally among groups and time points. The size of these fish did not allow us to obtain sufficient plasma for determination of ion concentrations and/or osmolality. For steady-state studies, fish were transferred by net to glass aquaria containing FW, SW and 2× SW for a period of up to 3 weeks; the minimum acclimation period used to establish a baseline in any of these conditions was 2 weeks. For long-term acclimation studies, fully acclimated fish were transferred directly from SW to FW, FW to SW, and SW to 2× SW and sampled after various time points up to 2 weeks; 6 fish that remained in SW and FW were sampled as pre-treatment controls (transferred from SW to SW and from FW to FW as sham controls). For short-term acclimation studies, fully acclimated fish were transferred directly from SW to 2× SW, 2× SW to SW, and 2× SW to 3× SW and sampled after various time points up to 5 h; 6 fish that remained in SW and 2× SW were sampled as pre-treatment controls (transferred from SW to SW and from 2× SW to 2× SW as sham controls). For all experiments, FW was obtained from a well at MDIBL, dechlorinated, and continuously filtered and aerated. The 2× and 3× SW were prepared by addition of Instant Ocean® (Spectrum Brands, Inc., Atlanta, GA, USA) to regular SW in 1× or 2× recommended amounts, and 80% water changes were performed on a weekly basis. Animals were killed by double-pithing. Animal care and experimentation were carried out in accordance with the Yale animal care facility (YACUC) and the animal care facility of MDIBL.
Tissue sampling
Immediately after the fish were killed, gills were rapidly excised, rinsed briefly in teleost Ringer solution composed of (in mmol l−1): 140 Na+, 5.4 K+, 1.2 Mg2+, 1.2 Ca2+, 124 Cl−, 25 Hepes, 2.4 HPO42− and 0.6 H2PO4− , pH 7.4, 300 mosmol l−1, adjusted with mannitol. Gills were then either processed immediately in acid-SDS (sample A) or incubated with 10 μmol l−1 forskolin diluted in teleost Ringer solution for 15 min prior to stopping with acid-SDS (sample B). As will be discussed below, sample B was utilized to obtain ‘total NKCC1 protein’ using the phospho-specific antibody R5, and sample A was used to measure fractional cotransporter phosphorylation in each animal (A/B). Unless otherwise noted, data from six fish in each condition and at each time point were analyzed.
For both dot blot and western blot analysis, gill samples A and B were denatured in 0.3 ml of 1 mol l−1 phosphoric acid/1% SDS in a tissue grinder for 1 min prior to neutralization (with 1 mol l−1 Tris/3 mol l−1 NaOH, volume predetermined to give pH 7.2–7.8), followed by further homogenization and boiling for 5–10 min. Samples were stored at −20°C prior to analysis; after thawing, samples were centrifuged at full speed (14,000 r.p.m.) for 10 min in a microcentrifuge (Eppendorf 5415C, Westbury, NY, USA) to remove insoluble debris, and supernatants were removed for further analysis.
Dot blotting
For dot-blotting experiments, aliquots of supernatant were pipetted into 13 96-well ‘master plates’, and diluted in transfer buffer (192 mmol l−1 glycine, 25 mmol l−1 Tris, pH 8.3, 0.1% SDS, 20% methanol) before dot blotting on a Millipore (Billerica, MA, USA) dot blotter, as previously described (Flemmer et al., 2002). Dot blots were probed with a phospho-specific NKCC1 antibody (R5). Use of the R5 rabbit polyclonal antibody has previously been demonstrated in mammalian cell lines and vertebrate tissues (Flemmer et al., 2002). R5 was raised against a diphosphopeptide containing T212 and T217 from the N-terminus of NKCC1 (human NKCC1 numbering), two phosphoregulatory sites that are conserved in all vertebrate NKCCs. Horseradish peroxidase (HRP)-conjugated secondary antibody was detected with chemiluminescent substrate (Supersignal West Dura, Pierce-Laboratories, Rockford, IL, USA), and light was captured with a cooled CCD-camera. Aliquots were also removed to other 96-well plates for determination of sample protein concentration (Bio-Rad DC Lowry assay, Hercules, CA, USA). Every dot blot was performed in triplicate, and a set of eight internal standards was used to standardize from one blot to another. The internal standards consisted of a dilution series prepared from a mixed sample of phosphorylated NKCC1, with no absolute reference point implied; all samples analyzed were within the scope of the linear range of this standard series. Experiments from two seasons were standardized to the same scale by re-analysis of a subset of the first season's samples along side the second season's samples.
SDS-PAGE western immunoblotting
Western immunoblotting was carried out for detection of total (forskolin-stimulated) and phosphorylated (non-stimulated) NKCC1 protein in killifish gills. In brief, gill supernatants were assayed for total protein (BCA protein assay, Pierce) and subsequently diluted in 4× Laemmli buffer (0.25 mol l−1 Tris, pH 6.8, 6% SDS, 40% glycerol, 0.04% Bromophenol Blue and 20% 2-mercaptoethanol). Samples were run on a 7.5% SDS-PAGE gel at 30 μg protein per lane. Following electrophoresis, proteins were transferred to immobilon PVDF transfer membranes (Millipore) at 40 V overnight in transfer buffer. PVDF membranes were blocked in phosphate-buffered saline with 0.1% Triton X-100 (PBST) and 5% non-fat dry milk for 30 min at room temperature and subsequently incubated with the primary antibody R5 (phospho-specific NKCC1) diluted 1:5000 in 5% milk in PBST overnight at 4°C. After rinsing 3× in PBST, blots were probed with HRP-conjugated goat anti-rabbit secondary antibody diluted 1:10,000 in 5% milk in PBST for 1 h at room temperature. PVDF membranes were developed with a chemiluminescent substrate (Supersignal West Dura, Pierce) for 5 min and subsequently exposed to autoradiography film (Amersham Hyperfilm, GE Healthcare, Bucks, UK). Film was developed using a Kodak X-OMAT 2000 processor (Carestream Health, Rochester, NY, USA).
Immunocytochemistry
For each fish, the left gills arches were removed, briefly rinsed in teleost Ringer solution, and immediately placed into PLP fixative (2% paraformaldehyde, 0.075 mol l−1 lysine, 0.04 mol l−1 sodium phosphate, 0.01moll−1 periodate), whereas the right gill arches were excised, briefly rinsed in teleost Ringer solution, incubated in 10 μmol l−1 forskolin in teleost Ringer solution for 15 min at room temperature, and subsequently placed in PLP fixative. Gills were fixed overnight in PLP fixative at 4°C, transferred to holding buffer (100 mmol l−1 phosphate buffer, 0.1% paraformaldehyde, 0.02% sodium azide), and stored at 4°C for later analysis. Fixed tissue was rinsed in PBS, held in PBS with 30% (w/v) sucrose at 4°C overnight, and frozen in embedding medium (Embed 812, Electron Microscopy Sciences, Hatfield, PA, USA). Tissue sections (5 μm) parallel to the long access of the filament epithelium were cut in a cryostat at −20°C. Sections were placed on slides (Probe on plus, Electron Microscopy Sciences), dried at room temperature, rinsed in PBS, and incubated in blocking solution (PBS, 0.1% bovine serum albumin, 10% goat serum) at room temperature for 30 min. Double staining of the same sections was performed for phosphorylated NKCC1 (R5) and Na+,K+-ATPase (α5) protein. Slides were incubated with α5 (1:1000) and R5 (NKCC, 1:1000) primary antibodies diluted in antibody dilution buffer (PBS, 0.1% bovine serum albumin, 10% goat serum) overnight at 4°C. After incubation, slides were rinsed 3× with PBS, exposed to secondary antibody for 2 h at room temperature, and again rinsed 3× with PBS. The secondary antibodies Alexa-Fluor 568 goat anti-mouse and Alexa-Fluor 488 goat anti-rabbit (Molecular Probes, Eugene, OR, USA) were used as appropriate. Antibody control experiments (secondary antibodies without primary) showed no specific staining and low background (data not shown). Sections were mounted in Vectashield (Vector Laboratories, Burlingame, CA, USA), covered by a coverslip, sealed with nailpolish, and examined with a Zeiss Axiophot fluorescence microscope with a mercury lamp.
Total RNA extraction, reverse transcription and quantitative PCR
Gills were removed from killifish immediately after they had been killed and stored overnight at 4°C in RNA later (Ambion, Austin, TX, USA). Total RNA was purified using Trizol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions, and cDNA was created by RT-PCR using random decamers (Superscript II, Invitrogen).
Quantitative PCR (qPCR) primers were designed for ~250 bp products, according to the killifish NKCC1a sequence (GI:47013796): CACAGCCATTGTGAACTACTTTATTG (forward), and TGTGGGTCAGCAAGGTCTCC (reverse); according to a partial sequence of killifish SPAK (obtained by degenerate PCR): GAGAAGATGGGTCTGTTCAGAT (forward) and GCAGAGTA AGCATTAAAACCTT (reverse); and according to the fugu GAPDH sequence (product confirmed by sequencing): CCTCCACC GGTGCAGCCAAG (forward) and GGAGACGACCTGGTGCTCAGTGTAT (reverse). qPCR was carried out using the Syber Green reagent (Qiagen, Valencia, CA, USA) on the iCycler qPCR machine (Bio-Rad). GAPDH mRNA levels were used as a controls. An efficiency of two was used for calculations, after confirmation of primers against a dilution series of a standard cDNA mixture; values obtained are averages of quadruplicate determinations.
Statistics
All data are presented as means ± s.e.m. A non-parametic one-way analysis of variance (ANOVA) on ranks was used to test for significant effects of salinity over time unless otherwise noted. When significant treatment effects (P<0.05) were established, differences among treatments were tested using Dunnett's post hoc test. Statistical analyses were performed using Statistica 7.0 (Statsoft, Inc., Tulsa, OK, USA).
RESULTS
Phosphorylation of NKCC1 in excised gills detected with the anti-phospho NKCC1 antibody (R5)
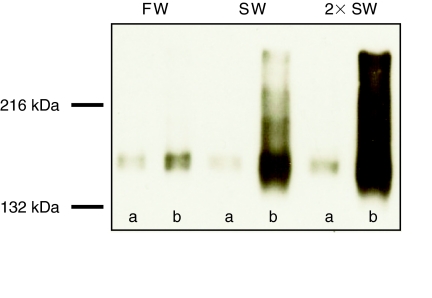
We examined phosphorylation of NKCC1 in the killifish gill upon incubation with forskolin using the anti-phospho NKCC1 antibody (R5) in both western immunoblotting and immunocytochemistry. In western immunoblots, the R5 antibody recognized a single strongly stained band centered at ~170 kDa (Fig. 1) which agrees well with the previously reported molecular mass of NKCC1 in Atlantic killifish (Scott et al., 2004); therefore it was possible to carry out further experiments using a dot-blot technique. Under non-stimulated conditions p-NKCC (R5 immunoreactivity) was low and no detectable differences between salinity treatments were observed (Fig. 1, lanes labeled a).
Fig. 1.
Representative western blot of gills taken from Atlantic killifish acclimated for ≥14 days to freshwater (FW), seawater (SW) or 2× SW. Gills were rapidly excised from fish, briefly rinsed in teleost Ringer solution, and incubated without (A) or with (B) 10 μmol l−1 forskolin for 15 min at room temperature. Each lane represents gill samples pooled from 6 individual fish. Equal amounts (30 μg) of SDS-solubilized protein were loaded in each lane and probed with anti-phospho Na+–K+–Cl− cotransporter antibody R5. The R5 antibody recognized a strongly stained band centered at ~170 kDa.
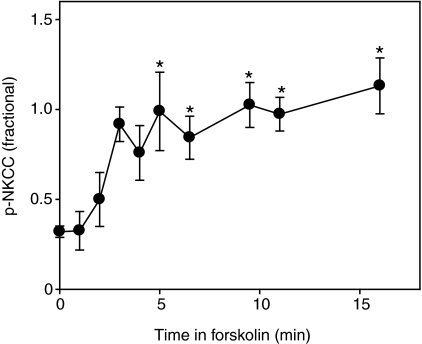
When excised gills were stimulated with 10 μmol l−1 forskolin for 15 min, all groups exhibited a large increase in p-NKCC signal, with stimulated p-NKCC levels being smallest in FW-acclimated fish, larger in SW-acclimated fish, and greatest in 2× SW-acclimated fish (Fig. 1, lanes labeled b). The time course of the ex vivo p-NKCC increase was analyzed by dot blot for SW-acclimated fish, with results illustrated in Fig. 2. It can be seen that incubation of excised gills with 10 μmol l−1 forskolin was effective in bringing about rapid phosphorylation of NKCC1 (Fig. 2). The fractional level of NKCC1 phosphorylation increased within ~5 min and remained at this level for the duration of the experiment (Fig. 2). This time course is similar to that of NKCC1 phosphorylation in the salt rectal gland of the shark (Lytle and Forbush, 1992) and in HEK-293 cells (Darman and Forbush, 2002). For the remainder of our experiments, we exploited a 15 min 10 μmol l−1 forskolin incubation to achieve presumed-maximal phosphorylation of NKCC1 in a paired sample for each gill. The phospho-specific R5 antibody signal in the presence of forskolin was thus taken as a measure of total NKCC1 protein. This approach has the advantage that it is internally controlled: fractional phosphorylation is immediately calculated as the ratio of two measurements from the same blot with the same antibody, without requiring external calibration.
Fig. 2.
Time course of NKCC1 cotransporter phosphorylation in excised killifish gills stimulated with forskolin. Gills (three pieces per fish) were excised from fish maintained in SW and incubated in teleost Ringer solution containing 10 μmol l−1 forskolin at room temperature. p-NKCC was analyzed on dot blots using the anti-p-NKCC antibody R5 and normalized for total protein. Data are means ± s.e.m. of 2–6 fish at a given time point. One-way ANOVA determined a significant effect (P<0.001) of time on p-NKCC. Asterisks indicate a significant difference (P<0.05) from time zero.
In all experimental groups, the α5 and R5 antibodies stained cells within the filament epithelium of killifish gills (Fig. 3). The size, shape and location of positively stained cells for both antibodies indicated that they were MRCs or chloride cells and no other cell type exhibited positive staining for either antibody. In FW-acclimated killifish gills without forskolin treatment, many MRCs were positively stained for Na+,K+-ATPase whereas few cells showed p-NKCC immunoreactivity (Fig. 3A), and only a small increase in signal was seen after forskolin stimulation. In SW- and 2× SW-acclimated killifish gills without forskolin treatment, many cells were positively stained for Na+,K+-ATPase but p-NKCC immunoreactivity was completely absent (Fig. 3B,C, upper rows). After maximal stimulation with forskolin, most MRCs in SW- and 2× SW-acclimated fish showed p-NKCC signal co-localizing with Na+,K+-ATPase (Fig. 3B,C, lower rows). Interestingly, in fish acclimated to 2× SW, the intensity of p-NKCC staining varied greatly between MRCs when NKCC1 phosphorylation was maximally stimulated with forskolin, with some cells exhibiting very bright R5 staining and others exhibiting weak R5 staining.
Fig. 3.
Representative immunofluorescence images showing Na+,K+-ATPase (α5, left column) and p-NKCC (R5, center column) immunoreactivity in the gills of Atlantic killifish (N=3–4) acclimated to FW (A), SW (B), and 2× SW (C) both with and without 10 μmol l−1 forskolin for 15 min at room temperature as indicated. The scale bar represents 50 μm.
The steady-state level of total NKCC1 and phospho-NKCC1 in the gills of killifish acclimated to water of varying salinity
We saw substantial variation in total NKCC1 protein expression among different experimental cohorts of fish, possibly due to environmental factors affecting our wild-caught populations; however, despite this variation, total NKCC1 levels were on average 5-fold greater in SW-acclimated fish relative to FW fish after at least 2 weeks (Fig. 4A). There was only a slight further increase in the level of total NKCC1 for fish in 2× SW, although an example of a modest increase will be pointed out in an acclimation time course, below, and we have observed greater increases in other experiments (data not shown).
Fig. 4.
‘Steady-state’ protein expression and fractional level of phosphorylation of NKCC1 in gills isolated from fish acclimated to FW, SW or 2× SW. NKCC1 was measured on dot blots probed with anti-phospho NKCC1 (R5) and normalized to protein content. (A) Total NKCC1, measured with anti-phospho NKCC1 in gill samples that were incubated with 10 μmol l−1 forskolin for 15 min in order to maximally stimulate NKCC1. Each bar represents the mean ± s.e.m. of 6 individual fish in a cohort of fish studied in one experiment. Results are shown relative to standards for four series of experiments with different cohorts of killifish. (B) p-NKCC in freshly excised gills as a ratio to the total level for the paired forskolin-stimulated gills shown in A. One-way ANOVA determined that salinity had a significant effect (P<0.007) on total NKCC1 in all experiments. One-way ANOVA determined that salinity had a significant effect (P<0.02) on p-NKCC in two of the four experiments. Asterisks indicate a significant difference (P<0.05) from FW within an experiment.
The fractional level of NKCC1 activation in fully acclimated killifish is presented in Fig. 4B. As described above, this is the ratio of phospho-antibody (R5) signal in freshly excised tissue, compared with a maximal level obtained by in vitro forskolin stimulation. For fish acclimated to FW, 55–75% of the transporter was phosphorylated and active. Interestingly, despite their greatly increased levels of total NKCC1 protein in response to the osmotically stressful conditions, fish in SW and 2× SW exhibited less than maximal phosphorylation of NKCC1. In SW- and 2× SW-acclimated fish, 19–66% and 23–52% of the transporter was phosphorylated and active, respectively (Fig. 4B).
Down-regulation of NKCC1 activity in the gills of killifish during acclimation to decreasing salinity
When SW-acclimated fish were transferred to FW, total NKCC1 in the gill decreased 4-fold after 2 days relative to pre-treatment SW values, and remained at this level for the duration of the experiment (Fig. 5A). The fractional level of phosphorylation of existing NKCC1 in these fish decreased 5.6-fold after 8 h, indicating that NKCC1 in the gill is rapidly de-activated in response to a decreased demand for salt secretion (Fig. 5B). Interestingly, the fractional level of NKCC1 phosphorylation in fish returned to pre-treatment levels after 2 days, as total NKCC1 protein was down-regulated (Fig. 5B).
Fig. 5.
Time course of total NKCC1 protein expression (A) and NKCC1 phosphorylation (B) after transition of fish from SW to FW. Each point represents the mean ± s.e.m. of 6 individual fish. One-way ANOVA determined a significant effect of time on both total NKCC1 (P<0.001) and p-NKCC (P=0.004). Asterisks indicate a significant difference (P<0.05) from pre-treatment control.
Up-regulation of NKCC1 activity in the gills of killifish during acclimation to increasing salinity
We observed an increase in total NKCC1 protein (approximately 5-fold in two experiments) when FW-acclimated fish were transferred to SW (Fig. 6A). The time course was quite variable, being complete in well under a day in two sets of experiments, but requiring >5 days in another. Significant changes in the fractional level of NKCC1 phosphorylation were obscured by variability in all three experiments (Fig. 6B). This observation may be explained by the finding of only weak expression of p-NKCC in FW fish.
Fig. 6.
Time course of total NKCC1 protein expression (A) and NKCC1 phosphorylation (B) after transition of fish from FW to SW. Each point represents the mean ± s.e.m. of 6 individual fish in a cohort of fish studied in one experiment. Different symbols represent different experiments. One-way ANOVA determined a significant effect of time on total NKCC1 in all three experiments (P<0.002), and a significant effect of time on p-NKCC in one experiment (P=0.023; open circles). Asterisks indicate a significant difference (P<0.05) from pre-treatment control within an experiment.
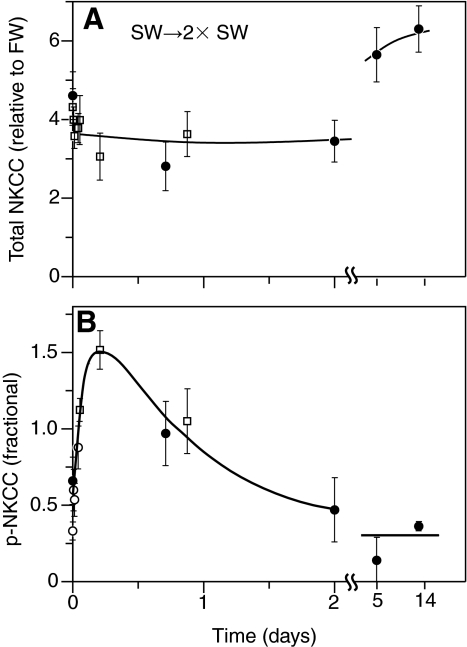
When SW-acclimated fish were transferred to 2× SW, one-way ANOVA revealed a significant effect (P=0.003) of time on total NKCC1 protein and there was a tendency for protein levels to be elevated after 5 and 14 days (Fig. 7A). One-way ANOVA also revealed an effect (P=0.004) of time on p-NKCC and despite a tendency for an increase in p-NKCC within the first hour, there were no significant post hoc comparisons (Fig. 7B). Note that the fractional level of NKCC1 phosphorylation reached a value of 1.5 in Fig. 7B, showing that phosphorylation levels in gills immediately removed from fish were actually higher than those of the internal controls. As in other experiments, the control values were obtained from companion gills after 15 min incubation with forskolin following dissection.
Fig. 7.
Time course of total NKCC1 protein expression (A) and NKCC1 phosphorylation (B) after transition of fish from SW to 2× SW. Each point represents the mean ± s.e.m. of 6 individual fish in a cohort of fish studied in one experiment. Different symbols represent different experiments. One-way ANOVA determined a significant effect of time on both total NKCC1 (P=0.003) and p-NKCC (P=0.004). Asterisks indicate a significant difference (P<0.05) from pre-treatment control within an experiment.
Short-term acclimation responses of NKCC1 phosphorylation during acclimation of killifish to varying salinity
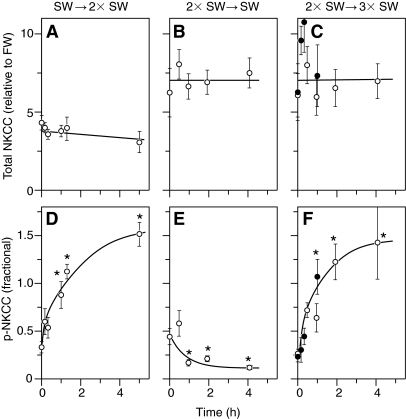
Short-term changes in NKCC1 phosphorylation are illustrated in Fig. 8 by data from three experiments in which fish were sampled during the first 5 h of the acclimation period. In each of these experiments there were no significant changes in total NKCC1 protein (Fig. 8A–C); however, substantial alterations in the fractional level of NKCC1 phosphorylation were observed within 1 h (Fig. 8D–F). The changes were in the direction that would be expected for an acclimation response to the osmotic challenge: on transfer from SW to 2× SW (Fig. 8D) and from 2× to 3× SW (Fig. 8F) greater than 3-fold increases in fractional NKCC1 phosphorylation were observed, whereas a rapid 2-fold decrease was seen after transfer from 2× to 1× SW (Fig. 8E).
Fig. 8.
Short-term changes in total NKCC1 protein expression (A–C) and NKCC1 phosphorylation (D–F) after transition of fish from SW to 2× SW (A,D), 2× SW to SW (B,E), and 2× SW to 3× SW (C,F). Each point represents the mean ± s.e.m. of 6 individual fish in a cohort of fish studied in one experiment. Different symbols represent different experiments. One-way ANOVA determined a significant effect of time on p-NKCC in all three transfer experiments (P<0.001). Asterisks indicate a significant difference (P<0.05) from pre-treatment control within an experiment. There was no effect of time on total NKCC1 protein in any of the three experiments (P>0.191).
SPAK and NKCC1a mRNA expression in FW- and SW-acclimated killifish gills
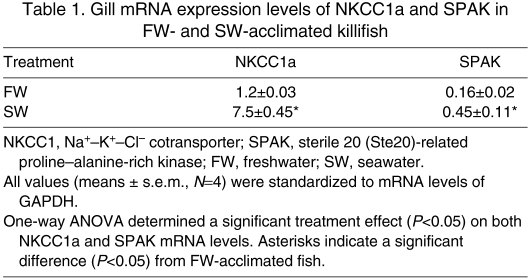
Lacking an appropriate antibody for killifish SPAK kinase, we examined mRNA expression levels using qPCR. We obtained the cDNA sequence for a killifish SPAK sequence from gill mRNA, using degenerate PCR in a region highly conserved between SPAK and oxidative stress-responsive kinase-1 (OSR1). Table 1 illustrates the results of an experiment in which killifish were fully acclimated to FW or SW, and the mRNA expression levels of NKCC1a and SPAK were determined by qPCR. It can be seen that the expression levels of SPAK and NKCC1a in the gill are 2.7-fold and 6.2-fold greater in SW-acclimated fish relative to FW fish, respectively.
Table 1.
Gill mRNA expression levels of NKCC1a and SPAK in FW- and SW-acclimated killifish
DISCUSSION
We sought to examine the role of NKCC1 phosphorylation in the regulatory processes of the killifish gill during salinity acclimation. Here we present our four major findings: (1) NKCC1 phosphorylation in the killifish gill is regulated via a cAMP–PKA pathway, (2) NKCC1 protein expression but not phosphorylation is regulated during long-term salinity acclimation, (3) NKCC1 phosphorylation is rapidly regulated during acute salinity acclimation, and (4) our results indicate that SW acclimation may involve the up-regulation of SPAK kinase in the killifish gill.
NKCC1 phosphorylation in the killifish gill is regulated via a cAMP–PKA pathway
The first objective of this study was to determine whether NKCC1 phosphorylation is regulated in the killifish gill. The overall process of salt and fluid secretion in Cl− secretory epithelia is generally stimulated by cAMP-dependent processes; investigations have supported a model in which cAMP stimulates PKA phosphorylation which enhances Cl− secretion by apically located Cl− channels (CFTR) by increasing both single channel activity and plasma membrane insertion. Enhanced Cl− secretion results in lowered intracellular Cl− concentration and cell shrinkage, which are both stimuli for phosphorylation and activation of basolaterally located NKCC1 (Gimenez, 2006). Thus forskolin, which stimulates adenylate cyclase raising intracellular cAMP, generally leads indirectly to the phosphorylation of NKCC1 in secretory epithelia. In the present study, incubation of excised gills with forskolin brought about a rapid increase in NKCC1 phosphorylation as determined by dot blotting, western immunoblotting and immunofluorescence studies employing a phospho-specific NKCC1 antibody (R5), demonstrating that this antibody is able to discriminate between phosphorylated (active) and non-phosphorylated (inactive) cotransporter. Further, this result is consistent with the conclusion that NKCC1 present in the killifish gill is activated by protein phosphorylation via a cAMP–PKA mediated pathway. Others have proposed a model for the rapid control of NKCC1 activity in the killifish gill incorporating this pathway (Marshall et al., 2005; Hoffmann et al., 2007); however, this is the first study to directly demonstrate the effect of a cAMP agonist on the phosphorylation state of NKCC1.
We also sought to determine the localization pattern of p-NKCC protein in the killifish gill in response to forskolin stimulation. To do this, we performed simultaneous immunofluorescence labeling on the gills of killifish acclimated to FW, SW and 2× SW with antibodies against Na+,K+-ATPase and p-NKCC. Co-labeling for Na+,K+-ATPase and p-NKCC revealed that both proteins were localized to MRCs in the filament epithelium. In FW-acclimated fish, the majority of MRCs showed no or very low p-NKCC immunoreactivity under basal conditions, and upon stimulation with forskolin, few MRCs became p-NKCC positive. In contrast, gills from SW- and 2× SW-acclimated fish exhibited a large up-regulation of p-NKCC signal upon forskolin stimulation. These results suggest that SW- and 2× SW-acclimated fish have a large capacity to rapidly turn on existing NKCC1 in the gill epithelium, whereas this capacity is limited in FW-acclimated fish.
NKCC1 protein expression but not phosphorylation is regulated in the killifish gill during long-term salinity acclimation
The second objective of this study was to examine the ‘steady-state’ level of NKCC1 phosphorylation in the gills of killifish fully acclimated to water of varying salinity. We observed that when fish were sampled ≥14 days after transfer, total NKCC1 protein in the gill was 5-fold greater in SW- and 2× SW-acclimated fish than in FW-acclimated fish. These results are strongly supportive of the hypothesis that the amount of ion transport proteins is dramatically increased in the teleost gill to cope with the secretory demands of salt and fluid handling in salt water, and are in agreement with a now large body of evidence to this effect (for reviews, see Marshall and Bryson, 1998; McCormick, 2001; Evans et al., 2005; Hwang and Lee, 2007).
In FW-acclimated fish, we observed that the majority of existing cotransporter in the gill is phosphorlyated and thus active. We hypothesize that FW-acclimated fish may maintain a high proportion of active NKCC1 for functions other than salt secretion, such as cell volume and/or acid–base regulation, as NKCC is known to be involved in these processes in other species (Gamba, 2005). It is interesting that when acclimated to FW, killifish maintain a limited capacity to turn on NKCC1 activity (as indicated by some stimulation with forskolin). It is likely that as an intertidal species killifish must maintain some ability to turn on salt secretion even when in FW, which may be necessary in the event of rapid SW exposure or ingesting an item with a high salt content. This idea is consistent with other studies which have demonstrated that after long-term acclimation to FW, killifish retain large numbers of MRCs in both the gill and opercular epithelia and high levels of Na+,K+-ATPase activity (Mancera and McCormick, 1998; Marshall et al., 1999). Interestingly, the fractional level of phosphorylated NKCC1 appears slightly lower in SW- and 2× SW-acclimated fish than in FW fish. Given the >14 day acclimation used in this experiment, it is not surprising that we did not observe large differences in the fractional level of phosphorylated cotransporter when comparing FW-, SW- and 2× SW-acclimated fish, as the processes of long-term salinity acclimation likely involve large changes in total protein levels but not changes in NKCC1 phosphorylation state. Together, our results support the idea that when in FW, euryhaline fish maintain a low level of NKCC1 protein that is mostly active, then during SW acclimation the fractional level of phosphorylated NKCC1 is decreased as newly synthesized protein becomes available.
NKCC1 phosphorylation is rapidly regulated in the killifish gill during acute salinity acclimation
Our third objective was to determine whether NKCC1 phosphorylation is rapidly regulated during acute salinity acclimation. In response to changing environmental salinity, we observed rapid (within 1 h) alterations in NKCC1 phosphorylation followed by later changes in total NKCC1 protein. This result is consistent with the existing hypothesis that salinity acclimation occurs in two phases: a first phase (seconds to hours) in which an osmotic stimulus such as increased plasma osmolality results in rapid phosphorylation and/or plasma membrane insertion of existing ion transporters, and a second phase (hours to days) during which increased protein synthesis, cell proliferation, differentiation and tissue reorganization take place (McCormick et al., 2003). Although plasma ions and osmolality were not measured in this study, previous work in killifish has shown that plasma Na+ levels and plasma osmolality respond rapidly (within 1 h) to changing environmental salinity (Marshall et al., 1999). Interestingly, in fish that were transferred from SW to FW and FW to SW we observed rapid decreases and increases, respectively, in total NKCC1 protein levels. This is somewhat surprising, as previous studies in fish have shown that significant changes in total NKCC1 protein levels do not typically occur until ≥3 days post-transfer (Scott et al., 2004; Tipsmark et al., 2004; Scott et al., 2005). It is possible that rapid decreases in total NKCC1 protein upon transfer from SW to FW are the result of processes such as MRC death (apoptosis and necrosis) and/or transporter endocytosis and degradation. On the other hand, rapid increases in NKCC1 protein upon transfer from FW to SW could result from the utilization of an existing pool of mRNA. Future studies are necessary to determine the role of NKCC1 translocation in the fish gill during acute salinity acclimation, as in mammalian systems it is likely that phosphorylation and translocation of NKCC1 act together to regulate cotransporter activity in response to osmotic stimuli (Gimenez and Forbush, 2003). Furthermore, it will be important to examine whether rapid regulation of NKCC activity occurs in other osmoregulatory tissues (NKCC2 in the gut and kidney) during acute salinity acclimation.
SW acclimation may involve the up-regulation of SPAK kinase in the killifish gill
Finally, we sought to examine whether mRNA expression of SPAK kinase is regulated in the killifish gill upon salinity acclimation. In mammals, an increasing body of evidence supports the hypothesis that SPAK (and the closely related OSR1) kinase is responsible for the direct regulation of NKCC1 (for reviews, see Delpire and Gagnon, 2006; Delpire and Gagnon, 2008). SPAK was identified as a potential partner in yeast two-hybrid experiments and a critical functional role was demonstrated by the striking effect of over-expression of a dominant-negative SPAK construct in HEK-293 cells expressing NKCC1 (Dowd and Forbush, 2003). The potential for SPAK/OSR1 to be the kinase which directly phosphorylates NKCC1 has recently been demonstrated in in vitro experiments (Vitari et al., 2005). The situation is not simple, however, as it is clear the SPAK binding site in the N-terminus of NKCC1 is not important for regulatory function, and there are complex interactions with the WNK family of kinases (Kahle et al., 2006). In this study we observed elevated levels of SPAK mRNA (along with elevated NKCC1a mRNA) in SW-acclimated killifish relative to FW-acclimated fish. Although we did not directly examine the role of SPAK kinase in regulating NKCC1 activity in the killifish gill in this study, we believe these data add to the growing body of evidence (Kultz and Avila, 2001; Marshall et al., 2005; Hoffmann et al., 2007) for a potential role of SPAK kinase in the regulation of salt secretion in the gills of teleosts. Future studies will be necessary to determine the time course of SPAK mRNA and protein changes (using species-specific antibodies) during SW acclimation, and to examine the presence of this kinase in other osmoregulatory tissues (gut, kidney) where different isoforms of NKCC are present. Finally, it will be interesting to examine the role that osmotic cues (hypertonicity and hypotonicity) and several ‘fast-acting’ hormones (arginine vasotocin, urotensin I and vasoactive intestinal peptide) play in regulating kinase activity in the fish gill.
CONCLUSIONS
In conclusion, we suggest that the mechanism for acute salinity acclimation in killifish involves rapidly turning on/off NKCC1 activity in the gill via phosphorylation whereas long-term salinity acclimation involves an increase in de novo synthesis of NKCC1 protein and not increases in the fractional level of protein phosphorylation. We also report that expression of SPAK kinase is up-regulated along with expression of NKCC1a during SW acclimation, indicating a potential role for SPAK as the regulatory kinase of NKCC1, and with a central role of phosphorylation in the regulatory process. Previous work has demonstrated that transfer from water of lower salinity to water of higher salinity results in the rapid activation of Na+,K+-ATPase in the killifish gill, but not in anadromous Atlantic salmon (Salmo salar), indicating that post-translational modifications may vary across species with different life history strategies (Mancera and McCormick, 2000). It would thus be valuable to compare the regulation of NKCC1 phosphorylation in the gill across different teleost species with a broad range in salinity tolerance to examine whether cotransporter phosphorylation plays a role in determining degree of euryhalinity.
ACKNOWLEDGEMENTS
We thank Frederique Dewaersegger, Grace Jones and Esther Bashi for technical assistance, and SueAnne Mentone for immunohistological assistance. This work was supported by NIH GM083340 to B.F. Deposited in PMC for release after 12 months.
REFERENCES
- Behnke R. J., Colon D. E., Zadunaisky J. A., Forbush B. (1999). Expression of the secretory Na-K-Cl cotransporter is increased during long term salt adaptation in the killifish, Fundulus heteroclitus. Bull. Mt. Desert Isl. Biol. Lab. Salisb. Cove Maine 38, 85-86 [Google Scholar]
- Berger H. A., Anderson M. P., Gregory R. J., Thompson S., Howard P. W., Maurer R. A., Mulligan R., Smith A. E., Welsh M. J. (1991). Identification and regulation of the cystic fibrosis transmembrane conductance regulator generated chloride channel. J. Clin. Invest. 88, 1422-1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher R. C., Larsen E. H. (1988). Comparison of ion-transport by cultured secretory and absorptive canine airway epithelia. Am. J. Physiol. 254, C535-C547 [DOI] [PubMed] [Google Scholar]
- Darman R. B., Forbush B. (2002). A regulatory locus of phosphorylation in the N terminus of the Na-K-Cl cotransporter, NKCC1. J. Biol. Chem. 277, 37542-37550 [DOI] [PubMed] [Google Scholar]
- Darman R. B., Flemmer A., Forbush B. (2001). Modulation of ion transport by direct targeting of protein phosphatase type 1 to the Na-K-Cl cotransporter. J. Biol. Chem. 276, 34359-34362 [DOI] [PubMed] [Google Scholar]
- Delpire E., Gagnon K. B. (2006). SPAK and OSR1, key kinases involved in the regulation of chloride transport. Acta Physiol. 187, 103-113 [DOI] [PubMed] [Google Scholar]
- Delpire E., Gagnon K. B. E. (2008). SPAK and OSR1: STE20 kinases involved in the regulation of ion homoeostasis and volume control in mammalian cells. Biochem. J. 409, 321-331 [DOI] [PubMed] [Google Scholar]
- Dowd B. F. X., Forbush B. (2003). PASK (proline-alanine-rich STE20-related kinase), a regulatory kinase of the Na-K-Cl cotransporter (NKCC1). J. Biol. Chem. 278, 27347-27353 [DOI] [PubMed] [Google Scholar]
- Eriksson O., Mayergostan N., Wistrand P. J. (1985). The use of isolated fish opercular epithelium as a model tissue for studying intrinsic activities of loop diuretics. Acta Physiol. Scand. 125, 55-66 [DOI] [PubMed] [Google Scholar]
- Evans D. H., Piermarini P. M., Choe K. P. (2005). The multifunctional fish gill: Dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol. Rev. 85, 97-177 [DOI] [PubMed] [Google Scholar]
- Flemmer A. W., Behnke R. J., Forbush B. (1999). Changes in phosphorylation state of the Na-K-Cl cotransporter (NKCC) in chloride cells of the gill of Fundulus heteroclitus (Killifish) during salt adaptation. Bull. Mt. Desert Isl. Biol. Lab. Salisb. Cove Maine 38, 80-82 [Google Scholar]
- Flemmer A. W., Gimenez I., Dowd B. F. X., Darman R. B., Forbush B. (2002). Activation of the Na-K-Cl cotransporter NKM detected with a phospho-specific antibody. J. Biol. Chem. 277, 37551-37558 [DOI] [PubMed] [Google Scholar]
- Foskett J. K., Scheffey C. (1982). The chloride cell – definitive identification as the salt-secretory cell in teleosts. Science 215, 164-166 [DOI] [PubMed] [Google Scholar]
- Gamba G. (2005). Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Phys. Rev. 85, 423-493 [DOI] [PubMed] [Google Scholar]
- Gimenez I. (2006). Molecular mechanisms and regulation of furosemide-sensitive Na-K-Cl cotransporters. Curr. Opin. Nephrol. Hy. 15, 517-523 [DOI] [PubMed] [Google Scholar]
- Gimenez I., Forbush B. (2003). Short-term stimulation of the renal Na-K-Cl cotransporter (NKCC2) by vasopressin involves phosphorylation and membrane translocation of the protein. J. Biol. Chem. 278, 26946-26951 [DOI] [PubMed] [Google Scholar]
- Hiroi J., Yasumasu S., McCormick S. D., Hwang P. P., Kaneko T. (2008). Evidence for an apical Na-Cl cotransporter involved in ion uptake in a teleost fish. J. Exp. Biol. 211, 2584-2599 [DOI] [PubMed] [Google Scholar]
- Hoffmann E. K., Schettino T., Marshall W. S. (2007). The role of volume-sensitive ion transport systems in regulation of epithelial transport. Comp. Biochem. Physiol. 148A, 29-43 [DOI] [PubMed] [Google Scholar]
- Hwang P. P., Lee T. H. (2007). New insights into fish ion regulation and mitochondrion-rich cells. Comp. Biochem. Physiol. 148A, 479-497 [DOI] [PubMed] [Google Scholar]
- Kahle K. T., Rinehart J., Ring A., Gimenez I., Gamba G., Hebert S. C., Lifton R. P. (2006). WNK protein kinases modulate cellular Cl-flux by altering the phosphorylation state of the Na-K-Cl and K-Cl cotransporters. Physiology 21, 326-335 [DOI] [PubMed] [Google Scholar]
- Katoh F., Lynch B. N. G., Cozzi R. R. F., Marshall W. S. (2007). Phosphorylation of focal adhesion kinase (FAK) at tyrosine 407 and integrin beta 1 regulate NKCC cotransporter in Fundulus heteroclitus. FASEB J. 21, A1337 [Google Scholar]
- Kultz D., Avila K. (2001). Mitogen-activated protein kinases are in vivo transducers of osmosensory signals in fish gill cells. Comp. Biochem. Physiol. 129B, 821-829 [DOI] [PubMed] [Google Scholar]
- Kurihara K., Moore-Hoon M. L., Saitoh M., Turner R. J. (1999). Characterization of a phosphorylation event resulting in upregulation of the salivary Na+-K+-2Cl(-) cotransporter. Am. J. Physiol. Cell Physiol. 277, C1184-C1193 [DOI] [PubMed] [Google Scholar]
- Lytle C., Forbush B. (1992). The Na-K-Cl cotransport protein of shark rectal gland.2. Regulation by direct phosphorylation. J. Biol. Chem. 267, 25438-25443 [PubMed] [Google Scholar]
- Lytle C., Forbush B. (1996). Regulatory phosphorylation of the secretory Na-K-Cl cotransporter: Modulation by cytoplasmic Cl. Am. J. Physiol. Cell Physiol. 39, C437-C448 [DOI] [PubMed] [Google Scholar]
- Mancera J. M., McCormick S. D. (1998). Evidence for growth hormone insulin-like growth factor I axis regulation of seawater acclimation in the euryhaline teleost Fundulus heteroclitus. Gen. Comp. Endocrinol. 111, 103-112 [DOI] [PubMed] [Google Scholar]
- Mancera J. M., McCormick S. D. (2000). Rapid activation of gill Na+,K+-ATPase in the euryhaline teleost Fundulus heteroclitus. J. Exp. Zool. 287, 263-274 [PubMed] [Google Scholar]
- Marshall W. S., Bryson S. E. (1998). Transport mechanisms of seawater teleost chloride cells: An inclusive model of a multifunctional cell. Comp. Biochem. Physiol. 119A, 97-106 [DOI] [PubMed] [Google Scholar]
- Marshall W. S., Emberley T. R., Singer T. D., Bryson S. E., McCormick S. D. (1999). Time course of salinity adaptation in a strongly euryhaline estuarine teleost, Fundulus heteroclitus: a multivariable approach. J. Exp. Biol. 202, 1535-1544 [DOI] [PubMed] [Google Scholar]
- Marshall W. S., Ossum C. G., Hoffmann E. K. (2005). Hypotonic shock mediation by p38 MAPK, JNK, PKC, FAK, OSR1 and SPAK in osmosensing chloride secreting cells of killifish opercular epithelium. J. Exp. Biol. 208, 1063-1077 [DOI] [PubMed] [Google Scholar]
- Marshall W. S., Main H. P., Katoh F., Cozzi R. R. F. (2007). Differential phosphorylation of focal adhesion kinase (FAK) in hypotonic regulation of NKCC cotransporter in NaCl secretion by Fundulus heteroclitus. FASEB J. 21, A1335 [Google Scholar]
- McCormick S. D. (2001). Endocrine control of osmoregulation in teleost fish. Am. Zool. 41, 781-794 [Google Scholar]
- McCormick S. D., O'Dea M. F., Moeckel A. M., Bjornsson B. T. (2003). Endocrine and physiological changes in Atlantic salmon smolts following hatchery release. Aquaculture 222, 45-57 [Google Scholar]
- Melvin J. E., Yule D., Shuttleworth T., Begenisich T. (2005). Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu. Rev. Physiol. 67, 445-469 [DOI] [PubMed] [Google Scholar]
- Nauntofte B. (1992). Regulation of electrolyte and fluid secretion in salivary acinar-cells. Am. J. Physiol. 263, G823-G837 [DOI] [PubMed] [Google Scholar]
- Pelis R. M., McCormick S. D. (2001). Effects of growth hormone and cortisol on Na+-K+-2Cl− cotransporter localization and abundance in the gills of Atlantic salmon. Gen. Comp. Endocr. 124, 134-143 [DOI] [PubMed] [Google Scholar]
- Riordan J. R., Forbush B., Hanrahan J. W. (1994). The molecular-basis of chloride transport in shark rectal gland. J. Exp. Biol. 196, 405-418 [DOI] [PubMed] [Google Scholar]
- Scott G. R., Richards J. G., Forbush B., Isenring P., Schulte P. M. (2004). Changes in gene expression in gills of the euryhaline killifish Fundulus heteroclitus after abrupt salinity transfer. Am. J. Physiol. Cell Physiol. 287, C300-C309 [DOI] [PubMed] [Google Scholar]
- Scott G. R., Claiborne J. B., Edwards S. L., Schulte P. M., Wood C. M. (2005). Gene expression after freshwater transfer in gills and opercular epithelia of killifish: insight into divergent mechanisms of ion transport. J. Exp. Biol. 208, 2719-2729 [DOI] [PubMed] [Google Scholar]
- Tabcharani J. A., Chang X. B., Riordan J. R., Hanrahan J. W. (1991). Phosphorylation-regulated Cl− channel in Cho cells stably expressing the cystic fibrosis gene. Nature 352, 628-631 [DOI] [PubMed] [Google Scholar]
- Tipsmark C. K., Madsen S. S., Seidelin M., Christensen A. S., Cutler C. P., Cramb G. (2002). Dynamics of Na+,K+,2Cl− cotransporter and Na+,K+-ATPase expression in the branchial epithelium of brown trout (Salmo trutta) and Atlantic salmon (Salmo salar). J. Exp. Zool. 293, 106-118 [DOI] [PubMed] [Google Scholar]
- Tipsmark C. K., Madsen S. S., Borski R. J. (2004). Effect of salinity on expression of branchial ion transporters in striped bass (Morone saxatilis). J. Exp. Zool. 301A, 979-991 [DOI] [PubMed] [Google Scholar]
- Vitari A. C., Deak M., Morrice N. A., Alessi D. R. (2005). The WNK1 and WNK4 protein kinases that are mutated in Gordon's hypertension syndrome phosphorylate and activate SPAK and OSR1 protein kinases. Biochem. J. 391, 17-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadunaisky J. A., Cardona S., Au L., Roberts D. M., Fisher E., Lowenstein B., Cragoe E. J., Spring K. R. (1995). Chloride transport activation by plasma osmolarity during rapid adaptation to high salinity of Fundulus heteroclitus. J. Membr. Biol. 143, 207-217 [DOI] [PubMed] [Google Scholar]