Abstract
Background
Insulin-like growth factor–I (IGF-I) and insulin stimulate cell proliferation in uterine leiomyoma (fibroid) tissue. We hypothesized that circulating levels of these proteins would be associated with increased prevalence and size of uterine fibroids.
Methods
Participants were 35–49-year-old, randomly selected members of an urban health plan who were enrolled in 1996–1999. Premenopausal participants were screened for fibroids with ultrasound. Fasting blood samples were collected. Associations between fibroids and diabetes, plasma IGF-I, IGF binding protein 3 (BP3), and insulin were evaluated for blacks (n = 585) and whites (n = 403) by using multiple logistic regression.
Results
IGF-I showed no association with fibroids in blacks, but in whites the adjusted odds ratios (aORs) for both mid and upper tertiles compared with the lowest tertile were 0.6 (95% confidence intervals [CI] = 0.3–1.0 and 0.4–1.1, respectively). Insulin and diabetes both tended to be inversely associated with fibroids in blacks. The insulin association was with large fibroids; aOR for the upper insulin tertile relative to the lowest was 0.4 (0.2–0.9). The aOR for diabetes was 0.5 (0.2–1.0). Associations of insulin and diabetes with fibroids were weak for whites. BP3 showed no association with fibroids.
Conclusions
Contrary to our hypothesis, high circulating IGF-I and insulin were not related to increased fibroid prevalence. Instead, there was suggestion of the opposite. The inverse association with diabetes, although based on small numbers, is consistent with previously reported findings. Future studies might investigate vascular dysfunction as a mediator between hyperinsulinemia or diabetes and possible reduced risk of fibroids.
Uterine leiomyomata, commonly called fibroids, are the leading indication for hysterectomy in the United States.1 The condition is especially common for blacks, a group with estimated cumulative hysterectomy rates for fibroids of 20% by age 45.2 Fibroids are benign smooth muscle tumors of clonal origin.3 Incidence increases with age from menarche to perimenopausal ages.4 Both estrogen and progesterone may stimulate their development. Although the mechanisms by which these hormones influence tumor onset and progression is not well understood (reviewed in Schwartz and Marshall5), their actions are mediated at least in part by growth factors.
Insulin-like growth factor–I (IGF-I) may be one of the growth factors that plays an important role in the pathogenesis of fibroids.6 IGF-I production can be stimulated by growth hormone, and its actions include cell proliferation and inhibition of apoptosis.7 In 1990 Boehm et al8 reported elevated IGF-I expression in fibroid compared with normal myometrium, and upregulation of gene expression and protein has been seen in several subsequent studies.9-17 Upregulation of IGF-I is also seen in fibroid tissue from the Eker rat, an animal model for fibroids.18 IGF-I stimulates fibroid cell mitosis in vitro.19,20 Based on human fibroid cell culture, Swartz et al6 demonstrated estrogen-dependent IGF-I upregulation and its linkage to transcription factors that increase the rate of cell cycle transition, thus providing a detailed description of IGF-I proliferative and anti-apoptotic effects in fibroids.
The extent to which circulating IGF-I may contribute to tumorigenesis is unknown. Most of the circulating IGF-I is produced in the liver. Higher levels have been associated with increased risk of breast cancer, another hormonally mediated tumor (reviewed by Renehan et al,21 but see also Baglietto et al22). Two studies have examined circulating levels of IGF-I in which women with and without uterine fibroids were compared. Neither showed differential levels, although sample sizes were small (cases and controls combined were 81 in 1 and 40 in the other).23,24
There are at least 6 high-affinity IGF binding proteins in the circulation that regulate the activity of IGF-I, the primary being binding protein 3 (BP3).25 BP3 may reduce IGF-I activity by preventing its binding to the receptor, but by binding IGF-I, it prolongs the half-life for IGF-I from minutes to hours. BP3 can also act independently of IGF-I, with antiproliferative effects.25 Both BP3 mRNA and protein are found in normal and fibroid smooth muscle cells.26 To our knowledge no prior study has examined the association between circulating levels of BP3 and fibroid status.
Insulin has also been hypothesized to play a role in fibroid pathogenesis.27 Insulin and IGF-I have similar growth-promoting activity, and both have weak binding affinity to the other's receptor.28 Experimental treatment of fibroid tissue with insulin can increase cell proliferation in vitro.29 In addition, hyperinsulinemia may stimulate increased ovarian hormone production, which might indirectly increase the development of fibroids.30
We hypothesized that increased circulating IGF-I and hyperinsulinemia would be associated with increased development of uterine fibroids. To test this hypothesis we measured IGF-I, BP3, and insulin in fasting blood samples taken from women in the National Institute of Environmental Health Sciences (NIEHS) Uterine Fibroid Study, a study that recruited randomly selected 35–49-year-old members of an urban health plan and screened them for fibroids with ultrasound, thus identifying both clinically diagnosed and subclinical cases.
Methods
Participants
The NIEHS Uterine Fibroid Study was designed to estimate prevalence and age-specific cumulative incidence of uterine fibroids and to investigate risk factors. Detailed methods have been described.31 Briefly, the computerized membership records of a prepaid health plan in Washington, DC were used to select randomly 35–49-year-old women. Those selected were screened for eligibility by telephone. Eligibility criteria were 1) the computerized listing had correctly identified 35–49-year-old woman with current membership at the study site, and 2) a telephone interview could be conducted in English. A total of 1323 black and white women agreed to participate (Fig. 1) (83% of those identified as eligible). The research was approved by the NIEHS and George Washington University Human Subject's Review Boards, and participants gave informed consent. The analysis is limited to premenopausal participants who made a study clinic visit at which time a fasting blood sample was drawn (Fig.1).
Figure 1.
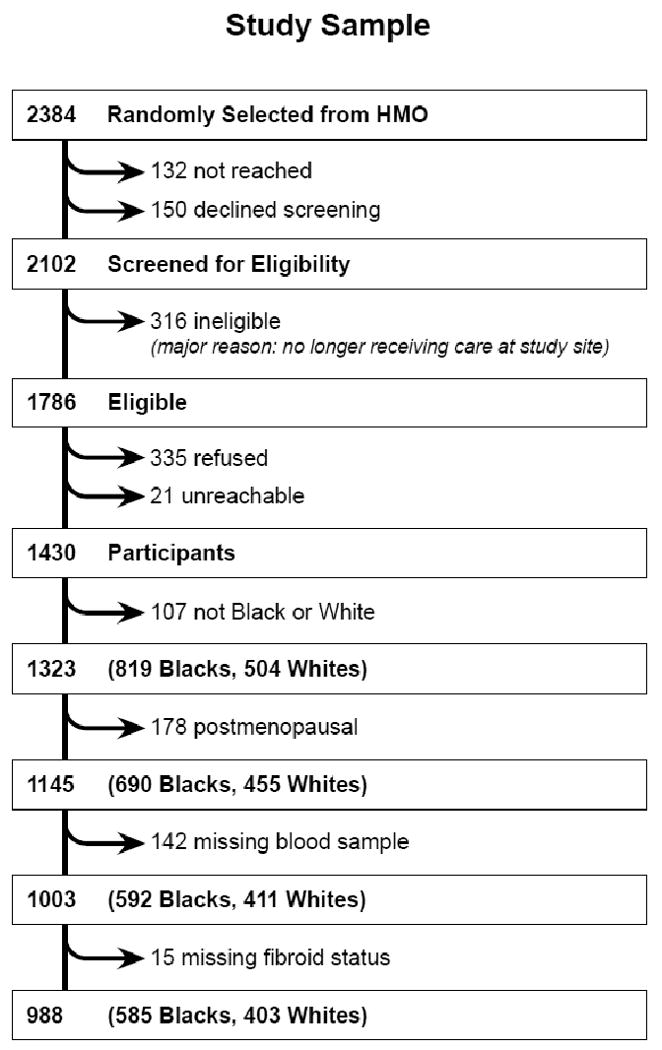
Flowchart showing the number of premenopausal black and white women in the NIEHS Uterine Fibroid Study from whom we collected blood and have data on fibroid status.
Determination of Fibroid Status
Fibroid status was determined for most participants by a transabdominal and transvaginal ultrasound screening examination. Participants who had recently undergone an ultrasound examination at the clinic for clinical purposes (21%) were not asked to repeat an examination for the study. We used ultrasounds from the prior 2 years if they showed “no fibroids”, and ultrasounds from the prior 5 years if they showed fibroids. For these women fibroid data were abstracted from the clinic radiology reports. The study ultrasound examinations were performed by sonographers who were certified by the American Registry of Diagnostic Medical Sonographers and were trained for the study to collect and record consistent data on fibroids (minimum diameter of 0.5 cm). They were under the direct supervision of 1 radiologist with fellowship training in ultrasound, and each study was checked at its completion by this radiologist. The examinations were performed on ultrasound units ATL HDI 9, Acuson 128 XP, and Diasonics DRF 400 by using transabdominal (3.5–5.0 mHz) and transvaginal (5.0–7.0 mHz) ultrasound probes. Sonographers completed a data collection form designed for this study that included data on uterine size (length, width, anterior to posterior diameter), heterogeneity of the echo pattern (none, diffuse, or focal), and size of the largest fibroid. Any questionable sonograms were reviewed by a single radiologist. Those uteri with diffuse heterogeneity but no focal fibroids (39 whites and 76 blacks) were categorized as having fibroids, but statistical analyses were also repeated without these women, and results did not change. For 18 participants who did not complete their ultrasound screening examinations and 1 whose ultrasound examination was indeterminate, we accepted self-report of a prior diagnosis. We did not rely on self-report of “no fibroids” because undiagnosed fibroids are common; about half of the undiagnosed women in our study were found to have fibroids at ultrasound screening.31
Assays
The NIEHS clinical chemistry laboratory measured IGF-I and BP3 in plasma that had been stored at -80°C. IGF-I was measured by extraction using radioimmunoassay kits (Nichols Institute Diagnostics San Juan Capistrano, CA). BP3 was measured by immunoradiometric assay kits (Diagnostic Systems Laboratories, Webster, TX). Insulin was measured in plasma at the Duke University Clinical and Research Laboratory. All intra- and interassay coefficients of variation were less than 10%.
Other Variables
Weight was measured at the clinic visit, and other data were collected during a telephone interview conducted by trained interviewers or from responses to a self-administered questionnaire. Diabetics were those responding “yes” to the question on the self-administered questionnaire “Has a doctor or health professional ever told you that you had diabetes, high blood sugar or “sugar,” not pregnancy induced?” Gestational diabetes was ascertained with the reproductive history questions in the telephone interview. For each delivery the participant was asked “Did you have any special medical problems during the pregnancy including (a list of 8 complications, 1 of which was) gestational diabetes (diabetes beginning during pregnancy)?”
Statistical Analyses
We evaluated the relation of IGF-I, BP3, insulin, and diabetes with uterine fibroids first using logistic regression, and then using a Bayesian multistate analysis.32 Plasma protein levels were divided into tertiles based on the combined sample of blacks and whites, and the lowest tertile was used as the reference group. Black women have a higher risk of fibroids and substantially different distributions of many covariates, so all analyses were performed separately for the 2 ethnic groups. Prior analyses of data from this study indicated that age, age of menarche, body mass index (BMI), physical activity, and number of deliveries after age 24 years were related to fibroid development.31,33 These potential confounders were included a priori.
Using logistic regression, we estimated the age-adjusted odds of fibroids and then the fully-adjusted odds of fibroids for tertiles of IGF-I, BP3, and insulin. The relation between diabetes and fibroids was also examined with age-adjusted and fully-adjusted logistic models. Sensitivity analyses were conducted to assess the impact of minor variations in our diagnostic definition of fibroids. For IGF-I, BP3, and insulin we also conducted logistic analyses based on size of the largest fibroid as the outcome (this analysis could not be done for diabetes because of the small numbers of diabetic women). The fibroid diameter, as a continuous variable, was not normally distributed, and various transformations did not produce normality. Therefore, we categorized the largest tumor diameter (small; <2cm; medium; >2 to <4 cm; or large; ≥4 cm), and compared each category as a separate outcome with women without fibroids (LOGISTIC procedure in SAS, SAS Institute, Cary, NC). Because fibroids are common, prevalence differences for the important associations from the logistic analyses were estimated with binomial regression34 to provide more meaningful estimates of effect.
The Bayesian analysis provided a method for evaluating effects on tumor onset compared with tumor progression, thus allowing us to begin to estimate associations with incidence in this cross-sectional study. We used a multistate modeling approach32 with a flexible stochastic model that incorporates data on age at any prior diagnosis, age at study ultrasound, and size of largest fibroid (if any were found) to characterize onset and subsequent progression of fibroids. Fibroid onset is defined as the time at which the tumors first grow large enough to be detectable by a sonogram. Even though a large percentage of participants with fibroids had never had a clinical diagnosis of fibroids, the true onset time is unknown. During a preclinical phase and even after clinical diagnosis, fibroids may continue to grow. Our Bayesian approach utilizes survival analysis techniques to integrate data on first diagnosis of fibroids (for those who had been diagnosed before study enrollment) and size of the largest fibroid. The analysis is implemented using a Markov chain Monte Carlo algorithm, after choosing noninformative prior distributions. Hypothesis tests of ordered trends in tumor onset and progression across categories of the variables of interest are based on estimated posterior probabilities. Posterior probabilities 0.95 or more are considered important.
Results
Characteristics of the study participants are shown in Table 1. Of the 585 blacks 427 had fibroids; of the 403 whites, 203 had fibroids. Insulin was log-normally distributed, and both the IGF-I and BP3 distributions were approximately normal. Blacks had higher levels of insulin and lower levels of BP3 than whites. As expected, insulin was highly correlated with BMI, IGF-I was inversely correlated with both age and BMI, and BP3 was correlated with IGF-I (see distributions and correlations in eAppendix, available with the online version of this article).
TABLE 1.
Characteristics of Premenopausal Participants in the NIEHS Uterine Fibroid Study with Blood Specimens and Fibroid Data.
| Characteristic | Blacks n = 585 No. (%) |
Whites n = 403 No. (%) |
|---|---|---|
| Fibroid statusa | ||
| None | 158 (27) | 200 (50) |
| Small | 95 (16) | 70 (17) |
| Medium | 191 (33) | 89 (22) |
| Large | 141 (24) | 44 (11) |
| Source of fibroid status data | ||
| Ultrasound | 564 (96) | 396 (98) |
| Surgical report | 11 (2) | 2 (1) |
| Self-report | 10 (2) | 5 (1) |
| Age (y) | ||
| 35–39 | 216 (37) | 136 (34) |
| 40–44 | 209 (36) | 136 (34) |
| 45–49 | 160 (27) | 131 (33) |
| Education | ||
| High school or less | 124 (21) | 12 (3) |
| Some beyond high school | 269 (46) | 33 (8) |
| College degree | 71 (12) | 67 (17) |
| Postbaccalaureate | 116 (20) | 281 (72) |
| Missing | 5 | 10 |
| BMI at enrollment | ||
| <25 | 149 (25) | 234 (58) |
| 25–29.99 | 180 (31) | 96 (24) |
| 30–34.99 | 115 (20) | 37 (9) |
| 35+ | 141 (24) | 36 (9) |
| Age of menarche (y) | ||
| <11 | 63 (11) | 17 (4) |
| 11 | 92 (16) | 61 (15) |
| 12 | 160 (27) | 111 (28) |
| 13 | 140 (24) | 135 (34) |
| 14 | 57 (10) | 43 (11) |
| >14 | 70 (12) | 34 (8) |
| Missing | 3 | 2 |
| Parous | ||
| No | 121 (21) | 236 (59) |
| Yes | 464 (79) | 167 (41) |
| No. of full-term pregnancies delivered after age 24 | ||
| 0 | 294 (50) | 255 (63) |
| 1 | 181 (31) | 56 (14) |
| 2 | 87 (15) | 81 (20) |
| 3+ | 22 (4) | 11 (3) |
| Smoking status | ||
| Never | 279 (48) | 234 (58) |
| Past | 136 (23) | 137 (34) |
| Current, < 10/d | 102 (18) | 14 (3) |
| Current, 10-19/d | 55 (9) | 12 (3) |
| Current, 20+/d | 9 (2) | 6 (1) |
| Alcohol (drinks/wk) | ||
| <0.5 | 301 (55) | 60 (16) |
| 0.5–2 | 122 (22) | 113 (30) |
| >2–<7 | 65 (12) | 102 (27) |
| 7+ | 60 (11) | 96 (26) |
| Missing | 37 | 32 |
Fibroid status was categorized based on the diameter of largest fibroid (<2 cm, small; >2->4 cm, medium; ≥4 cm, large).
The associations of IGF-I, BP3, and insulin with uterine fibroids based on logistic regression are shown in Figure 2. Age-adjusted and fully-adjusted models differ primarily because of adjustment for BMI in models of insulin and IGF-I, and adjustment for IGF-I in models of BP3. For IGF-I, there appeared to be an inverse association with fibroids in whites. When the association was examined by estimating prevalence differences, the estimates for the mid and upper tertiles of IGF-I compared with lower tertile were -11% (CI = -23% to 1%) and -9% (-23% to 4%), respectively. In fibroid size–specific models the reduction in fibroid development with IGF-I was seen for small and medium fibroids, but there was no evidence of reduced prevalence of large fibroids (Fig. 2). IGF-I was not associated with fibroids in blacks, and in an analysis of blacks and whites combined, the P-value for interaction by race was 0.07.
Figure 2.
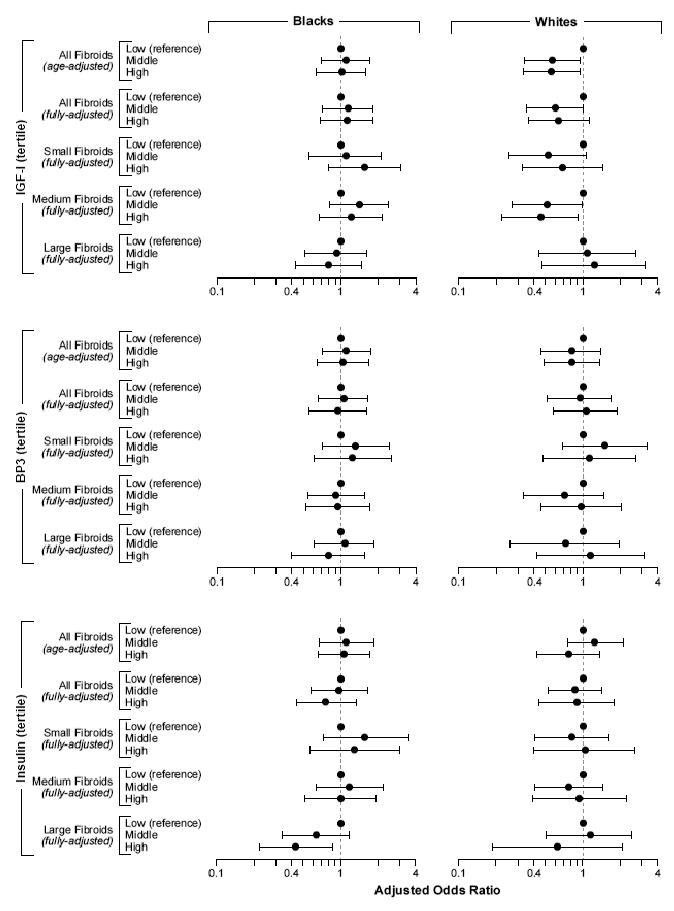
Relative odds of having fibroids (any sized fibroid, small: <2 cm diameter; medium: ≥2 to <4 cm diameter; and large fibroids: ≥4 cm diameter) associated with tertile of IGF-I, BP3, and insulin. The fully-adjusted ORs are adjusted for age, age of menarche, number of full-term pregnancies after age 24, BMI, and physical activity. There is also adjustment for BP3 in the fully-adjusted IGF-I models and for IGF-I in the fully-adjusted BP3 models. The horizontal lines show 95% confidence intervals.
For BP3, there was no evidence of an association with fibroids in any of the analyses. The molar ratio of IGF-I to BP3 was also examined, but it was highly correlated with IGF-I (r = 0.84 and 0.85 for blacks and whites, respectively) and showed similar, although slightly weaker, associations to those seen for IGF-I.
For insulin, neither age-adjusted nor fully-adjusted analyses showed convincing evidence for an association with presence of fibroids. When size of the largest fibroid was examined, there appeared a tendency for elevated insulin to be protective for large fibroids in both ethnic groups, and the association was stronger in blacks (Fig. 2). The estimated reductions in prevalence of large fibroids for blacks in mid and upper tertiles of insulin compared with the lower tertile were -12% (-22% to -1%) and -16% (-27% to -5%), respectively. When we excluded women who had been diagnosed with diabetes (43 blacks and 9 whites), there was little change in the relative odds of fibroids associated with insulin, IGF-I, and BP3.
There was an inverse association between diabetes and fibroids, especially for blacks (Table 2), where the estimated reduction in prevalence was -14% (0% to -29%). Black women who reported gestational diabetes but no other diagnosis of diabetes also tended to be less likely to have fibroids, but the confidence interval was broad (Table 2). All diabetes was adult onset with the exception of two black women who reported diagnoses at ages 13 and 16. Of those diagnosed with diabetes, 81% reported taking medication. Among blacks (the ethnic group with more diabetics) the inverse association between diabetes and fibroids was very similar for the 13 women who had been diagnosed at least 5 years prior to study enrollment compared with the 32 women who had more recent diagnoses (adjusted ORs = 0.47 and 0.52, respectively).
TABLE 2.
Age-adjusted and Fully-adjusted Relative Odds of Fibroids for Women with Diabetes Compared with Those without Among Premenopausal Blacks and Whites in the NIEHS Uterine Fibroid Study Enrolled 1996–1999
| Blacks | Whites | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No.a | ORb | (95% CI) | ORc | (95%CI) | No.a | ORb | (95%CI) | ORc | (95%CI) | |
| Diagnosis | ||||||||||
| None | 385:125 | 1.00 | Reference | 1.00 | Reference | 180:179 | 1.00 | Reference | 1.00 | Reference |
| Gestational | 21:10 | 0.74 | (0.34–1.62) | 0.69 | (0.31–1.57) | 4:3 | 0.72 | (0.16–3.29) | 1.15 | (0.24–5.52) |
| Diabetes | 30:15 | 0.67 | (0.35–1.29) | 0.50 | (0.25–1.02) | 5:4 | 0.96 | (0.24–3.76) | 0.83 | (0.21–3.38) |
Number with fibroids:number without fibroids.
Adjusted for age.
Adjusted for age, age of menarche, parity after age 24, BMI, and physical activity.
Bayesian analyses to examine the relation of IGF-I, insulin, and diabetes with tumor onset versus tumor progression controlled for the same factors included in multivariable logistic analyses. IGF-I in whites was inversely associated with tumor onset (posterior probability, pp = 0.97), not tumor progression (pp = 0.45). Insulin in blacks was inversely associated with tumor progression (pp = 0.98), not tumor onset (pp = 0.50). Diabetes was marginally protective of tumor onset in blacks (pp = 0.90), but the association with progression was weaker (pp = 0.62).
To evaluate the robustness of our results, we repeated the logistic analyses with minor changes in our definition of fibroids. First, we reassigned women who had no fibroids at ultrasound and no prior surgery for fibroids but had been previously told by a doctor that they had fibroids (32 blacks and 17 whites). They were moved from the “no fibroid” to the “fibroid” group. Second, we excluded women who had no focal fibroids but whose sonogram showed diffuse heterogeneity (75 blacks and 39 whites). These reanalyses showed results that were similar to those based on the original categorization of fibroid diagnosis. We also examined potential effect modification by BMI and exercise on the associations between insulin and IGF-I with presence of any sized fibroid. In none of these analyses was the interaction term important (p > 0.10).
Discussion
IGF-I and insulin have both been shown to increase cellular proliferation of fibroid tissue in culture.19,20,29 IGF-I expression or protein level is up-regulated in fibroids compared with normal myometrium,9-17 and IGF-I mediates proliferative actions of estrogen in fibroid tissue.6 Given these findings as well as the data linking IGF-I with breast cancer, we hypothesized that increased circulating levels of IGF-I and insulin would be associated with increased fibroid development. However, we found either no association or inverse effects for fibroids in both age-adjusted and fully-adjusted models of circulating IGF-I and insulin. Reduced prevalence of fibroids was seen for white women with mid or upper tertile values of IGF-I, whereas there was no association for black women. Blacks with mid or upper tertile values of insulin had a reduced prevalence of large fibroids, and consistent with the insulin association, blacks with diabetes also had a reduced prevalence of fibroids. Circulating BP3 was not associated with fibroid development.
To our knowledge, no previous large epidemiologic study has examined the association between circulating IGF-I or insulin levels and fibroid development, although 2 studies have reported on associations with diabetes. Based on very small numbers Faerstein et al16 found no association with diagnosed diabetes (OR = 0.9; CI = 0.4–2.2), but an increased relative odds of 2.0; (0.4–12.6) for the women taking medication for diabetes. Consistent with our findings, Wise et al35 found a reduced risk of fibroids among diabetics in the Black Women's Health Study (0.77; 0.60–0.98).
Circulating IGF-I comes primarily from the liver. It may be that factors such as fat metabolism that affect liver IGF-I transcription and secretion (thus determining circulating levels) have little influence on tissue levels in the uterus where transcription can occur in situ under the influence of estrogen.6 In situ expression may be a major difference between breast tissue and fibroid tissue. Few breast cancer cell lines express IGF-I.36 If IGF-I is a risk factor for breast cancer as some but not all studies suggest,22 the endocrine effects of circulating levels may be more important for breast cancer than for fibroids. The inverse association between circulating IGF-I levels and fibroid development in whites was surprising, but the reported associations between IGF-I and endometrial cancer are also generally inverse (reviewed in Lacey et al37). Circulating IGF-I has been positively correlated with vitamin D levels,38 which could possibly be a confounder, given its reported antiproliferative effects.
We had hypothesized that hyperinsulinemia would increase fibroid risk because of its proliferative effects on uterine smooth muscle in culture. However, there are also biologic pathways whereby hyperinsulinemia and diabetes might plausibly decrease fibroid development. Vascular dysfunction is part of the pathogenesis of diabetes and could inhibit tumor development. Insulin resistance and diabetes can be associated with cytotoxic effects both from accompanying hyperglycemia and from other circulating cytotoxic factors,39 and these factors could have direct antitumorigenic effects.
Our assessments of IGF-I, BP3, and insulin were based on measurements from a single blood sample. However, these factors track over time for individuals,40,41 and single measures have been predictive of disease incidence after several years of followup. The primary limitation of this study is the cross-sectional nature of data collection. IGF-I and insulin levels were measured in blood samples taken at the same clinic visit as the ultrasound screening for fibroids. A large percentage of fibroid cases were newly detected at screening (42% of black cases and 68% of white cases). Still, some fibroids were already large, and time of initial development is unknown because fibroids can remain asymptomatic for years. However, it seems unlikely that fibroids determine circulating IGF-I levels or prevent diabetes, so reverse causation is probably not an issue. Although numbers were limiting, we examined time-since-diabetes diagnosis within our black sample and found that the diabetes-related inverse association did not appear to vary by time-since-diagnosis, again suggesting that reverse causation is unlikely.
This analysis was also limited to premenopausal women with an intact uterus. This excluded a subset of women who had previously had hysterectomies for fibroids, and more blacks were excluded than whites. If IGF-I and insulin were related to more severe, early-onset disease, we could miss such an association by limiting to the premenopausal sample. However, when we examined tumor size, there was no suggestion that the exposures of interest increased the risk of large tumors, so this selection may have had little effect on results.
In summary, we found no evidence for increased risk of fibroids with high circulating IGF-I or insulin levels. In fact, both factors showed tendencies for protective associations.
Supplementary Material
Acknowledgments
Glenn Heartwell managed field study data collection. Deborah Cousins coordinated data management. Darlene Dixon, Shannon Laughlin and Jack Taylor reviewed an earlier draft of the manuscript, and Sue Edelstein prepared the figures.
Funding: Supported by the intramural program at the National Institute of Environmental Health Sciences with support from the Office of Research on Minority Health, National Institutes of Health, HHS.
Footnotes
eSupplemental material for this article is available with the online version of the journal at www.epidem.com; click on “Article Plus.”
References
- 1.Farquhar CM, Steiner CA. Hysterectomy rates in the United States 1990-1997. Obstet Gynecol. 2002;99:229–234. doi: 10.1016/s0029-7844(01)01723-9. [DOI] [PubMed] [Google Scholar]
- 2.Myers ER, Barber MW, Couchman GM, et al. Management of uterine fibroids (Evidence Report/Technology Assessment No. 34, contract 290-97-0014 to the Duke Evidence-based Practice Center). AHRQ Publication No. 01-E052. Rockville, MD: Agency for Healthcare Research and Quality; Jul, 2001. [Google Scholar]
- 3.Hashimoto K, Azuma C, Kamiura S, Kimura T, Nobunaga T, Kanai T, Sawada M, Noguchi S, Saji F. Clonal determination of uterine leiomyomas by analyzing differential inactivation of the X-chromosome-linked phosphoglycerokinase gene. Gynecol Obstet Invest. 1995;40:204–208. doi: 10.1159/000292336. [DOI] [PubMed] [Google Scholar]
- 4.Ross RK, Pike M, Vessey MP, Bull D, Yeates D, Casagrande JT. Risk factors for uterine fibroids: reduced risk associated with oral contraceptives. BMJ. 1986;293:359–362. doi: 10.1136/bmj.293.6543.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz SM, Marshall LM. Uterine leiomyomata. In: Goldman MB, Hatch MC, editors. Women and health. San Diego, California: Academic Press; 2000. [Google Scholar]
- 6.Swartz CD, Afshari CA, Yu L, Hall KE, Dixon D. Estrogen-induced changes in IGF-I, Myb family and MAP kinase pathway genes in human uterine leiomyoma and normal uterine smooth muscle cell lines. Mol Hum Reprod. 2005;11:441–450. doi: 10.1093/molehr/gah174. [DOI] [PubMed] [Google Scholar]
- 7.Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocrine Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 8.Boehm KD, Dalmon M, Gorodeski IG, Sheean LA, Utean WH, Ilan J. Expression of the insulin-like and platelet-derived growth factor genes in human uterine tissues. Mol Reprod Dev. 1990;27:93–101. doi: 10.1002/mrd.1080270203. [DOI] [PubMed] [Google Scholar]
- 9.Vollenhoven BJ, Herington AC, Healy DL. Messenger ribonucleic acid expression of the insulin-like growth factors and their binding proteins in uterine fibroids and myometrium. J Clin Endocrinol Metab. 1993;76:1106–1110. doi: 10.1210/jcem.76.5.7684390. [DOI] [PubMed] [Google Scholar]
- 10.Giudice LC, Irwin JC, Dsupin BA, Pannier EM, Jin IH, Vu TH, Hoffman AR. Insulin-like growth factor (IGF), IGF binding protein (IGFBP), and IGF receptor gene expression and IFGBP synthesis in human uterine leiomyomata. Hum Reprod. 1993;8:1796–1806. doi: 10.1093/oxfordjournals.humrep.a137937. [DOI] [PubMed] [Google Scholar]
- 11.van der Ven LTM, Roholl PJM, Gloudemans T, Van Buul-Offers SC, Welters MJ, Bladergroen BA, Faber JA, Sussenbach JS, Den Otter W. Expression of insulin-like growth factors (IGFs), their receptors and IGF binding protein-3 in normal, benign and malignant smooth muscle tissues. Br J Cancer. 1997;75:1631–1640. doi: 10.1038/bjc.1997.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Englund K, Lindblom B, Carlstrom K, Gustavsson I, Sjoblom P, Blanck A. Gene expression and tissue concentrations of IGF-I in human myometrium and fibroids under different hormonal conditions. Mol Hum Reprod. 2000;6:915–920. doi: 10.1093/molehr/6.10.915. [DOI] [PubMed] [Google Scholar]
- 13.Wolanska M, Bankowski E. An accumulation of insulin-like growth factor I (IGF-I) in human myometrium and uterine leiomyomas in various stages of tumor growth. Eur Cytokine Netw. 2004;15:359–363. [PubMed] [Google Scholar]
- 14.Kim DI, Lee TK, Lim IS, Kim H, Lee YC, Kim CH. Regulation of IGF-I production and proliferation of human leiomyomal smooth muscle cells by Scutellaria barbata D. Don in vitro: isolation of flavanoids of apigenin and luteolin as acting compounds. Toxicol Appl Pharmacol. 2005;205:213–224. doi: 10.1016/j.taap.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Wei JJ, Chiriboga L, Mittal K. Expression profile of the tumorigenic factors associated with tumor size and sex steroid hormone status in uterine leiomyomata. Fertil Steril. 2005;84:474–484. doi: 10.1016/j.fertnstert.2005.01.142. [DOI] [PubMed] [Google Scholar]
- 16.Wei J, Chiriboga L, Mizuguchi M, Yee H, Mittal K. Expression profile of tuberin and some potential tumorigenic factors in 60 patients with uterine leiomyomata. Mod Path. 2005;18:179–188. doi: 10.1038/modpathol.3800283. [DOI] [PubMed] [Google Scholar]
- 17.Dixon D, He H, Haseman JK. Immunohistochemical localization of growth factors and their receptors in uterine leiomyomas and matched myometrium. Environ Health Perspect. 2000;108(S5):795–802. doi: 10.1289/ehp.00108s5795. [DOI] [PubMed] [Google Scholar]
- 18.Burroughs KD, Howe SR, Okubo Y, Fuchs-Young R, LeRoith D, Walker CL. Dysregulation of IGF-I signaling in uterine leiomyoma. J Endocrinol. 2002;172:83–93. doi: 10.1677/joe.0.1720083. [DOI] [PubMed] [Google Scholar]
- 19.van der Ven LTM, Gloudemans T, Roholl PJM, van Buul-Offers SC, Bladergroen BA, Welters MJP, Faber JA, Sussenbach JS, Den Otter W. Growth advantage of human leiomyoma cells compared to normal smooth-muscle cells due to enhanced sensitivity toward insulin-like growth factor I. Int J Cancer. 1994;59:427–434. doi: 10.1002/ijc.2910590323. [DOI] [PubMed] [Google Scholar]
- 20.Strawn EY, Jr, Novy MJ, Burry KA, Bethea CL. Insulin-like growth factor I promotes leiomyoma cell growth in vitro. Am J Obstet Gynecol. 1995;172:1837–1844. doi: 10.1016/0002-9378(95)91420-x. [DOI] [PubMed] [Google Scholar]
- 21.Renehan AG, Harvie M, Howell A. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and breast cancer risk: eight years on. Endocrine-Related Cancer. 2006;13:273–278. doi: 10.1677/erc.1.01219. [DOI] [PubMed] [Google Scholar]
- 22.Baglietto L, English DR, Hopper JL, Morris HA, Tilley WD, Giles GG. Circulating insulin-like growth factor-I and binding protein-3 and the risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:763–768. doi: 10.1158/1055-9965.EPI-06-0960. [DOI] [PubMed] [Google Scholar]
- 23.Dawood MY, Khan-Dawood FS. Plasma insulin-like growth factor-I, CA-125, estrogen, and progesterone in women with leiomyomas. Fertil Steril. 1994;61:617–621. doi: 10.1016/s0015-0282(16)56635-7. [DOI] [PubMed] [Google Scholar]
- 24.Huang J, Zou J, Xu B, Zhang Y, Chen X, Lier D. Affect of insulin-like growth factor I and estradiol on the growth of uterine leiomyoma. Hunan Yi Ke Da Xue Xue Bao. 1999;24:29–32. [PubMed] [Google Scholar]
- 25.Rajaram S, Baylink DJ, Mohan S. Insulin-like growth factor-binding proteins in serum and other biological fluids: regulation and functions. Endocrine Rev. 1997;18:801–831. doi: 10.1210/edrv.18.6.0321. [DOI] [PubMed] [Google Scholar]
- 26.van der Ven LTM, Van Buul-Offers SC, Gloudemans T, Bloemen RJ, Roholl PJM, Sussenbach JS, Den Otter W. Modulation of insulin-like growth factor (IGF) action by IGF-binding proteins in normal, benign, and malignant smooth muscle tissues. J Clin Endocrinol Metab. 1996;81:3629–3635. doi: 10.1210/jcem.81.10.8855813. [DOI] [PubMed] [Google Scholar]
- 27.Faerstein E, Szklo M, Rosenshein NB. Risk factors for uterine leiomyoma: a practice-based case-control study. II. Atherogenic risk factors and potential sources of uterine irritation. Am J Epidemiol. 2001;153:11–19. doi: 10.1093/aje/153.1.11. [DOI] [PubMed] [Google Scholar]
- 28.Siddle K, Urso B, Niesler CA, Cope DL, Molina L, Surinya KH, Soos MA. Specificity in ligand binding and intracellular signaling by insulin and insulin-like growth factor receptors. Biochem Soc Trans. 2001;29:513–525. doi: 10.1042/bst0290513. [DOI] [PubMed] [Google Scholar]
- 29.Cramer SF, Robertson AL, Jr, Ziats NP, Pearson OH. Growth potential of human uterine leiomyomas: some in vitro observations and their implications. Obstet Gynecol. 1985;66:36–41. [PubMed] [Google Scholar]
- 30.Poretsky L, Kalin MF. The gonadotropic function of insulin. Endocrine Rev. 1987;8:132–141. doi: 10.1210/edrv-8-2-132. [DOI] [PubMed] [Google Scholar]
- 31.Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High incidence of uterine leiomyoma: Ultrasound evidence. Am J Obstet Gynecol. 2003;188:100–107. doi: 10.1067/mob.2003.99. [DOI] [PubMed] [Google Scholar]
- 32.Dunson DB, Baird DD. Bayesian modeling of incidence and progression of disease from cross-sectional data. Biometrics. 2002;58:813–822. doi: 10.1111/j.0006-341x.2002.00813.x. [DOI] [PubMed] [Google Scholar]
- 33.Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. Physical activity is associated with reduced uterine leiomyoma incidence. Am J Epidemiol. 2007;165:157–163. doi: 10.1093/aje/kwj363. [DOI] [PubMed] [Google Scholar]
- 34.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 35.Wise LA, Palmer JR, Stewart EA, Rosenberg L. Polycystic ovary syndrome and risk of uterine leiomyomata. Fertil Steril. 2007;87:1108–1115. doi: 10.1016/j.fertnstert.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moschos SJ, Mantzoros CS. The role of the IGF system in cancer: from basic to clinical studies and clinical applications. Oncology. 2002;63:317–332. doi: 10.1159/000066230. [DOI] [PubMed] [Google Scholar]
- 37.Lacey JV, Potischman N, Madigan MP, Berman ML, Mortel R, Twiggs LB, Barrett RJ, Wilbanks GD, Lurain JR, Fillmore CM, Sherman ME, Brinton LA. Insulin-like growth factors, insulin-like growth factor-binding proteins, and endometrial cancer in postmenopausal women: results from a U.S. case-control study. Cancer Epidemiol Biomarkers Prev. 2004;13:607–612. [PubMed] [Google Scholar]
- 38.Gomez JM. The role of insulin-like growth factor I components in the regulation of vitamin D. Curr Pharm Biotechnol. 2006;7:125–132. doi: 10.2174/138920106776597621. [DOI] [PubMed] [Google Scholar]
- 39.Pittenger GL, Malik RA, Burcus N, Boulton AJ, Vinik AI. Specific fiber deficits in sensorimotor diabetic polyneuropathy correspond to cytotoxicity against neuroblastoma cells of sera from patients with diabetes. Diabetes Care. 1999;22:1839–1844. doi: 10.2337/diacare.22.11.1839. [DOI] [PubMed] [Google Scholar]
- 40.Missmer SA, Speigelman D, Bertone-Johnson ER, Barbieri RL, Pollak MN, Hankinson SE. Reproducibility of plasma steroid hormones, prolactin, and insulin-like growth factor levels among premenopausal women over a 2- to 3-year period. Cancer Epidemiol Biomarkers Prev. 2006;15:972–978. doi: 10.1158/1055-9965.EPI-05-0848. [DOI] [PubMed] [Google Scholar]
- 41.Eckfeldt JH, Chambless LE, Shen YL. Short-term within-person variability in clinical chemistry test results. Experience from the Atherosclerosis Risk in Communities Study. Arch Pathol Lab Med. 1994;118:496–500. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


