Introduction
Deficits in higher cognitive functions such as memory have been well-documented in studies of normal aging and dementia using a variety of methods. Many functional neuroimaging investigations of memory have found deficits in hippocampus and prefrontal cortex when comparing young versus elderly age groups [e.g., (Cabeza et al., 2002; Grady et al., 2003; Grady, 2002)]. However, most of these studies did not carefully document the health of the elderly and data was typically averaged across individuals within a group (e.g., young vs old) which masks the rich individual variability often seen within groups. The current study examines individual variability within a group of elderly, while they performed a delayed auditory word recognition task, where some individuals had mild forms of pathology typically encountered within an elderly population. Correlations between the health of the elderly, their neural activation patterns, and their performance were examined.
Individual variability in the representation of language, another cognitive function, has already been demonstrated. For example, studies using electrical stimulation methods for localizing language functions have shown considerable variability with respect to brain anatomy and these differences in brain regions often correlated with verbal IQ (Ojemann et al., 1989). Similarly, a recent fMRI study showed that pre-existing individual differences in neural responses are important predictors of language learning (Mei et al., 2008). Price and Friston (2002; Friston and Price, 2003) also noted inter-subject variation when individuals perform a single cognitive task; they concluded that a particular cognitive function can be supported by more than one set of structural brain elements or networks. Together, these studies highlight that there are multiple ways of completing the same task and that some brain activation patterns are more efficient or adaptive than others.
We have previously shown that young and elderly, on average, utilize different strategies during a visual, delayed-match-to-sample task (visual-perceptual versus verbal-based strategies, respectively) while maintaining the same overall performance levels (Aine et al., 2006). Other functional imaging studies have also reported a posterior-to-anterior shift in brain patterns in young relative to elderly (Davis et al., 2008; Grady et al., 2003; Meulenbroek et al., 2004). Although it is generally concluded that compensation is occurring within the elderly (i.e., brain regions different from those invoked in the young group are recruited to maintain performance levels because neural regions have become deficient in some way; Cabeza et al., 2002), we question this notion since it is now known that white matter tracts continue to develop until at least the mid-forties. For example, myelination of frontal and temporal lobes, regions that are crucial for higher cognitive functions, continue to mature during this time (Bartzokis et al., 2001; Benes et al., 1994; Sowell et al., 2003). Therefore, one way to account for the apparent differences in visual-perceptual and verbal strategies used by young and elderly, respectively, is to suggest that new strategies evolve during protracted brain development, as neural pathways mature throughout middle age. From this perspective, young may rely on local visual-perceptual processes because frontal and temporal white matter tracts are not yet mature. Elderly, on the other hand, may rely on verbal abstraction once white matter tracts become mature because it is easier to remember a “red square in the lower left quadrant” (i.e., verbal chunking) than to remember the position of all colored squares making up the entire spatial array. If an elderly individual is instructed to keep the entire spatial array in mind, they can keep the array in memory; it just isn’t as natural for them to perform the task that way. Therefore, some elderly tend to use a different strategy from the young [see (Bor et al., 2003; Dror et al., 2005) for interesting discussions on the use of different strategies].
In the normal elderly population, however, pathological processes (e.g., hypertension, type 2 diabetes, mild forms of dementia) are equally likely to modify strategies applied to memory tasks in a non-volitional way as neural pathways become affected by a variety of insults. Again, most studies do not document the sub-clinical pathology that may exist within the elderly group, but rather depend on the participant’s self report of “normal.” However, recent radiological studies indicate that white matter hyperintensities (WMHs), typically associated with hypertension and type 2 diabetes, frequently target prefrontal lobes which affect executive control processes such as attention, working memory, and response inhibition (D’Esposito et al., 1995; Frackowiak, 1986; Gunning-Dixon and Raz, 2000; Hoffman, 1997; Jagust, 1994; Katzman, 1986; Petrides, 1994; Tullberg et al., 2004; Willott, 1997). That is, there appears to be an anterior-posterior gradient in the occurrence of white matter hyperintensities (Artero et al., 2004; Head et al., 2004). Since approximately 67% of adults aged 65–74 years have hypertension (CDC, National Center for Health Statistics) and approximately 1/3 of MRIs obtained from elderly participants show white matter changes (Inzitari, 2000), it is highly likely that elderly groups examined in normal aging studies have sufficient numbers of individuals with sub-clinical cognitive decline due to this “silent” underlying pathology. In addition to studies linking the presence of white matter hyperintensities (WMHs) with cognitive decline, recent epidemiological studies, such as the Framingham Heart Study, linked cardiovascular problems in midlife (e.g., hypertension) with the increased probability of developing dementia, including Alzheimer’s disease (AD), later in life [see review (Qiu et al., 2005)]. They show that higher systolic blood pressure or high serum cholesterol levels in midlife correlated with an increased risk for developing AD or mild cognitive impairment (MCI) later in life (Kivipelto et al., 2001; Schmidt et al., 2000).
In sum, only recently have investigators acknowledged that some elderly participants reveal no cognitive decline, even in high load and highly distracting conditions, compared to young (Gazzaley and D’Esposito, 2007) while other investigators using structural imaging methods acknowledge that vascular risk factors may exacerbate brain aging and account for some of the observed declines, since both prefrontal cortex and the hippocampus show selective vulnerability to hypertension (Raz et al., 2007). And finally, only recently are studies (Persson et al., 2006) beginning to suggest that cross-sectional elderly groups may contain individuals with undetected MCI or AD. Persson and colleagues examined a subset of older adults from an existing longitudinal study and split them into two groups. The groups did not differ when comparing their neuropsychological test results but they did differ on tests of memory obtained across a 10-year span (i.e., one group showed declines on their memory performance measures). The elders who showed a decline on memory tests also revealed either smaller hippocampal volumes or lower fractional anisotropy (FA) values on their diffusion tensor images, suggestive of disease processes including early stages of AD at baseline.
Our own work in aging and AD has emphasized the apparent influence that MR abnormalities seem to have on MEG signals, both single trials and averaged evoked responses. Bursts of slow-wave spindles or high frequency activity are often seen in our elderly participants who have abnormalities (e.g., volume loss and/or WMHs) evident from their MRIs. Both high frequency and slow-wave activity is most often seen over frontal and temporal regions, regions critical for higher cognitive functions. In our unpublished results, volume loss appears to affect amplitude more than latency. In contrast, WMHs tend to distort the overall pattern of time-courses, thereby resulting in more peaks or missing peaks; that is, peak latencies appear to be affected. These observations of linkages between MRI abnormalities and their correlations with changes in physiology motivate the present study.
Therefore, the aims of the present study are twofold. First, we wish to identify a range of different strategies for a relatively homogenous age group (elderly ≥ 64 years) performing a delayed, auditory verbal recognition task. By incorporating a wide range of abilities and health conditions, distinct strategies, both neural and behavioral, should be identified within this group of elderly. We use the term strategies, to refer to different ways of conducting the same task and operationally define strategies as reflecting different patterns of brain activation that correlate in some way with task performance. Effective strategies correlate with higher performance levels. The second aim is to show that pathology within the elderly group is associated with performance decrement. One prediction is that individuals showing evidence of moderate to severe WMHs on their MRIs or who have been diagnosed as MCI or probable AD use less effective strategies compared to the healthy elderly and that different neural patterns of activation can be identified for these individuals (e.g., different brain regions and/or increases in amplitudes and latencies of the responses) which correlate with poorer memory performance. Our diagnostic groups are determined by our neurologists’ clinical findings of probable AD or MCI and by our neuroradiologist’s MRI reports concerning volume loss and white matter ischemia. If we can demonstrate a clear linkage between pathology within our elderly group and performance decrement, then perhaps studies of aging that compare young versus elderly groups should remove the pathological cases from the elderly group, before group comparisons are made. Our premise is that age-related pathology correlates with cognitive decline and that most functional neuroimaging studies of aging have examined “normal aging,” complete with pathological processes normally found in community dwelling elderly such as hypertension, rather than “healthy successful aging” (i.e., no or only mild pathology) and that these two scenarios should be clearly differentiated from each other in order to better understand the status of cognitions across lifespan.
Materials and Methods
Participants
Thirty right-handed elderly participants (10 females and 20 males) ranging from 64 to 83 years of age (mean=74 years) participated in the study. Twelve of the participants were diagnosed as either amnestic MCI or probable AD. Individuals with a range of cognitive abilities were included to optimize the possibility of detecting different strategies applied to the delayed verbal recognition task and to assess which strategies are most efficient (i.e., best performance). All participants underwent a screening evaluation including a quantitative neurological examination, the Mini-Mental State Exam (MMSE), and the Satz-Mogel version of the Wechsler Adult Intelligence Scale-Revised. Verbal and visual memory were assessed with the California Verbal Learning Test (CVLT) and the Rey Osterreith Complex Figure Test (REY), both of which are well-validated measures used previously to examine effects of MCI/AD and WMHs on memory functions. Patients were diagnosed as amnestic MCI based on criteria modified from Petersen et al. (2004); they were not demented as defined in DSM-IV and cognitive impairment was not severe enough to impede routine activities of daily living. Patients in the AD group, identified from the memory disorders clinic at the New Mexico Veterans Health Care System, met DSM-IV and NINCDS-ADRDA criteria for probable diagnosis. All patients underwent structural neuroimaging and blood tests to exclude other causes of cognitive impairment. None of the patients had a history or exam findings of other chronic neurological conditions (e.g., Parkinson’s disease, epilepsy, focal neurological deficits, head trauma with persistent neurologic abnormalities). All particpants’ consent was obtained according to the Declaration of Helsinki; this research was approved by the University of New Mexico Human Research Review Committee and the New Mexico VA Health Care System Research and Development Committee.
Tasks
Participants engaged in two classification tasks (size discrimination and daily living) similar to the Warrington Recognition Memory Test (Warrington, 1984) where the same word list was presented before the delayed verbal recognition task. A size classification task was conducted first. A list of common nouns (n=104), constructed from lists of words used in the Rey Auditory Verbal Learning Test [RAVLT: see (Lezak, 1995)] was presented binaurally and participants were asked to decide whether the words, representing common objects, were larger than a television [a computer monitor was shown to them as an example], and they responded with a button press. Fifty-five percent of these words were single syllable words while 41% were two-syllable words and 4% were three-syllable words. The same list was presented a second time and participants determined if each object was used in daily living. Following a 20 minute delay period recognition memory was assessed using a list of 347 words and participants were instructed to respond with the left index finger if they heard the word previously (“Yes” response) and the right index finger if they did not (“No” response). The list of 243 foils (63% single syllable, 34% two-syllable, and 3% three-syllable new words) were obtained from the recognition list used in the RAVLT as well as nouns obtained from Word Association Norms (Palermo and Jenkins, 1964) and from the 1000 most commonly used words listed in The Teacher’s Word Book of 30,000 Words (Thorndike and Lorge, 1944). The resultant list of 347 words provided a target probability of 30%. The two lists of nouns (104 and 347) were matched across three categories, listed in order of importance: 1) word frequency; 2) number of syllables; and 3) similarity. Word lists were always presented in the same male voice and participants were not provided with information regarding list lengths.
A hearing test was conducted inside the MEG shielded room with the ear pieces in place before beginning the study; adjustments in intensity level were made for each participant based on the test results in order to achieve 60 dB SPL. Auditory tones (200 Hz, 500 Hz, 1000 Hz and 2000 Hz) were presented using NBS Presentation software (i.e., the smple hearing test provided by NBS was used) and were generated using a Creative Labs Soundblaster audio card. The sound was delivered to the subject’s ear canal using Etymotic Research ER-3A sound transducers connected with plastic tubing to foam earplugs. The order of tone frequency and ear to be stimulated was randomized. Initially, supra-threshold and sub-threshold tone volumes were established. Then in a random fashion, volumes for sub-threshold tones were gradually increased and the volumes of the supra-threshold tones were decreased in order to determine threshold values. The randomized presentation of tone frequencies, ear, and sub-/supra-threshold volumes helps to eliminate the possibility that participants can guess when they should hear the next tone. Since this system does not permit one to increase volume in one ear relative to the other, an average between left and right ear values was used to determine the average attenuation value. Practice on the task was provided to ensure that participants understood the instructions and felt comfortable with the task. A video camera in the room permitted viewing of the response buttons which provided additional confirmation that participants understood the instructions.
All behavioral responses were acquired using an optical response device developed at the MIND Research Network (The MIND Input Device, M. Doty, Patent #7,039,266). Five optical buttons were mounted inside a thermoplastic cast that was secured to the left and right forearms by Velcro straps to hold the hands on the response devices. Trials in which incorrect behavioral responses were made were not discarded since it was necessary to assure a sufficient number of “useable” trials for averaging responses for the MCI/AD group. That is, misses, false alarms and incorrect responses for “No” and “Yes” conditions were included in the averaged MEG data. It was predicted at the outset that MCI/AD patients and individuals with severe WMHs would make more errors than control participants, but the addition of extra trials to make up for errors would make the task too long for all of our elderly participants.
Prior to data collection three head position coils were attached to the subject. Electrodes were placed at the outer canthi and above/below the right eye for detecting eye-movements and blinks. A 3-D digitizer (Polhemus) registered the participants’ nasion, left and right tragus for purposes of establishing a 3-D coordinate head frame and all other points (typically 150) chosen to align the 3-D coordinate system with the MRIs. The coils were later localized (i.e., pulsed), and used to establish head position relative to the helmet. Magnetic fields were recorded with a CTF 275-channel MEG system (VSM MedTech, Ltd.) inside a mumetal/aluminum magnetically shielded room. The data were acquired as continuous data (digitized at 600 Hz, online filters at 0.1 to 200 Hz) with markers inserted at every stimulus event (verbal classification list 1, verbal classification list 2, and recognition list 3) for off-line averaging (filtered at 0.1 to 50 Hz). All MEG continuous data were preprocessed using CTF software for deletion of bad channels of data, digital filtering and artifact rejection. Epochs with MEG signals greater than 2000 fT were rejected from the offline average. Epochs from vertically located eye electrodes (monitoring blinks) with signals greater than 75 μV were rejected from the offline average. Past experience has shown that averages obtained from 80 or more trials provide a sufficient number of trials from which to conduct source localization. All “yes” data sets contained averaged responses from at least 80–104 trials.
The continuous data were further processed offline using MEGAN (E. Best, Los Alamos National Laboratory), an IDL-based program for displaying and writing all MEG data (e.g., head-shape points, sensor locations) to a standard netMEG file using the netCDF format (http://portal.mind.unm.edu/megsim/tools/MEGAN/MEGAN.html). MRIVIEW (Ranken et al., 2002; Ranken and George, 1993), an IDL-based software tool (http://www.lanl.gov/p/p21/mriview.shtml), was used for: 1) integrating volumetric MRI head data with MEG data; 2) launching the source localization algorithm (CSST: see below); and 3) setting up the best-fitting sphere head model. With the 3-D information from the Polhemus device, the coils provided information regarding the location of the fiducials relative to the biomagnetometer. By identifying the same fiducial points on the MRI and optimizing the fit with the Polhemus head shape points, a 3-D transformation was performed to align the MEG data with the MRI data, allowing for identification of the anatomical locations of the MEG sources following source analysis.
MEG source locations, strengths and orientations were characterized using CSST [Calibrated Start Spatio-temporal (Ranken et al., 2004)]. CSST is a semi-automated multidipole, spatio-temporal approach where estimation of time invariant parameters (locations) are conducted first, using nonlinear least squares minimization, followed by linear estimation of the associated time varying parameters (source magnitudes) (Mosher et al., 1992). CSST runs multiple instances of a downhill simplex search from random combinations of MR-derived starting locations from within the head volume on a Linux PC cluster. Both computer simulations and analysis of empirical data have demonstrated the accuracy and reliability of the multistart techniques which are used routinely in our MEG studies (Aine et al., 2005; Aine et al., 2000; Aine et al., 2006; Huang et al., 1998; Stephen et al., 2006a; Stephen et al., 2006b). General steps for processing the MEG and MRI data are shown in Figure 1. The criterion for a source to be labeled as a source for each cortical location was determined by examining source locations across subjects and choosing those within 1 cm radius of the average location relative to the underlying anatomy. Left and right hemisphere sources were collapsed for each cortical location.
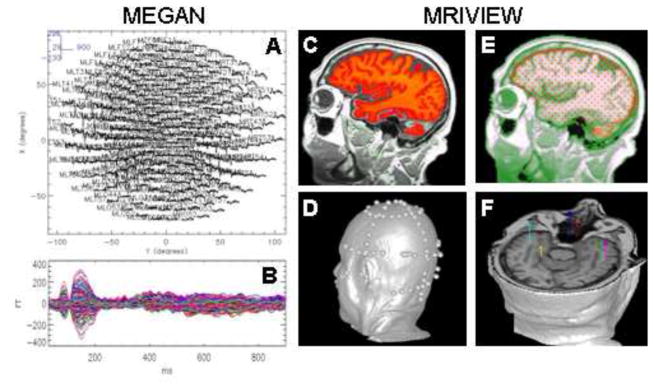
Fig. 1.
MEGAN was used for preprocessing the MEG data. These data were saved in netCDF format and used as input for the CSST algorithm (1.A: averaged responses to words - whole head array; 1.B: averaged responses to words—this is the interval of time that was analyzed). MRIVIEW was used for: 1) segmenting the cortical volume (1.C); 2) conducting a least squares fit between ~150 points digitized on the head surface and the reconstructed MR surface (1.D); 3) determining the starting locations (red dots) and best-fitting sphere head model (1.E); and 4) setting-up the CSST fits and displaying the CSST source localization results (1.F). Thousands of fits to the data are analyzed, as opposed to a single fit, enhancing the probability of reaching the global minimum and obtaining statistically adequate and accurate solutions.
We use semi-automated algorithms for several reasons. First, the user does not need to provide the software with initial starting parameters (guesses), which may bias the results toward previously reported areas of activity. Second, multistart algorithms analyze thousands of fits to the data, as opposed to a single fit, enhancing the probability of reaching the global minimum of the measure of goodness of fit and consequently obtaining statistically adequate and accurate solutions (e.g., 15,000–20,000 fits to the data were conducted for 6- and 7-dipole models with fewer numbers of fits for lower-order models). Third, data from all sensor locations (excluding channels with flux-jumps or artifact) were used in the unconstrained spatio-temporal fits (e.g., candidate solutions were not fixed in location nor constrained by cortical geometries determined via MRI).
The cost function adopted for the minimization procedure (i.e., minimizing the difference between the measured magnetic field distribution and the magnetic field calculated for an assumed model) is the χ2 where the noise estimated for each sensor location is taken into consideration. More specifically, the reduced chi-square value is used which is χ2 divided by the number of degrees of freedom (Bevington, 1969; Huang et al., 1998; Supek and Aine, 1993). Model adequacy was assessed by examining: 1) the resulting reduced-chi square values (Supek and Aine, 1997; 1993); 2) the clusters of location estimates to assess scatter [an indication of over-modeling, or fitting of noise (Supek and Aine, 1993)]; and 3) the residual waveforms (i.e., difference between the empirical data and the model) to assess whether additional signal remained [evidence of undermodeling (Huang et al., 1998)]. All three methods outlined above are used to determine the best model(s) for the data. The interval chosen for analysis does not begin at “0” ms since evoked activity is not evident during the initial 0–30 ms and attempts at fitting noise provides worse fits to the data. Therefore, 30–900 ms intervals were analyzed for all participants and a range of model orders (4–8 dipole models) was attempted for each participant. A semi-automated peak characterization routine created in MATLAB was used for deriving amplitude and latency measures (Stephen et al., 2006b).
Anatomical MRIs were acquired on a 1.5T Siemens Sonata MR scanner using the 8-channel phased array head coil at the Mind Research Network (MRN, Albuquerque, New Mexico). Two sequences were acquired with the following parameters: 1) T1-weighted MPRAGE, 1.5 mm slices, 128 slices per slab, TR = 12 ms, TE = 4.76 ms, FOV 25.6 cm, matrix 256 × 256; and 2) T2-weighted Turbo Spin Echo (TSE), 1.8 mm slices, 106 slices, TR = 9000 ms, TE = 64 ms, FOV 25.6 cm, matrix 256 × 256.
Image review was performed by a CAQ (Certificate of Added Qualification) fellowship-trained neuroradiologist. Anatomical images were reviewed electronically using an Osirix X (Mac) image viewer. All volume T1 weighted images were additionally reviewed using real-time multiplanar reformations in standard axial, coronal and sagittal planes. The radiologist was provided with basic demographic information including age and sex of the participant. The reviewer remained blinded to the status of the participant (case or control). Institutional IRB-mandated reporting of unexpected abnormalities was always performed. Additional abnormalities not requiring medical follow up (e.g., white matter lesions, atrophy, and vascular anomalies) were reported if, in the neuroradiologist’s experience, they were beyond what would be expected for a normal age-matched individual. This information was: 1) relayed to participants’ physicians if the participants wished to be informed of such findings, documented by another signature on the consent form and 2) used to establish MRI groupings.
Statistical Analyses
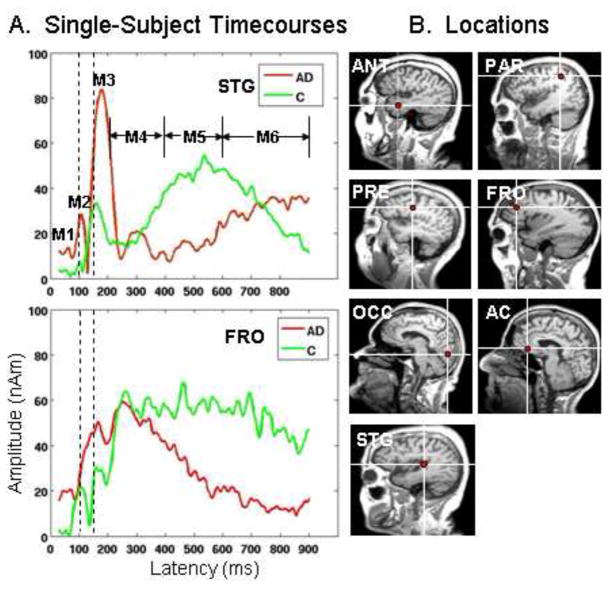
The time-course estimates for each region were quantified for each participant; peak amplitudes, relative to prestimulus baseline values, and maximum peak latencies for 6 time intervals were identified for the following intervals: 40–80 ms, 80–115 ms, 115–215 ms, 215–400 ms, 400–600 ms, and 600–900 ms (Figure 2A). Magnetic peaks 2 and 3 (M2 and M3) were readily identifiable in all single-subject data, consequently, repeated measures Analysis of Variance (ANOVA) on latency measures was conducted using Peaks 2 and 3 only to estimate the flow of information throughout brain regions responsive to auditory stimulation. The other intervals were chosen primarily to assess whether amplitudes were sustained across time as shown in Figure 2 in the time-courses for a normal control and an AD participant (green and red tracings, respectively). For example, time-courses in STG revealed greater amplitudes for the AD patient at M1-M3 (red tracings) while the slow wave (M5 and M6) was delayed relative to the normal control (green tracings). Similarly, the prefrontal (FRO) time-courses revealed early, higher amplitudes for the AD participant which did not remain sustained across the time interval as it did for the elderly control.
Fig. 2.
A. Averaged timecourses from two single participants, one elderly control and one AD patient are overlaid for superior temporal gyrus (STG) and lateral prefrontal cortex (FRO). Actual peaks (M1-M3) are shown for these 2 participants followed by 3 time intervals defining peaks 4–6. The vertical dashed lines aid in seeing differences in M2 and M3 latencies across the participants. Early differences in amplitude can be seen in STG (M2-M3) while late activity for the AD patient (red tracings) is later to onset (M5-M6). FRO reveals late sustained activity for the control participant at M5-M6 (green tracings), relative to the AD patient. 2.B. Seven source locations representative of those identified across participants are shown and are indicated by red dots at the white cross-hairs. ANT=Anterior Temporal Lobe; PAR=Superior Parietal Lobe; PRE=Premotor; FRO=Lateral Prefrontal Cortex; OCC=Occipital; AC=Anterior Cingulate; STG=Superior Temporal Gyrus.
Cluster groupings were accomplished using agglomerative hierarchical clustering as provided by PROC CLUSTER in SAS. While group comparisons were not a primary emphasis of this study, they were conducted to provide additional information regarding the make-up of the larger group of 30 individuals. Therefore, we began with a fully saturated model with all interactions and eliminated all insignificant higher-order interactions using PROC Mixed in SAS. We conducted Multivariate analyses with repeated measures; AD (MCI/AD vs. Controls) or MRI (1=no to mild abnormalities; 2=volume loss; 3=white matter ischemia) were grouping factors and time and yes/no responses were repeated factors. If the clinical report mentioned both WMHs and volume loss, the participant’s data was placed into the WMH group. The Multivariate analyses of amplitude enable one to document significant amplitude changes across time. Post hoc comparisons were conducted to isolate interaction effects. Pearson correlations were conducted on the behavioral data (reaction times and percent correct) for MCI/AD vs. Controls and MRI vs. Control groups and Task (yes/no). Pearson correlations were also conducted to determine if MEG activity (e.g., amplitude and peak latency) correlated with performance on the behavioral tasks (percent correct and RTs) and neuropsychological tests.
Results
Overall Behavioral and Source Localization Results
Behavioral results were obtained from 28 participants since equipment malfunction occurred for two participants (1 MCI and 1 control). Although responses to “Yes” conditions were generally more accurate and faster than responses to “No” conditions (mean Correct for yes=73.8%, STD=18.54, mean Correct for no=67.7%, STD=24.4, mean RT for yes=1231 ms, STD=171.2, mean RT for no=1298 ms, STD=159.3) this comparison did not reach statistical significance (percent correct, p=.08; RTs correct, p=.11). Because differences associated with “Yes” versus “No” responses were minimal and the same general regions were active for these two conditions, “Yes” responses were used for MEG MANOVA/ANOVA tests. MEG responses evoked by “Yes” and “No” conditions were used for correlations between amplitude/latency measures and performance (task and neuropsychological tests) across all participants.
Seven brain regions were most commonly seen across participants (Figure 2B): anterior temporal lobe (ANT: n=16); superior parietal (PAR: n=24); premotor (PRE: n=12); lateral prefrontal cortex (FRO: n=15); occipital cortex or lateral occipital gyrus (OCC: n=10); anterior cingulate (AC: n=10); and superior temporal gyrus (STG: n=28). PRE and STG source locations were the least variable across participants. OCC locations were the most variable across participants since some individuals showed activity in medial occipital cortex while others showed activity in the lateral occipital gyrus. All participants did not reveal the same pattern. Because each time interval does not necessarily contain a true “peak” only M2 and M3 latencies, latencies at which peaks can be identified for all 7 brain regions, were submitted to a multivariate analysis to determine the timing of activity across brain regions (i.e., which brain regions were active first). M2 and M3 peak latencies are shown in Table 1. Multivariate analyses for M2/M3 “Yes” responses (F=3.36, df=6,72, p<.006) indicate that activity in STG, PRE, and OCC had equivalent latencies while ANT and PAR were similar to each other but later than STG/PRE/OCC and earlier than AC and FRO.
Table 1.
*STG, PRE, OCC, †PAR & ANT, ‡AC & FRO. All Regions Multivariate for “Yes” Responses. Different symbols represent regions that were significantly different from each other at Peak latencies M2 (80–115 ms) and M3 (115–215 ms).
| Location/Peak Lat. | Mean/SD (ms) | Mean/SD (ms) |
|---|---|---|
| M2 | M3 | |
| STG | 97* (12) | 159* (19) |
| PRE | 99* (13) | 158* (14) |
| OCC | 99* (14) | 160* (28) |
| PAR | 105† (19) | 166† (18) |
| ANT | 104† (10) | 169† (20) |
| AC | 107‡ (13) | 183‡ (9) |
| FRO | 113‡ (17) | 171‡ (13) |
Word Recognition Strategies
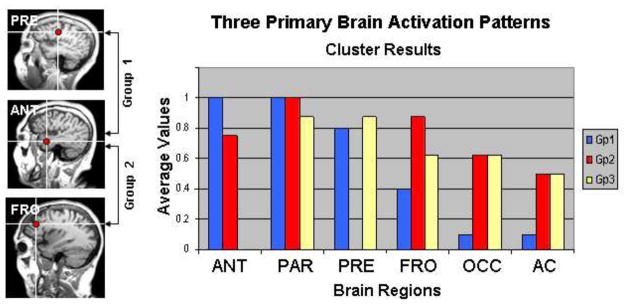
Three primary patterns of brain activation were observed when a cluster analysis was conducted on the presence or absence of locations across participants, regardless of diagnosis and MRI group. The bar graphs in Figure 3 reveal the relative weightings across 6 brain regions for each group. STG was not included in this analysis since most participants revealed activity in STG (auditory cortex); i.e., STG did not help differentiate between the cluster groups. Cluster group 1, revealing activity in ANT, PRE and PAR, had the highest percent correct (84.9%), the highest mean full scale IQ (134) and best performance on the delayed recall of the REY Complex Figure Test (REY), a test of visual memory (Table 2). The single most notable factor that separates this group from the other two groups is that the clear majority of its members had “no” or only “mild” abnormalities identified from their MRIs (n=8 out of 11). Cluster groups 2 and 3 were more similar to each other in showing widespread activity in FRO, PAR, OCC, and AC, but Group 2 did not reveal activity in PRE, as observed in Group 1, while Group 3 did not reveal activity in ANT. Percent Correct between Gp1 and Gp2 was significantly different (p<.05). RTs were not significantly different between groups. Full Scale IQ was significantly different between Gp1 and Gp2 (p<.05) and between Gp1 and Gp3 (p<.0005). REYD also differed between Gp1 and Gp2 (p<.01) and between Gp1 and Gp3 (p<.002). Performance differences between Group 2 and Group 3 were not significant although there was a trend in terms of IQ and delayed visual memory performance (Group 2 was higher).
Fig. 3.
A cluster analysis revealed 3 patterns of brain activity which correlated with IQ. MRIs at the left show differences between two clusters in terms of source locations. Anterior temporal (ANT) activation was common to both cluster groups shown at the left but all participants of Gp1 revealed ANT activity. PRE (premotor) was not evident for Gp2. Gp2 revealed more FRO (lateral prefrontal cortex) activity than Gp1. Bar graphs show the relative weights of the sources for each cluster group i.e., the number of participants from each cluster that revealed activity in the different regions. Most notable results are that Gp3 does not reveal ANT activity and Gp2 does not reveal PRE activity.
Table 2.
Cluster results reveal three primary brain activation patterns that correlate with IQ, visual memory (REYD), and percent correct. Asterisks indicate significant differences between groups. ANT=Anterior Temporal; PAR=Superior Parietal; PRE=Premotor; FRO=Lateral Prefrontal; FULL IQ=WAIS-R Full scale IQ; MMSE=Mini-mental status exam; EDU=Years of education; CVLT=California Learning Test Trial 5; REY=Complex Figure Test (visual memory) Delayed Recall. STD=Standard deviation, d′=d prime.
| Cluster Group | “Yes” Corr (STD) | “Yes” Corr d′ | “Yes” RT (STD) | Full IQ | MMSE | EDU | AGE | CVLT | REYD |
|---|---|---|---|---|---|---|---|---|---|
| 1 = ANT+PAR+PRE (n=11) | 84.9* (6.4) | 2.81 | 1209 (147.4) | 134* | 29.8 | 17 | 72.1 | 13 | 22.7* |
| 2 = PAR+FRO+ANT (n=8) | 62.2* (25.5) | 1.90 | 1258 (196.2) | 117* | 27.8 | 16 | 74.8 | 10.4 | 13.6* |
| 3 = PRE+PAR+FRO (n=9) | 78.2 (9.7) | 2.43 | 1200 (187.2) | 108* | 25.9 | 15 | 76.8 | 9 | 8.4* |
Table 3 summarizes the correlations across 28 individuals between the amplitude/latency measures for the brain regions deemed important for separating the groups identified in the cluster analysis (upper row) and task performance/neuropsychological test results (bottom row). ANT, PAR, and PRE measures correlated with accuracy while ANT and FRO measures correlated with RTs. ANT is the only region that showed correlations for both accuracy and RTs. It is interesting to note that “No” RTs for both ANT and FRO correlate with longer peak latencies while greater peak amplitudes in FRO correlate with shorter “Yes” RTs. Recall that RTs were generally faster for “Yes” responses but it did not reach statistical significance. It appears that peak latencies and amplitudes obtained from ANT and FRO are predictive of RTs. ANT and PAR measures also correlated with IQ and visual and verbal memory, respectively. PRE measures correlated with verbal and visual memory performance while FRO measures correlated with visual memory performance.
Table 3.
Correlations between amplitude/latency measures from brain regions identified as being important for differentiating between cluster groups, and task performance are shown in the upper portion of the columns. Negative correlations are identified by “−“ and positive correlations are identified by “+”. Correlations between amplitude/latency measures and neuropsychological test results are shown in the lower portion of each column. All correlations listed were at least p<.05. Amplitude and latency measures were taken from “yes” and “no” evoked response data. CVLT=Trial 5; CVLTD=Delayed Recognition; REYC=Copy of REY Figure; REYI= Immediate recall; REYD=Delayed Recall.
| Correlations for Amplitude/Latency Measures and Task/Neuropsychological Performance | |||
|---|---|---|---|
| ANT M3 & M4 (169–322 ms) | PAR M3 & M5 (166, 524ms) | PRE M2 (99 ms) | FRO M2-5 (113–768 ms) |
| Amp: -“No” RTs r=−.63 Lat: -“Yes” Correct r=−.73 +“No” RTs r=.59 |
Amp: -“Yes” Correct | r=−.47 Amp: -“No” Correct r=−.78 | Amp: -“Yes” RTs r=−.61 Lat: +“No” RTs r=.71 |
| Amp: -MMSE r=−.69 -EDU r=−.66 -PERF IQ r=−.71 -REYI r=−.64 -REYD r=−.64 Lat: -PERF IQ r=−.87 -VERB IQ r=−.79 -REYI r=−.64 |
Lat: -PERF IQ r=−.43 -VERB IQ r=−.48 -CVLT r=−.49 -CVLTD r=−.47 |
Amp: -MMSE r=−.67 -CVLT r=−68 -CVLTD r=−.81 -REYI r=−.71 -REYD r=−.65 |
Amp: +REYC r=.59 +REYI r=.76 +REYD r=.74 Lat: +REYC r=.78 |
MRI Abnormalities and MCI/AD
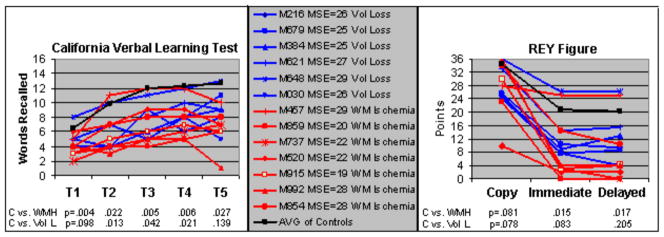
In our group of 30 elderly (28 participants had MRIs), 43% had abnormalities identified on their MRIs (WMHs and volume loss) rated moderate to severe by the neuroradiologist. None of the participants showed structural brain abnormalities other than WMHs and volume loss. We grouped the MRI abnormalities into two groups: 1) moderate to severe volume loss and 2) moderate to severe WMHs, consistent with chronic white matter (WM) ischemia. Figure 4 shows individual results on the California Verbal Learning Test (CVLT) and REY tests for the MRI groups compared to a control group free from moderate to severe MRI abnormalities; i.e., individuals with volume loss (blue lines) and WMHs (red lines) relative to the average of individuals revealing no or mild abnormalities and without evidence of MCI/AD (black line) are compared. The group with WM lesions performed worse than the other two groups on CVLT and the WMH group performed worse than the control group without MRI abnormalities on delayed recall of the REY figure. Individual MSE scores are also shown in the middle panel.
Fig. 4.
Performance on the verbal (California Verbal Learning Test or CVLT) and visual (REY Figure) memory tests by MRI groups (moderate to severe abnormalities). IDs for each participant, along with their mini-mental status exam (MSE) scores and group are shown in the middle panel (blue=volume loss, red=WMH). The black line in all boxes represents the average performance of individuals (n=13) without evidence of moderate to severe MRI abnormalities or MCI/AD. In general, participants with higher MSE scores performed best on the visual and verbal memory tests. T-test results between the controls and WMH group and between controls and the Volume Loss groups are shown for each trial of the CVLT and for the Copy, Immediate and Delayed recall of the REY Figure.
A one-way ANOVA conducted on the behavioral data for the 3 MRI groups (no abnormalities, volume loss, and white matter ischemia) revealed significant differences for “Yes” Correct between groups (F=4.6, df=2,22, p=.02) with group 3 (WMH) revealing fewer percent correct (Gp1=80.4%, STD=12.7 and Gp2=76.7%, STD=18.9; versus Gp3=56.2%, STD=22.6). There were no significant differences in RTs between MRI groups (Gp1=1208 ms, STD= 169.3; Gp2=1237 ms, STD=161.4; Gp3=1256 ms, STD=180.7). Many individuals with evidence of WM ischemia performed most poorly; 5 out of 7 of these individuals were diagnosed as probable AD. One member of the WMH group, recruited as a control subject, has shown a 6-word drop on the CVLT (Trial 5) and 7.5-point drop on the delayed recall of the REY figure across a 3 year span in our longitudinal study. Only one member of the WMH group (M457) has remained cognitively stable, longitudinally. Two members of the volume loss group were patients diagnosed with MCI while a third member was diagnosed as probable AD.
Table 4 presents general characteristics and performance measures for the different, overlapping sub-groupings of 30 elderly participants. In general, the group with WMHs and the amnestic MCI/AD group had lower IQ scores, and performed worse on the CVLT, on both immediate and delayed recall of the REY figure, and on the delayed auditory word recognition task (% correct).
Table 4.
Group characteristics and behavioral performance (means). T-tests were conducted between controls without moderate or several MRI abnormalities (n=13) and each group. Volume Loss (n=6), WMHs (n=7), and MCI/AD (n=12) groups overlapped.
| Group | Age | Edu | MMSE | CVLT5 | REYD | IQ | %Corr (STD) | d′ | RTs |
|---|---|---|---|---|---|---|---|---|---|
| Controls | 71.3 | 16.3 | 29.3 | 13.1 | 18.9 | 126.9 | 80.4 (12.7) | 2.44 | 1208 |
| Vol. Loss | 79.5* | 14.8 | 26.5* | 10.5 | 13.9 | 110.0 | 76.7 (18.9) | 2.68 | 1237 |
| WMHs | 78.3* | 13.6* | 26.0* | 8.3* | 7.2* | 106.3* | 56.2* (22.6) | 1.69 | 1256 |
| MCI/AD | 77.4* | 14.9 | 25.3† | 7.9† | 7.7† | 106.8* | 63.6* (21.9) | 2.10 | 1241 |
p<.05,
p<.005. EDU=Education level; MMSE=Mini-Mental Status Exam; CVLT5=percent correct on Trial 5; REYD=Delayed Recall of REY Figure; IQ=Full scale; %Corr=percent correct for “yes” responses on auditory task; STD=Standard deviations shown in parentheses for percent correct. RTs=Reaction times for “yes” responses on auditory task (ms).
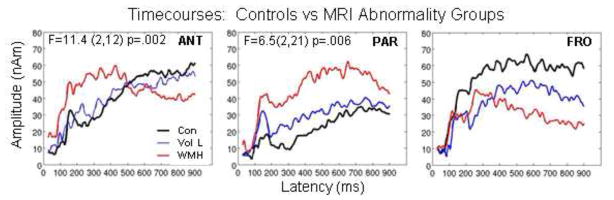
Time-courses from 3 brain regions are shown for the MRI groups in Figure 5. Multivariate results for MRI × Region for “Yes” responses on average across time revealed significant amplitude differences (F=3.99, 11,55, p<.0003). Analysis by region for amplitude indicated that amplitudes for the WMH group were greater on average across time in ANT (F=11.43, 2,12, p=.002) and PAR (F=6.5, 2,21, p=.006). Multivariate analyses for MRI × Region on M2 and M3 latencies were not significant. Although FRO amplitudes were not significant, this region is shown in Figure 5 since amplitude differences for the MCI/AD versus control group were significant (see below) and the timecourses looked remarkably similar between MCI/AD and WHM groups due to a substantial overlap in participants between these two groups.
Fig. 5.
Averaged timecourses are shown for 3 brain regions for the MRI groups: black tracings represent the average of normal controls, blue tracings represent the average for individuals with moderate to severe volume loss, and red tracings represent the average for individuals with chronic white matter ischemia (WMH). Multivariate results indicate significant differences for ANT and PAR. Frontal (FRO) regions showed the opposite trend.
Eight of our 11 MCI/AD with MRIs had at least one abnormality identified from their MRI rated as moderate to severe. Paired t-tests on subgroup behavioral data with unequal variances between MCI/AD and control groups revealed significant differences for “Yes” Correct (Control=80.4%, STD=12.7 and MCI/AD=63.6%, STD=21.9). Again, there were no significant differences for the RT data (Control=1208 ms, MCI/AD=1241 ms).
Task performance results indicate poor overall accuracy for the MCI/AD group. Verbal and visual memory, along with verbal fluency (Controlled Oral Word Association Test or COWA), digit symbol, and MMSE score were also significantly worse for the MCI/AD group [CVLT: t=3.83, 12.3, p<.002; CVLTD: t=3.98, 13.2, p<.002; REYI: t=2.79, 13.1, p<.02; REYD: t=2.76, 12.6, p<.02; COWA: t=2.78, 16.9, p<.01; DS: t=3.04, 17.9, p<.007; MSE: t=4.34, 12.1, p<.0009].
Because there was considerable overlap between WMH and MCI/AD groups, the time-courses for the 3 brain regions shown in Figure 5 for the MRI groups were very similar to the MCI/AD group and therefore are not shown. The only difference was that multivariate analyses indicated significant amplitude differences on average across time for ANT, PRE, and FRO, rather than for ANT and PAR (Region × AD: F=4.64, 6,65, p<.005). ANT and PRE revealed higher amplitudes for MCI/AD across time (ANT: F=8.4, 1,15, p=.01; PRE: F=22.9, 1,11, p=.0006) while FRO revealed the opposite pattern for “Yes” responses (F=8.08, 1,15, p=.01). Posthoc tests indicated significant amplitude differences particularly at M2 and M3. Differences were also notable for PRE at M4 and M5 and FRO at M6. There were no significant latency differences between MCI/AD and controls for M2 and M3.
Discussion
Different Strategies
The brain regions active in response to our delayed auditory verbal recognition task included the superior temporal gyrus (STG, in the vicinity of Heschl’s gryus), anterior temporal lobe (ANT), superior parietal lobe (PAR), premotor cortex (PRE), lateral prefrontal cortex (FRO), anterior cingulate (AC), and occipital cortex (OCC). These regions have been identified in auditory verbal memory studies previously. Cohen and colleagues (2004) identified what they refer to as an auditory word form area in left anterior superior temporal sulcus and note several studies showing left inferotemporal activations during the perception of auditory words as well. While some of our participants revealed activity in the lateral occipital gyrus, most revealed activity in medial occipital cortex similar to results found in our auditory incidental verbal learning study (Aine et al., 2003). Premotor activation has been shown previously and has been linked most often with subvocal verbal rehearsal processes (Binder et al., 2000; Braver et al., 1997; Herwig et al., 2003). Lateral prefrontal activation has been shown frequently in working memory studies and has been associated with maintenance of information, selection and organization of motor responses [e.g. (Muller and Knight, 2006; Postle et al., 1999)]. Consistent with the temporal properties of the BOLD response reported for a speech repetition study (Dehaene-Lambertz et al., 2006), activity in Heschl’s gyrus was active first, with later responses noted at the temporal poles. However, our results revealed significantly longer latencies for frontal regions relative to anterior temporal and parietal cortex, both of which had longer latencies than Heschl’s gyrus and premotor cortex.
A cluster analysis conducted on the full spectrum of participants (AD/MCI and MRI abnormalities) revealed three dominant patterns of activity that correlated with full scale IQ. Although STG was active in all groups, it did not correlate with task performance and therefore is not discussed further. As shown in Table 2, Cluster group 1 revealing activity in ANT, PAR and PRE had the highest percent correct, the highest mean full scale IQ (134) and best performance on the delayed recall of the REY Figure (visual memory). Correlations between the amplitude/latency measures and task performance and their correlations with performance on the neuropsychological tests, suggest that ANT, PAR, and PRE activity are related to accuracy [ANT latency is related to speed of response as well—see Table 3]. ANT and PAR are also highly correlated with IQ measures. All members of Group 1 revealed activation of ANT and PAR during the task and responded more accurately overall. Of special interest is the fact that early measures (e.g., beginning at ~100 ms) from PRE time-courses also correlated with recognition task accuracy (“No” Correct) as well as performance accuracy on verbal and visual memory tests. Group 1, which showed the greatest accuracy on the task, revealed activation of PRE while Group 2, which showed the worst accuracy scores on the task, did not reveal activation of PRE. Surprisingly, PRE activity did not correlate with RTs. Similarly, Herwig and colleagues (2003) found significantly higher error rates for a verbal working memory task when transcranial magnetic stimulation was applied to left premotor cortex. Multivariate analyses on latency measures indicate that activity in STG and PRE were indistinguishable.
Cluster groups 2 and 3 were more similar to each other in showing widespread activity in FRO, PAR, OCC, and AC, but Group 2 did not reveal activity in PRE as observed in Groups 1 and 3 while Group 3 did not reveal activity in ANT. OCC was active in several participants from both Groups 2 and 3 as if they engaged in visual imagery of the objects while they determined if they heard the word before (Aine et al., 2003). Performance differences between Group 2 and Group 3 were not significant although there was a trend in terms of IQ and delayed visual memory performance (Group 2 was higher). At this point it is impossible to determine whether the strategy used by Group 2 or Group 3 was more effective; however, the majority of MCI/AD patients (67%) were members of Group 3. Clearly data from more participants are needed in order to reach more definitive conclusions concerning the roles each of these brain regions play in verbal recognition memory. It is also important to note that an alternative interpretation for the different neural patterns reported on above may be due to the fact that trials in which incorrect responses were made were not discarded, in order to assure a sufficient number of useable trials for the MCI/AD group. Consequently, behavioral accuracy between cluster groups 1 and 2 was statistically different which may have contributed to differences in neural patterns evident between these two groups, rather than being due to strategy differences per se. Overall, the results do seem to suggest, however, that some ANT activation is linked to good task performance.
MRI Abnormalities and MCI/AD
WMHs and MCI/AD groups revealed poorer overall task accuracy compared to the elderly controls. Significant amplitude differences were evident particularly in the early peaks of the MEG time-courses (e.g., 100–160 ms) from ANT (WMHs and MCI/AD groups), PAR (WMHs), PRE (MCI/AD), and FRO (MCI/AD). ANT revealed higher amplitudes for the WMHs and MCI/AD groups on average across time compared to controls while FRO revealed lower amplitudes (significant in MCI/AD group). It appears that some activity in these regions is good, provided the activation does not exceed a certain threshold level. This statement is based on correlations between amplitude/latency measures across all participants that showed: 1) low amplitude activity in ANT correlated with better performance on neuropsychological test measures including IQ and verbal/visual memory; and 2) shorter peak latencies in ANT correlated with higher percent correct and shorter RTs on the task. This type of activation pattern does not differ much from that often found in fMRI studies where load-dependent effects in lateral prefrontal cortex were examined. That is, increased activity in lateral prefrontal cortex as a function of load is correlated with better performance but less of an increase was noted for subjects that performed faster and more accurately (Habeck et al., 2005; Rypma et al., 2002). Interestingly, in the current study, greater amplitudes and shorter peak latencies in FRO correlated with faster RTs.
Thus, it appears that some optimum level of activation is good for performance and that excessive activity in some brain regions may provide a preclinical indicator of pathology long before the complete absence of activity in these regions. For example, although changes in entorhinal cortex and hippocampus are typically focused upon when monitoring longitudinal changes in MCI and AD, several recent studies have noted the importance of mapping ANT activity. When monitoring cortical atrophy rates for MCI and AD in longitudinal designs these studies consistently note early changes in the anterior medial temporal lobe (Bozzali et al., 2006; Smith et al., 2007; Whitwell et al., 2007). Whitwell and colleagues (2007), for example, found changes in the anterior temporal lobe that occurred three years previous to a diagnosis of AD. At the time of diagnosis of AD, atrophy in the temporal lobes spread to include the middle temporal gyrus and the entire extent of the hippocampus. Similar conclusions were reached by Dickerson and colleagues (2008) in their review of fMRI studies demonstrating greater medial temporal lobe (MTL) activation in MCI patients compared to controls. They concluded that hyperactivation is a predictive marker in MCI while MTL hypoactivation occurs at a later stage of the disease. Bookheimer and colleagues (2000) also found greater magnitude/extent of blood oxygenation levels in a group of neurologically normal elders with a genetic risk for AD (carriers of APOE ε4 allele), in regions normally affected by AD.
Potential Consequences of WMHs
Epidemiological studies have recently revealed that cardiovascular problems in midlife (e.g., hypertension) increase the likelihood of developing dementia, including AD, later in life. There is considerable evidence now that in most elderly WMHs are strongly associated with cardiovascular disease (Kuo and Lipsitz, 2004). There is also strong evidence that hypertension and type 2 diabetes are major risk factors for severe WMHs (Artero et al., 2004; Cook et al., 2002; Dufouil et al., 2001; Inzitari, 2000; Kuo and Lipsitz, 2004). And finally, there is strong evidence that the presence of WMHs is associated with cognitive decline. The severity of decline relates to the density and locations of the lesions (Awad et al., 2004; De Groot et al., 2002; Inzitari, 2000; Kuo and Lipsitz, 2004; Manschot et al., 2006). WMHs are more abundant in frontal regions and are associated with frontal hypometabolism, prolonged processing times and executive dysfunction (Gunning-Dixon and Raz, 2000; Tullberg et al., 2004). Therefore, WMHs associated with hypertension frequently target prefrontal lobes, similar to the progression of aging deficits, which affect executive control processes such as attention, working memory, and response inhibition (D’Esposito et al., 1995; Frackowiak, 1986; Hoffman, 1997). A recent longitudinal study concluded: 1) that one likely contributing factor to declining performance in healthy elderly is ischemic WM disease and 2) that reducing cardiovascular risk factors such as high blood pressure and diabetes may be an important step toward optimizing cognitive stability (Kramer et al., 2007). Another recent study sought to compare the distribution of WMHs in participants diagnosed with MCI/AD and healthy controls and found greater WMH volumes in MCI/AD, relative to healthy controls, similar to our results, and the spatial distribution pattern was the same across groups (Holland et al., 2008). Since our sample of MCI/AD and our participants with WMHs overlapped significantly it will be important in future studies to examine a group with WMHs who are not diagnosed as MCI or probable AD to carefully examine a potential link between WMHs and MCI or AD. At present, it seems plausible that since prefrontal cortex is a collection of interconnected neocortical areas that sends and receives projections from virtually all cortical sensory systems, motor systems, and many subcortical structures (Miller and Cohen, 2001), disconnection of functionally related cortical and subcortical areas, caused by fiber tract demyelination and gliosis is a likely explanation for the cognitive impairment (Charlton et al., 2006; Inzitari, 2000).
Bartzokis (2003) and others (Braak et al., 2000; Roher et al., 2002) have already noted the possibility that myelin breakdown may be a contributing factor to AD and that frontal and temporal regions are most vulnerable since they are the last to myelinate. Braak and colleagues (2000) suggest that unmyelinated or incompletely myelinated axons are subjected to chronically high energy turnovers and as a result are more susceptible to influences of oxidative stress. It has been hypothesized that the loss of myelin would require a 5000-fold increase in neuronal energy expenditure to maintain neurotransmission levels (Nieuwenhuys, 1999). Is increased metabolic rate underlying the observed paradoxical increased amplitudes for both MCI/AD and the group with WMHs in anterior temporal regions? Qualitatively speaking, abnormally high MEG single trial activity occurs most frequently in frontal and temporal lobes as well. We intend to quantify this pattern in the near future.
We intentionally created a heterogeneous population of elderly by adding mild pathological cases to this group in order to demonstrate that different patterns of brain activity can be identified which correlate with behavioral performance measures and cognitive ability in general. Possible linkages between hypertension/type 2 diabetes and WMHs and even MCI/AD have been offered in this report because most neuroimaging studies of aging do not discard participant’s data that reveal greater than normal WMHs nor is this issue even discussed. Similarly, some studies will note that hypertensive subjects’ data were included in the analysis. The irony is that it is highly unlikely that investigators would knowingly include participants with diagnoses of MCI or AD into a group of normal elderly. Yet, many participants with moderate to severe cases of white matter ischemia do suffer from cognitive decline and some even have preclinical symptoms of AD, but their data may be unknowingly included in the elderly group. An interesting study by Haley and colleagues (2008) found reduced blood oxygenation levels in right inferior parietal cortex and inferior temporal gyrus for a group of healthy young adults (18–40 years) with a positive family history of hypertension, during a visuospatial 2-back working memory task. They concluded that family history of hypertension may be associated with subclinical dysfunction of visuospatial working memory performance. This group (Haley et al., 2007) already demonstrated a linkage between cardiovascular disease and lower levels of BOLD response to cognitive challenges, and lower levels of behavioral performance. In general, a group of randomly selected normal elderly will be more heterogeneous than a group of 20–29 year olds and the health of some elderly will be compromised (including cases of sub-clinical AD, hypertension and type 2 diabetes).
In conclusion, we have provided evidence that: 1) all elderly do not approach an auditory verbal recognition task in the same way and some combinations of brain regions or networks are more effective than others—the most effective network in this study did not include prefrontal cortex; 2) our MCI/AD sub-group tended to reveal either no activity in anterior temporal lobe or when they did, they revealed significantly higher amplitudes in both anterior temporal and premotor regions compared to controls—this enhanced activity was correlated with lower IQs and poorer performance on verbal/visual memory tests; 3) most of our MCI/AD group revealed moderate to severe abnormalities on their MRIs—these abnormalities correlated with poorer performance; 4) the group with WMHs resembled our MCI/AD group in terms of revealing increased amplitudes in anterior temporal regions and poor performance; and 5) reliance on self-report for health status of the elderly controls is inadequate—28% of our elderly controls had moderate to severe MRI abnormalities, even though we attempted to recruit healthy elderly. The literature provides very strong links now between WMHs and hypertension, diabetes and atherosclerosis. We have begun examining non-demented, middle-aged and elderly with hypertension and/or type 2 diabetes using FLAIR image sequences to quantify WMHs and blood tests to characterize the health of these patients, aiming to further investigate the structure-function relationships indicated by the present results. But, at present, these results suggest that one cannot rely on participants’ self-report as evidence of health in normal aging studies. On some occasions participants have suspected that they may have a memory problem and enrolled in the study to see if it was true. Therefore, independent measures of health should be acquired and used for exclusion purposes if the goal is to compare group-averaged measures for understanding healthy aging processes.
Acknowledgments
This work was supported by Award Number R01 AG020302-04 and R01 AG029495-01 from the National Institute On Aging. The content is soley the responsibility of the authors and does not necessarily represent the official views of the National Institute On Aging or the National Institutes of Health. This work was also supported in part by the Department of Energy under Award Number DE-FG02-99ER62764 to the Mind Research Network, the Radiology Department at UNM SOM, DHHS/NIH/NCRR/GCRC 5M01-RR-00997 awarded to UNM HSC, and the Research Service at the New Mexico VA Health Care System. We thank Megan Schendel for her help in acquiring the MEG data, Laura Lundy for her help with neuropsychological testing, and Selma Supek for her insightful comments on an earlier version of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aine C, Adair J, Knoefel J, Hudson D, Qualls C, Kovacevic S, Woodruff C, Cobb W, Padilla D, Lee R, et al. Temporal dynamics of age-related differences in auditory incidental verbal learning. Cognitive Brain Research. 2005;24:1–18. doi: 10.1016/j.cogbrainres.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Aine C, Huang M, Stephen J, Christner R. Multistart algorithms for MEG empirical data analysis reliably characterize locations and time courses of multiple sources. Neuroimage. 2000;12:159–72. doi: 10.1006/nimg.2000.0616. [DOI] [PubMed] [Google Scholar]
- Aine CJ, Stephen JM, Christner R, Hudson D, Best E. Task relevance enhances early transient and late slow-wave activity of distributed cortical sources. J Comput Neurosci. 2003;15:203–21. doi: 10.1023/a:1025864825200. [DOI] [PubMed] [Google Scholar]
- Aine CJ, Woodruff CC, Knoefel JE, Adair JC, Hudson D, Qualls C, Bockholt J, Best E, Kovacevic S, Cobb W, et al. Aging: Compensation or Maturation? NeuroImage. 2006;32:1891–1904. doi: 10.1016/j.neuroimage.2006.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artero S, Tiemeier H, Prins ND, Sabatier R, Breteler MM, Ritchie K. Neuroanatomical localisation and clinical correlates of white matter lesions in the elderly. J Neurol Neurosurg Psychiatry. 2004;75:1304–8. doi: 10.1136/jnnp.2003.023713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad N, Gagnon M, Messier C. The relationship between impaired glucose tolerance, type 2 diabetes, and cognitive function. J Clin Exp Neuropsychol. 2004;26:1044–80. doi: 10.1080/13803390490514875. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Nuechterlein KH, Edwards N, Mintz J. Age-related changes in frontal and temporal lobe volumes in men: a magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58:461–5. doi: 10.1001/archpsyc.58.5.461. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Cummings JL, Sultzer D, Henderson VW, Nuechterlein KH, Mintz J. White matter structural integrity in healthy aging adults and patients with Alzheimer disease: a magnetic resonance imaging study. Arch Neurol. 2003;60:393–8. doi: 10.1001/archneur.60.3.393. [DOI] [PubMed] [Google Scholar]
- Benes FM, Turtle M, Khan Y, Farol P. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Arch Gen Psychiatry. 1994;51:477–84. doi: 10.1001/archpsyc.1994.03950060041004. [DOI] [PubMed] [Google Scholar]
- Bevington P. Data reduction and error analysis for the physical sciences. New York: McGraw-Hill; 1969. [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Springer JA, Kaufman JN, Possing ET. Human temporal lobe activation by speech and nonspeech sounds. Cereb Cortex. 2000;10:512–28. doi: 10.1093/cercor/10.5.512. [DOI] [PubMed] [Google Scholar]
- Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, Small GW. Patterns of brain activation in people at risk for Alzheimer’s disease. N Engl J Med. 2000;343:450–6. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bor D, Duncan J, Wiseman RJ, Owen AM. Encoding strategies dissociate prefrontal activity from working memory demand. Neuron. 2003;37:361–7. doi: 10.1016/s0896-6273(02)01171-6. [DOI] [PubMed] [Google Scholar]
- Bozzali M, Filippi M, Magnani G, Cercignani M, Franceschi M, Schiatti E, Castiglioni S, Mossini R, Falautano M, Scotti G, et al. The contribution of voxel-based morphometry in staging patients with mild cognitive impairment. Neurology. 2006;67:453–60. doi: 10.1212/01.wnl.0000228243.56665.c2. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Schultz C, Braak E. Vulnerability of select neuronal types to Alzheimer’s disease. Ann N Y Acad Sci. 2000;924:53–61. doi: 10.1111/j.1749-6632.2000.tb05560.x. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Charlton RA, Barrick TR, McIntyre DJ, Shen Y, O’Sullivan M, Howe FA, Clark CA, Morris RG, Markus HS. White matter damage on diffusion tensor imaging correlates with age-related cognitive decline. Neurology. 2006;66:217–22. doi: 10.1212/01.wnl.0000194256.15247.83. [DOI] [PubMed] [Google Scholar]
- Cohen L, Jobert A, Le Bihan D, Dehaene S. Distinct unimodal and multimodal regions for word processing in the left temporal cortex. Neuroimage. 2004;23:1256–70. doi: 10.1016/j.neuroimage.2004.07.052. [DOI] [PubMed] [Google Scholar]
- Cook IA, Leuchter AF, Morgan ML, Conlee EW, David S, Lufkin R, Babaie A, Dunkin JJ, O’Hara R, Simon S, et al. Cognitive and physiologic correlates of subclinical structural brain disease in elderly healthy control subjects. Arch Neurol. 2002;59:1612–20. doi: 10.1001/archneur.59.10.1612. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The posterior-anterior shift in aging. Cereb Cortex. 2008;18:1201–9. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groot JC, De Leeuw FE, Oudkerk M, Van Gijn J, Hofman A, Jolles J, Breteler MM. Periventricular cerebral white matter lesions predict rate of cognitive decline. Ann Neurol. 2002;52:335–41. doi: 10.1002/ana.10294. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Dehaene S, Anton JL, Campagne A, Ciuciu P, Dehaene GP, Denghien I, Jobert A, Lebihan D, Sigman M, et al. Functional segregation of cortical language areas by sentence repetition. Hum Brain Mapp. 2006;27:360–71. doi: 10.1002/hbm.20250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Esposito M, Detre JA, Alsop DC, Shin RK, Atlas S, Grossman M. The neural basis of the central executive system of working memory. Nature. 1995;378:279–81. doi: 10.1038/378279a0. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Sperling RA. Functional abnormalities of the medial temporal lobe memory system in mild cognitive impairment and Alzheimer’s disease: insights from functional MRI studies. Neuropsychologia. 2008;46:1624–35. doi: 10.1016/j.neuropsychologia.2007.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dror IE, Schmitz-Williams IC, Smith W. Older adults use mental representations that reduce cognitive load: mental rotation utilizes holistic representations and processing. Exp Aging Res. 2005;31:409–20. doi: 10.1080/03610730500206725. [DOI] [PubMed] [Google Scholar]
- Dufouil C, de Kersaint-Gilly A, Besancon V, Levy C, Auffray E, Brunnereau L, Alperovitch A, Tzourio C. Longitudinal study of blood pressure and white matter hyperintensities: the EVA MRI Cohort. Neurology. 2001;56:921–6. doi: 10.1212/wnl.56.7.921. [DOI] [PubMed] [Google Scholar]
- Frackowiak RSJ. Measurement and imaging of cerebral function in ageing and dementia. In: Swaab D, Fliers E, Mirmiran M, Gool Wv, Haaren Fv, editors. Progress in Brain Research. 1986. pp. 69–85. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Price CJ. Degeneracy and redundancy in cognitive anatomy. Trends Cogn Sci. 2003;7:151–152. doi: 10.1016/s1364-6613(03)00054-8. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, D’Esposito M. Top-down modulation and normal aging. Ann N Y Acad Sci. 2007;1097:67–83. doi: 10.1196/annals.1379.010. [DOI] [PubMed] [Google Scholar]
- Grady C, McIntosh A, Craik F. Age-related differences in the functional connectivity of the hippocampus during memory encoding. Hippocampus. 2003;13:572–586. doi: 10.1002/hipo.10114. [DOI] [PubMed] [Google Scholar]
- Grady CL. Age-related differences in face processing: a meta-analysis of three functional neuroimaging experiments. Can J Exp Psychol. 2002;56:208–20. doi: 10.1037/h0087398. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology. 2000;14:224–32. doi: 10.1037//0894-4105.14.2.224. [DOI] [PubMed] [Google Scholar]
- Habeck C, Rakitin BC, Moeller J, Scarmeas N, Zarahn E, Brown T, Stern Y. An event-related fMRI study of the neural networks underlying the encoding, maintenance, and retrieval phase in a delayed-match-to-sample task. Brain Res Cogn Brain Res. 2005;23:207–20. doi: 10.1016/j.cogbrainres.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Haley AP, Gunstad J, Cohen RA, Jerskey BA, Mulligan RC, Sweet LH. Neural correlates of visuospatial working memory in healthy young adults at risk for hypertension. Brain Imaging and Behavior. 2008;2:192–199. [Google Scholar]
- Haley AP, Sweet LH, Gunstad J, Forman DE, Poppas A, Paul RH, Tate DF, Cohen RA. Verbal working memory and atherosclerosis in patients with cardiovascular disease: an fMRI study. J Neuroimaging. 2007;17:227–33. doi: 10.1111/j.1552-6569.2007.00110.x. [DOI] [PubMed] [Google Scholar]
- Head D, Buckner RL, Shimony JS, Williams LE, Akbudak E, Conturo TE, McAvoy M, Morris JC, Snyder AZ. Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the Alzheimer type: evidence from diffusion tensor imaging. Cereb Cortex. 2004;14:410–23. doi: 10.1093/cercor/bhh003. [DOI] [PubMed] [Google Scholar]
- Herwig U, Abler B, Schonfeldt-Lecuona C, Wunderlich A, Grothe J, Spitzer M, Walter H. Verbal storage in a premotor-parietal network: evidence from fMRI-guided magnetic stimulation. Neuroimage. 2003;20:1032–41. doi: 10.1016/S1053-8119(03)00368-9. [DOI] [PubMed] [Google Scholar]
- Hoffman JM. Positron emission tomorgraphy studies in dementia. In: Krishnan K, Doraiswamy P, editors. Brain Imaging in Clinical Psychiatry. New York: Marcel Dekker, Inc; 1997. pp. 533–573. [Google Scholar]
- Holland CM, Smith EE, Csapo I, Gurol ME, Brylka DA, Killiany RJ, Blacker D, Albert MS, Guttmann CR, Greenberg SM. Spatial distribution of white-matter hyperintensities in Alzheimer disease, cerebral amyloid angiopathy, and healthy aging. Stroke. 2008;39:1127–33. doi: 10.1161/STROKEAHA.107.497438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M, Aine CJ, Supek S, Best E, Ranken D, Flynn ER. Multi-start downhill simplex method for spatio-temporal source localization in magnetoencephalography. Electroencephalogr Clin Neurophysiol. 1998;108:32–44. doi: 10.1016/s0168-5597(97)00091-9. [DOI] [PubMed] [Google Scholar]
- Inzitari D. Age-related white matter changes and cognitive impairment. Ann Neurol. 2000;47:141–3. [PubMed] [Google Scholar]
- Jagust WJ. Neuroimaging in normal aging and dementia. In: Albert M, Knoefel J, editors. Clinical Neurology of Aging. 2. New York: Oxford University Press; 1994. pp. 190–213. [Google Scholar]
- Katzman R. Alzheimer’s disease. N Engl J Med. 1986;314:964–73. doi: 10.1056/NEJM198604103141506. [DOI] [PubMed] [Google Scholar]
- Kivipelto M, Helkala EL, Hanninen T, Laakso MP, Hallikainen M, Alhainen K, Soininen H, Tuomilehto J, Nissinen A. Midlife vascular risk factors and late-life mild cognitive impairment: A population-based study. Neurology. 2001;56:1683–9. doi: 10.1212/wnl.56.12.1683. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Mungas D, Reed BR, Wetzel ME, Burnett MM, Miller BL, Weiner MW, Chui HC. Longitudinal MRI and cognitive change in healthy elderly. Neuropsychology. 2007;21:412–8. doi: 10.1037/0894-4105.21.4.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo HK, Lipsitz LA. Cerebral white matter changes and geriatric syndromes: is there a link? J Gerontol A Biol Sci Med Sci. 2004;59:818–26. doi: 10.1093/gerona/59.8.m818. [DOI] [PubMed] [Google Scholar]
- Lezak M. Neuropsychological Assessment. New York: Oxford University Press; 1995. [Google Scholar]
- Manschot SM, Brands AM, van der Grond J, Kessels RP, Algra A, Kappelle LJ, Biessels GJ. Brain magnetic resonance imaging correlates of impaired cognition in patients with type 2 diabetes. Diabetes. 2006;55:1106–13. doi: 10.2337/diabetes.55.04.06.db05-1323. [DOI] [PubMed] [Google Scholar]
- Mei L, Chen C, Xue G, He Q, Li T, Xue F, Yang Q, Dong Q. Neural predictors of auditory word learning. Neuroreport. 2008;19:215–9. doi: 10.1097/WNR.0b013e3282f46ea9. [DOI] [PubMed] [Google Scholar]
- Meulenbroek O, Petersson KM, Voermans N, Weber B, Fernandez G. Age differences in neural correlates of route encoding and route recognition. Neuroimage. 2004;22:1503–14. doi: 10.1016/j.neuroimage.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mosher JC, Lewis PS, Leahy RM. Multiple dipole modeling and localization from spatio-temporal MEG data. IEEE Trans Biomed Eng. 1992;39:541–57. doi: 10.1109/10.141192. [DOI] [PubMed] [Google Scholar]
- Muller NG, Knight RT. The functional neuroanatomy of working memory: contributions of human brain lesion studies. Neuroscience. 2006;139:51–8. doi: 10.1016/j.neuroscience.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R. Structure and organization of fibre systems. In: Nieuwenhuys R, Tendokelaar HJ, Nicholson C, editors. The Central Nervous System of Vertebrates. Berlin: Springer; 1999. pp. 113–157. [Google Scholar]
- Ojemann G, Ojemann J, Lettich E, Berger M. Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. J Neurosurg. 1989;71:316–26. doi: 10.3171/jns.1989.71.3.0316. [DOI] [PubMed] [Google Scholar]
- Palermo D, Jenkins J. Word Association Norms. Minneapolis: University of Minnesota Press; 1964. p. 156. [Google Scholar]
- Persson J, Nyberg L, Lind J, Larsson A, Nilsson LG, Ingvar M, Buckner RL. Structure-function correlates of cognitive decline in aging. Cereb Cortex. 2006;16:907–15. doi: 10.1093/cercor/bhj036. [DOI] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–94. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Petrides M. Frontal lobes and behaviour. Curr Opin Neurobiol. 1994;4:207–11. doi: 10.1016/0959-4388(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Postle BR, Berger JS, D’Esposito M. Functional neuroanatomical double dissociation of mnemonic and executive control processes contributing to working memory performance. Proc Natl Acad Sci U S A. 1999;96:12959–64. doi: 10.1073/pnas.96.22.12959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Friston KJ. Degeneracy and cognitive anatomy. Trends Cogn Sci. 2002;6:416–421. doi: 10.1016/s1364-6613(02)01976-9. [DOI] [PubMed] [Google Scholar]
- Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4:487–99. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- Ranken D, Best E, Schmidt DM, George JS, Wood CC, Huang M. In: Nowak H, Jaueisen J, Giebler F, Huonker R, editors. MEG/EEG forward and inverse modeling using MRIVIEW; Proceedings of the 13th International Conference on Biomagnetism; 2002. pp. 785–787. [Google Scholar]
- Ranken D, George JS. MRIVIEW: an interactive computational tool for investigation of brain structure and function. Proceedings of the IEEE Visualization ‘93: IEEE Computer Society Press; 1993. pp. 324–331. [Google Scholar]
- Ranken DM, Stephen JM, George JS. MUSIC seeded multi-dipole MEG modeling using the Constrained Start Spatio-Temporal modeling procedure. Neurol Clin Neurophysiol. 2004;2004:80. [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Haacke EM. Brain aging and its modifiers: insights from in vivo neuromorphometry and susceptibility weighted imaging. Ann N Y Acad Sci. 2007;1097:84–93. doi: 10.1196/annals.1379.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roher AE, Weiss N, Kokjohn TA, Kuo YM, Kalback W, Anthony J, Watson D, Luehrs DC, Sue L, Walker D, et al. Increased A beta peptides and reduced cholesterol and myelin proteins characterize white matter degeneration in Alzheimer’s disease. Biochemistry. 2002;41:11080–90. doi: 10.1021/bi026173d. [DOI] [PubMed] [Google Scholar]
- Rypma B, Berger JS, D’Esposito M. The influence of working-memory demand and subject performance on prefrontal cortical activity. J Cogn Neurosci. 2002;14:721–31. doi: 10.1162/08989290260138627. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Schmidt H, Fazekas F. Vascular risk factors in dementia. J Neurol. 2000;247:81–7. doi: 10.1007/s004150050021. [DOI] [PubMed] [Google Scholar]
- Smith CD, Chebrolu H, Wekstein DR, Schmitt FA, Jicha GA, Cooper G, Markesbery WR. Brain structural alterations before mild cognitive impairment. Neurology. 2007;68:1268–73. doi: 10.1212/01.wnl.0000259542.54830.34. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–15. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Stephen JM, Ranken D, Aine CJ. Frequency-following and connectivity of different visual areas in response to contrast-reversal stimulation. Brain Topogr. 2006a;18:257–272. doi: 10.1007/s10548-006-0004-z. [DOI] [PubMed] [Google Scholar]
- Stephen JM, Ranken D, Best E, Adair J, Knoefel J, Kovacevic S, Padilla D, Hart B, Aine CJ. Aging changes and gender differences in response to median nerve stimulation measured with MEG. Clin Neurophysiol. 2006b;117:131–143. doi: 10.1016/j.clinph.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Supek S, Aine C. Spatio-temporal modeling of neuromagnetic data: I. Multisource location vs. timecourse estimation accuracy. Human Brain Mapping. 1997;5:139–53. doi: 10.1002/(SICI)1097-0193(1997)5:3<139::AID-HBM1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Supek S, Aine CJ. Simulation studies of multiple dipole neuromagnetic source localization: model order and limits of source resolution. IEEE Trans Biomed Eng. 1993;40:529–40. doi: 10.1109/10.237672. [DOI] [PubMed] [Google Scholar]
- Thorndike E, Lorge I. The Teacher’s Word Book of 30,000 Words. Oxford: Bureau of Publications, Teachers Co; 1944. [Google Scholar]
- Tullberg M, Fletcher E, DeCarli C, Mungas D, Reed BR, Harvey DJ, Weiner MW, Chui HC, Jagust WJ. White matter lesions impair frontal lobe function regardless of their location. Neurology. 2004;63:246–53. doi: 10.1212/01.wnl.0000130530.55104.b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington E. Recognition memory test. Windsor: NFER-Nelson; 1984. [Google Scholar]
- Whitwell JL, Petersen RC, Negash S, Weigand SD, Kantarci K, Ivnik RJ, Knopman DS, Boeve BF, Smith GE, Jack CR., Jr Patterns of atrophy differ among specific subtypes of mild cognitive impairment. Arch Neurol. 2007;64:1130–8. doi: 10.1001/archneur.64.8.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willott J. Neurogerontology: The aging nervous system. In: Ferraro K, editor. Gerontology: Perspectives and Issues. Springer; 1997. pp. 68–96. [Google Scholar]