Abstract
The existence of relative pitch perception in animals is difficult to demonstrate, since unlike humans, animals often attend to absolute rather than relative properties of sound elements. However, the results of the present study show that ferrets can be trained using relative pitch to discriminate two-tone sequences (rising vs. falling). Three ferrets were trained using a positive-reinforcement paradigm in which sequences of reference (one to five repeats) and target stimuli were presented, and animals were rewarded only when responding correctly to the target. The training procedure consisted of three training phases that successively shaped the ferrets to attend to relative pitch. In Phase-1 training, animals learned the basic task with sequences of invariant tone-pairs and could use absolute pitch information. During Phase-2 training, in order to emphasize relative cues, absolute pitch was varied each trial within a two-octave frequency range. In Phase-3 training, absolute pitch cues were removed, and only relative cue information was available to solve the task. Two ferrets successfully completed training on all three phases and achieved significant discriminative performance over the trained four-octave frequency range. These results suggest that ferrets can be trained to discern the relative pitch relationship of a sequence of tone-pairs independent of frequency.
INTRODUCTION
A significant amount of information is encoded in the contour patterns (rises and falls) of the pitch of acoustic signals, such as in speech and music. For example, human subjects can easily recognize sentence type on the basis of pitch contour alone in the absence of other information (Ladefoged, 1982). The frequency transpositions of a melody are readily recognized by most adult, and even infant, human listeners as the “same” and are perceived as structural equivalents of the original melody (Dowling and Fujitani, 1971; Demany and Armand, 1984; Trehub et al., 1984; Trehub and Hannon, 2006). Although human listeners can remember the exact musical intervals of familiar melodies, they appear to remember only the frequency contour of less familiar or novel stimuli (Dowling and Fujitani, 1971; Dowling, 1978; Bartlett and Dowling, 1980). Unlike humans, who attend chiefly to the relationships between sound elements, animals more heavily weight the absolute frequency of sound elements in their perceptual decisions and appear to be less attentive to relative pitch changes. Consequently, it has been difficult to train animals to attend to the relative pitch between sound elements, as D’Amato (1988) concluded after extensive behavioral research on monkeys and rats.
Most studies conducted with nonhuman species, including several species of birds (Hulse and Cynx, 1985, 1986; Ratcliffe and Weisman, 1986; Dooling et al., 1987; Page et al., 1989; Cynx, 1993; Weisman et al., 2004) and monkeys (D’Amato and Salmon, 1984; D’Amato, 1988; Izumi, 2001, 2003; Brosch et al., 2004, 2006), suggest that animals generally encode absolute pitch and have rather limited abilities to recognize the relative pitch contours of tonal stimuli. Songbirds have been shown to learn a relative pitch strategy, recognizing an ordinal rule for tone sequences that rise or fall in frequency regardless of the absolute frequency components. However, when the sequences were shifted out of the trained frequency range, they lost the discrimination. It then required as many trials to acquire a new discrimination as they needed to learn in the original discrimination (Hulse and Cynx, 1985; Cynx, 1993). Furthermore, songbirds failed to learn relative pitch discrimination when the absolute pitch cues were removed from the training (Page et al., 1989), indicating the primacy of absolute pitch perception in these species. Although a frequency range constraint was also noted in nonhuman mammals (Izumi, 2001, 2003), there are now two studies indicating that some nonhuman mammalian species are capable of relative pitch as measured by octave generalizations—rhesus monkeys (Wright et al., 2000) and dolphins (Ralston and Herman, 1995).
In a recent behavioral study, Walker et al. (2009) successfully trained ferrets on a two-alternative forced choice task to discriminate sounds that were higher or lower in pitch than a reference sound. Since the reference sound remained constant throughout a given session, the animal could use absolute strategies to solve the task. However, the result might also suggest that ferrets can be trained to utilize relative pitch cues in sequential sounds. The goal of the present study was to develop a new animal model to study the neural mechanisms underlying auditory pattern categorization based on direction of pitch changes (pitch contours) of tone sequences, and more generally to understand the neural basis and correlates of recognition and discrimination of spectrotemporally complex sounds. A training procedure which gradually directed animals to attend to the relative pitch change of two-tone sequences (rising vs falling) has been successfully developed, and the present report provides evidence of ferrets’ capability to categorize tonal patterns solely on the basis of these two-tone step changes over the trained frequency range.
METHODS
Subjects
Three naïve female adult ferrets, weighing 600–900 g, were used in this behavioral study. The animals were trained on a positive-reinforcement operant paradigm with water as reward. The ferrets were placed on a water-control protocol on which they were typically trained 5 days per week and obtained ad libitum water over the weekend. On training days, animals received one or two training sessions (∼100 trials in each session to satiation). All procedures conformed to the NIH policy on experimental animal care and use and were approved by the IACUC of the University of Maryland.
Experimental apparatus
Ferrets were tested in a customized-design wire mesh training cage (8 in. width×15 in. depth×9 in. height) which was placed within a single-walled, sound attenuated chamber (IAC). A lick sensitive waterspout (1 in.×1.5 in.) stood 5 in. above the floor of the training cage. The waterspout was connected to a computer controlled water dispenser (Crist Instrument Co., Inc., Maryland, USA). A loudspeaker (Manger MSW, Germany) was positioned 10 cm in front of the cage, and the animal’s behavior was monitored by video camera.
Basic behavioral paradigm
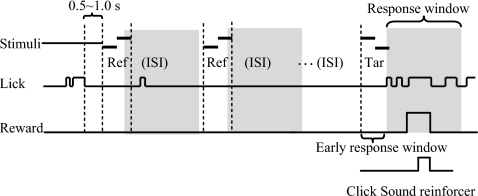
The positive-reinforcement operant paradigm used in these experiments differed substantially from previous behavioral studies in the laboratory that used a conditioning avoidance paradigm (Fritz et al., 2003). In the current study, ferrets were trained to lick a waterspout as the behavioral response to a target sound (unlike the conditioned avoidance paradigm, in which ferrets learned to refrain from ongoing licking when the target sound was presented). Each training session started with delivery of a drop of water (∼0.5 ml) to initiate licking of the waterspout. The first trial, and subsequent trials, began after the animal had consumed the water and then refrained from licking the spout for a minimum of 0.5 s, as illustrated in Fig. 1. A trial consisted of a sequence of one to five similar reference (non-target) sounds, followed by a different (target) sound. The inter-stimulus-interval between all references and target sound was 1.25 s. The animal was rewarded with a small drop of water when it licked the spout within a given time window after the target sound (last shaded area in Fig. 1). It received a 3–6 s timeout penalty if it did not lick during the target sound. The target reward drop volume (0.1–0.3 ml) was adjusted for each trial according to the licking pattern during the preceding reference stimuli. Specifically, the reward drop volume was inversely proportional to false-alarm rate of the trial in order to discourage licking during the reference stimuli. An additional click sound was played as a secondary “reinforcer” following water delivery during the early stages of training. The total number of reference stimuli presented in given trial varied from trial to trial and was selected from a pseudo-random sequence, in which there was an equiprobable likelihood that the target sound would be presented at each position in the sequence (from second to fifth position). A training session ended when the animal did not lick the spout in two consecutive trials.
Figure 1.
Positive-reinforcement operant paradigm. A trial was initiated when the animal refrained from licking the waterspout for 0.5–1.0 s. A reference sound (non-target) was presented and repeated randomly one to five times after trial initiation. A target sound followed the reference sounds. When the ferret licked the waterspout within a 1.2 s response time window after target onset (the shaded target period), its response was counted as a hit, which was followed by water reward. If the ferret licked the waterspout within a corresponding time window after reference sound onset (the shaded reference period), its response was counted as a false alarm, which caused reduction in reward volume. A miss of the target lead to a 3–6 s timeout penalty after completion of the trial. A click sound was played as a secondary reward reinforcer after water reward delivery.
Training procedure and stimuli
Training began with a 1–2 day habituation period during which animals were allowed to explore the training cage and learned to obtain water by licking the waterspout. Training was continued by a pre-training phase of approximately 2 weeks in which water delivery was associated with sounds.
All acoustic stimuli were two-tone sequences, 300 ms in duration, and ∼70 dB SPL. Each tone component in the two-tone sequence was 150 ms duration and was ramped with 5 ms rise-fall time. There was no silent gap between the tones. The frequency separation between the two tones was 1∕3 (Phase 1) or 1∕2 octaves (Phases 2 and 3).
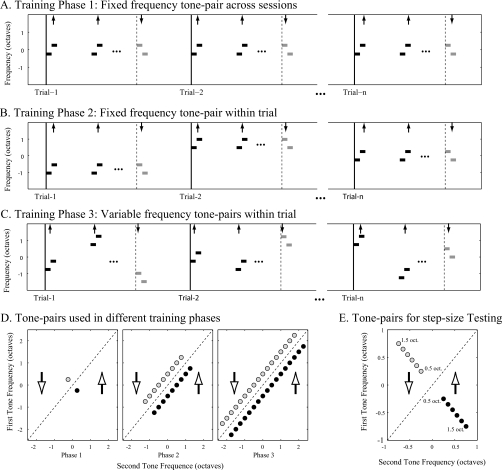
Two ferrets (H and J) were trained to discriminate the downward sequences (the target sequence) from the upward sequences (the reference sequence), and one ferret (M) was trained to discriminate upward sequences (the target sequence) from the downward sequences (the reference sequence). Animals underwent a three-phase training schedule [Figs. 2A, 2B, 2C] to be gradually directed to the final task requirements.
Figure 2.
Two-tone sequence discrimination task: Training procedures and stimuli sets. (A) Phase-1 training: The reference and target sequences were comprised of one tone-pair with fixed frequency across all training sessions. (B) Phase-2 training: The reference and target sequences for each trial were comprised of one tone-pair with fixed frequency, while the frequency of the tone-pair was changed randomly between successive trials. (C) Phase-3 training: Each of the reference or target sequences in a given trial was comprised of different tone-pairs for which the frequency of the tone-pair was chosen randomly from a set of 17 possible pairs. (D) Tone-pairs in different training phases: The rising and falling two-tone sequences were made from 1 tone-pair in Phase 1 (the left panel), 9 tone-pairs in Phase 2 (the middle panel), and up to 17 tone-pairs in Phase 3 (the right panel). The tone components of the two-tone sequence were 1∕2 octave apart in frequency, and the frequency contours of the sequences were roved up or down with 1∕4 octave increments. (E) Tone-pairs for step-size testing: The stimulus set varied the frequency separation between component tones, ranging from 0.5 up to 1.5 octaves. The solid vertical lines in (A)–(C) indicate the beginning of the trials. The vertical dashed lines indicate the target onset of each trial. The diagonal lines in (D) and (E) denote the iso-frequency line. The up and down arrows indicate directions of the reference and target sequence in (A)–(C) and of the quadrants in (D) and (E).
Initially, ferrets were trained with an easy version of the task (Phase-1 training), in which both the reference and target sequences were comprised of a fixed frequency tone-pair [the left panel in Fig. 2D]. The same tone-pair (with the same two tones arranged either upward or downward) was used during this entire training phase [Fig. 2A]. To perform the task, animals could either utilize the absolute frequencies of the tones (e.g., the initial and∕or terminal pitch of the sequence) and∕or relative properties of the tone sequence, specifically the direction of pitch change (rising vs falling). Once the behavioral criterion was achieved (see Sec. 2E), two animals received one or more additional tone-pairs in Phase-1 training. All animals then began Phase-2 training, in which the two-tone sequence for each trial consisted of a randomly picked tone-pair within a limited frequency range [Fig. 2B]. The frequency of the tone-pair was varied between trials over a frequency range that was gradually expanded up to two octaves around the initial frequency with 1∕4 octave increments, forming nine different rising or falling sequences at the end of this phase [the middle panel in Fig. 2D]. In the final stage, training Phase 3, all reference and target sequences within each trial were randomly chosen [Fig. 2C] so that the absolute frequency of the sequences varied, and the only fixed parameter was the direction of pitch change (rising or falling). The frequency range of the tone-pairs at this training stage was expanded to four octaves for a total of 17 rising or falling sequences [the right panel in Fig. 2D].
A different stimulus set was used to test the effect of the step-size of tone-pairs on the discriminative performance after completion of Phase-3 training. This stimulus set included six upward and six downward sequences, which were compromised of six tone-pairs whose frequencies were [1200 1697], [1120 1819], [1045 1949], [975 2089], [909 2239], and [849 2400] Hz, respectively. Therefore, the frequency separation (or interval) between component tones in those sequences varied from 0.5 to 1.5 octaves [see Fig. 2E].
Data analysis
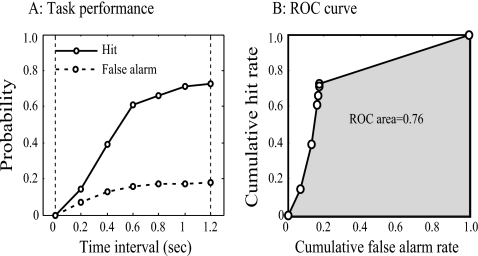
The timing of the first-lick after each of the references and target stimuli was recorded as the behavioral response to the stimulus on each trial. The task performance level was assessed by an analysis based on signal detection theory, in which both behavioral response accuracy and the behavioral response latency were exploited. The use of latency information in the analysis was useful in case of a difficult discrimination (Carterette et al., 1965; Emmerich et al., 1972) and also in obtaining a sufficient number of probability values for accurate determination of a receiver operating characteristic (ROC). A “first-lick” was defined as a hit or a false alarm depending on whether it was fallen in the response window following a target or non-target (the reference) sound (the shading zones after each sequence in Fig. 1). The probabilities of the hit (response after a target sound) and the false alarm (response after a non-target sound) were independently computed as function of the time intervals (from 0.0 to 1.2 s with an increment of 0.2 s) following the onset of the response window [Fig. 3A]. The ROC was then formed by the obtained probability functions (i.e., hit rate vs false-alarm rate function) [Fig. 3B]. The area under the ROC was taken as a measure of the task performance and was defined as the discriminative index (DI). This index yields a value of 0.5 for random performance, greater than 0.5 but less than 1.0 for nonrandom performance, and 1.0 for perfect performance. In each training session, the DI value was calculated from the original data and also from bootstrapped trials. In bootstrapped trials, the relationship between the behavioral responses (the First-lick time) and the stimulus tags (reference and target) was shuffled, and a shuffled-DI value was calculated. This process was repeated 50 times, and the mean value and the standard deviation of these shuffled-DI values were determined. A training session was considered to show significant discriminative performance if the obtained DI value was more than two standard deviations above the shuffled-DI mean. The behavioral criterion for achieving successful performance was defined as significant discriminative performance for a minimum of five consecutive training sessions. Animals could receive additional training on a given training phase after reaching criterion. Sessions with less than 40 trials were excluded from further analysis.
Figure 3.
Construction of ROC curve. (A) The probabilities for hit (solid line) and false alarm (dashed line) were independently computed at each of the time intervals from 0.0 to 1.2 s with 0.2 s increments following the onset of the response window after the target and reference. The vertical dashed lines indicate the start and the end of the response window. (B) The false-alarm probability function was plotted against hit probability function to construct the ROC curve (solid line). The area under the ROC curve (shaded area) was a measure of discriminative performance of the task.
RESULTS AND DISCUSSIONS
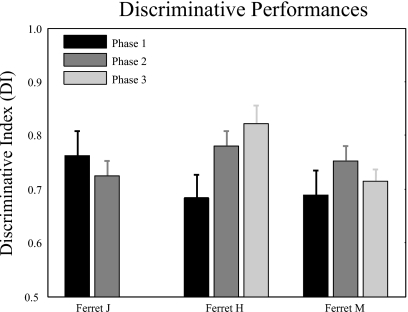
Three ferrets were trained on the two-tone discrimination task. Two of them completed all three phases of training. The third ferret was suspended from further training after completion of two phases (it was withdrawn from the study because it was suffering weight loss due to a severe and debilitating gastrointestinal infection). Figure 4 shows the mean performance of the ferrets across ten consecutive sessions after reaching behavioral criterion at each of the three training phases. All three animals reached behavioral criterion in the first two training phases and yielded mean DI values between 0.68–0.76 (M=0.71,s.d.=0.05) for Phase 1 and 0.72–0.77 (M=0.75,s.d.=0.03) for Phase 2. The performance of the two animals completing the final training phase (Phase 3) yielded DI values of 0.71 and 0.82, respectively (M=0.76,s.d.=0.08).
Figure 4.
Discriminative performances for all training phases. The bar plots show the average DI values, each of which was computed from ten consecutive sessions of Phase 1 (black), Phase 2 (dark gray), and Phase 3 (light gray) performances after reaching training criterion. The error bar indicates standard deviation.
Phase-1 training and frequency transposition
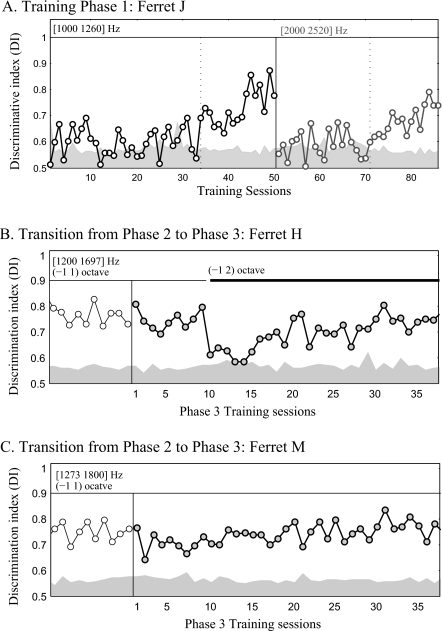
Training in Phase 1 took about 30–60 sessions before animals reached behavioral criterion. Since animals in Phase 1 could use either absolute or relative pitch to discriminate the target from reference sequences, if relative was being employed, then animals would easily generalize their training on the first tone-pair to a new tone-pair with different absolute frequencies. In order to test this conjecture, two ferrets were tested with a second tone-pair after learning the first tone-pair in Phase 1. The ferrets’ behavioral discrimination initially plummeted with the new tone-pair, and the animals performed at chance level. The example shown in Fig. 5A is from ferret J. In Phase 1, ferret J was trained on its first tone-pair (1000 and 1260 Hz) for 34 sessions until it reached behavioral criterion (the first vertical dashed line). Performance initially declined to a random level [the shaded area in Fig. 5A] when a new tone-pair (2000 and 2520 Hz) was introduced in Training Session 50. It took another 21 training sessions (the second vertical dashed line) to reach behavioral criterion for consistent performance for the second tone-pair, though ferret J learned this discrimination faster than for the first tone-pair. This behavioral pattern was tested and replicated in ferret M. These behavioral results, showing that the animals did not generalize to new tone-pair in Phase 1, indicate that the animals’ performance probably relied upon absolute pitch rather than relative pitch cues during Phase-1 training.
Figure 5.
The frequency transposition in Phase-1 training and the transition from Phase 2 to Phase 3. (A) The figure shows Phase-1 training data for ferret J. Phase-1 training was started (Training session=0) with a tone-pair at frequencies [1000 1260] Hz, and after learning the first pair, training was continued (Training session=50) with the second tone-pair at frequencies [2000 2520] Hz (the vertical solid line). [(B) and (C)] The animals maintained a high performance level when moved from Phase 2 (open circles) into Phase 3 (filled circles) when training within the same frequency range (indicated by the thin horizontal line on the top of each figure). In (B) the starting frequency range varied one octave above and below the initial frequency of the tone-pair [1200, 1697] Hz. Performance deteriorated when the frequency range of possible tone-pairs was extended an additional octave to two octaves above the initial tone-pair [indicated by the thick horizontal line in (B)] and regained after additional Phase-3 training. In (C) there was no change in frequency range during the transition from Phase 2 to Phase 3. The shaded area in (A)–(C) indicate the baseline performance (mean plus two standard deviation of the shuffled-DIs). A DI value above those dashed lines indicates a significant discriminative performance.
Transitioning from Phase 1 to Phase 2 and Phase 3: Learning relative pitch
Training in Phase 2 started after animals reached behavioral criterion on one (ferret H) or more tone-pairs (ferrets M and J) during Phase 1. In Phase-2 sessions, the reference and target stimuli in each trial consisted of upward and downward versions of the same tone-pair (randomly chosen for each trial from a small set of tone-pairs) that remained constant throughout a given trial [see Fig. 2B]. The training began with a set of three tone-pairs near the frequencies of the last tone-pair in Phase-1 training. The number of tone-pairs used in Phase-2 training sessions was gradually expanded up to nine tone-pairs, spanning a two-octave frequency range [see the middle panel in Fig. 2D]. All three ferrets reached significant discriminative performance within the two-octave frequency range after 24 (ferret H), 68 (ferret M), or 77 (ferret J) training sessions. Since the absolute frequencies of tone-pairs in the reference and target sequence of a given trial changed on a trial-by-trial basis, using absolute cues (as in Phase 1) was no longer an efficient strategy for task performance. It is more likely that animals used relative pitch to solve the task than the alternative that the animals memorized the absolute cues and responses for each of the nine possible tone-pairs.
Two ferrets (H and M) progressed to Phase-3 training, in which each reference and target sequence in a trial was randomly chosen from a set of up to 17 tone-pairs which spanned four octaves [see Fig. 2C and the right panel in Fig. 2D]. Both animals maintained a significant discriminative performance when transitioning from Phase-2 to Phase-3 training, as shown in Figs. 5B, 5C. These results indicate that the ferrets probably used the relative frequency contours of the sequence to solve the task in training Phase 2. However, even at this stage of training, animals did not generalize across all frequencies, and behavioral performance deteriorated when the frequency range of the tone-pairs expanded to a new frequency region, as indicated in Fig. 5B. Apparently, the animals had generalized their performance only in a two-octave frequency range which had been achieved during Phase-2 training, and hence additional training was necessary to extend the discriminative performance to a larger frequency range.
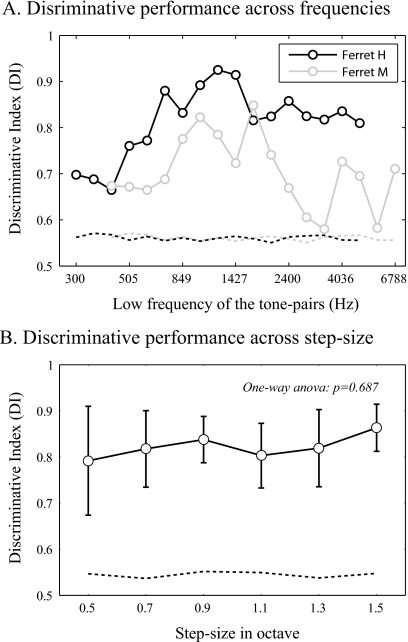
Both ferrets (H and M) achieved significant discriminative performance with a four-octave frequency range after additional Phase-3 training, as illustrated in Fig. 4 (the light gray bars show Phase-3 performance for each ferret) and Fig. 6A (the two curves show performance over the frequency range for each ferret). Figure 6A shows the ferrets’ discriminative performance for each of the tone-pairs after the animals reached performance criterion in Phase 3. In order to have sufficient behavioral data to compute the DI for each of the tone-pairs, the data in Fig. 6A were pooled from all ten sessions (the same data set as in Fig. 4). The significant discriminative performances were confirmed for all of the tone-pairs within the trained frequency range. The DIs from all tone-pairs within the four-octave frequency range [the lines with filled circle in Fig. 6A] were more than two standard deviations above the mean for shuffled-DIs [the dashed line in Fig. 6A]. The best performance was found at the tone-pairs in the middle frequency range for both ferrets. This result indicates that even though the ferrets could “partially” generalize the frequency contour categories within a wide frequency range after additional Phase-3 training, they were still subject to a frequency range constraint as in other nonhuman species (Hulse and Cynx, 1985; Izumi, 2001). Those profiles of behavioral performance across the frequency range might be explained by the training history of the animals, which showed a similar profile of experienced frequencies.
Figure 6.
Discriminative performance across frequencies and step-sizes of the tone-pairs. (A) Phase-3 data sets were the same as used in Fig. 4 for both ferrets M and H. The trials from all of those ten sessions were pooled together to compute the discriminative index for each of the tone-pairs. The significant discriminative performances were confirmed at all the tone-pairs within four-octave training frequency range. (B) Discriminative index at each frequency separation is represented as mean±standard deviation (N=6). There is no significant difference in discriminative performance across the frequency separations between the component tones of the sequence. The horizontal dashed lines in (A) and (B) indicate the baseline performance (mean plus two standard deviation of the shuffled-DIs). A DI value above those dashed lines indicates a significant discriminative performance.
The effect of frequency separation on task performance
In a separate set of experiments, the effects on task performance of the size of the frequency separation between the two component tones in the sequence were probed by a completely new set of two-tone sequences. As illustrated in Fig. 2E, this stimulus set had novel two-tone sequences with variable frequency separation (0.5–1.5 octaves). Each reference and target was randomly picked from the stimulus list. One ferret was tested with this stimulus set alternately with the fixed standard frequency separation of 1∕2 octave. There was no significant difference in behavioral performance across the different frequency separations [one-way ANOVA, p=0.687, Fig. 6B]. This result suggests that the animals did not attend to the interval size between the component tones in the sequence, but simply to the direction of pitch change of the two-tone sequence. This result was also seen in human subjects in melody recognitions. The recognition of randomly generated melodies (novel melodies) was dominated by contours, while for familiar melodies both the contours and intervals were critical for recognition (Dowling and Fujitani, 1971; Dowling, 1978).
GENERAL DISCUSSION
This study successfully demonstrated that ferrets are capable of relative pitch perception within a trained frequency range. With a three-phase training strategy, ferrets could learn to extract the relative pitch cue (the frequency contour) to discriminate between rising and falling two-tone sequences, independent of the absolute frequency of the tone sequence, over a four-octave range of frequencies.
During Phase-1 training, ferrets apparently used absolute pitch cues and discriminated target sequences from the reference sequences based on the initial or terminal pitch of the tone sequence. Transposition of the learned tone-pair to a different frequency caused a significant deterioration of performance, and animals needed to be retrained in order to master the new tone-pair. This result suggests that ferrets, like other nonhuman species, do not use relative pitch to solve contour discrimination tasks as a primary strategy, but are likely to use the absolute pitch of the tones in the sequence (particularly when the tone-pair frequencies are fixed).
However, ferrets were able to discriminate contour for multiple tone-pairs during Phase-2 training and appear to have learned to extract and utilize relative pitch cues in order to solve the task. Although the tone-pair frequencies remained constant within a given trial, and hence changes of the initial or ending pitch of the sequences could be used, ferrets appear to attend to the frequency contour within the sequence (relative cue) to solve the task. The behavioral evidence shown in Figs. 5B, 5C clearly indicates that performance on Phase 2 was very easily transferred to Phase 3, where the relative frequency contours were the only available cue. However, additional training was needed in Phase 3 in order to expand the frequency range over which the task was performed [indicated in Fig. 5B].
Sinnott et al. (1987) observed asymmetrical frequency discrimination in human subjects and some nonhuman primates and suggested that this asymmetrical sensitivity might relate to aspects of the species’ vocal communication signal. However, in D’Amato’s behavioral studies (reviewed in D’Amato, 1988), cebus monkeys and rats failed octave-generalization tests and also failed to demonstrate extraction of pitch contours. D’Amato concluded that perception of pitch contours requires specialized mechanisms that most animals lack and that “monkeys can’t hum a tune … because they don’t hear them” (D’Amato, 1988). This result was somewhat puzzling since birds and nonhuman primates are known to be capable of utilizing pitch cues and contours in vocal communication and recognition (Morton, 1977; Ratcliffe and Weisman, 1986; Weisman and Ratcliffe, 2004). In subsequent behavioral studies, rhesus monkeys were shown to generalize tonal melodies to whole one- and two-octave transpositions, but could not generalize over fractional transpositions, e.g., 0.5 octave or 1.5 octaves (Wright et al., 2000). These observations are very intriguing, but also raise additional questions since adult human subjects can generalize for both tonal and atonal melodies over both octave and non-octave transpositions (McDermott and Hauser, 2005), suggesting that there may be limitations in relative pitch in nonhuman primates. There is also some behavioral evidence for perception and generalization of frequency contours to octave transpositions in one bottlenose dolphin (Ralston and Herman, 1995), but these claims need replication and further study.
The failure of ferrets to fully generalize to untrained frequency ranges in the present study suggests that ferrets were also subject to a frequency range constraint as described in other nonhuman species, including songbirds, rats, and monkeys (D’Amato and Salmon, 1984; Hulse and Cynx, 1985, 1986; Dooling et al., 1987; Cynx, 1993; Wright et al., 2000; Izumi, 2001). These nonhuman species have been found to have difficulty generalizing contour categories to novel frequency ranges and contours. Ferrets, however, did acquire good discriminative performance in the new frequency range in just a few training sessions [Fig. 5B]. This acquisition was much faster than the case with songbirds that required as many trials as they had in learning the original discrimination (Hulse and Cynx, 1985; Cynx, 1993). The frequency range constraint revealed the extent of absolute pitch perception in relative pitch perception. Although the relationship between the relative and absolute pitch perception remains unclear, the failure to acquire relative discrimination when eliminating the absolute cues suggests that relative pitch in songbirds may depend on first identifying the patterns on the basis of their absolute pitches (Page et al., 1989). By contrast, in the present study, absolute pitch cues were available during only Phase-1 training, but not during the generalization training phases (Phases 2 and 3). Thus, animals learned the task with relative cues over a four-octave frequency range. These results indicate that ferrets, while not as good as humans, are somewhat better than songbirds in learning to utilize relative pitch cues.
Studies by Weisman et al. (1998) demonstrated that songbirds (even individuals reared in isolation) and parrots have highly accurate absolute pitch perception. In comparison, nonhuman mammals (such as rats) and humans exhibited only weak absolute pitch perception when classifying frequencies into ranges (Njegovan et al., 1995; Weisman et al., 1998, 2004). These findings lead Weisman to propose that there is a general difference in auditory processing in absolute and relative pitch perception between mammals (including humans) and songbirds (Weisman et al., 2004). The results in ferrets are consistent with this hypothesis. Along with other recent behavioral studies on birds and monkeys (Page et al., 1989; Wright et al., 2000; Izumi, 2001; Brosch et al., 2004), the present results provide evidence that animals can be trained to extract relative pitch when needed to perform a tonal pattern discrimination task.
CONCLUSIONS
The present study provides the evidence that ferrets can extract the tonal contour, independent of frequencies [Fig. 6A] and frequency separations [Fig. 6B] of the two-tone sequences. Appropriate task design and behavioral training procedures, such as the generalization training in Phase 2 in current study, are necessary to direct animal’s attention to the relational features of the sequences and to develop a relational solution of the task. Similarly, an early study on birds (Page et al., 1989) found that starlings extracted relative pitch from the pitch patterns only after acquiring a discrimination that permitted both absolute and relative pitch solutions. Although the natural tendency for animals may be to attend to the absolute properties of sound, the results of the present study suggest that they can still be trained to attend to relative pitch information.
ACKNOWLEDGMENT
This work was supported by R01DC005779 from NIDCD.
References
- Bartlett, J. C., and Dowling, W. J. (1980). “Recognition of transposed melodies: A key distance effect in development perspective,” J. Exp. Psychol. Hum. Percept. Perform. 6, 501–515. 10.1037/0096-1523.6.3.501 [DOI] [PubMed] [Google Scholar]
- Brosch, M., Oshurkova, E., Bucks, C., and Scheich, H. (2006). “Influence of tone duration and intertone interval on the discrimination of frequency contours in a macaque monkey,” Neurosci. Lett. 406, 97–101. 10.1016/j.neulet.2006.07.021 [DOI] [PubMed] [Google Scholar]
- Brosch, M., Selezneva, E., Bucks, C., and Scheich, H. (2004). “Macaque monkeys discriminate pitch relationships,” Cognition 91, 259–272. 10.1016/j.cognition.2003.09.005 [DOI] [PubMed] [Google Scholar]
- Carterette, E. C., Friedman, M. P., and Cosmides, R. (1965). “Reaction time distributions in the detection of weak signal in noise,” J. Acoust. Soc. Am. 38, 531–542. 10.1121/1.1909737 [DOI] [PubMed] [Google Scholar]
- Cynx, J. (1993). “Auditory frequency generalization and a failure to find octave generalization in a songbird, The European starling (Sturnus vulgaris),” J. Comp. Psychol. 107, 140–146. 10.1037/0735-7036.107.2.140 [DOI] [PubMed] [Google Scholar]
- D’Amato, M. R. (1988). “A search for tonal pattern perception in cebus monkeys: Why monkeys can’t hum a tune,” Music Percept. 5, 453–480. [Google Scholar]
- D’Amato, M. R., and Salmon, D. P. (1984). “Processing of complex auditory stimuli (tunes) by rats and monkeys (Cebus apella),” Anim. Learn Behav. 10, 126–134. [DOI] [PubMed] [Google Scholar]
- Demany, L., and Armand, F. (1984). “The perceptual reality of tone chroma in early infancy,” J. Acoust. Soc. Am. 76, 57–66. 10.1121/1.391006 [DOI] [PubMed] [Google Scholar]
- Dooling, R. J., Brown, S. D., Park, T. J., Okanoya, D., and Soli, S. D. (1987). “Perceptual organization of acoustic stimuli by budgerigars (Melopsittacus undulatus),” J. Comp. Psychol. 101, 139–149. 10.1037/0735-7036.101.2.139 [DOI] [PubMed] [Google Scholar]
- Dowling, W. J. (1978). “Scale and contour: Two components of a theory of memory for melodies,” Psychol. Rev. 85, 341–354. 10.1037/0033-295X.85.4.341 [DOI] [Google Scholar]
- Dowling, W. J., and Fujitani, D. A. (1971). “Contour, interval, and pitch recognition in memory for melodies,” J. Acoust. Soc. Am. 49, 524–531. 10.1121/1.1912382 [DOI] [PubMed] [Google Scholar]
- Emmerich, D. J., Gray, J., Watson, C., and Tanis, D. (1972). “Response latency confidence and ROCs in auditory signal detection,” Percept. Psychophys. 11, 65–72. [Google Scholar]
- Fritz, J. B., Shamma, S., Elhilali, M., and Klein, D. (2003). “Rapid task-related plasticity of spectrotemporal receptive fields in primary auditory cortex,” Nat. Neurosci. 6, 1216–1223. 10.1038/nn1141 [DOI] [PubMed] [Google Scholar]
- Hulse, S. H., and Cynx, J. (1985). “Relative pitch perception is constrained by absolute pitch in songbirds (Mimus, Molothrus and Sturnus),” J. Comp. Psychol. 99, 176–196. 10.1037/0735-7036.99.2.176 [DOI] [Google Scholar]
- Hulse, S. H., and Cynx, J. (1986). “Interval and contour in serial pitch perception by a passerine bird, The European starling (Sturnus vulgaris),” J. Comp. Psychol. 100, 215–228. 10.1037/0735-7036.100.3.215 [DOI] [Google Scholar]
- Izumi, A. (2003). “Effect of temporal separation on tone-sequence discrimination in monkeys,” Hear. Res. 175, 75–81. 10.1016/S0378-5955(02)00712-8 [DOI] [PubMed] [Google Scholar]
- Izumi, A. (2001). “Relative perception in Japanese monkeys (Macaca fuscata),” J. Comp. Psychol. 115, 127–131. 10.1037/0735-7036.115.2.127 [DOI] [PubMed] [Google Scholar]
- Ladefoged, P. (1982). A Course in Phonetics (Harcourt Brace Jovanovich, San Diego, CA: ). [Google Scholar]
- McDermott, J. H., and Hauser, M. (2005). “The origins of music: Innateness, uniqueness, and evolution,” Music Percept. 23, 29–59. 10.1525/mp.2005.23.1.29 [DOI] [Google Scholar]
- Morton, E. S. (1977). “On the occurrence and significance of motivation-structural rules in some bird and mammal sounds,” Am. Nat. 111, 855–869. 10.1086/283219 [DOI] [Google Scholar]
- Njegovan, M., Ito, S., Mewhort, D., and Weisman, R. (1995). “Classification of frequencies into ranges by songbirds and humans,” J. Exp. Psychol. Anim. Behav. Process 21, 33–42. 10.1037/0097-7403.21.1.33 [DOI] [PubMed] [Google Scholar]
- Page, S. H., Hulse, S. H., and Cynx, J. (1989). “Relative pitch perception in the starling (Sturnus vulgaris): Further evidence for an elusive phenomenon,” J. Exp. Psychol. Anim. Behav. Process 15, 137–146. 10.1037/0097-7403.15.2.137 [DOI] [PubMed] [Google Scholar]
- Ralston, J. V., and Herman, L. M. (1995). “Perception and generalization of frequency contours by a bottlenose dolphin (Tursiops truncatus),” J. Comp. Psychol. 109, 268–277. 10.1037/0735-7036.109.3.268 [DOI] [Google Scholar]
- Ratcliffe, L., and Weisman, R. G. (1986). “Song sequence discrimination in the black-capped chickadee (Parus atricapillus),” J. Comp. Psychol. 100, 361–367. 10.1037/0735-7036.100.4.361 [DOI] [PubMed] [Google Scholar]
- Sinnott, J. M., Owren, M. J., and Peterson, M. R. (1987). “Auditory frequency discrimination in primates: Species difference (cercopithecus, macaca, homo),” J. Comp. Psychol. 101, 126–131. 10.1037/0735-7036.101.2.126 [DOI] [Google Scholar]
- Trehub, S. E., Bull, D., and Thorpe, L. A. (1984). “Infant’s perception of melodies: The role of melodic contour,” Child Dev. 55, 821–830. [DOI] [PubMed] [Google Scholar]
- Trehub, S. E., and Hannon, E. E. (2006). “Infant music perception: Domain-general or domain-specific mechanisms?,” Cognition 100, 73–99. 10.1016/j.cognition.2005.11.006 [DOI] [PubMed] [Google Scholar]
- Walker, K. M. M., Schnupp, J. W. H., Hart-Schnupp, S. M. B., King, A. J., and Bizley, J. K. (2009). “Pitch discrimination by ferrets for simple and complex sounds,” J. Acoust. Soc. Am. 126, 1321–1335. 10.1121/1.3179676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman, R., Njegovan, M., Sturdy, C., Phillmore, L., Cotle, J., and Mewhort, D. (1998). “Frequency-range discriminations: Special and general abilities in zebra finches (Taeniopygia guttata) and humans (Homo sapiens),” J. Comp. Psychol. 112, 244–258. 10.1037/0735-7036.112.3.244 [DOI] [PubMed] [Google Scholar]
- Weisman, R. G., Njegovan, M. G., Williams, M. T., Cohen, J. S., and Sturdy, C. B. (2004). “A behavior analysis of absolute pitch: Sex, experience, and species,” Behav. Processes 66, 289–307. 10.1016/j.beproc.2004.03.010 [DOI] [PubMed] [Google Scholar]
- Weisman, R. G., and Ratcliffe, L. (2004). “Relative pitch and the song of black-capped chickadees,” Am. Sci. 92, 532–539. [Google Scholar]
- Wright, A. A., Rivera, J. J., Hulse, S. H., Shyan, M., and Neiworth, J. J. (2000). “Music perception and octave generalization in rhesus monkeys,” J. Exp. Psychol. Gen. 129, 291–307. 10.1037/0096-3445.129.3.291 [DOI] [PubMed] [Google Scholar]