Abstract
At present commercially available bilateral cochlear implants (CIs) improve their users’ speech understanding in noise but they employ two independent speech processors that cannot provide accurate and appropriate interaural level and time differences as seen binaurally in normal hearing (NH) listeners. Previous work suggests that binaural cues are accessible to bilateral CI users when presented to single pairs of pitch-matched electrodes, but the scope was limited and the mechanisms remained unclear. In this study, binaural masking level differences (BMLDs) were measured in five bilateral Nucleus-24 CI users over multiple pairs of pitch-matched electrodes. Average BMLD was 4.6±4.9 dB, but large individual variability prevented significance (p=0.09). Considering just the 125 Hz condition, as in previous work, phase (N0S0 vs N0Sπ) and electrode effects were significant. Compared with simulated bilateral CI users, actual bilateral CI users had proportionally higher thresholds for N0Sπ than N0S0. Together the present results suggest that the performance gap in BMLDs between CI and NH listeners is not due to a lack of sufficient acoustic cues in the temporal envelope domain but to a true binaural deficit related to a central mechanism in deprived binaural processing.
INTRODUCTION
Cochlear implant (CI) users can understand most speech in quiet, but the presence of noise can greatly challenge that understanding (i.e., Zeng and Galvin, 1999; Spahr and Dorman, 2004; Stickney et al., 2004). Although a second implant can benefit CI users for hearing in noise (Müller et al., 2002; Litovsky et al., 2004, 2006a; Buss et al., 2008; Litovsky et al., 2009), binaural sensitivity in these bilateral CI users falls short of normal hearing (NH) levels (van Hoesel et al., 1993; Buss et al., 2008; Nopp et al., 2004; Laszig et al., 2004; van Hoesel, 2004; Verschuur et al., 2005; Neuman et al., 2007; van Hoesel et al., 2008). In addition, there is a performance gap between testing the binaural sensitivity of CI users under ideal circumstances (i.e., Long et al., 2006) and their performance under real world conditions (i.e., van Hoesel et al., 2008). So far, measures of true binaural hearing, such as squelch, which requires computation of differences between sounds entering the left and right ears, appear to be of marginal benefit to bilateral CI users (Nopp et al., 2004; Laszig et al., 2004; Long et al., 2006; van Hoesel, 2004; Buss et al., 2008; van Hoesel et al., 2008). Uncoordinated stimulation timing produced by independent speech processors may be one limiting factor for binaural sensitivity. However, data from experiments using the Spear3 research interface (Hearworks, Pty, Melbourne, Australia), which can deliver highly synchronized stimulation pulses to both ears, still show lower than normal levels of binaural function in CI (Long et al., 2006; van Hoesel et al., 2008; Litovsky et al., 2010).
Binaural masking level difference (BMLD) is one measure of binaural sensitivity (Hirsh, 1948). Unlike head shadow cues or redundancy, BMLD requires precise computations of differences in the sounds entering the left and right ears (Durlach, 1963). For a signal-in-noise detection task, when the signal phase is inverted between the two ears, there is a higher likelihood for detection than when the signal phase is identical between the two ears. BMLD is calculated as the difference in threshold between the two conditions. In NH listeners this difference can be as large as 20 dB for tones (van de Par and Kohlrausch, 1997). Under similar stimuli conditions, BMLDs measured in bilateral CI users averaged ∼9 dB when stimulating with single pairs of electrodes (Long et al., 2006). Similar tests in children show 6.4 dB for CI and about 10 dB for NH (Van Deun et al., 2009a, 2009b). The cause of this gap between normal and CI listeners is not well understood.
Furthermore, BMLD in CI users appears to be much smaller for more complex sounds. An experiment that used speech under realistic listening conditions reported <1.5 dB of binaural unmasking (van Hoesel et al., 2008), which is significantly less than the 9 dB found in Long et al., 2006. In contrast, NH listeners typically had BMLD values that fell between the range seen for tones and speech (Levitt and Rabiner, 1967). This would suggest that in NH listeners binaural processing of BMLD for speech can be predicted by BMLD for tones. In the present study, we examined several factors that could potentially affect BMLDs in bilateral CI users under more realistic stimulation conditions than previously studied.
The first factor is that BMLD values may be influenced by electrode position along the cochlea. Complex stimuli such as speech have multiple frequency components, and in normal hearing listeners, BMLD decreases with increasing signal frequency (van de Par and Kohlrausch, 1997). BMLDs were shown to be similar between a 125 Hz sinusoid and a 125 Hz envelope in the form of “transposed” stimuli with a 4000 Hz carrier. This “transposition” of the stimuli places the 125 Hz signal at the 4000 Hz cochlear region. The similarity in BMLDs for NH listeners suggests that the 125 Hz sinusoid and the 125 Hz envelope of the transposed stimuli were processed similarly despite the activity at different cochlear regions (125 Hz vs 4 kHz). This is relevant to continuous interleaved sampling (CIS) based processing strategies in CI as sounds are typically transmitted via envelopes on top of a high pulse rate carrier (Wilson et al., 1991). For CI users, BMLDs were reported for basal, middle, and apical electrode pairs, but only one pair was tested in each subject (Long et al., 2006). Although BMLDs are expected to be similar over multiple cochlear regions in a single CI user as they are in NH listeners, it has not yet been experimentally verified.
A second factor that may influence BMLD levels in CI is the stimulus signal frequency. In normal hearing listeners, ∼10 dB BMLDs were reported with signal frequencies as high as 4000 Hz (van de Par and Kohlrausch, 1997). Existing studies show that CI users have difficulty discriminating higher pulse rates and modulation frequencies starting around 300 Hz under monaural (Zeng, 2002) and binaural (Carlyon et al., 2008) conditions. The 125 Hz signal frequency used in Long et al., 2006 is well within the temporal discrimination ability of CI users, but BMLD at higher signal frequencies has not been systematically addressed. If the mechanisms that cause this limitation play a role in binaural sensitivity, BMLD values may deteriorate with higher stimulus frequencies and with complex sounds that have higher frequency components.
Third, there is the possibility that the interaction between narrowband noise and a 125 Hz signal combined with the envelope extraction by the speech processor produces enough detectable difference cues between NmSm and NmS-m, where m and -m indicate monaural and the phase reversed monaural noise and signal stimuli. If these NmSm and NmS-m are discriminable monaurally, it could inflate BMLD values. For this reason, it is important to test whether BMLDs could be a result of monaural discrimination of the signal envelope.
Fourth, prior auditory deprivation can negatively impact binaural processing (Silman et al., 1984; Hall and Grose, 1993; Gray et al., 2009). It is known that the lack of auditory inputs can lead to neural degeneration in the periphery (Hinojosa and Lindsay, 1980). Furthermore, there is evidence, at least in children, that binaural processing continues to develop with experience and age (Hogan et al., 1996; Litovsky, 1997; Hall et al., 2004; Litovsky et al., 2006b), and a loss of binaural input could arrest this development.
A recent study of patients with unilateral hearing loss due to atresia suggested that there is a loss of 2 dB binaural advantage due to squelch for each decade of life with only unilateral hearing (Gray et al., 2009). The recovery of binaural sensitivity was not studied. However, it was earlier reported that adult patients with unilateral conductive hearing loss experience a blunting of binaural sensitivity, which eventually recovers to normal or near-normal levels by 1 year after surgical intervention (Hall and Grose, 1993). These subjects had normal cochlear function and once the conductive hearing loss was remedied, their hearing function was essentially restored to normal. Despite restoring the ability to hear, the cochlear implants do not restore normal hearing due to coarse frequency resolution, and furthermore, the use of independent speech processors virtually guarantees uncoordinated stimulation in pulse timing, thus providing abnormal interaural time difference (ITD) cues in the fine-structure of sound. Therefore even if the auditory system is capable of recovering normal binaural function, it would not be able to do so given abnormal and coarse frequency inputs provided by both CIs.
Auditory deprivation on unilateral cochlear implant use has been documented (Tyler and Summerfield, 1996), the effects of which on binaural function in adults has not been adequately explored and there are limited studies. The main effect is that some binaural function such as squelch is recovered over the course of 1 year (Buss et al., 2008). The evidence for bilateral hearing aids is a little clearer. Comparing two groups, one fitted unilaterally with hearing aids and the other bilaterally, there was a difference between ears in the unilaterally implanted group (Silman et al., 1984). As a result of auditory deprivation, it is unknown to what extent limited binaural processing is due to the implant and speech processor and how much is due to degeneration of binaural processing in the central auditory system. This may be addressed, in part, by comparing the performance of NH listeners under acoustic CI simulation to that of actual CI users.
The overall aim of this study is to investigate the extent of binaural sensitivity quantifiable by BMLD in bilateral CI users and why it falls short of normal performance on binaural listening tasks. Building upon the results from Long et al. (2006), the questions that this study will directly address are as follows: (1) How BMLDs compare across basal, middle, and apical pairs of pitch-matched electrodes; (2) how BMLDs change with signal frequency; (3) would masking level differences under monaural conditions predict binaural BMLDs; and (4) how do normal hearing listeners compare when subjected to acoustic CI simulations.
METHODS
Subjects
Five bilaterally implanted Nucleus 24 patients were tested (Table 1). Several of these patients wore hearing aids for a number of years. The two implantations were spaced 1 year apart in three subjects and 12 years in one subject. The fifth subject had both ears implanted simultaneously. Speech performance scores for these subjects are listed in Table 1. Sound input levels were set so that maximum stimulation levels were approximately 95% of most comfortable level (MCL) for that electrode pair.
Table 1.
CI subjects.
| Gender | Age | Age at deafness | Etiology | Age at hearing aid use | Left∕right implanted (years ago) | Basal pair (L∕R) | Middle pair (L∕R) | Apical pair (L∕R) | HINT speech scores quiet∕noise (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| CI1 | f | 62 | 26 | Ototoxicity | 26 | 6∕18 | 5∕5 | 12∕12 | 20∕21 | L:83∕32 |
| R:95∕65 | ||||||||||
| Bi: NA | ||||||||||
| CI2 | m | 51 | 3 | Congenital | 3 | 2∕3 | 5∕5 | 12∕12 | 22∕22 | L:0∕0 |
| R:0∕0 | ||||||||||
| Bi:0∕not tested | ||||||||||
| CI3 | f | 63 | 37 | Meniere’s | 43 | 4∕5 | 5∕5 | 12∕10 | 20∕20 | L:23∕not tested |
| R:91∕93 | ||||||||||
| Bi 99∕96 | ||||||||||
| CI4 | f | 72 | 26 | Otosclerosis | 38 | 3∕4 | 6∕6 | 11∕16 | 14∕16 | L: 91∕29 |
| R:95∕11 | ||||||||||
| Bi:99∕40 | ||||||||||
| CI5 | m | 68 | 34 | Progressive | 42 | 6∕6 | 5∕5 | 13∕12 | 20∕20 | L: 98∕39 |
| R: 98∕55 | ||||||||||
| Bi: 100∕58 | ||||||||||
For each subject, pairs of pitch-matched electrodes were found and balanced for loudness at MCL since binaural sensitivity is higher for pitch-matched pairs in CI (van Hoesel and Clark, 1997; Lawson et al., 1998; Long et al., 2003, 2007) and frequency-matched stimuli in NH (Henning, 1974; Nuetzel and Hafter, 1981). As an example, an apical pair (i.e., 20L, 20R) was selected and threshold and comfort levels were determined individually. Each electrode in a pair was played sequentially and the subject was asked to make a pitch judgment. If there was a perceived pitch difference, the next adjacent electrode on one side was selected, typically the more recently implanted ear, and the pitch was compared again. This search for a pitch-matched pair was iterated as many times as necessary until a suitable match was found. If needed, the loudness was balanced by lowering the MCL of the louder electrode to match. The pitch and loudness match was confirmed again. In a few cases, subjects reported that the similarity of pitch match changed when balancing loudness, requiring a search for a different pitch-matched pair. Three pairs of electrodes—apical, middle, and basal—were tested in each subject. The exact locations of these matched pairs are listed in Table 1. Finding and balancing each pair of pitch-matching electrodes could take anywhere from 30 min to over 1 h, and sometimes longer.
Stimulus and hardware configuration
All stimuli were generated digitally using MATLAB (Mathworks, Inc., Natick, MA) with 44.1 kHz sampling rate through a PC sound card. Stimuli consisted of 300 ms sinusoids at 125, 250, or 500 Hz. Masking sounds were 400 ms band-passed noise with bandwidths of ±25, 50, and 100 Hz, centered at each sinusoid, respectively. N0S0 signals denote masking noise that was identical for both ears, and sinusoids that had a zero-phase difference (also identical) between the two ears. N0Sπ denotes the same masking noise, but sinusoids that are 180° out of phase (inverted) between the two ears. The ratio of amplitude of the sinusoid to the noise is the signal to noise ratio (SNR) in decibel.
The signal processing algorithm is similar to that described in Long et al., 2006. The stimuli were presented through line-in jack, bypassing the microphone, on the Spear3 research interface (Hearworks, Pty, Melbourne, Australia) running a custom program that implemented a bilateral one-channel CIS program. Audio signals were digitized by the on-board analog-to-digital converters. The signal was half-wave rectified and low-passed filtered using a four-pole Butterworth filter. For the 125 and 250 Hz stimulus conditions, the signal was low-passed at 500 Hz, and the envelope of the signal was used to modulate a pulse train with a rate of 1000 pulses per second (pps). For the 500 Hz stimulus condition, the signal was low-passed at 1000 Hz before modulating a 2000-pps pulse train. A standard loudness growth map was used to compress the amplitude of the signal (as in Long et al., 2006). The stimulation mode used was MP1+2 (monopolar), with 25 μs per phase and an 8 μs pulse gap. The left and right pulsatile outputs of the Spear3 were verified to be synchronized to within 1 μs by viewing signals on an oscilloscope after being fed through an “implant-in-a-box” (Cochlear Pty, Lane Cove, Australia).
Implementation of acoustic CI simulation
Ten normal hearing subjects (20–26 years old) were tested using unprocessed stimuli along with several different vocoder configurations to acoustically simulate a bilateral CI listening condition. Only two subjects (NH1 and NH2) who were tested under unprocessed conditions returned for testing with the vocoder. A single-channel noise-excited vocoder was used to determine the extent to which envelope cues were preserved. Inputs were identical to those used for the experiments with CI listeners. Stimuli were half-wave rectified and low-passed at 500 Hz to extract the envelope. The resulting signal was used to modulate a broadband noise. Since the one-channel noise vocoder used here does not accurately simulate the conditions in which basal, middle, or apical pairs of electrodes were stimulated, a second vocoder model based on Gaussian-enveloped tones (Lu et al., 2007) was used to simulate synchronized and pulsatile stimulation for different electrode pairs. As with the noise vocoder, stimuli were half-wave rectified and low-pass filtered. The resulting envelope signal was used to modulate the amplitude of tone pulses that had Gaussian-shaped time-amplitude envelopes. The pulse rate was set at 1000 Hz for the Gaussian-enveloped tone pulses. The duration of each pulse was approximately 1 ms. The simulated basal, middle, and apical channels had center frequencies of 5367, 1426, and 369.5 Hz, based on Greenwood’s map (Greenwood, 1990).
Gaussian-enveloped tones have been used in binaural psychophysical experiments (Buell and Hafter, 1988; van den Brink and Houtgast, 1990), but its application in a vocoder has not been reported previously. It is similar to the transposed tones used in van de Par and Kohlrausch, 1997. Two important characteristics of cochlear implants, which can be modeled with the vocoder, are not accounted for in current noise-based CI simulations. The first is pulsatile stimulation. With CI the signal envelope is sampled at the pulse rate. Noise-excited vocoders have a continuous representation of the signal envelope, which at low stimulation rates, the deficiency to simulate well separated pulses becomes readily apparent. The spread of excitation can be controlled by the duration of the pulse, which can widen or narrow the spectral spread produced by each pulse. Since ITD sensitivity is better at lower stimulation rates, and pitch perception is affected by the spread of excitation, the ability to control these factors makes the Gaussian-enveloped tone vocoder a potentially useful platform for acoustically simulating bilateral CI use.
Testing procedure
Masking level thresholds were estimated by a three-interval, forced-choice adaptive procedure with a two-down∕one-up decision rule (Levitt, 1971). The custom software was implemented in MATLAB (Mathworks, Inc., Natick, MA). All three intervals contained N0. The signal S0 or Sπ was added to a single, randomly assigned interval; thus the target interval was either N0S0 or N0Sπ. The subject’s task was to identify which one of the three intervals contained the tone (N0S0 or N0Sπ vs N0). Two correct successive responses decreased the SNR for the next trial, while one incorrect response immediately increased the SNR for the next trial. Each change in direction of SNR (decreasing to increasing or vice versa) counted as a reversal. After eight total reversals, with stepsize decreasing from 5 to 1 dB after the initial three reversals, the SNR at which the last four reversals occurred were averaged to produce an estimate of the SNR threshold. Smaller values indicate better detection of the signal in noise compared to a larger SNR. Each run of the test consisted of two, randomly interleaved tracks, N0S0 and N0Sπ to help control for subject state between the two conditions. Once eight total reversals were observed in one track, the program presented stimuli exclusively from the other track until eight total reversals were also observed. With every subject, three threshold estimates were obtained for each phase (N0S0 vs N0Sπ), signal frequency, and electrode pair combination.
Data for monaural conditions were obtained by disconnecting either the left or right transmitting coil from the subject’s head and repeating the same test protocol described above. Normal hearing subjects were tested in a sound-proof chamber using calibrated headphones (HDA 200, Sennheiser, Old Lyme, CT). Sound levels were set to 70 dB sound pressure level (SPL), calibrated to a 1 kHz tone having the same rms level as the stimuli used in the experiment.
Data analysis
Average values were calculated for N0S0 and N0Sπ. The BMLD was calculated as the overall difference between the average N0S0 and N0Sπ values. An unpaired t-test was used to determine whether the three thresholds from N0S0 were statistically different from the three N0Sπ thresholds, with p<0.05 being the criterion for significance (see asterisk symbols).
For the pooled data, average thresholds and BMLD were calculated over all stimulus frequencies, electrode pairs, and subjects. A repeated-measures analysis of variance (r.m. ANOVA) was performed on the thresholds to determine the effect of phase (N0S0 and N0Sπ), electrode (basal, middle, and apical), and signal frequency (125, 250, and 500 Hz). A second r.m. ANOVA was repeated on just the data set containing 125 Hz.
RESULTS
Binaural masking level differences
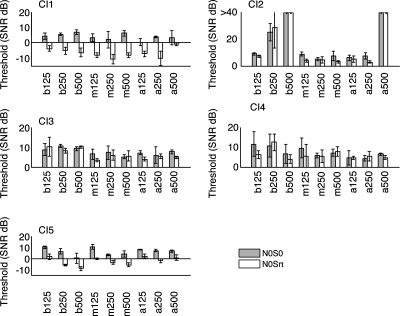
Masking level thresholds for various combinations of signal frequency and electrodes for N0S0 and N0Sπ are shown in Fig. 1. Masking level thresholds over subjects and conditions were all above 0 dB for N0S0, ranging from 0.3 to 11.6 dB SNR (signal to noise ratio), and both below and above 0 dB SNR (−10.6 to 12.5 dB) for N0Sπ. In fact, thresholds in the N0Sπ condition only reached negative values, where the amplitude of the signal was less than that of the noise, in two subjects (CI1 and CI5). On an individual basis, masking thresholds were generally lower for N0Sπ compared to N0S0, indicating a BMLD (see Table 2).
Figure 1.
Masking level thresholds for CI. Masking level thresholds are indicated by each bar. These indicate the smallest signal-to-noise ratio (in decibel) at which the subject was able to detect the signal in noise (see Sec. 2). Each group represents a different electrode pair and signal frequency condition. The labels designate the electrode pair (base∕middle∕apex) and stimulus condition (125∕250∕500 Hz). For example, a250 indicates apical pair of electrodes and stimulus condition of 250 Hz. In each group, the shaded bars represent the average of three trials from N0S0 while the unshaded bars designate the N0Sπ condition. The error bars represent standard deviation. For subject 2, some data points were truncated at 40 dB as the subject was unable to complete the signal detection task under those conditions.
Table 2.
Average thresholds and BMLDs over all stimulus conditions and electrode pairs.
| Subject | N0S0 (dB SNR) | N0Sπ (dB SNR) | BMLD (dB SNR) | R NmSm R NmS-m | L NmSm L NmS-m |
|---|---|---|---|---|---|
| CI1 | 3.9±2.1 | −6.7±3.1 | 10.7±3.4 | 3.1±2.2 | 3.2±3.0 |
| 3.6±2.3 | 1.7±1.1 | ||||
| CI2 | 16.4±14.6 | 15.0±16.3 | 1.5±2.8 | 28.3±14.5 | 25.7±15.9 |
| 25.0±16.6 | 26.3±15.6 | ||||
| CI3 | 7.6±1.7 | 6.4±2.5 | 1.2±1.8 | 22.7±14.8 | 23.38±18.2 |
| 24.8±12.9 | 23.39±18.2 | ||||
| CI4 | 7.4±2.6 | 6.2±2.6 | 1.2±2.4 | 24.2±17.4 | 34.3±6.6 |
| 24.3±17.3 | 31.2±10.3 | ||||
| CI5 | 6.2±3.3 | −2.6±3.9 | 8.8±2.2 | 6.8±1.6 | 5.8±2.6 |
| 6.6±2.1 | 6.5±1.8 | ||||
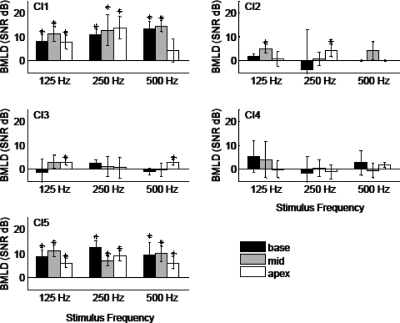
BMLD values are shown in Fig. 2, where vertical bars show the BMLD calculated for each electrode pair grouped by stimulus. Asterisks indicate statistically significant BMLDs (unpaired t-test, p<0.05), that is, higher average thresholds for N0S0 than N0Sπ. This comparison helps to identify cases in which there existed a meaningful difference in performance between the two conditions, regardless of the magnitude of the difference (i.e., small but significant vs large and insignificant BMLDs). For CI1 and CI5, BMLDs were statistically significant over almost all basal, middle, and apical electrode pairs and stimulus conditions. Average BMLDs (mean±s.d.) were 10.7±3.4 and 8.8±2.2 dB, respectively, for CI1 and CI5. BMLDs observed for CI2 and CI3 were generally lower (mean=1.5±2.8 and 1.2±1.8 dB, respectively), with only two out of nine conditions showing statistically significant BMLDs. CI4 showed no statistically significant BMLDs (mean=1.2±2.4 dB) under any stimulus conditions.
Figure 2.
Binaural masking level differences from five CI subjects. Each bar represents a BMLD value (y-axis) averaged over three trials, and is the mean difference of the N0S0 and N0Sπ thresholds in Fig. 1. BMLDs are grouped by stimulus condition along the x-axis. Data from different electrode pairs are indicated by the shading. The error bars represent the standard deviation. “∗” denote statistically significant BMLD values (unpaired t-test, p<0.05).
Over all subjects and conditions tested, the average BMLD was 4.6±4.9 dB. The main effect of phase on thresholds (N0S0 vs N0Sπ) was not significant (ANOVA, F1,4=4.954, p=0.09). There was no significant effect detected of either electrode or rate (ANOVA, p>0.05). The lack of significance in this case is most likely due to the large range of performance across individuals. It is worth noting that for each subject, thresholds for N0S0 were always higher than N0Sπ, but the standard deviations were always the same or higher for N0Sπ (see Table 2).
When only the 125 Hz condition was considered, as in Long et al., 2006, four of five subjects in the present study showed a significant BMLD in at least one cochlear region or electrode pair, with the average being 4.9±3.8 dB. Results from repeated-measures ANOVA showed a significant effect of phase (F1,4=10.130, p=0.033) and electrode (F2,8=13.366, p=0.003), but no interaction between the two factors (F2,8=3.844, p=0.068). Post-hoc t-tests with Bonferroni correction showed a significant difference (p=0.023) between average N0S0 (mean=7.1±3.2 dB) and N0Sπ (mean=2.24±5.2 dB) thresholds. Pair-wise comparisons of the electrodes revealed a significant difference (p=0.023) between thresholds of apical (mean=3.41±4.4 dB) and basal (mean=6.50±4.7 dB) electrodes. There were no significant differences between the thresholds of the middle electrode (4.2±5.5 dB) and either apical (p=0.82) or basal (p=0.71) electrodes. Although electrodes had an effect on threshold, there was no significant effect on BMLD as a function of cochlear region (r.m. ANOVA, F2,8=3.842, p=0.068).
Separate analysis of the 250 (mean=4.6±5.9 dB) and 500 Hz (mean=4.5±5.1 dB) conditions did not yield any statistical significance (ANOVA, p>0.05). Despite the large individual variability, these data suggest that at least some CI subjects can take advantage of binaural processing over multiple electrode pairs and signal frequency conditions.
Monaural detection thresholds
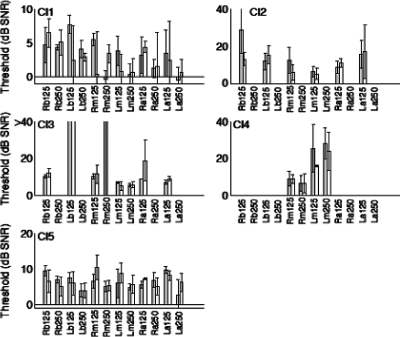
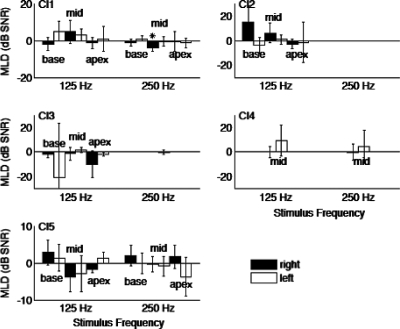
This study was also concerned with whether subjects can detect differences in amplitude modulation using a single ear. It is known that CI users can have very high sensitivity to amplitude modulations (Shannon, 1992). The performance under monaural conditions can reveal the contribution of binaural processing compared to what can be detected monaurally. Figure 3 shows masking level difference (MLD) thresholds from each ear separately for all five subjects. Only CI1 and CI5 were able to complete all test conditions, and thresholds for subjects C2–C4 were capped at 40 dB. Absolute thresholds tended to be higher than with the binaural condition, and MLDs between the two stimulus conditions, NmSm vs NmS-m, were not much different, where Nm indicates monaural noise, Sm is the monaural sinusoid, and S-m is the inverted monaural sinusoid. Under monaural conditions, no significant MLDs (unpaired t-test, p>0.05) were observed in any of the five subjects except for a single condition (CI1, 250 Hz) (Fig. 4).
Figure 3.
Monoaural masking level thresholds. Format is similar to Fig. 1. The labels along the x-axis include “R” and “L” to specify right or left ear for the monaural condition. Note that some bars representing high threshold values were truncated (e.g., CI3).
Figure 4.
Monaural MLDs. Format is similar to Fig. 2. For each group, shaded bars indicate data from the right ear, while unshaded bars are data from the left ear. Within each group, the left two bars are data from basal pair of electrodes, the third and fourth bars are from the middle pair of electrodes, and the last two bars are from the apical pair of electrodes.
Table 2 lists the average values obtained for monaural conditions. For CI1 and CI5, thresholds obtained for both right and left ears under monaural conditions are similar to the data from N0S0 condition, but not N0Sπ. These data from the monaural tests demonstrate that the BMLDs observed for the binaural condition cannot be explained by parallel but independent signal detection (via amplitude modulation of the signal envelope) between the left and right ears.
Binaural unmasking with acoustic simulation of CI
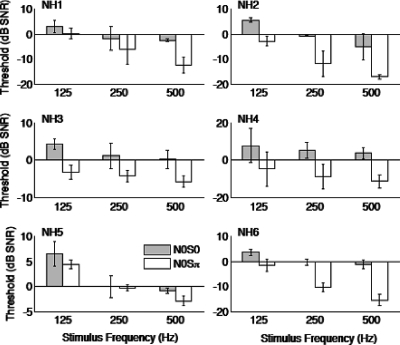
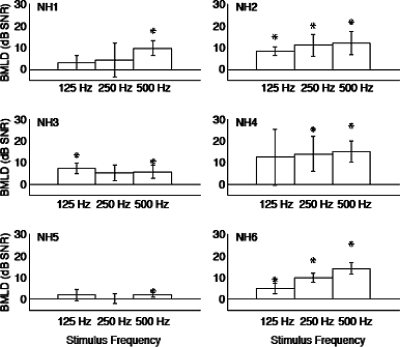
Because a prolonged lack of auditory input can have a negative impact on the normal functioning of the auditory system, the present manipulations were also conducted in normal hearing listeners using acoustic stimuli that were either unprocessed or with CI simulations. This approach would enable a distinction of issues stemming from CI-specific limitations related to the BMLD phenomenon. Thresholds, measured using unprocessed sounds, were on average 1.6±3.5 dB for N0S0 and −6.3±5.8 dB for N0Sπ (Fig. 5). BMLDs in unpracticed listeners ranged from 1.5±1.1 to 13.9±1.3 dB SNR (Fig. 6), which had a slightly wider range than that for CI listeners. Average BMLD was 7.9±4.7 dB (see Table 3 for individual values). There was a significant effect of both phase (ANOVA, F1,5=19.855, p=0.007) and signal frequency (F2,10=35.494, p⪡0.05), but no significant interaction. Considering that these NH subjects had minimal practice, the range data reported here are still consistent with that reported in other studies (i.e., van de Par and Kohlrausch, 1997).
Figure 5.
Masking thresholds for unprocessed stimuli in NH listeners. Format is the same as Fig. 1.
Figure 6.
BMLD for unprocessed stimuli in NH listeners. Format is the same as Fig. 2.
Table 3.
Average BMLDs over all stimulus conditions and electrode pairs for NH listeners.
| Subject | Unprocessed (dB SNR) | Noise vocoder (dB SNR) | Gaussian vocoder (dB SNR) |
|---|---|---|---|
| NH1 | 5.8±3.6 | 8.5±1.2 | 8.9±3.1 |
| NH2 | 10.6±1.9 | 9.3±4.5 | 11.7±6.3 |
| NH3 | 6.2±1.1 | ⋯ | ⋯ |
| NH4 | 13.9±1.3 | ⋯ | ⋯ |
| NH5 | 1.5±1.1 | ⋯ | ⋯ |
| NH6 | 9.8±4.6 | ⋯ | ⋯ |
| NH7 | ⋯ | 26.6±4.0 | 19.3±4.0 |
| NH8 | ⋯ | 21.8±15.4 | 15.9±9.8 |
| NH9 | ⋯ | 28.0±2.7 | 16.4±4.2 |
| NH10 | ⋯ | −7.9±14.4 | 3.1±5.8 |
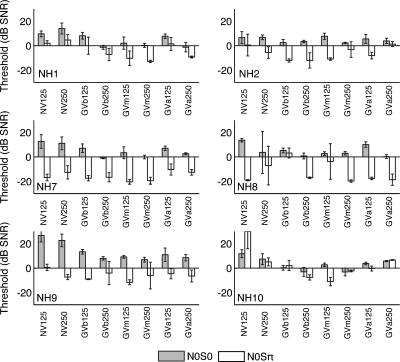
When tested with a noise-excited, one-channel acoustic CI simulation, thresholds tended to be higher than in the unprocessed condition and quite variable (Fig. 7). Average thresholds were 12.2±6.7 dB for N0S0 and −2.2±12.9 dB for N0Sπ. This indicated that subjects tended to have much more difficulty with N0S0. However, subjects showed a large drop in thresholds with N0Sπ as seen in NH7 and NH8 for the noise-excited vocoder with 125 Hz signal condition. Average BMLD was 14.4±14.7 dB. The effect of phase approached but did not reach statistical significance (ANOVA, F1,5=6.538, p=0.051), and signal frequency had no significant effect (F1,5=1.179, p=0.327).
Figure 7.
Masking thresholds for NH listeners using acoustic CI simulation. Format is the same as Fig. 1. Labels along the x-axis include NV-noise-excited vocoder, GVb—Gaussian-enveloped tone vocoder (basal), GVm (middle), and GVa (apical). See Sec. 2 for details on the Gaussian-enveloped tone vocoder.
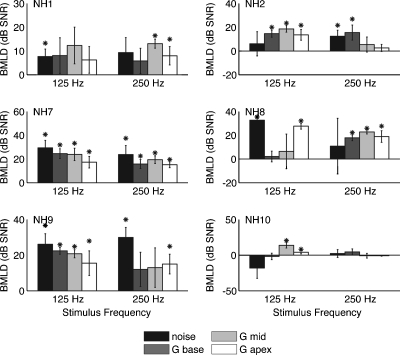
Since using a single-channel noise vocoder does not accurately simulate the single-electrode stimulus conditions with electrically pulsed signals under which the CI users were tested, a vocoder based on Gaussian-enveloped tones was used to test simulated effects of basal, middle, and apical pair stimulations (Fig. 8). Thresholds for the Gaussian-enveloped tone vocoder averaged 4.0±4.1 dB for N0S0 and −8.6±7.2 dB for N0Sπ. Phase showed a statistically significant effect (ANOVA, F1,5=27.080, p=0.003) but there was no effect of channel (F2,10=2.898, p=0.102) or signal frequency (F1,5=2.772, p=0.157). In addition, there were no significant interactions. Average BMLD was 12.6±7.8 dB. Although BMLD values from the Gaussian-enveloped tone vocoders were similar to the noise vocoder condition, average N0S0 threshold was nearly 8 dB higher for noise vocoder.
Figure 8.
BMLD for NH listeners subjected to acoustic CI simulation. Format is the same as Fig. 2.
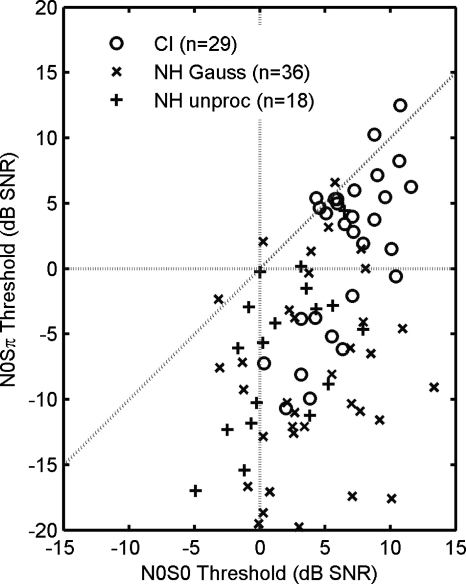
A comparison of the N0S0 and N0Sπ threshold values from CI and NH using vocoder stimuli is shown in Fig. 9. Each data point represents a single-electrode pair and signal frequency combination from the five CI subjects and six NH subjects. The mean values for CI subjects were 6.6±2.8 dB for N0S0 and 1.6±6.1 for N0Sπ. The mean values for simulated CI (Gaussian vocoders) with NH listeners were 4.0±4.1 dB for N0S0 and −8.6±7.2 for N0Sπ. The mean values for unprocessed stimuli with NH listeners were 1.6±3.5 dB for N0S0 and −6.3±5.8 for N0Sπ. These mean values of N0S0 were significantly different from each other (unpaired t-test, p<0.05). Mean values of N0Sπ were also significantly different (unpaired t-test, p⪡0.05), except between the simulated CI and unprocessed stimuli in NH listeners (unpaired t-test, p=0.26). Despite the large individual variability, Fig. 9 illustrates three important points. First, CI implant users tended to produce the highest (or worst) thresholds in both binaural conditions, particularly in the N0Sπ condition. Second, the CI simulations captured the best performance seen in the CI user group, but not the poorest performance (three of five subjects in the present study). Third, the similar performance between CI simulations and unprocessed conditions in NH listeners suggests that the acoustic cues in the envelope are adequate to support normal BMLD performance. These results have important implications for CI signal and neural processing and will be discussed next.
Figure 9.
Comparison of CI users to NH listeners with acoustic CI simulation. N0S0 thresholds are on the x-axis. N0Sπ thresholds are on the y-axis. Data points are from the 125 and 250 Hz stimulus conditions only. “O’s” are data from the five CI subjects. (Mean [N0S0; N0Sπ]=[6.6±2.8; 1.6±6.1] dB SNR.) “X’s” are data from six NH listeners using the Gaussian-enveloped tone vocoder. (Mean [N0S0; N0Sπ]=[4.0±4.1; −8.6±7.2] dB SNR.) “+’s” are data from six NH listeners with unprocessed stimuli. (Mean [N0S0; N0Sπ]=[1.6±3.5; −6.3±5.8] dB SNR.)
DISCUSSION
The purpose of this study was to establish BMLDs using an expanded set of electrodes and signal frequencies in order help understand why BMLDs are limited in bilateral CI users compared to NH listeners. The main findings of this study are that (1) BMLDs were measurable at multiple electrode locations for various signal∕masker conditions, (2) BMLDs were more likely to be observed for the 125 Hz signal condition than for 250 and 500 Hz, (3) MLDs obtained through monaural testing were insufficient to account for BMLD, and (4) NH listeners using an acoustic CI simulation performed better than CI users, particularly with the N0Sπ condition.
BMLDs and the related phenomenon of noise squelch in bilateral CI users have been reported in just a handful of studies (Nopp et al., 2004; Laszig et al., 2004; van Hoesel, 2004; Long et al. 2006; Buss et al., 2008; van Hoesel et al., 2008), and of these, only a few groups have investigated BMLDs using precisely controlled stimulation via single pairs of pitch-matched electrodes that are temporally synchronized through a research interface (van Hoesel, 2004; Long et al., 2006; van Hoesel et al., 2008) as opposed to using the patient’s own pair of clinical processors (Laszig et al., 2004; Schleich et al., 2004; Buss et al., 2008). The average BMLD obtained in this study, 4.6±4.9 dB falls within the range of BMLDs (1.5–9 dB) reported in literature for bilateral CI users (van Hoesel, 2004; Long et al., 2006; van Hoesel et al., 2008).
Although the stimuli used in this experiment followed that of Long et al. (2006), which reported an average BMLD of 9 dB, the data from this study’s subject pool displayed considerably more variability. Closer inspection of the data in Long et al., 2006 reveals that average thresholds (as opposed to BMLDs) were higher in this study (Fig. 9). N0S0 threshold was 6.6 dB compared to 3 dB in Long et al., 2006, and N0Sπ threshold was 1.6 dB compared to −6 dB. Individually, data from CI1 and CI5 were most similar to those in Long et al., 2006 where three of four subjects had N0S0 thresholds greater than 0 dB and N0Sπ thresholds near −9 dB. Their consistent performance over most electrode and signal frequency conditions suggests that binaural unmasking can be a useful mechanism for signal detection in noise. The smaller BMLD values from the other subjects, CI2–CI4, contributed to the lack of statistical significance (p=0.09) of BMLD over all stimulation conditions and underscore the fact that binaural unmasking may be of limited or no benefit to some bilateral CI users.
In comparing BMLDs with tones and speech, one methodological consideration is that, rather than using an out-of-phase signal as was done in both Long et al., 2006 and the present study, the speech stimuli in van Hoesel et al., 2008 were delayed by 700 μs, which may have contributed to a smaller unmasking effect. For a 125 Hz tone, 700 μs corresponds to a 31.5° phase shift. An out-of-phase signal maximizes the difference between two ears since sin(x)−sin(x+180°)≤2. In addition, since BMLDs for higher frequencies (250, 500 Hz) in this study were not statistically significant, having those higher frequencies present in speech would may not result in observable BMLDs for bilateral CI users. It remains unclear exactly how much contribution 700 μs makes as it is only one of multiple factors that could potentially impact BMLDs for speech.
Effect of signal frequency
If the analysis was restricted to just the 125 Hz signal condition (the only tested frequency in Long et al., 2006), the effect of signal phase was statistically significant (p=0.033). Because the higher signal frequencies were not tested in Long et al., 2006 it is unknown how their subjects would have performed at 250 and 500 Hz, although it could be speculated that BMLD levels may be similar to CI1 and CI5. With CI1 and CI5, BMLDs were observed for most electrode pair and signal frequency combinations, while two others had BMLDs for only a few combinations, which included 125 Hz. This suggests that the lower signal frequency may be more useful to bilateral CI users as a group, while some individuals could experience binaural unmasking at higher signal frequencies.
The limited BMLD at higher signal frequencies may be related to synchronous activation over broad regions at 300 Hz compared to lower rates (van Hoesel et al., 2009), or poor temporal discrimination of signals above 300 Hz (Zeng, 2002). The same temporal limitations apply even when the stimuli are presented diotically (Carlyon et al., 2008). Although the auditory nerves show highly synchronized responses to electrical stimulation (Hartmann et al., 1984), it is unclear where this temporal limitation is generated along the auditory pathway. If the temporal features are lost before the CI user’s auditory system can process the binaural signal, smaller BMLDs could result. In comparison, NH listeners have BMLDs near 10 dB at frequencies as high as 4 kHz (van de Par and Kohlrausch, 1997) as well as larger BMLDs for speech (Levitt and Rabiner, 1967). As speech has a broad spectral composition, the smaller BMLDs of CI users at higher signal frequencies may also help to explain smaller speech BMLDs.
Effect of electrode position
BMLDs were also analyzed by electrode position to look for any systematic changes with cochlear region. Although there was a significant effect of cochlear region on thresholds, there was no significant interaction between phase and electrode on thresholds for the 125 Hz condition, meaning that BMLD was not statistically different between electrodes. This indicated that cochlear region was likely not a limiting factor for speech BMLD. The data presented here showed that in at least two subjects, BMLDs were observed at all basal, middle, and apical pairs and could be 10 dB or higher. Since there was no significant effect of electrode position on BMLD, it follows that binaural unmasking was not limited by cochlear region. This result is consistent with NH listeners exhibiting binaural unmasking to the signal in envelope of a high frequency carrier (van de Par and Kohlrausch, 1997).
Peripheral mechanisms affecting BMLD
As demonstrated by the present data and prior studies, binaural unmasking can be observed in bilateral CI users, but even the best CI performers in these studies had BMLD values that fell below the 15–20 dB reported for NH listeners (van de Par and Kohlrausch, 1997). This could be due to either inadequate delivery of binaural cues to the peripheral nervous system, inadequate processing by the central nervous system, or a combination of both. It is thus important to consider what underlying mechanisms could be responsible for this performance gap.
The first possibility is that acoustic cues in the envelope are not sufficient to convey binaural information about N0S0 and N0Sπ. However, this is unlikely since NH listeners using the CI simulation had thresholds that were nearly the same as in the unprocessed condition (see Fig. 9). In NH listeners, BMLDs for both the unprocessed and transposed tones were nearly the same, but a reduction of about 6 dB for the transposed tone was noted for a 250 Hz signal (van de Par and Kohlrausch, 1997). In order for the results to be comparable, there must be sufficient acoustic cues remaining in the envelope since fine timing information is discarded by the CI simulation. Despite the same envelope extraction of the signal, actual CI users had higher thresholds than simulated CI, particularly with N0Sπ. For the unprocessed condition, the NH group had BMLD values lower than in literature. Some of this may be due to the minimal training and inexperience of the subjects, compared to those in van de Par and Kohlrausch, 1997, most of whom were reported to have experience in masking experiments.
A second possible mechanism is that the CI processor cannot adequately convey the acoustic cues in the envelope in time or amplitude. Under these experimental conditions, the pulse rate was set at 1000 and 2000 pps, which was fast enough to represent the signal frequencies used in this study. At high pulse rates (800 pps) ITD sensitivity to the pulses is very poor (van Hoesel and Tyler, 2003), and although this may contribute to some drop in performance, the cues in this study were all based on the envelope rather than in the interaural pulse timing.
A third possibility related to mechanism lies in the electrode-neural interface, such as the availability of suitably pitch-matched electrodes and effects of channel interactions. Because electrode array insertions are rarely identical, electrically elicited pitches are often offset between the two sides. The CI users tested here, and CI1, in particular, commented that when alternately switching between their own left and right processors they perceived there to be a noticeable pitch difference. Although pitch-matching was not directly addressed by the experiments in the present study, it has been shown psychophysically (Henning, 1974; Nuetzel and Hafter, 1981) and physiologically (e.g., Blanks et al., 2007) that the binaural system is most effective when the frequency of sounds between the left and right are nearly the same. In fact, in normal hearing listeners, ITD judgments deteriorate with mismatch in carrier frequency (Henning, 1974; Nuetzel and Hafter, 1981). For CI users, pitch-matched pairs of electrodes are necessary for ITD sensitivity (van Hoesel and Clark, 1997; Lawson et al., 1998; Long et al., 2003) and BMLD (Long et al., 2007).
Since it appeared that envelope was sufficient for transmitting cues for BMLD in this study, we examined whether there were others cues that were contributing to BMLD values. Despite the poor ability to discriminate modulation frequencies (Zeng, 2002), CI users can detect temporal fluctuations occurring up to 4000 Hz (Shannon, 1992). Measuring monaural performance can help to reveal the contribution of binaural processing. To be able to detect the signal in noise under these conditions puts more emphasis on temporal discrimination since a single channel was used and no binaural cues were available. Because MLDs were not significant in this study, BMLDs measured here likely result from true binaural hearing rather than independent and monaural discrimination of envelopes by each ear. Only when both ears were used was there a significant difference in threshold. Since the noise masker was randomly generated for each trial, there is no practical difference between NmSm and NmS-m.
Central auditory mechanisms affecting BMLD: Auditory deprivation
The issues discussed so far have been related to technical limitations of the cochlear implant system in processing and delivering binaural stimuli rather than the central auditory mechanisms that underlie binaural sensitivity. Even if normal peripheral processing could be restored in CI users, the central auditory system can still fail to effectively process binaural information. Both the limitations of CI users to discriminate high modulation frequencies (Zeng, 2002) and poor ITD sensitivity (van Hoesel et al., 2002; van Hoesel, 2007; Laback and Majdak, 2008; Litovsky et al., 2010) are examples where central auditory processing showed deficits despite temporally precise delivery of electrical stimuli to the auditory periphery.
In comparing NH listeners using simulations to actual CI users, there are two assumptions to be made about the simulation and the comparison. First is that the vocoder is an accurate simulation of what CI users hear. Gaussian-enveloped tones have not been used in the context of a vocoder, but they have been used for psychophysical, including binaural, experiments (Buell and Hafter, 1988; van den Brink and Houtgast, 1990). Although it would be possible to conduct a similar experiment with a one-channel noise-excited vocoder using band-limited noise, the Gaussian-enveloped tone vocoder may be a better choice. With a noise-excited vocoder, the carrier is noise. There may be interactions between noise in the carrier and any masking noise present in the envelope, particularly in the N0S0 condition. That is, the noise in the carrier may contribute to noise in the envelope such that for normal hearing listeners using the noise vocoder, this becomes a more difficult task without contributing additional insight to BMLD performance. The Gaussian tone pulses do not have this issue as the fine-structures of the tones are controlled and the amplitudes at each time point are defined algorithmically and not influenced by stochastic fluctuations in a noise carrier. For comparison, the N0S0 thresholds were higher for the noise vocoder than it was for the Gaussian-enveloped tone. Although the noise vocoder was implemented with broadband noise, the problem using a band-limited signal to simulate a specific channel remains the same since there is still an issue of a noise carrier interacting with masking noise in the envelope.
If the validity of the vocoder as a simulation is accepted, then it can be assumed that the peripheral auditory systems of NH and CI are receiving equivalent inputs. Therefore, performance differences between the two groups will depend on their ability to process binaural information. With an acoustic CI simulation, differences between NH and CI not explained by pitch-matching and channel interaction may point toward the central auditory system. As a population, the thresholds of CI users lagged behind NH listeners by nearly 10 dB for N0Sπ and but only 2.4 dB for N0S0. This suggests that the binaural performance difference may be in part due to physiological limitations of processing binaural information in the central auditory system. BMLDs for unprocessed stimuli reported here are smaller than those reported in literature (van de Par and Kohlrausch, 1997). Individually, at least one subject (NH4) had a BMLD of 13.9 dB for the unprocessed stimuli. The lower scores may be attributable to the inexperience of the test subjects (most were undergraduate students) compared to the more experienced subjects in van de Par and Kohlrausch, 1997, all but one of whom were indicated as laboratory colleagues.
Examining the clinical data, the best performers in this study, CI1 and CI5, had the longest experience with bilateral CI use and had prior experience with research testing. Their better performance would be consistent with having a longer recovery of binaural function (Laszig et al., 2004; Buss et al., 2008). The small BMLD values of CI2 may be related to the early onset of hearing impairment. CI3 had late onset of hearing loss, and only 1 year less bilateral experience than CI1 and CI5. Based on this subject’s clinical history larger BMLDs would have been expected. CI4 had 3 years of bilateral CI experience. There was some difficulty in finding suitably pitch-matched electrode pairs as noted by the right electrode being matched to apical and middle electrodes on the left over the course of the testing. Although the clinical data do appear to be on first inspection consistent with the BMLD levels of the subjects, due to the small sample size, the various causes of deafness, hearing aid history, and duration of implant use, it would be difficult to predict BMLD performance with any confidence from duration of deafness from the clinical data.
Future directions
Despite the large variability between subjects, BMLDs from these single pairs of electrodes (4.6 dB average, and ∼9 dB for the top two performers) were still larger than BMLDs for speech (<1.5 dB) reported in CI. The data presented here thus do not fully account for this performance gap. One direction that should be taken is to evaluate BMLDs with multiple and simultaneous masking electrodes. Would the binaural cues presented in one pair of electrode be sufficiently degraded by adjacent masking electrodes such that BMLDs are reduced? Addressing this issue would help to answer to what degree channel interactions affect binaural hearing and provide some clue as to what other factors are limiting BMLD in CI.
CONCLUSION
BMLDs were measured in five bilateral CI subjects using the Spear3 research processor. Stimuli were delivered though pitch-matched and loudness-balanced electrode pairs. The cochlear position of the electrode pair was varied as was the signal frequency. Normal hearing listeners were tested with unprocessed and acoustic CI simulations. The conclusions are as follows.
-
(1)
Average BMLD over all stimulus conditions was 4.6±4.9 dB. Significant BMLDs were observed in at least one electrode pair and signal frequency condition for four out of five subjects, with BMLDs varying no more than 3–5 dB over electrode pairs and stimulus conditions. Performance variability was high between subjects and average BMLD values per subject ranged from 1.2 to 10.7 dB;
-
(2)
For the subject group in this study, the effects of phase and electrode pair were significant when considering only the 125 Hz signal frequency condition, as in a prior study, suggesting that BMLDs are more likely to be observed for lower signal frequencies.
-
(3)
Monaurally detectable differences in NmSm and NmS-m were not sufficient to account for BMLDs.
-
(4)
NH listeners had similar unprocessed and simulated CI thresholds for N0S0 and N0Sπ. Actual CI users had higher thresholds than NH with simulated CI, but the difference was more pronounced for N0Sπ, implying a central binaural processing deficit.
The present results support prior studies that demonstrate binaural processing in CI users. However, a performance gap in BMLD still exists between even the best CI users in this study and NH listeners and may be related to a peripheral mechanism such as pitch mismatch, a central mechanism in a deprived auditory system, or a combination of both. The differences in actual and simulated CI performances point to a deficit in true binaural hearing that may have resulted from long-term deprivation of the auditory system.
ACKNOWLEDGMENTS
The authors would like to thank J. Yuan, M. Camilon, and P. Lin for assistance with data collection. This work was supported by NIH Grant Nos. 5 R01 DC003083 (R.L.) and P30 DC008369 (F.-G.Z.).
References
- Blanks, D. A., Roberts, J. M., Buss, E., Hall, J. W., and Fitzpatrick, D. C. (2007). “Neural and behavioral sensitivity to interaural time differences using amplitude modulated tones with mismatched carrier frequencies,” J. Assoc. Res. Otolaryngol. 8, 393–408. 10.1007/s10162-007-0088-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buell, T. N., and Hafter, E. R. (1988). “Discrimination of interaural differences of time in the envelopes of high-frequency signal: Integration times,” J. Acoust. Soc. Am. 84, 2063–2066. 10.1121/1.397050 [DOI] [PubMed] [Google Scholar]
- Buss, E., Pillsbury, H. C., Buchman, C. A., Pillsbury, C. H., Clark, M. S., Haynes, D. S., Labadie, R. F., Amberg, S., Roland, P. S., Kruger, P., Novak, M. A., Wirth, J. A., Black, J. M., Peters, R., Lake, J., Wackym, P. A., Firszt, J. B., Wilson, B. S., Lawson, D. T., Schatzer, R., D’Haese, P. S., and Barco, A. L. (2008). “Multicenter U.S. bilateral MED-EL cochlear implantation study: Speech perception over the first year of use,” Ear Hear. 29, 20–32. [DOI] [PubMed] [Google Scholar]
- Carlyon, R. P., Long, C. J., and Deeks, J. M. (2008). “Pulse-rate discrimination by cochlear-implant and normal-hearing listeners with and without binaural cues,” J. Acoust. Soc. Am. 123, 2276–2286. 10.1121/1.2874796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durlach, N. I. (1963). “Equalization and cancellation theory of binaural masking-level differences,” J. Acoust. Soc. Am. 35, 1206–1218. 10.1121/1.1918675 [DOI] [Google Scholar]
- Gray, L., Kesser, B., and Cole, E. (2009). “Understanding speech in noise after correction of congenital unilateral aural atresia: Effects of age in the emergence of binaural squelch but not in use of head-shadow,” Int. J. Pediatr. Otorhinolaryngol. 73, 1281–1287. 10.1016/j.ijporl.2009.05.024 [DOI] [PubMed] [Google Scholar]
- Greenwood, D. D. (1990). “A cochlear frequency-position function for several species—29 years later,” J. Acoust. Soc. Am. 87, 2592–2605. 10.1121/1.399052 [DOI] [PubMed] [Google Scholar]
- Hall, J. W., Buss, E., Grose, J. H., and Dev, M. B. (2004). “Developmental effects in the masking-level difference,” J. Speech Lang. Hear. Res. 47, 13–20. 10.1044/1092-4388(2004/002) [DOI] [PubMed] [Google Scholar]
- Hall, J. W., III, and Grose, J. H. (1993). “Short-term and long-term effects on the masking level difference following middle ear surgery,” J. Am. Acad. Audiol. 4, 307–312. [PubMed] [Google Scholar]
- Hartmann, R., Topp, G., and Klinke, R. (1984). “Discharge patterns of cat primary auditory fibers with electrical stimulation of the cochlea,” Hear. Res. 13, 47–62. 10.1016/0378-5955(84)90094-7 [DOI] [PubMed] [Google Scholar]
- Henning, G. B. (1974). “Detectability of interaural delay in high-frequency complex waveforms,” J. Acoust. Soc. Am. 55, 84–90. 10.1121/1.1928135 [DOI] [PubMed] [Google Scholar]
- Hinojosa, R., and Lindsay, J. R. (1980). “Profound deafness. Associated sensory and neural degeneration,” Arch. Otolaryngol. 106, 193–209. [DOI] [PubMed] [Google Scholar]
- Hirsh, I. J. (1948). “Binaural summation and interaural inhibition as a function of the level of masking noise,” Am. J. Psychol. 61, 205–213. 10.2307/1416966 [DOI] [PubMed] [Google Scholar]
- Hogan, S. C., Meyer, S. E., and Moore, D. R. (1996). “Binaural unmasking returns to normal in teenagers who had otitis media in infancy,” Audiol. Neuro-Otol. 1, 104–111. 10.1159/000259189 [DOI] [PubMed] [Google Scholar]
- Laback, B., and Majdak, P. (2008). “Binaural jitter improves interaural time-difference sensitivity of cochlear implantees at high pulse rates,” Proc. Natl. Acad. Sci. U.S.A. 105, 814–817. 10.1073/pnas.0709199105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laszig, R., Aschendorff, A., Stecker, M., Muller-Deile, J., Maune, S., Dillier, N., Weber, B., Hey, M., Begall, K., Lenarz, T., Battmer, R. D., Bohm, M., Steffens, T., Strutz, J., Linder, T., Probst, R., Allum, J., Westhofen, M., and Doering, W. (2004). “Benefits of bilateral electrical stimulation with the nucleus cochlear implant in adults: 6-month postoperative results,” Otol. Neurotol. 25, 958–968. 10.1097/00129492-200411000-00016 [DOI] [PubMed] [Google Scholar]
- Lawson, D. T., Wilson, B. S., Zerbi, M., van den Honert, C., Finley, C. C., Farmer, J. C., Jr., McElveen, J. T., Jr., and Roush, P. A. (1998). “Bilateral cochlear implants controlled by a single speech processor,” Am. J. Otol. 19, 758–761. [PubMed] [Google Scholar]
- Levitt, H. (1971). “Transformed up-down methods in psychoacoustics,” J. Acoust. Soc. Am. 49, 467–477. 10.1121/1.1912375 [DOI] [PubMed] [Google Scholar]
- Levitt, H., and Rabiner, L. R. (1967). “Predicting binaural gain in intelligibility and release from masking for speech,” J. Acoust. Soc. Am. 42, 820–829. 10.1121/1.1910654 [DOI] [PubMed] [Google Scholar]
- Litovsky, R., Parkinson, A., Arcaroli, J., and Sammeth, C. (2006a). “Simultaneous bilateral cochlear implantation in adults: A multicenter clinical study,” Ear Hear. 27, 714–731. 10.1097/01.aud.0000246816.50820.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovsky, R. Y. (1997). “Developmental changes in the precedence effect: Estimates of minimum audible angle,” J. Acoust. Soc. Am. 102, 1739–1745. 10.1121/1.420106 [DOI] [PubMed] [Google Scholar]
- Litovsky, R. Y., Johnstone, P. M., Godar, S., Agrawal, S., Parkinson, A., Peters, R., and Lake, J. (2006b). “Bilateral cochlear implants in children: Localization acuity measured with minimum audible angle,” Ear Hear. 27, 43–59. 10.1097/01.aud.0000194515.28023.4b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovsky, R. Y., Jones, G. L., Agrawal, S., and van Hoesel, R. (2010). “Effect of age at onset of deafness on binaural sensitivity in electric hearing in humans,” J. Acoust. Soc. Am. 127, 400–414. 10.1121/1.3257546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovsky, R. Y., Parkinson, A., and Arcaroli, J. (2009). “Spatial hearing and speech intelligibility in bilateral cochlear implant users,” Ear Hear. 30, 419–431. 10.1097/AUD.0b013e3181a165be [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovsky, R. Y., Parkinson, A., Arcaroli, J., Peters, R., Lake, J., Johnstone, P., and Yu, G. (2004). “Bilateral cochlear implants in adults and children,” Arch. Otolaryngol. Head Neck Surg. 130, 648–655. 10.1001/archotol.130.5.648 [DOI] [PubMed] [Google Scholar]
- Long, C. J., Carlyon, R. P., and Litovsky, R. Y. (2007). “Binaural unmasking with ‘transposed’ stimuli in bilateral cochlear implant users,” Conference on Implantable Auditory Prosthesis, Granlibakken, Tahoe City, CA (abstract).
- Long, C. J., Carlyon, R. P., Litovsky, R. Y., and Downs, D. H. (2006). “Binaural unmasking with bilateral cochlear implants,” J. Assoc. Res. Otolaryngol. 7, 352–360. 10.1007/s10162-006-0049-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, C. J., Eddington, D. K., Colburn, H. S., and Rabinowitz, W. M. (2003). “Binaural sensitivity as a function of interaural electrode position with a bilateral cochlear implant user,” J. Acoust. Soc. Am. 114, 1565–1574. 10.1121/1.1603765 [DOI] [PubMed] [Google Scholar]
- Lu, T., Carroll, J., and Zeng, F. -G. (2007). “On acoustic simulations of cochlear implants,” Conference on Implantable Auditory Prostheses (abstract), Lake Tahoe, CA, July 15–20.
- Müller, J., Schon, F., and Helms, J. (2002). “Speech understanding in quiet and noise in bilateral users of the MED-EL COMBI 40/40+ cochlear implant system,” Ear Hear. 23, 198–206. 10.1097/00003446-200206000-00004 [DOI] [PubMed] [Google Scholar]
- Neuman, A. C., Haravon, A., Sislian, N., and Waltzman, S. B. (2007). “Sound-direction identification with bilateral cochlear implants,” Ear Hear. 28, 73–82. 10.1097/01.aud.0000249910.80803.b9 [DOI] [PubMed] [Google Scholar]
- Nopp, P., Schleich, P., and D’Haese, P. (2004). “Sound localization in bilateral users of MED-EL COMBI 40/40+ cochlear implants,” Ear Hear. 25, 205–214. 10.1097/01.AUD.0000130793.20444.50 [DOI] [PubMed] [Google Scholar]
- Nuetzel, J. M., and Hafter, E. R. (1981). “Discrimination of interaural delays in complex waveforms: Spectral effects,” J. Acoust. Soc. Am. 69, 1112–1118. 10.1121/1.385690 [DOI] [Google Scholar]
- Schleich, P., Nopp, P., and D’Haese, P. (2004). “Head shadow, squelch, and summation effects in bilateral users of the MED-EL COMBI 40/40+ cochlear implant,” Ear Hear. 25, 197–204. 10.1097/01.AUD.0000130792.43315.97 [DOI] [PubMed] [Google Scholar]
- Shannon, R. V. (1992). “Temporal modulation transfer functions in patients with cochlear implants,” J. Acoust. Soc. Am. 91, 2156–2164. 10.1121/1.403807 [DOI] [PubMed] [Google Scholar]
- Silman, S., Gelfand, S. A., and Silverman, C. A. (1984). “Late-onset auditory deprivation: Effects of monaural versus binaural hearing aids,” J. Acoust. Soc. Am. 76, 1357–1362. 10.1121/1.391451 [DOI] [PubMed] [Google Scholar]
- Spahr, A. J., and Dorman, M. F. (2004). “Performance of subjects fit with the Advanced Bionics CII and Nucleus 3G cochlear implant devices,” Arch. Otolaryngol. Head Neck Surg. 130, 624–628. 10.1001/archotol.130.5.624 [DOI] [PubMed] [Google Scholar]
- Stickney, G. S., Zeng, F. G., Litovsky, R., and Assmann, P. (2004). “Cochlear implant speech recognition with speech maskers,” J. Acoust. Soc. Am. 116, 1081–1091. 10.1121/1.1772399 [DOI] [PubMed] [Google Scholar]
- Tyler, R. S., and Summerfield, A. Q. (1996). “Cochlear implantation: Relationships with research on auditory deprivation and acclimatization,” Ear Hear. 17, 38S–50S. 10.1097/00003446-199617031-00005 [DOI] [PubMed] [Google Scholar]
- van de Par, S., and Kohlrausch, A. (1997). “A new approach to comparing binaural masking level differences at low and high frequencies,” J. Acoust. Soc. Am. 101, 1671–1680. 10.1121/1.418151 [DOI] [PubMed] [Google Scholar]
- van den Brink, W. A., and Houtgast, T. (1990). “Spectro-temporal integration in signal detection,” J. Acoust. Soc. Am. 88, 1703–1711. 10.1121/1.400245 [DOI] [PubMed] [Google Scholar]
- Van Deun, L., van Wieringen, A., Francart, T., Scherf, F., Dhooge, I. J., Deggouj, N., Desloovere, C., Van de Heyning, P. H., Offeciers, F. E., De Raeve, L., and Wouters, J. (2009b). “Bilateral cochlear implants in children: Binaural unmasking,” Audiol. Neuro-Otol. 14, 240–247. 10.1159/000190402 [DOI] [PubMed] [Google Scholar]
- Van Deun, L., van Wieringen, A., Van den Bogaert, T., Scherf, F., Offeciers, F. E., Van de Heyning, P. H., Desloovere, C., Dhooge, I. J., Deggouj, N., De Raeve, L., and Wouters, J. (2009a). “Sound localization, sound lateralization, and binaural masking level differences in young children with normal hearing,” Ear Hear. 30, 178–190. 10.1097/AUD.0b013e318194256b [DOI] [PubMed] [Google Scholar]
- van Hoesel, R. J. (2007) “Sensitivity to binaural timing in bilateral cochlear implant users,” J. Acoust. Soc. Am. 121, 2192–2206. 10.1121/1.2537300 [DOI] [PubMed] [Google Scholar]
- van Hoesel, R., Bohm, M., Pesch, J., Vandali, A., Battmer, R. D., and Lenarz, T. (2008). “Binaural speech unmasking and localization in noise with bilateral cochlear implants using envelope and fine-timing based strategies,” J. Acoust. Soc. Am. 123, 2249–2263. 10.1121/1.2875229 [DOI] [PubMed] [Google Scholar]
- van Hoesel, R. J. M., Jones, G. L., and Litovsky, R. Y. (2009). “Interaural time-delay sensitivity in bilateral cochlear implant users: Effects of pulse-rate, modulation-rate, and place of stimulation,” J. Assoc. Res. Otolaryngol. 10, 557–567. 10.1007/s10162-009-0175-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hoesel, R., Ramsden, R., and Odriscoll, M. (2002). “Sound-direction identification, interaural time delay discrimination, and speech intelligibility advantages in noise for a bilateral cochlear implant user,” Ear Hear. 23, 137–149. 10.1097/00003446-200204000-00006 [DOI] [PubMed] [Google Scholar]
- van Hoesel, R. J. (2004). “Exploring the benefits of bilateral cochlear implants,” Audiol. Neuro-Otol. 9, 234–246. 10.1159/000078393 [DOI] [PubMed] [Google Scholar]
- van Hoesel, R. J., and Clark, G. M. (1997). “Psychophysical studies with two binaural cochlear implant subjects,” J. Acoust. Soc. Am. 102, 495–507. 10.1121/1.419611 [DOI] [PubMed] [Google Scholar]
- van Hoesel, R. J., Tong, Y. C., Hollow, R. D., and Clark, G. M. (1993). “Psychophysical and speech perception studies: A case report on a binaural cochlear implant subject,” J. Acoust. Soc. Am. 94, 3178–3189. 10.1121/1.407223 [DOI] [PubMed] [Google Scholar]
- van Hoesel, R. J., and Tyler, R. S. (2003). “Speech perception, localization, and lateralization with bilateral cochlear implants,” J. Acoust. Soc. Am. 113, 1617–1630. 10.1121/1.1539520 [DOI] [PubMed] [Google Scholar]
- Verschuur, C. A., Lutman, M. E., Ramsden, R., Greenham, P., and O’Driscoll, M. (2005). “Auditory localization abilities in bilateral cochlear implant recipients,” Otol. Neurotol. 26, 965–971. 10.1097/01.mao.0000185073.81070.07 [DOI] [PubMed] [Google Scholar]
- Wilson, B. S., Finley, C. C., Lawson, D. T., Wolford, R. D., Eddington, D. K., and Rabinowitz, W. M. (1991). “Better speech recognition with cochlear implants,” Nature (London) 352, 236–238. 10.1038/352236a0 [DOI] [PubMed] [Google Scholar]
- Zeng, F. G. (2002). “Temporal pitch in electric hearing,” Hear Res. 174, 101–106. 10.1016/S0378-5955(02)00644-5 [DOI] [PubMed] [Google Scholar]
- Zeng, F. G., and Galvin, J. J., III (1999). “Amplitude mapping and phoneme recognition in cochlear implant listeners,” Ear Hear. 20, 60–74. 10.1097/00003446-199902000-00006 [DOI] [PubMed] [Google Scholar]