Abstract
The treatment of moderate or severe hypertension in most cases requires the contemporaneous use of multiple antihypertensive agents. The most available two-drug combinations have an agent that addresses renin secretion and another one that is statistically more effective in renin-independent hypertension. The practice of combining agents that counteract different mechanisms is the most likely explanation for the fact that most available two-drug combinations have an agent that addresses renin secretion (beta-blocker, angiotensin converting enzyme inhibitor, angiotensin II receptor blocker or direct renin inhibitor) and another one that is more effective in renin-independent hypertension (diuretic, dihydropyridine or non-dihydropyridine calcium channel blocker). Based on these considerations, addition of hydrochlorothiazide to the combination of an antagonist of the renin-angiotensin system with a calcium channel blocker would constitute a logical approach. Inclusion of a diuretic in the triple combination is based on the evidence that these agents are effective and cheap, enhance the effect of other antihypertensive agents, and add a specific effect to individuals with salt-sensitivity of blood pressure. The benefit of triple combination therapy with amlodipine, valsartan and hydrochlorothiazide over its dual component therapies has been demonstrated, and the use of a single pill will simplify therapy resulting in better blood pressure control.
Keywords: valsartan, amlodipine, hydrochlorothiazide, HCTZ, blood pressure, hypertension
Introduction
Although a common and treatable risk factor for cardiovascular morbidity and mortality, hypertension (blood pressure ≥ 140/90 mmHg) is highly prevalent, affecting approximately one billion individuals worldwide.1,2 Concomitant with our progressive understanding of the epidemiology of hypertension and the beneficial effects of treatment, there has been a progressive lowering of the blood pressure values considered as the optimal treatment target. Even though awareness and treatment of hypertension have increased over the years, substantial improvements in blood pressure control rates are still lacking, with about two thirds of hypertensive adults aged 35–64 years failing to reach a blood pressure target of <140/90 mmHg,3 thus contributing to the negative result stating that the total control rates remain low, varying from 5% to 33%.3–5 Nowadays, it is postulated that hypertension must be considered a multifactorial disease, often requiring multiple drugs for its management, and the majority of hypertensive patients require two or more agents to reach a blood pressure goal. Particularly for those patients with stage 2 hypertension (or blood pressure > 20/10 mmHg above goal), it is recommended that treatment begins with a combination of two drugs from different classes.2,5,6 And, for patients who do not respond to dual therapy, the addition of a third drug is usually necessary. As a matter of fact, although numerous single-pill combinations with two drugs are already available, blood pressure remains largely uncontrolled, more so in elderly, black, diabetic, obese, and severely hypertensive patients.7–9 Data from different countries show that up to 85% of patients may need multiple medications to help control their blood pressure,10 and many need three or more.11
Combining drugs with complimentary modes of action is pragmatic, as it is more likely to achieve better blood pressure control, and also attenuate the adverse events (AEs) of the constituent monotherapy.12–14 Furthermore, the risk of non-compliance, one of the major reasons for failure of antihypertensive therapy, is reduced by 24% to 26% with the use of single-pill combination regimens. The absence of proper blood pressure control in the population is not only due to the patients’ non-compliance and/or non-adherence to treatment, but also a part of responsibility can be attributed to the physicians; none excluded!
From our point of view, the two most powerful elements with which physicians can increase blood pressure control rates are self-management of therapeutic inertia and appropriate selection of drug therapy. Therapeutic inertia can be defined as the failure to initiate or increase therapy when indicated, despite clearly-established guidelines and documented beneficial outcomes.15,16 This behavior is independent from patient issues with adherence or access to care, and most probably results from relying on “poor and weak” reasons for not modifying therapy, or a lack of organization. Productivity pressures that shorten the patient-physician interaction, and the multiple, sometimes conflicting, guidelines regarding therapy compound the issue. However, it has been argued that correcting inertia may not be enough to obtain blood pressure control in all the types of patients. A recent and interesting study employing a modification of survival17 demonstrated the diminished efficacy of antihypertensive therapy in the elderly. If therapeutic inertia by physicians is also increased, it would result in blood pressure control rates under 65%, even if the elderly were treated as aggressively as the youngest studied group.
Not only epidemiological or observational studies but also important and wide clinical trials, including ALLHAT (Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial), ACCOMPLISH (Avoiding Cardiovascular Events in Combination Therapy in Patients Living with Systolic Hypertension), INVEST (International Verapamil SR/Trandolapril Study), and LIFE (Losartan Intervention For Endpoint reduction in Hypertension), have reported that 23% to 54% of patients require three or more antihypertensive agents for blood pressure control and target level maintenance (<140/90 or <130/80 mmHg depending on cardiovascular risk).18–21 In patients with blood pressure ≥ 20/10 mmHg above goal, on average, 3.2 drugs might be needed to achieve blood pressure control.22 Thus, a single-pill combination of three drugs would be a desired treatment option for hypertension, and also because it is supposed to reduce the side effects of any single molecule.
Rationale for fixed combinations
Rather than a protracted vicious circle of ever-increasing monotherapy dosages, with the potential for more adverse events, the rapid achievement of blood pressure goals has the obvious advantage of reducing patient frustration. This therefore increases trust in the therapeutic relationship and, hence, adherence to treatment.23
Furthermore, regardless of the specific question posed in a particular clinical trial, studies exploring hypertension treatment outcomes have attempted to reach increasingly tighter blood pressure control levels. Coupled with the reduction of side effects, this leads to a significant increase in therapeutic compliance. Thus, the need for more drugs to achieve blood pressure control, the issues of convenience, and side effects affecting adherence led to the development of single-pill combination therapies involving almost all the newest classes of antihypertensive agents. The practice of combining agents that counteract different mechanisms is likely to be the reason why most available two-drug combinations have an agent that addresses renin secretion (beta-blocker, ACE-I [angiotensin converting enzyme inhibitor], ARB [angiotensin II receptor blocker] or DRI [direct renin inhibitor]) and another one that is more effective in renin-independent hypertension (diuretic, dihydropyridine, or non-dihydropyridine calcium channel blocker [CCB]). For these reasons, addition of hydrochlorothiazide (HCTZ) to the combination of an antagonist of the renin-angiotensin system (ACE inhibitor, ARB, or DRI) and a CCB would constitute a logical approach. Inclusion of a diuretic in the triple combination is based on the evidence that these agents are effective and cheap, enhance the effect of other antihypertensive agents, and add a specific effect for individuals with salt-sensitivity of blood pressure. This last mechanism is not yet fully understood, but there is strong evidence that deficient natriuretic derivatives of cytochrome p450 metabolism of arachidonic acid play an important role both in rodents24 and humans.25,26
The best known triple fixed combination available nowadays is the one which combines the CCB amlodipine, the ARB valsartan, and the diuretic HCTZ. Specifically, amlodipine blocks the contractile effects of calcium on cardiac and vascular smooth muscle cells; valsartan blocks the vasoconstriction and sodium retaining effects of angiotensin II on cardiac and vascular smooth muscle, adrenal and renal cells; and, finally, hydrochlorothiazide promotes the excretion of sodium and chloride in the kidney, leading to reductions in intravascular volume. A brief description of the mechanism of action of each individual component follows.
Amlodipine
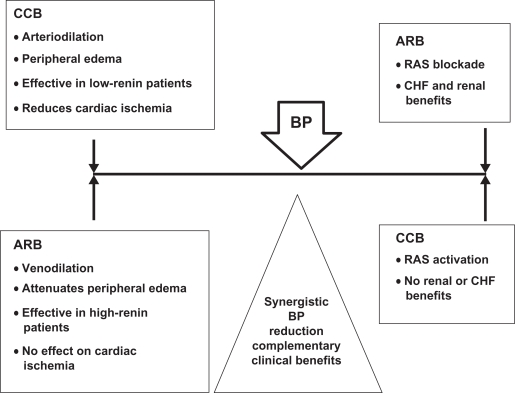
Amlodipine is a peripheral arterial vasodilator that acts directly on vascular smooth muscle to cause a reduction in peripheral vascular resistance and reduction in blood pressure. It is a dihydropyridine CCB that inhibits the transmembrane influx of calcium ions into vascular smooth muscle and cardiac muscle. The contractile processes of cardiac muscle and vascular smooth muscle are dependent upon the movement of extracellular calcium ions into these cells through specific ion channels. Amlodipine inhibits calcium ion influx across cell membranes selectively, with a greater effect on vascular smooth muscle cells than on cardiac muscle cells. Due to these mechanisms of action, amlodipine produces mostly arterial vasodilatation. Since the CCB does not promote a venous dilatation comparable to the arterial effect, a displacement of hydrostatic forces in peripheral capillaries is created which facilitates fluid extravasation into the interstitial space. This often results in lower extremities edema due to the force of gravity. However, when associated with valsartan, the presence of an ARB promotes both arterial and venous dilation, so balancing the hydrostatic pressure in peripheral capillaries and thus reducing fluid extravasation into the interstices. The end result is a difference in lower extremity edema (Figure 1).
Figure 1.
CCB–ARB: synergy of counter-regulation.
Abbreviations: ARB, angiotensin receptor blocker; BP, blood presssure; CCB, calcium channel blocker; CHF, congestive heart failure; RAS, renin-angiotensin system.
With chronic once-daily administration, antihypertensive effectiveness is maintained for at least 24 hours. Plasma concentrations correlate with effect in both young and elderly patients. The magnitude of reduction in blood pressure with amlodipine is also correlated with the pre-treatment blood pressure. Thus, individuals with moderate hypertension (diastolic pressure 105–114 mmHg) have about 50% greater response than patients with mild hypertension (diastolic pressure 90–104 mmHg). In hypertensive patients with normal renal function, therapeutic doses of amlodipine result in a decrease in renal vascular resistance and an increase in intraglomerular filtration rate and effective renal plasma flow without change in filtration fraction or proteinuria.
Valsartan
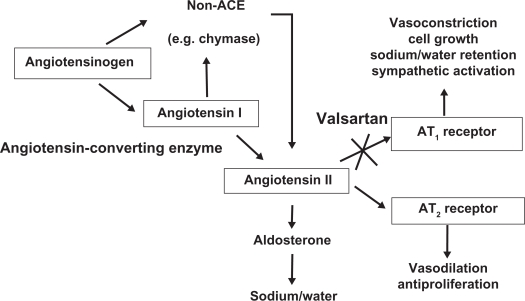
Valsartan’s action is to block the vasoconstrictor and aldosterone-secreting effects of angiotensin II. This is acheived by selectively blocking the binding of angiotensin II to the angiotensin II type 1 (AT1) receptor in many tissues, such as vascular smooth muscle and the adrenal gland. Thus, its action is independent of the pathways for angiotensin II synthesis. There is also an angiotensin II type 2 (AT2) receptor found in many tissues. But AT2 is not known to be linked with cardiovascular homeostasis. Valsartan has much greater affinity (about 20,000-fold) for the AT1 receptor than for the AT2 receptor. The increased plasma levels of angiotensin following AT1 receptor blockade with valsartan could produce a stimulation of the unblocked AT2 receptors (Figure 2). Because valsartan does not inhibit ACE (kininase II), it does not affect the response to bradykinin, as happens with the use of ACE inhibitors. It is well-known that the blockade of the angiotensin II receptor inhibits the negative regulatory feedback of angiotensin II on renin secretion. However, the resulting increased plasma renin activity and angiotensin II circulating levels do not overcome the effect of valsartan on blood pressure.
Figure 2.
Mechanism of action of valsartan.
Abbreviations: ACE, angiotensin converting enzyme; AT1, angiotensin II type 1; AT2, angiotensin II type 2.
In multiple-dose studies of hypertensive patients with stable renal insufficiency and patients with renovascular hypertension, valsartan had no clinically significant effects on glomerular filtration rate, filtration fraction, creatinine clearance, or renal plasma flow. Administration of valsartan to patients with essential hypertension results in a significant reduction of sitting, supine, and standing systolic blood pressure, usually with little or no orthostatic change.
Hydrochlorothiazide
HCTZ is a thiazide diuretic. Thiazides affect the renal tubular mechanisms of electrolyte resorption, directly increasing excretion of the ions sodium and chloride in approximately equivalent amounts. After oral administration of HCTZ, diuresis begins within 2 hours, peaks in about 4 hours, and lasts about 6 to 12 hours. Indirectly, the diuretic action of HCTZ reduces plasma volume, with consequent increases of plasma renin activity, aldosterone secretion, urinary loss, and decreases of serum potassium. The renin-aldosterone link is mediated by angiotensin II, so co-administration of an angiotensin II receptor-antagonist tends to equilibrate the potassium loss associated with these diuretics.
Pharmacokinetics
Following oral administration of the single-pill triple combination in normal healthy adults, peak plasma concentrations of amlodipine, valsartan and HCTZ are reached in about 6 hours, 3 hours, and 2 hours, respectively. The rates and extents of absorption of amlodipine, valsartan and HCTZ from the single-pill triple combination are the same as when administered as individual dosage forms, as confirmed by the results of a dedicated study.27 Peak plasma concentrations of amlodipine are reached 6–12 hours after administration of amlodipine alone. Absolute bioavailability has been estimated to be between 64% and 90%. Following oral administration of valsartan alone, peak plasma concentrations of valsartan are reached in 2 to 4 hours. Absolute bioavailability is about 25% (range 10%–35%). The pharmacokinetics of valsartan do not differ significantly between males and females. HCTZ is not metabolized but is eliminated rapidly by the kidney. At least 61% of the oral dose is eliminated as unchanged drug within 24 hours. The elimination half-life is between 5.8 and 18.9 hours.
Overview of recent clinical studies
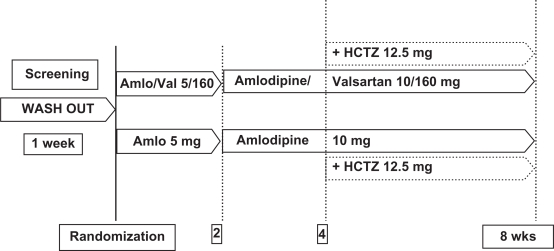
Most of the studies testing the triple combination of amlodipine/valsartan/HCTZ compare it to the double combination of valsartan/amlodipine or valsartan/HCTZ or amlodipine/HCTZ. In a recent study, our group showed that, in patients with stage 2 hypertension receiving HCTZ add-on if blood pressure > 130 mmHg, treatment with amlodipine/valsartan, with or without HCTZ, was more efficacious than treatment with amlodipine with or without HCTZ28 (Figure 3). The addition of HCTZ in this design followed the general clinical practice of adding another agent only if the patients are unable to achieve a target blood pressure level.
Figure 3.
Efficacy of amlodipine/valsartan compared with amlodipine monotherapy in patients with grade 2 + 3 hypertension: Study design.28
Abbreviations: Amlo, amlodipine; Val, valsartan; HCTZ, hydrochlorothiazide; wks, weeks.
Another quite recent and interesting randomized forced-titration study has demonstrated that patients with moderate-to-severe hypertension receiving a triple combination of amlodipine, valsartan and HCTZ had significantly greater blood pressure reductions and control rates compared to patients receiving the dual component therapies.29 Again, Deeks recently published the results of a large, 8-week, randomized, double-blind, multinational, phase III trial.30 The author demonstrated that in patients with moderate or severe hypertension, triple combination therapy with amlodipine/valsartan/HCTZ produces significantly greater reductions from baseline in mean sitting systolic blood pressure (msSBP) and mean sitting diastolic blood pressure (msDBP) than either valsartan/HCTZ, amlodipine/HCTZ, or amlodipine/valsartan. Furthermore, the proportion of patients achieving overall blood pressure control at endpoint was significantly greater with the triple combination regimen than with any of the dual regimens, with significantly more triple combination recipients achieving msSBP and msDBP control at each assessment throughout the trial, independent of age, race, gender and ethnicity.30
The study CVEA489A230231 played a very important role in obtaining the European Commission’s approval of the new 3-in-1 once-daily hypertension treatment. It is a multinational, randomized, double-blind, parallel-group, phase III study designed to compare the efficacy and safety of triple therapy (valsartan, amlodipine and HCTZ) with the various dual combinations of its components, such as valsartan/HCTZ, amlodipine/valsartan, or amlodipine/HCTZ, in patients with moderate-to-severe hypertension. The trial was conducted in 15 countries, with 2,271 patients randomized to double-blind treatment. Study CVEA489A2302 showed that triple therapy was more effective in reducing systolic and diastolic blood pressure than dual combinations of its components in patients with moderate-to-severe hypertension. Reductions in msSBP of 40 to 50 mmHg were achieved, with up to 58% more patients receiving triple therapy achieving overall blood pressure control (defined as <140/90 mmHg) versus dual therapy. Of particular interest is the fact that the maximum dose of triple therapy (valsartan/amlodipine/HCTZ 10/320/25 mg) demonstrated additional reductions of 18%–29% in systolic blood pressure and 19%–32% in diastolic blood pressure when compared to all dual combinations of its components at the same doses. Furthermore, ambulatory blood pressure monitoring showed that the blood pressure-lowering effect of triple therapy was maintained throughout the 24-hour period. In addition, this study showed that triple therapy was highly effective regardless of the patient’s age, gender, race, ethnicity or baseline blood pressure, and was generally well-tolerated compared with dual therapy.31
Discussion
In a press release issued by Novartis,32 David A Calhoun, MD (University of Alabama at Birmingham), commented that “the majority of people with hypertension will require more than 1 medication to control their blood pressure, and it is not uncommon for patients with severe hypertension and/or patients requiring stricter blood pressure control to need 3 or more medications. With a triple combination option, appropriate patients may experience a simpler routine of a convenient, once-daily pill to help them control their high blood pressure.”
Furthermore, we can also say that combination therapy is increasingly recommended for selected patients with hypertension to facilitate prompt attainment and maintenance of goal blood pressure. Clearly enough, the possibility of a single-pill combination therapy simplifies treatment and optimizes long-term compliance. Moreover, therapies combining drugs with complimentary mechanisms of action like amlodipine (a CCB), valsartan (an ARB) and HCTZ (a diuretic) have been shown to achieve better blood pressure control and attenuate AEs like peripheral edema and hyperkalemia.9,33,34 Also, it has been suggested that combining different drugs in a single pill may lead to better compliance and hence better blood pressure control.35,36 Most of the studies concerning combination therapy have shown that, although dual therapy with amlodipine/valsartan is still indicated as initial therapy in patients who are unlikely to be controlled with a single drug. and as second-line therapy in patients not responding adequately to monotherapy, triple combination therapy with amlodipine/valsartan/HCTZ has greater blood pressure-lowering efficacy and is well-tolerated compared to the dual therapy with amlodipine/HCTZ, and, in stage 2 hypertension, can provide additional benefits in patients who require more than 2 agents to reach their target blood pressure. In addition to covering major pathophysiological mechanisms and providing a treatment for resistant hypertension (currently defined as the failure to reach risk factor-tailored goal blood pressure, despite treatment with optimal doses of three pharmacological agents of different families, one of which is a diuretic), the agents used in this type of combination have outcome data to support their use in diverse patient populations.37
Mostly following some of the evidence mentioned above, the first fixed-dose combination pill containing 3 drugs was approved for use in the United States by the Food and Drug Administration (FDA)38 in April 2009. In fact, it combines the CCB amlodipine, the ARB valsartan, and the diuretic HCTZ.39 This triple combination has been indicated for the treatment of hypertension, but not as an initial therapy. Tablets are available in fixed dosages of amlodipine/valsartan/HCTZ of 5/160/12.5 mg, 10/160/12.5 mg, 5/160/25 mg, 10/160/25 mg, and 10/320/25 mg. Dosing is once-daily. According to the FDA, a patient may be switched to the triple or the triple may be added if blood pressure is not adequately controlled with any 2 antihypertensive drugs from the classes of CCBs, ARBs, and diuretics. In conclusion, if we are to effectively deal with the worldwide cardiovascular epidemic, we believe that even with well-chosen double and triple combinations, there will still be a substantial number of patients who will need additional antihypertensive therapy. Thus, we (the physicians) should never feel satisfied with our job just because we are using a new class or a new combination of molecules. Our aim must be to achieve an effective and rapid reduction of blood pressure, always adapting our beliefs to the effective results obtained with our patients.
Footnotes
Disclosures
Dr Destro reports receiving consulting and lecture fees from Novartis Pharma, Boehringer Ingelheim, AstraZeneca, Servier, Menarini IFR, Schering-Plough, Guidotti, Pfizer, Knoll, Bayer, Chiesi, Sankyo, Merck Sharp & Dohme, Malesci and Errekappa; he has received research support as a study investigator from Novartis Pharma, Boehringer Ingelheim, Menarini IFR, Guidotti, Bayer and Chiesi. Dr Preti reports receiving consulting and lecture fees and research support as a study investigator from Novartis Pharma. Dr Cagnoni and Dr Rossi Ricci report receiving research support as a study co-investigator from Novartis Pharma. No other potential conflict of interest relevant to this article was reported.
References
- 1.Kearney P, Whelton M, Reynolds K, Muntner P, Whelton P, He J. Global burden of hypertension: Analysis of worldwide data. Lancet. 2005;365(9455):217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 2.Chobanian A, Bakris G, Black H. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 3.Wolf-Maier K, Cooper R, Kramer H, et al. Hypertension treatment and control in five European countries, Canada, and the United States. Hypertension. 2004;43:10–17. doi: 10.1161/01.HYP.0000103630.72812.10. [DOI] [PubMed] [Google Scholar]
- 4.Antikainen R, Moltchanov V, Chukwuma C, et al. Trends in the prevalence, awareness, treatment and control of hypertension: The WHO MONICA Project. Eur J Cardiovasc Prev Rehabil. 2006;13:13–29. doi: 10.1097/00149831-200602000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Mancia G, De Backer G, Dominiczak A, et al. for ESH-ESC Task Force on the Management of Arterial Hypertension 2007 ESH-ESC practice guidelines for the management of arterial hypertension. J Hypertens. 2007;25(9):1751–1762. doi: 10.1097/HJH.0b013e3282f0580f. [DOI] [PubMed] [Google Scholar]
- 6.Mancia G, Laurent S, Agabiti-Rosei E, et al. Reappraisal of European guidelines on hypertension management: A European Society of Hypertension Task Force document. J Hypertension. 2009;27:923–934. doi: 10.1097/HJH.0b013e328333146d. [DOI] [PubMed] [Google Scholar]
- 7.UK Prospective Diabetes Study Group Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317(7160):703–713. [PMC free article] [PubMed] [Google Scholar]
- 8.Mancia G, Brown M, Castaigne A, et al. Outcomes with nifedipine GITS or co-amilozide in hypertensive diabetics and nondiabetics in Intervention as a Goal in Hypertension (INSIGHT) Hypertension. 2003;41(3):431–436. doi: 10.1161/01.HYP.0000057420.27692.AD. [DOI] [PubMed] [Google Scholar]
- 9.Smith TR, Philipp T, Vaisse B, et al. Amlodipine and valsartan combined and as monotherapy in stage 2, elderly, and black hypertensive patients: Subgroup analyses of 2 randomized, placebo-controlled studies. J Clin Hypertens. 2007;9(5):355–364. doi: 10.1111/j.1524-6175.2007.06689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pepine CJ, Handberg EM, Cooper-DeHoff RM, et al. A calcium antagonist vs a non-calcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil-Trandolapril Study (INVEST): A randomized controlled trial. JAMA. 2003;290(21):2805–2816. doi: 10.1001/jama.290.21.2805. [DOI] [PubMed] [Google Scholar]
- 11.Grosso A, Jin J, Chen J. An 8-week, multi-centre, randomized, double-blind, parallel-group study to evaluate the efficacy and safety of the combination of valsartan/HCTZ/amlodipine compared to valsartan/HCTZ, valsartan/amlodipine, and HCTZ/amlodipine in patients with moderate to severe hypertension. Novartis Full Clinical Study Report. 2008 [Google Scholar]
- 12.Dahlof B, Sever PS, Poulter NR, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): A multicentre randomised controlled trial. Lancet. 2005;366(9489):895–906. doi: 10.1016/S0140-6736(05)67185-1. [DOI] [PubMed] [Google Scholar]
- 13.Mochizuki S, Dahlöf B, Shimizu M, et al. Valsartan in a Japanese population with hypertension and other cardiovascular disease (Jikei Heart Study): A randomised, open-label, blinded endpoint morbidity-mortality study. Lancet. 2007;369:1431–1439. doi: 10.1016/S0140-6736(07)60669-2. [DOI] [PubMed] [Google Scholar]
- 14.Poldermans D, Glazes R, Kargiannis S, et al. Tolerability and blood pressure-lowering efficacy of the combination of amlodipine plus valsartan compared with lisinopril plus hydrochlorothiazide in adult patients with stage 2 hypertension. Clin Ther. 2007;29(2):279–289. doi: 10.1016/j.clinthera.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Elliott WJ. What factors contribute to the inadequate control of elevated blood pressure? J Clin Hypertens. 2008;10(1 Suppl 1):20–26. doi: 10.1111/j.1524-6175.2007.08028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phillips L, Branch W, Cook C, et al. Clinical inertia. Ann Intern Med. 2001;135:825–834. doi: 10.7326/0003-4819-135-9-200111060-00012. [DOI] [PubMed] [Google Scholar]
- 17.Bailey K, Grossardt B, Graves J. Novel use of Kaplan-Meier methods to explain age and gender differences in hypertension control rates. Hypertension. 2008;51:841–847. doi: 10.1161/HYPERTENSIONAHA.107.101659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jamerson K, Weber MA, Bakris GL, et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359(23):2417–2428. doi: 10.1056/NEJMoa0806182. [DOI] [PubMed] [Google Scholar]
- 19.Devereux RB, de Faire U, Fyhrquist F, et al. Blood pressure reduction and antihypertensive medication use in the losartan intervention for endpoint reduction in hypertension (LIFE) study in patients with hypertension and left ventricular hypertrophy. Curr Med Res Opin. 2007;23(2):259–270. doi: 10.1185/030079906X162854. [DOI] [PubMed] [Google Scholar]
- 20.Bangalore S, Messerli FH, Cohen JD, et al. Verapamil-sustained release-based treatment strategy is equivalent to atenolol-based treatment strategy at reducing cardiovascular events in patients with prior myocardial infarction: An INternational VErapamil SR-Trandolapril (INVEST) substudy. Am Heart J. 2008;156(2):241–247. doi: 10.1016/j.ahj.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 21.Cushman WC, Ford CE, Cutler JA, et al. Success and predictors of blood pressure control in diverse North American settings: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT) J Clin Hypertens. 2002;4(6):393–404. doi: 10.1111/j.1524-6175.2002.02045.x. [DOI] [PubMed] [Google Scholar]
- 22.Turnbull F. Effects of different blood-pressure-lowering regimens on major cardiovascular events: Results of prospectively-designed overviews of randomised trials. Lancet. 2003;362(9395):1527–1535. doi: 10.1016/s0140-6736(03)14739-3. [DOI] [PubMed] [Google Scholar]
- 23.Gradman A, Acevedo C. Evolving strategies for the use of combination therapy in hypertension. Curr Hypertens Rep. 2002;4:343–349. doi: 10.1007/s11906-002-0062-x. [DOI] [PubMed] [Google Scholar]
- 24.Ma Y, Schwartzman M, Roman R. Altered renal P-450 metabolism of arachidonic acid in Dahl salt-sensitive rats. Am J Physiol. 1994;267:R579–589. doi: 10.1152/ajpregu.1994.267.2.R579. [DOI] [PubMed] [Google Scholar]
- 25.Laffer C, Gainer J, Waterman M, et al. The T8590C polymorphism of CYP4A11 and 20-hydroxyeicosatetraenoic acid in essential hypertension. Hypertension. 2008;51:767–772. doi: 10.1161/HYPERTENSIONAHA.107.102921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laffer C, Laniado-Schwartzman M, Wang M, Nasjletti A, Elijovich F. Differential regulation of natriuresis by 20-hydroxyeicosatetraenoic acid in human salt-sensitive versus salt-resistant hypertension. Circulation. 2003;107:574–578. doi: 10.1161/01.cir.0000046269.52392.14. [DOI] [PubMed] [Google Scholar]
- 27.Novartis CVEA489A2104: A multi-center, multiple dose, open-label, four-cohort, parallel study to assess the pharmacokinetic drug interaction following co-administration of valsartan, hydrochlorothiazide and amlodipine in patients with hypertension Novartis Clinical Trials Results Database 2009. Available from: http://www.novctrd.com/ctrdWebApp/clinicaltrialrepository/public/login.jsp
- 28.Destro M, Luckow A, Samson M, Kandra A, Brunel P. Efficacy and safety of amlodipine/valsartan compared with amlodipine monotherapy in patients with stage 2 hypertension: a randomized, double-blind, multicenter study: The EX-EFFeCTS Study. J Am Soc Hypertens. 2008;2:294–302. doi: 10.1016/j.jash.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Calhoun DA, Lacourciere Y, Chiang YT, Glazer RD. Triple antihypertensive therapy with amlodipine, valsartan, and hydrochlorothiazide: A randomized clinical trial. Hypertension. 2009;54:32–39. doi: 10.1161/HYPERTENSIONAHA.109.131300. [DOI] [PubMed] [Google Scholar]
- 30.Deeks E. Amlodipine/valsartan/hydrochlorothiazide: Fixed-dose combination in hypertension. Am J Cardiovasc Drugs. 2009;9:411–418. doi: 10.2165/11204350-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 31.Novartis CVEA489A2302: An 8-week, multicenter, randomized, double-blind, parallel-group study to evaluate the efficacy and safety of the combination of valsartan/HCTZ/amlodipine compared to valsartan/HCTZ, valsartan/amlodipine, and HCTZ/amlodipine inpatients with moderate to severe hypertendion Novartis Clinical Trials Results Database 2009. Available from: http://www.novctrd.com/ctrdWebApp/clinicaltrialrepository/public/login.jsp
- 32.Novartis FDA approves Exforge HCT® – the only high blood pressure treatment to combine three medications in a single pill 2009. Available from: http://www.novartis.com/newsroom/media-releases/en/2009/1310474.shtml Accessed June 3, 2009.
- 33.Schrader J, Salvetti A, Calvo C, et al. The combination of amlodipine/valsartan 5/160 mg produces less peripheral oedema than amlodipine 10 mg in hypertensive patients not adequately controlled with amlodipine 5 mg. Int J Clin Pract. 2009 Feb;63(2):217–225. doi: 10.1111/j.1742-1241.2008.01977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kjeldsen SE AT, Sierra ADL, et al. Amlodipine and valsartan: calcium channel blockers/angiotensin II receptor blockers combination for hypertension. Drug Eval. 2007;4:31–40. [Google Scholar]
- 35.Gerbino PP, Shoheiber O. Adherence patterns among patients treated with fixed-dose combination versus separate antihypertensive agents. Am J Health Syst Pharm. 2007;64(12):1279–1283. doi: 10.2146/ajhp060434. [DOI] [PubMed] [Google Scholar]
- 36.Bramley TJ, Gerbino PP, Nightengale BS, Frech-Tamas F. Relationship of blood pressure control to adherence with antihypertensive monotherapy in 13 managed care organizations. J Manag Care Pharm. 2006;12(3):239–245. doi: 10.18553/jmcp.2006.12.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Destro M, Crikelair N, Yen J, Glazer R. Triple therapy with amlodipine, valsartan, and HCTZ in Stage 2 hypertensive patients. Curr Med Res Opin. doi: 10.2147/vhrm.s11522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yusuf SW, Ali SS, Swafford J, et al. Culture-positive and culture-negative endocarditis in patients with cancer: A retrospective observational study, 1994–2004. Medicine. 2006;85(2):86–94. doi: 10.1097/01.md.0000208503.06288.7b. [DOI] [PubMed] [Google Scholar]
- 39.Novartis Pharmaceuticals Corporation (East Hanover NJ) Exforge HCT®: Highlights of prescribing information 2009. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/022314lbl.pdf Accessed April 30, 2009.