Abstract
Mast cells can function as effector and immunoregulatory cells in IgE-associated allergic disorders, as well as in certain innate and adaptive immune responses. This review will focus on exciting new developments in the field of mast cell biology published within the last year. It will highlight advances in the understanding of FcεRI-mediated signaling and mast cell activation events, as well as in the use of genetic models to study mast cell function in vivo. Finally, we will discuss newly identified roles of mast cells or individual mast cell products, such as proteases and IL-10, in host defense, cardiovascular disease and tumor biology, and in settings in which mast cells have anti-inflammatory or immunosuppressive functions.
Mast cells are derived from hematopoietic progenitor cells but do not ordinarily circulate in mature form; instead, differentiation and maturation of mast cells occurs locally, following migration of their precursors to the vascularized tissues or serosal cavities in which mast cells will ultimately reside1–6. Mast cells are key effector cells in IgE-associated immune responses, including allergic disorders and certain protective immune responses to parasites2, 6–8. IgE-dependent mast cell activation leads to the secretion of three classes of mediators; degranulation results in secretion of preformed mediators that are stored in the cells’ cytoplasmic granules (e.g., vasoactive amines and neutral proteases), proinflammatory lipid mediators are synthesized de novo, and growth factors, cytokines, and chemokines are synthesized and secreted. However, mast cells can be activated to express important effector and immunomodulatory functions by many mechanisms that are independent of IgE, and the kinetics, amounts and/or spectrum of mediators released can be stimulus-dependent6, 7, 9.
FcεRI signalling events
Mast cells can respond to many different stimuli, and thereby participate in a wide variety of physiological and pathological processes, as a result of their activation by any of an array of receptors. However, the best-studied mechanism by which mast cells perform immunologically specific function is through antigen- and IgE-dependent aggregation of the high affinity IgE receptor, FcεRI7, 10–15 (Fig. 1).
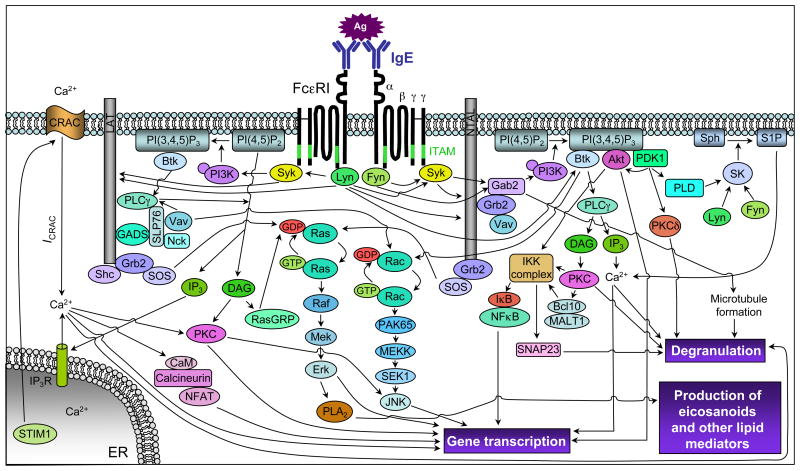
Figure 1.
Simplified scheme of early FcεRI-mediated signaling events. Ag-induced crosslinking of FcεRI induces activation of Lyn, which phosphorylates FcεRI ITAMs (green) and activates Syk following ITAM binding, and Fyn, which phosphorylates the adaptor Gab2 to activate the PI3K pathway. Lyn and Syk phosphorylate many adaptor molecules, e.g., LAT and NTAL, and enzymes to regulate activation of the Ras, PLCγ, PI3K and other pathways. Grb2 and SOS activate the Ras/Erk pathway, which regulates transcription factor activation and arachidonic acid metabolism (through PLA2 activation). PLCγ can either be activated through the coordinated function of LAT/Gads/SLP-76/Vav and Btk or independently of LAT through a PI3K/Btk-dependent pathway. PLCγ activation regulates classical PKC activation (through DAG generation) and calcium responses (through the generation of IP3). IP3 binding to the IP3R triggers Ca2+ release from the ER; STIM1 couples ER Ca2+ store depletion with the activation of CRAC channels, leading to the influx of extracellular Ca2+ and ICRAC. The PI3K product, PI(3,4,5)P3, is an important lipid mediator that regulates the activity of various enzymes, e.g., Btk, Akt, PDK1, PLD and SK, and the formation of other lipid mediators, e.g., DAG and S1P. S1P can act intracellulary, to regulate Ca2+ influx and degranulation (independently of PLC and IP3), and extracellularly (following secretion from the cell) by binding to surface S1P1 or S1P2 receptors and thereby inducing cytoskeletal rearrangement or enhancing degranulation, respectively. The IKK complex consists of two catalytic subunits, IKKα/IKK1 and IKKβ/IKK2, and a regulatory subunit, NEMO/IKKγ; this complex phosphorylates IκB to activate the transcription factor NFκB. IKKβ/IKK2 also phosphorylates SNAP23 to facilitate SNARE complex formation. Arrows indicate the contributions of these signaling pathways toward mast cell degranulation, arachidonic acid metabolism, and cytokine/chemokine/growth factor production. Note: some arrows do not indicate direct interactions or targets. Bcl10, B cell lymphoma 10; Btk, Bruton’s tyrosine kinase; Ca2+, calcium; CaM, calmodulin; CRAC, Ca2+ release activated calcium channel; DAG, diacylglycerol; Gab2, Grb2 associated binding protein 2; GADS, Grb2 related adaptor downstream of Shc; ER, endoplasmic reticulum; Erk, extracellular signal-regulated kinase; ICRAC, Ca2+ release activated current; IκB, inhibitor of κB; IKK, IκB kinase; IP3, inositol 1,4,5-trisphosphate; IP3R, IP3 receptor; ITAM, immunoreceptor tyrosine based activation motif; LAT, linker for activation of T cells; MALT1, mucosa associated lymphoid tissue lymphoma translocation protein 1; NEMO, NFκB essential modulator; NFAT, nuclear factor of activated T cells; NFκB, nuclear factor κB; NTAL, non-T cell activation linker; PI3K, phosphoinositide 3-kinase; PI(3,4,5)P3, phosphatidylinositol 3,4,5-trisphosphate; PKC, protein kinase C; PL, phospholipase; RasGRP, Ras guanyl nucleotide-releasing protein; S1P, sphingosine 1 phosphate; SK, sphingosine kinase; SLP-76, SH2-domain containing leukocyte protein of 76 kDa; SOS, son of sevenless homolog; Sph, sphingosine; STIM1, stromal interaction molecule 1.
FcεRI is expressed on mast cells as a heterotetrameric receptor comprised of an IgE-binding α subunit, the membrane tetraspanning β subunit, and two identical disulphide linked γ subunits (which are important for initiating signalling events downstream of this receptor because they each contain one immunoreceptor tyrosine-based activation motif [ITAM])7, 11, 12, 14. The stability of FcεRI on the mast cell surface, which is a major determinant of FcεRI expression levels, can be influenced both by external factors (e.g., IgE binding2, 7)and intracellular molecules (e.g., Rabaptin-516). Antigen- and IgE-induced crosslinking of cell surface FcεRI induces activation of Lyn, which phosphorylates FcεRI ITAMs and activates Syk following ITAM binding. Lyn and Syk phosphorylate several adaptor molecules and enzymes to regulate mast cell activation. In addition to Lyn, FcεRI aggregation activates a second Src family kinase, Fyn, which phosphorylates the adaptor Gab2 to activate the PI3K pathway.
In addition to its aforementioned signal initiating activity, Lyn also negatively regulates FcεRI-induced signalling events (including Fyn activation)12. Indeed, Lyn knockout mast cells and mice are hyperresponsive to IgE plus antigen stimulation12, 17, 18. Hong et al.19 reported that a third Src family kinase, Hck, plays a positive regulatory role in FcεRI-induced mast cell degranulation and cytokine release by both Lyn-dependent and Lyn-independent mechanisms (both of which are dependent, at least in part, on phosphorylation of the FcεRI β chain). The Lyn-dependent mechanism involves Hck-mediated suppression of Lyn’s negative regulatory kinase activity (i.e., Lyn activity and the phosphorylation of various Lyn targets [e.g., SHIP] were increased, while the phosphorylation of various positive regulatory molecules [e.g., Syk] was reduced, in hck−/− bone marrow-derived cultured mast cells [BMCMCs])19. The authors proposed a hierarchical relationship among the Src family kinases downstream of FcεRI: Hck negatively regulates Lyn, which negatively regulates Fyn19. Additional studies are required to understand more fully the interplay among Src family kinases downstream of FcεRI (e.g., why was Fyn activity normal in hck−/− BMCMCs despite increased Lyn activity?).
PLCγ hydrolyzes PI-4,5-P2 (phosphatidyl inositol 4,5-bisphosphate) to form soluble IP3 (inositol 1,4,5-trisphosphate) and membrane bound DAG (diacylglycerol) 7, 11–14; IP3 binding to its receptor in the endoplasmic reticulum (ER) rapidly induces the first stage of calcium (Ca2+) mobilization, the transient release of Ca2+ from ER stores, which in turn induces prolonged influx of Ca2+ through store-operated calcium release-activated calcium (CRAC) channels in the plasma membrane (Fig. 1). The recent identification of STIM1, a sensor of ER Ca2+ concentrations that couples depletion of ER Ca2+ stores with activation of CRAC channels20, 21, and CRACM1 (aka Orai1), the pore-forming subunit of the CRAC channel22–25, has increased our understanding of CRAC currents at the molecular level. Using STIM1-deficient mice, Baba et al.26 showed that STIM1 is required for FcεRI-induced Ca2+ influx, degranulation, transcription factor (i.e., NFκB and NFAT) activation, and IgE-dependent anaphylaxis in vivo and Vig et al.27 used CRACM1-deficient mice to show that CRACM1 is required for FcεRI-induced degranulation, lipid mediator synthesis, cytokine release and IgE-dependent allergic responses in vivo. These studies demonstrate conclusively that the second stage of FcεRI-induced Ca2+ mobilization, the influx of Ca2+ mediated by STIM1 and CRACM1, is essential for mast cell activation in vitro and in vivo26,27.
Recent work indicates that canonical transient receptor potential channels (TRPC), which are Ca2+-permeable nonselective cation channels, may associate with STIM1 and CRACM1 to enhance Ca2+ entry28, 29. Using inhibitory RNA in a rat mast cell (RBL-2H3) line, Ma et al.30 showed that TRPC5, in addition to STIM1 and CRACM1, is required for optimal FcεRI-induced Ca2+ influx and degranulation. They proposed that strontium-permeable TRPC5 associates with STIM1 and CRACM1 in a stoichiometric manner to enhance FcεRI-induced Ca2+ influx and degranulation in mast cells30.
The rate of Ca2+ influx through store-operated channels is also dependent on the membrane potential, which is regulated by calcium-activated nonselective (CAN) cation channels31, such as TRPM4 (transient receptor potential cation channel, subfamily M, member 4). Using TRPM4-deficient mice, Vennekens et al.32 showed that TRPM4 activates a CAN current which depolarizes membrane potential and limits the driving force for Ca2+ entry through CRAC channels in BMCMCs; FcεRI-induced degranulation, leukotriene release and TNF (but not IL-6) production was increased in TRPM4-deficient BMCMCs and TRPM4-deficient mice exhibited more severe acute (but not late-phase) inflammation during IgE-mediated passive cutaneous anaphylaxis responses. Accordingly, the authors proposed that TRPM4 acts as a “molecular brake” on Ca2+ influx after FcεRI-induced mast cell activation in vitro and in vivo32.
Downstream of early FcεRI-induced signaling events (such as Ca2+ influx), the final stages of mast cell degranulation require membrane fusion events. The exocytosis of mast cell granules, or secretory lysosomes, is mediated by membrane fusion proteins called SNAREs (soluble N-ethyl-maleimide-sensitive factor [NSF] attachment protein receptors)33–37; these are divided into t-SNAREs, localized on the target membrane (e.g., syntaxins and soluble NSF attachment proteins [SNAPs]) and v-SNAREs, localized on the vesicle membrane (e.g., vesicle-associated membrane proteins [VAMPs]). Murine rodent and human mast cells express VAMP-2, -3, -7 and -8, and both VAMP-7 and -8 colocalize with secretory granules in RBL-2H3 cells35, 38. Two groups recently showed that FcεRI-induced exocytosis is reduced in VAMP-8-deficient mast cells39, 40. Puri & Roche39 reported that this defect was limited to a distinct subset of secretory granules (i.e., those containing serotonin and cathepsin D). They did not observe any defects in the regulated exocytosis of granules containing histamine39. By contrast, Tiwari et al.40 reported an approximately 50% reduction in FcεRI-induced β-hexosaminidase and histamine release in vitro in the absence of VAMP-8. Moreover, they observed reduced blood histamine levels in VAMP-8-deficient mice during passive systemic anaphylaxis40. Although these groups used different VAMP-8-deficient mice, the reason for the discrepancies in their findings remains to be determined. Finally, Sander et al.41 reported that inhibition of syntaxin 4, SNAP-23, VAMP-7 or VAMP-8, but not VAMP-2 or VAMP-3, inhibited FcεRI-induced histamine release in primary human mast cells.
The t-SNAREs syntaxin 4 and SNAP23 regulate FcεRI-induced exocytosis from mast cells33, 35 and the phosphorylation of SNAP23 (on Ser120 and Ser95) has been shown to modulate exocytic events42. Suzuki & Verma43 recently reported that IKKβ (inhibitory κB kinase β; also termed IKK2), one of two catalytically active subunits of the IKK complex, phosphorylates SNAP23 on Ser120 and Ser95. Although the IKK complex is best known for its role in activating the transcription factor NFκB, this IKKβ/IKK2-mediated phosphorylation of SNAP23 was shown to upregulate FcεRI-induced degranulation in vitro in an NFκB-independent manner43. Moreover, they showed that IKKβ/IKK2 in mast cells plays a critical role in enhancing IgE-mediated acute local or systemic reactions, as well as an example of a cutaneous late phase reaction (in an NFκB-dependent manner), in vivo. These exciting results suggest that IKKβ/IKK2 may have additional substrates that allow this kinase to regulate NFκB-independent mast cell activation events, such as SNARE complex formation.
In addition to SNAREs, Rab GTPases regulate exocytic events34, 36, 37; for example, Rab27a and its effector, Munc13–4, enhance FcεRI-induced mast cell degranulation44. Higashio et al.45 reported that Doc2α, which was thought to be a brain specific isoform of the Doc2 family that regulates Ca2+-dependent synaptic vesicle exocytosis, is expressed in mast cells. They showed that Doc2α colocalized with Munc13–4 on secretory granules in RBL-2H3s and that FcεRI-induced Ca2+-dependent secretory lysosome exocytosis was reduced in BMCMCs from Doc2α-deficient mice45.
Negative regulation of FcεRI-dependent mast cell activation
Several negative intracellular regulators can diminish FcεRI-induced signaling events (e.g., the lipid phosphatase SHIP; Fig. 2). Some signaling molecules initiate both activating and inhibitory signals (e.g., Lyn phosphorylates FcεRI ITAMs as well as inhibitory receptor immunoreceptor tyrosine based inhibitory motifs [ITIMs], the latter leads to recruitment of inhibitory signaling molecules, such as SHIP). Other signaling molecules can negatively regulate FcεRI-induced mast cell activation events by altering the rate of FcεRI internalization (e.g., one function of RabGEF1 is to enhance FcεRI internalization)46, 47. Bansal et al.48 recently identified regulator of G protein signaling (RGS) 13 as a novel negative regulator of FcεRI-induced degranulation (but not cytokine [TNF, IL-6 or IL-13] production) in vitro and IgE-dependent passive cutaneous or systemic anaphylaxis in vivo. RGS proteins typically inhibit G protein coupled receptor (GPCR) signaling events through GTPase-accelerating protein (GAP) activity on Gα subunits. Although GPCR signaling can amplify FcεRI-mediated responses through activation of PI3Kγ49, RGS13-mediated inhibition of FcεRI-induced activation occurs independently of RGS13’s GAP activity48. Instead, Bansal et al.48 proposed that RGS13, which is upregulated following antigen stimulation, binds to the p85α subunit of PI3K and disrupts its association with an FcεRI-activated signaling complex containing Gab2 and Grb248 (Fig. 2).
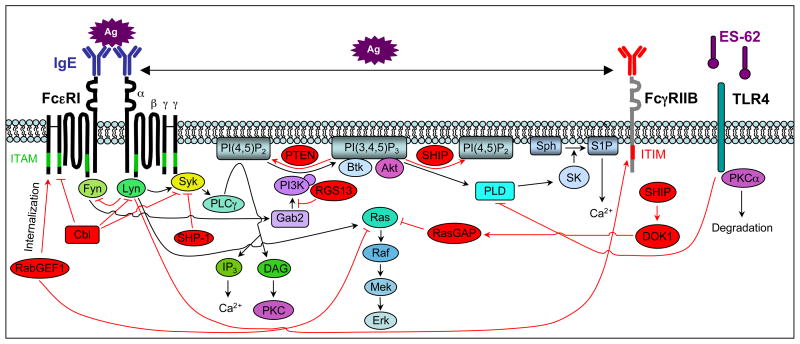
Figure 2.
Negative regulation of FcεRI-mediated signaling events. FcεRI aggregation activates a number of proteins that negatively regulate the positive signaling pathways activated downstream of this receptor. For example, Lyn, which initiates both activating and inhibitory signals, negatively regulates Fyn activity and, thus, Gab2 phosphorylation. Other negative regulators include c-Cbl (which facilitates the ubiquitination of FcεRI, Lyn and Syk), the tyrosinse phosphatase SHP-1 (which dephosphorylates Syk), the lipid phosphatases SHIP (which catalyzes the hydrolysis of PI(3,4,5)P3 to PI(3,4)P2) and PTEN (which catalyzes the hydrolysis of PI(3,4,5)P3 to PI(4,5)P2), RasGAP (which enhances the intrinsic GTPase activity of Ras), RabGEF1 (which enhances FcεRI internalization and can bind to GTP-bound Ras), and RGS13 (which binds to the p85α subunit of PI3K and disrupts its association with Gab2 and Grb2). Ag-induced coaggregation of FcεRI with FcγRIIB inhibits FcεRI-induced signaling events and mast cell activation via Lyn mediated phosphorylation of the FcγRIIB ITIM (red) and the subsequent recruitment of SHIP and DOK1. Finally, ES-62, a glycoprotein secreted by filarial nematodes, forms a complex with TLR4 (which causes the sequesteration and subsequent proteosome-independent degradation of PKCα) to block FcεRI-induced PLD-coupled, SK-mediated Ca2+ flux and NFκB activation. DOK1, docking protein 1; Gab2, Grb2 associated binding protein 2; ITAM, immunoreceptor tyrosine based activation motif; ITIM, immunoreceptor tyrosine based inhibititory motif; NFκB, nuclear factor κB; PI3K, phosphoinositide 3-kinase; PKC, protein kinase C; PLD, phospholipase D; PTEN, phosphatase and tensin homolog; RabGEF, Rab5 guanine nucleotide exchange factor; RasGAP, GTPase activating protein; RGS, regulator of G protein signaling; SHIP, Src homology 2 (SH2) domain-containing inositol 5′-phosphatase; SHP-1, SH2 domain-containing tyrosine phosphatase-1; SK, sphingosine kinase; TLR4, toll like receptor 4.
In addition to negative intracellular regulators, signalling events initiated by FcεRI and other ITAM-containing immunoreceptors are negatively regulated by their coaggregation with ITIM-containing receptors. Mast cells express several inhibitory receptors, including FcγRIIB, gp49B1, MAFA and PIR-B7, 14. FcγRIIB, the first identified ITIM-containing receptor, is an attractive therapeutic target for mast cell activation events because it recruits the lipid phosphatase SHIP following coaggregation with FcεRI in vivo50 (Fig. 2).
Melendez et al.51 reported that ES-62, a glycoprotein secreted by filarial nematodes, might also be investigated as a novel therapeutic for allergy. They showed that ES-62, which inhibits the activation of many immune cells (e.g., B and T cells, dendritic cells and macrophages), also inhibited FcεRI-induced degranulation, arachidonic acid metabolism, and the production of TNF, IL-3 and IL-6 (but not IL-13 or IL-5) in human BMCMCs. ES-62 mediated these effects by forming a complex with Toll-like receptor 4, which caused sequestration and proteasome-independent degradation of PKCα, resulting in decreased phospholipase D-coupled, sphingosine kinase-mediated Ca2+ influx and NFκB activation51 (Fig. 2). Moreover, they showed that ES-62 significantly reduced the magnitude of both a model of cutaneous immediate-type hypersensitivity to oxazolone and a model of OVA-induced airway hyperreactivity to Methacholine, and airway allergic inflammation, in mice sensitized to OVA admixed with aluminum hydroxide as an adjuvant. The authors suggested that ES-62-mediated suppression of mast cell activation may contribute to the reduced incidence of allergic disorders in people harboring worms and, since ES-62 appears to be well tolerated by millions of infected people, that ES-62 derivatives might represent a new type of therapeutic agent for diseases such as asthma.
New developments in mast cell models
Human mast cell populations and mouse mast cells derived from bone marrow (BMCMCs), other hematopoietic tissues (e.g., fetal liver; FLCMCs) or embryonic stem cells (ESCMCs) are powerful tools for investigating the mechanisms by which mast cells might influence various immunological or other biological responses in vitro. Although there are many similarities between mast cell populations in humans and in mice, there are also some differences in their anatomical distribution, phenotype and function that may influence the particular roles of mast cells in various biological responses in the two species2, 52. However, the ability to manipulate human mast cell numbers, phenotype or function in vivo is quite limited. Accordingly, many investigators have attempted to analyze mast cell function using more tractable experimental species, especially the mouse.
The in vivo relevance and biological importance of in vitro observations about mast cell function, as well as the contributions of mast cells towards the expression of particular biological responses in vivo, can be assessed using c-kit mutant mice (e.g., WBB6F1-KitW/W-v or C57BL/6-KitW-sh/W-sh mice) that virtually lack mast cell populations53–58. The mast cell deficiency of these mice can be selectively repaired by the adoptive transfer of genetically compatible, in vitro derived mast cells from congenic wild-type mice or various transgenic or mutant mice55, 57, 58 or from mouse embryonic stem cells56, or mast cells that have been transduced with short hairpin (sh)RNA to reduce expression of proteins of interest59. These mast cell knockin mice are now widely used to assess the contributions of mast cells or specific mast cell products in diverse biological responses in vivo.
C57BL/6-KitW-sh/W-sh mice are gaining popularity for such studies because these mice have fewer, or less severe, phenotypic abnormalities than are observed in WBB6F1-KitW/W-v mice. For example, unlike WBB6F1-KitW/W-v mice, C57BL/6-KitW-sh/W-sh mice are neither anemic nor sterile, and they appear to have normal numbers of bone marrow and blood neutrophils57, 60. It is important to consider the genetic background of the mice and the effects of the different c-kit mutations on other cell lineages when studying various disease models. For example, while Lee et al.61 showed that WBB6F1-KitW/W-v mice were resistant to the development of joint inflammation in one model of autoantibody-induced arthritis, Zhuo et al.60 recently reported that mast cell-deficient C57BL/6-KitW-sh/W-sh, but not mast cell-deficient WBB6F1-KitW/W-v, mice developed autoantibody-mediated, neutrophil-dependent immune complex arthritis. They attributed this difference to the relative neutrophil deficiency observed in WBB6F1-KitW/W-v mice60.
Two groups recently reported the generation of mast cell-specific Cre mice. Scholten et al.62 generated transgenic mice expressing Cre recombinase under the control of the mast cell protease (Mcpt) 5 promoter; using ROSA26-EYFP mice, they showed efficient Cre-mediated recombination in mast cells from the peritoneal cavity and skin while only minimal reporter gene expression was detected outside the mast cell compartment. Musch et al.63 expressed Cre recombinase under the control of the baboon α-chymase promoter; using ROSA26R mice, they showed efficient Cre-mediated recombination in lung and colon tissue mast cells, but not in mast cells isolated from the peritoneal cavity or in vitro generated BMCMCs. It will be important to exercise care both in characterizing the phenotypic features of such mice (as expressed in mast cells and possibly in other cell types) and in interpreting the results of experiments using such animals. However, we think that validated mast cell-specific Cre mice, and inducible mast cell-specific Cre mice, may well become powerful genetic models for investigating the contributions of mast cells or mast cell-specific products to health and disease.
Mast cell proteases
Helping out in host defense
Mast cells are strategically located very near sites where the body comes in contact with the external environment; a prime location for the initiation and modulation of innate immune responses. Indeed, Malaviya et al.64 and Echtenacher et al.65 showed that mast cells can contribute importantly to innate bacterial clearance, at least in part by enhancing the recruitment of neutrophils to the site of infection. Since then, many in vitro or in vivo studies have provided additional evidence that mast cells can enhance host defense through direct effects on pathogens, by initiating and modulating the inflammation associated with innate immune responses, and perhaps by initiating adaptive immune responses to pathogens.
Another protective function of mast cells during innate responses to bacterial infection is to limit the toxicity of certain products generated by the host, which can have adverse effects at high concentrations. For example, mast cells can limit the toxicity of the peptide endothelin (ET)-1, whose levels are markedly elevated during acute bacterial peritonitis and sepsis, by releasing proteases stored in their granules that can degrade this peptide66, 67. ET-1 exhibits high homology with sarafotoxins (the most toxic components of Israeli mole viper [Atractaspis engaddensis] venom), and Metz et al.59 showed that mast cells can substantially enhance resistance to the pathology and mortality induced in mice by the venoms of A. engaddensis and two other poisonous snakes, and that of the honeybee. Metz et al.59 used shRNA and pharmacological methods to show that this mast cell-mediated reduction in endogenous (ET-1) and exogenous (sarafotoxin) toxic peptides was dependent on carboxypeptidase A3 (CPA3) activity; however, mast cells that lack CPA3 concomitantly lack mast cell protease (MCP)-559, 67. In an elegant study, Schneider et al.67 generated a mutant mouse (Mc-cpaY356L,E378A) bearing two amino acid mutations that rendered CPA3 catalytically inactive without affecting expression of other proteases. Using this mutant, they confirmed that mast cell-mediated innate defense against ET-1 and sarafotoxin is dependent on CPA3 activity and defined the molecular mechanism by which CPA3 inactivates these toxins67.
In addition to ET-1, Piliponsky et al.68 recently reported that levels of neurotensin (NT; a peptide known to induce hypotension) are increased in a mouse model of sepsis. They showed that, in mice, NT can contribute to sepsis-related mortality, that mast cells can reduce NT levels in vivo, that mast cells can degrade NT via the protease neurolysin, and that mast cells can reduce NT-induced hypotension, as well as sepsis-related mortality68. Moreover, in a pilot study of human patients with sepsis (or with cardiogenic shock), they found that plasma concentrations of NT were elevated to levels similar to those observed in mice with acute bacterial peritonitis68. Because sepsis is the most common cause of death in intensive care units in the United States, there is considerable interest in identifying additional biomarkers and therapeutic targets in this disorder. The findings of Piliponsky et al.68 raise the possibility that NT might contribute to the pathology in patients with sepsis; therefore, inhibiting the pathological actions of NT may confer benefit in this setting.
Although IL-15 is known to play a critical role in innate immunity, Orinska et al.69 found that IL-15-deficient mice are actually less susceptible to sepsis-related mortality. Moreover, WBB6F1-KitW/W-v mice engrafted with IL-15-deficient mast cells survived better than those engrafted with wild-type mast cells. Orinska et al.69 showed that mast cells express intracellular IL-15 (both constitutively and following stimulation with lipopolysaccharide), which appears to function as a negative transcriptional regulator of a mast cell chymase, MCP-2. The authors proposed that this IL-15-mediated repression of MCP-2 activity limits the bactericidal activity of mast cells and the recruitment of neutrophils needed to clear the bacterial infection.
Further highlighting the importance of mast cell proteases in innate host defense, Thakurdas et al.70 reported that the mast cell tryptase, MCP-6 (also known as tryptase β2 [Tpsb2]), can play a critical protective role in bacterial infections (i.e., MCP-6/Tpsb2-deficient mice have reduced ability to clear Klebsiella pneumoniae injected into their peritoneal cavity, probably because of diminished recruitment of neutrophils. The authors suggested that MCP-6/Tpsb2 is the primary preformed granule mediator of mast cells that can protect mice during acute bacterial infections70; however, the mechanism by which MCP-6/Tpsb2 induces neutrophil recruitment to the site of infection remains to be determined.
Mast cell proteases also can contribute to resistance to parasite infections. For example, Knight et al.71 reported delayed expulsion of the adult helminth and increased deposition of muscle larvae in MCP-1-deficient mice. Shin et al.72 recently showed that MCP-6 is important for clearance of chronic Trichinella spiralis infection; i.e., the recruitment of eosinophils to T. spiralis larvae and the elimination of larvae in chronically infected skeletal muscle were decreased in MCP-6/Tpsb2-deficient mice. Because eosinophil infiltration around T. spiralis larvae was also decreased in IgE-deficient mice, the authors suggested that mast cells and, more specifically, MCP-6/Tpsb2 link adaptive and innate immunity in the chronic phase of T. spiralis infection72.
Helping out in allergic reactions
Mast cells are typically thought of as troublesome cells due to their prominent role in IgE-dependent allergic hypersensitivity reactions, such as allergic rhinitis (hay fever), atopic dermatitis (eczema), allergic (or “atopic”) asthma, and some food allergies. However, Rauter et al.73 showed that β-tryptase, a protease released by mast cells, can cleave IgE. They detected IgE cleavage products in tissue fluids collected from sites of allergic inflammation and showed that protamine (an inhibitor of heparin-dependent proteases) enhanced IgE-mediated allergic skin inflammation induced by skin prick testing in human subjects73. Although protamine treatment may have had other effects that influenced the magnitude of the biological responses analyzed, the findings suggest that mast cell protease-dependent degradation of IgE may help to limit this type of allergic inflammation. However, the extent to which this mechanism can contribute to the reduction of local (or systemic) IgE levels during other examples of allergic inflammation remains to be determined.
Immunomodulatory roles of mast cells
Slowing things down
Mast cells can exert positive or negative immunomodulatory functions on immune cells (i.e., influence the recruitment, survival, development, phenotype or function of immune cells) and thereby enhance or suppress the initiation, magnitude and/or duration of immune responses6. Because they might at first seem counter intuitive, given the mast cell’s well-deserved reputation as a promoter of inflammation, we will first discuss some negative immunomodulatory functions of this cell. Hart et al.74 showed that the ability of ultraviolet B (UVB) irradiation of the skin to induce systemic immunosuppression of contact hypersensitivity (CHS) responses was mast cell dependent. Byrne et al.75 recently showed that UV-induced mast cell migration from the skin to the draining lymph nodes, which is mediated by CXCR4 on mast cells, is a key step in the induction of UV-induced immunosuppression. Mast cells have also been shown to mediate immunosuppressive functions following Anopheles mosquito bites76, and in peripheral tolerance to skin allografts (which requires the participation of CD4+CD25+FoxP3+ TReg cells)77; however, the mechanism(s) by which mast cells mediate immunosuppressive functions in each of these studies remains to be elucidated.
Grimbaldeston et al.78 showed that mast cells can mediate negative immunomodulatory functions in vivo by producing IL-10. Mast cells and mast cell-derived IL-10 limited the magnitude, and promoted the resolution, of CHS responses induced in response to the hapten 2,4-dinitro-1-fluorobenzene (DNFB) or urushiol (Fig. 3), which is the hapten-containing sap of poison ivy (Toxicodendron radicans) or poison oak (T. diversilobum)78. Mast cells and mast cell-derived IL-10 also suppressed innate cutaneous responses to chronic low-dose UVB-irradiation78. Although mast cells limited multiple aspects of these responses, including inflammation, epidermal hyperplasia, and skin ulceration, the pathways that link mast cell-derived IL-10 (or other mast cell mediators that are relevant in this setting) to the observed tissue changes remain to be defined (i.e., mast cells and mast cell-derived IL-10 may influence these responses through a complex combination of direct and indirect effector and immunomoregulatory functions).
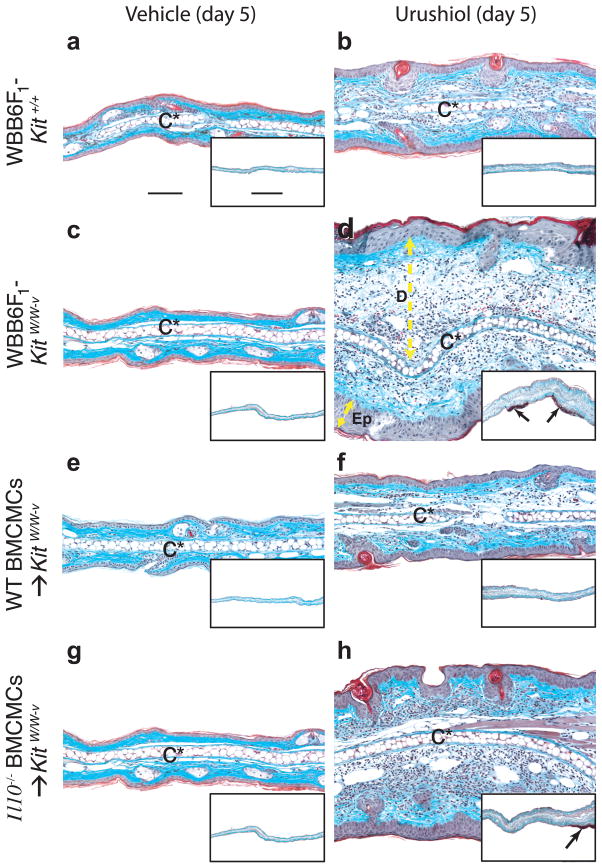
Figure 3.
Mast cell limit the pathology associated with CHS to urushiol. Cross-sections of ears (stained with Masson’s Trichrome) from WBB6F1-Kit+/+ (wild-type) mice (a,b), WBB6F1-KitW/W-v mice (c,d) or WBB6F1-KitW/W-v mice engrafted 8 weeks before the experiment with WT BMCMCs (WT BMCMC → KitW/W-v mice) (e,f) or Il10−/− BMCMCs (Il10−/− BMCMC → KitW/W-v mice) (g,h) were obtained 5 d after challenge with vehicle (100% acetone) only (a,c,e,g) or 5 mg/ml of urushiol (b,d,f,h). Focal full thickness necrosis of the epidermis and/or ulceration occurred in association with CHS responses to urushiol at 5 d after challenge in 8/10 of the mast cell-deficient WBB6F1-KitW/W-v mice and in 8/8 Il10−/− BMCMC → KitW/W-v mice but in none of the 10 wild-type or 7 WT BMCMC → KitW/W-v mice; *P < 0.05 by Chi-square test for all comparisons between rates of epidermal necrosis and ulceration in wild-type (WBB6F1-Kit+/+) mice or WT BMCMC → KitW/W-v mice and the corresponding mast cell-deficient WBB6F1-KitW/W-v mice or Il10−/− BMCMC → KitW/W-v mice. Similar findings were observed in association with CHS responses to urushiol in 5 of 7 C57BL/6-KitW-sh/W-sh mice in response to DNFB. By contrast, epidermal necrosis and ulceration occurred in none of the corresponding congenic wild-type mice or WT BMCMC-engrafted C57BL/6-KitW-sh/W-sh mice (10 or 8 for urushiol and 19 or 16 for DNFB, respectively); *P < 0.05 by Chi-squre test for all comparisons between rates of epidermal ulceration in wild-type or WT BMCMC-engrafted C57BL/6-KitW-sh/W-sh mice and the corresponding mast cell-deficient C57BL/6-KitW-sh/W-sh mice. C*: cartilage; double-headed arrows show thickness of dermis (D) or epidermis (Ep); arrows in insets: ulcers with adherent exudates (red). Scale bar in a = 100 μm & in inset in a = 1000 μm. Photomicrographs are representative of the findings observed in each of the 3 experiments performed (n = 3–7 mice/group per experiment). Taken from74.
Revving things up
Mast cells are involved in the development of various T cell-associated immune responses in mice, including models of multiple sclerosis (i.e., experimental autoimmune encephalomyelitis)79 and bullous pemphigoid80, and mast cells (and mast cell-derived TNF) were shown to contribute to disease pathology in a model of TH17 cell-dependent, neutrophil-associated lung inflammation in ovalbumin (OVA)-challenged, OVA-specific T cell receptor transgenic mice81. Moreover, mast cells contribute to the development of delayed type CHS (aka allergic contact dermatitis) under some, but not all, experimental conditions5, 82, 83. Using different immunizing doses of the hapten oxazolone, Norman et al.84 recently showed that mast cells can act to reduce or enhance the inflammatory response in CHS reactions depending on the concentration of hapten used for immunization.
Several mast cell-derived products can influence T cell development, recruitment, phenotype, proliferation and activation in vitro and in vivo5, 6, 79, 81, 85, 86 and mast cells can promote T cell activation indirectly through the stimulation of antigen-presenting cells (APCs) in vivo (i.e., mast cells induce the migration of dendritic cells86 and Langerhans cells85, 87 to draining lymph nodes where antigen presentation occurs). Kambayashi et al.88 reported that antigen incorporated into mast cells via FcεRI can activate antigen-specific T cell responses in vitro; this mechanism is independent of mast cell MHC class II expression, but requires that such mast cells undergo apoptosis and then ingestion by APCs.
Because mast cells can help to initiate adaptive immune responses by inducing or enhancing the migration of APCs to draining lymph nodes, and via lymphocyte activation, McLachlan et al.89 hypothesized that the administration of small-molecule mast cell activators (e.g., compound 48/80) with vaccine antigens might enhance the development of a protective antigen-specific immune response. Indeed, they found that subcutaneous or nasal administration of these activators enhanced dendritic cell and lymphocyte trafficking to draining lymph nodes and increased antigen-specific serum IgG responses89. Moreover, nasal administration of compound 48/80 with B5R poxvirus protein, but not B5R poxvirus protein alone, protected the immunized mice against infection with vaccinia virus in vivo. The authors showed that mast cells and mast cell-derived TNF were required for the enhancement of immune responses in WBB6F1-KitW/W-v mice that had been engrafted with mast cells in the footpad and then vaccinated at the same site, however, their efforts to engraft mast cells in the nasal cavity of mast cell-deficient mice were unsuccessful. As noted above, WBB6F1-KitW/W-v mice have other defects besides a profound deficiency of mast cells, including neutrophil defects, and mast cell activators can have effects on cell types other than mast cells. However, from a clinical perspective, if this novel vaccination approach can be shown to be effective and, as importantly, safe (since mast cell activation in the context of vaccination could result in clinical toxicities), this approach may be of considerable value even if the method works because of effects on cells in addition to (or other than) mast cells.
Mast cells in models of disease
Cardiovascular disorders
Several lines of evidence have implicated mast cells in the development of a variety of chronic inflammatory disorders, including cardiovascular diseases. Because mast cells are found in the heart and, in humans, around coronary arteries and within atherosclerotic lesions, several groups have proposed that mast cells may contribute to the pathogenesis of atherogenesis90–92. Indeed, Bot et al.90 showed that targeted activation of perivascular mast cells promoted atherogenesis and plaque destabilization in apolipoprotein E-deficient mice. By crossing atherosclerosis-prone low-density lipoprotein receptor-deficient mice with C57BL/6-KitW-sh/W-sh mice, Sun et al.92 provided in vivo evidence that mast cells can contribute to atherosclerosis; i.e., they observed smaller lesions with fewer inflammatory cell (macrophage and T cell) infiltrates in the absence of mast cells. They provided evidence that mast cells promote atherosclerosis in this setting by releasing proinflammatory cytokines (IL-6 and IFNγ), which augment the expression of matrix-degrading proteases. This group also reported that mast cells contribute to the pathogenesis of elastase-induced abdominal aortic aneurysms (AAA) in mice (i.e., C57BL/6-KitW-sh/W-sh mice failed to develop AAA)93. They showed that AAA formation in this model required mast cell-derived IL-6 and IFNγ, but not TNF, and that mast cells increased matrix-degrading protease expression, smooth muscle cell apoptosis, and microvessel growth93. Similarly, Tsuruda et al.94 showed that AAA formation following periaortic application of calcium chloride (accompanied by increased numbers of mast cells and T cells, activation of matrix metalloproteinase 9, and angiogenesis in the aortic tissue) was impaired in mast cell-deficient Ws/Ws rats.
Cancer
The importance of a possible functional link between chronic inflammation and cancer has long been recognized; for example, treatment with non-steroidal anti-inflammatory drugs, which can inhibit chronic inflammation, reduces the risk of several cancers95. The majority of tumors contain inflammatory cells, including mast cells, which have potential effects that might either benefit the tumor or contribute to tumor resistance or rejection. Using WBB6F1-KitW/W-v mice, Coussens et al.96 provided evidence that mast cells can facilitate angiogenesis during early stages of skin carcinogenesis. Soucek et al.97 recently used pharmacological (cromolyn) and genetic (C57BL/6-KitW-sh/W-sh mice) approaches in vivo to provide evidence that mast cells are required for angiogenesis and macroscopic expansion of Myc-induced pancreatic β-cell tumors. Although cromolyn is widely characterized as a ‘mast cell-stabilizer’ (i.e., an agent that blocks the release of mast cell mediators following appropriate activation of the cell) that can suppress mouse mast cell function in vivo, its molecular targets are neither fully defined nor restricted to mast cells6. Moreover, because Kit signaling has been shown to be important for angiogenesis98 and cell lineages other than mast cells are affected by the c-kit mutation in C57BL/6-KitW-sh/W-sh mice, it will be of interest to assess whether engraftment of the C57BL/6-KitW-sh/W-sh mice with mast cells can restore wild-type responsiveness in this model of tumor progression. However, taken together, these results and others indicate that certain tumors may hijack certain functions of mast cells which facilitate angiogenesis and contribute to tumor survival.
Mutations of the tumor suppressor, adenomatous polyposis coli (APC) gene, are necessary and sufficient for the initiation of hereditary and many spontaneous human colorectal cancers99, 100. However, angiogenesis and tissue remodeling are also required for tumor expansion. Gouaris et al.99 recently reported that mast cells accumulate in adenomatous polyps (in a lympocyte-independent manner) and are required for polyp formation, the initiating step of colon cancer. Polyp-prone APC mutant (APCΔ468) mice reconstituted with bone marrow from wild-type, C57BL/6-KitW-sh/W-sh, or Cd34−/−Cd43−/− mice showed a tight correlation between the number of mast cells and mast cell progenitors with the frequency and size of polyps (wild-type > C57BL/6-KitW-sh/W-sh > Cd34−/−Cd43−/−) and blood vessel density (wild-type > Cd34−/−Cd43−/−)99. TNF was required for adenomatous polyp growth, and the authors proposed that mast cell-derived TNF acts in an autocrine fashion to amplify the local mast cell pool at the site of tumor formation99. They concluded that mast cells contribute importantly to the development of colon cancer. These results are consistent with those in a study reporting the reduced susceptibility of WBB6F1-KitW/W-v mice to chemically-induced intestinal tumors101. Engraftment of the WBB6F1-KitW/W-v mice with wild-type bone marrow cells increased carcinogen-induced tumorigenesis to nearly wild-type levels, consistent with a role of mast cells in this process. However, engraftment of wild-type BMCMCs failed to result in mucosal mast cells in WBB6F1-KitW/W-v recipients, and also failed to “normalize” the animals’ response to the carcinogen101.
Although other evidence also suggests that mast cells can promote tumorigenesis and tumor progression, there are some tumor models in which mast cells appear to have roles that favor the host. For example, Sinnamon et al.100 reported a protective role for mast cells in colorectal tumorigenesis. They crossed C57BL/6-KitW-sh/W-sh with Min (multiple intestinal neoplasia; a model for early intestinal tumorigenesis) mice and reported that the frequency and size of adenomas was increased in such mice, whereas tumor cell apoptosis and eosinophil infiltration were decreased100. Sinnamon et al.100 suggested that the net contributions of mast cells in various tumor models may favor the host or the tumor, depending on the specific tumor models, genetic variables (both germline and tumor-specific) and microenvironmental factors (e.g., in the case of gastrointestinal tumors, intestinal flora). The story is likely to be as (if not more) complicated in humans, given that humans, as well as their colonic neoplasms, are so diverse.
Conclusions
The new evidence indicating that mast cells can contribute to the pathology of cardiovascular diseases and certain cancers (at least in rodents) continues to tarnish the reputation of this enigmatic cell. However, this “bad guy” image is increasingly being challenged, and to some extent overshadowed, by the identification of an impressive number of protective roles that mast cells can play in both innate and adaptive immune responses, and even in host responses to some tumors (Fig. 4). But many questions in mast cell biology remain to be resolved. For example, certain features of mouse mast cell phenotype and/or function can vary substantially among different strains of mice102, 103. It will be of interest to define how, and to what extent, various genetic factors can influence aspects mast cell biology in humans. However, this is likely to be a challenging topic to investigate. Another current goal is to understand how the mast cell lineage can perform so many distinct functions, e.g., depending on the setting either promoting or limiting innate or adaptive immune responses6, 104, 105. Can mast cells, like T cells, generate developmentally, phenotypically and functionally distinct “subsets”, or do individual mast cells have sufficient plasticity to exhibit distinct features based on their responsiveness to particular local and/or systemic environmental signals? Do both mechanisms occur? Finally, it will be important to assess how our understanding of mast cell biology can be exploited clinically. Defining to what extent one can safely enhance the positive functions of mast cells, or inhibit their harmful activities, will continue to represent important goals, both to achieve a fuller understanding of this fascinating cell and to exploit such knowledge to reduce disease and promote health.
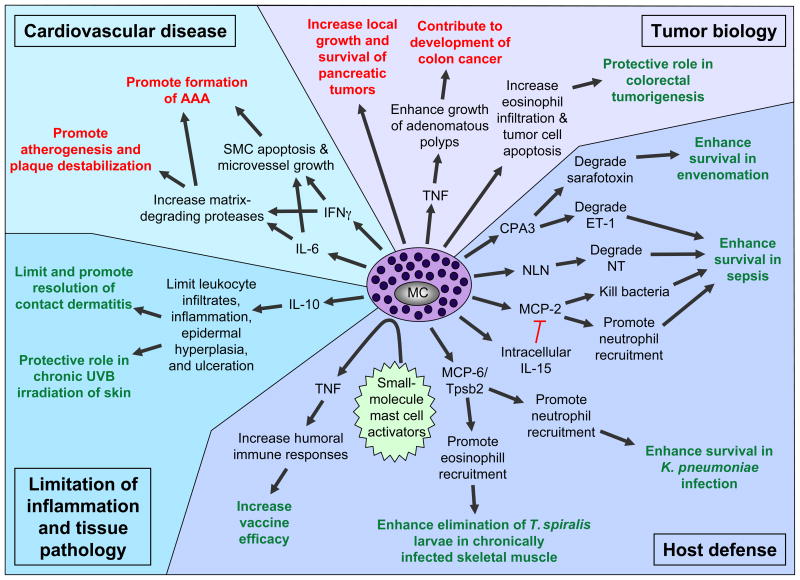
Figure 4.
Newly identified protective (green) or detrimental (red) roles of mast cells and mast cell products in biological responses in mice. AAA, abdominal aortic aneurysm; CPA3, carboxypeptidase A3; ET-1, endothelin-1; IgE, immunoglobulin E; IL, interleukin; MC, mast cell; MCP, mast cell protease; NLN, neurolysin; NT, neurotensin; SMC, smooth muscle cell; TNF, tumor necrosis factor; Tpsb2, tryptase β2.
References
- 1.Kitamura Y. Heterogeneity of mast cells and phenotypic change between subpopulations. Annu Rev Immunol. 1989;7:59–76. doi: 10.1146/annurev.iy.07.040189.000423. [DOI] [PubMed] [Google Scholar]
- 2.Mekori YA, Metcalfe DD. Mast cells in innate immunity. Immunol Rev. 2000;173:131–40. doi: 10.1034/j.1600-065x.2000.917305.x. [DOI] [PubMed] [Google Scholar]
- 3.Kawakami T, Galli SJ. Regulation of mast-cell and basophil function and survival by IgE. Nat Rev Immunol. 2002;2:773–86. doi: 10.1038/nri914. [DOI] [PubMed] [Google Scholar]
- 4.Marone G, Galli SJ, Kitamura Y. Probing the roles of mast cells and basophils in natural and acquired immunity, physiology and disease. Trends Immunol. 2002;23:425–7. doi: 10.1016/s1471-4906(02)02274-3. [DOI] [PubMed] [Google Scholar]
- 5.Metz M, Grimbaldeston MA, Nakae S, Piliponsky AM, Tsai M, Galli SJ. Mast cells in the promotion and limitation of chronic inflammation. Immunol Rev. 2007;217:304–28. doi: 10.1111/j.1600-065X.2007.00520.x. [DOI] [PubMed] [Google Scholar]
- 6.Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat Rev Immunol. 2008 doi: 10.1038/nri2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005;23:749–86. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- 8.Gurish MF, Austen KF. The diverse roles of mast cells. J Exp Med. 2001;194:F1–5. doi: 10.1084/jem.194.1.f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rivera J, Gilfillan AM. Molecular regulation of mast cell activation. J Allergy Clin Immunol. 2006;117:1214–25. doi: 10.1016/j.jaci.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 10.Nishida K, Yamasaki S, Ito Y, Kabu K, Hattori K, Tezuka T, Nishizumi H, Kitamura D, Goitsuka R, Geha RS, Yamamoto T, Yagi T, Hirano T. Fc{epsilon}RI-mediated mast cell degranulation requires calcium-independent microtubule-dependent translocation of granules to the plasma membrane. J Cell Biol. 2005;170:115–26. doi: 10.1083/jcb.200501111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast-cell activation. Nat Rev Immunol. 2006;6:218–30. doi: 10.1038/nri1782. [DOI] [PubMed] [Google Scholar]
- 12.Rivera J, Gilfillan AM. Molecular regulation of mast cell activation. J Allergy Clin Immunol. 2006;117:1214–25. doi: 10.1016/j.jaci.2006.04.015. quiz 1226. [DOI] [PubMed] [Google Scholar]
- 13.Kambayashi T, Koretzky GA. Proximal signaling events in Fc epsilon RI-mediated mast cell activation. J Allergy Clin Immunol. 2007;119:544–52. doi: 10.1016/j.jaci.2007.01.017. quiz 553–4. [DOI] [PubMed] [Google Scholar]
- 14.Kraft S, Kinet JP. New developments in FcepsilonRI regulation, function and inhibition. Nat Rev Immunol. 2007;7:365–78. doi: 10.1038/nri2072. [DOI] [PubMed] [Google Scholar]
- 15.Rivera J, Olivera A. Src family kinases and lipid mediators in control of allergic inflammation. Immunol Rev. 2007;217:255–68. doi: 10.1111/j.1600-065X.2007.00505.x. [DOI] [PubMed] [Google Scholar]
- 16.Rios EJ, Piliponsky AM, Ra C, Kalesnikoff J, Galli SJ. Rabaptin-5 regulates receptor expression and functional activation in mast cells. Blood. doi: 10.1182/blood-2008-04-152660. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hernandez-Hansen V, Smith AJ, Surviladze Z, Chigaev A, Mazel T, Kalesnikoff J, Lowell CA, Krystal G, Sklar LA, Wilson BS, Oliver JM. Dysregulated FcepsilonRI signaling and altered Fyn and SHIP activities in Lyn-deficient mast cells. J Immunol. 2004;173:100–12. doi: 10.4049/jimmunol.173.1.100. [DOI] [PubMed] [Google Scholar]
- 18.Odom S, Gomez G, Kovarova M, Furumoto Y, Ryan JJ, Wright HV, Gonzalez-Espinosa C, Hibbs ML, Harder KW, Rivera J. Negative regulation of immunoglobulin E-dependent allergic responses by Lyn kinase. J Exp Med. 2004;199:1491–502. doi: 10.1084/jem.20040382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong H, Kitaura J, Xiao W, Horejsi V, Ra C, Lowell CA, Kawakami Y, Kawakami T. The Src family kinase Hck regulates mast cell activation by suppressing an inhibitory Src family kinase Lyn. Blood. 2007;110:2511–9. doi: 10.1182/blood-2007-01-066092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–45. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–41. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–3. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–85. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 24.Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O, Cahalan MD. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–9. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–3. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 26.Baba Y, Nishida K, Fujii Y, Hirano T, Hikida M, Kurosaki T. Essential function for the calcium sensor STIM1 in mast cell activation and anaphylactic responses. Nat Immunol. 2008;9:81–8. doi: 10.1038/ni1546. [DOI] [PubMed] [Google Scholar]
- 27.Vig M, DeHaven WI, Bird GS, Billingsley JM, Wang H, Rao PE, Hutchings AB, Jouvin MH, Putney JW, Kinet JP. Defective mast cell effector functions in mice lacking the CRACM1 pore subunit of store-operated calcium release-activated calcium channels. Nat Immunol. 2008;9:89–96. doi: 10.1038/ni1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liao Y, Erxleben C, Yildirim E, Abramowitz J, Armstrong DL, Birnbaumer L. Orai proteins interact with TRPC channels and confer responsiveness to store depletion. Proc Natl Acad Sci U S A. 2007;104:4682–7. doi: 10.1073/pnas.0611692104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat Cell Biol. 2007;9:636–45. doi: 10.1038/ncb1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma HT, Peng Z, Hiragun T, Iwaki S, Gilfillan AM, Beaven MA. Canonical transient receptor potential 5 channel in conjunction with Orai1 and STIM1 allows Sr2+ entry, optimal influx of Ca2+, and degranulation in a rat mast cell line. J Immunol. 2008;180:2233–9. doi: 10.4049/jimmunol.180.4.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohr FC, Fewtrell C. Depolarization of rat basophilic leukemia cells inhibits calcium uptake and exocytosis. J Cell Biol. 1987;104:783–92. doi: 10.1083/jcb.104.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vennekens R, Olausson J, Meissner M, Bloch W, Mathar I, Philipp SE, Schmitz F, Weissgerber P, Nilius B, Flockerzi V, Freichel M. Increased IgE-dependent mast cell activation and anaphylactic responses in mice lacking the calcium-activated nonselective cation channel TRPM4. Nat Immunol. 2007;8:312–20. doi: 10.1038/ni1441. [DOI] [PubMed] [Google Scholar]
- 33.Guo Z, Turner C, Castle D. Relocation of the t-SNARE SNAP-23 from lamellipodia-like cell surface projections regulates compound exocytosis in mast cells. Cell. 1998;94:537–48. doi: 10.1016/s0092-8674(00)81594-9. [DOI] [PubMed] [Google Scholar]
- 34.Jahn R, Sudhof TC. Membrane fusion and exocytosis. Annu Rev Biochem. 1999;68:863–911. doi: 10.1146/annurev.biochem.68.1.863. [DOI] [PubMed] [Google Scholar]
- 35.Paumet F, Le Mao J, Martin S, Galli T, David B, Blank U, Roa M. Soluble NSF attachment protein receptors (SNAREs) in RBL-2H3 mast cells: functional role of syntaxin 4 in exocytosis and identification of a vesicle-associated membrane protein 8-containing secretory compartment. J Immunol. 2000;164:5850–7. doi: 10.4049/jimmunol.164.11.5850. [DOI] [PubMed] [Google Scholar]
- 36.Blank U, Cyprien B, Martin-Verdeaux S, Paumet F, Pombo I, Rivera J, Roa M, Varin-Blank N. SNAREs and associated regulators in the control of exocytosis in the RBL-2H3 mast cell line. Mol Immunol. 2002;38:1341–5. doi: 10.1016/s0161-5890(02)00085-8. [DOI] [PubMed] [Google Scholar]
- 37.Logan MR, Odemuyiwa SO, Moqbel R. Understanding exocytosis in immune and inflammatory cells: the molecular basis of mediator secretion. J Allergy Clin Immunol. 2003;111:923–32. quiz 933. [PubMed] [Google Scholar]
- 38.Hibi T, Hirashima N, Nakanishi M. Rat basophilic leukemia cells express syntaxin-3 and VAMP-7 in granule membranes. Biochem Biophys Res Commun. 2000;271:36–41. doi: 10.1006/bbrc.2000.2591. [DOI] [PubMed] [Google Scholar]
- 39.Puri N, Roche PA. Mast cells possess distinct secretory granule subsets whose exocytosis is regulated by different SNARE isoforms. Proc Natl Acad Sci U S A. 2008;105:2580–5. doi: 10.1073/pnas.0707854105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tiwari N, Wang CC, Brochetta C, Ke G, Vita F, Qi Z, Rivera J, Soranzo MR, Zabucchi G, Hong W, Blank U. VAMP-8 segregates mast cell-preformed mediator exocytosis from cytokine trafficking pathways. Blood. 2008;111:3665–74. doi: 10.1182/blood-2007-07-103309. [DOI] [PubMed] [Google Scholar]
- 41.Sander LE, Frank SP, Bolat S, Blank U, Galli T, Bigalke H, Bischoff SC, Lorentz A. Vesicle associated membrane protein (VAMP)-7 and VAMP-8, but not VAMP-2 or VAMP-3, are required for activation-induced degranulation of mature human mast cells. Eur J Immunol. 2008;38:855–63. doi: 10.1002/eji.200737634. [DOI] [PubMed] [Google Scholar]
- 42.Hepp R, Puri N, Hohenstein AC, Crawford GL, Whiteheart SW, Roche PA. Phosphorylation of SNAP-23 regulates exocytosis from mast cells. J Biol Chem. 2005;280:6610–20. doi: 10.1074/jbc.M412126200. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki K, Verma IM. Phosphorylation of SNAP-23 by IκB kinase2 (IKK2/β) regulates anaphylactic reactions: NF-κB-independent regulation of mast cell exocytosis. Cell. (in press) [Google Scholar]
- 44.Neeft M, Wieffer M, de Jong AS, Negroiu G, Metz CH, van Loon A, Griffith J, Krijgsveld J, Wulffraat N, Koch H, Heck AJ, Brose N, Kleijmeer M, van der Sluijs P. Munc13–4 is an effector of rab27a and controls secretion of lysosomes in hematopoietic cells. Mol Biol Cell. 2005;16:731–41. doi: 10.1091/mbc.E04-10-0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Higashio H, Nishimura N, Ishizaki H, Miyoshi J, Orita S, Sakane A, Sasaki T. Doc2{alpha} and Munc13–4 Regulate Ca2+-Dependent Secretory Lysosome Exocytosis in Mast Cells. J Immunol. 2008;180:4774–84. doi: 10.4049/jimmunol.180.7.4774. [DOI] [PubMed] [Google Scholar]
- 46.Tam SY, Tsai M, Snouwaert JN, Kalesnikoff J, Scherrer D, Nakae S, Chatterjea D, Bouley DM, Galli SJ. RabGEF1 is a negative regulator of mast cell activation and skin inflammation. Nat Immunol. 2004;5:844–52. doi: 10.1038/ni1093. [DOI] [PubMed] [Google Scholar]
- 47.Kalesnikoff J, Rios EJ, Chen CC, Alejandro Barbieri M, Tsai M, Tam SY, Galli SJ. Roles of RabGEF1/Rabex-5 domains in regulating Fc epsilon RI surface expression and Fc epsilon RI-dependent responses in mast cells. Blood. 2007;109:5308–17. doi: 10.1182/blood-2007-01-067363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bansal G, Xie Z, Rao S, Nocka KH, Druey KM. Suppression of immunoglobulin E-mediated allergic responses by regulator of G protein signaling 13. Nat Immunol. 2008;9:73–80. doi: 10.1038/ni1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laffargue M, Calvez R, Finan P, Trifilieff A, Barbier M, Altruda F, Hirsch E, Wymann MP. Phosphoinositide 3-kinase gamma is an essential amplifier of mast cell function. Immunity. 2002;16:441–51. doi: 10.1016/s1074-7613(02)00282-0. [DOI] [PubMed] [Google Scholar]
- 50.Saxon A, Kepley C, Zhang K. “Accentuate the negative, eliminate the positive”: engineering allergy therapeutics to block allergic reactivity through negative signaling. J Allergy Clin Immunol. 2008;121:320–5. doi: 10.1016/j.jaci.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 51.Melendez AJ, Harnett MM, Pushparaj PN, Wong WS, Tay HK, McSharry CP, Harnett W. Inhibition of Fc epsilon RI-mediated mast cell responses by ES-62, a product of parasitic filarial nematodes. Nat Med. 2007;13:1375–81. doi: 10.1038/nm1654. [DOI] [PubMed] [Google Scholar]
- 52.Bischoff SC. Role of mast cells in allergic and non-allergic immune responses: comparison of human and murine data. Nat Rev Immunol. 2007;7:93–104. doi: 10.1038/nri2018. [DOI] [PubMed] [Google Scholar]
- 53.Kitamura Y, Go S, Hatanaka K. Decrease of mast cells in W/Wv mice and their increase by bone marrow transplantation. Blood. 1978;52:447–52. [PubMed] [Google Scholar]
- 54.Lyon MF, Glenister PH. A new allele sash (Wsh) at the W-locus and a spontaneous recessive lethal in mice. Genet Res. 1982;39:315–22. doi: 10.1017/s001667230002098x. [DOI] [PubMed] [Google Scholar]
- 55.Nakano T, Sonoda T, Hayashi C, Yamatodani A, Kanayama Y, Yamamura T, Asai H, Yonezawa T, Kitamura Y, Galli SJ. Fate of bone marrow-derived cultured mast cells after intracutaneous, intraperitoneal, and intravenous transfer into genetically mast cell-deficient W/Wv mice. Evidence that cultured mast cells can give rise to both connective tissue type and mucosal mast cells. J Exp Med. 1985;162:1025–43. doi: 10.1084/jem.162.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsai M, Wedemeyer J, Ganiatsas S, Tam SY, Zon LI, Galli SJ. In vivo immunological function of mast cells derived from embryonic stem cells: an approach for the rapid analysis of even embryonic lethal mutations in adult mice in vivo. Proc Natl Acad Sci U S A. 2000;97:9186–90. doi: 10.1073/pnas.160254997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grimbaldeston MA, Chen CC, Piliponsky AM, Tsai M, Tam SY, Galli SJ. Mast cell-deficient W-sash c-kit mutant KitW-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol. 2005;167:835–48. doi: 10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wolters PJ, Mallen-St Clair J, Lewis CC, Villalta SA, Baluk P, Erle DJ, Caughey GH. Tissue-selective mast cell reconstitution and differential lung gene expression in mast cell-deficient KitW-sh/KitW-sh sash mice. Clin Exp Allergy. 2005;35:82–8. doi: 10.1111/j.1365-2222.2005.02136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Metz M, Piliponsky AM, Chen CC, Lammel V, Abrink M, Pejler G, Tsai M, Galli SJ. Mast cells can enhance resistance to snake and honeybee venoms. Science. 2006;313:526–30. doi: 10.1126/science.1128877. [DOI] [PubMed] [Google Scholar]
- 60.Zhou JS, Xing W, Friend DS, Austen KF, Katz HR. Mast cell deficiency in Kit(W-sh) mice does not impair antibody-mediated arthritis. J Exp Med. 2007;204:2797–802. doi: 10.1084/jem.20071391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee DM, Friend DS, Gurish MF, Benoist C, Mathis D, Brenner MB. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science. 2002;297:1689–92. doi: 10.1126/science.1073176. [DOI] [PubMed] [Google Scholar]
- 62.Scholten J, Hartmann K, Gerbaulet A, Krieg T, Muller W, Testa G, Roers A. Mast cell-specific Cre/loxP-mediated recombination in vivo. Transgenic Res. 2008;17:307–15. doi: 10.1007/s11248-007-9153-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Musch W, Wege AK, Mannel DN, Hehlgans T. Generation and characterization of alpha-chymase-Cre transgenic mice. Genesis. 2008;46:163–6. doi: 10.1002/dvg.20378. [DOI] [PubMed] [Google Scholar]
- 64.Malaviya R, Ikeda T, Ross E, Abraham SN. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature. 1996;381:77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- 65.Echtenacher B, Mannel DN, Hultner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 1996;381:75–7. doi: 10.1038/381075a0. [DOI] [PubMed] [Google Scholar]
- 66.Maurer M, Wedemeyer J, Metz M, Piliponsky AM, Weller K, Chatterjea D, Clouthier DE, Yanagisawa MM, Tsai M, Galli SJ. Mast cells promote homeostasis by limiting endothelin-1-induced toxicity. Nature. 2004;432:512–6. doi: 10.1038/nature03085. [DOI] [PubMed] [Google Scholar]
- 67.Schneider LA, Schlenner SM, Feyerabend TB, Wunderlin M, Rodewald HR. Molecular mechanism of mast cell mediated innate defense against endothelin and snake venom sarafotoxin. J Exp Med. 2007;204:2629–39. doi: 10.1084/jem.20071262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Piliponsky AM, Chen CC, Nishimura T, Metz M, Rios EJ, Dobner PR, Wada E, Wada K, Zacharias S, Mohanasundaram UM, Faix JD, Abrink M, Pejler G, Pearl RG, Tsai M, Galli SJ. Neurotensin increases mortality and mast cells reduce neurotensin levels in a mouse model of sepsis. Nat Med. 2008;14:392–8. doi: 10.1038/nm1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Orinska Z, Maurer M, Mirghomizadeh F, Bulanova E, Metz M, Nashkevich N, Schiemann F, Schulmistrat J, Budagian V, Giron-Michel J, Brandt E, Paus R, Bulfone-Paus S. IL-15 constrains mast cell-dependent antibacterial defenses by suppressing chymase activities. Nat Med. 2007;13:927–34. doi: 10.1038/nm1615. [DOI] [PubMed] [Google Scholar]
- 70.Thakurdas SM, Melicoff E, Sansores-Garcia L, Moreira DC, Petrova Y, Stevens RL, Adachi R. The mast cell-restricted tryptase mMCP-6 has a critical immunoprotective role in bacterial infections. J Biol Chem. 2007;282:20809–15. doi: 10.1074/jbc.M611842200. [DOI] [PubMed] [Google Scholar]
- 71.Knight PA, Wright SH, Lawrence CE, Paterson YY, Miller HR. Delayed expulsion of the nematode Trichinella spiralis in mice lacking the mucosal mast cell-specific granule chymase, mouse mast cell protease-1. J Exp Med. 2000;192:1849–56. doi: 10.1084/jem.192.12.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shin K, Watts GF, Oettgen HC, Friend DS, Pemberton AD, Gurish MF, Lee DM. Mouse mast cell tryptase mMCP-6 Is a critical link between adaptive and innate Immunity in the chronic phase of Trichinella spiralis Infection. J Immunol. 2008;180:4885–4891. doi: 10.4049/jimmunol.180.7.4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rauter I, Krauth MT, Westritschnig K, Horak F, Flicker S, Gieras A, Repa A, Balic N, Spitzauer S, Huss-Marp J, Brockow K, Darsow U, Behrendt H, Ring J, Kricek F, Valent P, Valenta R. Mast cell-derived proteases control allergic inflammation through cleavage of IgE. J Allergy Clin Immunol. 2008;121:197–202. doi: 10.1016/j.jaci.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 74.Hart PH, Grimbaldeston MA, Swift GJ, Jaksic A, Noonan FP, Finlay-Jones JJ. Dermal mast cells determine susceptibility to ultraviolet B-induced systemic suppression of contact hypersensitivity responses in mice. J Exp Med. 1998;187:2045–53. doi: 10.1084/jem.187.12.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Byrne SN, Limon-Flores AY, Ullrich SE. Mast cell migration from the skin to the draining lymph nodes upon ultraviolet irradiation represents a key step in the induction of immune suppression. J Immunol. 2008;180:4648–55. doi: 10.4049/jimmunol.180.7.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Depinay N, Hacini F, Beghdadi W, Peronet R, Mecheri S. Mast cell-dependent down-regulation of antigen-specific immune responses by mosquito bites. J Immunol. 2006;176:4141–6. doi: 10.4049/jimmunol.176.7.4141. [DOI] [PubMed] [Google Scholar]
- 77.Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, Scott ZA, Coyle AJ, Reed JL, Van Snick J, Strom TB, Zheng XX, Noelle RJ. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 78.Grimbaldeston MA, Nakae S, Kalesnikoff J, Tsai M, Galli SJ. Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat Immunol. 2007;8:1095–104. doi: 10.1038/ni1503. [DOI] [PubMed] [Google Scholar]
- 79.Gregory GD, Raju SS, Winandy S, Brown MA. Mast cell IL-4 expression is regulated by Ikaros and influences encephalitogenic Th1 responses in EAE. J Clin Invest. 2006;116:1327–36. doi: 10.1172/JCI27227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen R, Ning G, Zhao ML, Fleming MG, Diaz LA, Werb Z, Liu Z. Mast cells play a key role in neutrophil recruitment in experimental bullous pemphigoid. J Clin Invest. 2001;108:1151–8. doi: 10.1172/JCI11494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nakae S, Suto H, Berry GJ, Galli SJ. Mast cell-derived TNF can promote Th17 cell-dependent neutrophil recruitment in ovalbumin-challenged OTII mice. Blood. 2007;109:3640–8. doi: 10.1182/blood-2006-09-046128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Askenase PW, Van Loveren H, Kraeuter-Kops S, Ron Y, Meade R, Theoharides TC, Nordlund JJ, Scovern H, Gerhson MD, Ptak W. Defective elicitation of delayed-type hypersensitivity in W/Wv and SI/SId mast cell-deficient mice. J Immunol. 1983;131:2687–94. [PubMed] [Google Scholar]
- 83.Galli SJ, Hammel I. Unequivocal delayed hypersensitivity in mast cell-deficient and beige mice. Science. 1984;226:710–3. doi: 10.1126/science.6494907. [DOI] [PubMed] [Google Scholar]
- 84.Norman MU, Hwang J, Hulliger S, Bonder CS, Yamanouchi J, Santamaria P, Kubes P. Mast cells regulate the magnitude and the cytokine microenvironment of the contact hypersensitivity response. Am J Pathol. 2008 doi: 10.2353/ajpath.2008.070559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jawdat DM, Albert EJ, Rowden G, Haidl ID, Marshall JS. IgE-mediated mast cell activation induces Langerhans cell migration in vivo. J Immunol. 2004;173:5275–82. doi: 10.4049/jimmunol.173.8.5275. [DOI] [PubMed] [Google Scholar]
- 86.Suto H, Nakae S, Kakurai M, Sedgwick JD, Tsai M, Galli SJ. Mast cell-associated TNF promotes dendritic cell migration. J Immunol. 2006;176:4102–12. doi: 10.4049/jimmunol.176.7.4102. [DOI] [PubMed] [Google Scholar]
- 87.Bryce PJ, Miller ML, Miyajima I, Tsai M, Galli SJ, Oettgen HC. Immune sensitization in the skin is enhanced by antigen-independent effects of IgE. Immunity. 2004;20:381–92. doi: 10.1016/s1074-7613(04)00080-9. [DOI] [PubMed] [Google Scholar]
- 88.Kambayashi T, Baranski JD, Baker RG, Zou T, Allenspach EJ, Shoag JE, Jones PL, Koretzky GA. Indirect involvement of allergen-captured mast cells in antigen presentation. Blood. 2008;111:1489–96. doi: 10.1182/blood-2007-07-102111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McLachlan JB, Shelburne CP, Hart JP, Pizzo SV, Goyal R, Brooking-Dixon R, Staats HF, Abraham SN. Mast cell activators: a new class of highly effective vaccine adjuvants. Nat Med. 2008 doi: 10.1038/nm1757. [DOI] [PubMed] [Google Scholar]
- 90.Bot I, de Jager SC, Zernecke A, Lindstedt KA, van Berkel TJ, Weber C, Biessen EA. Perivascular mast cells promote atherogenesis and induce plaque destabilization in apolipoprotein E-deficient mice. Circulation. 2007;115:2516–25. doi: 10.1161/CIRCULATIONAHA.106.660472. [DOI] [PubMed] [Google Scholar]
- 91.Kovanen PT. Mast cells and degradation of pericellular and extracellular matrices: potential contributions to erosion, rupture and intraplaque haemorrhage of atherosclerotic plaques. Biochem Soc Trans. 2007;35:857–61. doi: 10.1042/BST0350857. [DOI] [PubMed] [Google Scholar]
- 92.Sun J, Sukhova GK, Wolters PJ, Yang M, Kitamoto S, Libby P, MacFarlane LA, Mallen-St Clair J, Shi GP. Mast cells promote atherosclerosis by releasing proinflammatory cytokines. Nat Med. 2007;13:719–24. doi: 10.1038/nm1601. [DOI] [PubMed] [Google Scholar]
- 93.Sun J, Sukhova GK, Yang M, Wolters PJ, MacFarlane LA, Libby P, Sun C, Zhang Y, Liu J, Ennis TL, Knispel R, Xiong W, Thompson RW, Baxter BT, Shi GP. Mast cells modulate the pathogenesis of elastase-induced abdominal aortic aneurysms in mice. J Clin Invest. 2007;117:3359–68. doi: 10.1172/JCI31311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tsuruda T, Kato J, Hatakeyama K, Kojima K, Yano M, Yano Y, Nakamura K, Nakamura-Uchiyama F, Matsushima Y, Imamura T, Onitsuka T, Asada Y, Nawa Y, Eto T, Kitamura K. Adventitial mast cells contribute to pathogenesis in the progression of abdominal aortic aneurysm. Circ Res. 2008 doi: 10.1161/CIRCRESAHA.108.173682. [DOI] [PubMed] [Google Scholar]
- 95.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Coussens LM, Raymond WW, Bergers G, Laig-Webster M, Behrendtsen O, Werb Z, Caughey GH, Hanahan D. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999;13:1382–97. doi: 10.1101/gad.13.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Soucek L, Lawlor ER, Soto D, Shchors K, Swigart LB, Evan GI. Mast cells are required for angiogenesis and macroscopic expansion of Myc-induced pancreatic islet tumors. Nat Med. 2007;13:1211–8. doi: 10.1038/nm1649. [DOI] [PubMed] [Google Scholar]
- 98.Kopp HG, Ramos CA, Rafii S. Contribution of endothelial progenitors and proangiogenic hematopoietic cells to vascularization of tumor and ischemic tissue. Curr Opin Hematol. 2006;13:175–81. doi: 10.1097/01.moh.0000219664.26528.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gounaris E, Erdman SE, Restaino C, Gurish MF, Friend DS, Gounari F, Lee DM, Zhang G, Glickman JN, Shin K, Rao VP, Poutahidis T, Weissleder R, McNagny KM, Khazaie K. Mast cells are an essential hematopoietic component for polyp development. Proc Natl Acad Sci U S A. 2007;104:19977–82. doi: 10.1073/pnas.0704620104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sinnamon MJ, Carter KJ, Sims LP, Lafleur B, Fingleton B, Matrisian LM. A protective role of mast cells in intestinal tumorigenesis. Carcinogenesis. 2008;29:880–6. doi: 10.1093/carcin/bgn040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wedemeyer J, Galli SJ. Decreased susceptibility of mast cell-deficient Kit(W)/Kit(W-v) mice to the development of 1, 2-dimethylhydrazine-induced intestinal tumors. Lab Invest. 2005;85:388–96. doi: 10.1038/labinvest.3700232. [DOI] [PubMed] [Google Scholar]
- 102.Nakae S, Suto H, Iikura M, Kakurai M, Sedgwick JD, Tsai M, Galli SJ. Mast cells enhance T cell activation: importance of mast cell costimulatory molecules and secreted TNF. J Immunol. 2006;176:2238–48. doi: 10.4049/jimmunol.176.4.2238. [DOI] [PubMed] [Google Scholar]
- 103.Yamashita Y, Charles N, Furumoto Y, Odom S, Yamashita T, Gilfillan AM, Constant S, Bower MA, Ryan JJ, Rivera J. Cutting edge: genetic variation influences Fc epsilon RI-induced mast cell activation and allergic responses. J Immunol. 2007;179:740–3. doi: 10.4049/jimmunol.179.2.740. [DOI] [PubMed] [Google Scholar]
- 104.Christy AL, Brown MA. The multitasking mast cell: positive and negative roles in the progression of autoimmunity. J Immunol. 2007;179:2673–9. doi: 10.4049/jimmunol.179.5.2673. [DOI] [PubMed] [Google Scholar]
- 105.Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008;454:445–54. doi: 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]