Abstract
OBJECTIVES
This prospective study examined if changes in traditional and novel coronary heart disease (CHD) risk factors are greater within a year of the final menstrual period (FMP), relative to changes that occur before or after that interval, in a multi-ethnic cohort.
BACKGROUND
Understanding the influence of the menopause on CHD risk remains elusive and has been evaluated primarily in Caucasian samples.
METHODS
The Study of Women’s Health across the Nation (SWAN) is a prospective study of the menopausal transition in 3302 minority (African American, Hispanic, Japanese, or Chinese) and Caucasian women. After 10 annual exams, 1054 women had achieved a FMP not due to surgery and without HT use prior to FMP. Measured CHD risk factors included lipids and lipoproteins, glucose, insulin, blood pressure, fibrinogen, and C-reactive protein. We compared which of two models provided a better fit to the observed risk factor changes over time in relation to FMP: a linear model, consistent with chronological aging, or a piece-wise linear model, consistent with ovarian aging.
RESULTS
Only total cholesterol, LDL-C, and apolipoprotein-B demonstrated substantial increases within the 1 year interval before and after FMP, consistent with menopause-induced changes. This pattern was similar across ethnic groups. The other risk factors were consistent with a linear model, indicative of chronological aging.
CONCLUSIONS
Women experience a unique rise in lipids at the time of FMP. Monitoring lipids in perimenopausal women should enhance primary prevention of CHD.
Keywords: Menopause, risk factors, lipids, inflammation, race
Introduction
Understanding the influence of the menopausal transition on women’s risk for coronary heart disease (CHD) remains elusive (1). Women who have an early menopause, especially due to surgical oophorectomy, are at elevated risk for CHD events, compared to age-matched premenopausal women (2). Further, adverse risk factor profiles can account for the elevated risk for CHD events associated with hysterectomy and oophorectomy in the Women’s Health Initiative (3), and for an earlier age at menopause in the Framingham cohort (4). Women’s rates of CHD do not increase dramatically during the perimenopause, but start to climb exponentially in the postmenopausal years (1). On the other hand, the postmenopausal rise in CHD rates may reflect the cumulative impact of earlier alterations in CHD risk factors beginning during the menopausal transition. Taken together, these disparate findings have contributed to controversy about whether menopause accelerates CHD risk or if elevated CHD risk leads to an earlier menopause (5).
One approach to understanding the directionality of the effect is to evaluate changes in risk factors for CHD during the peri-and early post-menopause. Epidemiological studies suggest that the menopause is associated with increases in total cholesterol and low density lipoprotein cholesterol (LDL-C), while increases in blood pressure and weight during midlife are more linear and appear to reflect chronological aging (6–11). However, almost all studies were based on Caucasian samples and did not measure risk factors simultaneously with changes in menopausal status. Thus, the designs were not optimal to determine associations between changes in menopause status and changes in risk factors. Many of the studies were also conducted prior to the identification of emerging risk factors, such as C-reactive protein (CRP) and fibrinogen. The aim of this report is to describe the changes in a wide array of risk factors, including lipids and lipoproteins, blood pressure, insulin, glucose, and hemostatic and inflammatory factors in women before and after their final menstrual period (FMP), and to ascertain whether changes in these risk factors were related to ovarian aging as opposed to chronological aging. The sample was composed of African American, Chinese, Japanese, Hispanic, and Caucasian women, allowing for a secondary aim of evaluating ethnic differences in the patterns of risk factor change over time in relation to FMP.
Methods
Participants
The study sample was composed of 1054 women who achieved a FMP by the end of 9 years of follow-up in the Study of Women’s Health across the Nation (SWAN), a longitudinal, multi-site community-based study of 3302 initially pre- and early peri-menopausal women (12). A screening survey was conducted in 16,065 women in 1995–1997 to assess eligibility and to collect health, reproductive, demographic and lifestyle data. Each of seven sites recruited at least 450 eligible women into the longitudinal cohort, Caucasian women and women from one specified minority group (African Americans in Pittsburgh, PA, Boston, MA, Detroit area, MI, and Chicago, IL; Japanese in Los Angeles, CA; Chinese in the Oakland East Bay region, CA; and Hispanic women in Newark, NJ). Eligibility criteria for the longitudinal cohort were age 42–52 years, having an intact uterus, having at least one menstrual period and not using exogenous reproductive hormones in the 3 months prior to the baseline interview, and having self-identified with the site's designated race/ethnic groups. The Institutional Review Boards at all participating sites approved the study protocol.
Women’s menstrual bleeding status, including the date of the most recent menstrual period, was assessed annually. The FMP was defined as the date of the participant’s last menstrual period prior to 12 consecutive months of amenorrhea and was assigned retrospectively, once at least 12 months of amenorrhea had been transpired. For each annual observation, years remaining until the FMP or years elapsed since the FMP were computed for observations occurring prior to or after the FMP, respectively. For a woman with one or two consecutive missed annual visits that encompassed her FMP, the date of the FMP was imputed based on her menopause status at the preceding annual visit. Omitted from the analysis were 189 women with missing FMP date or covariates; 645 women lost to follow-up prior to FMP; and 1034 women with an indeterminant date of FMP due to hormone therapy (HT) use (N=801) or surgical menopause (N=233; some because of both) prior to final menses (those with HT use or surgical menopause after FMP were included in the analytic sample). Relative to the 645 women lost to follow-up prior to FMP, women in the analytic sample were less likely to be Hispanic and more likely to be Chinese or Japanese; the site that followed Hispanics was closed due to administrative reasons unrelated to the study’s scientific goals.
Procedures
SWAN participants were questioned annually about health, lifestyle and psychosocial factors. Anthropometric measurements and phlebotomy procedures were obtained with standardized protocols. At each examination, the fasting blood draw was targeted to the early follicular phase of the menstrual cycle (days 2 – 5) in menstruating women and prior to 10 a.m. to allow for a standardized hormonal milieu. All samples were maintained at 4° C until separated and then were frozen at −80 ° C and shipped on dry ice to a central certified laboratory (Medical Research Laboratories, Highland Heights, Kentucky, USA. Assays were not undertaken at follow-up years 2, 8, and 9 for all risk factors, and, in addition, for fibrinogen and Factor VIIc at follow-up years 4 and 6 for budget reasons.
Cardiovascular Risk Factor Measurement
Risk factors were selected for measurement in SWAN if they were predictors of CHD in women or if they were influenced by ovarian hormones. All lipid and lipoprotein fractions were analyzed on EDTA treated plasma. Total cholesterol and triglycerides were (13) analyzed by enzymatic methods and high-density lipoprotein cholesterol (HDL-C) was isolated using heparin-2M manganese chloride (14,15). Serum insulin was measured using RIA (DPC Coat-a-count, Los Angeles, CA) procedure and monitored as part of the monthly quality assurance program by the Diabetes Diagnostic Laboratory at the University of Missouri. Glucose was measured using a hexokinase-coupled reaction on a Hitachi 747-200 (Boehringer Mannheim Diagnostics, Indianapolis, IN). Lp(a) was quantified by competitive ELISA. Fibrinogen and Factor VIIc were measured in frozen citrated plasma using a clot-based turbidometric detection system, with the Factor VII assay using Factor VII deficient plasma in preparing the standard curve. Tissue-type plasminogen activator antigen (tPA-ag) was measured in plasma using a double antibody in an enzyme-linked immunosorbant assay (American Diagnostica, Greenwich, CT), with a human single chain tPA-ag as a standard calibrated against an international standard (National Institute for Biological Standards and Control, UK). Plasminogen activator inhibitor Type 1 (PAI-1) was measured using a solid phased monoclonal antibody and a second enzyme-labeled goat antiserum for detection (American Diagnostica, Greenwich, CT). CRP measured using an ultra-sensitive rate immunoephelometry (Dade-Behring, Marburg, Germany).
Other Variables
Age, race/ethnicity, and education (≤ high school degree, some college/vocational training, college degree or more) were obtained at the baseline examination. Dietary data were collected at year 0 and 5 (16); the within-woman correlation for dietary variables ranged from 0.45 for percent calories from fat to 0.66 for calories from alcohol. Physical activity associated with routine activity, sports/leisure, and household/ childcare was collected at baseline, years 3, 5, and 6 (17). The within-woman correlation for these variables ranged from 0.57 for household and child care-related activity to 0.67 for sports-related activity. Medication use, including HT, health history, smoking (current, past, never), menopausal status, and BMI were obtained at each examination. BMI (kg/m2) was calculated from measurements of weight and height, which were obtained with calibrated scale and a stadiometer.
Statistical Analyses
The analytic sample was compared using chi-square and t-tests with the subgroup of SWAN participants who were still candidates for a FMP at the ninth annual follow-up visit, i.e. no prior or current use of HT, no hysterectomy or bilateral oophorectomy, and fewer than 12 consecutive months of amenorrhea.
We evaluated the magnitude of change in each risk factor by dividing the transition into three segments relative to FMP. The three segments were based on the timing of changes seen for follicle stimulating hormone (FSH), which is tightly linked to the menopausal transition, independent of chronological age: 1) more than 12 months before the FMP; 2) within 12 months before and after the FMP; and 3) more than 12 months after the FMP. A risk factor change (slope) that is similar across all three segments is consistent with chronological aging, with no impact of the menopausal transition. Differences in rates of risk factor change across the three segments, with a steeper slope in segment 2 than in 1 and 3, as observed for FSH (18,19), is consistent with changes driven by ovarian aging, independent of chronological aging.
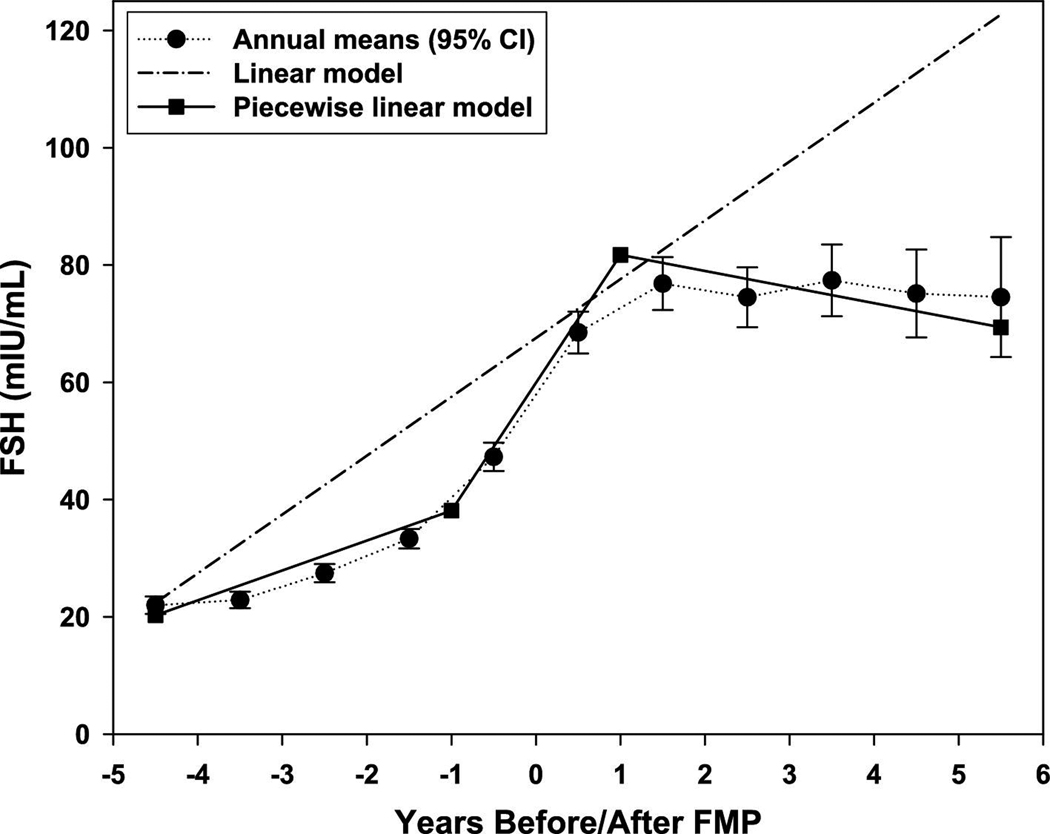
To test this statistically, we fit two longitudinal models for each risk factor: 1) a linear model, which assumes a constant slope across the three time periods; and 2) a piece-wise linear model, which allows the slope to differ across the three segments. To demonstrate, Figure 1 shows the annual mean SWAN values of FSH from five years prior to five years after the FMP, as well as the estimates from both the linear and piece-wise linear models. The piece-wise linear model tracks the annual mean values closely, with a steep positive slope in segment 2 that differs significantly from the slopes in segments 1 and 3. Because the model fit is better for the piece-wise linear model than for the linear model, we conclude that changes in FSH are being driven by ovarian aging rather than solely by chronological aging.
Figure 1. FSH annual and estimated means.
Pattern of FSH across the SWAN follow-up period showing the annual covariate adjusted mean values compared to estimated values by the better fitting piece-wise linear model (menopause-related) and the linear model (aging-related); relative AIC fit for piece-wise linear vs linear models (95% CI) is 0.6640 (0.6096, 0.7184).
Separate longitudinal mixed models were estimated for each risk factor as a function of years before/after FMP, separately for the linear and piece-wise linear models (20); this technique accommodates within-subject correlation, unequal numbers of observations per subject, and unequal intervals between measures. Model fit was assessed using the Akaike Information Criterion (AIC), with the best fit indicated by the lowest AIC (21).Relative fit compared the AIC values of the linear model and the piece-wise model (22). Confidence intervals indicate whether the relative fit is statistically different from 1 (i.e., equivalent fit for the two models) at p < .05. For risk factors in which the piece-wise linear model provided a better fit than the linear model, the slopes in the three segments based on time intervals surrounding FMP were compared.
Models adjusted for age at FMP, ethnicity, site, baseline height, baseline log weight and change in log weight, concurrent smoking (never, past, current), concurrent relevant medication use (antihypertensive use for analyses of blood pressure and pulse pressure, insulin use for analyses of glucose and insulin, lipid-lowering medications for analyses of lipids and lipoproteins), and total calories, percent calories from fat, and calories from alcohol, and physical activity due to routine activity, sports/leisure, and household/child care from the most recent prior measurement. With the exception of smoking, which was used as a time-invariant covariate because of its stability (baseline smoking), all other annually measured variables were used as time-varying covariates. Right-skewed outcomes were log-transformed for analyses and back-transformed for presentation. Analyses excluded observations concurrent with HT use or a 6-month HT washout period; note that in the analytic sample, any of these deleted observations would have occurred only after a woman’s FMP because women who used HT or had a hysterectomy/bilateral oophorectomy prior to FMP were not included in the analytic sample. The total number of observations available for analysis ranged from 4507 (LDL-C) to 5739 (blood pressure). Figures presented below are truncated at −5 and +6 years around the FMP because of the smaller number of observations at the extremes and the larger standard errors. We tested whether risk factor changes with respect to the FMP varied significantly by ethnicity, after first estimating ethnic-specific models to confirm that the same functional form for the model – linear or piece-wise linear -- was appropriate for each ethnic group. These analyses were conducted in the full sample and repeated in the largest ethnic groups only (African Americans and Caucasians). Power to detect interactions by ethnicity showed that an annual difference of 0.08 standard deviation in LDL-C could be detected between the smallest groups (Hispanics and Japanese) and 0.03 standard deviation between the largest ethnic groups. We also tested for interactions between baseline weight and status for those CV risk factors that fit the piece-wise linear models because in the full SWAN cohort, women who were in the highest tertile of baseline weight did not show effect of menopausal status on lipids through year 07 (23).
Results
Baseline Characteristics of Women who had a Final Menstrual Period
The 1054 women who had an observed FMP were about 47 years of age at baseline and on average had their FMP approximately 3 years after study entry (Table 1). The proportion of ethnic groups with a FMP was generally consistent with proportions recruited in those groups at baseline, with the largest proportion Caucasian, followed by African Americans and the remaining ethnic groups. Relative to 380 women who were still candidates for FMP after visit 09, women in the analytic sample at baseline were older, leaner, and more often current smokers, and reported lower sports-related physical activity; they also had higher HDL-C and lower CRP levels. No other differences in age-adjusted risk factors were observed.
Table 1.
Age-Adjusted Baseline Characteristics of the Analytic Sample With the Final Menstrual Period by Follow-up 09 Compared With Women Not Yet Postmenopausal
| Characteristic | Baseline analytic sample (N=1054) |
Women not yet postmenopausal (N=380) |
|---|---|---|
| Ethnicity: % | ||
| African American | 29.0 | 26.6 |
| Caucasian | 44.9 | 46.5 |
| Chinese | 10.8 | 10.7 |
| Hispanic | 4.7 | 0.0 |
| Japanese | 11.1 | 14.2 |
| Age in years: Mean (95% CI) (a) * | 47.1 (46.9, 47.2) | 44.3 (44.1, 44.6) |
| Smoking Status: % * | ||
| Never | 57.3 | 65.7 |
| Past | 23.9 | 26.1 |
| Current | 18.8 | 8.2 |
| Weight (kg): Mean (95% CI) * | 72.3 (71.0, 73.5) | 74.5 (72.3, 76.7) |
| Total dietary intake in kilocalories: Mean (95% CI) |
1827.6 (1784.9, 1870.3) | 1838.4 (1763.3, 1913.5) |
| % calories from fat: Mean (95% CI) | 32.7 (32.3, 33.2) | 31.8 (31.0, 32.6) |
| Any alcohol consumption: % | 49.4 | 47.4 |
| Calories from alcohol: Mean (95% CI)(b) |
42.3 (38.3, 46.8) | 44.9 (37.6, 53.7) |
| Physical activity: Mean (95% CI) | ||
| Sports * | 2.6 (2.5, 2.7) | 2.8 (2.7, 2.9) |
| Leisure / routine | 2.4 (2.4, 2.5) | 2.4 (2.3, 2.5) |
| Household / child care | 2.7 (2.6, 2.7) | 2.7 (2.6, 2.8) |
| Lipid-lowering medication: % (N) | 0.9 (9) | 0.3 (1) |
| Antihypertensive medication: % (N) | 10.1 (106) | 8.5 (32) |
| Age at Final Menstrual Period (years): Mean (95% CI) |
51.2 (51.0, 51.4) | -- |
| Lipids and Lipoproteins: Mean (95% CI) |
||
| Cholesterol (mg/dl) (c) | 191.8 (189.8, 193.8) | 193.0 (189.4, 196.5) |
| LDL (mg/dl) (c) | 113.6 (111.7, 115.4) | 115.8 (112.6, 119.1) |
| Apo-B (mg/dl) (c) | 108.1 (106.4, 109.9) | 110.5 (107.4, 113.6) |
| HDL (mg/dl) (c) * | 57.5 (56.6, 58.4) | 55.4 (53.9, 57.0) |
| ApoA1 (mg/dl) (c) | 151.0 (149.5, 152.5) | 148.0 (145.3, 150.7) |
| Triglycerides (mg/dl) (c) | 90.2 (87.5, 93.0) | 95.1 (90.2, 100.3) |
| Lp(a): Mean (95% CI) | 14.6 (13.5, 15.9) | 15.5 (13.3, 17.9) |
| Glucose and Insulin: Mean (SE) | ||
| Glucose (mg/dl) (c) | 91.6 (91.0, 92.3) | 92.3 (91.2, 93.4) |
| Insulin (uIU/mL) (c) | 8.8 (8.5, 9.1) | 8.8 (8.3, 9.3) |
| Blood Pressure: Mean (95% CI) | ||
| Systolic blood pressure (mmHg) (c) |
114.9 (113.9, 116.0) | 114.2 (112.4, 116.0) |
| Diastolic blood pressure (mmHg) (c) |
74.1 (73.4, 74.8) | 73.7 (72.5, 74.8) |
| Hemostatic Factors: Mean (95% CI) |
||
| tPA-ag (ng/mL) | 6.8 (6.6, 7.0) | 6.9 (6.5, 7.2) |
| PAI-1 (ng/mL) | 19.1 (18.2, 20.0) | 20.4 (18.7, 22.2) |
| Fibrinogen (mg/mL) | 283.4 (279.5, 287.4) | 288.0 (281.0, 295.1) |
| Factor VIIc(%) | 113.7 (111.9, 115.6) | 114.0 (110.8, 117.3) |
| CRP-hs (mg/L) * | 1.4 (1.3, 1.5) | 1.6 (1.4, 1.9) |
Not adjusted for age
Drinkers only
Untreated measurements only
p-value for between-group difference < 0.05, using logistic regression for categorical variables and analysis of covariance for continuous variables
Changes in Cardiovascular Risk Factors in Relation to Final Menstrual Period
Lipids and Lipoproteins
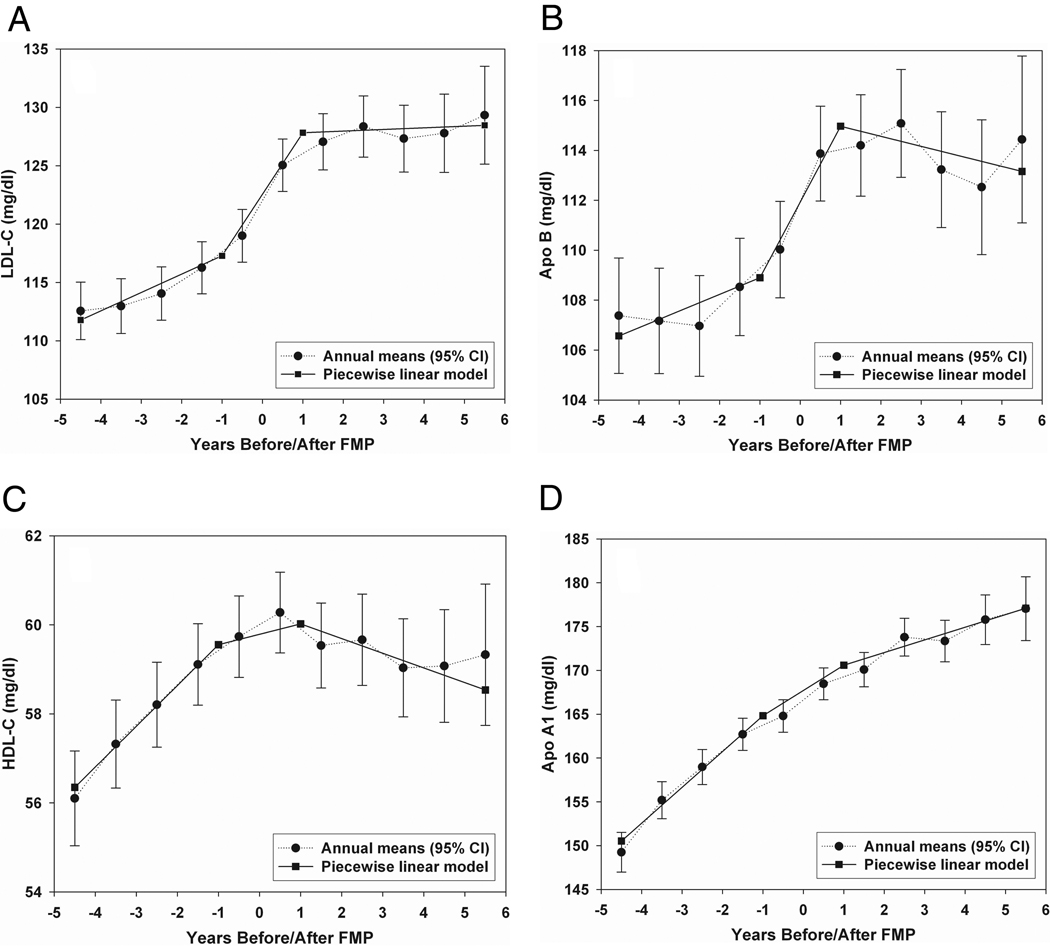
The mean adjusted changes across time in total cholesterol, LDL-C, Apo-B, HDL-C, and ApoA1 were fit better by the piece-wise linear model than by the linear model (Figure 2). The increases in total cholesterol, LDL-C, and Apo-B were substantial around the FMP and were significantly greater than the increases in total cholesterol, LDL-C and Apo-B, prior to and after the FMP interval (Table 2). The greatest increase in HDL-C and ApoA1 levels occurred prior to the 1 year interval surrounding the FMP and then leveled off or declined. The absence of interactions between ethnicity and the slopes generated from the piece-wise linear model indicated that all ethnic groups changed in a similar manner around the FMP; tests only in African American and Caucasian women also showed no significant interaction effects. A linear model fit log-transformed triglycerides across time, consistent with the effects of chronologic aging rather than ovarian aging.
Figure 2. Lipids annual and estimated means.
Pattern of LDL-C, Apo-B, HDL-C, and ApoA1 across the SWAN follow-up period showing the annual covariate adjusted mean values compared to estimated values by the better fitting piece-wise linear model (menopause-related); relative AIC fit for piece-wise linear vs linear models (95% CI), 1.0430 (1.0130, 1.0730), 1.0340 (1.0092, 1.0588), 1.0486 (1.0203, 1.0769), and1.0255 (1.0036, 1.0474), respectively.
Table 2.
Segment-specific slopes for years before/after FMP for cardiovascular risk factors where piece-wise linear trajectory fits better than linear trajectory (relative fit > 1)
| Segment-specific slope (standard error) |
p-value for pair-wise difference in segment-specific slopes |
||||||
|---|---|---|---|---|---|---|---|
| Outcome, mg/dl (No. Observations/ No. Women) |
Segment 1: > 12 months before FMP |
Segment 2: Within 12 months of FMP |
Segment 3: > 12 months after FMP |
Segment 1 vs. Segment 2 |
Segment 1 vs. Segment 3 |
Segment 2 vs. Segment 3 |
Overall p- value for differences in segment- specific slopes |
| Total cholesterol (4725/1022) |
2.98 (0.37) |
6.47 (0.62) |
−0.16 (0.36) |
<0.0001 | <0.0001 | <0.0001 | <0.0001 |
| LDL-C (4507/1012) |
1.57 (0.32) |
5.20 (0.55) |
0.14 (0.32) |
<0.0001 | 0.0007 | <0.0001 | <0.0001 |
| Apo-B (4709/1021) |
0.67 (0.28) |
3.24 (0.45) |
−0.40 (0.25) |
<0.0001 | 0.0025 | <0.0001 | <0.0001 |
| HDL-C (4724/1022) |
0.92 (0.13) |
0.40 (0.21) |
−0.33 (0.12) |
0.0823 | <0.0001 | 0.0142 | <0.0001 |
| ApoA1 (4709/1021) |
4.09 (0.32) |
2.16 (0.54) |
1.44 (0.29) |
0.0102 | <0.0001 | 0.3398 | <0.0001 |
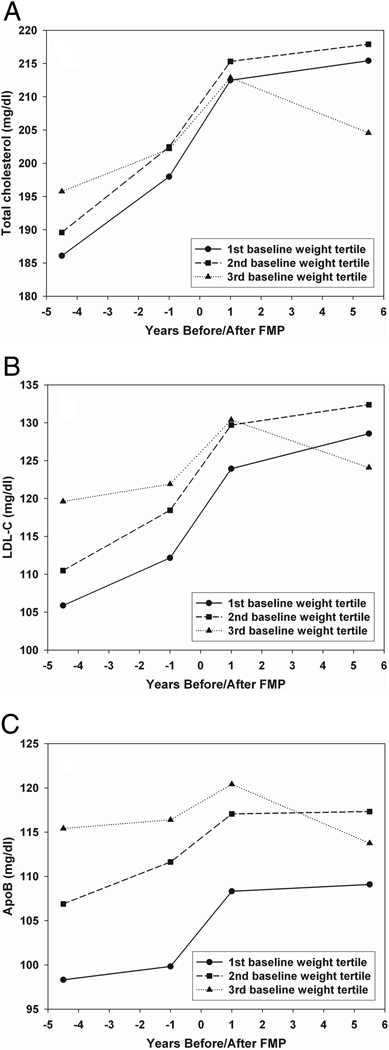
Effect modification by baseline weight was statistically significant for total and LDL cholesterol and Apo-B, due largely to a flattening of lipids in heavier women after the FMP. The steep upward incline around the FMP occurred in all three weight tertiles (Figure 2).
Glucose, Insulin, Blood Pressure, Lp(a), and Hemostatic Factors
Linear models provided a better fit than did piece-wise models for the changes across time of glucose, tPA-ag, PAI-1, and fibrinogen, relative AIC fits < 1. Linear and piece-wise models were of equivalent fit for SBP, DBP, Lp(a), insulin, Factor VII-c, and CRP trajectories, relative AIC fits included 1. Examination of percent annual change showed that the following increased: triglycerides (1.75%), Lp(a) (2.48%), insulin (2.84%), and Factor VII-c (1.40%); SBP increased on average 0.26 mmHg annually. Glucose (−2.21%) and PAI-1 (−6.75%) declined. DBP, tPA-ag, fibrinogen, and CRP did not change across time.
Discussion
The aims of the study were to evaluate the change in CHD risk factors in relation to the FMP, independent of age and other confounders, among women who have experienced a natural menopause; and to examine the extent of ethnic differences in those patterns. The results showed significant increases in total cholesterol, LDL-C, and Apo-B within a year of the FMP, relative to the observed increase over time intervals before or after. These FMP-related increases were similar in women classified into tertiles by baseline weight. Importantly, the rate of change relative to FMP did not vary by ethnicity, suggesting that the menopause had a uniform influence on lipids. The influence of the interval surrounding FMP on total cholesterol, LDL-C and Apo-B was independent of age as well as the many covariates that vary by ethnicity, including weight, weight gain, and medications. The present analysis is the first to document effects of the natural menopausal transition anchored prospectively by FMP with sufficient follow-up, annual assessment of risk factors, accurate and detailed timing of menopause, and extensive covariate adjustment. It is also the first to report the absence of detectable ethnic differences in the influence of menopause on lipids and lipoproteins. These findings are consistent with the hypothesis that the increase in CHD in postmenopausal women may in part be related to the earlier changes in lipids associated with the menopausal transition. The analyses showed no evidence of FMP on blood pressure, insulin, glucose, Lp(a), and hemostatic and inflammatory factors. These null menopause results are consistent with other SWAN analyses of relationship between reproductive hormones and changes in risk factors, and with earlier reports of changes in menopause based on bleeding patterns (6–10, 24). Taking these findings together, it is unlikely that the acceleration of CHD risk in the postmenopausal years is due to menopause-induced increases in these factors.
Like SWAN, the Melbourne Midlife Women’s Health Project reported HDL-C increased steadily up to the FMP, peaked at the FMP, and declined thereafter, resulting in little net change in HDL-C across the transition (25,26). Thus, the acute increase in HDL-C prior to LMP is unlikely to be protective for women. In contrast to HDL-C, log-transformed triglycerides increased in a linear fashion across the study period, perhaps because HDL-C and log-transformed triglycerides had different associations with log weight gain over time in our sample, r = −.06, and r = .20, respectively. Changes in HDL and very low density particle concentration with aging may also play a role (27).
Study limitations
Results are based on women who had a natural FMP by 9 years. These women, like in other studies (23, 28–32), were smokers and leaner. The bias that this may introduce is not clear, given that smoking status, weight, change in weight, medications, and many other relevant covariates were adjusted for at each year before and after FMP. Second, the results are based on the subset of women who did not use HT prior to the FMP or who had not reported hysterectomy. Women who take HT or have hysterectomy or bilateral oophorectomy are undoubtedly different than women who have a natural menopause. Third, approximately 25% of women were lost to follow-up. One site that followed Hispanics was closed due to administrative reasons. Finally, financial constraints precluded the assay of stored samples in years 2, 8, and 9. Only blood pressure was measured annually.
Clinical implications
Our findings have potential clinical implications because identification of modifiable risk factors and early intervention may reduce women’s increased risk of CHD after the menopause. While an increase in LDL-C and a decline in HDL-C between pre- and post-menopause has been previously reported in healthy Caucasian women (8), our data specifically identify the critical time period, the year immediately around the FMP, as the time of the most adverse changes in the lipid profile in all ethnic groups. This study underscores the need to closely monitor lipid profiles of pre- and peri-menopausal women, and the importance of emphasizing proven lifestyle measures and therapeutic interventions prior to the menopause transition to counter and possibly prevent this adverse change in lipids associated with menopause itself. While absolute levels of LDL-C were within what is considered the normal range, even lipids in the normal range during the pre- and peri-menopausal transition predict later coronary calcification and carotid intima media thickness (33–35). Whether the threshhold levels for lipid-lowering therapy should change around the time of menopause, or whether the absolute or relative degree of change in lipids (independent of premenopause levels) predicts future CHD events merits further study. The menopause-associated changes in total cholesterol, LDL-C and Apo-B observed herein may play an important role in women’s increased risk for CHD in the postmenopausal years.
Figure 3. Systolic Blood Pressure and Plasminogen Activator Inhibitor Type 1 Lipid Annual Means according to Baseline Weight.
Pattern of total cholesterol, LDL-C and Apo-B across SWAN follow-up period showing the annual covariate adjusted mean values in women categorized by baseline weight tertiles. Slopes around the FMP interval were identical in the three groups for total cholesterol, LDL-C, and ApoB, Ps >0.28, respectively, whereas slopes more than 1 year after the FMP differed, Ps < 0.01, respectively.
ACKNOWLEDGMENTS
The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants NR004061; AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495). The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
Dr. Matthews reports that she had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Abbreviation List
- CHD
Coronary heart disease
- LDL-C
Low density lipoprotein cholesterol
- CRP
C-reactive protein
- HDL-C
High density lipoprotein cholesterol
- Apo
Apolipoprotein
- Lp(a)
Lipoprotein(a)
- tPA-ag
Tissue-type plasminogen activator antigen
- PAI-1
Plasminogen activator inhibitor Type 1
- BP
Blood pressure
- FSH
Follicle Stimulating Hormone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors report no conflicts of interest.
References
- 1.Tunstall-Pedoe H. Myth and paradox of coronary risk and the menopause. Lancet. 1998;351:1425–1427. doi: 10.1016/S0140-6736(97)11321-6. [DOI] [PubMed] [Google Scholar]
- 2.Colditz GA, Willet WC, Stampfer MJ, Rosner B, Speizer FE, Hennekens CH. Menopause and the risk of coronary heart disease in women. N Engl J Med. 1987;316:1105–1110. doi: 10.1056/NEJM198704303161801. [DOI] [PubMed] [Google Scholar]
- 3.Howard BV, Kuller L, Langer R, et al. Risk of cardiovascular disease by hysterectomy status, with and without oophorectomy: the Women’s Health Initiative Observational Study. Circulation. 2005;111:1462–1470. doi: 10.1161/01.CIR.0000159344.21672.FD. [DOI] [PubMed] [Google Scholar]
- 4.Kok HS, van Asselt KM, van der Schouw YT, et al. Heart disease risk determines menopausal age rather than the reverse. J Am Coll Cardiol. 2006;47:1976–1984. doi: 10.1016/j.jacc.2005.12.066. [DOI] [PubMed] [Google Scholar]
- 5.Bittner V. Menopause and cardiovascular risk: cause or consequence? J Am Coll Cardiol. 2006;47:1984–1986. doi: 10.1016/j.jacc.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 6.Matthews KA, Kuller LH, Sutton-Tyrrell K. Changes in cardiovascular risk factors during the peri- and post-menopausal years. In: Bellino F, editor. Biology of Menopause. Norwell, MA: Serono Symposia USA Inc; 2000. pp. 147–148. [Google Scholar]
- 7.Lindquist O. Intraindividual changes of blood pressure, serum lipids and body weight in relation to menstrual status: results from a prospective population study of women in Goteborg, Sweden. Prev Med. 1982;11:162–172. doi: 10.1016/0091-7435(82)90015-9. [DOI] [PubMed] [Google Scholar]
- 8.Matthews KA, Meilahn E, Kuller LH, Kelsehy SF, Caggiula AW, Wing RR. Menopause and risk factors for coronary heart disease. N Engl J Med. 1989;321:641–646. doi: 10.1056/NEJM198909073211004. [DOI] [PubMed] [Google Scholar]
- 9.Guthrie JF, Dennerstein L, Dudley EC. Weight gain and the menopause: a 5-year longitudinal study. Climacteric. 1999;2:205–211. doi: 10.3109/13697139909038063. [DOI] [PubMed] [Google Scholar]
- 10.Bonithon-Kopp C, Scarabin PY, Carne B, Malmejac A, Guize L. Menopause-related changes in lipoproteins and some other cardiovascular risk factors. Int J Epidemiol. 1990;19:42–48. doi: 10.1093/ije/19.1.42. [DOI] [PubMed] [Google Scholar]
- 11.Sowers MF, Zheng H, Tomey K, Symons J, et al. Changes in body composition in women over six years at midlife: ovarian and chronological aging. J Clin Endocrinol Metabol. 2007;92:894–901. doi: 10.1210/jc.2006-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sowers MF, Crawford S, Sternfeld B, et al. Design, survey sampling and recruitment methods of SWAN: a multi-center, multi-ethnic, community-based cohort study of women and the menopausal transition. In: Wren J, Lobo RA, Kelsey J, Marcus R, editors. Menopause: Biology and Pathobiology. Academic Press; 2000. pp. 175–188. [Google Scholar]
- 13.Myers GL, Cooper GR, Winn CL, Smith SJ. The Centers for Disease Control – National Heart, Lung, and Blood Institute lipid standardization program: an approach to accurate and precise lipid measurements. Clin Lab Med. 1989;9:105–135. [PubMed] [Google Scholar]
- 14.Steiner P, Freidel J, Bremner W, Stein E. Standardization of micromethods for plasma cholesterol, triglyceride and HDL-cholesterol with the lipids clinics’ methodology. J Clin Chem. 1981;19:850. [Google Scholar]
- 15.Warnick G, Albers J. A comprehensive evaluation of the heparin manganese precipitation procedure for estimating high-density lipoprotein cholesterol. J Lipid Res. 1978;19:65–76. [PubMed] [Google Scholar]
- 16.Huang MH, Schocken M, Block G, et al. Variation in nutrient intakes by ethnicity: results from the Study of Women’s Health Across the Nation (SWAN) Menopause. 2002;9:309–319. doi: 10.1097/00042192-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Sternfeld B, Ainsworth BA, Quesenberry CP., Jr Physical activity patterns in a diverse population of women. Prev Med. 1999;28:313–323. doi: 10.1006/pmed.1998.0470. [DOI] [PubMed] [Google Scholar]
- 18.Wilson JD, Foster DW, editors. William’s Textbook of Endocrinology. 8th ed. Philadelphia: Saunders; 1992. [Google Scholar]
- 19.Sowers MF, Zheng H, McConnell D, Nan B, Harlow S, Randolph JF. Follicle stimulating hormones (FSH) and its rate of change to define menopause transition stages. J Clin Endocrinol Metab. 2008;93 doi: 10.1210/jc.2008-0482. 3958-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. New York: John Wiley & Sons; 2004. [Google Scholar]
- 21.Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr. 1974;AC-19:716–723. [Google Scholar]
- 22.Agresti A, Caffo B. Computational Statistics and Data Analysis. Compu Stat Data An. 2002;39:127–136. [Google Scholar]
- 23.Derby CA, Crawford SL, Pasternak RC, Sowers MF, Sternfeld B, Matthews KA. Change in lipids during the menopause transition in relation to age and weight: the Study of Women’s Health across the Nation (SWAN) Am J Epidemiol. 2009;169:1352–1361. doi: 10.1093/aje/kwp043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sutton-Tyrrell K, Wildman RP, Matthews KA, et al. Sex hormone-binding globulin and the free androgen index are related to cardiovascular risk factors in multiethnic premenopausal and perimenopausal women enrolled in the Study of Women Across the Nation (SWAN) Circulation. 2005;111:1242–1249. doi: 10.1161/01.CIR.0000157697.54255.CE. [DOI] [PubMed] [Google Scholar]
- 25.Do KA, Green A, Guthrie, Dudley EC, Burger HG, Dennerstein L. Longitudinal study of risk factors for coronary heart disease across the menopausal transition. Am J Epidemiol. 2000;151:584–593. doi: 10.1093/oxfordjournals.aje.a010246. [DOI] [PubMed] [Google Scholar]
- 26.Guthrie JR, Ball M, Dudley EC, et al. Impaired fasting glycaemia in middle-aged women: a prospective study. Int J Obesity. 2001;25:646–651. doi: 10.1038/sj.ijo.0801569. [DOI] [PubMed] [Google Scholar]
- 27.Freedman DS, Otvos JD, Jeyarajah EJ, et al. Sex and age differences in lipoprotein subclasses measured by nuclear magnetic resonance spectroscopy. Clin Chem. 2004;50:1189–1200. doi: 10.1373/clinchem.2004.032763. [DOI] [PubMed] [Google Scholar]
- 28.Gold EB, Bromberger J, Crawford S, et al. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol. 2001;153:865–874. doi: 10.1093/aje/153.9.865. [DOI] [PubMed] [Google Scholar]
- 29.McKinlay SM, Bifano NL, McKinlay JB. Smoking and age at menopause in women. Ann Intern Med. 1985;103:350–356. doi: 10.7326/0003-4819-103-3-350. [DOI] [PubMed] [Google Scholar]
- 30.Bromberger JT, Matthews KA, Kuller LH, et al. Prospective study of the determinants of age at menopause. Am J Epidemiol. 1997;145:124–133. doi: 10.1093/oxfordjournals.aje.a009083. [DOI] [PubMed] [Google Scholar]
- 31.Santoro N, Brockwell S, Johnston J, et al. Helping midlife women predict the onset of the final menses – SWAN, the Study of Women’s Health Across the Nation. Menopause. 2007;14 doi: 10.1097/gme.0b013e31802cc289. 425-4. [DOI] [PubMed] [Google Scholar]
- 32.Cooper GS, Baird DD, Darden FR. Measures of menopausal status in relation to demographic, reproductive, and behavioral characteristics in a population-based study of women aged 35–49 years. Am J Epidemiol. 2001;153:1159–1165. doi: 10.1093/aje/153.12.1159. [DOI] [PubMed] [Google Scholar]
- 33.Matthews KA, Kuller LH, Sutton-Tyrrell K, Chang YF. Changes in cardiovascular risk factors during the perimenopause and postmenopause and carotid artery atherosclerosis in healthy women. Stroke. 2001;32:1104–1111. doi: 10.1161/01.str.32.5.1104. [DOI] [PubMed] [Google Scholar]
- 34.Matthews KA, Kuller LH, Chang Y, Edmundowicz D. Premenopausal risk factors for coronary and aortic calcification: a 20-year follow-up in the Healthy Women Study. Prev Med. 2007;45:302–308. doi: 10.1016/j.ypmed.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuller LH, Matthews KA, Edmundowicz D, Chang Y. Incident coronary artery calcium among postmenopausal women. Atherosclerosis. 2008;200:278–285. doi: 10.1016/j.atherosclerosis.2007.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]