Abstract
Disturbances in Ca2+ homeostasis have been implicated in a variety of neuropathological conditions including Parkinson’s disease (PD). However, the importance of store-operated Ca2+ entry (SOCE) channels in PD remains to be investigated. In the present study, we have scrutinized the significance of TRPC1 in 1-methyl- 4-phenyl-1, 2, 3, 6 -tetrahyrdro-pyridine (MPTP)-induced PD using C57BL/6 animal model or PC12 cell culture model. Both sub-acute and sub-chronic treatments of MPTP significantly reduced TRPC1, and tyrosine hydroxylase levels, but not TRPC3, along with increased neuronal death. Furthermore, MPTP induces mitochondrial dysfunction, which was associated with reduced mitochondrial membrane potential, decreased level of Bcl2, Bcl-xl, and an altered Bcl-xl/Bax ratio thereby initiating apoptosis. Importantly, TRPC1 overexpression in PC12 cells showed significant protection against MPP+ induced neuronal apoptosis, which was attributed to the restoration of cytosolic Ca2+ and preventing loss of mitochondrial membrane potential. Silencing of TRPC1 or addition of TRPC1 channel blockers decreased mitochondrial membrane potential, whereas activation of TRPC1 restored mitochondrial membrane potential in cells overexpressing TRPC1. TRPC1 overexpression also inhibited Bax translocation to the mitochondria and thereby prevented cytochrome c release and mitochondrial mediated apoptosis. Overall, these results provide compelling evidence for the role of TRPC1 in either onset/progression of PD and restoration of TRPC1 levels could limit neuronal degeneration in MPTP mediated PD.
INTRODUCTION
Neuronal protection or prevention of neuronal degeneration has been identified as therapeutic targets for many neurodegenerative diseases. Parkinson’s disease (PD) is the second most common neurodegenerative disease and afflicts ~1.8 % of the population by age 65 years (1). The neuropathological hallmark of PD is an irreversible loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) and their terminals in the striatum (2). Although, the majority (>85%) of PD cases are sporadic and the underlying molecular causes are unknown, postmortem studies have showed the involvement of several predisposing factors including viral infection and environmental toxins such as MPTP (3, 4). MPTP, a potent neurotoxin that selectively destroys the nigrostriatal dopaminergic neurons in humans, sub-human primates, and lower animals, mimics PD like symptoms (5, 6). MPTP is a highly lipophilic molecule, which is able to cross the blood brain barrier (7). Once inside the brain, the pro-toxin MPTP is rapidly converted into 1-methyl-4-phenylpyridinium ions (MPP+), the active neurotoxin, by monoamine oxidase B (8). Furthermore, MPP+ is selectively accumulated in dopaminergic neurons and is concentrated within mitochondria where it acts to inhibit electron transport chain, decreases mitochondrial membrane potential, and induces disturbances in Ca2+ homeostasis, which could eventually lead to neuronal loss (9–11). However, precisely how these neurons die and the factors involved are not yet established.
Changes in intracellular Ca2+ levels have been associated with both triggering/regulating and inhibiting neuronal apoptosis (12). In most cell type’s release of ER Ca2+ leads to the activation of store-operated calcium channels (SOCC), which initiates Ca2+ entry from the external media via the store operated calcium entry (SOCE) mechanism (13). Although, the molecular identity of the SOCE channel is not yet determined, TRPC1 has been demonstrated to be activated by store depletion per se (14, 15). Moreover, TRPC1 is important for critical processes such as cell proliferation, axonal guidance, and apoptosis. Besides TRPC1 is also ubiquitously expressed, including many regions of the brain such as cerebral cortex, hippocampus, cerebellum, amygdale and in the substantia nigra (16–22), which indicates that TRPC1 could have significance in the survival and function of neuronal cells. Thus, the present study was undertaken to not only identify the possible role of TRPC1 in neuronal growth and survival particularly in PD, but also to establish the mechanism of MPTP mediated neuronal loss. The data presented here demonstrate that MPTP induces neuronal loss by decreasing TRPC1 levels followed by the initiation of apoptosis mainly by disrupting mitochondrial membrane potential. Furthermore, TRPC1 overexpression prevented MPP+-induced cellular death by preserving loss of mitochondrial membrane potential, which in turns inhibits apoptosis.
MATERIALS AND METHODS
Animals
Male C57BL/6 mice, (1, 6 and 12 months) were obtained from Charles River Laboratories (Wilmington, MA) and 8–10 month older male C57BL/6J mice were obtained from Jackson Laboratory (Maine, USA). All animals were housed in a temperature controlled room under a 12/12-hour light/dark cycle with ad libitum access to food and water. All animal experiments were carried out as per the institutional guidelines for the use and care of animals. All efforts were made to minimize animal suffering, to reduce the number of animals used, and to utilize alternatives to in vivo techniques if available.
MPTP treatment
MPTP was dissolved in sterile saline and injected intraperitoneally (i.p). All the mice were subdivided into two groups (n=6; six mice in each group); each received different doses and schedules of MPTP administration. Age groups (1, 6 and 12 months) received only subacute MPTP injections, since sub-chronic MPTP treated mice (12 months) died during the procedure. For Sub-acute MPTP treatment animals were further sub-divided into two groups (n=6). Group I served as a control and received equivalent i.p. injections of saline. Group II mice received 25 mg kg−1 of MPTP (i.p.) for 5 consecutive days at 24h intervals. Animals were sacrificed 7 days after the last MPTP-injection. For Sub-chronic MPTP treatment animals were also sub-divided into two groups (n=8). Group I served as a control and received equivalent i.p. injections of saline, whereas group II mice received 15 mg kg−1 of MPTP (i.p.) on 10 consecutive days at 24h interval. Animals were sacrificed 7 days after the last MPTP-injection. The brain was removed from the skull and placed dorsal side up. Using a scalpel blade, a coronal cut was made adjacent to the inferior colliculi approximately at bregma −6.36 mm. A second cut was made approximately at bregma −2.54 mm, based on the mouse brain atlas (23). The ventral midbrain was dissected to ensure that there was no contamination of the hippocampus, cortex or cerebellum. Brain regions from 2–3 animals were pooled for each experiment.
Immunohistochemistry
Animals were anesthetized with ether after 7 days of MPTP treatment and perfused transcardially with phosphate-buffered saline (PBS) followed by paraformaldehyde (4%, w/v) in PBS and post-fixed in paraformaldehyde. The fixed tissue was serially sectioned at 40 µm with a freezing microtome (Microm HM400, Richard Allen Scientific, Kalamazoo, MI) throughout the entire midbrain region and used for immunohistochemical analysis of tyrosine hydroxylase (TH), caspase 3, Bcl-xl (Cell Signaling Technology, Danvers, MA) and TRPC1 (Santa Cruz Biotechnology, CA). A kit (Vector ABC Elite kit) from Vector Laboratories (Burlingame, CA) was used along with 3′3′ diaminobenzoic acid as a chromogen to develop the reaction in the presence of H2O2. For quantitative measurements, persons blind to the treatment counted the number of tyrosine hydroxylase and TRPC1 positive neurons in the substantia nigra pars compacta (SNpc). Measurements from four to six sections per brain were averaged to obtain one value per subject. For Nissl staining the sections were mounted onto gelatin-coated slides and rehydrated for 45 min in 0.1M phosphate-buffered saline (PBS), pH 7.2. The sections were incubated with the NeuroTrace blue fluorescent Nissl stains (Molecular Probes, Eugene, OR) and mounted with Vectashield mounting medium for fluorescence with DAPI.
Cell culture, MPP+ treatment, Transfection and Reagents
PC12 cells (adrenal gland; Pheochromocytoma) were obtained from American Type Culture Collection (ATCC, Manassas, VA). They were maintained in poly-d lysine coated dishes (BD Biocoat, MA) in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with heat-inactivated 10% horse serum (HS) (Gibco, MD) and 5% fetal bovine serum (FBS) (Gibco, MD), 100 units/ml penicillin, 100 µg/ml streptomycin in a water-saturated atmosphere of 5% CO2 at 37 °C. The cells were cultured in the presence of MPP+ for 24 h to mimic PD like conditions. Following the above cell treatment protocol, cell viability was measured by using the Vybrant MTT cell proliferation assay kit (Molecular Probes, Eugene OR). Absorbance was read at 570 nm (630 nm as a reference) on a microplate reader (Molecular Device, Sunnyvale, CA). Cell viability was expressed as a percentage of the control culture. For adenoviral expression PC12 cells were infected with a MOI of 5 pfu of AdTRPC1 (24). For transient transfection (TRPC1 shRNA & TRPC3 shRNA), cells were transfected in OptiMEM with Lipofectamine reagent 2000 (Invitrogen, Carlsbad, CA) using standard procedures and were used 48-h post-transfection (25).
Western blot analyses
To perform Western blot analyses, crude membrane and cytosolic fraction were prepared from tissues and PC12 cells by using Fraction PREP cell fractionation kits (Biovision, CA). Mitochondrial fractions were isolated using mitochondrial isolation kits (Sigma-Aldrich). Protein concentrations were determined, using the Bradford reagent (Bio-Rad), and resolved on NuPAGE 4–12% Bis-Tris gel (Invitrogen, CA), transferred to polyvinylidene difluoride (PVDF) membranes, and probed with respective antibodies. A 1:200 dilution of the primary antibody was used to probe for TRPC1 (15) and TRPC3 (Sigma-Aldrich). The remaining antibodies were used at a 1:1000 dilution except actin (1:5000, Calbiochem, NJ). Peroxidase-conjugated respective secondary antibodies were used to label the proteins and detected using ECL reagent and Bio-Rad Quantity One (CA) system.
Assessment of apoptosis
The vibrant apoptosis assay kit was used to assess apoptosis as per the manufacturer instructions (Molecular Probes, Eugene, OR). This kit distinguishes apoptotic and necrotic cells by lipid dye (YO-PRO-1) and propidium iodide (PI) staining. The cells were visualized at 40× using a fluorescence microscope with filters appropriate for distinguishing fluorescein (FITC) and rhodamine (TRITC) emission spectra. The dead and necrotic cells show bright red rhodamine fluorescence, whereas apoptotic cells exhibit green fluorescence. The total number of apoptotic and necrotic cells were counted, and the percentages of cells exhibiting apoptosis/necrosis were calculated.
Confocal Microscopy
For immunofluorescence, PC12 cells were grown on coverslips, washed with PBS and fixed for 30 min using 4% paraformaldehyde. The cells were then permeabilized using cold methanol and blocked for 1 hour with horse serum (5% in PBS). For TRPC1 staining, cells were treated with TRPC1 antibody at 1:100 dilution, washed and labeled with FITC-linked anti-rabbit secondary antibody (1:100 dilutions). Confocal images were collected using an MRC 1024-krypton/argon laser scanning confocal equipped with a Zeiss LSM 510 Meta photomicroscope.
Calcium Measurements
PC12 cells were cultured on glass bottom dishes for 24 h and were treated for another 24 h with MPP. After incubation cells were incubated with 2 µM fura-2 (Molecular Probes) for 45 min at 37 °C under an atmosphere of 5% CO2-95% air. The cells were washed twice with Ca2+ free buffer (10 mM HEPES, 120 mM NaCl, 5.4 mM KCl, 1 mM MgCl2, 10 mM glucose, pH 7.4). For fluorescence measurements, the fluorescence intensity of Fura-2-loaded control cells was monitored with a CCD camera-based imaging system (Compix) mounted on an Olympus XL70 inverted microscope equipped with an Olympus 40× (1.3 NA) fluor objective. A monochrometer dual wavelength enabled alternative excitation at 340 and 380 nm, whereas the emission fluorescence was monitored at 510 nm with an Okra Imaging camera (Hamamatsu, Japan). The images of multiple cells collected at each excitation wavelength were processed using the C imaging, PCI software (Compix Inc., Cranbery, PA), to provide ratios of Fura-2 fluorescence from excitation at 340 nm to that from excitation at 380 nm (F340/F380). Analog plots of the fluorescence ratio (340/380) from an average of more than 50 cells are shown.
Measurement of mitochondrial membrane potential
Measurements of mitochondrial potential were carried out using lipophilic cation 5,5',6,6'-tetrachloro-1,1',3,3'-tetraethyl-benzimidazolcarbocyanine iodide (JC-1) dye as per the manufacture instructions (Molecular Probes, Eugene, OR). The dye undergoes a reversible change in fluorescence emission from green to red as the mitochondrial membrane potential increases. Thus, the JC-1 monomer emits green cytoplasmic fluorescence in inactive mitochondria, while the aggregated form emits a red fluorescence in mitochondria with high membrane potential and functional capacity. PC12 cells were loaded with JC-1 (1 µg/ml) for 10 min in the same medium and conditions Confocal images were collected using an MRC 1024-krypton/argon laser scanning confocal equipped with a Zeiss LSM 510 Meta photomicroscope. Images were analyzed using the J-image software. Mitochondria depolarization is specifically indicated by a decrease in the red to green fluorescence intensity ratio. Regions of interest stained by JC-1 were selected corresponding to at least twenty individual cells per independent experiment.
Mitochondrial transmembrane potential was also measured using fluorescent cationic dye rhodamine 123. It has been shown that the uptake of rhodamine 123 into mitochondria is a function of mitochondrial transmembrane potential. Depolarization of mitochondrial membrane potential results in the loss of Rh123 from the mitochondria and a decrease in intracellular fluorescence (26). PC12 cells (4×104) were incubated with MPP+ (24 h) followed by 20 min incubation with 10 µM of rhodamine123 at 37 °C in DMEM media. The fluorescence signals of the dye were measured immediately at an excitation wavelength of 488 nm and an emission wavelength of 510 nm using a fluorescence microplate reader. At the initial readings, FCCP (1.5 µM) and oligomycin (0.25 µg/mL) were added, in order to make the maximum retention of Rh123, and the fluorescence signals were measured in 488/510 nm. The difference between the fluorescence monitored before and after the addition of FCCP and oligomycin was used to evaluate the ψmit. The results were expressed as percentage of increase or decrease above control fluorescence.
Statistical analysis
Data analysis was performed using Origin 7.0 (OriginLab, Northampton, MA). Statistical comparisons were made using Student’s t test and analysis of variance (ANOVA) followed by Duncan’s multiple range test (DMRT). Experimental values are expressed as means ± S.D. Differences in the mean values were considered to be significant at p < 0.05.
RESULTS
Vulnerability of dopaminergic neurons in SNpc to MPTP
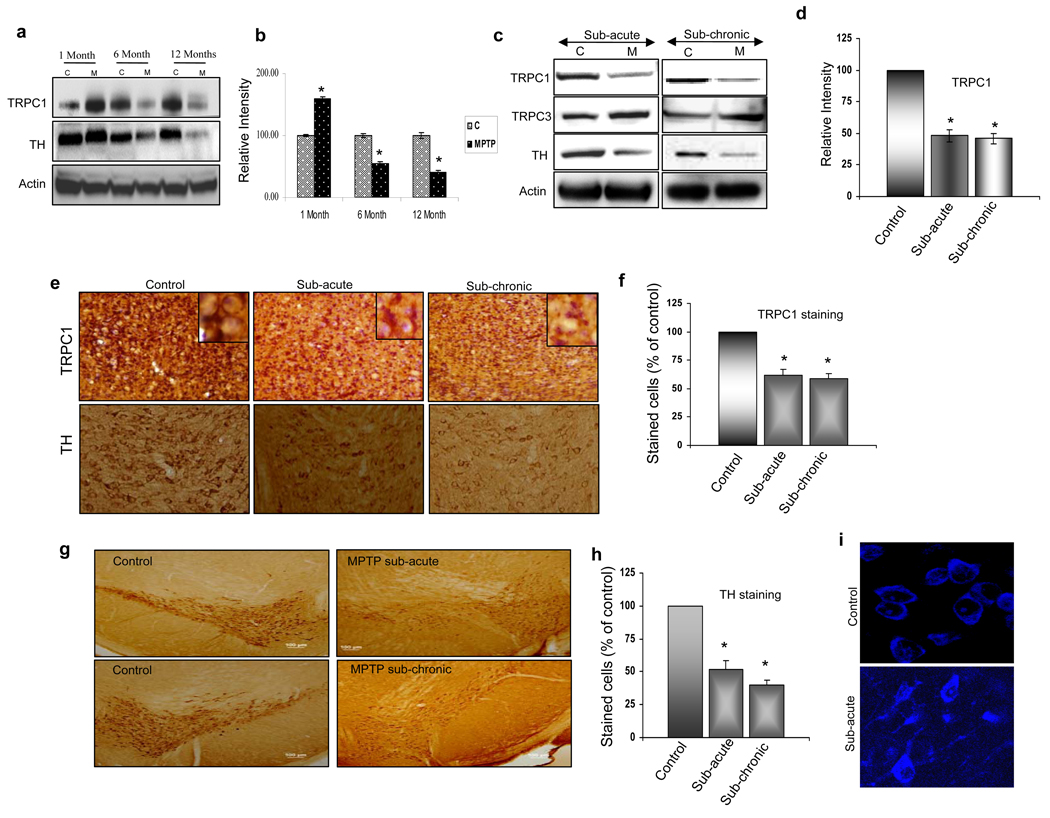
To define the role of age in amending neuronal loss in sub-acute MPTP treatment, we first compared the MPTP-induced neuronal degeneration between 3 different aged mice with respect to tyrosine hydroxylase (TH) expression. TH is the rate limiting enzyme of dopamine synthesis, and is a marker for dopaminergic neurons (27). As indicated in Fig. 1a, progressive loss in TH was only observed in the SNpc region of 6 and 12 month, but not in 1 month old MPTP-treated mice (Fig. 1a statistical analysis is shown in 1b). Age matched mice injected with saline were used as their respective controls. Similarly, TRPC1 levels, but not TRPC3 (data not shown), was decreased in 6 and 12 month old MPTP-treated mice. Furthermore, a significant increase in TRPC1 levels was observed in 1 month old MPTP-treated mice. Whereas, no change in actin protein levels was observed in all age group mice. These results are consistent with other studies, which have demonstrated that older animals are much more susceptible to MPTP than younger ones (28). Based on these results, we chose to use 8–10 month old mice for the remaining studies.
Figure 1. Sub-acute and sub-chronic treatments of MPTP decreased TRPC1 and TH levels in SNpc.
(a) Western blot on crude membranes and cytosolic fractions prepared from substantia nigra of age different (1, 6 & 12 months) control (C) and sub-acute MPTP treated mice (M). The upper blot (crude membranes that are representative of 2–3 independent experiments) was probed with anti-TRPC1 antibodies, the lower portion of the same blot was probed with anti-actin antibodies and the middle blot (cytosolic fraction) was probed with anti-tyrosine hydroxylase (anti-TH) antibodies. (b) Bar graph indicating the quantitative analysis of TRPC1 protein.*, values that are significantly different from their respective controls (p<0.05). (c) Western blot performed on crude membranes and cytosolic fraction prepared from substantia nigra of control (C; 8–10 month older), and sub-acute or sub-chronic MPTP treated mice (M). The crude membranes were probed with anti-TRPC1 and anti-TRPC3 antibodies and the lower portion of the same blot was probed with anti-actin antibodies. Cytosolic fraction was probed with anti-TH antibody. (d) Bar graph indicating the quantitative analysis of TRPC1 protein from 3–4 independent experiments.*, values that are significantly different from their respective controls (p<0.05). (e) TRPC1-immunoreactivity in the substantia nigra pars compacta (SNpc). Mice were received either saline (control) or a sub-acute or sub-chronic MPTP treatment (× 20). Bar graph indicating the quantitative analysis of TRPC1-immunopositive neurons is shown in (f). *, indicates values that are significantly different from their respective controls (p<0.05). (g) TH-immunoreactivity in the SNpc, mice were received either saline (control) or a sub-acute or sub-chronic MPTP treatment (× 20). (h) Bar graph indicates the quantitative analysis of TH-immunopositive neurons.*, denotes values that are significantly different from their respective controls (p<0.05 using date from 3–4 independent experiments). (i) Nissl staining of SNpc. Mice were received either saline (control) or sub-acute MPTP treatment (× 63).
To further establish the conditions, both sub-acute and sub-chronic MPTP treatment was performed on C57BL/6J, 8–10 month old mice. Importantly, both sub-acute and sub-chronic MPTP treatments showed a significant decrease in cytosolic TH levels along with a reduction in TRPC1 levels in dopaminergic neurons of the SNpc (Fig. 1c). In contrast, TRPC3 levels were increased after MPTP treatment, whereas no change was observed in actin levels. Quantification of the data indicated a more than 50% decrease in TRPC1 levels under both sub-acute and sub-chronic conditions (Fig. 1d). Immunohistochemistry results further confirmed the impact of MPTP treatment and the diminution of TRPC1 levels on the degeneration process, where both TH (Fig. 1e, f, g & h) and TRPC1-positive neurons were significantly decreased upon MPTP-treatment when compared to their respective controls (Fig. 1e & f). Higher magnifications for plasma membrane TRPC1 staining are shown as insets, which indicated the loss of TRPC1 membrane staining in both sub-acute and sub-chronic treated mice. Nissl staining in the SNpc was also used, which showed that MPTP treatment severely affect dopaminergic neurons. Neuronal morphology was significantly altered; mainly the cells appeared shrunken with creased cell membranes along with diffused Niss1 staining, which is consistent with neuropathology (Fig. 1i).
MPTP induces apoptotic degeneration of dopaminergic neurons in SNpc
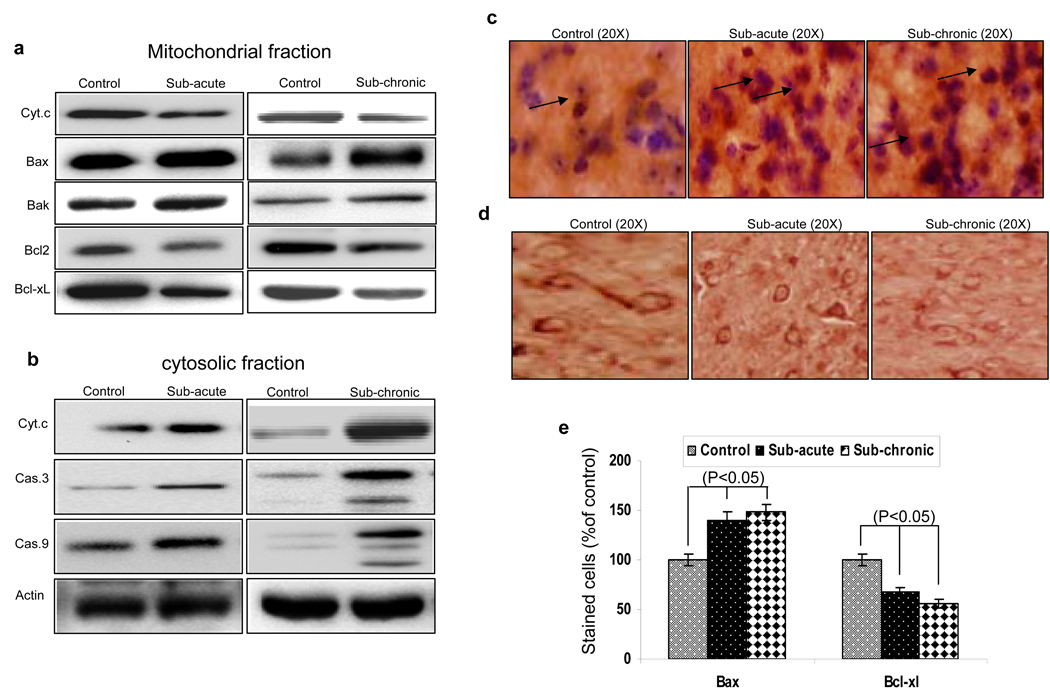
Mitochondrial membrane potential (ψmit) has been shown to play a critical role in cell death. To establish if loss of mitochondrial membrane potential due to MPTP treatment increases cell death, levels of pro- and anti-apoptotic proteins in both cytosolic and mitochondrial fractions were assessed. As indicated in Fig. 2a and 2b, increased Bax and Bak translocation/levels were observed in mitochondrial fractions in both sub-acute and sub-chronic MPTP treatments. Consistent with this cytochrome c level were decreased in mitochondrial fractions, with subsequent increase in the cytosolic fractions isolated from MPTP-treated mice. Importantly anti-apoptotic proteins such as Bcl2 and Bcl-xl levels were decreased in the mitochondrial fractions (Fig. 2a), indicating that MPTP initiates apoptosis. To test whether caspases are involved in the mitochondrial-mediated apoptotic pathway, cytosolic fractions of control and MPTP-treated SNpc were immunobloted for both caspase-9 and its downstream partner caspase-3. Although, caspase-3 and caspase-9 along with the presence of the two cleaved products of about 32–35 kDa were observed only in sub-chronic MPTP treatment, a significant increase in pro-caspase levels (caspase 3 & 9) were observed in sub-acute MPTP treatment. To further confirm these results immunohistochemistry was performed on control and MPTP treated animals. As indicated in Fig. 2c and 2e, a significant increase in the Bax expression was observed in both sub-acute and sub-chronic MPTP treated animals. Similarly, levels of anti-apoptotic protein Bcl-xl (Fig. 2d & 2e) and Bcl2 (data not shown) were decreased in the SNpc region upon MPTP treatment.
Figure 2. MPTP induces cell death by initiating apoptosis.
(a) Western blot performed on mitochondrial fraction prepared from substantia nigra of control and sub-acute or sub-chronic MPTP treated mice. The blots were probed with anti-cytochrome c, anti-Bax, anti-Bak, anti-Bcl2 and anti-Bcl-xl antibodies. (b) Western blot performed on cytosolic fraction prepared from substantia nigra of control and sub-acute or sub-chronic MPTP treated mice. The blots were probed with anti-cytochrome c, anti-caspase 3, anti-caspase 9 and anti-actin antibodies. The immunostaining of Bax (c), and Bcl-xl (d) are shown in SNpc both with and without MPTP treatment. Bar graphs indicating the quantitative analysis of Bax and Bcl-xl immunopositive neurons are shown in (g). *, indicates values that are significantly different from their respective controls (p<0.05)
Significance of TRPC1 in dopaminergic neurons survival
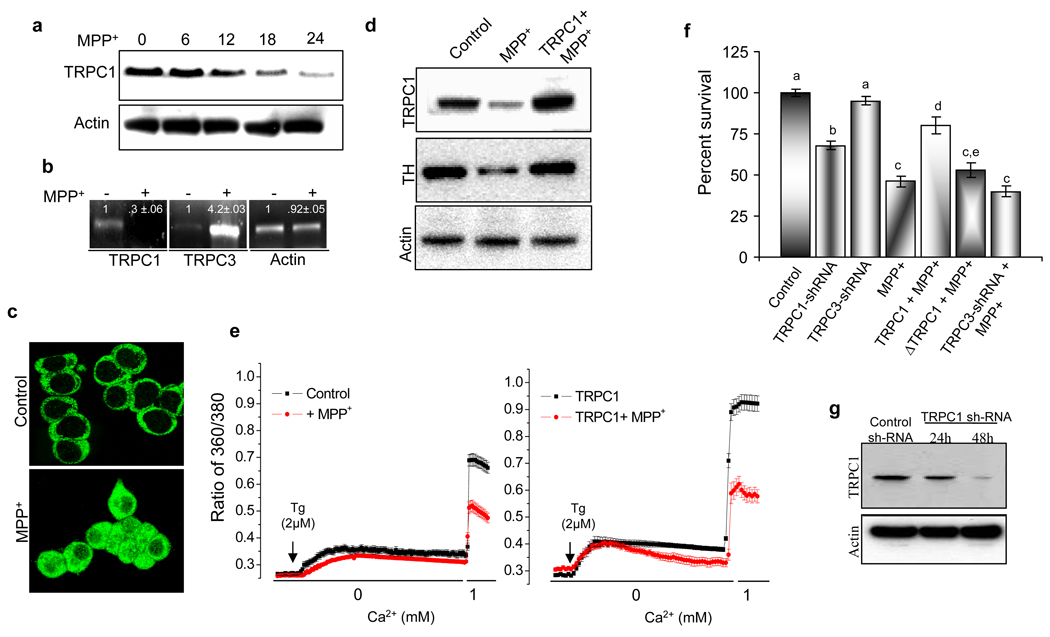
To validate our in vivo animal evidence and to determine the possible significance of TRPC1 in mediating neuronal survival, we further evaluated whether similar conclusions would be obtained in cell culture model. To address this, we first examined the effects of MPP+ on membrane TRPC1 protein levels in PC12 cells. As depicted in Fig. 3a, no significant changes were observed in membrane TRPC1 levels when incubated with 250 µM MPP+ for an initial period of 6 h. However, prolonged incubation with MPP+ (12–24 h) showed a significant decrease in TRPC1 protein and mRNA levels. In contrast, an increase in TRPC3 mRNA levels, but no significant change in actin levels was observed indicating that MPP+ specifically targeted TRPC1 (Fig. 3b). These results are in agreement with our previous studies, where TRPC1 levels were decreased upon MPP+ treatment in SH-SY5Y cells (22). Further, immunocytochemical studies showed that the TRPC1 was expressed in the plasma membrane; whereas treatment with 250 µM MPP+ for 24 h significantly decreased plasma membrane staining of TRPC1, with most of staining now observed in the cytosol (Fig. 3c). Incubation of PC12 cells without the TRPC1 antibody did not show any membrane staining (data not shown).
Figure 3. TRPC1 overexpression promotes cell survival.
(a) Western blot on crude membranes prepared from PC12 cells treated with MPP+ (250 µM) in a time-dependent manner (0, 6, 12, 18, and 24 h). The upper blot was probes with anti-TRPC1 antibody, and the lower blot was probed with anti-actin antibody. (b) Representative RT-PCR analysis of TRPC1, TRPC3, and actin using their respective primers (quantification of the bands is shown in the figure). (c) Localization of endogenous TRPC1 protein in control and MPP+-treated PC12 cells. (d) Western blot on crude membranes and cytosolic fraction prepared from control PC12 cells, MPP+-treated cells and TRPC1 over-expressing cells (transiently transfected with Ad-TRPC1 encoding virus for 24 h) treated with MPP+ for 24 h. The crude membranes blots were probed with anti-TRPC1 and anti-actin antibodies. The cytosolic fraction blot was probed with anti-TH antibody. Blots from crude membranes and cytosolic fraction are representative of two independent experiments. (e) Calcium imaging was performed in the presence of thapsigargin (2 µM) in control, Control+ MPP+, TRPC1 overexpressing cells, and TRPC1 over-expressing cells treated with MPP+. Calcium entry was measured by the addition of 1mM calcium and traces shown here are the mean of 50–60 cells. (f) MTT assays performed on control PC12 cells, MPP+-treated cells, TRPC1 silenced cells, TRPC1 over-expressing cells treated with MPP+, ΔTRPC1 transfected cells treated with MPP+ and TRPC3 silenced cells treated with MPP+. Values not sharing a common superscript differ significantly at p<0.05 (DMRT). (g) Western blots of control and TRPC1 sh-RNA (24–48 hours) probed with TRPC1 or actin antibodies.
To examine the effect of TRPC1 expression on the survival of PC12 cells, we transiently overexpressed TRPC1 in PC12 cells and its role in MPP+ toxicity was determined. A multiplicity of infection 5 for Adnovirus-TRPC1 was used to infect PC12 cells; we have previously used this virus to overexpress TRPC1 in SH-SY5Y and human submandibular gland (HSG) cells (22, 24). Addition of MPP+ in control cells for 24 hours again decreased TRPC1 levels which are consistent with the results demonstrated in Fig. 3a. In contrast, TRPC1 levels were restored (similar to control) in MPP+ treated PC12 cells that overexpress TRPC1 (Fig. 3d). Furthermore, MPP+ treatment significantly reduced TH level, whereas TRPC1 overexpression prevented MPP+ mediated decrease of TH (Fig.3d). Re-probing the same blots with anti-actin antibodies showed no decreases in actin protein levels, indicating the specificity of the loss or gain of particular proteins (TRPC1 and TH) under these conditions. To determine the effect of MPP+ on store-operated Ca2+ entry (SOCE), we treated PC12 cells with thapsigargin (Tg, a SERCA pump blocker) in Ca2+-free medium. As shown in figure 3e, PC12 cells treated with Tg showed a rapid release of Ca2+ from the ER, followed by Ca2+ influx through the plasma membrane (upon addition of 1 mM Ca2+). Importantly, a striking difference was observed in PC12 cells treated with MPP+, where the amount of both Ca2+ release from the ER as well as Ca2+ influx across the plasma membrane were decreased (traces indicate average data from at least 50 individual cells), which could be due to the loss of TRPC1. To demonstrate that indeed TRPC1 is important for SOCE in PC12 cells, overexpression of TRPC1 significantly increased SOCE. Although, TRPC1 overexpressing cells treated with MPP+ showed a decrease in Ca2+ influx (as compared with TRPC1 alone), this decrease was not significant than control untreated cells (Fig. 3e). Furthermore, TRPC1+MPP+ treated cells showed an increased SOCE when compared with control+ MPP+ treated cells, indicating that overexpression of TRPC1 can restore SOCE.
We then investigated the function of TRPC1 mediated Ca2+ influx in PC12 cells. TRPC1 has been shown to play a key role in cell proliferation and survival (21, 22, 29), thus we anticipated that perhaps TRPC1 would be necessary for the survival of these cells. To confirm our hypothesis we assess viability (using MTT assay) on control and TRPC1 overexpressing cells, which were treated with MPP+. Cells treated with MPP+ showed a significant decrease in cell survival as compared with control untreated cells (Fig. 3f). In contrast, TRPC1 overexpressing cells showed resistance to cell death induced by MPP+ and showed increased survival. Importantly, overexpression of ΔTRPC1 (truncated TRPC1 after S5 (aa 567–793)), which has been shown to decrease SOCE (30), showed no protection against MPP+-induced cell death. These results confers that TRPC1 mediated Ca2+ influx is necessary for cell survival upon MPP+-induction. Furthermore, TRPC1 silencing also showed a decrease in cell survival (Fig. 3f), whereas no significant decrease was observed in TRPC3 silenced PC12 cells. Although, TRPC3 silenced cells treated with MPP+ showed a decrease in cell survival, this decrease was not significantly different from control MPP+ treated cells (Fig. 3f).
TRPC1 protects the cells against MPP+ mediated death by preserving mitochondrial membrane potential
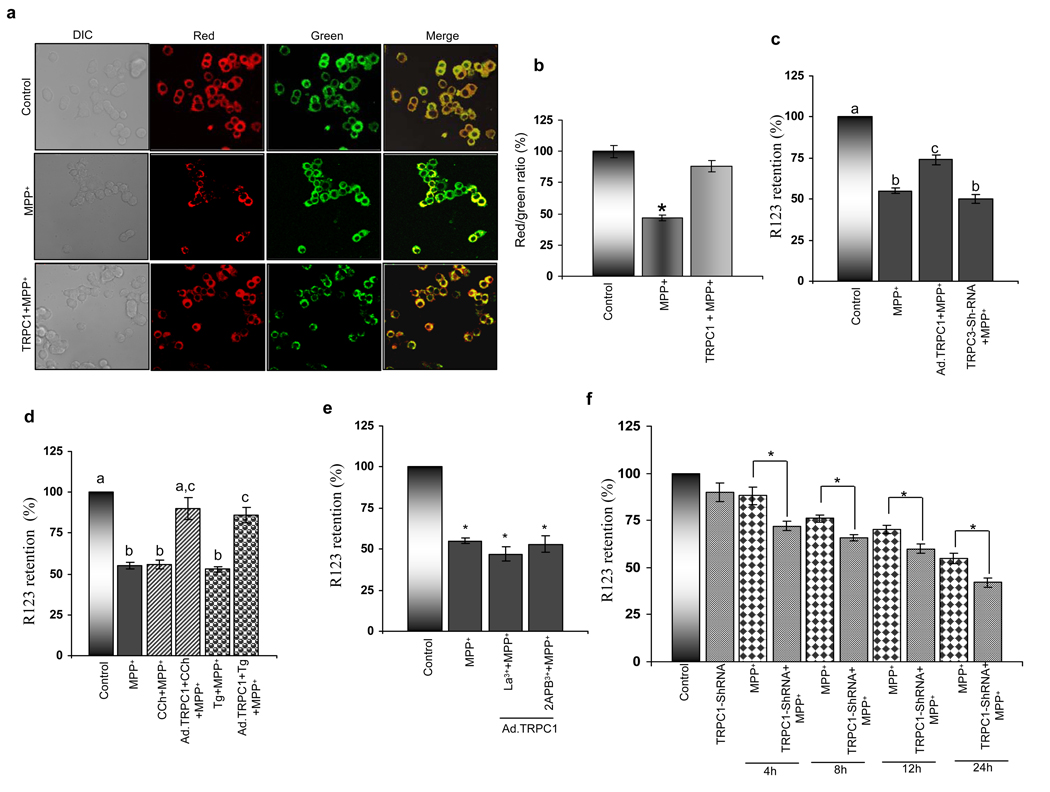
Mitochondrial dysfunction is an early event induced by MPTP. Thus, we investigated the role of TRPC1 overexpression on MPP+ mediated mitochondrial membrane potential (Δψmit) using either rhodamine 123 or JC-1 dyes. We used JC-1, since it is capable of entering selectively into mitochondria and can reversibly change its color from green to red as the membrane potentials increases, whereas cells with low Δψmit, maintain or re-acquire monomeric form of JC-1, thus showing only green fluorescence. Thus the ratio of red versus green fluorescence is an indicative of change in Δψmit. Consistent with previous report (18) cells treated with MPP+ demonstrated a decreased ratio (red/green), indicating a decrease in mitochondrial membrane potential (Fig. 4a & 4b). In contrast, overexpression of TRPC1 increases this ratio (compared to MPP+ treated cells), suggesting that TRPC1 overexpression restores membrane potential and could inhibit mitochondrial dysfunction.
Figure 4. Calcium influx via TRPC1 improves mitochondrial membrane potential upon MPP+ treatment.
(a) JC-1 dye was used to measure the mitochondrial membrane potential of control PC12 cells, MPP+-treated and TRPC1 overexpressing cells treated with MPP+. Pictures shows bright field, green channel fluorescence, red channel fluorescence and green/red merge images of JC-1 in PC12 cells (b) Ratios of fluorescence intensities in the red and green channels of the control PC12 cells, MPP+-treated cells and TRPC1 over-expressing cells treated with MPP+. (c) Mitochondrial membrane potential was evaluated by the capacity of mitochondria from PC12 cells to take up the fluorescent cationic dye Rh123. (d) Cells were incubated with MPP+ in the presence or absence of TRPC channel agonist carbachol, thapsigargin, and (e) TRPC channel antagonists 2-APB and La3+. CCh, Tg, La3+ and 2APB were added 10 minutes prior to the addition of MPP+. Values not sharing a common superscript differ significantly at p<0.05 (DMRT). (f) The effect of TRPC1 knockdown and MPP+ treatment in TRPC1 knockdown cells on mitochondrial membrane potential; the bar graph represents three combined experiments. *, indicates values that are significantly different from their respective controls (p<0.05)
To have more conclusive evidence we also used rhodamine 123 to elucidate the role of TRPC1 in regulating MPP+ mediated loss of Δψmit. As expected, MPP+ treatment resulted in reduction of Δψmit as compared with control untreated cells (Fig. 4c). In contrast, cells overexpressing TRPC1 showed significant increase in membrane potential than MPP+ alone treated cells. However, MPP+-induced mitochondrial depolarization was not significantly different in TRPC3 shRNA transfected cells. Importantly, activation of TRPC1 by carbachol (CCh) or thapsigargin (Tg) significantly enhanced the membrane potential in TRPC1 overexpressing cells, but not in control cells (Fig. 4d). Addition of CCh or Tg in control cells without MPP+ treatment did show any significant loss in Δψmit (data not shown). Furthermore, blocking of TRPC1 channel activity either by La3+ (TRPC1 channel blocker) or 2-APB (which indirectly effect TRPC1 function), inhibited TRPC1 mediated restoration of Δψmit (Fig. 4e). To demonstrate if silencing of TRPC1 itself leads to loss in mitochondrial membrane potential, we silenced TRPC1 (using sh-TRPC1 for 48 hours; Fig. 3g) and mitochondrial membrane potential was determined. Although, loss of TRPC1 by itself did not cause any significant change in the Δψmit, addition of MPP+ in TRPC1 knockdown cells showed a significant decrease in mitochondrial membrane potential at every time point tested when compared with MPP+ alone (4–24 hours) (Fig. 4f). Interestingly, treatment of MPP+ for 4–8 hours (condition which does not decrease TRPC1 levels, Fig. 3a) in control cells also showed a significant decrease in Δψmit, which was further decreased in TRPC1-silenced cells, indicating that knockdown of TRPC1 increases susceptible to MPP+ mediated toxicity. Together, the results presented here suggest that TRPC1 restores MPP+ mediated loss in Δψmit, which could be critical for the protection of neuronal cells.
TRPC1 overexpression protects PC12 cells against MPP+ induced apoptosis
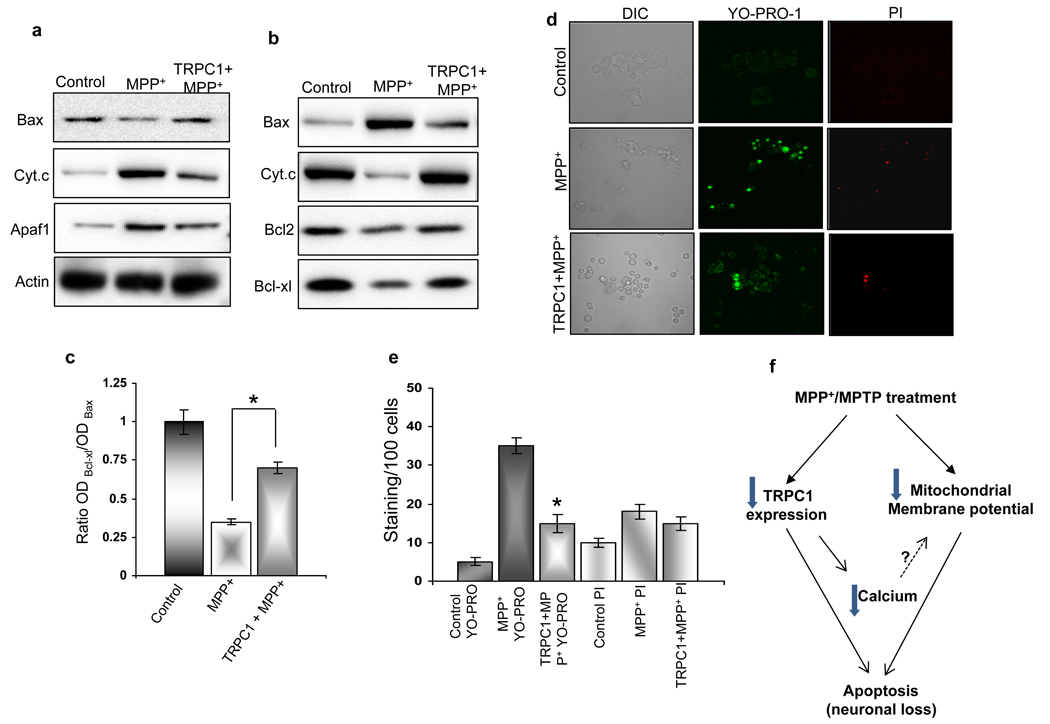
A change in membrane potential usually alters the equilibrium between the pro- and anti-apoptotic proteins in the mitochondria. Therefore, we sought to confirm the anti-apoptotic effects of TRPC1 by studying the proteins involved in the mitochondrial-mediated apoptotic process. The apoptotic pathway is largely mediated through Bcl2 family proteins, which include both pro-apoptotic members such as Bax, Bak, and BNIP3 that promote mitochondrial permeability and anti-apoptotic members such as Bcl2 and Bcl-xl that inhibit their effects (31). The disruption of mitochondrial transmembrane potential and the subsequent cytochrome c release have been considered as the early phenomena in the apoptotic process (32). As expected, addition of MPP+ for 24 hrs, showed elevated Bax translocation from cytosol to the mitochondria (Fig. 5a & 5b), along with subsequent decrease in mitochondrial Bcl2 and Bcl-xl protein levels (Fig. 5b). The ratio of Bcl-xl to Bax is shown in Fig. 5c, which again indicates that MPP+ treatment increases pro-apoptotic proteins. The Bax translocation into mitochondria and release of cytochrome c upon MPP+ treatment was demonstrated in the subcellular fractionation using anti-Bax and anti-cytochrome c antibodies. Also the APAF1 protein level in cytosolic fractions was significantly higher in MPP+ treated cells than untreated control cells. Importantly, Bax translocation to the mitochondria and cytochrome c release were significantly attenuated in TRPC1 overexpressing cells. Furthermore, TRPC1 overexpressing cells showed restored levels of mitochondrial Bcl2 and Bcl-xl and decreased cytosolic APAF1 levels when compared with MPP+ treated cells, while no significant changes were observed in actin levels (Fig. 5a & 5b). Cumulatively, these results suggest that the reduction in Bax translocation and cytochrome c release due to TRPC1 overexpression limits the activation of other downstream members of the apoptotic cascade.
Figure 5. TRPC1 promotes cell survival by restoring mitochondrial membrane potential and inhibiting apoptosis.
Western blot performed on cytosol (a) and mitochondrial fraction (b) of control PC12 cells, MPP+-treated cells and TRPC1 overexpressing cells treated with MPP+. Cytosolic fractions were probed with anti-Bax, anti-cytochrome c, anti-Apaf1 and anti-actin antibodies. The mitochondrial fractions were probed with anti-Bax, anti-cytochrome c, anti-Bcl2 and anti-Bcl-xl antibodies. (c) Bcl-xl/Bax ratio. Blots from cytosolic and mitochondrial fractions are representative of three independent experiments. (d) Marker for apoptosis (YO-PRO-1) and necrosis (PI) were used to stain control PC12 cells, MPP+-treated cells and TRPC1 over-expressing cells treated with MPP+. Fluorescence images were taken immediately using 40× objectives and the red and green cells were counted along with total number of cells. (e) Mean bar graph from 500–1000 cells in each group. *, denotes values significantly different from its counterpart (p<0.05 values are from at least 4 independent experiments). (f) Proposed model for the role of TRPC1 in MPTP/MPP+ mediated cell death.
To confirm that indeed TRPC1 protects PC12 by apoptosis, we used vibrant staining, which differentiates between necrotic and apoptotic mediated cell death. A concomitant increase in cells undergoing apoptosis (shown by YO-PRO-1 staining) was observed in cells treated with MPP+ (Fig. 5d; average data are shown in Fig. 5e). In contrast, cell death through the necrotic pathway was insignificant when compared with control without MPP+ treatment (Fig. 5d, e). These results are consistent to previous findings showing that MPP+ causes cell death via the apoptotic pathway (33, 34). Importantly, PC12 cells overexpressing TRPC1 showed a remarkable decrease in cell death via apoptosis upon MPP+ treatment, whereas no significant difference was observed in cell death via necrosis (Fig. 5d & 5e). Taken together these results suggest that TRPC1 protects neuronal cells against apoptosis induced by MPP+.
DISCUSSION
Ca2+ homeostasis plays a central role in neuronal signaling. Disturbances in neuronal Ca2+ homeostasis have been implicated in variety of neuropathological conditions including PD (35, 36). The ER provides a large intracellular Ca2+ store which releases Ca2+ under various conditions. Store operated Ca2+ entry is a mechanism devised by the cells to ensure optimal refilling of the internal Ca2+ stores and to maintain a prolonged increase in cytosolic Ca2+ upon stimulation that is vital for protein synthesis, cell growth and proliferation. However, no information is available on the identity of the channel(s) and its role in neuronal survival has not been established, even though abnormal functioning of SOCE channels has been linked to several diseases (37). Previous studies have shown that TRPC1 is important for SOCE (14, 15) and thus could help in neuronal survival. Moreover the importance of TRPC1 in cell survival is supported by other observations. First, up-regulation of TRPC1 increases cell proliferation and restricts apoptosis in keratinocytes (29). Second, down regulation or inhibition of SOC channels resulted in enhancement of ER depletion-induced apoptosis in prostate cancer cells and osteoclasts (38, 39). However, the mechanisms that underlie the protective effect of TRPC1 on neuronal degeneration especially in PD have not been identified.
Using MPTP, which is one of the best in vivo models for PD (40, 41), we have elucidated the role of TRPC1 in degeneration of dopaminergic neurons. The systemic administration of sub-acute and sub-chronic MPTP led to the death of dopaminergic neurons in the SNpc, which was confirmed by the level of TH and Nissl staining. The level of TH was significantly decreased in SNpc after MPTP treatments demonstrating the neurodegenerative effects of MPTP. Also, our results indicate that MPTP and MPP+-treatments significantly decrease TRPC1 levels and TRPC1-mediated Ca2+ influx. This decrease was not a generalized affect, since actin or TRPC3 levels were not decreased upon MPTP and MPP+-treatments. TRPC3 levels on the other hand, were actually increased, which could be due to compensatory mechanism, but still failed to show protection upon MPP+ treatment. Moreover, TRPC3 silencing did not show any loss in cell survival; however, at present we cannot rule out the significance of TRPC3 in neuronal survival. Importantly, MPP+ not only decreases TRPC1 levels in the plasma membrane but also effects its localization. Interestingly, TRPC1 overexpressing cells showed a significant increase in membrane TRPC1 levels, Ca2+ influx and consequently showed more resistance to MPP+-induced cell death. In contrast, silencing of TRPC1 decreased cell survival. Interestingly, deletion of TRPC1 pore region (ΔTRPC1567–793) that fails to bring Ca2+ (30), also failed to protect cells upon MPP+ treatment. Based on these findings, we postulated that the decrease in membrane TRPC1 levels upon MPTP and MPP+-treatment would contribute to altered Ca2+ homeostasis thereby leading to cell death. In addition, ER stress caused due to improper Ca2+ homeostasis (decreased SOCE) may contribute further along with inhibition of mitochondrial respiration, since Ca2+ is also critical for energy metabolism in the mitochondria. This is substantiated by a recent report, which demonstrated that ER stress can produce mitochondrial dysfunction by affecting various components of cytochrome c oxidase (42). Based on our results, we hypothesize that TRPC1 overexpression would facilitate replenishment of the ER Ca2+ pool following MPP+ treatment, thus minimizing/ inhibiting cell death. Our proposition is consistent with recent study from Chigurupati et al (43) and they reported that MPP+-induces cell death by depleting ER Ca2+ pool and Herp (Homocysteine-inducible ER stress protein) overexpression confers protection by blocking ER Ca2+ depletion. However additional experiments will be needed to validate our hypothesis.
Mitochondrial dysfunction is strongly implicated in the pathogenesis of PD, either as a cause or downstream hallmark of dopaminergic degeneration (44). Once inside the neurons, MPP+ rapidly accumulates in the mitochondrial matrix and inhibits mitochondrial respiration and disrupts its membrane potential (Δψmit). Using live imaging technique we show that MPP+ causes a reduction in the basal Δψmit in PC12 cells, which is consistent with animal studies that also showed decrease in Δψmit upon MPTP treatment. Furthermore, overexpression of TRPC1 in PC12 cells restores loss of Δψmit and silencing of TRPC1 decreases Δψmit in response to MPP+ treatment. This could be due to maintaining the Ca2+ homeostasis in TRPC1 overexpressing cells, since treatments aimed to block TRPC1 function also showed a decrease in Δψmit. Furthermore, maintaining of ER Ca2+ homeostasis by TRPC1 could prevent translocation of Bax into mitochondria. Thus inhibiting the release of cytochrome c from mitochondria, which will block it’s binding to APAF1-caspase-9-dATP complex thereby inhibiting the activation of caspases (45). Alternatively, Ca2+ entry via TRPC1 could also facilitate mitochondrial function since Ca2+ is needed for proper functioning of mitochondria (46).
A strong correlation between SOCE and apoptosis was found by Jayadev et al. (47) indicating that lack of Ca2+ entry initiates apoptosis. In contrast, excessive increase in cytosolic Ca2+ via the voltage gated L-type Ca2+ channels have also been shown to lead to neuronal loss (48), thus a tight balance in Ca2+ homeostasis is important to maintain neuronal survival. Our results clearly show that loss of dopaminergic neurons in PD was mainly due to apoptosis, which was confirmed using PD animal models. Upon MPTP –induction, pro-apoptotic Bax and Bak proteins were increased in mitochondrial fractions, which can initiate mitochondrial-mediated cell death by sequestering anti-apoptotic members such as Bcl2 and Bcl-xl. Bax and Bak protein have been shown to function in concert as essential gateways to intrinsic cell death pathways operating at mitochondria (49). In response to stress Bax is translocated to the mitochondria, which results in permeabilization of the outer mitochondrial membrane and release of pro-apoptotic factor cytochrome c into the cytosol, thereby leading to the activation of various caspases (50).
Our cell culture studies showed that overexpression of TRPC1 decreases Bax activation, reduced cytochrome c leakage, and promote cell survival. Bax expression is known to be critical for neuronal apoptosis, since Bax−/− mice do not undergo apoptosis (51). The mechanism by which Bax induces apoptosis is still unclear, but evidence suggests that Bax translocation could play a significant role. Pan et al. (52) showed that depletion of intracellular Ca2+ storage can accelerate the Bax translocation from cytosol to mitochondria and since TRPC1 is important for refilling of the ER pool and maintaining Ca2+ homeostasis, thus it could inhibit MPP+ mediated Bax translocation and prevent the activation of other downstream molecules. Although, exactly how TRPC1 overexpression protects these cells from apoptosis is presently unknown, we propose two possibilities by which TRPC1 could protect these cells against MPP+ induced apoptosis. First it can prevent the activation and translocation of Bax, by restoring ER-Ca2+ homeostasis and mitochondrial membrane potential, thereby reducing the leakage of cytochrome c. The second possibility is TRPC1 could inhibit apoptosome complex formation, which is also dependent on external Ca2+, thereby inhibiting the activation of downstream caspases. Although, both the possibilities are valid, our results indicate that regulation of mitochondrial membrane potential is critical in TRPC1 mediated protection. However at present we cannot rule out the other possibility and further research is needed to confirm/refute this hypothesis.
In summary, we have used both animal and cell culture models to establish the significance of TRPC1 in PD. Our results indicate that plasma membrane TRPC1 is down regulated upon treatment with MPTP and MPP+. Moreover, decrease of TRPC1 function upon MPTP/MPP+ treatment makes the cells more vulnerable to apoptosis, mainly due to reduced mitochondrial membrane potential, increased activation of Bax translocation, thereby increases cytochrome c leakage followed by the activation of downstream apoptotic pathway (see model in Fig. 5f). Importantly, overexpression of TRPC1 shows increased protection by preventing MPP+-mediated loss of mitochondrial membrane potential and inhibiting Bax translocation. Taken together our results suggest that TRPC1 expression and function is important for dopaminergic neuronal survival. However since TRPC1 overexpression did not completely rescue Δψmit and apoptosis, it may not be the sole reason for neuronal death and future research is needed to tease out the details of TRPC1 mediated protection against MPTP/MPP+.
ACKNOWLEDGEMENTS
We specially thank the confocal facility at UND and Virginia Achen for her help with immunofluorescence. We duly acknowledge grant support from the National Science Foundation (0548733) and the National Institutes of Health (DE017102, 5P20RR017699).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
I hereby acknowledge that Drs. Selvaraj, Watt (co-authors on this manuscript) and myself, do not have financial or other conflict and approve this manuscript for publication.
Contributor Information
Senthil Selvaraj, Department of Biochemistry & Molecular Biology, School of Medicine & Health Sciences, University of North Dakota, Grand Forks, North Dakota-58201..
John A Watt, Department of Anatomy and Cell Biology, School of Medicine & Health Sciences, University of North Dakota, Grand Forks, North Dakota-58201..
Brij B Singh, Department of Biochemistry & Molecular Biology, School of Medicine & Health Sciences, University of North Dakota, Grand Forks, North Dakota-58201..
REFERENCES
- 1.de Rijk MC, Launer LJ, Berger K, Breteler MM, Dartigues JF, Baldereschi M, Fratiglioni L, Lobo A, Martinez-Lage J, Trenkwalder C, Hofman A. Prevalence of Parkinson's disease in Europe: A collaborative study of population-based cohorts. Neurology. 2000;54:S21–S23. [PubMed] [Google Scholar]
- 2.Burke RE. Programmed cell death and Parkinson's disease. Mov. Disord. 1998;13 Suppl 1:17–23. [PubMed] [Google Scholar]
- 3.Schapira AH, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD. Mitochondrial complex I deficiency in Parkinson's disease. J. Neurochem. 1990;54:823–827. doi: 10.1111/j.1471-4159.1990.tb02325.x. [DOI] [PubMed] [Google Scholar]
- 4.Kristensson K. Potential role of viruses in neurodegeneration. Mol. Chem. Neuropathol. 1992;16:45–58. doi: 10.1007/BF03159960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burns RS, Chiueh CC, Markey SP, Ebert MH, Jacobowitz DM, Kopin IJ. A primate model of parkinsonism: selective destruction of dopaminergic neurons in the pars compacta of the substantia nigra by N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Proc. Natl. Acad. Sci. USA. 1983;80:4546–4550. doi: 10.1073/pnas.80.14.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langston JW, Irwin I. MPTP: current concepts and controversies. Clin. Neuropharmacol. 1986;9:485–507. [PubMed] [Google Scholar]
- 7.Chiueh CC, Markey SP, Burns RS, Johannessen JN, Jacobowitz DM, Kopin IJ. Neurochemical and behavioral effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in rat, guinea pig, and monkey. Psychopharmacol. Bull. 1984;20:548–553. [PubMed] [Google Scholar]
- 8.Kitahama K, Denney RM, Maeda T, Jouvet M. Distribution of type B monoamine oxidase immunoreactivity in the cat brain with reference to enzyme histochemistry. Neuroscience. 1991;44:185–204. doi: 10.1016/0306-4522(91)90260-u. [DOI] [PubMed] [Google Scholar]
- 9.Lotharius J, Dugan LL, O'Malley KL. Distinct mechanisms underlie neurotoxin-mediated cell death in cultured dopaminergic neurons. J. Neurosci. 1999;19:1284–1293. doi: 10.1523/JNEUROSCI.19-04-01284.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Javitch JA, D'Amato RJ, Strittmatter SM, Snyder SH. Parkinsonism-inducing neurotoxin, N-methyl-4-phenyl-1,2,3,6 -tetrahydropyridine: uptake of the metabolite N-methyl-4-phenylpyridine by dopamine neurons explains selective toxicity. Proc. Natl. Acad. Sci. USA. 1985;82:2173–2177. doi: 10.1073/pnas.82.7.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vila M, Jackson-Lewis V, Vukosavic S, Djaldetti R, Liberatore G, Offen D, Korsmeyer SJ, Przedborski S. Bax ablation prevents dopaminergic neurodegeneration in the 1-methyl- 4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson's disease. Proc. Natl Acad. Sci. USA. 2001;98:2837–2842. doi: 10.1073/pnas.051633998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi DW. Calcium: still center-stage in hypoxic-ischemic neuronal death. Trends Neurosci. 1995;18:58–60. [PubMed] [Google Scholar]
- 13.Putney JW., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 14.Ambudkar IS. TRPC1: a core component of store-operated calcium channels. Biochem. Soc. Trans. 2007;35:96–100. doi: 10.1042/BST0350096. [DOI] [PubMed] [Google Scholar]
- 15.Liu X, Cheng KT, Bandyopadhyay BC, Pani B, Dietrich A, Paria B, Singh BB, Birnbaumer L, Ambudkar IS. Attenuation of store-operated Ca2+ current impairs salivary gland fluid secretion in TRPC1(−/−) mice. Proc. Natl. Acad. Sci. USA. 2007;104:17542–17547. doi: 10.1073/pnas.0701254104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riccio A, Medhurst AD, Mattei C, Kelsell RE, Calver AR, Randall AD, Benham CD, Pangalos MN. mRNA distribution analysis of human TRPC family in CNS and peripheral tissues. Brain Res. Mol. Brain Res. 2002;109:95–104. doi: 10.1016/s0169-328x(02)00527-2. [DOI] [PubMed] [Google Scholar]
- 17.Kim SJ, Kim YS, Yuan JP, Petralia RS, Worley PF, Linden DJ. Activation of the TRPC1 cation channel by metabotropic glutamate receptor mGluR1. Nature. 2003;426:285–291. doi: 10.1038/nature02162. [DOI] [PubMed] [Google Scholar]
- 18.Wu Y, Shang Y, Sun S, Liu R. Antioxidant effect of erythropoietin on 1-methyl-4-phenylpyridinium-induced neurotoxicity in PC12 cells. Eur. J. Pharmacol. 2007;564:47–56. doi: 10.1016/j.ejphar.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 19.Wang GX, Poo MM. Requirement of TRPC channels in netrin-1-induced chemotropic turning of nerve growth cones. Nature. 2005;434:898–904. doi: 10.1038/nature03478. [DOI] [PubMed] [Google Scholar]
- 20.Shim S, Goh EL, Ge S, et al. XTRPC1-dependent chemotropic guidance of neuronal growth cones. Nat. Neurosci. 2005;8:730–735. doi: 10.1038/nn1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fiorio Pla A, Maric D, Brazer SC, Giacobini P, Liu X, Chang YH, Ambudkar IS, Barker JL. Canonical transient receptor potential 1 plays a role in basic fibroblast growth factor (bFGF)/FGF receptor-1-induced Ca2+ entry and embryonic rat neural stem cell proliferation. J. Neurosci. 2005;25:2687–2701. doi: 10.1523/JNEUROSCI.0951-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bollimuntha S, Singh BB, Shavali S, Sharma SK, Ebadi M. TRPC1-mediated inhibition of 1-methyl-4-phenylpyridinium ion neurotoxicity in human SH-SY5Y neuroblastoma cells. J. Biol. Chem. 2005;280:2132–2140. doi: 10.1074/jbc.M407384200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paxions G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. San Diego, CA, USA: Academic Press; 2001. [Google Scholar]
- 24.Singh BB, Zheng C, Liu X, Lockwich T, Liao D, Zhu M, Birnbaumer L, Ambudkar IS. Trp1-dependent enhancement of salivary gland fluid secretion: role of store-operated calcium entry. FASEB J. 2001;15:1652–1654. doi: 10.1096/fj.00-0749fje. [DOI] [PubMed] [Google Scholar]
- 25.Ong HL, Cheng KT, Liu X, Bandyopadhyay BC, Paria BC, Soboloff J, Pani B, Gwack Y, Srikanth S, Singh BB, Gill DL, Ambudkar IS. Dynamic assembly of TRPC1-STIM1-Orai1 ternary complex is involved in store-operated calcium influx. Evidence for similarities in store-operated and calcium release-activated calcium channel components. J. Biol. Chem. 2008;282:9105–9116. doi: 10.1074/jbc.M608942200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferreiro E, Oliveira CR, Pereira CMF. The release of calcium from the endoplasmic reticulum induced by amyloid-beta and prion peptides activates the mitochondrial apoptotic pathway. Neurobiol. Dis. 2008;30:331–342. doi: 10.1016/j.nbd.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 27.O'Callaghan JP, Miller DB, Reinhard JF., Jr 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced damage of striatal dopaminergic fibers attenuates subsequent astrocyte response to MPTP. Neurosci. Lett. 1990;117:228–233. doi: 10.1016/0304-3940(90)90149-4. [DOI] [PubMed] [Google Scholar]
- 28.Ricaurte GA, Irwin I, Forno LS, DeLanney LE, Langston E, Langston JW. Aging and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced degeneration of dopaminergic neurons in the substantia nigra. Brain Res. 1987;403:43–51. doi: 10.1016/0006-8993(87)90120-x. [DOI] [PubMed] [Google Scholar]
- 29.Pani B, Cornatzer E, Cornatzer W, Shin DM, Pittelkow MR, Hovnanian A, Ambudkar IS, Singh BB. Up-regulation of transient receptor potential canonical 1 (TRPC1) following sarco(endo)plasmic reticulum Ca2+ ATPase 2 gene silencing promotes cell survival: a potential role for TRPC1 in Darier's disease. Mol. Biol. Cell. 2006;17:4446–4458. doi: 10.1091/mbc.E06-03-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu X, Singh BB, Ambudkar IS. TRPC1 is required for functional store-operated Ca2+ channels. Role of acidic amino acid residues in the S5–S6 region. J.Biol. Chem. 2003;278:11337–11343. doi: 10.1074/jbc.M213271200. [DOI] [PubMed] [Google Scholar]
- 31.Antonsson B, Conti F, Ciavatta A, et al. Inhibition of Bax channel-forming activity by Bcl-2. Science. 1997;277:370–372. doi: 10.1126/science.277.5324.370. [DOI] [PubMed] [Google Scholar]
- 32.Chandra J, Samali A, Orrenius S. Triggering and modulation of apoptosis by oxidative stress. Free. Radic. Biol. Med. 2000;29:323–333. doi: 10.1016/s0891-5849(00)00302-6. [DOI] [PubMed] [Google Scholar]
- 33.Itano Y, Nomura Y. 1-methyl-4-phenyl-pyridinium ion (MPP+) causes DNA fragmentation and increases the Bcl-2 expression in human neuroblastoma, SH-SY5Y cells, through different mechanisms. Brain Res. 1995;704:240–245. doi: 10.1016/0006-8993(95)01120-x. [DOI] [PubMed] [Google Scholar]
- 34.Noda Y, Sumino T, Fujisawa Y, Miyata N, Kaiya T, Kohda K. 1-amino-4-phenyl-1,2,3,6-tetrahydropyridine and 1-amino-4-phenylpyridinium salt, the 1-amino analogues of neurotoxins, MPTP and MPP+, induce apoptosis in PC12 cells: detection of apoptotic cells by Comet assay and flow cytometric analysis. In Vivo. 2004;18:561–569. [PubMed] [Google Scholar]
- 35.Paschen W, Doutheil J. Disturbances of the functioning of endoplasmic reticulum: a key mechanism underlying neuronal cell injury? J. Cereb. Blood. Flow. Metab. 1999;19:1–18. doi: 10.1097/00004647-199901000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Tymianski M, Tator CH. Normal and abnormal calcium homeostasis in neurons: a basis for the pathophysiology of traumatic and ischemic central nervous system injury. Neurosurgery. 1996;38:1176–1195. doi: 10.1097/00006123-199606000-00028. [DOI] [PubMed] [Google Scholar]
- 37.Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol. Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 38.Vanden Abeele F, Roudbaraki M, Shuba Y, Skryma R, Prevarskaya N. Store-operated Ca2+ current in prostate cancer epithelial cells. Role of endogenous Ca2+ transporter type 1. J. Biol. Chem. 2003;278:15381–15389. doi: 10.1074/jbc.M212106200. [DOI] [PubMed] [Google Scholar]
- 39.Mentaverri R, Kamel S, Brazier M. Involvement of capacitive calcium entry and calcium store refilling in osteoclastic survival and bone resorption process. Cell Calcium. 2003;34:169–175. doi: 10.1016/s0143-4160(03)00080-0. [DOI] [PubMed] [Google Scholar]
- 40.Schober A. Classic toxin-induced animal models of Parkinson's disease: 6-OHDA and MPTP. Cell Tissue Res. 2004;318:215–224. doi: 10.1007/s00441-004-0938-y. [DOI] [PubMed] [Google Scholar]
- 41.Sonsalla PK, Heikkila RE. The influence of dose and dosing interval on MPTP-induced dopaminergic neurotoxicity in mice. Eur. J. Pharmacol. 1986;129:339–345. doi: 10.1016/0014-2999(86)90444-9. [DOI] [PubMed] [Google Scholar]
- 42.Hori O, Ichinoda F, Tamatani T, et al. Transmission of cell stress from endoplasmic reticulum to mitochondria: enhanced expression of Lon protease. J. Cell Biol. 2002;157:1151–1160. doi: 10.1083/jcb.200108103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chigurupati S, Wei Z, Belal C, et al. The Homocysteine-inducible Endoplasmic Reticulum Stress Protein Counteracts Calcium Store Depletion and Induction of CCAAT Enhancer-binding Protein Homologous Protein in a Neurotoxin Model of Parkinson Disease. J. Biol. Chem. 2009;284:18323–18333. doi: 10.1074/jbc.M109.020891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abou-Sleiman PM, Muqit MM, Wood NW. Expanding insights of mitochondrial dysfunction in Parkinson's disease. Nat. Rev. Neurosci. 2006;7:207–219. doi: 10.1038/nrn1868. [DOI] [PubMed] [Google Scholar]
- 45.Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998;391:43–50. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- 46.Chan CS, Gertler TS, Surmeier DJ. Calcium homeostasis, selective vulnerability and Parkinson's disease. Trends Neurosci. 2009;32:249–256. doi: 10.1016/j.tins.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jayadev S, Petranka JG, Cheran SK, Biermann JA, Barrett JC, Murphy E. Reduced capacitative calcium entry correlates with vesicle accumulation and apoptosis. J. Biol. Chem. 1999;274:8261–8268. doi: 10.1074/jbc.274.12.8261. [DOI] [PubMed] [Google Scholar]
- 48.Chan CS, Guzman JN, Ilijic E, J.N, et al. Rejuvenation protects neurons in mouse models of parkinson's disease. Nature. 2007;28:1081–1086. doi: 10.1038/nature05865. [DOI] [PubMed] [Google Scholar]
- 49.Wei MC, Zong WX, Cheng EH, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Budihardjo I, Oliver H, Lutter M, Luo X, Wang X. Biochemical pathways of caspase activation during apoptosis. Annu. Rev. Cell Dev. Biol. 1999;15:269–290. doi: 10.1146/annurev.cellbio.15.1.269. [DOI] [PubMed] [Google Scholar]
- 51.Miller TM, Moulder KL, Knudson CM, Creedon DJ, Deshmukh M, Korsmeyer SJ, Johnson EM., Jr Bax deletion further orders the cell death pathway in cerebellar granule cells and suggests a caspase-independent pathway to cell death. J. Cell. Biol. 1997;139:205–217. doi: 10.1083/jcb.139.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pan Z, Bhat MB, Nieminen AL, Ma J. Synergistic movements of Ca(2+) and Bax in cells undergoing apoptosis. J. Biol. Chem. 2001;276:32257–32263. doi: 10.1074/jbc.M100178200. [DOI] [PubMed] [Google Scholar]