Abstract
This study investigated the effects of acute psychosocial stress on trapezius single motor unit discharge behaviors. Twenty-one healthy women performed feedback-controlled isometric contractions under conditions of low and high psychosocial stress in the same experimental session. Psychosocial stress was manipulated using a verbal math task combined with social evaluative threat which significantly increased perceived anxiety, heart rate, and blood pressure (P<0.001). Motor unit discharge behaviors including the threshold and discharge rate at recruitment (7.7 (5.7) %MVC and 7.3 (6.8) pps, P>0.121, N=103) and derecruitment (6.0(4.4) %MVC and 6.5(4.1) pps, P>0.223, N=99), the mean (11.3 (2.3) pps, P=0.309, N=106) and variability (2.5 (0.91) pps, P=0.958, N=106) of discharge rate, and the proportion of motor units exhibiting double discharges (21%, P=0.446) did not change across stress conditions. Discharge rate modulation with changes in contraction intensity was highly variable and similar across stress conditions (P>0.308, N=89). Rate-rate modulation of concurrently active motor units was also highly variable (r=−0.84–1.00, N=75). Estimates of ΔF for motor unit pairs with rate-rate modulation ≥0.7 were positive and similar across stress conditions (4.7(2.0) pps, P=0.405, N=16). Results indicate that acute psychosocial stress does not alter trapezius motor unit discharge behaviors during a precisely controlled motor task in healthy women.
Keywords: Motoneuron, Anxiety, Discharge rate modulation, Intrinsic activation, EMG, Muscle activation
Introduction
Musculoskeletal disorders have a high prevalence in Western society, with the neck region identified as one of the most common sites of pain (Cote et al., 1998; Urwin et al., 1998; Picavet & Schouten, 2003). Psychosocial stress has been consistently identified as a risk factor for the development of neck pain (Bernard, 1997; Ariens et al., 2001; Andersen et al., 2003; Bongers et al., 2006) especially among women (Ostergren et al., 2005). The trapezius muscle is known to be active at low levels for prolonged periods over the course of a day (Westgaard et al., 2001; Holte & Westgaard, 2002), and this prolonged activity has been linked to the development of neck pain (Veiersted et al., 1993). Interestingly, psychosocial stress has been shown to increase activity of the trapezius muscle during intended periods of rest (Lundberg et al., 2002) and during the performance of motor tasks that are commonly encountered in the workplace (McLean & Urquhart, 2002; Nilsen et al., 2007; Schleifer et al., 2008). Some investigators have suggested that these stress-evoked increases in muscle activity may directly contribute to the development of neck pain through prolonged activation of low threshold motor units (Hagg, 1991; Lundberg, 2002), while others have proposed that stress may induce an autonomic response that generates parallel increases in motor activity and pain (Westgaard, 1999).
Several studies have used surface electromyography (EMG) to evaluate muscle responses to a controlled laboratory stressor during a variety of motor tasks. The majority of these studies observed global increases in trapezius muscle activity during acute stress exposure (Lundberg et al., 1994; Laursen et al., 2002; Lundberg et al., 2002; McLean & Urquhart, 2002; Visser et al., 2004; Nilsen et al., 2007; Schleifer et al., 2008). Although one study reported that stress did not alter the amplitude of trapezius muscle activity, the ability of the muscle to relax was impaired under stressful conditions (Blangsted et al., 2004). Despite multiple observations of elevated surface EMG in response to stress, there has been limited research on changes in the discharge behaviors of low threshold motor units that underlie this motor response. Lundberg et al. (2002) reported that the same motor units were recruited during contractions performed in the presence and absence of mental stress for a majority of subjects, and Westgaard et al. (2006) observed that respiratory modulation of discharge rates was less common in motor units activated by mental stress than in motor units active during voluntary contractions. However, neither of these studies investigated changes in motor unit recruitment and derecruitment thresholds, discharge rates, or discharge rate variability which might be expected to contribute to previously observed increases in surface EMG (Lundberg et al., 1994; Lundberg et al., 2002; McLean & Urquhart, 2002; Nilsen et al., 2007; Schleifer et al., 2008) and force fluctuations (Noteboom et al., 2001a; Noteboom et al., 2001b; Christou et al., 2004) in response to stress. One study found no difference in the mean discharge rate of a small sample of trapezius motor units examined during a typing task performed with and without mental stress (Westad et al. 2004). This study also reported atypical discharge behaviors including limited modulation of discharge rates with changes in contraction intensity, however, these behaviors were not compared between low and high stress conditions. Other studies have reported atypical discharge behavior of trapezius motor units studied in the absence of stress, including limited discharge rate modulation (Westgaard & De Luca, 2001; Westad et al., 2004) and abrupt changes in recruitment threshold (Westad et al., 2003). The extent to which these motor unit discharge behaviors are affected by exposure to acute psychosocial stress is not known. Therefore, the aim of this study was to compare the discharge behaviors of low threshold trapezius motor units during exposure to low and high levels of acute psychosocial stress. We expected to find that psychosocial stress would exacerbate atypical discharge behaviors in trapezius motor units, and alter patterns of recruitment and rate coding consistent with the previously observed effects of psychosocial stress on surface EMG recordings in the trapezius muscle. Preliminary findings from this investigation have been published in abstract form (Stephenson & Maluf, 2008).
Methods
Twenty-seven healthy women ranging from 23 to 57 years of age (mean (SD) 37.4 (10.9) years) volunteered to participate in the study. All subjects were free of neck pain at the time of the study and reported no history of neck pain and no other neurological or orthopedic impairment in the upper limbs. Subjects were excluded if they reported that they were pregnant, or that they had a recent history of heart problems, high blood pressure, or cognitive or psychological disorders. All subjects provided informed consent in accordance with the guidelines of the Colorado Multiple Institutional Review Board.
Experimental setup
Subjects were seated upright with their hip and knee joints flexed at approximately 90 degrees and their feet resting comfortably on a foot rest. The arms were abducted approximately 45 degrees and positioned in line with the trunk, with the elbows flexed and the forearms supported on arm rests parallel to the floor. Custom made restraints were placed over each shoulder. Each restraint consisted of a padded force transducer mounted on a vertical metal restraint that was adjusted to fit securely over the acromion with the shoulders in a relaxed position. Elevation of the shoulders exerted a compressive force on the restraints. The force exerted by the left shoulder was displayed in real-time on a feedback screen positioned at eye level approximately one meter in front of the subject.
Experimental Protocol
Subjects participated in an initial familiarization session to practice the motor tasks and become accustomed to the laboratory setting, and then returned within two weeks for a single experimental session during which all data were collected. During the experimental session subjects performed a maximal voluntary isometric contraction (MVC) in which they were instructed to gradually increase shoulder elevation force from zero to maximum over a period of 3 s, and then maintain maximal force for 2–3 s while receiving strong verbal encouragement. Subjects performed several trials, separated by at least 60 s of rest, until peak forces from two trials agreed within 5%. The greater of these two values was considered the shoulder elevation MVC force. After several minutes rest the subjects then performed submaximal test contractions whereby they were required to steadily increase and decrease the intensity of the isometric contraction, matching the shoulder elevation force to a triangular template displayed on the feedback screen. The specific dimensions of the triangular template were determined individually for each subject to optimize recordings from identified motor units, with peak elevation force ranging from 4 to 48 % MVC force (mean (SD) 16 (12) %MVC) and the rate of force increase ranging from 1.3 to 7.9 %MVC/s (mean (SD) 3.0 (1.5) %MVC/s). After customizing the dimensions of the force template for each subject, the same template was used throughout all test conditions for that subject. Subjects were instructed to perform all tasks bilaterally, but force data were collected and displayed from the left side only. One block of test contractions consisted of five triangular templates, each separated by 60 s rest. Subjects were instructed to relax their shoulders at the start of each condition and during all rest periods between voluntary contractions. Subjects performed one block of test contractions under low stress conditions (low pre) followed by one block under high stress conditions (high). Immediately after the high stress condition, subjects were fully debriefed and asked to rest quietly until heart rate and mean arterial blood pressure recovered to pre stress levels (≥ 15 min). Following this recovery period, subjects repeated a block of test contractions under low stress conditions (low post).
Stress Manipulation
Levels of psychosocial stress were manipulated using a verbal math task (Noteboom et al., 2001b; Lundberg et al., 2002) combined with social evaluative threat (Dickerson & Kemeny, 2004). Subjects were informed that the purpose of the study was to examine the effects of mental concentration and therefore remained naïve to the stress manipulation until the debriefing that followed the high stress condition. To control for attention effects during the low stress condition, subjects were asked to verbally count backwards by five from a four-digit number that was evenly divisible by five. Subjects were told that these were practice trials in which their performance would not be monitored. Encouragement and positive feedback were provided during the low stress condition regardless of actual performance. This block of trials was then followed by the high stress condition in which the difficulty of the math task was increased by asking the subject to verbally count backwards by a randomly selected one- or two-digit number from a randomly selected four-digit number. Prior to this condition, subjects were informed that it was extremely important to perform the math test as fast and accurately as possible. Subjects were instructed that their performance would be videotaped and graded against other participants, and were offered a monetary incentive for good performance. The high stress condition was administered by a different examiner with whom the subject was not familiar, and no positive feedback was provided regardless of performance. Immediately after completing the high stress condition all subjects were fully debriefed regarding the purpose of the stress manipulation. Subjects were assured that their performance had not been permanently recorded on videotape, and that they would receive the full monetary compensation regardless of performance. After the debriefing and recovery period (≥ 15 min) subjects repeated the low stress condition.
Data acquisition
The vertical force exerted during shoulder elevation was measured during all contractions using a force transducer (1112 N range; 7.6 mV/N; P310, Cooper Instruments, Warrenton, VA, USA) positioned over the acromion, A/D converted at 100 samples/s (Power 1401, 16-bit resolution, Cambridge Electronic Design, Cambridge, UK) and displayed as real-time feedback of contraction intensity on a monitor placed at eye-level in front of the subject. The interference electromyography (EMG) signal from the left upper trapezius muscle was recorded using biploar surface electrodes (silver-silver chloride; 8 mm electrode diameter; In Vivo Metric, Healdsburg, CA, USA). Electrodes were placed with 15 mm interelectrode distance, centered 20 mm lateral to the midpoint between C7 and the posterior lateral border of the acromion, and the reference electrode was placed over a bony portion of the clavicle. Bioamplifiers (Coulbourn Instruments, Allentown, PA, USA) were used to amplify (×1000) and band-pass filter (13–1000 Hz) the surface EMG signals prior to sampling and storage at 2000 samples/s.
Single motor unit activity was recorded from the left upper trapezius muscle during the test contractions using a bipolar intramuscular electrode. Intramuscular electrodes were custom made and consisted of two Formvar-insulated stainless steel wires (50 µm diameter; California Fine Wire Co., Grover Beach, CA, USA) with the cross sectional area exposed for recording. After the subject had performed the MVC contractions a 30-gauge needle was used to insert the wires between the two surface electrodes placed over the left trapezius muscle. The needle was removed after placement of the intramuscular wires, and the signals were examined online to verify the detection of motor unit action potentials. If necessary, small adjustments were made to the placement of the wires to enhance signal quality. The reference electrode for the intramuscular recordings was a surface electrode placed over a bony portion of the clavicle. Intramuscular EMG signals were amplified (×1000) and band-pass filtered (20–8000 Hz) prior to sampling and storage at 20000 samples/s.
Physiologic arousal was assessed at the beginning of the experimental session (baseline) and at the end of each block of test contractions using an automated oscillometric cuff (Coulbourn V series module) placed around the right arm to measure heart rate and blood pressure. Perceived anxiety was assessed at baseline and at the end of each block of test contractions using the state anxiety portion of the Spielberger State-Trait Anxiety Index (STAI; Speilberger et al. 1970).
Data analysis
An example of outcome variables calculated from data collected during the experimental session is presented in Fig. 1. All offline analyses were performed with custom software written in Matlab (v7.5.0.342, MathWorks Inc., Natick, MA, USA). Consistent with previous studies of the trapezius muscle (e.g. Westad et al. 2003; Westgaard and De Luca 2001; 1999) surface EMG rather than force was used as an indication of contraction intensity in the trapezius muscle because several muscle groups potentially contribute to net shoulder elevation force (Hamilton & Luttgens, 2002). Surface EMG values were root mean square (RMS) processed using a 0.5 s window. Maximal EMG was calculated as the RMS amplitude of the surface EMG signal over a 0.5 s interval centered about the peak surface EMG achieved in the MVC trials, and the RMS amplitude of surface EMG during the test contractions was normalized to maximal EMG. The amplitude of trapezius muscle activity during each feedback-controlled triangular contraction was characterized by the peak value of the RMS processed surface EMG. Contraction and relaxation rates were calculated as the slope of the RMS surface EMG during the ascending and descending limbs of the contraction respectively (Fig. 1).
Figure 1.
Example data from one subject in the low pre condition showing, from bottom to top, shoulder elevation force (solid line) and target force (dashed line), RMS surface EMG, raw intramuscular EMG, and the instantaneous discharge rate profiles of two concurrently active motor units expressed in pulses per second (pps). Recruitment and derecruitment thresholds were assessed as the magnitude of the RMS surface EMG at the point at which regular discharge of motor unit action potentials began and ceased, as indicated by the vertical dotted lines for the lower threshold reference motor unit. The discharge rates at recruitment and derecruitment were calculated as the mean instantaneous discharge rate over the first five action potentials following recruitment and the last five motor unit action potentials preceding derecruitment respectively, as indicated by the black data points in the discharge rate profiles. Motor unit discharge behavior throughout the contraction was characterized by the mean and range of instantaneous discharge rates, with variability assessed as the RMS error from a 5th order polynomial fit to the discharge rate profile, as indicated for the reference motor unit. The PIC magnitude was estimated by ΔF, calculated as the difference between the discharge rate of the reference motor unit at the time of recruitment and derecruitment of the test motor unit. In this example the test motor unit was recruited when the reference motor unit was discharging at 12.3 pps, and derecruited when the reference motor unit was discharging at 7.3 pps, giving a ΔF of 5.0 pps (12.3 – 7.3 pps). The positive ΔF value indicates that the discharge rate of the reference motor unit was lower at test motor unit derecruitment than it was at test motor unit recruitment.
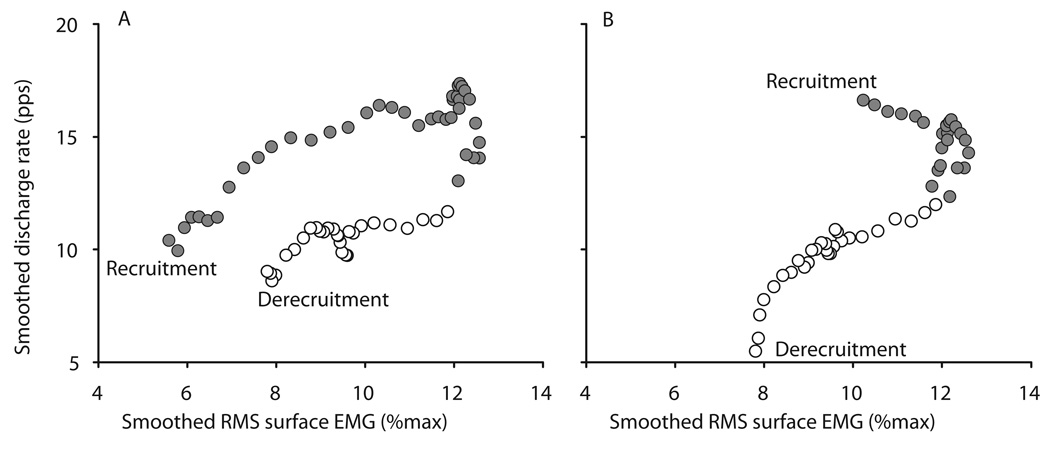
Single motor unit action potentials were discriminated from the intramuscular EMG recording using the spike-sorting algorithm in Spike2 software (v5.14; Cambridge Electronic Design, Cambridge, UK). Motor unit action potentials were automatically identified based on waveform shape and were manually verified on a spike-by-spike basis to increase discrimination accuracy. It was not possible to reliably track the same motor unit across all three test conditions, presumably due to slight movements of the intramuscular electrode between blocks of test contractions. Different motor units were therefore recorded in each condition, and were treated as independent samples. Recruitment of a motor unit was taken as the point at which regular discharge (defined as successive discharges within 600 ms) of motor unit action potentials began, and recruitment threshold was assessed as the magnitude of the RMS surface EMG at the time of recruitment. Similarly, the derecruitment threshold of the motor unit was assessed as the magnitude of the RMS surface EMG at the time at which regular discharge of motor unit action potentials ceased (see Fig. 1).
The instantaneous discharge rate of each motor unit was determined as the reciprocal of the interval between each motor unit action potential and the motor unit action potential preceding it, and was expressed in pulses per second (pps). Instantaneous discharge rates >50 pps were classified as double discharges (Partanen & Lang, 1978), and were considered to represent a distinct motor control strategy. The number of double discharges was therefore quantified as a distinct outcome, and then double discharges were removed before further analyses were performed to prevent the distortion of instantaneous discharge rate profiles by extreme values. The discharge rates at recruitment and derecruitment were calculated as the mean instantaneous discharge rate over the first five action potentials following recruitment and the last five motor unit action potentials preceding derecruitment respectively (Van Cutsem et al., 1998). Motor unit discharge behavior throughout the contraction was characterized by the mean and range of instantaneous discharge rates, with the variability assessed as the RMS error from a 5th order polynomial fit to the discharge rate profile. This measure of variability was selected because the discharge rate profile often did not change in a linear fashion across time, which precluded the use of a linear detrending procedure to calculate the coefficient of variation of the discharge rate. Similar to the procedure described by De Luca and Erim (1994), discharge rate modulation was assessed by smoothing the motor unit discharge rate profile and the RMS surface EMG or shoulder elevation force over 1 s intervals using a 100 ms moving window, and then quantifying the slope of the linear relation between these variables (see Fig. 4).
Figure 4.
An example from a single subject of the relation between the smoothed discharge rate profile and RMS surface EMG for two concurrently active motor units during the low pre condition. Discharge rate modulation was calculated as the slope of this relation for the ascending (filled circles) and descending (open circles) limbs of the contraction.
For the majority of trials there was more than one motor unit active during the voluntary contraction, enabling us to examine the association between the instantaneous discharge rates of concurrently active motor units. As described in detail by Gorassini et al. (2002), the discharge rate of each motor unit was averaged over consecutive 500 ms windows spanning the interval during which both motor units were active. Rate-rate modulation was then calculated as the correlation between the mean discharge rate of the two motor units within each 500ms window. Additionally, the paired motor unit analysis was used to calculate ΔF for a subset of motor unit pairs. As illustrated in Fig. 1, ΔF was calculated as the difference between the discharge rate of a lower threshold reference motor unit at the time of recruitment and derecruitment of a higher threshold test motor unit (Bennett et al., 2001; Gorassini et al., 2002; Gorassini et al., 2004). The discharge rate of the reference motor unit was smoothed with a 5th order polynomial prior to the calculation of ΔF. This analysis has been proposed as an indirect method of estimating the magnitude of intrinsic activation of the test motor unit by persistent inward currents using the discharge rate of the reference motor unit as an index of the level of synaptic input to the motoneuron pool (Gorassini et al. 2002).
Statistical analysis
Normality of data was assessed using the Kolmogorov-Smirnov test. Heart rate, mean arterial blood pressure, perceived anxiety, the mean and RMS error of instantaneous discharge rate profiles, rate-rate modulation and ΔF values were not significantly different from the normal distribution and are reported as mean (SD) in the text and tables, and as mean ± 95% CI in the figures. Measures of physiologic stress and perceived anxiety were compared across test conditions using a repeated measures one-way analysis of variance (ANOVA) for Test Condition with four levels (baseline; low pre; high; low post). Mean and RMS error of instantaneous discharge rate profiles, rate-rate modulation and ΔF values were compared across test conditions using separate independent one-way ANOVA tests for Test Condition with three levels (low pre; high; low post).
The contraction intensity, rate of change in contraction intensity, threshold and discharge rate of motor units at recruitment and derecruitment, range in instantaneous discharge rate and the discharge rate modulation of motor units with respect to RMS surface EMG and force were not normally distributed and are reported as median (interquartile range) in the text and tables. These variables were compared across test conditions using a Kruskal-Wallis test with three levels (low pre; high; low post). A separate Wilcoxon signed rank test was used for each test condition to compare discharge rate and threshold between recruitment and derecruitment, and to compare the rate of change in contraction intensity and discharge rate modulation between ascending and descending limbs of the contraction. The proportion of motor units exhibiting double discharges was compared across test conditions using a 3×2 chi-square test, and the number of double discharges exhibited by these motor units was compared using an independent one-way ANOVA for Test Condition with three levels (low pre; high; low post). The level of significance for all comparisons was set at P<0.05. When the ANOVA yielded a significant effect post hoc analyses were performed using t-tests with Bonferonni correction for multiple comparisons. All statistical analyses were performed with SPSS software (v16.0.1, Chicago, IL, USA).
Results
Of the 27 subjects enrolled in the study, five were withdrawn because reliable motor unit recordings could not be obtained and one subject withdrew consent. Results are presented from the 21 subjects for whom successful intramuscular EMG recordings were obtained. These subjects were aged 37.3 (11.1) years (range 23 – 55 years) and had a trait anxiety score of 30 (5) (range 23 – 38) on the Spielberger STAI. These characteristics do not differ from those of subjects who did not complete the study. The experimental manipulation successfully increased heart rate (P<0.001), mean arterial blood pressure (P<0.001) and perceived anxiety (P<0.001) during the high stress condition (Fig. 2). Only trials with one or more clearly identified motor units and smoothly controlled increases and decreases in RMS surface EMG in which the rate of relaxation was equal to (within 1% max EMG/s) or faster than the rate of contraction were selected for analysis. This procedure is recommended to avoid overestimating ΔF due to differences in the rate of muscle contraction and relaxation (c.f. Gorassini et al., 2002). These criteria yielded contractions from 20 subjects in the low pre condition, 17 subjects in the high condition, and 13 subjects in the low post condition. The lower number of contractions in the low post condition occurred due to the long recovery period (≥ 15 min) that preceded this condition, during which the intramuscular electrode was sometimes displaced from a successful recording area. The intensity of contractions selected for analysis was similar across test conditions, with peak RMS surface EMG values of 14.3 (9.5) %max in low pre, 17.1 (16.5) %max in high, and 16.1 (14.2) %max in low post conditions (P=0.676). The rate of change in RMS surface EMG on the ascending limb of the contraction was similar across test conditions at 1.9 (0.7), 2.4 (1.1) and 1.9 (1.5) %maxEMG/s in the low pre, high, and low post conditions respectively (P=0.534). The rate of change in RMS surface EMG on the descending limb was also similar across test conditions (1.6 (1.5), 1.8 (1.7) and 2.4 (1.8) %maxEMG/s respectively; P=0.485). There was no difference in the rate of change in RMS surface EMG between the ascending and descending limbs of the contraction for any test condition (P=0.218, 0.149 and 0.311 for low pre, high and low post respectively).
Figure 2.
Mean arterial blood pressure, heart rate and perceived anxiety at the beginning of the experimental session (baseline) and after each experimental condition. Data points represent mean ± 95% CI. Asterisks indicate significant differences across conditions (P<0.001).
Motor unit discharge behavior
Forty-four motor units were discriminated from 20 subjects in the low pre condition, 35 motor units from 17 subjects in the high condition, and 27 motor units from 13 subjects in the low post condition. The mean, range, and variability of the discharge rate for these motor units did not differ across test conditions (Table 1). Motor units were recruited at a higher contraction intensity than at which they were derecruited (P=0.002, 0.001 and 0.010 for low pre, high and low post respectively). There was a tendency for motor units to be recruited with a higher discharge rate compared to derecruitment but this was only significant for the low pre condition (P=0.008, 0.080 and 0.107 for low pre, high and low post respectively). Similar results were obtained in paired statistical analyses for a subset of 10 motor units from 8 subjects which could be reliably discriminated in both the low pre and high conditions.
TABLE 1.
Motor Unit Characteristics During Isometric Triangular Ramp Contractions Under Conditions of Low and High Stress
| Low Pre | High | Low Post | Effect of Condition |
|
|---|---|---|---|---|
| Motor unit recruitment | ||||
| Threshold (% maxEMG)* |
7.1 (5.7); N = 44 |
8.5 (5.8); N = 34 |
8.3 (7.4); N = 25 |
P = 0.223 |
| Discharge rate (pps)* | 7.9 (6.9); N = 44 |
7.5 (7.7); N = 34 |
5.7 (4.5); N = 25 |
P = 0.121 |
| Motor unit derecruitment | ||||
| Threshold (% maxEMG)* |
5.7 (4.6); N = 42 |
6.4 (5.4); N = 33 |
5.3 (4.5); N = 24 |
P = 0.324 |
| Discharge rate (pps)* | 6.5 (4.5); N = 42 |
7.4 (5.0); N = 33 |
5.6 (2.0); N = 24 |
P = 0.223 |
| Discharge rate modulation (pps/% maxEMG) | ||||
| Ascending limb* | 0.3 (0.6); N = 44 |
0.4 (0.6); N = 34 |
0.3 (0.3); N = 27 |
P = 0.738 |
| Descending limb* | 0.4 (0.4); N = 35 |
0.4 (0.7); N = 31 |
0.2 (0.6); N = 23 |
P = 0.308 |
| Discharge rate during contraction (pps) | ||||
| Mean | 11.4 (2.1); N = 44 |
11.6 (2.7); N = 35 |
10.7 (2.1); N = 27 |
P = 0.309 |
| Range* | 15.0 (7.9); N = 44 |
16.4 (10.5); N = 35 |
16.4 (11.1); N = 27 |
P = 0.837 |
| RMS error | 2.5 (1.0); N = 44 |
2.5 (0.9); N = 35 |
2.5 (0.8); N = 27 |
P = 0.958 |
These variables are non-normally distributed and are presented as median (interquartile range). All other variables are normally distributed and are presented as mean (standard deviation).
Double discharges occurred in several motor units, as illustrated for a representative subject in Fig. 3A. The proportion of motor units exhibiting double discharges did not differ across test conditions (27% in low pre, 20% in high, 15% in low post; P=0.446), nor did the number of double discharges observed in these motor units (4.8 (4.8) in low pre, 4.1 (2.5) in high and 2.8 (1.7) in low post respectively; P=0.681). There were also occurrences where a distinct jump in the discharge rate of a motor unit was observed, without similar increases in concurrently active motor units. One example of this is shown in Fig. 3B.
Figure 3.
Panel A shows an example of consecutive double discharge from a single subject during the high stress condition. The top panel shows the intramuscular EMG, with the section outlined by vertical dotted lines depicted below on an expanded timescale. The bottom panel shows the instantaneous discharge rate of the motor unit discharge, expressed in pulses per second (pps). Panel B shows an example from a different subject during the high stress condition of a discrete jump in the discharge rate of one motor unit (upper panel) while a concurrently active motor unit (lower panel) shows no change in discharge rate.
The modulation of discharge rate with respect to RMS surface EMG was highly variable, and did not differ for ascending and descending limbs of the contraction (Table 1; P=0.115, 0.318 and 0.129 for low pre, high and low post respectively). Fig. 4 shows the discharge rate modulation of two motor units that were concurrently active during the low pre condition. The motor unit recruited at 5.6 %maxEMG (Fig. 4A) showed an expected pattern of rate modulation by increasing its discharge rate with increases in contraction intensity at a rate of 0.6 pps/%maxEMG. For the descending limb of the contraction, there was a period where discharge rate modulation was minimal yet the overall slope of the relation was still positive (0.5 pps/%maxEMG). In contrast, the motor unit recruited at 10.2 %maxEMG (Fig. 4B) displayed an unexpected decrease in discharge rate with increasing contraction intensity, resulting in a discharge rate modulation of −1.0 pps/%maxEMG. Discharge rate modulation varied from negative to positive values across all three test conditions (Fig. 5A) and did not differ across test conditions (P=0.738 and P=0.308 for ascending and descending limbs of the contraction respectively; Table 1). These results did not change when discharge rate modulation was assessed with respect to shoulder elevation force rather than RMS surface EMG (P=0.694 and 0.455 for differences across test conditions on the ascending and descending limbs of the contraction respectively).
Figure 5.
Panel A shows the discharge rate modulation on the ascending (filled boxes) and descending (open boxes) limbs of the contraction for the low pre, high and low post conditions. The boundaries of the boxes represent the 25th and 75th percentiles, while the line within the box represents the median value. Error bars above and below the box indicate the 90th and 10th percentiles respectively, and individual data points outside these values are represented by filled circles. Panel B shows the rate-rate modulation (r) of concurrently active motor unit pairs from low pre (triangles), high (circles) and low post (squares) test conditions. The dashed horizontal line indicates rate-rate modulation of 0.7, the minimum value required for inclusion in ΔF calculations (see text for more details).
Thirty-six pairs of concurrently active motor units were identified from 16 subjects in the low pre condition, 20 motor unit pairs from 13 subjects in the high condition, and 19 motor unit pairs from 10 subjects in low post condition. The rate-rate modulation of these 75 motor units pairs was highly variable (Fig. 5B) and did not differ across test conditions (P=0.524). Rate-rate modulation showed no relation to the difference in recruitment threshold between the two motor units (r=−0.017; P=0.921 for low pre; r=0.059; P=0.803 for high and r=0.039; P=0.874 for low post) however it is evident from Fig. 5B than no motor unit pairs with a difference in recruitment threshold of >10 %maxEMG had rate-rate modulation of greater than moderate strength (r≥0.7; horizontal dashed line on Fig. 5B). Seventeen motor unit pairs were not eligible for the paired motor unit analysis because the lower threshold reference motor unit stopped firing before the higher threshold test motor unit. Motor unit pairs that were excluded based on this criterion were evenly distributed across low and high stress conditions, and were not significantly closer in recruitment threshold (2.9 (3.3) vs. 5.4 (4.4) %maxEMG; P=0.115) or recruitment time (1.3 (1.9) vs. 2.5 (2.0) s; P=0.314) than motor units pairs for which the higher threshold test motor unit was derecruited first. Two motor unit pairs were not eligible for the paired motor unit analysis because the test motor unit continued to fire into the rest period, and a further two pairs were excluded because the test motor unit was recruited during a phase of steep acceleration in the discharge rate of the reference motor unit. The ΔF estimates were calculated for the remaining 54 pairs corresponding to 27 pairs from 16 subjects in low pre, 13 pairs from 9 subjects in high, and 14 pairs from 7 subjects in low post. Estimates of ΔF were positive (3.9 (2.2) pps for low pre, 4.2 (2.9) pps for high and 3.1 (1.5) pps for low post) and did not differ across test conditions (P=0.443). Many of these pairs had low rate-rate modulation. Estimating ΔF only for motor unit pairs with rate-rate modulation ≥0.7, as recommended by Powers et al. (2008), required exclusion of more than two thirds of the sample (38 of 54 motor unit pairs; Fig. 5B). The resulting ΔF values remained positive (4.7 (2.0) pps) and did not differ across test conditions (P=0.405, N=16).
Discussion
The purpose of this study was to determine whether discharge behaviors of low threshold trapezius motor units are altered by acute psychosocial stress during voluntary contractions. Results confirm previous reports of atypical discharge behaviors observed in trapezius motor units under low stress conditions (Westgaard & De Luca, 2001; Westad et al., 2003; Westad et al., 2004), and further indicate that these behaviors are not altered by acute increases in stress during feedback-controlled voluntary contractions in healthy women.
Discharge Behavior of Trapezius Motor Units
The motor units examined in this study exhibited several discharge behaviors that are atypical of motor unit behavior in the more commonly studied limb muscles. The most striking of these behaviors was a highly variable pattern of discharge rate modulation. In limb muscles the discharge rate of active motor units typically increases linearly with contraction intensity (see De Luca and Erim 1994). Quantification of discharge rate modulation with respect to contraction intensity (net force and surface EMG) revealed that this stereotypical modulation was often absent in trapezius motor units, supporting previous qualitative reports of dissociation between surface EMG and motor unit discharge rate profiles (Westgaard & De Luca, 2001). The trapezius muscle is thought to consist of distinct compartments in which the motor units may be independently controlled (Falla et al., 2007; Falla & Farina, 2008a, b). Differential firing behaviors of motor units in different compartments may contribute to dissociation between the discharge rate of individual motor units and the interference EMG signal. However, we observed different patterns of discharge rate modulation for motor units active within the same location of the muscle, suggesting that the dissociation between discharge rate and contraction intensity cannot be fully explained by compartmentalization of the muscle. This is supported by low and negative values of rate-rate modulation, which indicate that motor units active in the same region of the muscle often displayed discordant patterns of discharge rate modulation. This behavior occurred even for motor units with similar recruitment thresholds which are thought to receive similar synaptic inputs (Binder et al., 1996).
We observed several instances whereby concurrently active motor units were derecruited in the same order that they were recruited, i.e. the earlier recruited (lower threshold) unit stopped firing before the later recruited (higher threshold) unit. This occurred in 17 out of 75 (23%) motor unit pairs and contravenes the size principle of orderly recruitment (Henneman, 1957) which is typically observed during isometric contractions in other muscles (De Luca & Erim, 1994). Motor units of the trapezius (Westgaard & de Luca, 1999) and distal limb muscles (Bawa et al., 2006) have been reported to display rotation, whereby a higher threshold unit is recruited and a lower threshold motor unit ceases to fire, however this has only been observed after several minutes of prolonged muscle contraction. Westad et al. (2003) reported similar changes in the threshold of trapezius motor units without direct evidence of rotation but this observation was also made during prolonged contractions. Motor unit rotation is thought to represent a motor control strategy to reduce overuse and fatigue in motor units active for a prolonged period of time (Eken, 1998; Westad et al., 2003). Our findings indicate that changes in recruitment and derecruitment order in trapezius motor units can occur during short- as well as long-duration contractions. This behavior may reflect a unique functional role for some trapezius motor units in posture (Alexander et al., 2007) or respiration (Westgaard et al., 2006). Combined with observations of discordant discharge rate modulation among concurrently active motor units, these results may indicate that trapezius motor units located within the same region of the muscle can subserve distinct functional roles.
Double discharges were observed in approximately 21% of the motor units studied. The proportion of motor units exhibiting double discharges was similar to previous reports from the trapezius (Kudina & Alexeeva, 1992) and other (Bawa & Calancie, 1983; Kudina & Churikova, 1990) muscles. Although Denslow (1948) observed double discharges in a much higher proportion of trapezius motor units, it is difficult to directly compare these results as the muscle activity was induced by light pin scratches and not voluntary contractions. It has been suggested that double discharges may occur in fast movements that require the rapid generation of a relatively high force (Sjogaard et al., 2001; Sogaard et al., 2001), however the observation of double discharges in the trapezius muscle during slowly varying low-intensity contractions may indicate an alternative functional role for this behavior. Consistent with previous reports, double discharges occurred not only at the beginning and end of motor unit firing, but were also interspersed with single discharges (Denslow, 1948; Kudina & Alexeeva, 1992) and appeared in long trains (Bawa & Calancie, 1983; Kudina & Alexeeva, 1992; Westad et al., 2004). The maximum occurrence of double discharges we observed in a single motor unit was 18 out of 57 discharges (32%). Although there is some evidence that double discharges can increase muscle force (Burke et al., 1976; Zajac & Young, 1980) and slow the development of fatigue (Binder-Macleod & Barker, 1991) when they occur at the beginning of a contraction, the functional relevance of long trains of double discharges is not clear. In animal motoneurons, double discharges have been associated with a delayed depolarization (Calvin & Schwindt, 1972) that originates in the dendrites (Kernell, 1964; Nelson & Burke, 1967) and causes a hump in the afterhyperpolarization of the motoneuron. If this hump is large enough to cross the threshold for spike generation a second action potential will be generated. There is evidence that double discharges in human motor units arise from a similar central origin (Kudina & Churikova, 1990), and that the mechanism is related to the intrinsic properties of the motoneuron (Kudina & Alexeeva, 1992). Our results confirm previous observations of long trains of double discharges in trapezius motor units (Kudina & Alexeeva, 1992; Westad et al., 2004), yet suggest that the incidence of double discharges is not altered during acute stress.
Many of the discharge behaviors observed in trapezius motor units are consistent with the presence of persistent inward currents (PICs) in the motoneurons. For example, it has previously been suggested that abrupt changes in recruitment threshold may be due to the activation and inactivation of PICs in the motoneuron (Westad et al., 2003; Bawa et al., 2006) and that dissociation in the firing rate of concurrently active motor units may be caused by differential activation of PICs in the individual motoneurons (Eken, 1998). Furthermore, the sudden jump in discharge rate that we observed in some motor units is characteristic of a PIC that is activated after the motor unit has begun firing (Hounsgaard et al. 1988; Eken & Kiehn, 1989). Although there is indirect evidence that human motoneurons are capable of generating PICs (Kiehn & Eken, 1997; Gorassini et al., 1998; Collins et al., 2001, 2002; Gorassini et al., 2002; Walton et al., 2002; Gorassini et al., 2004; Nickolls et al., 2004; Kamen et al., 2006), there are currently no direct measures of PIC activation that can be used to verify the existence of PICs in humans. The paired motor unit analysis has been proposed as an indirect method to estimate the magnitude of PIC contributions to normal motor behaviors in the human tibialis anterior muscle (Bennett et al., 2001; Gorassini et al., 2002). Persistent inward currents contribute to the depolarization of the motoneuron and reduce the magnitude of synaptic input required to maintain regular discharge of action potentials. The paired motor unit analysis estimates the magnitude of PIC contributions to motor unit firing by using the discharge rate of a lower threshold reference motor unit to estimate the level of synaptic input to a higher threshold test motor unit. A change in the level of synaptic input during recruitment and derecruitment of the test motor unit is quantified as ΔF, and is thought to reflect the magnitude of any PIC in the motoneuron. The positive ΔF values we observed in this study concur with previous reports of positive ΔF values in the tibialis anterior muscle (Gorassini et al., 2002; Gorassini et al., 2004), and may provide the first evidence to support the presence of PICs in human trapezius motoneurons. However, as with all indirect methods, there are several assumptions underlying the paired motor unit analysis that must be considered.
A central tenet of this analysis is that the two motor units receive similarly varying synaptic input which is reflected in the discharge rate of the lower threshold reference motor unit. Low values of rate-rate modulation in the current study indicate that this assumption was often violated for trapezius motor units. Thus, discordant modulation of concurrently active motor units may limit the use of the paired motor unit technique in this muscle. Although positive ΔF values were still observed after exclusion of motor unit pairs with low rate-rate modulation (r<0.7) we cannot be certain that these estimates were not confounded by factors other than PICs which may also contribute to hysteresis in the firing rates of active motor units (Powers et al., 2008). Therefore, although many of the discharge behaviors of trapezius motor units are consistent with the presence of PICs in spinal motoneurones, this hypothesis remains difficult to verify with currently available techniques.
Absence of Stress Effects on the Discharge Behavior of Trapezius Motor Units
The majority of studies using surface EMG have reported increases in global activity of the trapezius muscle in response to acute psychosocial stress (Lundberg et al., 1994; Laursen et al., 2002; Lundberg et al., 2002; McLean & Urquhart, 2002; Visser et al., 2004; Nilsen et al., 2007; Schleifer et al., 2008). In contrast to these studies, we observed no difference in the discharge behavior of trapezius motor units across conditions of low and high psychosocial stress. This finding is in agreement with one previous study that found no effect of psychosocial stress on the magnitude of interference EMG recordings from the trapezius muscle (Blangsted et al., 2004). The absence of a stress effect on motor unit discharge behavior in the present study is unlikely to be related to the potency of the stressor, as changes in physiologic arousal were at least as large as those previously reported (Lundberg et al., 1994; Lundberg et al., 2002). Furthermore, the study population was similar to that in which stress responses have previously been observed (Lundberg et al., 1994; Laursen et al., 2002; Lundberg et al., 2002; McLean & Urquhart, 2002; Visser et al., 2004; Nilsen et al., 2007; Schleifer et al., 2008). A fundamental difference between this and previous studies is the motor task that subjects were asked to perform. Elevated activity of the trapezius muscle has previously been observed in response to stress when subjects were instructed to rest (Lundberg et al., 2002), maintain an elevated arm position (Lundberg et al., 1994), type on a computer keyboard (Laursen et al., 2002; McLean & Urquhart, 2002; Nilsen et al., 2007; Schleifer et al., 2008) or use a computer mouse (Laursen et al., 2002; Visser et al., 2004). These tasks contrast to the motor task performed in the present study, which required a high degree of accuracy with the use of real-time visual feedback to generate precisely controlled shoulder elevation forces that matched a triangular force template.
The mechanisms underlying stress-evoked increases in trapezius muscle activity during natural motor behaviors are not known. Recent evidence suggests that the peripheral nervous system does not contribute to this motor response (Nilsen et al., 2008), however a direct role of the central nervous system remains possible. The spinal cord receives noradrenergic innervation from the locus coeruleus (Holstege & Kuypers, 1987; Proudfit & Clark, 1991), which increases its activity in response to stress (Tsigos & Chrousos, 2002; Valentino & Van Bockstaele, 2008). At the cellular level it has been shown that noradrenaline potentiates the generation of PICs in spinal motoneurons (Conway et al., 1988; Lee & Heckman, 1999), providing a direct pathway by which stress might increase the intrinsic excitability of motoneurons. Large variability in the PIC estimates for different motor units and the relatively low number of motor unit pairs included in this analysis may have reduced our ability to detect a significant effect of the stress condition on ΔF. However, the absence of a significant change in ΔF is consistent with the lack of stress effects on other discharge behaviors evaluated for a large sample of trapezius motor units in this study (Table 1). Alternatively, the absence of a significant increase in PIC estimates during the high stress condition may be explained by a task-dependent model. Any increase in PIC magnitude under stressful conditions would increase depolarization of the motoneuron. Situations in which the output of the muscle does not have to be tightly controlled would lead to elevated muscle activity, and may be seen as advantageous in a ‘flight or flight’ stress response. However, any additional depolarization contributed by the PIC would be expected to have a detrimental effect on the performance of a motor task requiring a high degree of accuracy, and it is plausible that the central nervous system may compensate by increasing inhibition to the motoneuron pool in order to control net muscle force and allow successful performance of the motor task. Although this suggestion is speculative, it raises the possibility that understanding the task conditions under which stress-evoked increases in muscle activity occur may inform our understanding of the mechanisms underlying this response.
Conclusions
Trapezius motor units display atypical discharge behaviors, including a high incidence of double discharges, abrupt changes in recruitment and derecruitment threshold, and discharge rate modulation that is dissociated both from net changes in contraction intensity and from the discharge rates of neighboring motor units. These behaviors are similar to those previously described for low threshold trapezius motor units studied in the absence of stress, and are not altered when a precision motor task is performed under stressful conditions that elevate cardiovascular responses and perceived anxiety. The absence of changes in motor unit discharge behavior in response to acute psychosocial stress may reflect high precision demands of the motor task that was performed. Further study is required to determine the effects of stress on single motor unit behavior during functional motor tasks.
Acknowledgements
This research was supported by NIH award R21-AR054181 to KSM
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander C, Miley R, Stynes S, Harrison PJ. Differential control of the scapulothoracic muscles in humans. J Physiol. 2007;580:777–786. doi: 10.1111/j.1469-7793.2000.t01-1-02034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen JH, Kaergaard A, Mikkelsen S, et al. Risk factors in the onset of neck/shoulder pain in a prospective study of workers in industrial and service companies. Occupational and environmental medicine. 2003;60:649–654. doi: 10.1136/oem.60.9.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariens GA, van Mechelen W, Bongers PM, Bouter LM, van der Wal G. Psychosocial risk factors for neck pain: a systematic review. American journal of industrial medicine. 2001;39:180–193. doi: 10.1002/1097-0274(200102)39:2<180::aid-ajim1005>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Bawa P, Calancie B. Repetitive doublets in human flexor carpi radialis muscle. J Physiol. 1983;339:123–132. doi: 10.1113/jphysiol.1983.sp014707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bawa P, Pang MY, Olesen KA, Calancie B. Rotation of motoneurons during prolonged isometric contractions in humans. Journal of neurophysiology. 2006;96:1135–1140. doi: 10.1152/jn.01063.2005. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Li Y, Harvey PJ, Gorassini M. Evidence for plateau potentials in tail motoneurons of awake chronic spinal rats with spasticity. Journal of neurophysiology. 2001;86:1972–1982. doi: 10.1152/jn.2001.86.4.1972. [DOI] [PubMed] [Google Scholar]
- Bernard BP. Musculoskeletal disorders and workplace factors: A critical review of epidemiologic evidence for work-related musculoskeletal disorders of the neck, upper extremity, and low back. Cincinnati, OH: U.S. Department of Health & Human Services, National Institute for Occupational Safety and Health; 1997. Publication No. 97–178628, 1997. [Google Scholar]
- Binder-Macleod SA, Barker CB., 3rd Use of a catchlike property of human skeletal muscle to reduce fatigue. Muscle & nerve. 1991;14:850–857. doi: 10.1002/mus.880140909. [DOI] [PubMed] [Google Scholar]
- Binder MD, Heckman CJ, Powers RK. The physiological control of motoneuron activity. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology Section 12, Exercise: Regulation and Integration of Multiple Systems. Oxford University Press; 1996. pp. 3–53. [Google Scholar]
- Blangsted AK, Sogaard K, Christensen H, Sjogaard G. The effect of physical and psychosocial loads on the trapezius muscle activity during computer keying tasks and rest periods. European journal of applied physiology. 2004;91:253–258. doi: 10.1007/s00421-003-0979-z. [DOI] [PubMed] [Google Scholar]
- Bongers PM, Ijmker S, van den Heuvel S, Blatter BM. Epidemiology of work related neck and upper limb problems: psychosocial and personal risk factors (part I) and effective interventions from a bio behavioural perspective (part II) Journal of occupational rehabilitation. 2006;16:279–302. doi: 10.1007/s10926-006-9044-1. [DOI] [PubMed] [Google Scholar]
- Burke RE, Rudomin P, Zajac FE., 3rd The effect of activation history on tension production by individual muscle units. Brain research. 1976;109:515–529. doi: 10.1016/0006-8993(76)90031-7. [DOI] [PubMed] [Google Scholar]
- Calvin WH, Schwindt PC. Steps in production of motoneuron spikes during rhythmic firing. Journal of neurophysiology. 1972;35:297–310. doi: 10.1152/jn.1972.35.3.297. [DOI] [PubMed] [Google Scholar]
- Christou EA, Jakobi JM, Critchlow A, Fleshner M, Enoka RM. The 1- to 2-Hz oscillations in muscle force are exacerbated by stress, especially in older adults. J Appl Physiol. 2004;97:225–235. doi: 10.1152/japplphysiol.00066.2004. [DOI] [PubMed] [Google Scholar]
- Collins DF, Burke D, Gandevia SC. Large involuntary forces consistent with plateau-like behavior of human motoneurons. J Neurosci. 2001;21:4059–4065. doi: 10.1523/JNEUROSCI.21-11-04059.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DF, Burke D, Gandevia SC. Sustained contractions produced by plateau-like behaviour in human motoneurones. J Physiol. 2002;538:289–301. doi: 10.1113/jphysiol.2001.012825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway BA, Hultborn H, Kiehn O, Mintz I. Plateau potentials in alpha-motoneurones induced by intravenous injection of L-dopa and clonidine in the spinal cat. J Physiol. 1988;405:369–384. doi: 10.1113/jphysiol.1988.sp017337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote P, Cassidy JD, Carroll L. The Saskatchewan Health and Back Pain Survey. The prevalence of neck pain and related disability in Saskatchewan adults. Spine. 1998;23:1689–1698. doi: 10.1097/00007632-199808010-00015. [DOI] [PubMed] [Google Scholar]
- De Luca CJ, Erim Z. Common drive of motor units in regulation of muscle force. Trends in neurosciences. 1994;17:299–305. doi: 10.1016/0166-2236(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Denslow JS. Double discharges in human motor units. Journal of neurophysiology. 1948;11:209–215. doi: 10.1152/jn.1948.11.3.209. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Eken T. Spontaneous electromyographic activity in adult rat soleus muscle. Journal of neurophysiology. 1998;80:365–376. doi: 10.1152/jn.1998.80.1.365. [DOI] [PubMed] [Google Scholar]
- Eken T, Kiehn O. Bistable firing properties of soleus motor units in unrestrained rats. Acta physiologica Scandinavica. 1989;136:383–394. doi: 10.1111/j.1748-1716.1989.tb08679.x. [DOI] [PubMed] [Google Scholar]
- Falla D, Farina D. Motor units in cranial and caudal regions of the upper trapezius muscle have different discharge rates during brief static contractions. Acta Physiol (Oxf) 2008a;192:551–558. doi: 10.1111/j.1748-1716.2007.01776.x. [DOI] [PubMed] [Google Scholar]
- Falla D, Farina D. Non-uniform adaptation of motor unit discharge rates during sustained static contraction of the upper trapezius muscle. Experimental brain research Experimentelle Hirnforschung. 2008b;191:363–370. doi: 10.1007/s00221-008-1530-6. [DOI] [PubMed] [Google Scholar]
- Falla D, Farina D, Graven-Nielsen T. Spatial dependency of trapezius muscle activity during repetitive shoulder flexion. J Electromyogr Kinesiol. 2007;17:299–306. doi: 10.1016/j.jelekin.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Gorassini M, Yang JF, Siu M, Bennett DJ. Intrinsic activation of human motoneurons: possible contribution to motor unit excitation. Journal of neurophysiology. 2002;87:1850–1858. doi: 10.1152/jn.00024.2001. [DOI] [PubMed] [Google Scholar]
- Gorassini MA, Bennett DJ, Yang JF. Self-sustained firing of human motor units. Neuroscience letters. 1998;247:13–16. doi: 10.1016/s0304-3940(98)00277-8. [DOI] [PubMed] [Google Scholar]
- Gorassini MA, Knash ME, Harvey PJ, Bennett DJ, Yang JF. Role of motoneurons in the generation of muscle spasms after spinal cord injury. Brain. 2004;127:2247–2258. doi: 10.1093/brain/awh243. [DOI] [PubMed] [Google Scholar]
- Hagg GM. Static work loads and occupational myalgia--a new explanation model. In: Anderson PA, Hobart DJ, Danhoff JV, editors. Electromyographical Kinesiology. North-Holland: Elsevier Science Publishers; 1991. pp. 141–144. [Google Scholar]
- Hamilton N, Luttgens K. Kinesiology: scientific basis of human motion. Boston: McGraw-Hill; 2002. [Google Scholar]
- Henneman E. Relation between size of neurons and their susceptibility to discharge. Science (New York, NY) 1957;126:1345–1347. doi: 10.1126/science.126.3287.1345. [DOI] [PubMed] [Google Scholar]
- Holstege JC, Kuypers HG. Brainstem projections to spinal motoneurons: an update. Neuroscience. 1987;23:809–821. doi: 10.1016/0306-4522(87)90160-6. [DOI] [PubMed] [Google Scholar]
- Holte KA, Westgaard RH. Daytime trapezius muscle activity and shoulder-neck pain of service workers with work stress and low biomechanical exposure. American journal of industrial medicine. 2002;41:393–405. doi: 10.1002/ajim.10039. [DOI] [PubMed] [Google Scholar]
- Kamen G, Sullivan R, Rubinstein S, Christie A. Evidence of self-sustained motoneuron firing in young and older adults. J Electromyogr Kinesiol. 2006;16:25–31. doi: 10.1016/j.jelekin.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Kernell D. The Delayed Depolarization in Cat and Rat Motoneurones. Progress in brain research. 1964;12:42–55. doi: 10.1016/s0079-6123(08)60616-0. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Eken T. Prolonged firing in motor units: evidence of plateau potentials in human motoneurons? Journal of neurophysiology. 1997;78:3061–3068. doi: 10.1152/jn.1997.78.6.3061. [DOI] [PubMed] [Google Scholar]
- Kudina LP, Alexeeva NL. Repetitive doublets of human motoneurones: analysis of interspike intervals and recruitment pattern. Electroencephalography and clinical neurophysiology. 1992;85:243–247. doi: 10.1016/0168-5597(92)90112-o. [DOI] [PubMed] [Google Scholar]
- Kudina LP, Churikova LI. Testing excitability of human motoneurones capable of firing double discharges. Electroencephalography and clinical neurophysiology. 1990;75:334–341. doi: 10.1016/0013-4694(90)90111-v. [DOI] [PubMed] [Google Scholar]
- Laursen B, Jensen BR, Garde AH, Jorgensen AH. Effect of mental and physical demands on muscular activity during the use of a computer mouse and a keyboard. Scandinavian journal of work, environment & health. 2002;28:215–221. doi: 10.5271/sjweh.668. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Enhancement of bistability in spinal motoneurons in vivo by the noradrenergic alpha1 agonist methoxamine. Journal of neurophysiology. 1999;81:2164–2174. doi: 10.1152/jn.1999.81.5.2164. [DOI] [PubMed] [Google Scholar]
- Lundberg U. Psychophysiology of work: stress, gender, endocrine response, and work-related upper extremity disorders. American journal of industrial medicine. 2002;41:383–392. doi: 10.1002/ajim.10038. [DOI] [PubMed] [Google Scholar]
- Lundberg U, Forsman M, Zachau G, Eklof M, Palmerud G, Melin B, Kadefors R. Effects of experimentally induced mental and physical stress on motor unit recruitment in the trapezius muscle. Work & Stress. 2002;16:166–178. [Google Scholar]
- Lundberg U, Kadefors R, Melin B, Palmerud G, Hassmen P, Engstrom M, Dohns IE. Psychophysiological stress and EMG activity of the trapezius muscle. International journal of behavioral medicine. 1994;1:354–370. doi: 10.1207/s15327558ijbm0104_5. [DOI] [PubMed] [Google Scholar]
- McLean L, Urquhart N. The influence of psychological stressors on myoelectrical signal activity in the shoulder region during a data entry task. Work & amp; Stress. 2002;16:138–153. [Google Scholar]
- Nelson PG, Burke RE. Delayed depolarization in cat spinal motoneurons. Experimental neurology. 1967;17:16–26. doi: 10.1016/0014-4886(67)90118-5. [DOI] [PubMed] [Google Scholar]
- Nickolls P, Collins DF, Gorman RB, Burke D, Gandevia SC. Forces consistent with plateau-like behaviour of spinal neurons evoked in patients with spinal cord injuries. Brain. 2004;127:660–670. doi: 10.1093/brain/awh073. [DOI] [PubMed] [Google Scholar]
- Nilsen K, Sand T, Stovner L, Leistad R, Westgaard R. Autonomic and muscular responses and recovery to one-hour laboratory mental stress in healthy subjects. BMC musculoskeletal disorders. 2007;8:81. doi: 10.1186/1471-2474-8-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen KB, Sand T, Borchgrevink P, Leistad RB, Ro M, Westgaard RH. A unilateral sympathetic blockade does not affect stress-related pain and muscle activity in patients with chronic musculoskeletal pain. Scandinavian journal of rheumatology. 2008;37:53–61. doi: 10.1080/03009740701716850. [DOI] [PubMed] [Google Scholar]
- Noteboom JT, Barnholt KR, Enoka RM. Activation of the arousal response and impairment of performance increase with anxiety and stressor intensity. J Appl Physiol. 2001a;91:2093–2101. doi: 10.1152/jappl.2001.91.5.2093. [DOI] [PubMed] [Google Scholar]
- Noteboom JT, Fleshner M, Enoka RM. Activation of the arousal response can impair performance on a simple motor task. J Appl Physiol. 2001b;91:821–831. doi: 10.1152/jappl.2001.91.2.821. [DOI] [PubMed] [Google Scholar]
- Ostergren PO, Hanson BS, Balogh I, et al. Incidence of shoulder and neck pain in a working population: effect modification between mechanical and psychosocial exposures at work? Results from a one year follow up of the Malmo shoulder and neck study cohort. Journal of epidemiology and community health. 2005;59:721–728. doi: 10.1136/jech.2005.034801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partanen VS, Lang AH. An analysis of double discharges in the human electromyogram. Journal of the neurological sciences. 1978;36:363–375. doi: 10.1016/0022-510x(78)90044-8. [DOI] [PubMed] [Google Scholar]
- Picavet HS, Schouten JS. Musculoskeletal pain in the Netherlands: prevalences, consequences and risk groups, the DMC(3)-study. Pain. 2003;102:167–178. doi: 10.1016/s0304-3959(02)00372-x. [DOI] [PubMed] [Google Scholar]
- Powers RK, Nardelli P, Cope TC. Estimation of the contribution of intrinsic currents to motoneuron firing based on paired motoneuron discharge records in the decerebrate cat. Journal of neurophysiology. 2008;100:292–303. doi: 10.1152/jn.90296.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfit HK, Clark FM. The projections of locus coeruleus neurons to the spinal cord. Progress in brain research. 1991;88:123–141. doi: 10.1016/s0079-6123(08)63803-0. [DOI] [PubMed] [Google Scholar]
- Schleifer LM, Spalding TW, Kerick SE, Cram JR, Ley R, Hatfield BD. Mental stress and trapezius muscle activation under psychomotor challenge: a focus on EMG gaps during computer work. Psychophysiology. 2008;45:356–365. doi: 10.1111/j.1469-8986.2008.00645.x. [DOI] [PubMed] [Google Scholar]
- Sjogaard G, Sogaard K, Finsen L, Olsen HB, Christensen H. Doublets in motor unit activity of human forearm muscle during simulated computer work. Acta physiologica et pharmacologica Bulgarica. 2001;26:83–85. [PubMed] [Google Scholar]
- Sogaard K, Sjogaard G, Finsen L, Olsen HB, Christensen H. Motor unit activity during stereotyped finger tasks and computer mouse work. J Electromyogr Kinesiol. 2001;11:197–206. doi: 10.1016/s1050-6411(00)00053-5. [DOI] [PubMed] [Google Scholar]
- Stephenson JL, Maluf KS. Effects of psychosocial stress on intrinsic activation of human trapezius motoneurons. Washington DC, USA: Society for Neuroscience Abstracts; 2008. [Google Scholar]
- Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. Journal of psychosomatic research. 2002;53:865–871. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- Urwin M, Symmons D, Allison T, et al. Estimating the burden of musculoskeletal disorders in the community: the comparative prevalence of symptoms at different anatomical sites, and the relation to social deprivation. Annals of the rheumatic diseases. 1998;57:649–655. doi: 10.1136/ard.57.11.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele E. Convergent regulation of locus coeruleus activity as an adaptive response to stress. European journal of pharmacology. 2008;583:194–203. doi: 10.1016/j.ejphar.2007.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cutsem M, Duchateau J, Hainaut K. Changes in single motor unit behaviour contribute to the increase in contraction speed after dynamic training in humans. J Physiol. 1998;513:295–305. doi: 10.1111/j.1469-7793.1998.295by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiersted KB, Westgaard RH, Andersen P. Electromyographic evaluation of muscular work pattern as a predictor of trapezius myalgia. Scandinavian journal of work, environment & health. 1993;19:284–290. doi: 10.5271/sjweh.1472. [DOI] [PubMed] [Google Scholar]
- Visser B, De Looze M, De Graaff M, Van Dieen J. Effects of precision demands and mental pressure on muscle activation and hand forces in computer mouse tasks. Ergonomics. 2004;47:202–217. doi: 10.1080/00140130310001617967. [DOI] [PubMed] [Google Scholar]
- Walton C, Kalmar JM, Cafarelli E. Effect of caffeine on self-sustained firing in human motor units. J Physiol. 2002;545:671–679. doi: 10.1113/jphysiol.2002.025064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westad C, Mork PJ, Westgaard RH. Firing patterns of low-threshold trapezius motor units in feedback-controlled contractions and vocational motor activities. Experimental brain research Experimentelle Hirnforschung. 2004;158:465–473. doi: 10.1007/s00221-004-1925-y. [DOI] [PubMed] [Google Scholar]
- Westad C, Westgaard RH, De Luca CJ. Motor unit recruitment and derecruitment induced by brief increase in contraction amplitude of the human trapezius muscle. J Physiol. 2003;552:645–656. doi: 10.1113/jphysiol.2003.044990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westgaard RH. Muscle activity as a releasing factor for pain in the shoulder and neck. Cephalalgia. 1999;19 Suppl 25:1–8. doi: 10.1177/0333102499019s2501. [DOI] [PubMed] [Google Scholar]
- Westgaard RH, Bonato P, Westad C. Respiratory and stress-induced activation of low-threshold motor units in the human trapezius muscle. Experimental brain research Experimentelle Hirnforschung. 2006;175:689–701. doi: 10.1007/s00221-006-0587-3. [DOI] [PubMed] [Google Scholar]
- Westgaard RH, de Luca CJ. Motor unit substitution in long-duration contractions of the human trapezius muscle. Journal of neurophysiology. 1999;82:501–504. doi: 10.1152/jn.1999.82.1.501. [DOI] [PubMed] [Google Scholar]
- Westgaard RH, De Luca CJ. Motor control of low-threshold motor units in the human trapezius muscle. Journal of neurophysiology. 2001;85:1777–1781. doi: 10.1152/jn.2001.85.4.1777. [DOI] [PubMed] [Google Scholar]
- Westgaard RH, Vasseljen O, Holte KA. Trapezius muscle activity as a risk indicator for shoulder and neck pain in female service workers with low biomechanical exposure. Ergonomics. 2001;44:339–353. doi: 10.1080/00140130119649. [DOI] [PubMed] [Google Scholar]
- Zajac FE, Young JL. Properties of stimulus trains producing maximum tension-time area per pulse from single motor units in medial gastrocnemiu muscle of the cat. Journal of neurophysiology. 1980;43:1206–1220. doi: 10.1152/jn.1980.43.5.1206. [DOI] [PubMed] [Google Scholar]