Abstract
Leukemia is one of the cancers most susceptible to induction by ionizing radiation, but the effects of lower doses delivered over time have not been adequately quantified. Following the Chornobyl (Chernobyl) accident in Ukraine in April 1986, several hundred thousand workers who were involved in cleaning up the site and its surroundings received fractionated exposure, primarily from external gamma radiation. To increase our understanding of the role of protracted low-dose radiation exposure in the etiology of leukemia, we conducted a nested case-control study of leukemia in a cohort of cleanup workers identified from the Chornobyl State Registry of Ukraine. The analysis is based on 71 cases of histologically confirmed leukemia diagnosed in 1986–2000 and 501 age- and residence-matched controls selected from the same cohort. Study subjects or their proxies were interviewed about their cleanup activities and other relevant factors. Individual bone marrow radiation doses were estimated by the RADRUE dose reconstruction method (mean dose=76.4 (SD=213.4) milligray (mGy)). We used conditional logistic regression to estimate leukemia risks. The excess relative risk of total leukemia was 3.44 per Gy (95% confidence interval 0.47–9.78, p<0.01). The dose-response was linear and did not significantly differ by calendar period of first work in the 30-km Chornobyl zone, duration or type of work. We found a similar dose-response relationship for chronic and non-chronic lymphocytic leukemia.
INTRODUCTION
Studies of individuals exposed to moderate doses of radiation, generally at high dose-rates, such as survivors of the atomic bombings in Japan, have demonstrated that leukemia is one of the cancers most susceptible to induction by ionizing radiation and that it can occur very soon after radiation exposure (1–3). There remains, however, considerable interest in the relationship between protracted exposure to low doses of radiation and leukemia, because these types of exposure are most likely to be encountered by the general public and radiation workers (1).
The accident at the Chornobyl (Chernobyl) nuclear power plant in northern Ukraine in April 1986, as well as being a public health, social and economic disaster for the countries most affected, also provided an opportunity to evaluate the relationship between leukemia and low dose and low dose-rate radiation (4). After the accident, several hundred thousand workers, who were involved in cleaning up the site and its surroundings, received fractionated whole-body doses, primarily from external radiation (1). To date, only studies of workers from the Russian Federation have attempted to quantify the risk of leukemia among Chornobyl cleanup workers (5–8). Data from these studies suggest an association between leukemia and radiation exposure, but the magnitude of the radiation effect is unclear due to substantial uncertainty in dose estimates (1). Buzunov et al. reported an increased risk of leukemia among Ukrainian cleanup workers, but dose estimates were not available and evaluation was based only on the year first worked at the accident site (9).
To increase our understanding of the role of protracted low-dose radiation exposure in the etiology of leukemia, we conducted a nested case-control study of leukemia in Ukrainian cleanup workers.
MATERIALS AND METHODS
We provided a full description of the study in Paper 1 (10) which we briefly summarize below.
Cases
We identified a cohort of 110,645 workers from the Chornobyl State Registry of Ukraine (SRU) who participated in Chornobyl cleanup activities before 1991 and who were initially registered in one of five oblasts1 (Chernihiv, Cherkasy, Kharkiv, Kyiv and Dnipropetrovsk) or Kyiv City. The selected geographical area allowed for easy access by study investigators and included a large number of cleanup workers. The cohort represents 46% of all cleanup workers included in the SRU.
We ascertained potential cases of leukemia occurring within the cohort between 1986 and 2000 through computerized linkage (11) of cohort records and a Provisional Leukemia Registry (10). We established an expert international panel of five hematologists and hematopathologists to review all diagnoses (12). The Panel was given 128 potential leukemia cases to review (111 with a preliminary diagnosis of leukemia and 17 with a preliminary diagnosis of MDS) and they confirmed 87 cases of leukemia (Table 1). The Panel initially classified the leukemia cases using the French-American-British (FAB) system but changed to the WHO system of classification in 2007(13). Complete medical records, including a description of the histologic confirmation of the diagnosis were available for all cases. In 56 (64.4%) of 87 confirmed cases diagnosis was supported by biological material. Sixteen cases were excluded from risk analysis because their doses could not be calculated reliably (2 proved to be ineligible, 7 could not be traced, 4 refused to complete the dosimetry questionnaire, and for 3 the quality of interview was inadequate). Thus, our analysis included 71 cases of the 87 (81.6%) confirmed cases of leukemia, and, as shown in Table 1, this participation rate was similar for all leukemia cases excluding chronic lymphocytic leukemia (CLL) and CLL cases separately (p=0.58).
Table 1.
Distribution of Cases of Leukemia Among Ukrainian Chornobyl Cleanup Workers by Cell Type, 1986–2000.
| Cell Type | Cases confirmed by the International Hematology Panel | Cases with estimated doses (% confirmed cases) |
|---|---|---|
| Acute lymphocytic leukemia | 4 | 3 (75.0) |
| Acute myeloid leukemia | 6 | 5 (83.3) |
| Acute leukemia otherwise not specified | 9 | 6 (66.7) |
| Chronic myeloid leukemia | 15 | 14 (93.3) |
| Other chronic leukemiaa | 4 | 4 (100) |
| Sub-total non-CLL | 38 | 32 (84.2) |
| Chronic lymphocytic leukemia | 49 | 39 (79.6) |
| TOTAL | 87 | 71 (81.6) |
These cases were identified as large granular lymphocytic leukemia and were verified by immunophenotypic surface markers as 2 cases of the T-cell and 2 cases of the NK cell type.
Controls
For each potential leukemia case, to achieve a 5:1 matching ratio, we randomly selected five to nine control subjects from members of the cohort who were alive and at risk at the time of the case’s diagnosis (incidence density sampling) and matched on oblast or Kyiv City and year of birth. Of the selected 792 controls, 536 were interviewed, 101 refused to participate, 133 could not be traced, and 22 moved out of the study regions (response rate of 71.6 % for alive controls, 60.2 % for next-of-kin and 66.1 % for colleagues responding for deceased controls). Originally, 348 controls were selected for 71 leukemia cases and the remaining 188 controls had been selected and interviewed for cases which were not included in the study because their initial diagnosis was not confirmed by the study hematologist, their final diagnosis was not confirmed by the international panel, or they failed to participate in this study. Of the latter ones, only 153 (81.3%) could be matched to the 71 cases and, thus, the total number of controls used in this analysis is 501. Match on year of birth was achieved for 442 controls (88.2%). The remaining controls were matched within 2 years of birth (n=35 or 7.0%), or within 5 years of birth (n=24 or 4.8%).
Dosimetry
The RADRUE dosimetry method was used to estimate individual Chornobyl-related bone marrow doses for all cases and controls (14). The method uses detailed interviews with study subjects, or if they were deceased with their next-of-kin for demographic and medical data and co-worker proxies for the details of clean-up activities, carried out by trained interviewers to ascertain Chornobyl work and residential history. The interview included questions on workers’ activities during cleanup, location of places of work and residence, types of work, transportation routes to and from work and corresponding dates. An expert dosimetrist used the questionnaire data in combination with a data base of field exposure measurements to estimate the total Chornobyl-related dose for each subject (including both cleanup activities and residence in the highly-contaminated areas). Investigators have tested and validated the RADRUE dose estimation methodology (14, 15).
Due to the high mortality rate of leukemia patients and since we conducted interviews between 2002 and 2004 for cases diagnosed in 1986–2000, we had to interview proxy respondents for 60 percent of case subjects, mainly next-of-kin for personal, residential and medical history and coworkers for Chornobyl work history. In contrast, since most control subjects were alive, we relied on proxy interviews for only 7.2 percent of controls.
The RADRUE method was used to calculate annual bone marrow dose estimates for 1986–1990 for each study subject by generating ten thousand realizations of a dose prediction equation by random sampling from assumed distributions of model parameters. We based dose-response analyses on cumulative dose estimates for each worker derived as the sum of the arithmetic means of 10,000 annual bone marrow dose estimates (mean=76.4, standard deviation (SD)=213.4 mGy, 2-year lag).
Statistical analysis
We used standard conditional logistic regression for matched sets for all analyses. We computed odds ratios (OR) to estimate relative risks (RR) in four dose categories (0–1.9, 2.0–19.9, 20.0–149.9, 150.0–3220 milligray (mGy)) based on the categorization of the case dose distribution approximately into quarters. We fit an excess relative risk (ERR) model for continuous doses,
| (1) |
where β is the ERR per Gray (ERR/Gy), Zi represents potential modifying factors and γi their corresponding parameters In this equation, the effect of dose multiplies the background risk and by adding 1.0 to the ERR, one obtains the relative risk at 1 Gy of radiation. Model 1 is a linear model in dose, although we evaluated several alternative forms, including linear-quadratic, power and exponential models. For these analyses, we used the PECAN module from the EPICURE suite of programs (16) to derive point and confidence interval (CI) estimates for all parameters based on maximum likelihood estimation procedures, and used likelihood ratio tests for tests of hypotheses. All p-values are two-sided. We conducted analyses for all leukemias and separately for CLL and non-CLL cases.
We investigated calendar period first worked in the 30-km Chornobyl zone (categorized into April–May 1986, June–December 1986, 1987, and 1988–1990), duration of mission, i.e., total time worked within the zone (up to 1, 2–3, 4–5, and 6+ months), number of missions (1, 2, 3, and 4+), type of work performed in the zone during the first mission (grouped into early responders, military personnel, professional nuclear power workers, drivers and construction workers), as well as smoking, alcohol consumption, education, and urban/rural residence as possible independent risk factors of leukemia after adjustment for radiation exposure. We retained adjustment variables in the model if they significantly improved model fit or changed the risk estimate by more than 10 percent.
We also evaluated age at exposure, number of missions within the Chornobyl zone, year of first mission, type of work performed, total duration in the zone and source of information (subject or proxy respondent) as possible effect modifiers of the dose effect.
We assessed lag interval, a period of recent exposure assumed unrelated to disease, for the calculation of cumulative dose from 1986 to 1990 in one year increments between 0 and 10 years. The deviance, a measure of model fit, was minimized by two standard deviations, i.e. 3.84, for a lag of 2 years for all cases and for CLL and non-CLL cases separately. We therefore used a lag of two years for the calculation of cumulative dose in all analyses.
RESULTS
Table 2 shows selected descriptive characteristics of study subjects. Case and control subjects did not differ by year of birth, geographic area, type (urban/rural) of residence, or educational level. Among the 71 cases used in the analysis, the International Hematology Panel classified 39 cases as CLL (55%) and 32 as non-CLL (45%).
Table 2.
Descriptive Characteristics of Cases and Controls Identified From the Cohort of Ukrainian Chornobyl Cleanup Workers During Follow-Up (1986–2000).
| Cases | (%) | Controls | (%) | DOFb | pvalueb | |
|---|---|---|---|---|---|---|
| Total | 71 | 100.0 | 501 | 100.0 | ||
| Year of birth | 4 | 0.89 | ||||
| 1923–1929 | 4 | 5.6 | 35 | 7.0 | ||
| 1930–1939 | 24 | 33.8 | 144 | 28.7 | ||
| 1940–1949 | 18 | 25.4 | 149 | 29.7 | ||
| 1950–1959 | 21 | 29.6 | 146 | 29.1 | ||
| 1960–1965 | 4 | 5.6 | 27 | 5.4 | ||
| Areas of study | 5 | 0.89 | ||||
| Cherkasy oblast | 3 | 4.2 | 40 | 8.0 | ||
| Chernihiv oblast | 6 | 8.5 | 44 | 8.8 | ||
| Dnipropetrovsk oblast | 16 | 22.5 | 96 | 19.2 | ||
| Kharkiv oblast | 8 | 11.3 | 67 | 13.4 | ||
| Kyiv oblast | 14 | 19.7 | 102 | 20.4 | ||
| Kyiv City | 24 | 33.8 | 152 | 30.3 | ||
| Type of residence | 1 | 0.69 | ||||
| Urban | 52 | 73.2 | 405 | 80.8 | ||
| Rural | 14 | 19.7 | 96 | 19.2 | ||
| Unknown | 5 | 7.0 | 0 | 0.0 | ||
| Education | 3 | 0.33 | ||||
| 8 years or less | 9 | 12.7 | 74 | 14.8 | ||
| High school | 21 | 29.6 | 210 | 41.9 | ||
| Trade school | 18 | 25.4 | 112 | 22.4 | ||
| College | 18 | 25.4 | 102 | 20.4 | ||
| Unknown | 5 | 7.0 | 3 | 0.6 | ||
| Proxy interviews | 1 | <0.01 | ||||
| No | 29 | 40.8 | 465 | 92.8 | ||
| Yes | 42 | 59.2 | 36 | 7.2 | ||
| Cell Type | ||||||
| Non–CLLa | 32 | 45.1 | NAd | |||
| CLL | 39 | 54.9 | ||||
CLL, chronic lymphocytic leukemia
DOF, degrees of freedom from the chi-square test
p value of the chi-square test
NA, not applicable
After adjustment for dose, the odds ratio (OR) by calendar period first worked April/May 1986, was 1.64 relative to first worked between 1988 and 1990, but this difference was not statistically significant (Table 3). ORs for duration of cleanup work at Chornobyl were close to unity and there was no clear trend. Similarly, number of missions and type of work performed in the 30-km zone showed no variation in risk after adjustment for dose.
Table 3.
Odds Ratios and 95% Confidence Intervals For All Leukemia by Chornobyl Cleanup Work In the 30-km Zone.
| Cases | (%) | Controls | (%) | ORa, b | 95%CIc | DOFd | p-valuee | |
|---|---|---|---|---|---|---|---|---|
| Calendar period first worked in the 30-km Chornobyl zone | 3 | 0.56 | ||||||
| April/May 1986 | 37 | 52.1 | 200 | 39.9 | 1.64 | 0.60–4.45 | ||
| June/Dec 1986 | 17 | 23.9 | 154 | 30.7 | 1.06 | 0.39–2.90 | ||
| 1987 | 10 | 14.1 | 78 | 15.6 | 1.12 | 0.39–3.18 | ||
| 1988–1990 | 7 | 9.9 | 69 | 13.8 | 1 | |||
| Type of work performed in the Chornobyl 30-km zone during the first mission | 3 | 0.93 | ||||||
| early responders | 14 | 19.7 | 92 | 18.4 | 1 | |||
| military personnel | 25 | 35.2 | 198 | 39.5 | 0.88 | 0.36–2.11 | ||
| professional nuclear power workers | 5 | 7.0 | 21 | 4.2 | 1.15 | 0.37–3.63 | ||
| drivers and construction workers | 27 | 38.0 | 190 | 37.9 | 1.11 | 0.54–2.31 | ||
| Duration of mission, months | 3 | 0.75 | ||||||
| <=1 | 42 | 59.2 | 296 | 59.1 | 1 | |||
| 2–3 | 17 | 23.9 | 135 | 26.9 | 1.02 | 0.52–1.98 | ||
| 4–5 | 6 | 8.5 | 28 | 5.6 | 1.7 | 0.63–4.57 | ||
| 6+ | 6 | 8.5 | 42 | 8.4 | 0.8 | 0.33–2.23 | ||
| Number of missions | 3 | 0.78 | ||||||
| 1 | 55 | 77.5 | 384 | 76.6 | 1 | |||
| 2 | 13 | 18.3 | 83 | 16.6 | 1.08 | 0.54–2.17 | ||
| 3 | 2 | 2.8 | 18 | 3.6 | 0.64 | 0.14–2.95 | ||
| 4+ | 1 | 1.4 | 16 | 3.2 | 0.47 | 0.06–3.66 | ||
OR, odds ratios for background variables from conditional logistic regression model adjusted for cumulative doses lagged by 2 years
cases and controls matched on year of birth and oblast
CI, confidence interval
DOF, degrees of freedom from the likelihood ratio test
p-values for test of homogeneity of odds ratios
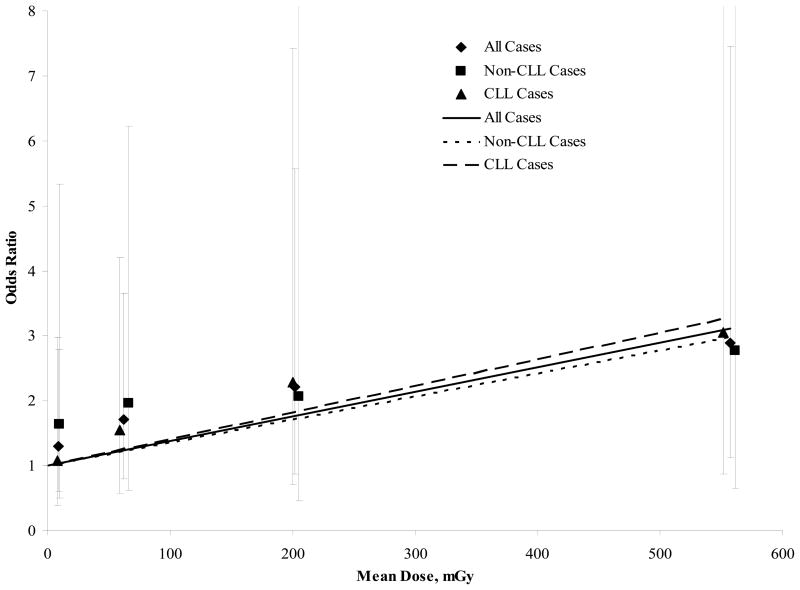
As shown in table 4, the OR for total leukemia increased with dose categories (p=0.03 for test of linear trend). Although based on few cases, we further divided the highest dose category (Fig. 1) (150.0–3220.0 mGy) into two and found a somewhat higher risk in the upper dose category (OR = 2.21; 95% CI: 0.87–5.57 and OR=2.89; 95% CI: 1.12–7.46 for categories of 150.0–274.9 and 275.0–3220.0 mGy, respectively). Analyses were also done separately for CLL and non-CLL, and we observed consistent trends for the two subtypes (p=0.04 and 0.25 for test of linear trend, respectively), but the number of cases was small and the p-value for trend did not reach statistical significance for the non-CLL cases.
Table 4.
Odds Ratios and 95% Confidence Intervals by Categories of Cumulative Dose and Type of Leukemia.
| Dose Range, mGya | Mean Dose, mGy | Cases | % | Controls | % | ORb, c | 95%CId | DOFe | p-valuef |
|---|---|---|---|---|---|---|---|---|---|
| All Cases | |||||||||
| 0–1.9 | 0.6 | 17 | 23.9 | 157 | 31.3 | 1 | 1 | 0.03 | |
| 2.0–19.9 | 8.8 | 17 | 23.9 | 143 | 28.5 | 1.28 | 0.59–2.75 | ||
| 20.0–149.9 | 62.2 | 20 | 28.2 | 131 | 26.1 | 1.71 | 0.80–3.64 | ||
| 150.0–3220.0 | 377.4 | 17 | 23.9 | 70 | 14.0 | 2.50 | 1.17–5.33 | ||
| Total | 76.4 | 71 | 100.0 | 501 | 100.0 | ||||
| Non-CLL Cases | |||||||||
| 0–1.9 | 0.5 | 8 | 25.0 | 76 | 32.6 | 1 | 1 | 0.25 | |
| 2.0–19.9 | 9.4 | 8 | 25.0 | 65 | 27.9 | 1.61 | 0.49–5.25 | ||
| 20.0–149.9 | 66.3 | 9 | 28.1 | 59 | 25.3 | 1.95 | 0.61–6.19 | ||
| 150.0–3220.0 | 409.9 | 7 | 21.9 | 33 | 14.2 | 2.40 | 0.72–7.99 | ||
| Total | 81.6 | 32 | 100.0 | 233 | 100.0 | ||||
| CLL Cases | |||||||||
| 0–1.9 | 0.6 | 9 | 23.1 | 81 | 32.5 | 1 | 1 | 0.04 | |
| 2.0–19.9 | 8.3 | 9 | 33.3 | 78 | 29.9 | 1.07 | 0.39–2.93 | ||
| 20.0–149.9 | 58.7 | 11 | 30.8 | 72 | 30.2 | 1.55 | 0.57–4.21 | ||
| 150.0–2600.0 | 349.7 | 10 | 12.8 | 37 | 7.5 | 2.60 | 0.98–6.87 | ||
| Total | 72.0 | 39 | 100 | 268 | 100 | ||||
mGy, cumulative doses in milligray lagged by 2 years
odds ratios from conditional logistic regression model
cases and controls matched on year of birth and oblast
CI, confidence interval
DOF, degrees of freedom from the likelihood ratio test
p-value from the linear trend test where the score for each category is the mean value for the cumulative dose
Figure 1.
Plot of the Odds Ratios of Leukemia by Mean Dose for Each of Five Dose Categories and a Fitted Dose-Response Line Constructed Using the Least Squares Method.
With continuous dose, we estimated an ERR/Gy of 3.44 for all leukemias combined (95% CI: 0.47–9.78, p<0.01) (Table 5). The dose-response parameters for CLL (ERR/Gy = 4.09; 95% CI: undefined-14.41) and non-CLL (ERR/Gy = 2.73; 95% CI:undefined-13.50) were consistent. A formal test of homogeneity between the two slopes yielded a p-value of 0.75, indicating no significant difference in the effects for non-CLL and CLL cases. We found no evidence that the dose-response estimates for total leukemia or leukemia subtypes was confounded by smoking, alcohol, education, urban/rural residence, occupation or exposure to chemicals (results not shown).
Table 5.
Excess relative risk models of leukemia for dose and interactions with age at exposure, year of diagnosis, duration of missions and number of missions.
| Description | N cases | ERR per Gya, b, c | Lower Bound | Upper Bound | p-valued |
|---|---|---|---|---|---|
| All cases | 71 | 3.44 | 0.47 | 9.78 | <0.01 |
| Cell type | |||||
| Non-CLL | 32 | 2.73 | n.e.f | 13.50 | 0.75 |
| CLLe | 39 | 4.09 | n.e. | 14.41 | |
| Proxy interviews | |||||
| No | 29 | 6.20 | n.e. | 27.11 | 0.47 |
| Yes | 42 | 2.45 | n.e. | 9.46 | |
| Age at exposure, years | |||||
| 21–44 | 36 | 0.03 | n.e. | 6.03 | 0.07 |
| 45–63 | 35 | 8.83 | 1.52 | 32.78 | |
| Year of diagnosis | |||||
| < 1993 | 19 | 4.67 | n.e. | 38.00 | 0.81 |
| >=1993 | 52 | 3.19 | 0.21 | 10.21 | |
| Duration of missions, months | |||||
| <=1 | 42 | 4.47 | 0.78 | 13.24 | 0.46 |
| >1 | 29 | 1.92 | n.e. | 9.86 | |
| Number of missions | |||||
| 1 | 55 | 4.31 | 0.76 | 12.60 | 0.52 |
| >1 | 16 | 2.03 | n.e. | 10.88 | |
ERR, excess relative risk per gray (Gy)
conditional logistic regression model with cumulative doses lagged 2 years
cases and controls matched on year of birth and oblast
p-value from the likelihood ratio test for interaction effects
CLL, chronic lymphocytic leukemia
n.e., could not be estimated
When we excluded subjects with doses above 500 mGy to assess the influence of subjects with extremely high doses, we found a comparable estimate of effect (N cases = 67, ERR/Gy=3.54, 95% CI: undefined, 11.1, p=0.08). The inclusion of quadratic, exponential or power terms in dose did not improve model fit (p-values of 0.77, 0.73, and 0.33, respectively), indicating no evidence of curvilinearity in the dose-response.
To assess the validity of a 2-year lag period, we analyzed the 52 cases diagnosed since 1993 (all cleanup work ceased in 1990). The results were very similar to those for all study subjects, i.e., an ERR of more than three per gray, an association which approaches statistical significance (p=0.06, not shown). Comparative analyses of annual doses and cumulative doses lagged by 2 years provided evidence that risk arose primarily from doses received in 1986 (not shown).
Table 5 shows the risk estimates for directly interviewed cases and for deceased cases for whom proxy interviews were necessary. Since there were very few proxy-interviewed controls, we included all controls in these analyses. The ERR/Gy for directly interviewed cases was 2.5-fold that for proxy cases, although a test of interaction for source of interview data yielded a p-value of 0.47. We did not observe statistically significant interaction for categories of year of diagnosis, duration of missions or number of missions. However, the ERR/Gy was non-significantly lower for workers with longer durations of exposure and greater numbers of missions, suggesting a reduced effect with lower dose-rate. The ERR/Gy for workers first exposed before age 45 years (median age at exposure) was smaller than for those exposed at later ages (p=0.07) and a higher ERR/Gy at older ages at exposure was also seen for CLL and non-CLL cases when we analyzed them separately (not shown).
DISCUSSION
In a nested case-control study of Chornobyl cleanup workers in Ukraine, we observed a significant association between Chornobyl-related radiation dose and increased risk of leukemia. Our risk estimate for Chornobyl clean-up workers exposed to protracted radiation was comparable to the one from the Life Span Study of atomic bomb survivors exposed to high-dose-rate ionizing radiation (3). However, while differences were not statistically significant (p>0.5), the estimates of ERR/Gy for workers exposed for longer durations or from multiple missions were about half those for workers who received their exposure within one month or during one mission. These findings provide limited support for the hypothesis that protracted delivery of radiation dose over time decreases risk.
The strengths of this study are many and include the relatively large number of cases and controls compared to other studies of cleanup workers, selection of cases and controls from within a large cohort of cleanup workers from Ukraine, the wide and rigorous search for diagnoses of leukemia and 99 ancillary diagnoses in all medical institutions treating leukemia in the target geographic areas, and confirmation of diagnoses for all study cases by the International Hematology Panel consisting of hematologists and hematopathologists which reviewed medical records for all cases and biological material for a majority of cases.
Furthermore, individual bone marrow doses were estimated for all study subjects by the RADRUE dosimetric method which allows for the possibility of dose reconstruction for deceased cases and was validated in other studies (14, 15). The RADRUE doses have been shown to be superior to the ‘official’ doses from the SRU which were found to be available for less then a third of cohort members and which are subject to substantial uncertainties. Cumulative individual bone marrow radiation doses were higher than in most studies of nuclear workers although still in the low-dose range (76.4 mGy vs 19.4 in Cardis et al.(17)).
Another strength of the study is the high interview participation rates, both for cases and controls and for alive and deceased study subjects. To minimize potential biases, interviewers were not aware of subjects’ case-control status and were carefully trained not to ask probing questions beyond those listed on the questionnaire. Similarly, doses were estimated without knowledge of subjects’ case-control status and members of the International Hematology Panel did not know the radiation dose of cases under review. Finally, the information collected during interviews allowed the investigation of the effects of a number of potential confounders not generally available in other studies of cleanup workers.
A limitation of the study was that the number of cases who died and, thus for whom proxy interviews were necessary, was sizeable. While the quality of data from the proxy interviews was more uncertain than the data collected directly from subjects, it was deemed sufficient for dose estimation based on the results of re-interviews and interviews of several co-workers for deceased cases (15). Measurement errors in dosimetry were complex, but because they were random, they probably attenuated the estimates of effect. To account for uncertainties in the dose estimation, we used Monte-Carlo procedures and estimated 10,000 random realizations of bone marrow dose for each subject. An average of these was used in the dose-response analysis. Thorough analysis of dose uncertainties and their potential effects on risk estimates is planned.
While there are study limitations, the observed association between radiation and leukemia is unlikely to be due to chance given the consistency of the dose-response relationships observed in both categorical and continuous analyses, for annual and cumulative doses, and in the entire dose range, as well as for doses less than 500 mGy, when adjusting for other measures of exposure at Chornobyl and for different leukemia cell subtypes. However, it must be recognized that recall bias, i.e., that cases could either preferentially recall their Chornobyl experience or else exaggerate such experiences leading to an overestimation of their dose, cannot be ruled out. The higher ERR seen for non-proxy compared with proxy cases (6.20 vs 2.45 per Gy, respectively) could be an indication of recall bias or, more likely, it could reflect greater error in estimating doses for proxy cases.
The modifying effect of age at exposure on risk of leukemia seen in the present study is in the opposite direction expected a priori from studies such as the atomic bomb survivors study (3), i.e. the risk was higher for workers exposed after age 45 compared to those less than 45, although the difference was not statistically significant. However, a similar effect has been observed in some studies of nuclear workers exposed to low-dose protracted radiation (18).
Most published studies of Chornobyl cleanup workers report an elevated risk of leukemia (1, 4), with much of the evidence coming from studies of Russian cleanup workers who received average doses of 100–200 mGy (5–8). Based on the dose and follow-up information for 168,000 workers from the Russian National Medical and Dosimetric Registry, Ivanov et al. (5) reported an increased risk of all leukemia with an ERR of 4.3 per Gy (n=48). Risk estimation was based on comparison of observed incidence with the national incidence of leukemia for males from the same age groups. Methodological concerns prompted Boice and Holm to question the validity of this analysis (19). In a more recent cohort analysis of 42 cases of non-CLL leukemia among 71,870 workers from the same registry, Ivanov et al. (6) reported a significantly increased ERR of 6.7 per Gy. Two earlier case-control studies from the same registry initially showed no significant trend with dose for all leukemia, leukemia excluding CLL or liquidators who worked in the 30-km zone in 1986–1987 (6), but a later analysis estimated significant ERRs ranging from 0.28 to 15.59 per Gy for essentially the same groups (7). The reasons for the differences in estimates are not clear, but the large uncertainties in “official” doses from the Chornobyl Registry and absence of rigorous histopathologic case verification are a concern.
Buzunov et al. (9) conducted an ecologic study of leukemia occurrence among approximately 175,000 liquidators in Ukraine using data from the State Registry of Ukraine and national leukemia morbidity statistics. Leukemia incidence rates for workers first employed in 1986, when doses were relatively high, were double those for workers employed in 1987, when doses were lower.
Our findings also can be compared with studies of nuclear workers who were exposed to low doses of radiation at low dose-rates (15,16). In a pooled analysis of workers from 15 countries, approximately 400,000 nuclear workers were monitored for external radiation. Despite the large number of workers, the confidence interval for the nearly 2-fold ERR per Gy for leukemia remained wide and included unity (ERR = 1.93 per Gy, 95%CI: <0, 8.47) (18). A recent analysis of leukemia mortality in the cohort of U.S. shipyard workers exposed to protracted low-level gamma radiation (20), also found a non-significant increase in risk with increasing radiation dose.
Among males exposed to acute radiation from the atomic bombs between the ages of 20 and 60 (similar to the present study), the ERR per Gy for non-CLL leukemia is approximately two-fold (based on the linear term of a linear quadratic dose-response relationship) (1). Because radiation-related leukemia risk has been shown to decrease with time since exposure, it is reasonable to predict that during the first 10–20 years of follow-up after the Chornobyl accident excess risk would be higher or approximately three-four-fold (2, 21). Thus, our results for non-CLL appear to be consistent with those from the atomic bomb survivor study and indicate no measurable difference in leukemia risk following acute or protracted radiation exposure.
A likely cause of the high proportion of CLL cases in our study (55%) compared with only about 40% reported by population-based cancer registries is the difference in the level of medical monitoring and diagnostic tools used (22, 23). Zent et al. (24) suggested that, due to the rather indolent nature of CLL, tumor registries may be missing as much as 38% of CLL compared with the incidence of CLL detected using sophisticated measures such as flow cytometric immunophenotypic analysis. Because annual medical exams including blood tests and a visit to a hematologist are mandatory for all cleanup workers registered in the SRU (25, 26), it would be expected that a large number of cases would be detected that would not have been diagnosed among people receiving routine medical care. Indeed, Gluzman et al. (26) showed a larger percent of CLL cases among Ukrainian Chernobyl cleanup workers 10–20 years after the accident compared with the age-and sex comparable general population of Ukraine (49% and 44%, respectively). The over-representation of CLL cases may also be due to the more benign clinical course and longer survival that led to a greater likelihood of ascertainment (a type of length-bias sampling) using our thorough case-finding protocol. Under-ascertainment of acute leukemia cases who died prior to being properly diagnosed or whose diagnoses could not be confirmed due to lack of histological materials, could also have resulted in over-representation of CLL cases. However, the potential over diagnosis of CLL and under diagnosis of non-CLL cannot account for our observed positive radiation dose-response relation for CLL since neither situation should be related to dose and because doses were estimated for similar proportions of CLL and non-CLL cases confirmed by the panel (79.6 and 84.2%, respectively, p=0.58, see Table 1).
The generally similar radiation effects we found for CLL and non-CLL is somewhat surprising in view of the lack of significantly increased radiation risks for CLL observed in most other studies (1, 17, 27, 28). One explanation is that the higher proportion of proxies interviewed for non-CLL cases compared with CLL cases (69 vs 51 %, respectively, p-value=0.14) could have resulted in less precise dose estimates for the non-CLL cases and therefore a reduction in the dose-response.
Another explanation may be related to the fact that most other studies are based on mortality data. Analyzing data from atomic bomb survivors, Ron et al. showed that incidence data had greater diagnostic accuracy than mortality data and provided more complete information on relatively nonfatal cancers (29). Finch and Linet have suggested that over a quarter of all cases of CLL may be asymptomatic for many years, and even after diagnosis survival is significantly longer compared to other types of leukemia (30). Thus, mortality data would underestimate, possibly substantially, the occurrence of CLL. Not surprisingly, recent mortality studies that evaluated dose-response for CLL separately had either negative findings (17, 31) or positive findings with a negative dose-response trend (20, 28, 32). Two recent incidence-based studies of radiation workers have shown an association between CLL and occupational radiation exposure (33, 34), with one study (33) reporting a significant increase in CLL among Czech uranium miners presumably due to a gamma radiation component of exposure and radon in the mines, and the other (34) reporting an elevated risk among radiologic technologists who worked during the early years when occupational doses were presumably high. In contrast, high-dose studies of populations treated with radiotherapy for a first primary cancer showed no increase in the incidence of CLL, whereas a significant increase was demonstrated for all other types of leukemia (35, 36). Due to the very low CLL incidence in Japan (2), data on the relation with radiation are not available from studies of atomic bomb survivors.
Some earlier genetic and molecular studies have shown that lymphatic malignancies differ from other types of leukemia, possibly explaining the apparent variation in response to radiation in the two types of leukemia (37, 38). However, in a recent review of the latest molecular, clinical, and epidemiologic evidence for radiation-associated risks of CLL, Richardson et al. (39) argue that the somatic mutations involved in CLL etiology are similar to those of other lymphatic neoplasms, and that the assumption that CLL is an exception to the principles of radiation carcinogenesis is without firm foundation. It is also notable that marked differences between the clinical course and morphological features of CLL diagnosed in Chornobyl cleanup workers and other populations have been demonstrated (25, 40, 41). Chornobyl-associated CLL cases were characterized by younger age, advanced stage of disease at presentation and rapid progression. Cleanup workers with large radiation doses had CLL characterized by high mutation rates in several genes associated with poor disease prognosis (25, 40).
In summary, we found a significant linear dose-response between Chornobyl-related radiation exposure among Ukrainian cleanup workers and risk of leukemia. Our finding of an association between CLL and ionizing radiation adds new information to the controversy regarding the effects of radiation on CLL (41–43). To further clarify these issues, we are extending the case-control study to ascertain cases for another six years (2001–2006).
IN MEMORIAM
We would like to dedicate this article to the memory of Drs. Gilbert Beebe and Geoffrey R. Howe and to acknowledge that without their tireless efforts over many years this study would not have been possible. We would also like to acknowledge Dr. Howe’s contributions to the establishment of the Ukrainian Cancer Registry in the early 1990s, which will be a lasting resource for Chornobyl research studies.
Acknowledgments
This research was supported by the Intramural Research Program of the U.S. National Cancer Institute, NIH, DHHS, and the Department of Energy. The U.S. Nuclear Regulatory Commission and the French Institute of Radioprotection and Nuclear Safety provided the initial funds for purchase of equipment. The authors are grateful to the Ministry of Health Care and the Academy of Medical Sciences of Ukraine for issuing special orders supporting the project activities in the area of interest.
Footnotes
Oblast is an administrative unit similar in size to a state or province.
References
- 1.UNSCEAR. (United Nations Scientific Committee on the Effects of Atomic Radiation) Effects. II. New York: United Nations; 2000. Report to the General Assembly, with Scientific Annexes. [Google Scholar]
- 2.Preston DL, Pierce DA, Shimizu Y, Cullings HM, Fujita S, Funamoto S, Kodama K. Effect of recent changes in atomic bomb survivor dosimetry on cancer mortality risk estimates. Radiat Res. 2004;162:377–89. doi: 10.1667/rr3232. [DOI] [PubMed] [Google Scholar]
- 3.Preston DL, Kusumi S, Tomonaga M, Izumi S, Ron E, Kuramoto A, Kamada N, Dohy H, Matsuo T, et al. Cancer incidence in atomic bomb survivors. Part III. Leukemia, lymphoma and multiple myeloma, 1950–1987. Radiat Res. 1994;137:S68–97. [PubMed] [Google Scholar]
- 4.Howe GR. Leukemia following the Chernobyl accident. Health Phys. 2007;93:512–5. doi: 10.1097/01.HP.0000281178.75068.e3. [DOI] [PubMed] [Google Scholar]
- 5.Ivanov VK, Tsyb AF, Gorsky AI, Maksyutov MA, Rastopchin EM, Konogorov AP, Korelo AM, Biryukov AP, Matyash VA. Leukaemia and thyroid cancer in emergency workers of the Chernobyl accident: estimation of radiation risks (1986–1995) Radiat Environ Biophys. 1997;36:9–16. doi: 10.1007/s004110050049. [DOI] [PubMed] [Google Scholar]
- 6.Ivanov VK, Tsyb AF, Gorsky AI, Maksioutov MA, Khait SE, Preston D, Shibata Y. Elevated leukemia rates in Chernobyl accident liquidators. BMJ. 2003 [Google Scholar]
- 7.Ivanov VK, Tsyb AF, Konogorov AP, Rastopchin EM, Khait SE. Case-control analysis of leukemia among Chernobyl accident emergency workers residing in the Russian Federation, 1986–1993. J Radiol Prot. 1997;17:137–157. [Google Scholar]
- 8.Konogorov AP, Ivanov VK, Chekin SY, Khait SE. A case-control analysis of leukemia in accident emergency workers of Chernobyl. J Environ Pathol Toxicol Oncol. 2000;19:143–51. [PubMed] [Google Scholar]
- 9.Buzunov V, Omelyanetz N, Strapko N, et al. Chernobyl NPP accident consequences cleaning up participants in Ukraine - health status epidemiologic study - main results. In: Karaoglou A, Desmet G, Kelly GN, et al., editors. The Radiological Consequences of the Chernobyl Accident. Minsk, Belarus: European Commission 16544 EN; 1996. pp. 871–8. [Google Scholar]
- 10.Romanenko AY, Bebeshko V, Hatch M, Bazyka D, Finch SC, Dyagil I, Reiss RF, Chumak VV, Bouville A, et al. The Ukrainian-American Study of Leukemia and Related Disorders Among Chornobyl Cleanup Workers from Ukraine: I. STUDY METHODS. Rad Res. 2008 doi: 10.1667/RR1402.1. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howe GR. Use of computerized record linkage in cohort studies. Epidemiol Rev. 1998;20:112–21. doi: 10.1093/oxfordjournals.epirev.a017966. [DOI] [PubMed] [Google Scholar]
- 12.Dyagil I, Adam M, Beebe GW, Burch JD, Gaidukova SN, Gluzman D, Gudzenko N, Klimenko V, Peterson L, et al. Histologic verification of leukemia, myelodysplasia, and multiple myeloma diagnoses in patients in Ukraine, 1987–1998. Int J Hematol. 2002;76:55–60. doi: 10.1007/BF02982719. [DOI] [PubMed] [Google Scholar]
- 13.Jaffe ES, Harris NL, Stein H, Vardiman JW. WHO Classification of Tumours. Lyon: Intl Agency for Research on Cancer; 2001. Pathology and Genetics: Tumous of Haematopoietic and Lymphoid Tissues; p. 352. [Google Scholar]
- 14.Chumak VV, Voilleque P, Bakhanova E, Golovanov I, Luckyanov N, Sholom S, Kryuchkov V, Bouville A. The Ukrainian-American Study Of Leukemia And Related Disorders Among Cleanup Workers From Ukraine: II. ESTIMATION OF BONE-MARROW DOSES. Rad Res. 2008 doi: 10.1667/RR1403.1. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kryuchkov V, Chumak VV, Anspaugh L, Cardis E, Bakhanova E, Golovanov I, Luckyanov N, Kesminiene A, Maceika E, et al. RADRUE method for reconstruction of external doses to Chernobyl liquidators in questionnaire-based studies. Health Phys. 2008 doi: 10.1097/HP.0b013e3181ac9306. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Preston DL, Lubin JH, Pierce DA, McConney ME. EPICURE User’s guide. Seattle, WA: Hirosoft International Corporation; 1993. [Google Scholar]
- 17.Cardis E, Vrijheid M, Blettner M, Gilbert E, Hakama M, Hill C, Howe G, Kaldor J, Muirhead CR, et al. The 15-Country Collaborative Study of Cancer Risk among Radiation Workers in the Nuclear Industry: estimates of radiation-related cancer risks. Radiat Res. 2007;167:396–416. doi: 10.1667/RR0553.1. [DOI] [PubMed] [Google Scholar]
- 18.Cardis E, Vrijheid M, Blettner M, Gilbert E, Hakama M, Hill C, Howe G, Kaldor J, Muirhead CR, et al. Risk of cancer after low doses of ionising radiation: retrospective cohort study in 15 countries. Bmj. 2005;331:77. doi: 10.1136/bmj.38499.599861.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boice JD, Jr, Holm LE. Radiation risk estimates for leukemia and thyroid cancer among Russian emergency workers at Chernobyl. Radiat Environ Biophys. 1997;36:213–4. [PubMed] [Google Scholar]
- 20.Matanoski GM, Tonascia JA, Correa-Villasenor A, Yates KC, Fink N, Elliott E, Sanders B, Lantry D. Cancer Risks and Low-Level Radiation in U. S. Shipyard Workers. J Radiat Res (Tokyo) 2007 doi: 10.1269/jrr.06082. [DOI] [PubMed] [Google Scholar]
- 21.Boice JD. Leukaemia, Chernobyl and Epidemiology. J Radiol Prot. 1997;17:129–133. [Google Scholar]
- 22.Dores GM, Anderson WF, Curtis RE, Landgren O, Ostroumova E, Bluhm EC, Rabkin CS, Devesa SS, Linet MS. Chronic lymphocytic leukaemia and small lymphocytic lymphoma: overview of the descriptive epidemiology. Br J Haematol. 2007;139:809–19. doi: 10.1111/j.1365-2141.2007.06856.x. [DOI] [PubMed] [Google Scholar]
- 23.Fedorenko ZP, Gorokh YL, Pushkar LO, Koutsenko LB. Bulletin of National Cancer Registry of Ukraine. Kyiv: Institute of Oncology of Academy of Medical Sciences of Ukraine; 2005. Cancer incidence in Ukraine, 2003–2004. [Google Scholar]
- 24.Zent CS, Kyasa MJ, Evans R, Schichman SA. Chronic lymphocytic leukemia incidence is substantially higher than estimated from tumor registry data. Cancer. 2001;92:1325–30. doi: 10.1002/1097-0142(20010901)92:5<1325::aid-cncr1454>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 25.Abramenko I, Bilous N, Chumak A, Davidova E, Kryachok I, Martina Z, Nechaev S, Dyagil I, Bazyka D, et al. Chronic lymphocytic leukemia patients exposed to ionizing radiation due to the Chernobyl NPP accident-With focus on immunoglobulin heavy chain gene analysis. Leuk Res. 2008;32:535–45. doi: 10.1016/j.leukres.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 26.Gluzman D, Imamura N, Sklyarenko L, Nadgornaya V, Zavelevich M, Machilo V. Patterns of hematological malignancies in Chernobyl clean-up workers (1996–2005) Exp Oncol. 2006;28:60–3. [PubMed] [Google Scholar]
- 27.Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation (BEIR VII-phase II) Health risks from exposure to low levels of ionizing radiation. Washington, DC: National Academy Press; 2005. [PubMed] [Google Scholar]
- 28.Schubauer-Berigan MK, Daniels RD, Fleming DA, Markey AM, Couch JR, Ahrenholz SH, Burphy JS, Anderson JL, Tseng CY. Chronic lymphocytic leukaemia and radiation: findings among workers at five US nuclear facilities and a review of the recent literature. Br J Haematol. 2007;139:799–808. doi: 10.1111/j.1365-2141.2007.06843.x. [DOI] [PubMed] [Google Scholar]
- 29.Ron E, Preston DL, Mabuchi K, Thompson DE, Soda M. Cancer incidence in atomic bomb survivors. Part IV: Comparison of cancer incidence and mortality. Radiat Res. 1994;137:S98–112. [PubMed] [Google Scholar]
- 30.Finch SC, Linet MS. Chronic leukaemias. Baillieres Clin Haematol. 1992;5:27–56. doi: 10.1016/s0950-3536(11)80034-x. [DOI] [PubMed] [Google Scholar]
- 31.Shilnikova NS, Preston DL, Ron E, Gilbert ES, Vassilenko EK, Romanov SA, Kuznetsova IS, Sokolnikov ME, Okatenko PV, et al. Cancer mortality risk among workers at the Mayak nuclear complex. Radiat Res. 2003;159:787–98. doi: 10.1667/0033-7587(2003)159[0787:cmrawa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 32.Boice JD, Cohen SS, Mumma MT, Dupree Ellis E, Eckerman KF, Leggett RW, Boecker BB, Brill AB, Henderson BE. Mortality among radiation workers at Rocketdyne (Atomics International), 1948–1999. Radiat Res. 2006;166:98–115. doi: 10.1667/RR3582.1. [DOI] [PubMed] [Google Scholar]
- 33.Rericha V, Kulich M, Rericha R, Shore DL, Sandler DP. Incidence of leukemia, lymphoma, and multiple myeloma in Czech uranium miners: a case-cohort study. Environ Health Perspect. 2006;114:818–22. doi: 10.1289/ehp.8476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Linet MS, Freedman DM, Mohan AK, Doody MM, Ron E, Mabuchi K, Alexander BH, Sigurdson A, Hauptmann M. Incidence of haematopoietic malignancies in US radiologic technologists. Occup Environ Med. 2005;62:861–7. doi: 10.1136/oem.2005.020826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kleinerman RA, Boice JD, Jr, Storm HH, Sparen P, Andersen A, Pukkala E, Lynch CF, Hankey BF, Flannery JT. Second primary cancer after treatment for cervical cancer. An international cancer registries study. Cancer. 1995;76:442–52. doi: 10.1002/1097-0142(19950801)76:3<442::aid-cncr2820760315>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 36.Boivin JF, Hutchison GB, Evans FB, Abou-Daoud KT, Junod B. Leukemia after radiotherapy for first primary cancers of various anatomic sites. Am J Epidemiol. 1986;123:993–1003. doi: 10.1093/oxfordjournals.aje.a114351. [DOI] [PubMed] [Google Scholar]
- 37.Boice JD, Jr, Inskip PD. Radiation-induced leukemia. In: Henderson ES, Lister TA, Greaves MF, editors. Leukemia. Philadelphia: W.B. Saunders; 1996. pp. 195–209. [Google Scholar]
- 38.Caligaris-Cappio F, Hamblin TJ. B-cell chronic lymphocytic leukemia: a bird of a different feather. J Clin Oncol. 1999;17:399–408. doi: 10.1200/JCO.1999.17.1.399. [DOI] [PubMed] [Google Scholar]
- 39.Richardson DB, Wing S, Schroeder J, Schmitz-Feuerhake I, Hoffmann W. Ionizing radiation and chronic lymphocytic leukemia. Environ Health Perspect. 2005;113:1–5. doi: 10.1289/ehp.7433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kryachok I, Polyshchuk O, Dyagil I, Abramenko I, Bazyka D, Bebeshko V. Comparative analysis of CLL in persons who suffered after Chernobyl accident and in unexposed CLLpatients. Haematologica. 2005;90:454A. [Google Scholar]
- 41.Hamblin TJ. Have we been wrong about ionizing radiation and chronic lymphocytic leukemia? Leuk Res. 2008;32:523–5. doi: 10.1016/j.leukres.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 42.Silver SR, Hiratzka SL, Schubauer-Berigan MK, Daniels RD. Chronic lymphocytic leukemia radiogenicity: a systematic review. Cancer Causes Control. 2007;18:1077–93. doi: 10.1007/s10552-007-9048-y. [DOI] [PubMed] [Google Scholar]
- 43.Linet MS, Schubauer-Berigan MK, Weisenburger DD, Richardson DB, Landgren O, Blair A, Silver S, Field RW, Caldwell G, et al. Chronic lymphocytic leukaemia: an overview of aetiology in light of recent developments in classification and pathogenesis. Br J Haematol. 2007;139:672–86. doi: 10.1111/j.1365-2141.2007.06847.x. [DOI] [PubMed] [Google Scholar]