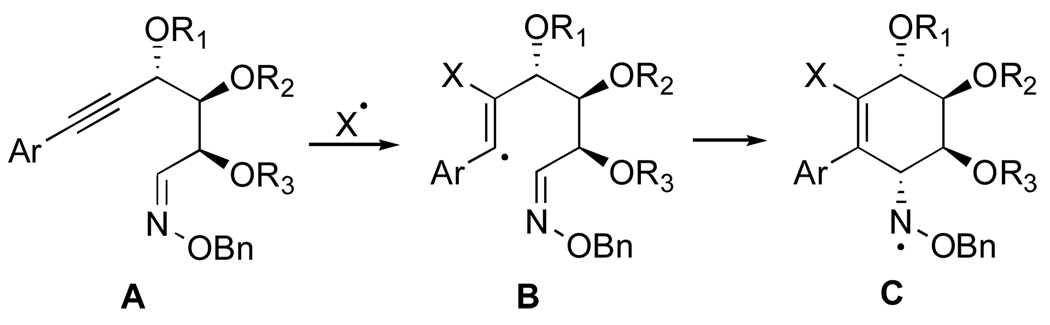
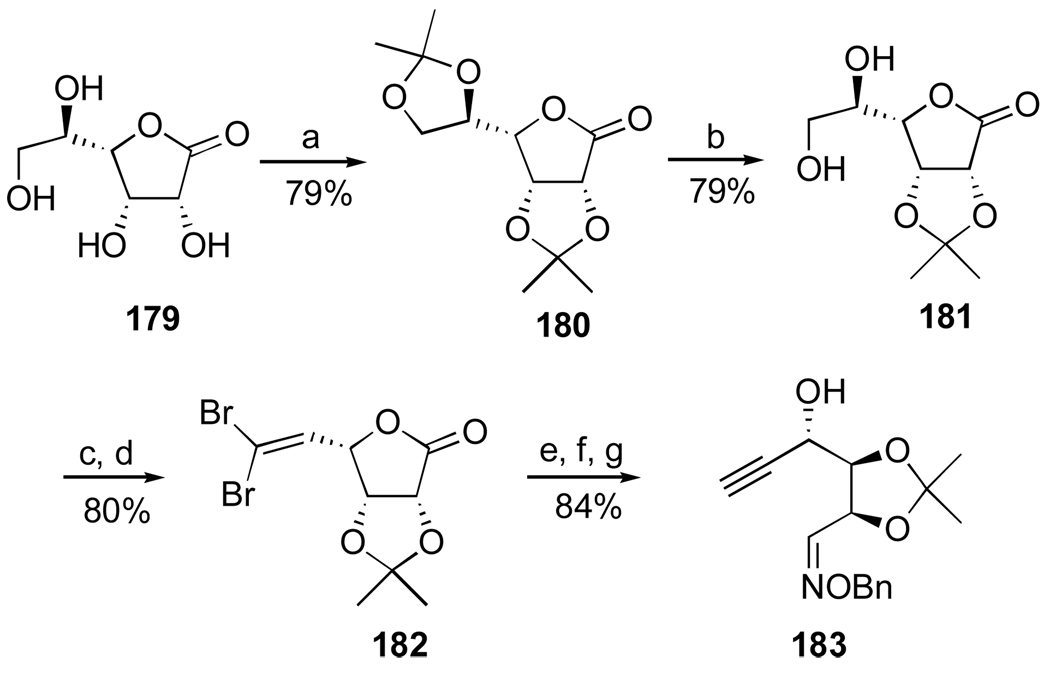
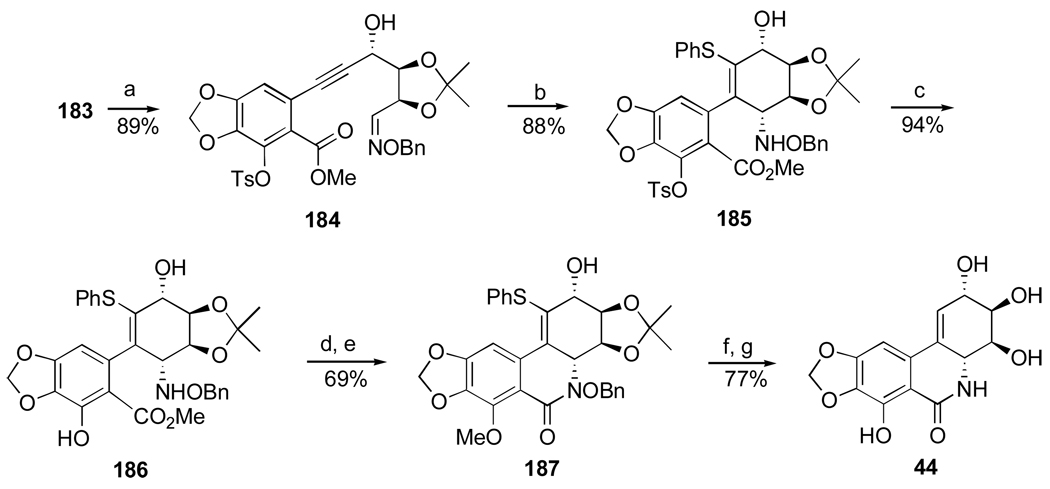
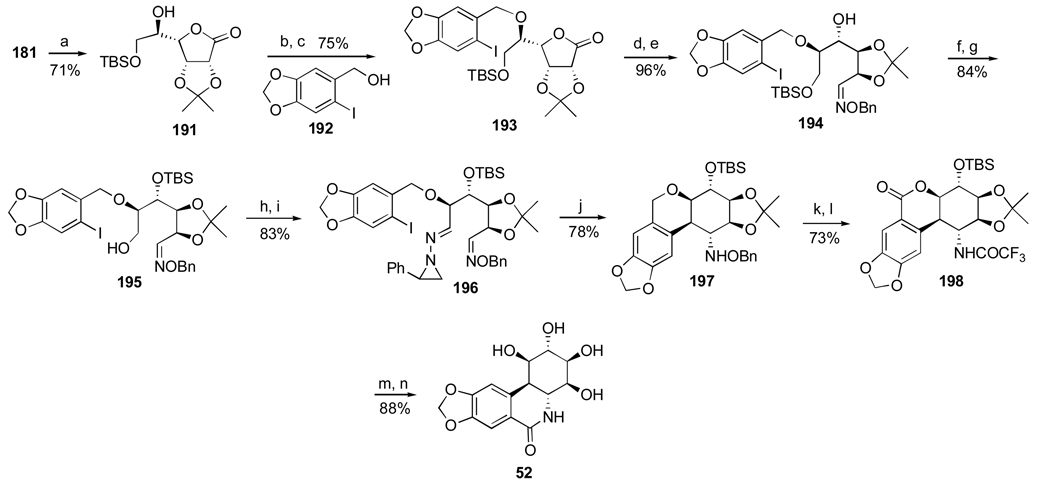
1. Introduction
Ornamental flower growers know that placing a cut daffodil (a.k.a. narcissus) in a vase with other flowers has a negative effect on the quality of those flowers and significantly shortens their vase life. Furthermore, a common horticultural practice for the cultivation of narcissus flowers involves the introduction of cuts on the bulbs before immersing them into water. The mucilage that leaches out from the cuts is constantly removed by frequent changing of water and this leads to sprouting. These observations raise speculation that specific components in the mucilage of the narcissus bulbs may have powerful growth-inhibitory effects. Historical use of narcissus flowers, as well as at least thirty other plants of the Amaryllidaceae family, in folk medicine for the management of cancer1 speaks volumes to validate this conjecture.
Indeed, powerful anticancer properties of Narcissus poeticus L. were already known to the Father of Medicine, Hippokrates of Kos (ca. B.C. 460–370), who recommended a pessary prepared from narcissus oil for the treatment of uterine tumors.2 His successors, the ancient Greek physicians Pedanius Dioscorides (ca. A.D. 40–90) and Soranus of Ephesus (A.D. 98–138) continued using this therapy in the first and second centuries A.D.3,4 In addition, the topical anticancer uses of extracts from this plant5,6 as well as from N. pseudonarcissus7–9 were recorded in the first century A.D. by the Roman natural philosopher Gaius Plinius Secundus, (A.D. 23–79), better known as Pliny the Elder.10 Even the Bible provides multiple references to the Mediterranean N. tazetta L., which has a long history of use against cancer.11 The applications of narcissus oil in cancer management continued in the middle ages in Chinese, North African, Central American and Arabian medicine.1,12 The uses of other genera of the Amaryllidaceae family were also common, e. g. Hymenocallis caribaea (L. emend Gawler) Herbert, utilized by early European medical practitioners for inflammatory tumors.13
More recently, the plants of the Amaryllidaceae have been under intense scrutiny for the presence of the specific metabolites responsible for the medicinal properties associated with this plant family. The study began in 1877 with the isolation of alkaloid lycorine from Narcissus pseudonarcissus14 and since then more than 100 alkaloids, exhibiting diverse biological activities, have been isolated from the Amaryllidaceae plants.
Based on the present scientific evidence, it is likely that isocarbostyril constituents of the Amaryllidaceae, such as narciclasine, pancratistatin and their congeners, are the most important metabolites responsible for the therapeutic benefits of these plants in the folk medical treatment of cancer. Notably, N. poeticus L. used by the ancient Greek physicians, as was eluded before, is now known to contain some 0.12 g of narciclasine per kg of fresh bulbs.15 Continuing along this intriguing path, the focus of the present review is a comprehensive literature survey and discussion of the chemistry and biology of these compounds as specifically relevant to their potential use in medicine. The examination of the synthetic organic chemistry, more specifically the total synthesis efforts inspired by the challenging chemical structures of narciclasine, pancratistatin and their congeners, will be reduced to a minimum in view of the two very recent excellent reviews published on this subject.16,17
2. Plant Sources and Isolation Methods
2.1 Amaryllidaceae Plants in Traditional Medicine
Plants, belonging to the Amaryllidaceae family, are herbaceous perennials that grow from bulbs. The family consists of about sixty genera, whose eight hundred species are widely distributed in several countries in the world. They are also cultivated as ornamental plants for their beautiful flowers and for the production of volatile oil. Amaryllidaceae plants are extensively used in traditional medicine in different countries and their pharmacological effects are frequently associated with several typical alkaloids they synthesize. The therapeutic action of a range of wild plants, although not scientifically proven, has been discovered by indigenous people over centuries. Developing countries are often subject to shortages of funds, medical facilities and newly developed medicine, which make them more dependent on their natural sources. Among these, various African, Asian and Polynesia communities still use traditional remedies for primary health care.18
The majority of compounds found in the Amaryllidaceae family are alkaloids, which are uniquely associated with its members. The study of Amaryllidaceae alkaloids began in 1877 with the isolation of lycorine (3, Figure 1) from N. pseudonarcissus,14 and the interest around this group of naturally occurring compounds has increased in time because of their effective antitumoral and antiviral activities. Lycorine is a pyrrolo[de]phenathridine ring-type alkaloid extracted from different Amaryllidaceae genera, whose structure was elucidated by Nagakawa et al. in 1956.19 However, because of increasing attention attracted by lycorine in the last few decades and in particular the ability of 3 to inhibit ascorbic acid synthesis in vivo,20 the chemical and biological aspects of this interesting alkaloid are being further investigated.21
Figure 1.
Ring types and representative Amaryllidaceae alkaloids (see Table 1).
Hundreds of new alkaloids isolated from different parts and in different vegetative phases of ca. 150 species belonging to 36 genera can be grouped into 12 distinct ring-types (Table 1). The structures of a representative alkaloid of each ring type are shown in Figure 1, Figure 2, Figure 3 and Figure 7.22 The advances made in the isolation as well as chemical and biological characterization of such alkaloids have been extensively reviewed.5,14, 21–28
Table 1.
| ring type | alkaloid | structure | |
|---|---|---|---|
| I | N-(3,4-dioxybenzyl)-4-oxyphenethylamine | norbelladine | 1 |
| II | N-(3.4-dioxybenzyl)-3,4-dioxyphenethylamine | rystilline | 2 |
| III | pyrrolo[de]phenanthridine | lycorine | 3 |
| IV | lycorenine | hippeastrine | 10 |
| V | galanthamine | narwedine | 4 |
| VI | 5,10b-ethanophenanthridine | haemanthamine | 5 |
| VII | 1,2-epoxy-5,10b-ethanophenanthridine | 1,2-β-epoxyambelline | 6 |
| VIII | pretazettine | pretazettine | 7 |
| IX | tetrahydroisoquinoline | cherylline | 8 |
| X | phenanthridone/lignoid | crinasiadine | 43 |
| XI | clivimine | clivimine | 18 |
| XII | ismine | ismine | 9 |
Figure 2.
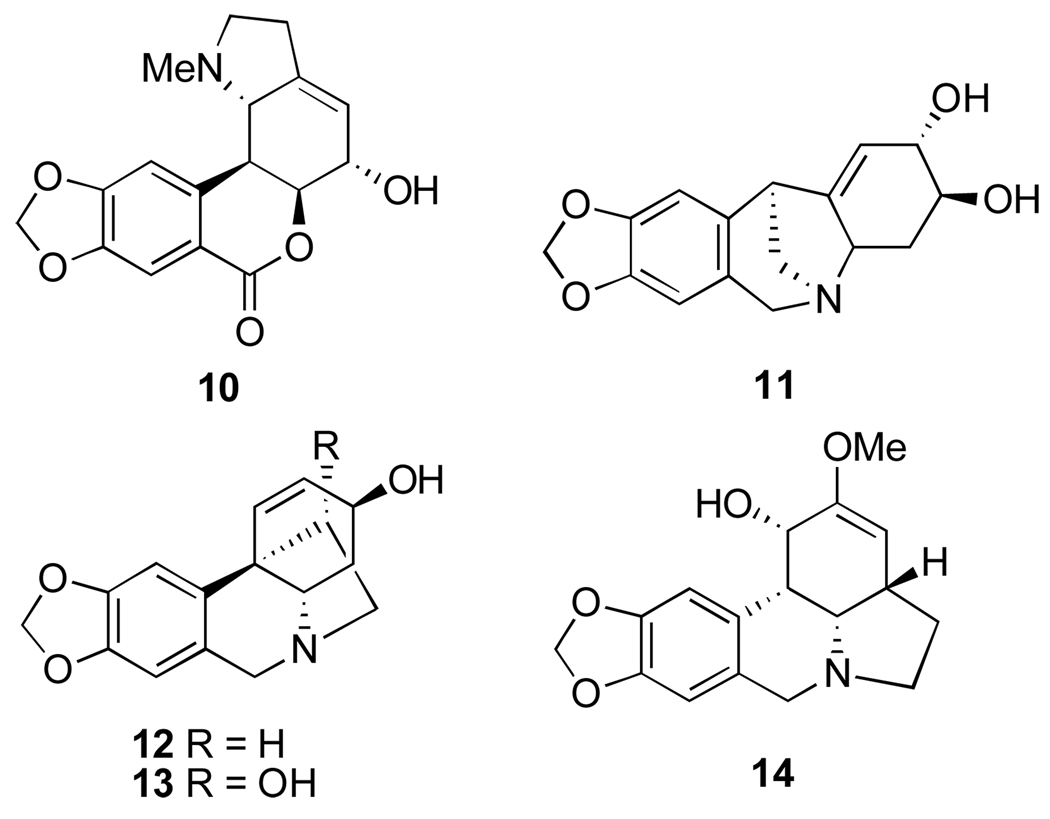
Alkaloids from Amaryllis belladonna L.: hippeastrine (10), pancracine (11), vittatine (12), 11-hydroxyvittatine (13) and amarbellisine (14).
Figure 3.
Alkaloids from Clivia spp.: clivonine (15), clivatine (16), clivimine (18), nobilisine (19), nobilisitine A (20) and B (17), and (+)-8-demethylmaritidine (21).
Figure 7.
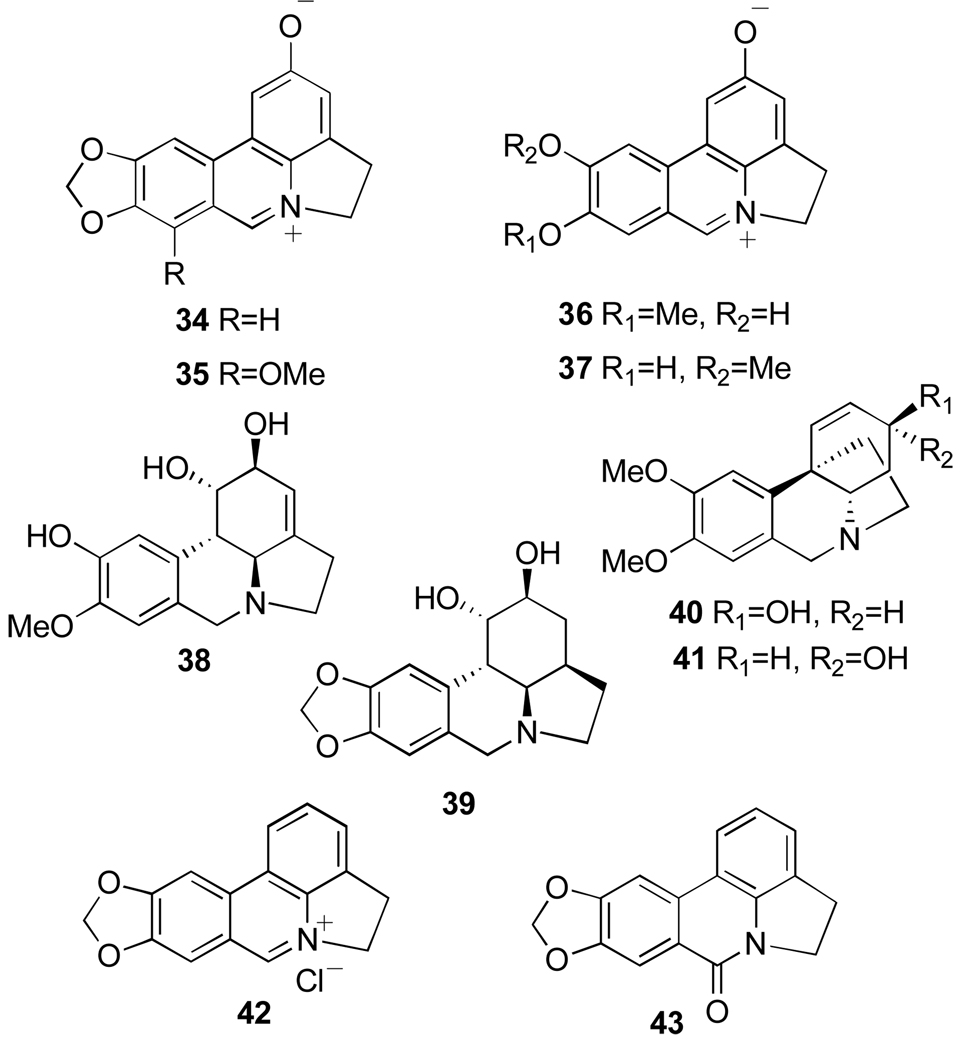
Phenanthridiunium betaine alkaloids from Pancratium and Zephyranthes: ungeremine (34), zeflabetaine (35), zefbetaine (36), iso-zefbetaine (37), pseudolycorine (38), α-dihydrolycorine (39), maritidine (40) and (+)-epi-maritidine (41), as well as lycorine oxidation derivatives anhydrolycorinium chloride (42) and anhydrolycorine lactam (=crinasiadine, 43).
The initially discovered analgesic activity exhibited by the Amaryllidaceae alkaloids generated a lot of interest and it was attributed to their resemblance to morphine and codeine skeletons. In this respect, the alkaloids of the pyrrolo[de]phenanthridine group are considered more promising than the alkaloids of galanthamine and 5,10b-ethanophenathridine groups due to reduced toxicity.29 Narwedine (4, Figure 1) potentiates the pharmacological effects of caffeine, carbazole, arecoline, and to a lesser extent of nicotine, in laboratory animals. In addition, narwedine and vittatine (12, Figure 2) potentiate the analgesic effects of sub-optimal doses of morphine.29
Earlier studies also showed that a number of Amaryllidaceae alkaloids cause a transient fall in blood pressure in laboratory animals in high dose. Indeed, narwedine, galanthamine and epi-galanthamine (4, Figure 1, 32 and 33, Figure 6) were reported to produce significant hypotensive effects in mice.29,30 Another area of interest is acetylcholinesterase inhibitory activity of galanthamine and its ability to amplify the nerve-muscle transfer.30 Extracts of several Amaryllidaceae plants were also found to possess pronounced antibacterial and antifungal activities.31
Figure 6.
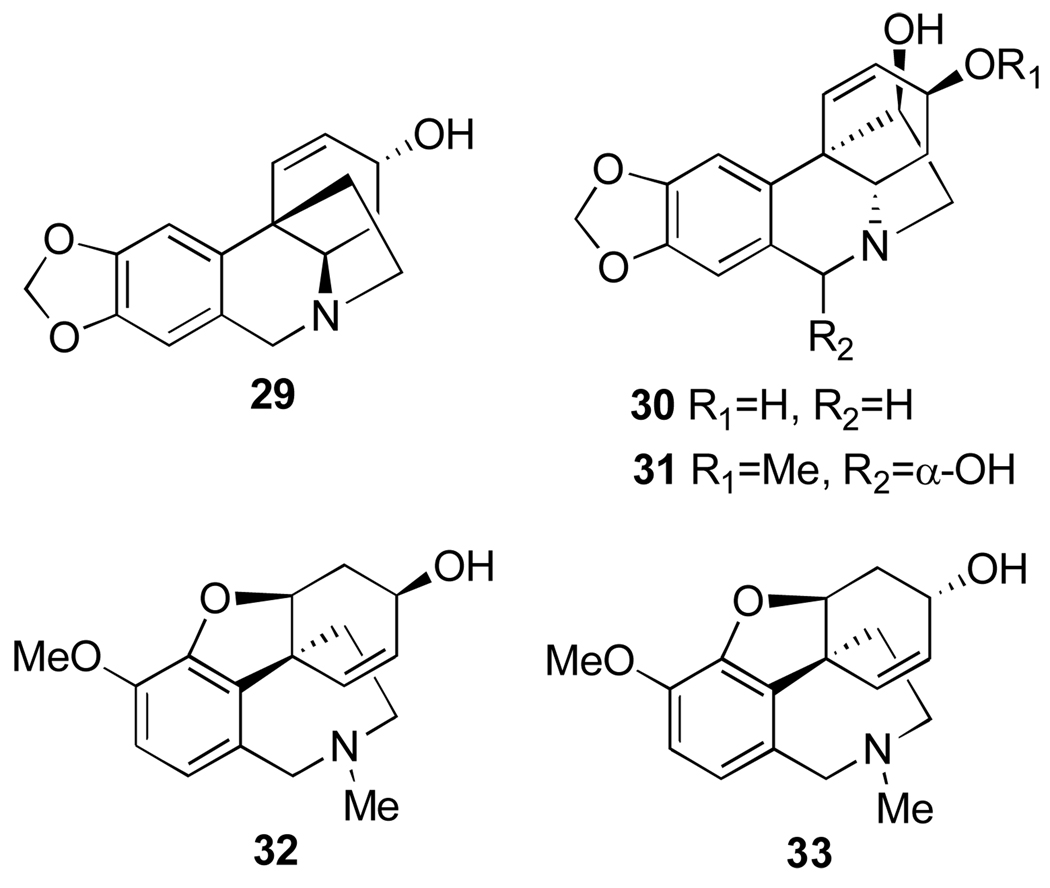
Alkaloids from Leucojum aestivum, N. tazetta ssp. tazetta and Pancratium maritimum exhibiting antimalarial activity: crinine (29), 3-epi-hydroxybulbospermine (30), 6-hydroxyhaemanthamine (31), galanthamine (32) and 3-epi-galanthamine (33).
Amaryllidaceae alkaloids have repeatedly shown antitumor potential and, among other characteristics, exhibited in vivo activity against various human viruses.18 Several members of the Amaryllidaceae family are toxic and cause symptoms such as headaches, excessive salivation, nausea, dizziness, heartbeat irregularities, visual disturbance and dermatitis. Some toxic principles include lycorine and haematim, and the inappropriate use of a number of species can be fatal. Nevertheless, other species are administered orally as medicine to children or eaten in porridge by local people.18
A number of species of the genus Amaryllis have been used in folk medicine, including Amaryllis belladonna L. (also named Hippeastrum equestre), which is cultivated in Egypt as an ornamental plant.32 Six alkaloids have been isolated from the bulbs of this species, namely: lycorine (3, Figure 1), hippeastrine, pancracine, vittatine, 11-hydroxyvittatine and amarbellisine (10–14, Figure 2).33 The results of antifungal and antibacterial screening showed that amarbellisine, pancracine, vittatine and 11-hydroxyvittatine have antibacterial activity against Gram-negative Escherichia coli, while pancracine also showed activity against Pseudomonas aeruginosae. Furthemore, all alkaloids especially lycorine, amarbellisine and hippeastrine showed antifungal activity against Candida albicans.33
Some members of Ammocharis, a widely distributed genus, generally inhabit seasonal wet places. A. coronica, a species which contains biochemicals such as alkaloids and triterpenoids in its bulbs, is known to be toxic. Therefore, instead of oral administration, fresh, wet scales are cooked and used as enemas for blood cleansing or applied topically to open wounds or boils.18
Plants of Bophane genus are known for their large bulbs and production of characteristic alkaloids. B. disticha (L.f.) Herb. is known as a toxic plant, containing compounds with alleged hallucinogenic potential. However, bulb scales or infusion are used on septic wounds and external sores, as well as for rheumatism and relief of pain. Decoctions are also used for the treatment of headaches, cramps and internal pains.18
The Brunsvigia genus produces large bulbs containing a number of alkaloids, shown to have significant antimalarial, cytotoxic, and antineoplastic activity. The bulbs are applied as antiseptic dressings on fresh wounds, whereas bulb decoctions are administered for the treatment of coughs, colds as well as abdominal, renal and liver complaints.18
Clivia species, namely C. miniata and C. nobilis are cultivated in Egypt as ornamental plants for their beautiful flowers. Root infusion of C. miniata (Lindl.) Regel is used to treat snakebites and wounds. In addition, its roots and leaves are taken by South African women during pregnancy and child birth. Aqueous leaf extracts have proven to augment or induce labor. Bulb decoctions are also used against infertility and urinary complaints.18 A number of Clivia species have been reported to contain Amaryllidaceae alkaloids represented by the 3a,4-dihydrolactone[2]benzopyrano[3,4-g]indole ring system. This includes clivonine (15, Figure 3), isolated from C. miniata,34 and clivatine,35 clivimine,36 nobilisine,35 as well as nobilisitine A and B37 (16, 18, 19, 20 and 17, Figure 3) isolated from C. nobilis. (+)-8-Demethylmaritidine (21, Figure 3), a crinine-type alkaloid, was also isolated from this species. The antimicrobial activity of the alkaloid extract from C. nobilis was tested against Gram-positive (Staphylococcus aureus) and Gram-negative (E. coli and Pseudomonas aeruginosa) bacterial strains as well as and fungi (C. albicans). All alkaloids with the exception of clivimine showed antibacterial activity against Gram-positive S. aureus. The same compounds, in particular, nobilisitine B, exhibited antifungal activity against C. albicans.36
Species of Crinum genus are large, showy plants with umbels of like flowers. They are found in tropical and subtropical regions throughout the world, and for centuries they have been used traditionally to cure ailments and diseases.38 One of the earliest recorded uses of Crinum is as a powerful emetic. In Asia and America the bulbs were used to “hasten the ripening of indolent tumors,” while C. zeylanicum was used in the Moluccas as a strong poison.38 C. asiaticum is known throughout southeast Asia and Polynesia as an emetic and diaphoretic, while C. bulbispermum is a general medicinal herb. In southern Sotho different parts of this plant are used to treat colds, coughs, and as an external application or wash for wounds, scrofula and hemorrhoids. This plant is also used by Zulu, Xhosa and Sotho people as a gynecological remedy and charm. In Lesotho it is used as a medicine to increase milk flow and in India as a rubefacient in the treatment of rheumatism. C. delagoense plants are used in Zhulu and Xhosa traditional medicine to treat urinary problems, while in Australia, the aborigines obtain starch from C. flaccidum to eat as a kind of gruel. C. giganteum is a Congo leprosy remedy. C. jagus is used as a veterinary medicinal plant in Cameroon, while in Nigeria it is used to treat open sores and in an anticonvulsant preparation. In Tanganyika the fruits and the inner bulb scales of C. kirkii are used as a purgative and the outer bulb scales as a rat poison. C. macowanii plants are used by traditional medical practitioners in Zimbabwe to treat backache and also in a number of principal uses, namely: pus disease, blood cleansing, kidney and bladder disease, glandular swelling, fever, infected sores, boils and acne. C. moorei bulbs in a decoction are used for swelling and urinary problems, while C. pratense is used in Indian medicine for intestinal diseases such as diarrhea and dysentery.38 C. latifolium grows abundantly in the upper Gangetic Plain and is also cultivated as a garden flower. Extracts of different parts of this species are used in popular medicine for a variety of purposes, e.g. a rubefacient in rheumatism and piles, and for abscess treatment to promote suppuration. Two new pyrrolophenanthridone alkaloids, pratorimine and pratosine (24 and 25, Figure 4) were isolated from the bulbs of C. latofolium and characterized on the basis of comprehensive spectral analysis, chemical transformations and synthesis.39 The bulbs of C. jagus and C. glaucum are used in traditional medicine in southern Nigeria for memory loss and other mental symptoms associated with ageing. Alkaloidal extracts of bulbs from each species showed inhibition of acetylcholinesterase, an activity exploited therapeutically to raise the depressed levels of acetylcholine in the brain associated with Alzheimer’s disease. A number of alkaloids were isolated from this extract and their activity quantified. The most active alkaloids isolated were hyamine (23, Figure 4) and lycorine (3, Figure 1), while other alkaloids were comparatively inactive, such as haemanthamine and crinamine (5, Figure 1 and 22, Figure 4). Cholinesterase activity appears to be associated with the presence of two free hydroxyl groups in this structural type of Amaryllidaceae alkaloids.40
Figure 4.
Selected alkaloids from Crinum spp.: crinamine (22), hyamine (23), pratorimine (24) and pratosine (25).
The use of Crinum extends to animals, such as treatment of weight loss, low milk production, milk loss or for healthy calves and placenta retention in the cattle.38 The reason for the use of Crinum species for similar medicinal purposes in different countries is possibly due to their alkaloidal constituents, which in some instances are common to many species. Phytochemical analysis has recently yielded a vast array of compounds, including 150 different alkaloids.39 The structures of the Crinum alkaloids, although diverse, are derived from three fundamental nuclei, viz. N-(3,4-dioxybenzyl)-4-oxyphenethylamine (e.g. norbelladine, 1, Figure 1), pyrrolo[de]phenanthridine (e.g. lycorine, 3, Figure 1) and 5,10b-ethanophenanthridine (e.g. vittatine or crinine, 12, Figure 2 and 29, Figure 6). Various trivial names of Crinum alkaloids, their distribution and biological activities have been reviewed by Ghosal et al.22
Species belonging to the genus Cyrtanthus are among the plants used in South African traditional medicine. C. mackenii is used as a protective charm against storms and evil. Other related species are used to treat diseases like scrofula, chronic coughs, headache, cystitis and leprosy as well as during pregnancy and child birth. The distribution of the genus Cyrtanthus, comprising 51 species in southern Africa, encompasses tropical Africa. In South Africa, Cyrtanthus species are mainly distributed in the Western and Eastern Cape province with smaller distribution in Transvaal and KaZulu-Natal.41
Gethyllis genus comprises 32 species found mainly in southern Africa (Namibia and South Africa). They are widely distributed in the Western and Northern Cape. The genus is endangered and little is known about its chemistry and biological activities. Gethyllis species are used traditionally in South African folk medicine. G. ciliaris is used in the Cape as a remedy for colic, flatulence and indigestion. The fruit pods of many Gethyllis species are boiled or administered as an alcohol infusion of brandy for stomach disorders, while flower decoctions are used for toothache.41 Organic extracts of Cyrtanthus species show antibacterial and anti-inflammatory activities, with the most potent obtained from leaves and/or roots of C. falcatus and G. ciliaris.42
Haemanthus genus produces several alkaloids.22 These perennial plants are used against coughs, dropsy asthma and as topical antiseptics. Some species have shown RNA antiviral (e.g. Poliovirus) and antineoplastic activities, but are also reported for their toxicity, probably due to the presence of alkaloids.18
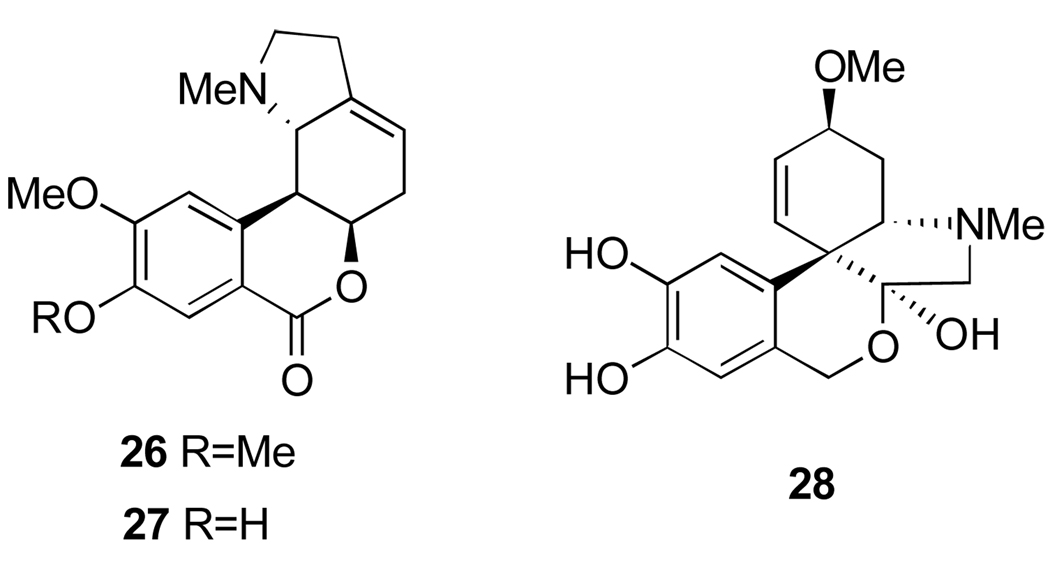
Narcissus tazetta L. grows widely in Egypt. It is also cultivated as an ornamental plant and for the production of volatile oil. A number of alkaloids were isolated5,23,24,25 from this species, which are of great interest because of their effective antitumor and antiviral activities, particularly against the choriomeningitis virus.42 Wild plants were found to contain lycorine (3, Figure 1), homolycorine, 9-O-demethylhomolycorine and tazettine (26, 27 and 28, Figure 5).43,44
Figure 5.
Alkaloids from Narcissus tazetta L.: homolycorine (26), its 9-O-demethyl derivative (27) and tazettine (28).
The alkaloids isolated from N. tazzetta ssp. tazetta as well as Pancratium maritimum and Leucojum aestivum appear to belong to four groups, namely lycorine, crinine, tazettine, and galanthamine types. Isolated lycorine, haemanthamine (1 and 5, Figure 1), tazettine (28, Figure 5), crinine, 3-epi-hydroxybulbispermine and 6-hydroxyhaemanthamine (29–31, Figure 6) were tested for antimalarial activity against Plasmodium falciparum. All four groups of alkaloids exhibited antimalarial activity, albeit at different potencies. 6-Hydroxyhaemanthamine, haemanthamine and lycorine were found to be the most potent inhibitors of P. falciparum, while galanthamine and tazettine were least potent.45
Pancratium genus has attracted considerable attention due to the complex structural types of its alkaloids25 and significant therapeutic properties of some of these compounds.46 Earlier investigation on the Egyptian P. maritimum L., resulted in the isolation of lycorine, tazettine, pancracine, lycorenine, galanthamine, sickenbergine, homolycorine, haemanthidine, hippadine, demethylhomolycorine and trispheridirine47 and then haemanthamine, pseudolycorine and 11-hydroxyvittatine.48,49 Successively, from the same plant were isolated two 2-oxyphenathridinium betaine-type alkaloids: ungeremine and zefbetaine50 (34 and 36, Figure 7), known for their very interesting biological activities (i.e. cytotoxic, antibiotic51–53 and plant growth-regulatory51). The corrected structure of zefbetaine, deduced from a more accurate spectroscopic and chemical analysis, appeared in contrast with that reported in its first isolation from Zephyranthes flava.51 In fact, this structure determination was also supported by the partial synthesis of zefbetaine from pseudolycorine (38, Figure 7), an alkaloid isolated from P. maritimum L. together with α-dydrolycorine (39, Figure 7)48 and by comparison with its unnatural isomer iso-zefbetaine (37, Figure 7)50,54 Ungeremine is also found in Ungernia minor,55 Crinum americanum,56 C. asiaticum57 and Z. flava.51 Interestingly, this natural product can be obtained by microbial transformation of lycorine performed by a Pseudomonas sp. isolated from the rizosphere of S. lutea. Together with ungeremine and zefbetaine, two other phenanthridinuim derivatives were isolated and characterized as anhydrolycorinium chloride (42, Figure 7) and the dihydroderivative of ungeremine. The evidence obtained demonstrates that the transformation occurs through oxidative aromatization of lycorine.53 Many of these microbially aromatized compounds, including lactam 43 (criasiadine, Figure 7) showed good antibiotic activity when tested against Corynebacterium fascians.53
In the genus Zephyrantus, Z. parulla Killip appears in the history of Peru (by Padre Cobo, 1653) for treating tumors, and Z. Rosea (Spreng.) Lindll. has found use in China for treating breast cancer.1 The leaves of Z. candida (Lindl.) Herb. have been employed in Africa as a treatment for diabetes mellitus.58 The bulbs from a 1964 Hong Kong collection of these plants produced an extract, which showed good anticancer activity (cell line from human epidermoid carcinoma of nasopharynx, KB system) in the U.S. National Cancer Institute’s (NCI) research programs. Z. flava Roem & Schult is a deciduous herb native to America, which is naturalized in India. The plant grows abundantly in the upper Gangetic plains and bears yellow flowers during rainy season (July–August). The extract of its bulbs is used in the indigenous system of medicine for a variety of therapeutic purposes, e.g. in the treatment of diabetes, for ear and chest ailments, and against viral infections. The bulbs contain a complex mixture of alkaloids and non-nitrogenous aromatic compounds.59 Investigations carried out on the fresh mature fruits allowed the identification of the following compounds: eight tertiary alkaloids, crinamine, haemanthamine, lycorine, maritidine, methylpseudolycorine, pretazettine, haemanthidine and pseudolycorine, two lactam alkaloids, narciclasine and pratorimine, three glucosyloxy alkaloids, kalbreclasine, lycorine-1-O-β-D-glucoside, pseudolycorine-1-O-β-D-glucoside, and four phenanthridinuim betaine alkaloids, criasbetaine, ungeremine, zefbetaine and zeflabetaine (35, Figure 7).51 Z. rosae similarly grows in the upper Gangetic plain as well as the Sikkim region of the Eastern Himalayas up to 1000 m., and it is also grown in gardens as an ornamental flowering plant and for medicinal purposes. Extracts of flowers and bulbs are used for a variety of therapeutic purposes, which can be described in modern terms as immunomodulators. From the extract of fresh bulb were isolated four alkaloids: crinamine (22, Figure 4), haemanthamine (5, Figure 1), maritidine and (+)-epi-maritidine (40 and 41, Figure 7).60
2.2. Future Perspectives
Only 15% of the world’s known plant resources have been screened for their therapeutic values.61 Natural products provide mankind with more environmentally friendly alternatives to commercially produced medicines. Bulbous plants, though studied less intensively than herbs and trees for medicinal potential, have proven to contain a range of unique biologically active compounds. Valuable uses include their analgesic, anticancer, antimutagenic, immuno-stimulatory, anti-infective, antimalarial, cardiovascular and respiratory system effects.38,62 Traditional uses of some bulbous species, mainly belonging to the Amaryllidaceae and Hyacinthaceae, could provide useful leads in novel pharmaceutical developments. Since the biochemical content and pharmacological action of many of these species still remains poorly understood, future research should be invaluable in this respect. Some of the medicinally important Amaryllidaceae plants discussed in Section 2.1 are shown in Figure 8.
Figure 8.
Representative amaryllidaceae plants used in traditional medicine. Reprinted with with permission of Springer Science and Business Media (Reference 28).
2.3. Narciclasine and its Naturally Occurring Analogues: Plant Sources and Isolation Methods
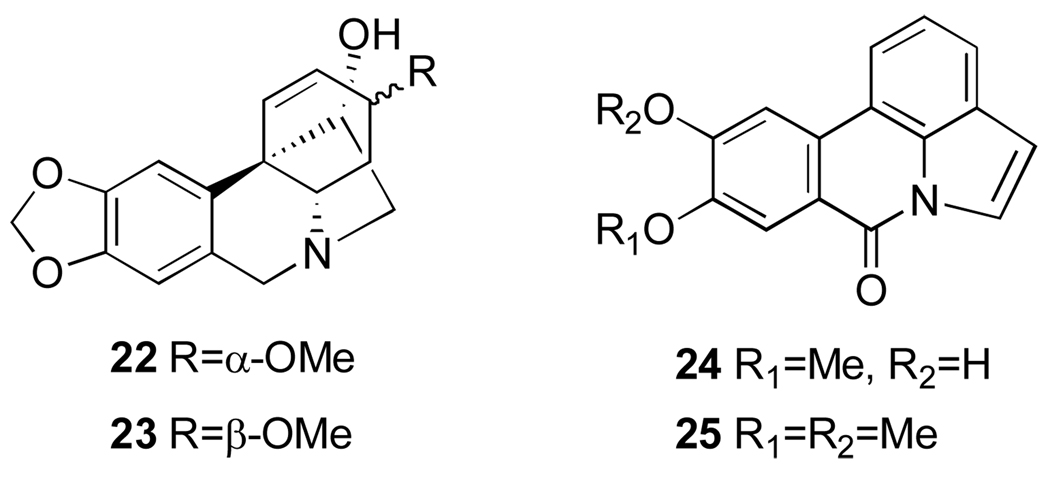
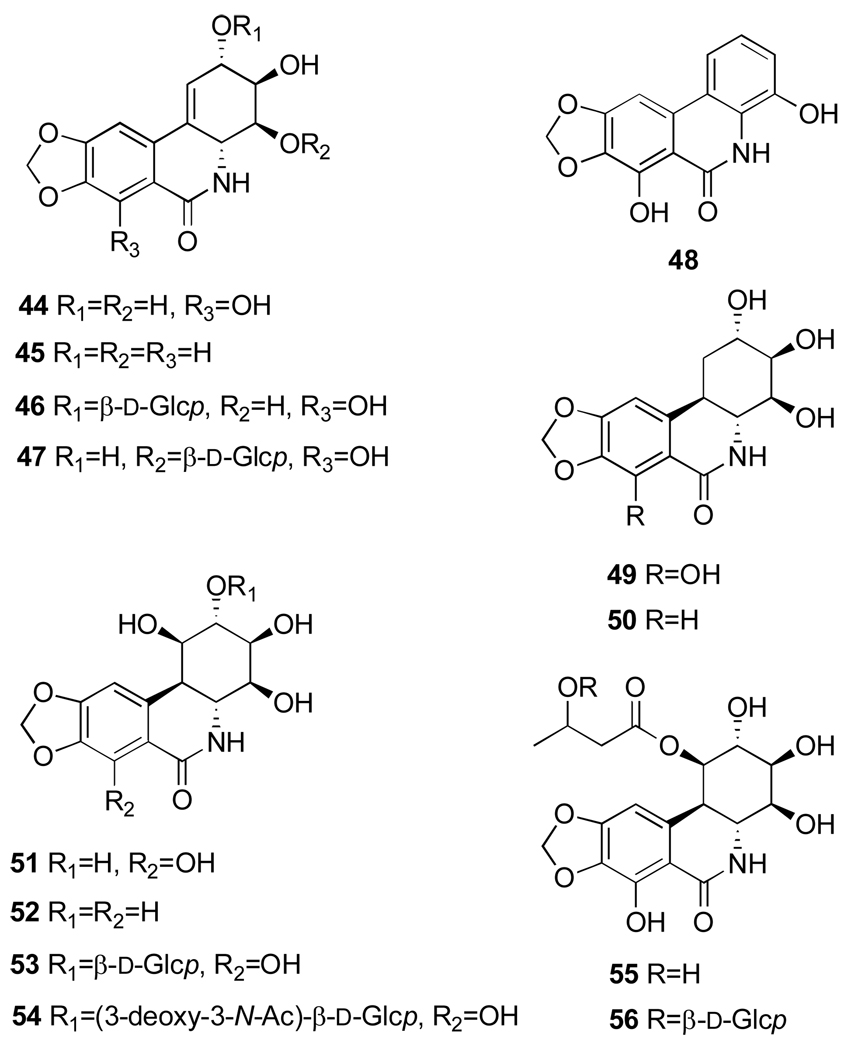
Narciclasine (1,3,4,5-Tetrahydro-2,3,4,7-tetrahydroxy[1,3]dioxolo[4,5-j]phenanthridin-6(2H)-one, 44, Figure 9) is not a basic metabolite, since the nitrogen is amidic in character, but its structure is closely related to the Amaryllidaceae lycorine type alkaloids. Narciclasine, isolated for the first time from different varieties of Narcissus bulbs63 in a systematic program aimed at the detection of antigrowth factors in plants, showed potent antimitotic activity.64 The structure of narciclasine was then revised by a chemical study65 and unambiguously assigned including the absolute stereochemistry by an X-ray analysis of the corresponding tetraacetate.66 Narciclasine is identical to lycoricidinol, independently isolated from Lycoris radiata.67 From the bulbs of the same Narcissus species a closely related metabolite was also isolated and named narciprimine (48, Figure 9).63 The early structure of narciprimine was successively revised by a chemical study65 and found to be identical to arolycoricidinol obtained by acid treatment lycoricidinol.63,67 Later, narciprimine and narciclasine were also isolated together from Zephyranthes tubispatha68 and Lycoris sanguinea.69
Figure 9.
Narciclasine (44), pancratistatin (51) and their corresponding naturally occurring analogues (45–50) and (52–56).
Promising activity of narciclasine has stimulated much effort toward the isolation and chemical characterization of its naturally occurring analogues from different Amaryllidaceae species. 7-Deoxynarciclasine (45, Figure 9, often referred to as lycoricidine in the literature) was isolated from L. radiata67 and L. sanguinea69 as well as Pancratium littorale70 (later reidentified as Hymenocallis littoralis, vide infra) and Haemanthus kalbreyeri.71 The last two species were also found to contain narciclasine and a new phenanthridone pancratistatin (51, Figure 9).70–73 Haemanthus kalbreyeri also produces 7-deoxypancratistatin and 2-O-β-d-glucoside of pancratistatin (named pancratiside) (52 and 53, Figure 9).71 Two other congeners of narciclasine, trans-dihydronarciclasine and its 7-deoxy derivative (49 and 50, Figure 9) were isolated correspondingly from Z. candida74 and Hymenocallis littoralis, H. caribea, H. latifolia.72 Thus, it appears that all three subtypes of these phenanthridones, namely narciclasine, pancratistatin and trans-dihydronarciclasine occur in both 7-hydroxy and 7-deoxy forms. Interestingly, narciclasine, pancratistatin and ungeremine were also found in lubber grasshopper Brachystola magna. This is the only isolation of isocarbostyril and phenanthridinuim alkaloids from an insect.75
Finally, a number of glycosyl derivatives of narciclasine and pancratistatin were isolated from different Amaryllidaceae species. As mentioned above, pancratiside is isolated H. kalbreyeri,71 kalbreclasine and 4-O-β-d-glucosylnarciclasine (46 and 47, Figure 9) are isolated respectively from Z. flava,51 H. kalbreyeri76 and P. maritimum.77 In addition, three others derivatives of pancratistatin, 1-O-(3-deoxy-3-N-Ac)-β-d-glucopyranosyl-pancratistatin (54, Figure 9), named telastaside, 1-O-(3-hydroxybutyryl)-pancratistatin and 1-O-(3-O-β-d-glucopyranosylbutyryl)-pancratistatin (55 and 56, Figure 9) were isolated from the smokey-grey moth Polytela gloriosa (54)78 and Z. carinata (55, 56).79
Narciclasine and its naturally occurring analogues were isolated from different commercially available, wild and cultivated in botanical gardens Amaryllidaceae species as summarized in Table 2.
Table 2.
The occurrence of narciclasine and its natural congeners in the Amaryllidaceae
| compound (structure) | Amaryllidaceae | content mg/kga |
ref. |
|---|---|---|---|
| narciclasine (lycoricidinol, 44) | Brachystola magna | 1.5 | 75 |
| Galanthus nivalis L. | 10 | 15 | |
| G. elwesii Hook | 25 | “ | |
| Narcissus spp. | 30–200 | “ | |
| Haemanthus puniceus L. | < 5 | “ | |
| H. kalbreyeri | 4.4b | 71 | |
| Hymenocallis littoralis | ND | 63 | |
| H. speciosa | 2.0 | 82 | |
| H. expansa | 3.0 | “ | |
| Leucojum aestivum L. | 30 | 15 | |
| L. Vernum L. | 50 | “ | |
| Lycoris radiata Herb. | 41.6 | 67 | |
| L. saguinea | ND | 69 | |
| N. tazetta L. | 65 | 81 | |
| Pancratium littorale | ND | 72 | |
| P. maritimum L. | 50 | 15 | |
| Sprekelia formosissima (L.) Herb | > 5 | “ | |
| Sternbergia lutea (L.) Ker-Gawl | < 5 | “ | |
| Vallota speciosa L’Her | < 5 | “ | |
| Zephyranthes flava | 135.0c | 51 | |
| Z. tubispatha | ND | 68 | |
| narciprimine (48) | L. sanguinea | ND | 69 |
| Narcissus spp. | 3–5 | 63 | |
| Z. tubispatha | ND | 68 | |
| 7-deoxynarciclasine (lycoricidine, 45) | Lycoris radiata Herb. | 31.9 | 67 |
| L. sanguinea | ND | 69 | |
| H. littoralis | 222.2 | 70,72 | |
| H. kalbreyeri | 1.7b | 71 | |
| trans-dihydronarciclasine (49) | Z. candida | ND | 74 |
| 7-deoxy-trans-dihydronarciclasine (50) | H. littoralis | 33.0 | 72 |
| pancratistatin (51) | B. magna | 2.5 | 75 |
| H. kalbreyeri | 15.8b | 71 | |
| H. littoralis | 144.4 | 70,72,73 | |
| H. expansa | 4.0 | 82 | |
| H. pedalis | 28.9 | “ | |
| H. sonorensis | 3.0 | “ | |
| H. speciosa | 3.0 | “ | |
| H. variegata | 6.0 | “ | |
| P. maritimum | 6.0 | “ | |
| Z. grandiflora | 19.1 | 80 | |
| Z. carinata | 7.5d | 79 | |
| 7-deoxypancratistatin (52) | H. kalbreyeri | 2.7b | 71 |
| kalbreclasine (46) | H. kalbreyeri | 3.1b | 76 |
| Z. flava | 165.0c | 51 | |
| 4-O-β-D-glucopyranosylnarciclasine (47) | P. maritimum | 3.3 | 77 |
| pancratiside (53) | H. kalbreyeri | 19.6b | 71 |
| telastaside (54) | Polytela gloriosa | 400 | 78 |
| 1-O-(3-hydroxybutyryl)pancratistatin (55) | Z. carinata | 23.7d | 78 |
| 1-O-(3-O-β-D-glucopyranosylbutyryl) pancratistatin (56) | Z. carinata | 43.8d | “ |
Fresh bulbs or insects (B. magna and P. gloriosa);
Fresh roots;
Fresh seeds;
Air dried bulbs
ND = Not determined
The processes used for the extraction and purification of these isocarbostyril plant constituents differ in the methodology and yields of products, which are frequently obtained in crystalline form (Table 2). The major stages of these processes include extraction of the fresh plant material with a single or a mixture of solvents, purification of the crude organic extract or the extract liberated from bases (including essentially alkaloids) by chromatography, followed by a final crystallization of the isocarbostyril compounds. The established processes could be summarized as follows.
Method A
The fresh grounded bulbs of the analyzed Amaryllidaceae plant are extracted with 95% ethanol and left to macerate for 1 day. The ethanol extract is filtered, concentrated, subjected to a complex process of clearing and then extracted with n-butanol. The n-butanol extract is fractionated by a silica gel column and crude phenanthridones so obtained are crystallized from a suitable solvent.63
Method B
The fresh ground bulbs of the analyzed Amaryllidaceae plant are extracted with methanol for a longer period of time (3 days). The crude concentrated extract is subjected to successive extractions with ethyl acetate and finally fractionated by silica gel column. The crude phenanthridones are crystallized from a suitable solvent mixture.67 Alternatively, the residual aqueous phase of the initial methanol extract is extracted in succession with petrol, ethyl acetate and n-butanol. The residue of n-butanol extract is dissolved in a small volume of methanol-dioxane, filtered and the filtrate is kept at room temperature until a brown solid separates. The latter is dissolved in methanol and fractionated by a silica gel column to yield crude phenanthridones, which are crystallized from a suitable solvent.76
Method C
The fresh plant material is macerated in aqueous methanol in a high-speed blender, filtered and the filtrate evaporated under reduced pressure to give a viscous brown slurry. This was triturated with hot petrol to remove fatty acid materials and weakly polar alkaloids. The petrol-insoluble fraction is treated with aqueous acetic acid, and a brown solid that separates is collected. The cleared acid solution is extracted with ethyl ether, then basified and extracted in succession with chloroform, ethyl acetate and n-butanol. These organic fractions are independently worked on to purify alkaloids, phenanthridones as well as phenanthridinium and glycosyl alkaloids, respectively.51
Method D
This method involves an initial extraction of ground plant material (essentially bulbs) with a methanol-methylene chloride (1:1 v/v) for a long (15–20 days) period of time. The methylene chloride phase is separated by addition of water. The aqueous phase is adjusted by addition of further amounts of methanol and methylene chloride and the bulbs are re-extracted for the same amount of time. Decantation and addition of water separates the methylene chloride phase. The aqueous phase is concentrated, centrifuged and the resulting clear solution is extracted with n-butanol. The n-butanol extract is concentrated and its aliquot is dissolved in methanol. The addition of acetone allows frequently insoluble material to separate. The treatment of this precipitate with methanol causes pancratistatin to separate.70,72,80
Method E
Such an alternative method utilizes mucilage secreted from bulbs. The plant bulbs are chopped in small pieces and immersed in distilled water for 30 hours to allow the mucilage to leach out and then washed four times. The mucilage solutions are combined, lyophilized and the residual powder is extracted with 70% ethanol using a Soxhlet. The extract is concentrated, extracted with ethyl acetate and the corresponding crude extract is fractionated by a silica gel column yielding a crystalline isocarbostyril constituent.81
Method A was applied to extract narciclasine from thirty two species and varieties of the Narcissus genus resulting in the isolation of the pure phenanthridone in the range of 30–200 mg/kg.15 The same method was used to extract narciclasine from other Amaryllidaceae genera (Table 2),15 but a more simplified procedure was used when the extraction was carried out on daffodils particularly rich in narciclasine avoiding essentially the column chromatographic fractionation.63 As observed for other Amaryllidaceae alkaloids (e.g. the alkaloids extracted from A. belladonna L.)33 the content may vary during the year at the different stages of plant growth: in N. incomparabilis Mill var. helios 200 mg/kg of narciclasine were found in march at the flowering stage, but only 100–120 mg/kg in November.15 The same method was used to extract and purify in very low yield narciprimine from Narcissus spp. (Table 2).63
Method B was used to extract narciclasine and 7-deoxynarciclasine from L. radiata Herb.; the yields for each isocarbostyril metabolite, crystallized respectively from water and methanol,67 are given in Table 2. Similarly, narciclasine, kalbreclasine and 7-deoxypancratistatin were isolated from H. kalbreyeri71,76 applying a minor modification of this method.
Method C was used to purify narciclasine and kalbreclasine from Z. flava seeds. In this case, the residue obtained from the ethyl acetate extraction was treated as in Method B.51
Method D was used to purify narciclasine and some of its congeners, namely pancratistatin, 7-deoxynarciclasine and 7-deoxy-trans-dihydronarciclasine from Z. grandiflora,80 P. maritima82 and some Hymenocallis spp., which represent a broad geographical distribution, such as H. littoralis (Hawaii), H. caribea and H. latifolia (Singapore)72 and H. speciosa and H. variegated (Singapore), H. pedalis (Seychelles), H. expansa (Bermuda), and H. sonoranesis (Mexico).82 A similar procedure was also used to isolate narciclasine, pancratistatin and ungeremine from the insect Brachystola magna.75 This method of extraction followed by purification of the n-butanol extract by a silica gel preparative TLC was used to isolate telastaside (54) from P. gloriosa along with several Amaryllidaceae alkaloids belonging to the subgroups of pyrrolo- and 5,10b-ethano-[de]phenanthridine (Table 1). The larva of this insect is a specialized herbivore, which uses Amaryllidaceae plants, avoided by other insects, as an ecological niche.78 Telastaside represents the first isolation of an isocarbostyril derivative from an insect. An improved laboratory and pilot plant scale techniques have also been developed for the isolation of pancratistatin from the difficult-to-separate mixture of narciclasine and its 7-deoxy derivative occurring in H. littoralis. This method eliminates the use of n-butanol extraction and Sephadex LH-20 chromatography steps that are very effective for laboratory scale isolation of pancratistatin, but costly on a large scale. The method proved efficient and rapid.83 Finally, a scale-up method for the isolation of phenanthridones (pancratistatin and related isocarbostyrils) from H. littoralis was developed. The bulbs were subdivided and placed in six a big steel containers (120 L). Technical grade methanol was added to each vessel. After extraction for 20–48 days, the methanol phase was removed and evaporated to give a water concentrated solution. A second extraction was carried out with the same solvent for 16–24 days. The concentrated aqueous phases were combined and extracted five times with methylene chloride. The organic phase were concentrated to dryness and the residue partitioned with ethyl acetate and then with n-butanol. These fractions were evaporated to dryness and dissolved in methanol, and the addition of acetone allowed lycorine to separate. The soluble fraction was concentrated and dissolved in methanol, filtered and fractioned by gel-permeation using a Sephadex LH-20 column chromatography eluted with methanol. Some fractions contained the above mentioned phenanthridones pancratistatin, narciclasine and its 7-deoxy and 7-deoxy-trans-dihydro derivatives.84
Method E was applied to isolate narciclasine from the bulbs of N. tazetta L.81 The phenanthridone was obtained in the same yield when bulbs of the same plant were extracted with 95% ethanol according the classical method A.63
Method F
An alternative improved method F in respect to all the above described procedures was developed by Evidente. It avoids the use of organic solvents (ethanol, methanol or methanol combined with methylene chloride). The method is based on the acidic properties of the amidic nitrogen of narciclasine. The air dried and milled plant material is extracted with an aqueous solution of sodium hydroxide. The mixture is left to macerate overnight at room temperature and then it is filtered and centrifuged. The aqueous solution is acidified at pH 2 and extracted three times with ethyl acetate. The organic extracts are combined, dried and evaporated under reduced pressure and the residue is purified by silica gel column. Narciclasine is crystallized from acetic acid.85 When this procedure was applied to S. lutea Ker-Gawl bulbs, narciclasine was obtained in higher yield (20 mg/kg) than that (<5 mg/kg) obtained using the classical ethanol extraction method A.63,15
3. Biosynthetic Studies
3.1. Biosynthesis of Amaryllidaceae Alkaloids
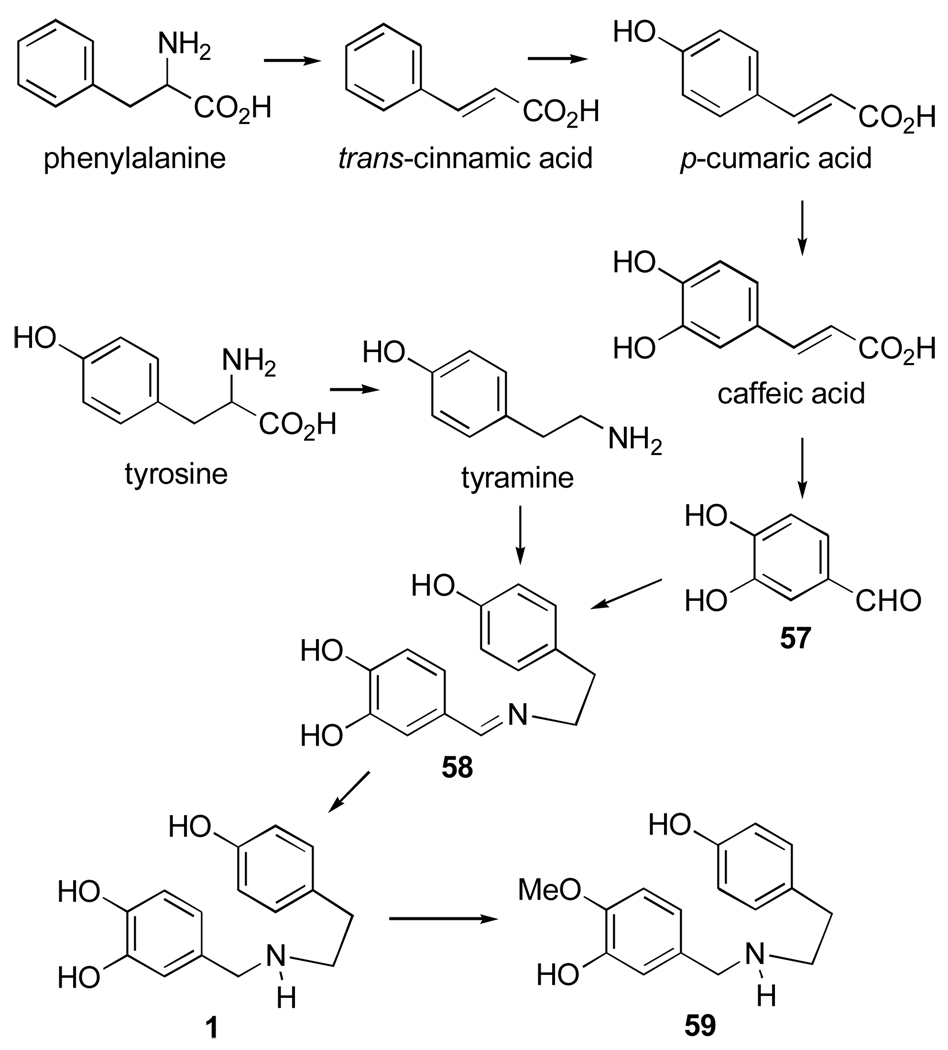
Several studies on the biosynthesis of Amaryllidaceae alkaloids belonging to different ring-type subgroups have been carried out and reviewed.86 O-methylnorbelladine (59, Figure 10), which plays a central role in the biosynthesis of these alkaloids, is formed from phenylalanine and tyrosine by the route illustrated in Figure 10. Phenylalanine is a precursor of protocatechuic aldehyde (57), which condenses with tyramine, derived from tyrosine, to yield Shiff base 58. Reduction of the latter affords norbelladine (1), which is methylated to O-methylnorbelladine (59).
Figure 10.
Biosynthesis of O-methylnorbelladine (59).
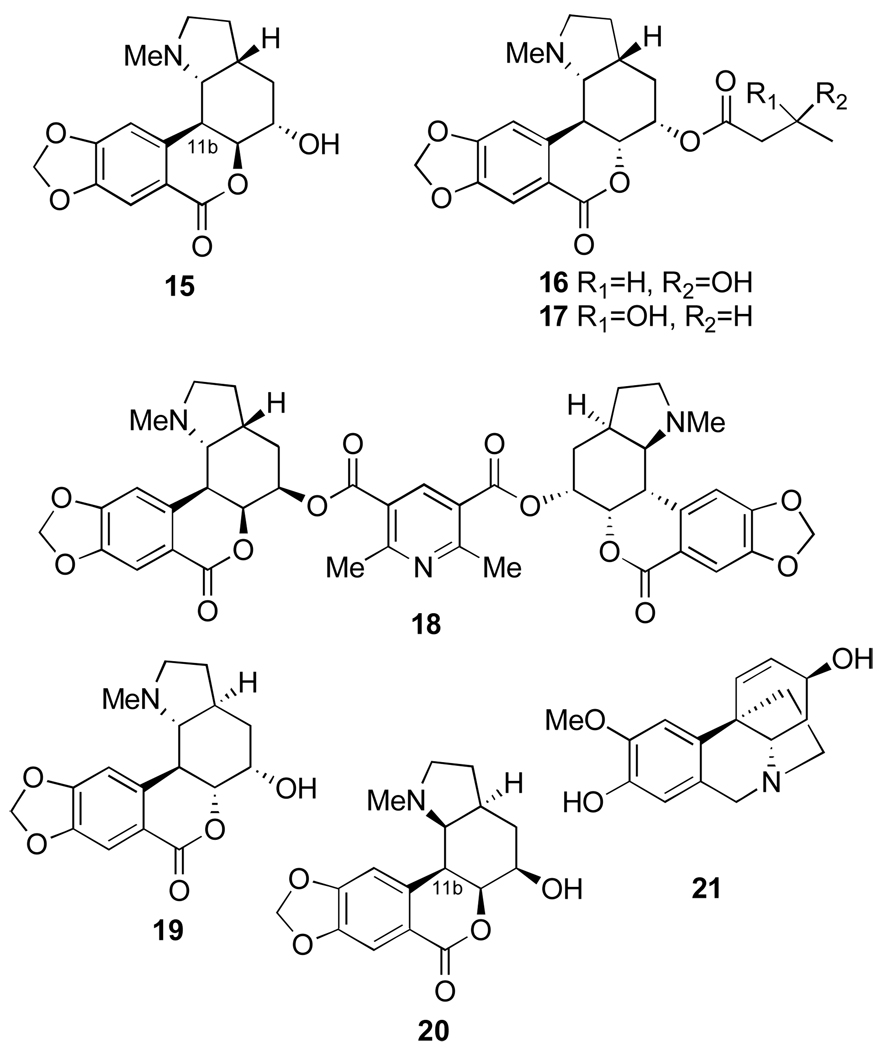
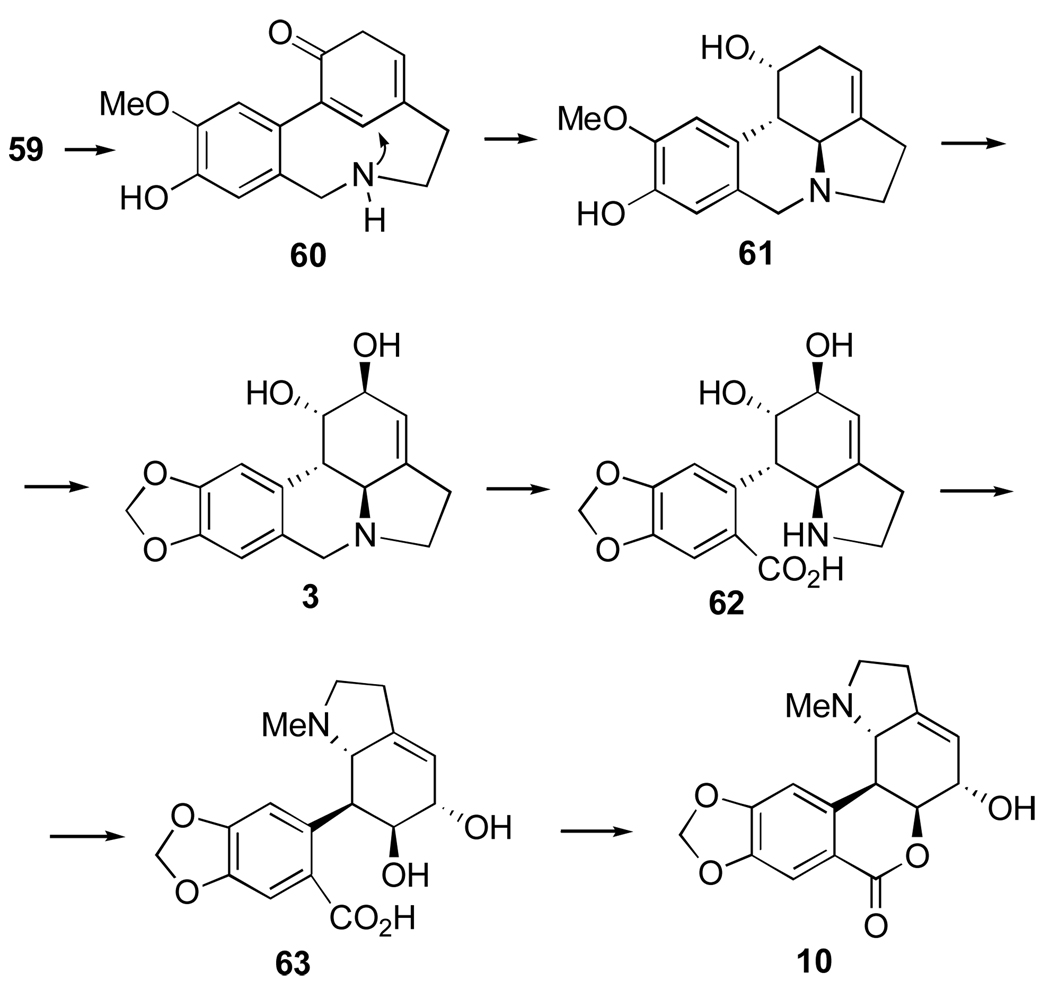
Para-ortho oxidative coupling of O-methylnorbelladine (59) yields the transient intermediate 60 (Figure 11). Nucleophilic attack of the amino group on the dienone produces norpluvine (61). This, in turn, by allylic oxidation and formation of the methylenedioxy group from the O-methyl group of 5987 yields lycorine (3). This mechanism is supported by the studies involving appropriately labelled precursors.88–90 Lycorine is the precursor of hippeastrine (10) and the plausible intermediates are 62 and 63 (Figure 11).
Figure 11.
Biosynthesis of Amaryllidaceae alkaloids by para-ortho oxidative coupling of O-methylnorbelladine.
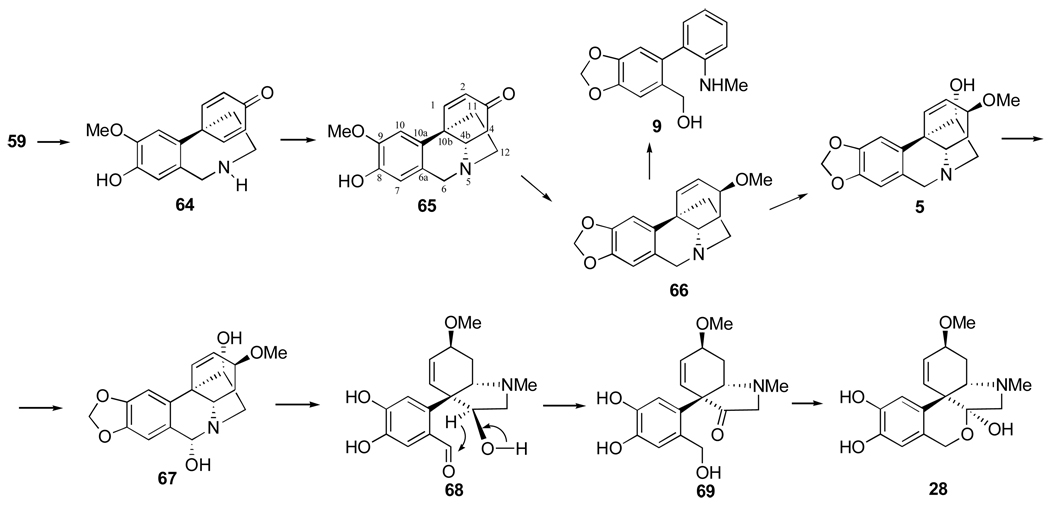
Para-para coupling of O-methylnorbelladine leads to dienone intermediate 64 (Figure 12), which by intramolecular Michael addition leads to noroxomaritidine (65).90 The latter by a series of successive steps, including the conversion of the O-methyl ether of 5987 into the methylenedioxy group, leads to alkaloids such as buphanisine (66). Further oxidation yields haemanthamine (5),91–94 which has been shown to be a precursor of haemanthidine (67) and tazettine (28). The conversion of intermediate 68 to 69 involves an intramolecular hydride transfer.95
Figure 12.
Biosynthesis of Amaryllidaceae alkaloids by para-para oxidative coupling of O-methylnorbelladine.
The non-heterocyclic alkaloid ismine (9), which possesses a C-13 skeleton similarly to narciclasine, is present in trace amounts in a number of Amaryllidaceae plants. Feeding experiments showed biological derivation of ismine from 59 by para-para coupling, followed by a late elimination of the “ethano” bridge from the C-15 crinane skeleton. Further evidence was obtained by incorporation in Sprekelia formosissima of triply labelled [6,10-3H2; 12-14C]noroxomaritidine (65) into 9 as well as 5 and 67.96 In addition, a possible link between the biosynthesis of haemanthamine, haemanthidine and that of ismine was demonstrated. Thus, using a doubly labelled [6R,10-3H2]noroxomaritidine as a precursor, evidence of the stereospecificity of the benzylic oxidation in the biosynthesis of 9 was established, in that a pro-R-hydrogen from C-6 of noroxomaritidine is removed, which was also observed in the conversion of 5 into 67.96
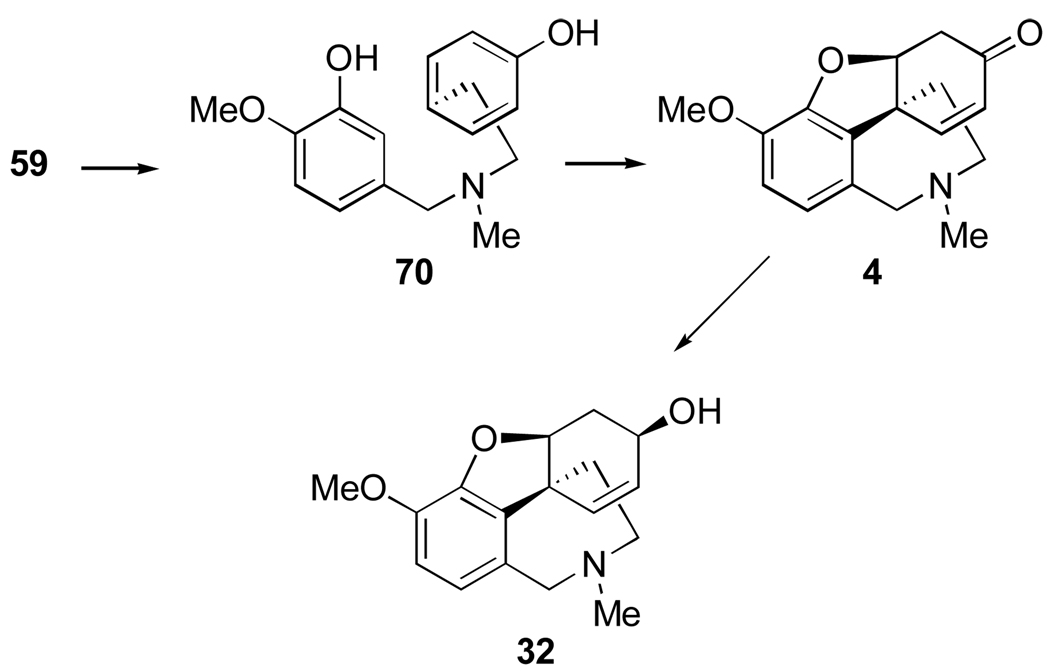
The inverse ortho-para oxidative coupling of O-methylnorbelladine represents a third biosynthetic pathway and it leads to narwedine (4) and galanthamine (32) as illustrated in Figure 13. In this case the N-methylation to the intermediate 70 is apparently essential prior to oxidative coupling.92,97–99
Figure 13.
Biosynthesis of Amaryllidaceae alkaloids by a third mode of oxidative coupling of O-methylnorbelladine.
3.2. Biosynthesis of Narciclasine
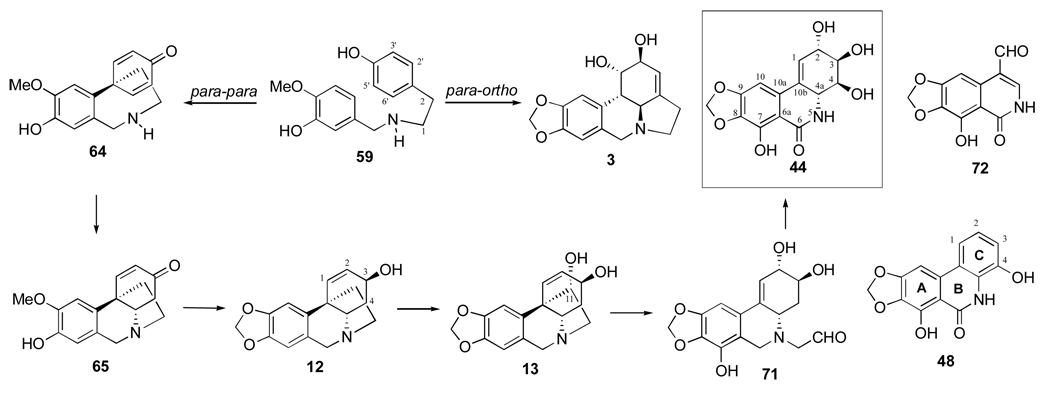
Several studies have been carried out to determine the biosynthesis pathway, which leads from O-methylnorbelladine (59) to narciclasine (44), using different and specifically labeled precursors.25
As discussed above, the biosynthesis of several Amaryllidaceae alkaloids involves different phenolic couplings, which generate, through independent pathways, alkaloids that belong to different subgroups, such as lycorine (3) and haemanthamine (5). Since alkaloids from both of these subgroups are often isolated from the same plants as narciclasine, the latter might arise by either para-ortho (Figure 11) or para-para (Figure 12) pathways with a late degradation of the C-15 skeleton (Figure 14).
Figure 14.
Biosynthesis of narciclasine (44).
The first experiment involved the study of tritium incorporation from a suitable ortho-labeled [3,’5’-3H2; 14C-O-methyl]-59 in daffodils into lycorine, haemanthamine and narciclasine, followed by chemical investigations of specific sites of incorporation in the radioactive narciclasine and haemanthamine. Lycorine, haemanthamine and narciclasine showed a 50, 100 and 75% tritium label retention, respectively. The 75% retention observed in narciclasine compared with the expected 50% retention shown by lycorine pointed to the derivation of this isocarbostyril from 59 following the para-para coupling pathway.25 Indeed, narciclasine, upon aromatization to narciprimine (48) in acidified heavy water, lost ca. 30% of the 3H activity without picking up of deuterium from the solvent. Since in this conversion the hydrogen atoms at positions C4 and C4a of narciclasine are removed, the tritium, which is lost, should be located at one or both of the above mentioned positions. However, because of the lack of radioactivity of narciclasic aldehyde (72, Figure 14), which retains the C4a hydrogen, the removed 3H atom was assigned to position C4.25 The results support the derivation of lactam 44 from 59 along the para-para oxidative coupling pathway.
Further evidence was obtained by incorporation a meta-labeled [2’,6’-3H2; 14C-O-methyl]-59 in narciclasine, haemanthamine and norpluvine, followed by similar chemical investigations of the specific incorporation sites in radioactive narciclasine. Narciclasine (44) and haemanthamine (5) incorporated the precursor without significant 3H loss, while norpluvine (61) showed ca. 15% tritium loss. The 3H labels were assigned to positions C1 and C4a of narciclasine because narciclasic aldehyde (72) retained all the 3H activity and narciprimine (48) formed with 50% 3H loss. The latter lost all of the 3H activity after the hydrogens ortho and para to the phenolic group of the C ring were substituted for deuterium by base-catalyzed exchange of a suitable derivative.25
These tracer experiments confirmed that narciclasine is biosynthesized from O-methylnorbelladine by para-para coupling followed by a late elimination of two carbon atoms.100 Further work has been carried out to define the nature of the intermediates between 59 and narciclasine. It involved the use of suitably labeled intermediates, such as racemic [1,4b-3H2] noroxomaritidine (65) and racemic [3-3H3]vittatine (12). The radioactive labels of both noroxomaritidine and vittatine were incorporated into narciclasine, which was subjected to chemical degradation to investigate the specific sites of radioactivity incorporation. The labeling pattern of radioactive narciclasine was determined by degradation to narciprimine (48), which, after methylation, was brominated at positions C1 and C2 as well as by oxidation of narciclasine to narciclasic aldehyde (72). The obtained relative 3H-molar activities of the degradation series point to the following conclusions: i) the conversion of 59 into narciclasine proceeds through its phenol-coupled product 65, and through intermediates bearing a pseudoaxial hydroxyl group at the position C3 of the crinane skeleton; ii) the hydrogen removal from position C3 during the biosynthesis does not occur; iii) enzymes which are able to form a methylendioxy group of the crinane skeleton irrespectively of the oxidation level at C-3 are involved.101
Additional experiments showed tritium incorporation from suitably labeled [2,4-3H2]11-hydroxyvittatine (13) in P. maritimum into narciclasine (5%) and haemanthamine (3%). Radioactive 13 had been previously obtained from P. maritimum in several feeding experiments with [3’,5’-3H2]-59. The labeling pattern of the radioactivity of narciclasine was determined by chemical degradation of narciclasine into narciprimine (48), which in confirmation of the previous findings, retained about 70% of the starting tritium activity. The results obtained establish the intermediacy of 13 in the biosynthesis of narciclasine and confirm the hydroxylation β to the nitrogen in the degradation of the alkaloidal skeleton.102 These findings also provide experimental evidence for the relevant steps in the biosynthetic route leading from 59 to narciclasine involving the loss of the “ethano” bridge from the intermediates possessing the crinane skeleton. 11-hydroxyvittatine (13) could be the last intermediate, possessing the “ethano” bridge, and it might undergo the C-C bond cleavage in a mechanistically acceptable manner. Alkaloid 13 might undergo a retro-Prins reaction to give an intermediate like 71, which in a few oxidative steps could give narciclasine.103 A similar mechanism should occur in the biosynthesis of the alkaloid ismine (9), which possesses a C-13 skeleton as does narciclasine, and as mentioned above, is derived from oxocrinine through elimination of the “ethano” bridge.
3.3 Biosynthesis of Narciclasine’s Congeners
There are still now no reports in the literature on the biosynthesis of the narciclasine and pancratistatin analogues. Some indirect results on the biosynthesis of pancratistatin were obtained by developing a biotechnological approach, which involved a large culture tissue cloning operation of H. littoralis bulbs. These “seedlings” replicas were transplanted to potting soil under greenhouse conditions and the final planting in native soil, exposing the plant to light and temperature conditions typical of arid Tempe, Arizona.104 The plant tissue culture → greenhouse → field production sequence was successively utilized to increase an original 1.5 kg of wild H. littoralis bulbs to some 60,000 bulbs at present. In Sonoran Desert (central Arizona), the tropical H. littoralis was found to reach maximum pancratistatin content in October and a minimum in May. Generally pancratistatin was accompanied by lesser yields of narciclasine, 7-deoxynarciclasine and its trans-dihydroderivative.83
4. Synthetic Modifications of Narciclasine and its Congeners
4.1 Early Studies
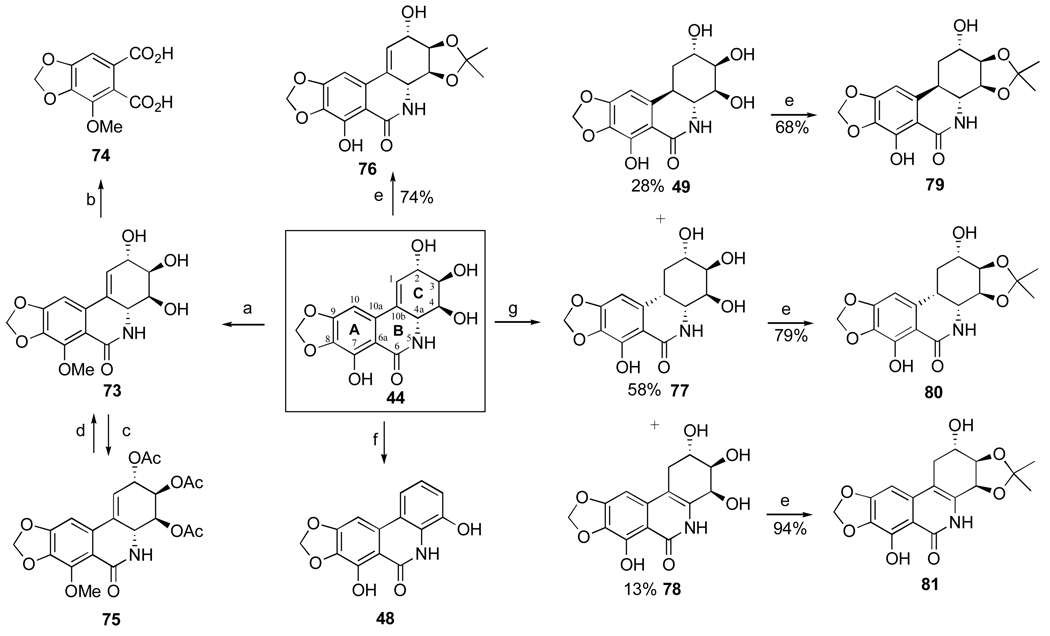
The first investigation of narciclasine’s chemistry was conducted by Piozzi et al.63 and Mondon et al.105 to confirm the structure of this important natural product and generate a small library of analogues for biological structure-activity studies. Thus, narciclasine is selectively methylated at the phenolic hydroxyl with diazomethane in ethanol (44 → 73, Scheme 1). The resulting 7-methoxy derivative is easily oxidized with cold KMnO4 to cotarnic acid (74). Acetylation of the three hydroxyl groups in 73 is achieved with acetic anhydride in pyridine and triacetate 75 is converted back to 73 when reacted with Ba(OH)2 in methanol. Treatment of 44 with concentrated HCl leads to elimination of two molecules of water in ring C providing aromatic analogue 48, which is a natural product narciprimine and it is found in narcissus bulbs in small quantities (Section 2.3).
Scheme 1.a.
a Reagents and conditions: (a) CH2N2 (excess), EtOH, 48 h; (b) KMnO4, H2O, 0 °C, 48 h; (c) Ac2O, py, 48 h, rt; (d) Ba(OH)2, MeOH, reflux; (e) acetone, HC(OEt)3, TsOH, reflux, 15 h; (f) conc. HCl; (g) H2, Pd/CaCO3, EtOH, 3 h.
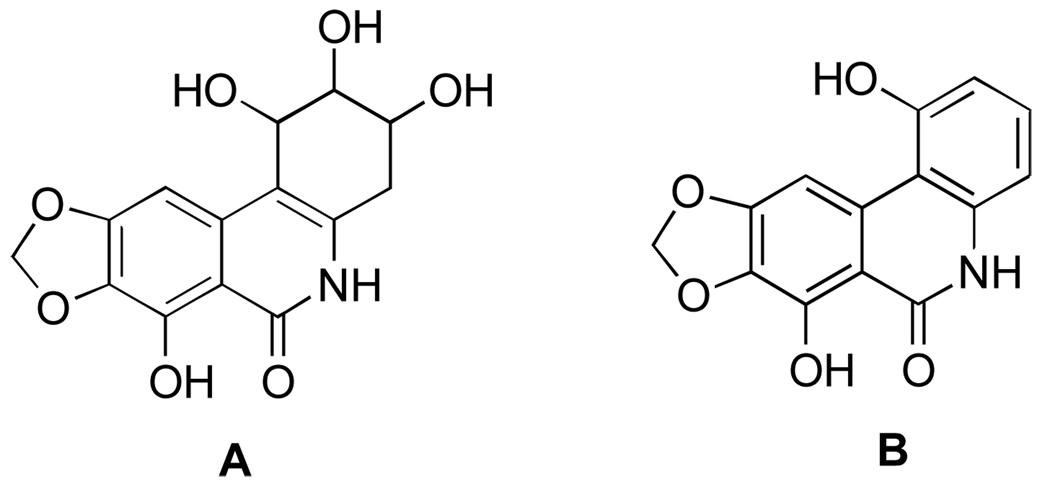
While these transformations were later used by other investigators to make narciclasine’s derivatives, the yields for these reactions were not provided in the original report.63 Furthermore, the positioning of the hydroxyl groups in narciclasine was erroneously established as in A, and thus the structure of narciprimine was incorrectly deduced as B (Figure 15).
Figure 15.
Incorrect original structure assignment of narciclasine (A) and narciprimine (B).
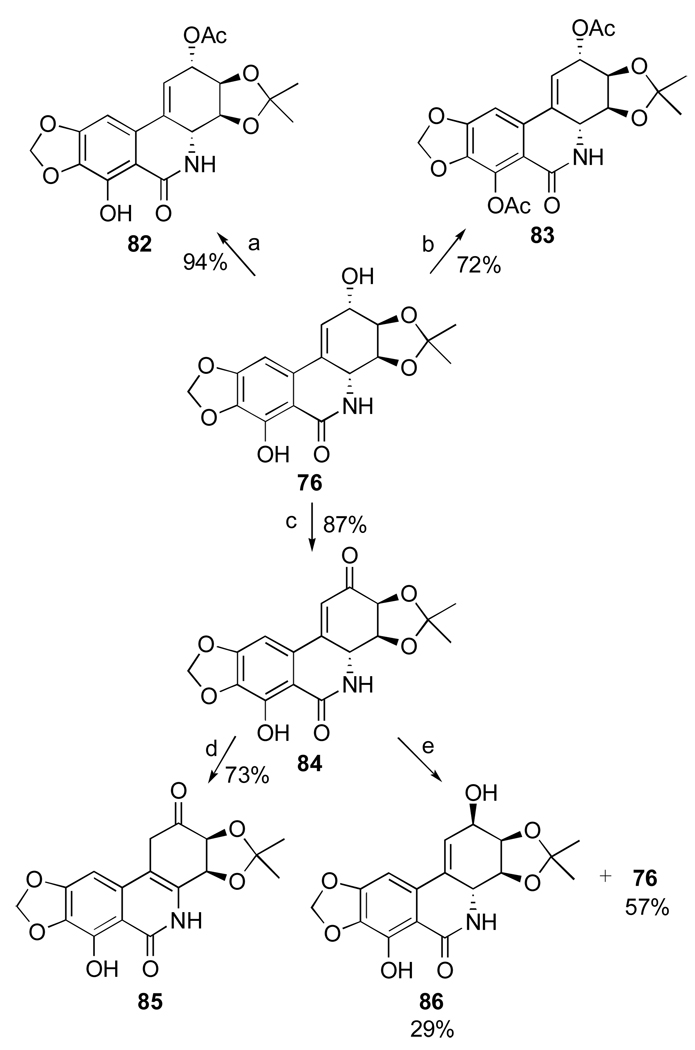
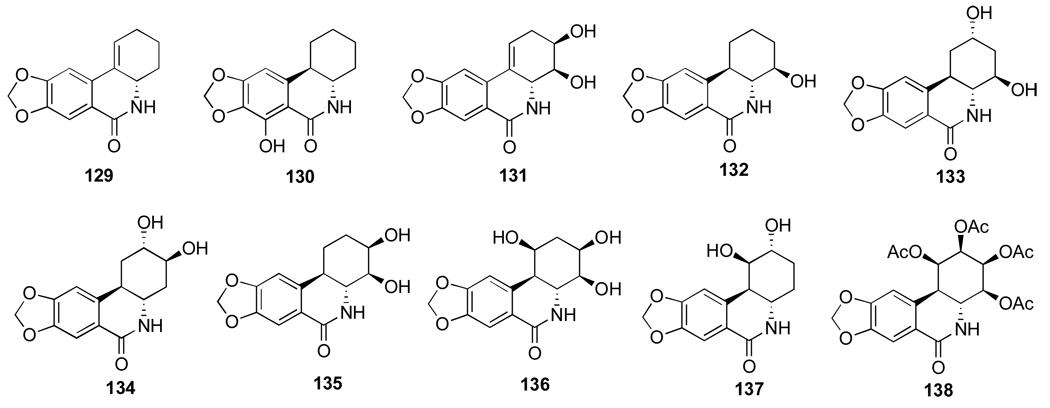
Mondon and Krohn105 studied hydrogenation of the double bond in 44 and found that along with expected trans and cis products 49 and 77 the reaction results in the formation of isomeric compound 78, named isonarciclasine. trans-Dihydronarciclasine 49 is a naturally occurring narciclasine analogue and it was later isolated from Z. candida as was discussed in Section 2.3. Selective functionalization of the hydroxyl groups in these products as well as narciclasine itself is achieved through the exclusive formation of C3,C4-acetonides 76, 79, 80, 81 when the starting tetraols are treated with triethylorthoformate and p-TsOH in acetone. Fast acetylation of the C2-hydroxyl in acetonide 76 permits facile differentiation between the two remaining hydroxyl groups (Scheme 2). Although the reaction utilizes excess of acetic anhydride in pyridine, it is stopped after 3 hours of stirring at room temperature to give monoacetylated product 82 in excellent yield. When the components are allowed to react for 3 days, diacetyl acetonide 83 is formed. In addition, the C2-hydroxyl is smoothly oxidized to the corresponding ketone 84 with MnO2 in THF. This compound is thermodynamically unstable and undergoes double bond isomerization to give 85. Reduction of the keto function with sodium borohydride in ethanol proceeds non-selectively producing two isomeric secondary alcohols 86 and 76. These can be easily separated and, therefore, this transformation allows for the preparation of C2-epi-narciclasine.
Scheme 2.a.
a Reagents and conditions: (a) Ac2O, py, 3 h; (b) Ac2O, py, 3 d; (c) MnO2, THF, 2.5 h; (d) silica gel, THF; (e) NaBH4, EtOH, 0.5 h, rt.
4.2 Studies by Pettit’s Group and Semisynthesis of Pancratistatin from Narciclasine
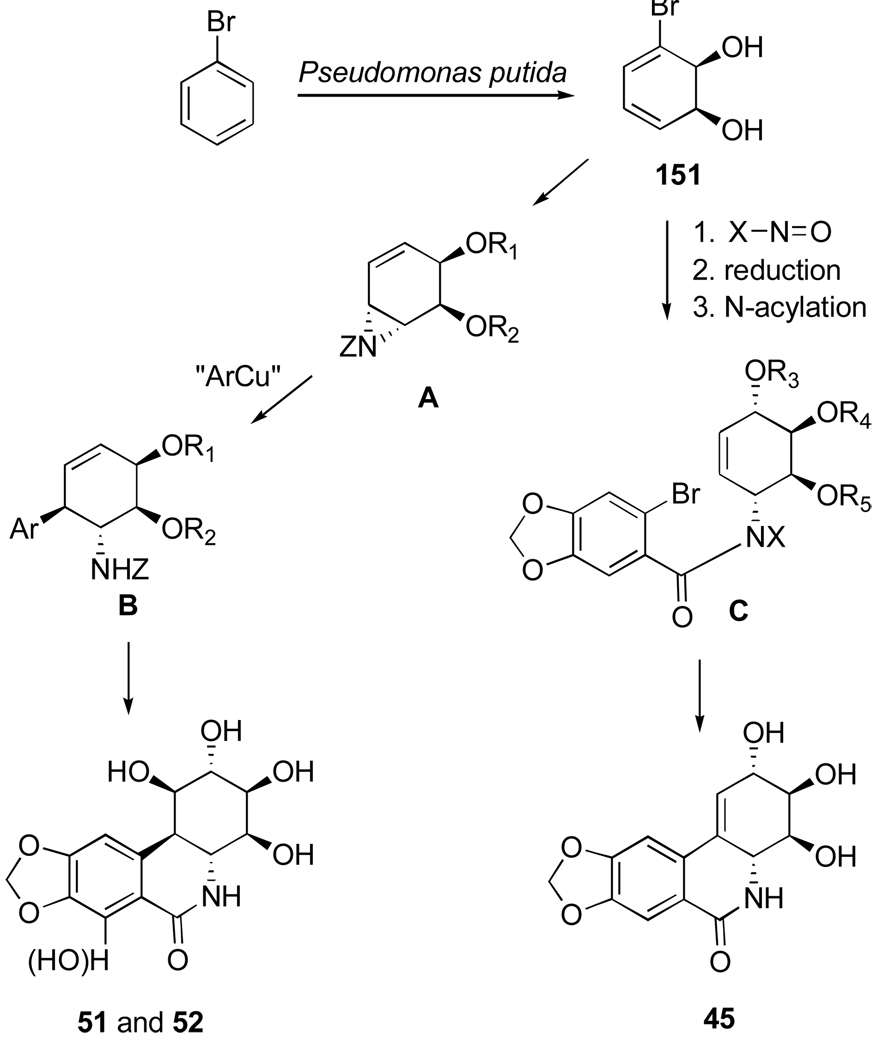
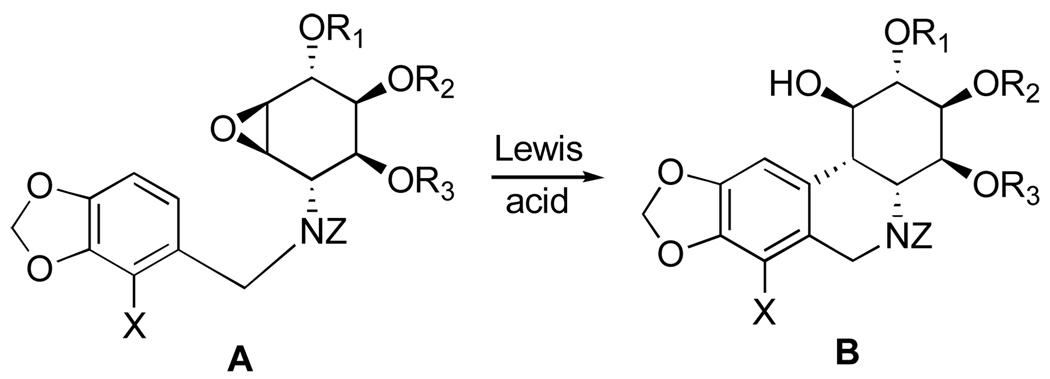
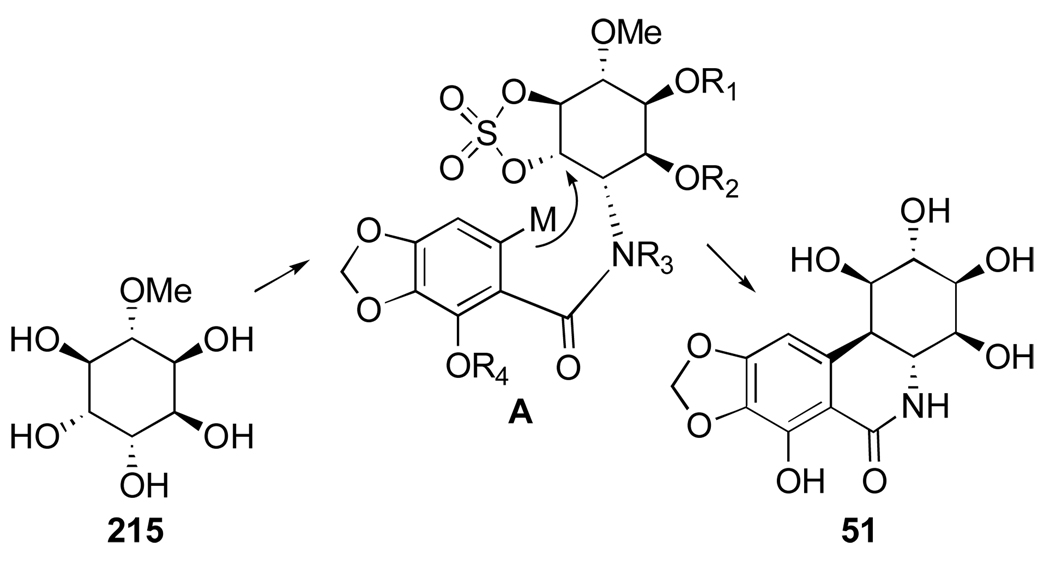
As part of an effort to develop a semisynthesis of pancratistatin from the more naturally abundant narciclasine, Pettit’s group has extensively studied chemistry associated with narciclasine, generating a number of interesting analogues and eventually accomplishing such a relay synthesis of pancratistatin.
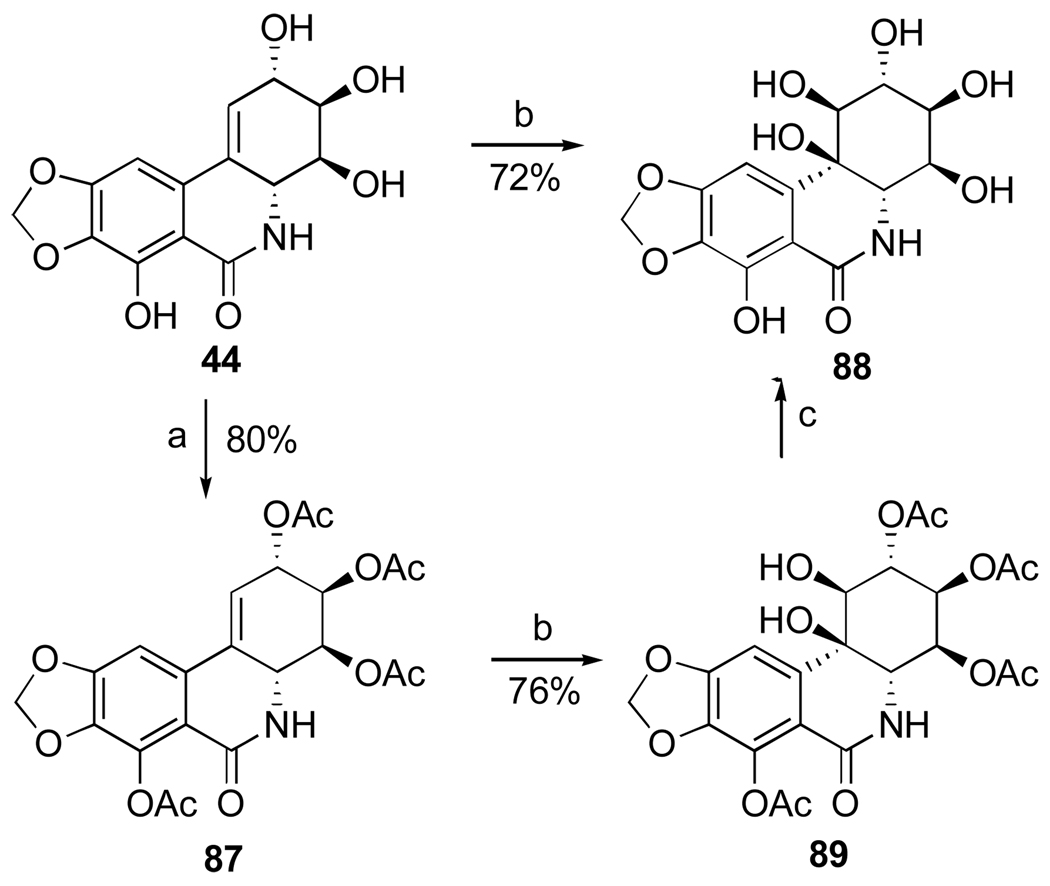
Thus, the double bond in narciclasine (44) as well as in its tetraacetate derivative (87) undergoes exclusive β-face dihydroxylation with catalytic OsO4 in the presence of hydroquinine 4-chlorobenzoate (HQ) to give diols 88 and 89 respectively (Scheme 3). Interestingly, the use of the pseudoenantiomeric catalyst hydroquinidine 4-chlorobenzoate (HQD) does not change this stereochemical outcome. Deacetylation of 89 with methanolic ammonia gives 10b-R-hydroxypancratistatin (88).106,107
Scheme 3.a.
a Reagents and conditions: (a) Ac2O, py; (b) OsO4 (cat.), NMO, HQ, DMF/H2O; (c) NH3/MeOH, CH2Cl2.
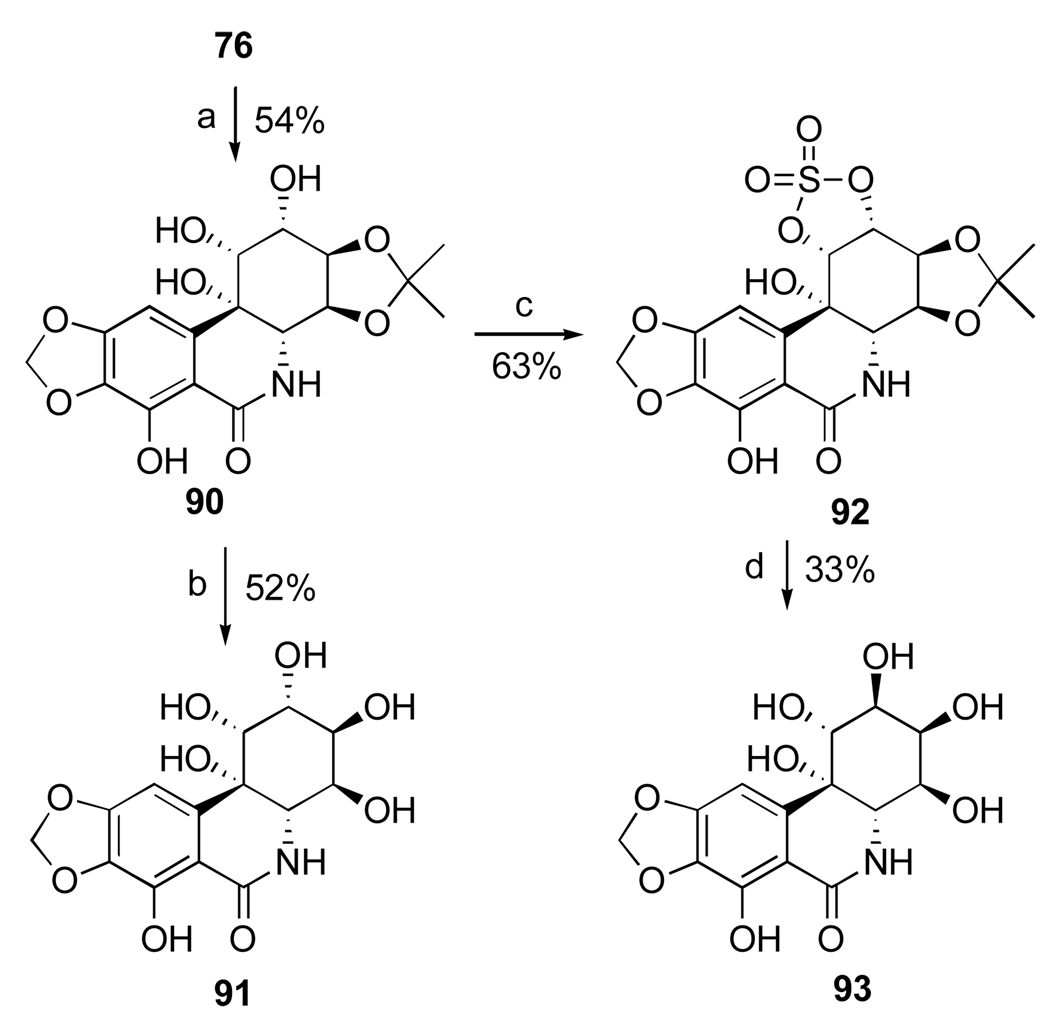
The investigators found that the facial preference of the dihydroxylation is affected by the protection pattern of narciclasine. Thus, narciclasine acetonide (76) undergoes attack by the osmium reagent from the α-face of the double bond preferentially, giving diol 90 and the corresponding β-diastereomer in 2:1 ratio (Scheme 4). The acetonide group in 90 is smoothly removed with a solution of sulfuric acid in H2O/THF to afford 10b-S-1-epi-pancratistatin (91). In an attempt to invert the configuration at C1 to that corresponding to natural pancratistatin, acetonide 90 was converted to cyclic sulfate 92 by treatment with thionyl chloride and subsequent oxidation of the intermediate sulfite with RuCl3·3H2O/NaIO4. The ring-opening with cesium benzoate, however, occurred instead at C2, affording after deprotection hexaol 93.107
Scheme 4.a.
a Reagents and conditions: (a) OsO4 (cat.), NMO, (DHQD)2PHAL, DMF/H2O; (b) H2SO4, THF/H2O; (c) SOCl2, Et3N, THF; RuCl3·3H2O/NaIO4, MeCN/CCl4/H2O; (d) PhCO2H, Cs2CO3, DMF; H2SO4, THF/H2O, 60 °C; K2CO3, MeOH.
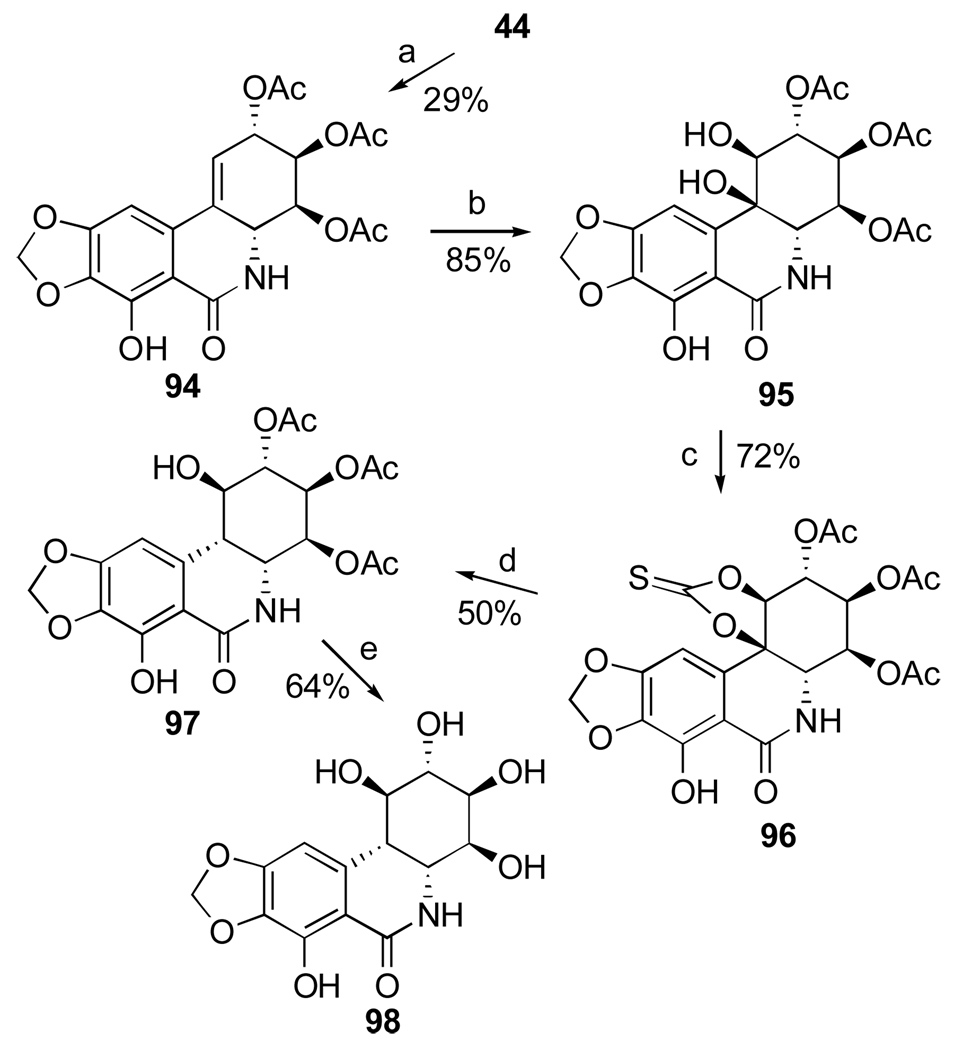
Removal of the C10b tertiary benzylic alcohol by either hydrogenolysis or dehydration/hydrogenation in hexaol 88 was expected to furnish pancratistatin. All attempts, however, have not met with success. Instead, Pettit and coworkers investigated radical a deoxygenation approach utilizing a C10b-C1 cyclic thiocarbonate, with the expectation that the reaction would proceed with the correct regiochemistry due to stability of the tertiary benzylic radical at C10b.108 Although acetylation of narciclasine with 3.25 equivalents of Ac2O results in a mixture of acetates, the desired triacetate 94 can be isolated by column chromatography in modest yield (Scheme 5). Highly selective β-dihydroxylation of the double bond in 94 provides triol 95, which reacts with N,N’-thiocarbonyldiimidazole in 2-butanone to afford cyclic thiocarbonate 96 in good yield. Although successful, radical deoxygenation of 96 by heating with Bu3SnH and AIBN in toluene gives the undesired cis-isomer 97. Deacetylation of the latter compound results in 10b-S-pancratistatin (98), the cis-isomer of the natural isocarbostyril.
Scheme 5.a.
a Reagents and conditions: (a) Ac2O (3.25 equiv.), py; (b) OsO4 (cat.), NMO, (DHQ)2PHAL, DMF/H2O; (c) N,N’-thiocarbonyldiimidazole, 2-butanone; (d) Bu3SnH, AIBN, PhCH3, 80 °C, 16 h; (e) K2CO3, H2O/MeOH/CH2Cl2.
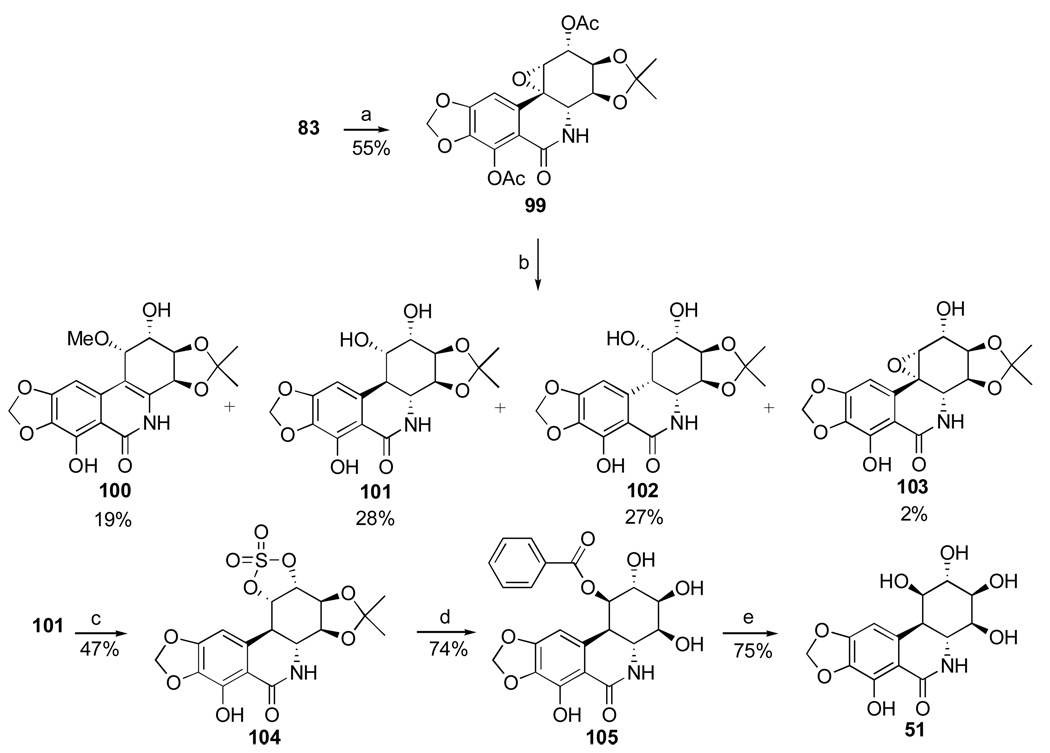
The successful strategy for the conversion of narciclasine to pancratistatin involves selective α-epoxidation of the double bond in diacetate 83, followed by hydrogenolysis of C10b-C1 epoxide 99 (Scheme 6).109 The reaction results in the formation of a mixture of four products, including the desired trans diol 101 in modest yield. The inversion of the C1 stereocenter is required, and this time it is achieved uneventfully by the formation of C1–C2 cyclic sulfate 104 and nucleophilic ring-opening at C1 with cesium benzoate. Methanolysis of the benzoate ester in 105 gives pancratistatin (51) in good yield. Thus, pancratistatin is obtained from narciclasine in ten steps and overall yield of 3.6%.
Scheme 6.a.
a Reagents and conditions: (a) MCPBA, phosphate buffer; (b) H2, 10% Pd/C; K2CO3, MeOH/H2O (c) SOCl2, Et3N, THF; RuCl3·3H2O/NaIO4, MeCN/CCl4/H2O; (d) PhCO2H, Cs2CO3, DMF; H2SO4, THF/H2O, 70 °C; (e) K2CO3, MeOH.
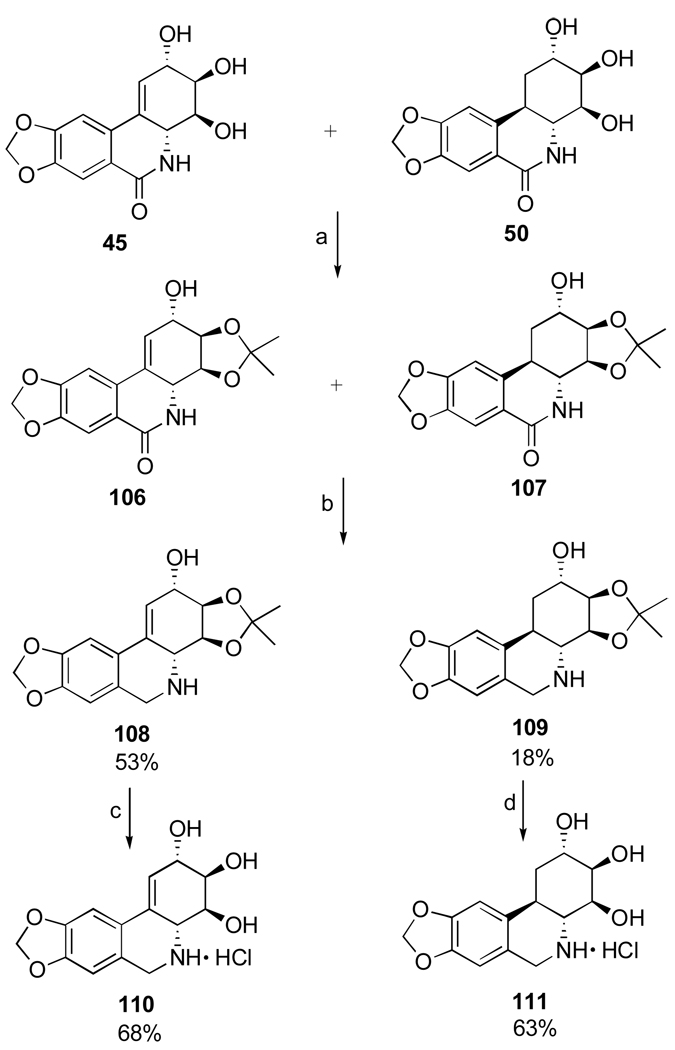
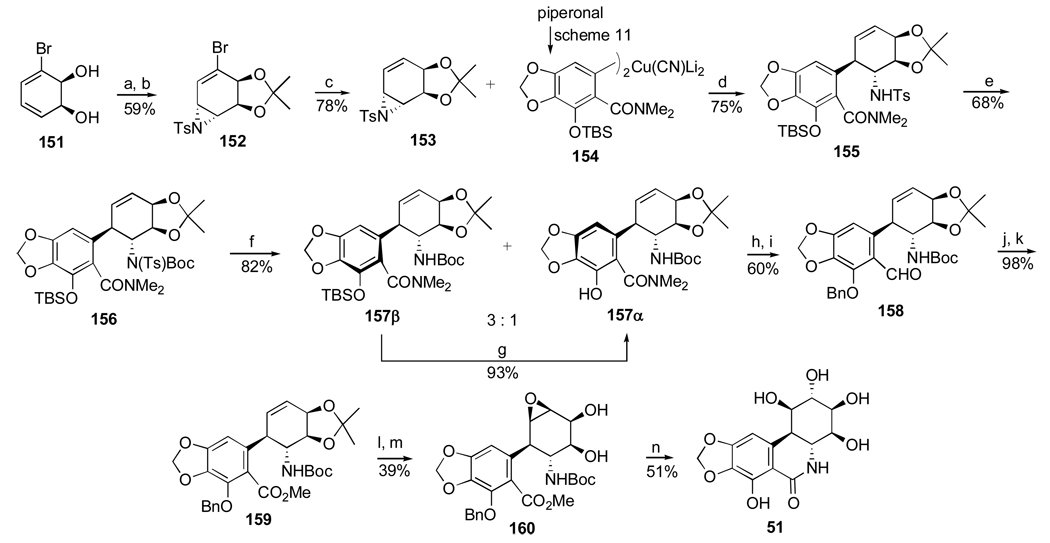
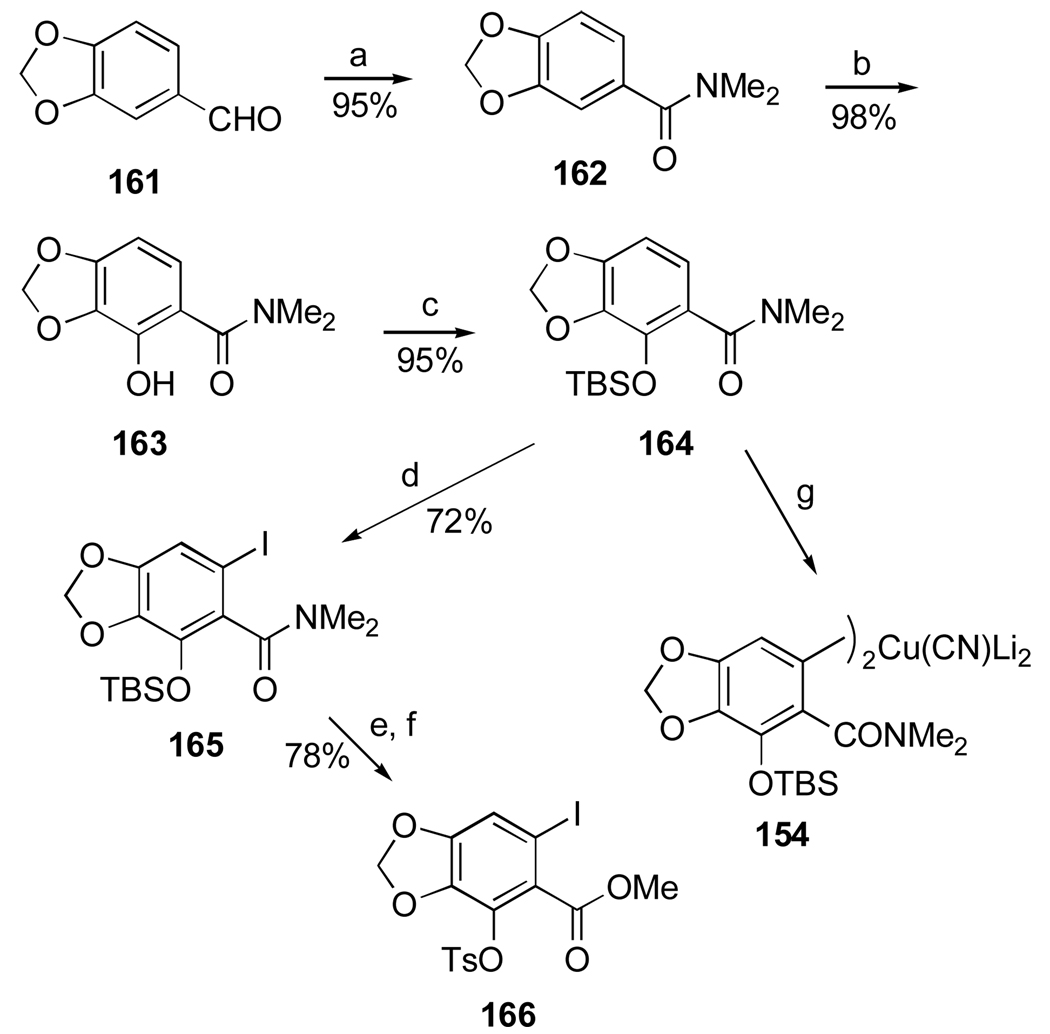
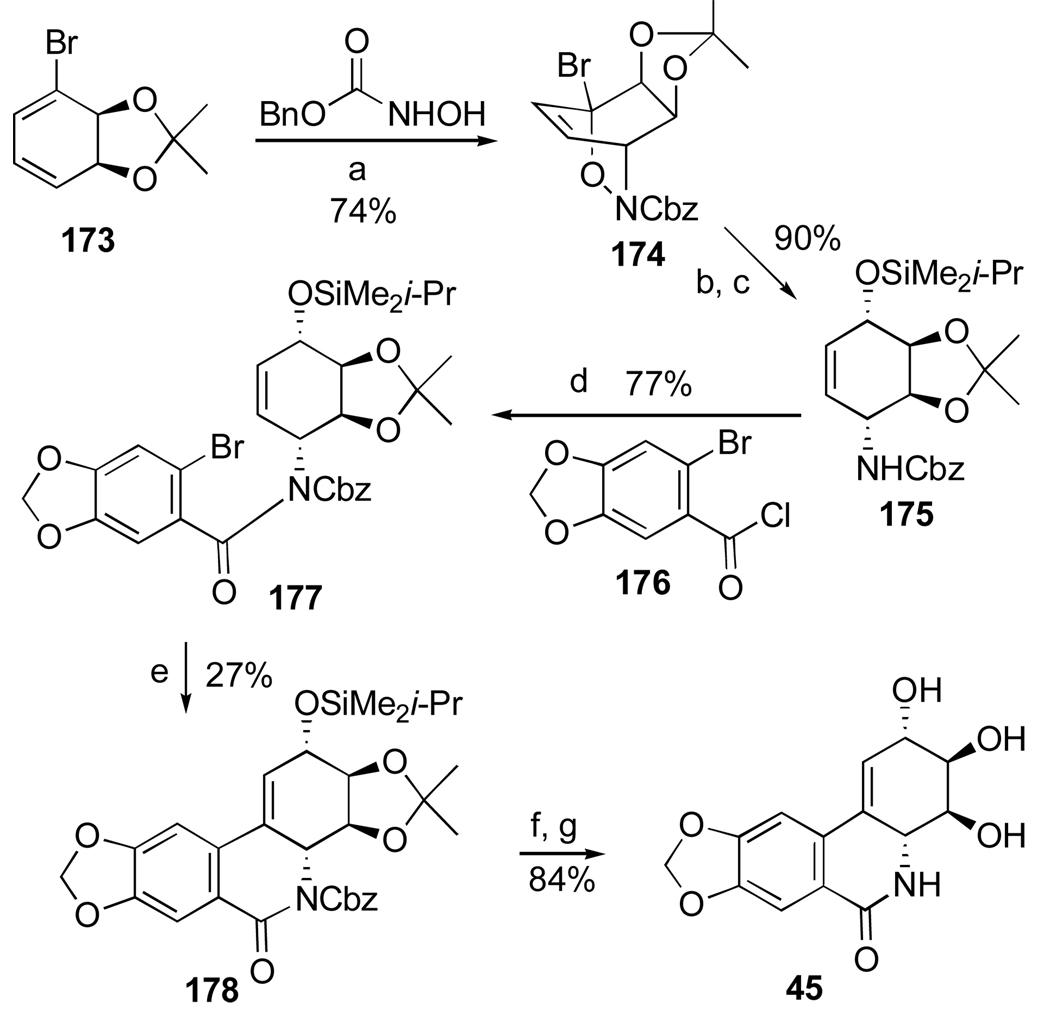
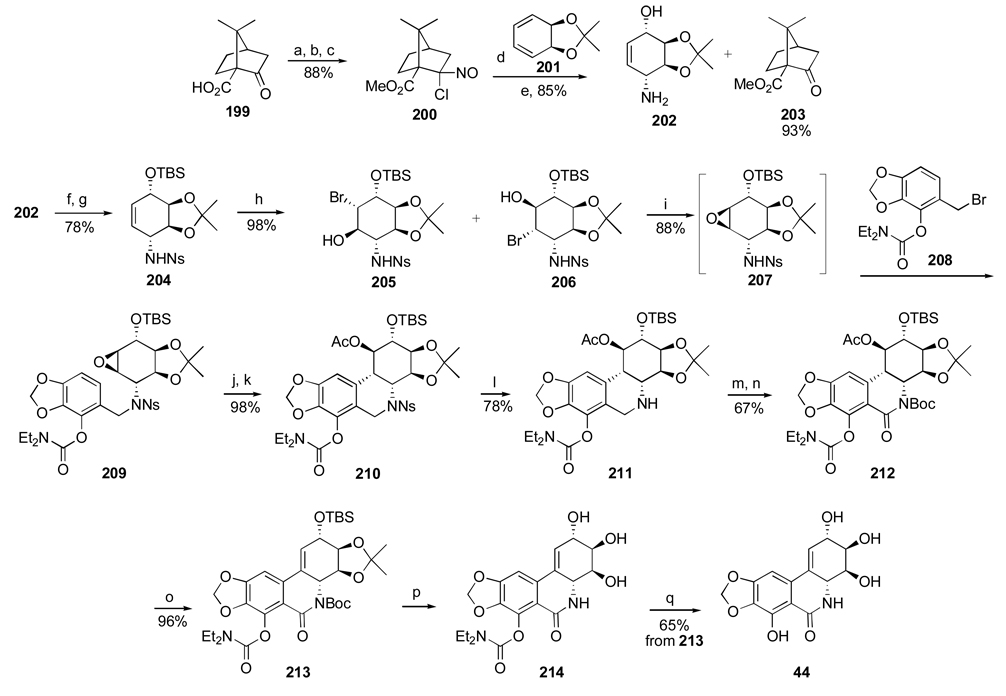
Another important investigation resulting in the synthesis of useful analogues of the natural isocarbostyril 7-deoxynarciclasine (45) and 7-deoxy-trans-dihydronarciclasine (50, Section 2.3) has also been reported by Pettit’s group.84 The study was prompted by a practical problem of separation of 45 and 50 after their isolation as a chromatographically inseparable mixture from P. littorale bulbs. It was hoped that various derivatization reactions might lead to easily separable compounds that could be converted back to the original isocarbostyrils or give rise to interesting analogues of these natural products. Thus, a fraction from P. littorale containing triols 45 and 50 was treated with dimethoxypropane in DMF to give a mixture of corresponding acetonides 106 and 107 (Scheme 7). Protection of C2 hydroxyl in both compounds as TBS ether, reduction of the amide functionality with LiAlH4 followed by removal of the silyl group gave easily separable by column chromatography acetonides 108 and 109 in respective yields of 53% and 18%. Removal of the acetonide protection led to the preparation of amine analogues 110 and 111 of the natural isocarbostyrils as their hydrochloride salts.
Scheme 7.a.
a Reagents and conditions: (a) Me2CH(OMe)2, p-TsOH, DMF; (b) TBSCl, imidazole, DMF; LiAlH4, ether; TBAF, THF; (c) aq. H2SO4, THF/CH2Cl2; NaHCO3; HCl, MeOH (d) HCl, MeOH.
5. Biological Mechanism of Action
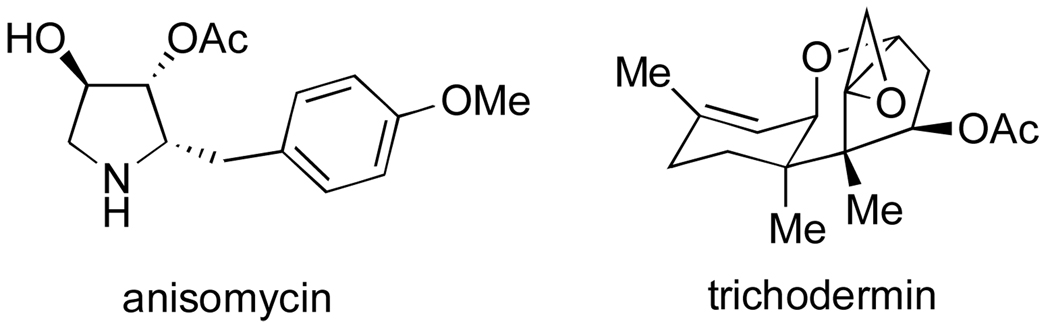
Preliminary biological evaluation of narciclasine, which was performed by Ceriotti immediately following its isolation from narcissus bulbs, already indicated a tremendous potential of this isocarbostyril to affect various biological systems.64 Thus, Ceriotti reported that narciclasine strongly inhibited the growth of wheat grain radicles at very low concentrations and showed potent antimitotic activity against the cells of sarcoma 180 in ascites form when administered subcutaneously in low dosages. Studies by a number of laboratories, most prominently by Spanish investigators at the Instituto de Biologia in Madrid, established that these as well as many other biological activities of narciclasine can be attributed to its potent inhibition of eukaryotic ribosomal protein biosynthesis. Carrasco et al. first reported that narciclasine inhibited poly U-directed [14C]phenylalanine incorporation in yeast polysomes and rabbit reticulocyte (immature red blood cells) ribosomes.110 The authors further went on to show that the isocarbostyril inhibited the association of both [3H]anisomycin and [14C]trichodermin with both yeast and rabbit reticulocyte ribosomes. Anisomycin (Figure 16), an antibiotic produced by Streptomyces griseolus, and trichodermin, an antifungal metabolite, are both known to inhibit peptide bond formation by eukaryotic ribosomes by association with the peptidyl transferase catalytic center integrated with the 60S ribosomal subunit.
Figure 16.
Structures of anisomycin and trichodermin.
Furthermore, Jimenez et al. showed that the mutant Saccharomyces cerevisiae TR1 strain, which possesses a ribosomal alteration in the peptidyl transferase center of the 60S subunit and is resistant to both anisomycin and trichodermin, shows cross-resistance to narciclasine. While the isocarbostyril inhibits the uptake of [3H]leucine into the proteins of the wild type Saccharomyces cerevisiae strain Y166, similar concentrations of narciclasine fail to produce the same effect with trichodermin and anisomycin-resistant cells.111,112 These and additional experiments prompted the investigators to conclude that narciclasine interacts with the 60S eukaryotic ribosome subunit and inhibits peptide bond formation step in eukaryotic protein biosynthesis.
The same investigators further proceeded to evaluate comparatively the effects of narciclasine and seventeen Amaryllidaceae alkaloids on (a) HeLa cell growth and (b) DNA, RNA and protein syntheses in intact Krebs ascites cells.113 DNA, RNA and protein syntheses were studied by incorporation of [14C]thymidine, [14C]uridine and [14C]proline respectively. Narciclasine (44) and five of the alkaloids, namely lycorine (3), haemanthamine (5), dihydrolycorine (39), pseudolycorine (38) and pretazettine (7) exhibit potent growth-inhibitory activity against HeLa cells (Table 3).
Table 3.
Comparative biological evaluation of selected Amaryllidaceae constituents used in the study by Jimenez et al.
| HeLa cell growth inhibition |
protein synthesis inhibition |
DNA synthesis inhibition |
RNA synthesis inhibition |
|
|---|---|---|---|---|
| compound | MIC µM | IC50 µM | IC50 µM | IC50 µM |
| 44 | 0.1 | 0.07 | ~ 1 | > 1 |
| 39 | 100 | 300 | ~ 1000 | > 1000 |
| 3 | 6 | 4 | 20 | > 20 |
| 7 | 1 | 3 | > 100 | > 100 |
| 5 | 4 | 1 | ~ 10 | > 10 |
| 38 | 25 | 8 | 30 | > 100 |
The same six compounds inhibit in vivo protein synthesis and to a much lesser extent DNA synthesis, while RNA synthesis is unaffected. The concentration ranges required for the inhibition of protein synthesis are in good agreement with the MIC values defined as concentrations required for complete inhibition of HeLa cell growth. The authors proposed that the weak inhibition of the DNA synthesis is a secondary effect of the potent inhibition of protein synthesis, since translation is required for DNA synthesis. These results further corroborate the idea that the inhibition of protein biosynthesis is the primary mode of action of narciclasine as well as a number of other Amaryllidaceae plant constituents in eukaryotic cells.
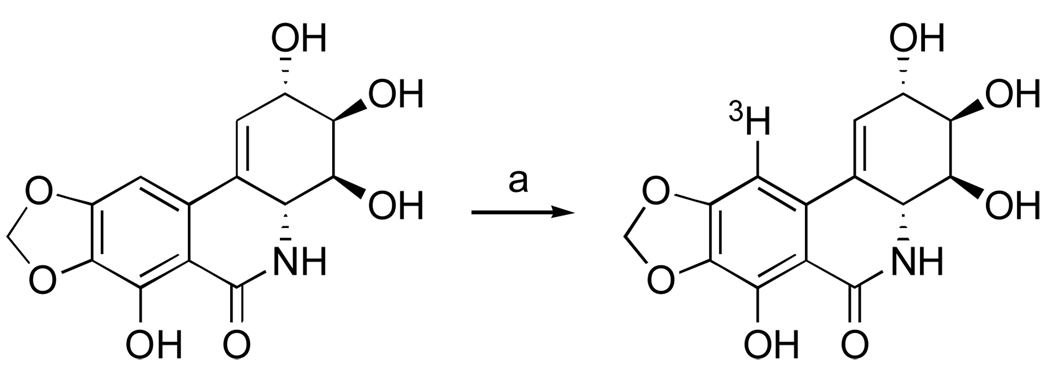
To obtain direct evidence that narciclasine indeed interacts with eukaryotic ribosomes Baez et al. prepared a tritium labeled narciclasine derivative (Scheme 8).114 Although the site of tritium incorporation in narciclasine was not reported by the investigators, it is reasonable that under the described reaction conditions the electrophilic aromatic substitution takes place to give C10-labeled compound.
Scheme 8.a.
a Reagents and conditions: (a) 3H2O, Pd/C, 60 °C, 23 h, sealed ampoule; co-evaporation with ethanol (5 times).
Using [3H]narciclasine, the investigators confirmed that the isocarbostyril specifically interacts with 60S ribosome subunits of both Saccharomyces cerevisiae and rabbit reticulocytes, but not with 40S subunits. Furthermore, narciclasine failed to bind to the ribosomes from Escherichia coli, explaining an earlier observation of narciclasine’s protein synthesis inhibition specifically in eukaryotic cells and not in those of E. coli.110
[3H]Narciclasine was used by Baez and Vazquez to perform a structure-activity relationship study of narciclasine derivatives.115 More specifically, various synthetic narciclasine derivatives were assayed for their propensity to inhibit the poly U-directed poly[14C]phenylalanine synthesis by yeast ribosomes and the activity patterns were correlated with the ability of these compounds to inhibit the binding of [3H]narciclasine to yeast ribosomes (Table 4).
Table 4.
SAR study of narciclasine derivatives
| concen- tration |
[14C]Phe incorpo- ration |
concen- tration |
[3H]nar- ciclasine binding |
|
|---|---|---|---|---|
| compound | mM | % control | mM | % control |
| 44 | 0.1 | 6 | N/A | N/A |
| 1 | 0 | |||
| 49 | 0.1 | 8 | 0.01 | 25 |
| 1 | 5 | 1 | 4 | |
| 79 | 0.1 | 8 | 0.01 | 44 |
| 1 | 6 | 1 | 5 | |
| 77 | 0.1 | 67 | 0.1 | 96 |
| 1 | 52 | 1 | 90 | |
| 73 | 0.1 | 49 | 0.1 | 95 |
| 1 | 43 | 1 | 70 | |
| 78 | 0.1 | 21 | 0.1 | 68 |
| 1 | 12 | 1 | 16 | |
Thus, trans-dihydronarciclasine (49), trans-dihydronarciclasine acetonide (79) and isonarciclasine (78) inhibit both polyphenylalanine synthesis and [3H]narciclasine binding, suggesting that these compounds interact with the same ribosomal site as narciclasine. It is also possible that less potent cis-dihydronarciclasine (77) and O-methylnarciclasine (73) have the same binding sites as narciclasine, but their affinities for the ribosome are much lower since they have little inhibitory effect on [3H]narciclasine binding. Of interest is the significant difference in activities of trans- and cis-dihydronarciclasines as well as the fact that methylation of the 7-OH group in narciclasine diminishes the activity, but does not completely abolish it. Not shown in Table 4 due to complete lack of activity in both assays are narciclasine acetonide (76), trans-dihydronarciclasine tetracetate, cis-dihydronarciclasine tetracetate, O,N-dimethylnarciclasine, 7-deoxynarciclasine triacetate and O-methyl-cis-dihydronarciclasine.
6. Effects of Narciclasine and its Congeners on Plant Physiology
6.1 The “Vase Effect”
The ornamental flower growers know that placing cut daffodils in a vase with other cut flowers has a negative effect on the flower quality and significantly shortens their vase life. Barendse provided more specific descriptions of such visual observations.116 For example, when ten cut roses (var. Baccara) were placed in a vase together with ten daffodils (var. Carlton) for 4 hours at 2 °C, the rose flowers desiccated, petals changed from red to bluish red and the leaves abscised. Daffodils inhibited flower opening in Iris (var. Ideal), freesia (var. Golden Melody) and anemones just after a short exposure in the vase. Tulips (var. Parade) underwent stem bending, precocious tepal bluing and leaf shriveling. Furthermore, a common horticultural practice for cultivation of narcissus flowers involves the removal of the old scales and roots of the bulbs followed by introduction of cuts on the bulbs before immersing them into water. The mucilage that leaches out from the cuts is constantly removed by frequent changing of water. This procedure leads to sprouting and raises a speculation that an inhibitor is present in the mucilage of the narcissus bulbs. van Doorn studied the daffodil “vase effect” on roses and tulips in some detail (Table 5) and concluded that the physiological reasons for shortening of the vase lives of these two flowers were different.117 Daffodils or their stem mucilage negatively affect water uptake in roses and result in water stress symptoms (wilting, lack of flower opening) in these flowers. In contrast, the water uptake by tulips was only little affected, and, instead of water stress symptoms, precocious leaf yellowing was observed. The latter effect was ascribed by the investigator to the toxic properties of the alkaloids in daffodil mucilage.
Table 5.
Effect of daffodils (var. Carlton) and their stem mucilage (1% v/v) on the vase life and the rate of water uptake of roses (Rosa hybrida Sonia) and tulips (Tulipa gesneriana Apeldoorn)
| treatment | vase life | water uptake |
|---|---|---|
| days | mL/stem/h | |
| one rose per vase | ~ 11 | ~ 9 |
| two roses per vase | ~ 10 | ~ 9 |
| one rose, one daffodil | ~ 3 | ~ 4 |
| one rose, daffodil mucilage | ~ 3 | ~ 3 |
| one tulip per vase | ~ 7 | ~ 5 |
| two tulips per vase | ~ 7 | ~ 5 |
| one tulip, one daffodil | ~ 4 | ~ 4 |
| one tulip, daffodil mucilage | ~ 4 | ~ 4 |
In a recent study van Doorn et al.118 obtained evidence pointing to narciclasine, a constituent of daffodil mucilage, as a primary component responsible for the daffodil “vase effect.” The investigators studied the effect of delayed senescence manifested by Iris (var. Hollandica) flowers when the latter are placed in a vase containing a Carlton daffodil stem. Senescence in Iris flowers is normally characterized by inward rolling of the flag tepals and color change from purple to yellowish white, followed by desiccation. The exposure of the Iris flowers to a daffodil stem results in remarkable delay of tepal senescence; the flowers eventually wilt while retaining the color. The same effect was observed when either daffodil mucilage (0.3 mL/L) or purified narciclasine (0.6 µM) were added to the water. The investigators hypothesized that narciclasine may inhibit the synthesis of enzymes required for the senescence-associated degradation processes. Indeed, narciclasine significantly attenuates the increase in protease activity and inhibits lipid breakdown in tepal edges of cut Iris flowers.118
6.2 Plant Growth-Inhibitory Properties
A number of reports describe plant growth-inhibitory properties of narciclasine. It inhibits the growth of coleoptiles of wheat embryos and significantly decreases the number of mature spikelets of Lolium temulentum.119 The isocarbostyril interferes with the elongation growth of Avena coleoptiles rice seedlings,67 etiolated pea epicotyls,120 cress roots,121 and inhibits the callus growth of cultured tabacco piths67 as well as radicle growth of wheat.64
Inhibition of protein biosynthesis by narciclasine could account for most, if not all, of these and related observations. The studies of antagonistic effects of narciclasine on auxin action in plants are particularly revealing in this respect. Various auxin bioassays in L. radiata122 and N. pseudonarcissus123 failed to produce growth-promoting effect and this may be attributed to the presence of a protein inhibitor, such as narciclasine, in the tissue extracts. Indeed, Kang et al. reported that narciclasine strongly suppresses auxin-induced ethylene production in mung bean (Vigna radiata Wilczek) seedlings, while potently inhibiting [14C]leucine incorporation into protein in the tissue.124 The investigators further showed that narciclasine does not inhibit ethylene synthesis from its immediate precursor, 1-aminocyclopropane-1-carboxylic acid (ACC) during short term incubation, but the activity of ACC synthase and, therefore, the amount of ACC in the tissue treated with indole-3-acetic acid (IAA) is significantly diminished by narciclasine. Taken together, these results suggest that the auxin-induced ethylene formation is not due to a specific interaction of narciclasine with auxin, but rather results from the general inhibition of protein synthesis by narciclasine. A similar conclusion was made by Sasse et al., who investigated the inhibitory effect of narciclasine on fusicoccin and IAA-induced growth of dwarf pea (Pisum sativum L.) hooks and tips.125 Narciclasine completely suppresses IAA-induced growth, whereas fussicoccin-induced growth is only partially inhibited. Since it is known that IAA effect on plant growth fully depends on protein synthesis and fussicoccin action is only partially dependent on it, general inhibition of protein synthesis by narciclasine in this plant tissue provides a satisfactory explanation for these observations.
6.3 Other Effects
Other noteworthy effects of narciclasine on plant physiology include inhibition of auxin transport in Citrullus edulis seedlings,126 blocking of chlorophyll biosynthesis and chloroplast formation in wheat (Triticum aestivum) seedlings,127 and possible phytoalexin action.81 Both narciclasine and 7-deoxynarcilasine were found to be main antifeedants in the bulbs of L. radiata.128 Narciclasine appeared to be the most potent inhibitor of ascorbic acid biosynthesis in vivo among forty Amaryllidaceae alkaloids and their synthetic derivatives tested in potato tubers, as was reported by Evidente et al.129
Lastly, most of narciclasine congeners, particularly 7-deoxynarciclasine, pancratistatin and 7-deoxypancratistatin have been shown to have similar plant growth-inhibitory properties to those of narciclasine. In this connection, growth-stimulatory effects of 2-O-β-glucosides of narciclasine (kalbreclasine, 46) and pancratistatin (pancratiside, 53) are intriguing.71 Thus, Ghosal et al. detailed such contrasting behavior for the pancratistatin/pancratiside pair in respect to the germination of seeds and the development of roots of Zephyranthes flava. While under normal incubation conditions 40–50% of the control seeds were germinated after 36 hours, 100% of the pancratiside-treated (0.1 mM) and none of the pancratistatin-treated seeds were germinated. Similarly, growth and elongation of roots of Z. flava were enhanced by pancratiside (235% and 177% over control), while pancratistatin inhibited these processes (70% and 95% over control).71 Apparently, such phenomenon is general for these classes of Amaryllidaceae constituents, since similar observations were reported for lycorine/lycorine-1-O-β-glucoside pair.130,131 Further investigation of these effects will undoubtedly shed more light on plant growth regulatory mechanisms.
7. Effects of Narciclasine and its Congeners Potentially Beneficial to Human Health
7.1 Anticancer Activities
Early studies by Ceriotti on biological activities of narciclasine revealed that this constituent of the daffodil bulbs possesses potent antimitotic properties against the highly malignant mouse sarcoma cells (Sarcoma 180) passaged in the ascites form.64 Narciclasine was administered by intraperitoneal or subcutaneous injections or via oral route. Subcutaneous injection was most effective and resulted in complete disappearance of mitoses after 4 hours of administration at the doses of 0.9 mg/kg. In addition, an LD50 value of 5 mg/kg in mice was established for the subcutaneous injection administration route. Working independently at a about the same time, Okamoto et al. reported toxic effects of narciclasine and 7-deoxynarciclasine toward the cells of Ehrlich Carcinoma, with the former isocarbostyril being more potent.67 Mondon and Krohn reported the first SAR investigation of narciclasine and its derivatives by assessing their toxicities to HeLa and HEPH cell lines as models of human cervical and laryngeal carcinomas respectively.105 Narciclasine, O-methylnarciclasine (73) and trans-dihydronarciclasine (49) were most potent exhibiting toxicities at concentrations of 0.1 µg/mL. Significantly less potent were cis-dihydronarciclasine (77) and O,N-dimethylnarciclasine, while narciclasine acetonide (76) and isonarciclasine (78) were found to be inactive. These SAR data are in good agreement with the results of Baez and Vazquez (Table 4, vide supra)115 of the suppression of poly U-directed poly[14C]phenylalanine synthesis as well as inhibition of binding of [3H]narciclasine to yeast ribosomes. The only exception is the total lack of toxicity of isonarciclasine (78) to the intact cells, although it is quite potent in the cell free ribosomal assays. Membrane permeability appears to be an important consideration for comparing the outcomes of these two assays.
Many bioactivity-guided isolations of narciclasine congeners from their respective plant sources utilized murine P-388 lymphocytic leukemia in vitro and in vivo (PS system) bioassays.132,133 The efficacies of the purified natural products were also initially assessed using this cell line. Although the in vitro potencies reported in the literature vary, narciclasine (44), 7-deoxynarciclasine (45), trans-dihydronarciclasine (49), 7-deoxy-trans-dihydronarciclasine (50), and pancratistatin (51) have all registered GI50 values as low as 0.01 µg/mL.70,74,75,84 In addition, pancratistatin (51) was effective in vivo against this cancer (38–106% life extension at 0.75–12.5 mg/kg) as well as murine M-5076 ovary sarcoma (53–84% life extension at 0.38–3.0 mg/kg).70,73 These initial promising data warranted the evaluation of the above five narciclasine congeners as well as synthetic cis-dihydronarciclasine (77), 7-deoxy-cis-dihydronarciclasine (112, obtained by hydrogenation of 45)134 and isonarciclasine (78) in the NCI sixty human cancer cell line in vitro screen.135 The GI50 values, as reported Pettit et al.,72 are averaged across the entire sixty cell line panel and are given in Table 6. Table 6 also lists the “Compare Correlation Coefficients,” which are generated by a computerized pattern recognition algorithm and serve as an indication of similarities in differential cellular sensitivities or characteristic fingerprints for each compound.135 The synthetic analogues 77, 78 and 112 are only weakly active or inactive in this in vitro screen, while the natural isocarbostyrils (44, 45, 49–51) exhibit nanomolar growth-inhibitory potencies. These results are consistent with the data from the SAR studies of the inhibition of eukaryotic protein biosynthesis (Table 4) and provide further support for this mechanism of action for narciclasine and its congeners in eukaryotic cells. Furthermore, the “Compare Correlation Coefficients” show that the differential cellular response profiles are very similar for these five compounds, further corroborating a uniform mode of action. Importantly, the differential cellular sensitivity profile associated with these isocarbostyrils does not show good correlation with any of the known anticancer agents. Melanoma subgroup of cancer cell lines is the most responsive, while selected cell lines from within such subpanels as NCS lung, colon, brain and renal are more than 1000 times more responsive than the least sensitive cell lines within these subpanels. Pettit and coworkers concluded their analysis of NCI screening data with the following statement: “Indeed, it seems likely that pancratistatin and related trans-dihydrocarbostyrils, as well as narciclasine, constitute the most important antineoplastic components of Amaryllidaceae species used the folk medical treatment of cancer.”72
Table 6.
Results of comparative anticancer evaluation of narciclasine and related isocarbostryrils in the NCI sixty cell line screen
| compound | mean GI50 |
compare correlation coefficient |
|---|---|---|
| µM | ||
| narciclasine (44) | 0.016 | 0.90 |
| 7-deoxynarciclasine (45) | 0.15 | 0.90 |
| trans-dihydronarciclasine (49) | 0.013 | 0.92 |
| 7-Deoxy-trans-dihydronarciclasine (50) | 0.068 | 0.89 |
| pancratistatin (51) | 0.091 | 1 |
| cis-dihydronarciclasine (77) | 3.8 | < 0.6 |
| 7-deoxy-cis-dihydronarciclasine (112) | 95.5 | < 0.6 |
| isonarciclasine (78) | 11.8 | < 0.6 |
Additionally, potent cytotoxic/antitumor activities were reported for narciclasine-4-O-β-D-glucopyranoside (47).77 Using the brine shrimp (Artemia salina) assay Abou-Donia et al. obtained the LD50 value of 0.88 µg/mL for 47 (LD50 = 0.29 µg/mL was obtained for 44 in this assay). Both 44 and 47 exhibited antitumor activity in the potato disc antitumor assay by inhibiting crown gall tumor initiation (62% inhibition for 44, 53% inhibition for 47). Also, C1-esterified naturally occurring pancratistatin derivatives 55 and 56 showed cytotoxicities comparable to pancratistatin itself when tested against HeLa, KB and P-388-D1 (models for human cervical and epidermoid carcinomas as well as murine lymphocytic leukemia respectively) cell lines.79 In addition, both non-glucosylated (55) and glucosylated (56) pancratistatin C-1 esters, as well as pancratistatin itself, were later shown to cause cell cycle arrest and apoptosis in promyelocytic leukemia HL-60RG cells.136 7-Deoxypancratistatin (52), originally reported to have plant growth-inhibitory properties,71 was later evaluated against human cancer cell lines and found to be ca. 10 times less potent than its 7-hydroxy congener.137
Recently, Pandey and coworkers reported the results of investigations of the effects of pancratistatin on normal and cancer cells, providing evidence supporting selective toxicity of this isocarbostyril to cancer cells. Thus, pancratistatin was found to selectively induce apoptosis using non-genomic targets in Jurkat (model for human T-cell leukemia) cell line as opposed to normal nucleated blood cells.138 While pancratistatin is cytotoxic at submicromolar concentrations to a number of human cancer cell lines, normal human fibroblasts (NHF) and human umbilical vein endothelial cells (HUVEC) are unaffected by the same treatments. In contrast, such chemotherapeutic drugs as paclitaxel and etoposide show toxicity to both of these non-cancerous cell lines.139 Finally, the authors speculated that pancratistatin may take advantage of the differences between mitochondria in cancerous and non-cancerous cells to selectively induce apoptosis in the former cell types.140 Further studies will be necessary to understand the nature of this effect in detail and reconcile these findings with the inhibition of protein biosynthesis as the established mode of action for narciclasine and its congeners. These experiments, however, bode well for the clinical development of pancratistatin, narciclasine and their analogues.
Pettit and coworkers have tested all of the synthetic intermediates shown in Scheme 3, Scheme 4, Scheme 5, Scheme 6 and Scheme 7, which represent a large selection of analogues accessible from the natural isocarbostyrils, against murine P-388 lymphocytic leukemia cells as well as a minipanel of human cancer cell lines including pancreas (BXPC-3), breast (MCF-7), CNS (SF268), NCS lung (NCI-H460), colon (KM20L2) and prostate (DU-145) in vitro cancer models. All C10b-hydroxyanalogues of pancratistatin, including those with cis (88) and trans (91, 93) B/C ring junction, are significantly less potent than the natural compound or are inactive.107 The C10b-epimer of pancratistatin (98), possessing the cis B/C ring fusion, is less active than the natural isocarbostyril by a factor of 10.108 An intriguing discovery is high potency of C1-benzoate ester pancratistatin derivative (105), which exceeds that of pancratistatin by 10–100 times depending on the cell type.109 Whether the benzoate moiety enhances the activity by facilitating the intracellular delivery and is subsequently hydrolyzed within cells, or by serving as an important part of the cytotoxic pharmacophore is not clear. Further studies are anticipated. Finally, replacing the amide functionality by an amino group in 110 and 111 reduces the toxicities 10–100 times relative to the natural isocarbostyrils.84
Synthetic efforts by many research groups, aimed at preparation of analogues inaccessible from the natural isocarbostyrils, have been invaluable in defining the cytotoxic pharmacophore(s) associated with these compounds to a much greater extent. The analogues, prepared from commercially available starting materials, can be divided into three categories based on whether the major structural differences with the natural compounds are located in rings A, B or C.
As has been already discussed, removal of the 7-hydroxyl group in ring A as in 7-deoxypancratistatin reduces the potency of pancratistatin 10-fold.137 The inspection of the NCI 60 cell line primary screening data in Table 6, as well as the results from independent testing by Pettit’s group,84 indicates that the similar effect is present in narciclasine/7-deoxynarciclasine and trans-dihydronarciclasine/7-deoxy-trans-dihydronarciclasine pairs. Further, Hudlicky et al. have synthesized a singly oxygenated ring A analogue of pancratistatin (113, Figure 17)141 and showed that removal of another oxygen in ring A of pancratistatin further lowers the potency 10-fold relative to 7-deoxypancratistatin (100-fold relative to pancratistatin).
Figure 17.
Ring A analogues.
Bioisosteric replacement of methylenedioxybenzene moiety with the indole ring is a common practice in drug design142 and, therefore, Hudlicky’s group performed an ab initio computational comparative analysis of such β-carboline-1-one mimic of pancratistatin (114).143 Indeed, the results of the computational study were encouraging showing a significant amount of overlapping parameters, including favorable geometries and electrostatic potential distribution, between 114 and pancratistatin containing methylenedioxybenezene moiety. The investigators developed an efficient nine-step synthesis of 114.143,144 Disappointingly, the indole-containing compound showed only weak activity against the murine P388 cells and was completely inactive against a panel of human cancer cell lines. Finally, bis-silylated ring A analogue 115, an intermediate resulting from the synthetic efforts by Hudlicky’s group,137,145 was found to be inactive as well.
The stereochemistry of the B/C ring junction appears to be an important element affecting the analogues’ potency. Trans ring junction as in natural carbostyrils 49, 50, 51, 52 or the sp2 character of the C10b position as in 44 or 45 seem to be critical for cytotoxicity. The inversion of the C10b stereocenter resulting in the cis B/C fusion stereochemistry leads to reduction or complete loss of potency. This has been demonstrated with the synthetic analogues cis-dihydronarciclasine (77) and 7-deoxy-cis-dihydronarciclasine (112) in the 60 cancer cell line screen (Table 4) as well as the C10b-epimer of pancratistatin (98) as has been discussed above. Furthermore, Hudlicky et al. synthesized the C10b-epimer of 7-deoxypancratistatin (116, Figure 18) and showed that it is inactive as well.146 It is of interest that none of these cis-analogues has been isolated from the natural sources, although many of the isolations are bioactivity-guided and these compounds could have been easily missed by natural product chemists. To date, no variation in ring B has resulted in improved activity. Lactone analogues of 7-O-methylnarciclasine (117) and 7-deoxynarciclasine (118), synthesized by Chapleur and coworkers were found to be inactive.147 As was mentioned above, analogues 110 and 111, lacking the amide functionality in ring B, are significantly less potent than the parent isocarbostyrils. In this connection, N-tosyl analogue (119), containing significant structural variations in both rings A and B was found to have low micromolar growth-inhibitory potencies against a panel of human cancer cell lines, as was reported by Hudlicky and coworkers.137 Due to such profound differences in structure, it is likely that this compound works through a different mechanism than the parent isocarbostyril. Further, Chapleur and coworkers reported a synthesis of seco-analogues 120 and 121, lacking the C10a-C10b bond.148 The compounds were shown to be inactive, demonstrating the importance of the rigid geometry with the intact ring B. Fessner and coworkers described an interesting biocatalytic synthesis of a carbohydrate-type analogue 122, containing variations in both rings B and C.149 The results of biological testing of this compound have not been reported but, since the lactone ring B analogues are inactive, it will be hard to assess the effect of the endocyclic oxygen in ring C on cytotoxic activity. Lastly, Kornienko and coworkers reported the preparation of arylcyclitol libraries (123 – 128), which lack the lactam moiety but incorporate the C10a-C10b bond with the correct configuration.150,151 The compounds were found to be inactive, results that complement the findings of Chapleur on the importance of the intact ring B.
Figure 18.
Ring B analogues.
7-Deoxy-trans-dihydronarcilasine (50) is a nanomolar cancer cell growth-inhibitory compound and it represents the natural isocarbostyril with the fewest number of the hydroxyl groups. Several research groups have invested much effort to elucidate the minimum requirement for cytotoxicity in terms of the oxygen functionality in the molecule. Compounds 129 and 130, completely lacking any hydroxyl groups in ring C were prepared, but unfortunately not evaluated for biological activity.152–154 Similarly, Banwell and coworkers prepared 2-deoxy analogue of 7-deoxynarciclasine (131), but did not report any biological data for this interesting test compound.155
The synthesis and biological evaluation of compounds 132, 133, 134 by McNulty and coworkers156,157 as well as 135 by Hudlicky and coworkers,141 have clearly indicated the importance of having at least three hydroxyl groups in ring C for potent cytotoxic activity. 132 and 133 were found inactive, 134 is on average 2–3 orders of magnitude less potent than pancratistatin in the NCI 60 cell line screen, while 135 only showed low micromolar growth-inhibitory potency against the murine P388 cell line but was completely inactive against a panel of human cancer cell lines. Unfortunately, no biological testing data were provided for compound 136, synthesized by Pandey et al.158 It would be instructive to learn how the transposition of the hydroxyl group from C2 (as in 50) to C1 (as in 136) affects the cytotoxicity. Lastly, diol 137 and tetraacetate 138, the latter being C2-epimeric to 7-deoxypancratistatin, have been synthesized both in racemic form by DeShong’s159 and Alonso’s160 groups respectively. However, no biological activity data were provided.
Finally, Keck’s and Hudlicky’s groups prepared unnatural optical antipodes of 7-deoxynarciclasine (139) and 7-deoxypancratistatin (140) respectively (Figure 20).161–163
Figure 20.
Structures of ent-7-deoxynarciclasine and ent-7-deoxypancratistatin.
While the results of biological testing of ent-7-deoxynarciclasine haven’t been disclosed yet, rather surprisingly ent-7-deoxypancratistatin exhibits cytotoxic potency, which is only 10-fold lower than the corresponding natural enantiomer, when the latter compound was evaluated in a minipanel of human cancer cell lines.146 Interestingly, the inspection of the stereochemical relationships between different Amaryllidaceae anticancer constituents reveals pseudo-enantiomeric nature of some of them. Thus, relative configurations of the four stereocenters in the structure of lycorine (3, Figure 1) are similar to those of pancratistatin (positions C2, C1, C10b and C4a), while being opposite in the absolute sense. Given the fact the lycorine and narciclasine as well as their congeners are believed to exert their growth-inhibitory properties by the inhibition of protein biosynthesis in eukaryotes, preparation and biological testing of the optical antipodes of narciclasine, pancratistatin and trans-dihydronarciclasine is intriguing with respect to further elucidation of the origin of the activity associated with these molecules as well as development of their analogues that could be less toxic to normal cells.
7.2 Antiviral Activities
Narciclasine and its natural and synthetic analogues were evaluated for antiviral activity in the drug discovery program of the US Army Medical Research Institute of Infectious Diseases (USAMRIID).164,165 The analogues were tested against tropical RNA viruses, which cause hemorrhagic, encephalitic or febrile disease in large areas of the world. First, the evaluation was performed in cell culture against flaviviruses (Japanese encephalitis, yellow fever and dengue-4), bunyaviruses (Punta Toro, Rift Valley fever and sandfly fever), alphavirus (Venezuelan equine encephalomyelitis), lentivirus (HIV type 1) and a DNA-containing vaccinia virus. Activity was found only with flaviviruses and bunyaviruses, but even that was observed at concentrations within 10-fold of those causing toxicity to uninfected control cells. Narciclasine, pancratistatin, isonarciclasine, trans- and cis-dihydronarciclasine as well as 7-deoxy analogues of these compounds showed in vitro activity against all three flaviviruses, Japanese encephalitis, yellow fever and dengue-4. All of these compounds are also active against two bunyaviruses, Punta Toro and Rift Valley fever, whereas only 7-deoxy analogues of narciclasine, pancratistatin and cis-dihydronarciclasine exhibit antiviral effect against sandfly fever virus. Among the analogue series, antiviral activity (expressed as IC50) generally closely parallels toxicity to uninfected cells (expressed as TC50) producing therapeutic indices (TI) values ranging between 3 and 8. Since viruses utilize the cellular protein biosynthetic machinery, it is not unlikely that the observed antiviral effect is solely due to the inhibition of this process by the narciclasine-type compounds. Further work is necessary to evaluate whether there is a contribution of a specific antiviral component different from the general inhibition of cellular protein biosynthesis. Notwithstanding, narciclasine, pancratistatin and 7-deoxypancratistatin show good chemotherapeutic efficacy (> 80% survival) in mice infected with low-dose challenge Japanese encephalitis virus. The investigators conclude: “There apparently is a narrow therapeutic margin or poor selectivity. In spite of this, to our knowledge, this provides the most significant example of effective, non-immunomodulatory, antiviral chemotherapy of Japanese encephalitis viral infections in mice.”165
The activity of narciclasine against the Punta Toro virus was further confirmed in an independent study by Sidwell et al., who evaluated 75 compounds in both in vitro and in vivo models of this infection.166 Additionally, narciclasine was shown to be active against Rauscher leukemia virus in an in vitro model in NIH/3T3 cells, however the therapeutic window is again very narrow due to its toxicity to host cells at effective antiviral concentrations.167
7.3 Other Potentially Useful Activities
Ceriotti reported bacteriostatic activity for narciclasine when the isocarbostyril was evaluated against Escherichia coli HpR.64 The GI50 value of 8 µg/mL was established. These data are intriguing given the fact that narciclsaine does not inhibit protein biosynthesis in E. coli,110 nor does it bind to E. coli ribosomes, as was established using experiments with [3H]narciclasine (vide supra).114,115 Pettit et al. also reported marginal antibacterial activity associated with narciclasine derivatives 87 and 92 against Neisseria gonorrhoeae (MIC = 32–62 µg/mL).107 The mechanism of action for these antibacterial effects remains to be elucidated.
Related areas of promise are antifungal activity of narciclasine (Cryptococcus neoformans, MIC 8–16 µg/mL)107 and antiparasitic activity associated with pancratistatin and 7-deoxynarciclasine. More specifically, the latter two isocarbostyrils were reported to inhibit the in vitro proliferation of Encephalitozoon intestinalis, a microsporidian, which causes intestinal and systemic infections in immunocompromised patients. The IC50 values are 0.18 and 0.2 µM, eight times lower than the corresponding IC50 numbers for toxicity to host cells.168
As has been discussed in Section 6.3, 2-O-β-glucosides of narciclasine (kalbreclasine, 46) and pancratistatin (pancratiside, 53) have unusual effects on plant physiology stimulating growth as opposed to the growth-inhibitory action of the non-glycosylated isocarbostyrils. It turns out that the growth-stimulatory effects of the glucosylated analogues are also observed in animal test models. Kalbreclasine exhibits potent mitogenic action on splenic lymphocytes in healthy adult mice, stimulating their extensive proliferation at concentration of 20 µg/mL and higher. The effect is comparable to the known mitogen, concanavalin A.76
8. Preclinical Studies of Narciclasine and its Congeners
The preclinical evaluation of narciclasine started with the seminal work by Ceriotti, who demonstrated the isocarbostyril’s powerful anti-metastatic activity in mice (vide supra).64 Subsequent studies have been proceeding at a slow pace due to a number of significant challenges. Along with the issue of availability of sufficient quantities of material, which is a general problem for natural product-based drugs, the other important obstacle hampering the clinical development of narciclasine-type isocarbostyrils is poor water solubility.169 A significant effort has been invested in investigating suitable pharmaceutical formulation methods for these agents. For example, methods for entrapment of narciclasine into poly(2-hydroxyethyl)methacrylate matrices for subcutaneous implantation have been reported.170,171 Pancratistatin’s poor aqueous solubility (53 µg/mL) can be enhanced by the addition of organic solvents, such as DMSO or DMF. Additionally, complex formation with nicotinamide can increase pancratistatin’s solubilization to 1 mg/mL. However, the use of organic solvents as formulation components is not desirable, while the anticipated clinical doses of pancratistatin would require unacceptably high doses of nicotinamide.172 It was also reported that the use of a 40% aqueous solution of hydroxypropyl-β-cyclodextrin (HPCD) solubilizes pancratistatin up to 1.2 mg/mL.173 Yet, such solubility is still not sufficient for clinical use. The failure to achieve an acceptable pharmaceutical formulation has led to research efforts aimed at preparation of water-soluble prodrugs. Pettit’s group has reported a number of such successful strategies involving the preparations of phosphate derivatives of the natural isocarbostyrils susceptible to the action of human non-specific phosphatases, which would release the active drug in vivo. Pancratistatin-7-O-phosphate has been prepared using two different methods for the introduction of the phosphate moiety (Scheme 9).174,175 The C-ring hydroxyl groups are selectively protected by the preparation of pentaacetate 141 and the subsequent selective hydrolysis of the phenolic acetate by refluxing 141 in water/pyridine. The introduction of the phosphite ester on the phenolic hydroxyl with i-Pr2NP(OBn)2 and its subsequent oxidation to the corresponding phosphate proved to be irreproducible due to the instability of the phosphorous reagent.174 An improvement of this method involves the reaction of 142 with dibenzylphosphite and carbon tetrachloride in presence of i-Pr2NEt and DMAP, which gives dibenzyl phosphate ester 143 directly.175 Hydrogenolytic removal of the benzyl protection and deacetylation in either sequence (e or f) affords the desired pancratistatin phosphate prodrug as the disodium salt 144.
Scheme 9.a.
a Reagents and conditions: (a) Ac2O, DMAP, py; (b) H2O, py, reflux; (c) i-Pr2NP(OBn)2, tetrazole, CH2Cl2; MCPBA, CH2Cl2; (d) (BnO)2P(O)H, DIPEA, DMAP, CCl4/MeCN; (e) H2, 10% Pd/C, ethanol; NaOH, H2O; (f) NaOMe, HOMe; H2, 10% Pd/C, ethanol; NaOMe, HOMe.
Yet, the mutistep prodrug preparation starting from the scarcely available natural product prompted Pettit’s group to continue their investigation and a general one-step strategy applicable to the prodrug synthesis for all potent natural isocarbostyrils was found. The method involves reacting the corresponding isocarbostyril with tetrabutylammonium dihydrogenphosphate and dicyclohexylcarbodiimide in pyridine at 80 °C.176–178 These conditions result in highly selective formation of 3,4-cyclic phosphates in all cases. The initially formed pyridinium salts are passed through a sodium form of Dowex ion exchange resin to afford sodium salts of the desired prodrugs. C1-benzoate ester pancratistatin derivative (105), whose growth-inhibitory activity exceeds that of pancratistatin by 10–100 times (vide supra), was converted to soluble cyclic phosphate derivative (146) as well. The structures and yields of the synthesized prodrugs are given in Figure 21.
Figure 21.
Structures of 3,4-cyclic phosphate prodrugs.
All the synthesized phosphate prodrugs were evaluated against human cancer cell lines and found to have slightly weaker growth-inhibitory activities than the corresponding parent compounds as would be expected due to incomplete dephosphorylation of these compounds under the in vitro testing conditions.176–178 The cytotoxity profiles among different cell lines are very similar to the corresponding isocarbostyrils, demonstrating that the activity results from the dephosphorylated compounds and the dephosphorylation process is expected to be much more efficient in vivo. The clinical development of the phosphate prodrugs is anticipated. Intriguing results of preclinical evaluation of pancratistatin prodrugs 144 and 145 were reported by Pettit’s group. Compound 144 was investigated for its activity against saphenous vein endothelial cells (SVEC) in culture and a murine colon cancer in vivo model using intraperitoneal injection (12.5 mg/kg).179 In an independent report, cyclic phosphate 145 was tested for its efficacy against human colon adenocarcinoma (DLD-1) in a subcutaneous xenograft model in nude mice using intravenous administration mode (100 mg/kg).180 Both prodrugs cause an irreversible disruption of cellular cytoskeleton and trigger vascular shutdown in the treated tumors. Disruption of the tumor blood flow results in significant tumor growth inhibition and hemorrhagic necrosis within the tumors. An exciting possibility of the antivascular/antiangiogenesis mechanism for the in vivo action of both pancratistatin phosphates is emerging and bodes well for the forthcoming clinical testing. Similar experiments with prodrugs 146–150 are awaited.
9. Selected Total Synthesis Efforts
With an excellent solution to the water solubility problems of the Amaryllidaceae anticancer isocarbostyrils in hand, the issue of supply remains the only major obstacle for completion of preclinical evaluation and advancement of these agents to clinical trials. Pettit and coworkers developed a biotechnological route to pancratistatin, narciclasine, 7-deoxynarciclasine and 7-deoxy-trans-dihydronarciclasine involving an initial tissue culture cloning of H. littoralis bulbs.83,104 Although this effort led to the isolation of ca. 15 grams of pancratistatin and 12 grams of 7-deoxynarciclasine, these quantities are only sufficient to satisfy an interim demand for these agents. In general, due to the low natural abundance of the Amaryllidaceae anticancer isocarbostyrils (Table 2), the isolation from plants cannot be viewed with optimism as a solution to the supply problem. A practical chemical synthesis, on the other hand, could potentially provide a viable alternative. Several dozen of completed total syntheses of these compounds are a clear indication of the interest shown by the synthetic community in the challenge presented by these natural products (Table 7). Unfortunately, most of these endeavors focus on showcasing synthetic methodology developed by the investigators, and the majority of the synthetic routes are too long or low yielding to be of practical value. Most of this chemistry has been reviewed16,17,181 and, therefore, here the discussion will center on the approaches that upon further optimization can be utilized for the efficient preparation of these medicinally promising agents. The criteria for evaluation of practicality of synthetic efforts have been discussed in an excellent review by Hudlicky,182 and they are used in our critical analysis of the relevant synthetic pathways.
Table 7.
Reported total syntheses of the Amaryllidaceae isocarbostyrils
| isocarbostyril | research group | publication year | number of stepsa | overall yielda | reference |
|---|---|---|---|---|---|
| (+)-narciclasine | Rigby | 1997 | 23 | 0.2% | 183, 184 |
| (+)-narciclasine | Hudlicky | 1999 | 9 | 0.6% | 185, 146 |
| (+)-narciclasine | Keck | 1999 | 14 | 16% | 162 |
| (+)-narciclasine | Yan | 2002 | 13 | 17% | 186 |
| (±)-pancratistatin | Danishefsky | 1989 | 27 | 0.16% | 187 |
| (+)-pancratistatin | Hudlicky | 1995 | 14 | 2% | 188, 189 |
| (+)-pancratistatin | Trost | 1995 | 19 | 8% | 190 |
| (+)-pancratistatin | Haseltine | 1997 | 24 | 0.97% | 191 |
| (+)-pancratistatin | Magnus | 1998 | 22 | 1.2% | 192, 193 |
| (+)-pancratistatin | Rigby | 2000 | 23 | 0.35% | 184 |
| (+)-pancratistatin | Pettit | 2001 | 10 | 3.6% | 109b |
| (±)-pancratistatin | Kim | 2002 | 21 | 4% | 194, 195 |
| (+)-pancratistatin | Li | 2006 | 13 | 9% | 196 |
| (±)-7-deoxynarciclasine | Ohta, Kimoto | 1975 | 20 | 1.5% | 197, 198, 199 |
| (+)-7-deoxynarciclasine | Paulsen | 1982 | 12 | 4% | 200, 201 |
| (±)-7-deoxynarciclasine | Schubert | 1987 | 18 | 7.2% | 202c |
| (+)-7-deoxynarciclasine | Ogawa | 1991 | 23 | 0.04% | 203, 204 |
| (+)-7-deoxynarciclasine | Hudlicky | 1992 | 8 | 12% | 205, 206 |
| (±)-7-deoxynarciclasine | Martin | 1993 | 11 | 4% | 207 |
| ent-7-deoxynarciclasine | Keck | 1996 | 14 | 11% | 161, 162 |
| (+)-7-deoxynarciclasine | Keck | 1999 | 11 | 27% | 162 |
| (+)-7-deoxynarciclasine | Yan | 2002 | 15 | 11% | 208 |
| (±)-7-deoxynarciclasine | Padwa | 2006 | 15 | 10% | 209, 210 |
| (+)-7-deoxypancratistatin | Paulsen | 1982 | 9 | 6.5% | 200, 201 |
| (+)-7-deoxypancratistatin | Keck | 1995 | 22 | 4% | 211, 212 |
| (+)-7-deoxypancratistatin | Hudlicky | 1995 | 12 | 2.6% | 213 |
| (+)-7-deoxypancratistatin | Hudlicky | 1995 | 10 | 3% | 213, 189 |
| (+)-7-deoxypancratistatin | Chida | 1996 | 29 | 0.03% | 214 |
| (+)-7-deoxypancratistatin | Keck | 1998 | 15 | 12% | 215 |
| ent-(−)-7-deoxypancratistatin | Hudlicky | 1999 | 14 | 1% | 163 |
| (+)-7-deoxypancratistatin | Plumet | 2000 | 21 | 3% | 216 |
| (+)-7-deoxypancratistatin | Madsen | 2006 | 13 | 1.4% | 217 |
| (+)-7-deoxypancratistatin | Madsen | 2006 | 15 | 4.3% | 217 |
| (±)-7-deoxypancratistatin | Padwa | 2006 | 23 | 3% | 218, 210 |
| (±)-trans-dihydronarciclasine | Cho | 2007 | 15 | 11% | 219 |
| (±)-7-deoxy-trans-dihydronarciclasine | Tsuda | 1978 | 16 | 1.1% | 220 |
| (+)-7-deoxy-trans-dihydronarciclasine | Chida | 1996 | 24 | 0.18% | 214 |
| (+)-7-deoxy-trans-dihydronarciclasine | Iwabuchi | 2005 | 24 | 1.4% | 221 |
Number of steps and overall yield from commercially available starting material for the longest linear sequence. One-pot procedures are counted as one step. If the utilized starting material is not commercially available, but had been reported previously by other investigators, the number of steps and yields were taken from the cited sources.
Relay synthesis from (+)-narciclasine.
Improvement of the synthesis by Ohta and Kimoto (Ref. 201).
Of the many strategies described in the literature, the chemoenzymatic approach by Hudlicky’s group and radical cyclization chemistry developed by Keck’s group are quite general and can potentially lead to efficient preparations of pancratistatin, narciclasine, 7-deoxynarciclasine and 7-deoxypancratistatin. Additionally, syntheses of (+)-narciclasine and (+)-pancratistatin reported by Yan’s and Li’s groups respectively are short and high yielding. Unfortunately, efficient synthetic routes to trans-dihydronarciclasine and 7-deoxy-trans-dihydronarciclasine remain elusive. The only synthesis of trans-dihydronarciclasine was recently reported by Cho and coworkers.219 Although relatively short and high yielding, the synthesis leads to racemic material, and therefore, rather low yielding hydrogenation of (+)-narciclasine (Scheme 1) is the only preparation of the enantiomerically pure trans-dihydronarciclasine at the time of this writing.
9.1 Chemoenzymatic Strategy to (+)-Pancratistatin, (+)-7-Deoxypancratistatin and (+)-7- Deoxynarciclasine by Hudlicky and Coworkers
The chemoenzymatic approach to the Amaryllidaceae anticancer isocarbostyrils by Hudlicky and coworkers is based on the oxidation of bromobenzene by a bacterial dioxygenase enzyme of Pseudomonas putida in the whole cell laboratory fermentation apparatus.222 Although relatively expensive, diol 151 (Figure 22) is now available commercially (201 USD / 5 grams, Aldrich). Regioselective aziridination (A) and aziridine ring opening with an appropriately functionalized cuprate reagent provides B, possessing four of the six stereocenters in pancratistatin and 7-deoxypancratistatin with the correct relative and absolute stereochemistries. Lactamization and trans-diol installation through olefin epoxidation and epoxide trans-diaxial ring opening gives the targets 51 and 52. Alternatively, Diels-Ader reaction of appropriately protected 151 with an acyl nitroso compound followed by N–O bond cleavage and N-acylation to introduce the aromatic residue give C. The intramolecular Heck reaction then affords the target 45.
Figure 22.
Chemoenzymatic strategy to (+)-pancratistatin, (+)-7-deoxypancratistatin and (+)-7-deoxynarciclasine.
The strategy was implemented in the synthesis of pancratistatin as follows.188,189 Diol 151 is protected as acetonide and then treated with (N-tosylimino)phenyliodinane and Cu(acac)2 catalyst to generate aziridine 152 in a highly regioselective manner (Scheme 10). Radical debromination leads to 153, which is reacted with the highly functionalized arylcuprate 154 in an SN2 fashion (as opposed to SN2’) affording the coupled product 155 in good yield. Such rapid build-up of complexity and installation of four key stereocenters present in the target molecule make this sequence extremely efficient. However, the subsequent troublesome formation of atropoisomers and the difficulties posed by the stable tosylamide and dimethylamide functionalities greatly diminish the practicality of the overall synthetic sequence. Reductive removal of tosylamide is facilitated by the introduction of the Boc moiety to give 156, which exists as a mixture of atropoisomers that undergo reductive detosylation at different rates and even with different outcomes. Thus, the major detosylated compound 157β retains the TBS ether group after treatment with Na/anthracene, while the minor 157α is obtained with the free phenolic hydroxyl. Therefore, 157β is further desilylated to converge on the 157α atropoisomer. Removal of the dimethylamide group is achieved by reduction of the latter to the aldehyde moiety with sodium bis(methoxyethoxy)aluminum hydride and subsequent oxidation and methylation with diazomethane to obtain ester 159. Unmasking of the allylic hydroxyl in 159 allows for β-selective epoxidation to give 160. Finally, reflux in aqueous sodium benzoate solution accomplishes a number of transformations, namely epoxide ring opening, cleavage of the Boc protection, lactamization and debenzylation of the phenolic hydroxyl, giving the target molecule directly in 51% yield.
Scheme 10.a.
a Reagents and conditions: (a) Me2C(OMe)2, p-TsOH, CH2Cl2; (b) PhI=NTs, Cu(acac)2, MeCN; (c) Bu3SnH, AIBN, THF; (d) THF, −78 °C to rt; (e) s-BuLi, THF, (Boc)2O; (f) Na/anthracene, DME, −78 °C; (g) TBAF, THF; (h) NaAl(MeOCH2CH2O)2H2, morpholine, −45 °C, THF; (i) BnBr, K2CO3, DMF; (j) NaClO2, KH2PO4, 2-methyl-2-butene, t-BuOH, H2O; (k) CH2N2; (l) AcOH, THF, H2O, 60 °C; (m) t-BuOOH, VO(acac)2, PhH, 60 °C; (n) BzONa (cat.), H2O, 100 °C.
This short 14-step synthesis proceeds in a rather low 2% overall yield due to the impediments mentioned above. Further optimization is undoubtedly required to reduce this promising strategy to a practical preparation of (+)-pancratistatin.
The highly functionalized arylcuprate 154 is prepared from piperonal by utilizing a clever directed metalation sequence. Both Hudlicky’s189 and later Keck’s162 groups have shown this efficient way of construction of such regiochemically complex benzene derivativatives, which in principle can be generally applicable to any pancratistatin or narciclasine syntheses (Scheme 11).
Scheme 11.a.
a Reagents and conditions: (a) MnO2, NaCN, Me2NH, i-PrOH; (b) n-BuLi, TMEDA, −104 °C; B(OMe)3, THF; AcOH, H2O2; (c) TBSCl, imidazole; (d) n-BuLi, TMEDA, −104 °C, Et2O, I2; (e) Me3OBF4, Na2HPO4, MeCN; (f) TsCl, py; (g) i. s-BuLi, TMEDA, THF, −90 °C; ii. CuCN, −90 to −20 °C.
The aldehyde group of piperonal (161) is converted to dimethylamide 162 by a one step procedure involving treatment of the former with MnO2 in the presence of Me2NH. First ortho-lithiation directed by the amide functionality is followed by the treatment of the aryl lithium intermediate with trimethylborate and oxidation of the resulting boronate with hydrogen peroxide to yield phenol 163. The latter is protected as TBS ether to afford tetrasubstituted benzene 164. Second lithiation, now at the other ortho-position of the dimethylamide functionality, affords aryl iodide 165 after the aryl lithium is quenched with iodine or results in the formation of higher order cyanocuprate 154 when the aryl lithium is treated with copper (I) cyanide. Lastly, Keck and coworkers showed that the amide can be converted to the corresponding ester upon treatment with trimethyloxonium tetrafluoroborate.162 The freed under the reaction conditions phenolic hydroxyl can be further protected with a group capable of modulating the electronic properties of the benzene ring, e. g. tosyl ester 166 optimized for Sonogashira coupling in their synthesis of (+)-narciclasine (vide infra).
The second generation synthesis of (+)-7-deoxypancratistatin by Hudlicky’s group was modified to avoid problems encountered in their route toward pancratistatin, namely abstaining from the use of dialkylamide and sulfonamide functionalities.189 Thus, instead of N-tosyl aziridine 152, the investigators prepared N-carbomethoxy aziridine 167 (Scheme 12). Radical debromination followed by the SN2 addition of cuprate 169 gives carbamate 170. The latter is deprotected and subjected to allylic hydroxyl-directed β-epoxidation to give 171, which undergoes smooth trans-diaxial epoxide opening affording carbamate 172. Finally, exhaustive acetylation, regioselective Bischler-Napieralski cyclization and deacetylation result in the target natural isocarbostyril 52.
Scheme 12.a.
a Reagents and conditions: (a) Me2C(OMe)2, p-TsOH, CH2Cl2; (b) p-O2NPhSO3NHCO2Me, K2CO3, CH2Cl2; (c) Bu3SnH, AIBN, THF; (d) BF3·Et2O, THF, −78 °C to −30 °C; (e) AcOH, THF, H2O, 65 °C; (f) t-BuOOH, VO(acac)2, PhH, 60 °C; (g) BzONa (cat.), H2O, 100 °C; (h) Ac2O, DMAP, py; (i) Tf2O, DMAP, CH2Cl2, 5 °C; (j) MeONa, MeOH.
This very short (10 steps from diol 151 or 11 steps from bromobenzene) synthesis unfortunately proceeds in rather low overall yield of 3%. The key steps that undermine the yield are aziridination, radical debromination and aziridine ring opening. Given a large number of aziridination protocols available in the literature and flexibility of cuprate chemistry, it is highly possible that these impediments can be overcome to provide a scalable synthetic route to this natural product.
The chemoenzymatic strategy allows for a very efficient synthesis of (+)-7-deoxynarciclasine (Scheme 13).205,206 Diels-Alder reaction of diene 173 with in situ generated BnOCON=O gives bicyclic oxazine 174, in a highly stereo- and regioselective manner. Na(Hg)-promoted reductive cleavage of first C–Br and then N–O bonds gives syn-aminoalcohol, which is silylated to afford carbamate 175 in good yield over three steps. N-acylation of 175 with acid chloride 176 leads to the coupled product 177, which undergoes a palladium-catalyzed Heck-type ring-closure providing a modest yield of phenanthridone 178. Finally, transfer hydrogenolysis of the Cbz group and removal of the acetonide protection with CF3CO2H gives the target isocarbostyril 45.
Scheme 13.a.
a Reagents and conditions: (a) Bu4NIO4, CH2Cl2; (b) Al(Hg), THF; (c) i-PrMe2SiCl, imidazole, CH2Cl2; (d) BuLi, THF, −78 °C; (e) Pd(OAc)2, Tl(OAc), DIPHOS, anisole; (f) Pd/C, cyclohexene, EtOH; (g) CF3CO2H, 0 °C.
This pathway constitutes the shortest synthesis of (+)-7-deoxynarciclasine (8 steps from diol 151, 9 steps from bromobenzene) reported to date in excellent (12%) overall yield and it should be well-suited for a large scale preparation of this anticancer natural product. The weakest step is the cyclization of 177 to 178. This unconventional Heck-type transformation involving a formal anti β-hydride elimination is reasonably well precedented, but is still poorly understood.223,224 Further study of its mechanism is warranted and it should directly impact the efficiency of this and many other syntheses.
9.2 Radical Cyclization Strategies to (+)-Narciclasine, (+)-7-Deoxynarciclasine and (+)-7-Deoxypancratistatin by Keck and Coworkers
Keck and coworkers have explored a number of radical cascade cyclizations for the construction of the phenanthridone skeleton associated with the Amaryllidaceae anticancer constituents. These efforts resulted in efficient syntheses (+)-narciclasine, (+)-7-deoxynarciclasine and (+)-7-deoxypancratistatin. Their strategy for the syntheses of (+)-narciclasine and (+)-7-deoxynarciclasine is shown in Figure 23.
Figure 23.
Radical cyclization strategy to (+)-narciclasine and (+)-7-deoxynarciclasine.
Regioselective addition of a suitable radical to the alkyne moiety of A was expected to give an intermediate vinyl radical B, which would undergo cyclization onto the C=N bond of the pendant oxime functionality. The strategy was implemented as follows.162
Readily available D-gulonolactone (179, 79 USD / 25 grams, Aldrich) is converted to diacetonide derivative 180, in which the C5, C6 diol is selectively unmasked upon treatment with mild acid to give 181 (Scheme 14). Periodate cleavage of the diol functionality followed by Corey-Fuchs reaction result in dibromoalkene 182. The lactone is further reduced to the corresponding lactol, which is converted to O-benzyl oxime and then treated with BuLi to form terminal alkyne 183 in excellent yield over the three-step sequence.
Scheme 14.a.
a Reagents and conditions: (a) Me2C(OMe)2, p-TsOH; (b) AcOH, H2O; (c) NaIO4, CH2Cl2; (d) CBr4, PPh3, NEt3; (e) L-selectride, Et2O, −78 °C; (f) HCl·H2NOBn, py; (g) n-BuLi, Et2O, −90 °C.
Alkyne 183 serves as a common precursor for the synthesis of both isocarbostyrils. To reach narciclasine the investigators performed Sonogashira coupling of terminal alkyne in 183 with aryl iodide 166 to give 184 and this sets the stage for the key radical cyclization reaction (Scheme 15). Phenylthiyl radical adds highly regioselectively to the triple bond of 184 (presumably because of the benzylic stabilization) and the resulting intermediate vinyl radical undergoes ring-closure with the O-benzyl oxime moiety acting as the final radical acceptor. Chemoselective reduction of the tosylate in 185 and reprotection of the phenol as methyl ether are followed by the treatment with Me3Al to induce cyclization to a fully assembled narciclasine skeleton in 187. Finally, SmI2 reduction results in N–O bond cleavage and the removal of the PhS group with the concomitant loss of the phenolic methyl to give narciclasine acetonide. The latter is converted to narciclasine (44) by the treatment with trifluoroacetic acid.
Scheme 15.a.
a Reagents and conditions: (a) 166, Pd(OAc)2, CuI, NEt3, PPh3, THF; (b) PhSH, hν, PhMe, 27 °C; (c) SmI2, THF, H2O, 0 °C; (d) MeI, K2CO3, DMF; (e) Me3Al, THF; (f) SmI2, MeOH, THF, 0 °C; (g) TFA, 0 °C.
Overall, the synthesis proceeds in 14 steps from gulonolactone (179) and an excellent 16% yield. Although the phenolic protection strategies in the form of tosylate ester and methyl ether are optimized for the palladium-catalyzed coupling of 183 and 166, radical cyclization of 184 and the cascade reduction processes of 187, it should be possible to considerably shorten the synthesis by further investigating and possibly limiting the phenolic protection/deprotection manipulations.
Sonogashira coupling of 183 with aryl iodide 188 gives 189 and sets the stage for the radical cyclization leading to (+)-7-deoxynarciclasine (Scheme 16). In the event, phenylthiyl radical-promoted cyclization proceeds as cleanly and regioselectively as the corresponding process used in the construction of the narciclasine skeleton. Reduction of the cyclized product 190 with SmI2 leads to N–O and C–S bonds cleavage and lactamization. Finally, acetonide removal furnishes the target compound 45.
Scheme 16.a.
a Reagents and conditions: (a) Pd(OAc)2, CuI, NEt3, PPh3, THF; (b) PhSH, hν, PhMe, 27 °C; (c) SmI2, THF; (d) TFA, 0 °C.
Overall, the synthesis proceeds in just 11 steps from gulonolactone and excellent 27% yield. It is noteworthy that C2-hydroxyl (narciclasine numbering) needs no protection in the second half of the synthesis, enhancing the efficiency of the synthetic route.
Keck and coworkers have also applied such radical cyclization approaches to the synthesis of (+)-7-deoxypancratistatin. Their first generation synthesis is based on the cyclization of the benzylic radical B onto a pendant oxime moiety giving lactol structure C, which is further converted to the target isocarbostyril by rearrangement to the corresponding lactam (Figure 24).
Figure 24.
Radical cyclization strategy in the first generation synthesis of (+)-7-deoxypancratistatin of Keck and coworkers.
Unfortunately, such strategy resulted in a rather long 22 step synthetic sequence.211,212 A significantly improved second generation synthesis utilizes Kim cascade radical cyclization process involving the use of an aziridine imine moiety (A, Figure 25). The intramolecular addition of aryl radical to the C=N bond of the aziridine imine (B) leads to the loss of nitrogen and styrene regenerating a carbon based benzylic radical C, which undergoes a second cyclization onto the C=N bond of the oxime functionality completing the construction of the desired multicyclic skeleton D.215
Figure 25.
Kim cascade radical cyclization strategy in the second generation synthesis of (+)-7-deoxypancratistatin of Keck and coworkers.
Such second generation synthesis was accomplished as follows (Scheme 17). Gulonolactone-derived diol 181 is selectively silylated at the primary hydroxyl and then subjected to benzyl ether formation under acidic conditions to give 193. Reduction of the lactone to the corresponding lactol moiety, followed by oxime formation results in 194. Silylation of the secondary hydroxyl in 194 and selective cleavage of the primary silyl ether affords alcohol 195. Oxidation of the latter to the corresponding aldehyde is followed by its conversion to the desired N-aziridinylimine 196. The key radical cascade cyclization event proceeds cleanly with the use of Ph3SnH to give 197 as a single diastereomer in excellent yield for such a complex transformation. The completion of the synthesis involves N–O bond cleavage with SmI2, conversion of the resulting amine to trifluoroacetamide, followed by PCC oxidation of the benzylic position to give 198. The latter is subjected to treatment with BF3·Et2O in CH2Cl2 resulting in removal of the acetonide and silyl ether protecting groups. Lactone to lactam rearrangement promoted by potassium carbonate in methanol likely proceeds via the intermediacy of methyl ester and gives the target isocarbostyril 52 in excellent yield.
Scheme 17.a.
a Reagents and conditions: (a) TBSCl, imidazole; (b) 192, NaH, Cl3CCN, 0 °C; (c) TfOH, THF, 0 °C; (d) L-selectride, CH2Cl2, −78 °C; (e) HCl·H2NOBn, py; (f) TBSOTf, 2,6-lutidine, CH2Cl2, 0 °C; (g) HF·py, THF; (h) TPAP, NMO, 4Å MS; (i) 1-amino-2-phenylaziridine, EtOH, 0 °C; (j) Ph3SnH, AIBN, PhH, 78 °C; (k) SmI2, THF, then TFAA; (l) PCC, CH2Cl2, 55 °C; (m) BF3·Et2O, CH2Cl2; (n) K2CO3, MeOH.
Overall the synthesis requires 15 linear steps from D-gulonolactone and proceeds in very good 12% overall yield. It should be noted that the syntheses of (+)-7-deoxynarciclasine and (+)-7-deoxypancratistatin utilizing the radical cyclization strategies are slightly longer, but higher yielding than the chemoenzymatic synthetic pathways to these natural products discussed above. Both of these approaches are very general, permitting access to a number of the Amaryllidaceae anticancer isocarbostyrils, and with further optimization they can be well-suited for practical preparation of these agents for clinical evaluation.
9.3 Synthesis of (+)-Narciclasine by Yan and Coworkers
Yan and coworkers described an efficient synthesis of (+)-narciclasine186 based on an intramolecular Lewis acid promoted arene-epoxide coupling following an established precedent from the work of Hudlicky et al.146,225 on the synthesis of 10b-epi-7-deoxypancratistatin (Figure 26).
Figure 26.
Strategy to (+)-narciclasine based on intramolecular arene-epoxide ring opening.
The preparation of aminoconduritol 202, possessing all four stereocenters in the structure of narciclasine with the correct relative and absolute stereochemistries, is accomplished by the asymmetric cycloaddition the chloronitroso compound 200 with diene 201, followed by the reductive N–O cleavage with Al-Hg (Scheme 18). 200 is derived from ketopinic acid (199, 65 USD/1 gram, Aldrich). Although the starting material is somewhat expensive, ketoester 203 is isolated by simple extraction of the aqueous reaction mixture in 93% yield and can be recycled. Protection of the hydroxyl and amino groups in aminoconduritol 202 (> 99% ee) as TBS ether and sulfonamide respectively gives 204. The introduction of the required epoxide at the β-face of olefin 204 is achieved through non-regioselective bromohydrin formation, possibly via the intermediacy of a bromonium ion at the convex α-face of the molecule followed by the unselective attack by water. Both bromohydrins, 205 and 206, lead to the same β-epoxide 207 when their 1:1 mixture is treated with potassium carbonate in MeCN. In situ addition of bromide 208 to the reaction mixture results in concomitant N-piperonylation giving 209 in excellent overall yield for this complex transformation. The crucial arene-epoxide cyclization is cleanly promoted by tin tetrachloride affording a quantitative yield of 210 after acetylation of the cyclized material. The completion of the synthesis involves nitrogen reprotection and oxidation of the benzylic position to form lactam 212. This is followed by the syn-elimination of the acetoxy group with DBU in hot benzene and complete deprotection to give the target molecule.
Scheme 18.a.
a Reagents and conditions: (a) SOCl2, MeOH; (b) HCl·NH2OH, MeOH, AcONa; (c) t-BuOCl, CH2Cl2; (d) CH2Cl2, 0 °C, 12 h; (e) Al-Hg, H2O/MeCN; (f) p-O2NPhSO2Cl, MeCN, NEt3; (g) TBSCl, DBU; (h) NBS, H2O/acetone; (i) MeCN, K2CO3, 60 °C, then 208; (j) 20 mol% SnCl4, CH2Cl2; (k) Ac2O, K2CO3; (l) HSCH2CO2H, LiOH, DMF; (m) Boc2O, MeCN; (n) RuCl3, NaIO4, H2O; (o) DBU, PhH, 70 °C; (p) HCO2H, THF, 60 °C; (q) LiAlH4, THF.
The synthesis is very efficient requiring 13 steps to reach the target compound in an excellent 17% overall yield. Although the starting material, ketopinic acid (199) is not inexpensive, the procedure involving its recycling mitigates the cost problem. Further improvements can be made by reducing the number of protecting groups as well as protection/deprotection steps (e.g. 210 → 212).
9.4 Synthesis of (+)-Pancratistatin by Li and Coworkers
Li and coworkers have developed an efficient route to (+)-pancratistatin utilizing a strategy based on intramolecular cyclic sulfate ring opening with an arylmetal nucleophile (A, Figure 27).196 Pinitol (215, 34 USD / 0.5 grams, Aldrich) represents somewhat expensive, but attractive starting material as it already incorporates four stereogenic centers found in (+)-pancratistatin.
Figure 27.
Strategy to (+)-pancratistatin based on intramolecular ring opening of a cyclic sulfate moiety.
Selective protection of the trans (215 → 216) and cis (216 → 217) diols leaves C6-hydroxyl in compound 217 available for the replacement with a nitrogen functionality, which is present in pancratistatin at this position with the same stereochemistry (Scheme 19). This is accomplished by Mitsunobu inversion with methylsulfonic acid and the subsequent substitution with azide ion giving azide 218 with the overall retention of configuration. Removal of the silyl protection, introduction of the cyclic sulfate at the unmasked trans-diol moiety and Staudinger azide reduction yield cyclic sulfate-containing amine 219, ready for coupling with an aromatic residue.
Scheme 19.a.
a Reagents and conditions: (a) TIPDSCl2, imidazole, DMAP; (b) Me2C(OMe)2, p-TsOH; (c) PPh3, DEAD, MeSO3H, CH2Cl2; (d) NaN3, DMF, 60 °C; (e) TBAF, THF; (f) SOCl2, Et3N, CH2Cl2; (g) NaIO4, RuCl3, aq. MeCN; (h) PPh3, aq. THF.
The aromatic acid chloride coupling partner is prepared from bromide 220 by removal of the PMB protecting group and treatment of the resulting phenol with MgBr2·OEt2 and phosgene in ether (Scheme 20). This procedure gives the presumed acid chloride complex 221, which is reacted in situ with amine 219 affording the coupled product 222. MOM protection of the NH and OH groups gives 223 and sets the stage for the crucial ring forming intramolecular cyclic sulfate ring opening reaction. The investigators found that treatment of bromide 223 with t-BuLi, followed by the addition of anhydrous CeCl3 leads to the desired transformation via the presumed arylcerium intermediate. Finally, complete deprotection is accomplished by treating 224 with BBr3 in CH2Cl2, followed by the addition of MeOH under careful temperature control to avoid the loss of the methylenedioxy unit.
Scheme 20.a.
a Reagents and conditions: (a) AcOH, 100 °C; (b) MgBr2·OEt2, COCl2, ether, 219; (c) K2CO3, MOMCl, DMF; (d) t-BuLi, CeCl3, ultrasound, THF, −78 °C to rt; (e) BBr3, CH2Cl2, −78 °C, then MeOH, −78 °C to rt.
This synthesis represents the shortest (13 steps from pinitol) and the most high yielding (9% overall) route form commercially available starting material to (+)-pancratistatin reported so far. At the time of this writing the results have been disclosed in a communication format only, and the full article, detailing the experimental nuances is eagerly awaited.
10. Concluding Remarks
The use of Amaryllidaceae plants, particularly of the genus Narcissus, for cancer management beginning with Hippokrates in the forth century B.C., later by medical practitioners of the middle ages throughout the world, and currently through ongoing research efforts has advanced our knowledge to the point of having identified the specific small molecule constituents of these plants that possess potent anticancer properties. As most researchers now believe these constituents include isoquinolines narciclasine, pancratistatin, trans-dihydronarciclasine and their corresponding 7-deoxy analogues. The chemical structures of these non-basic metabolites have been elucidated by spectroscopic means and supported by independent syntheses. Their chemical properties as well as their effects on plant and human physiology have been under intense scrutiny in the last few decades and significant knowledge has been gained. Yet, none of these compounds has advanced to human clinical trials for treating cancer and there is still a lot to be done before this happens. We finish our review by identifying further possible research directions that will bring us closer to the implementation of these compounds and/or their derivatives in human medicine.
The apparent major impediment thwarting further developments in the preclinical evaluation of these compounds is the issue of supply. Since isolated amounts of these isoquinolines from the discovered plant sources are small, further search for plants synthesizing these metabolites is essential. In this context, the identification of narciclasine and pancratistatin in the lubber Grasshopper B. Magna, collected in Texas, is intriguing.75 It is believed that insects select their diet to obtain plant constituents important for their own chemical defense.226 Surprisingly, species of the Amaryllidaceae family have not been recorded among this Grasshopper’s preferred foods.227 This finding may have potentially significant implications in the discovery of new plant sources for these anticancer isocarbostyrils.
Additionally, a practical chemical synthesis from a commercially available inexpensive starting material constitutes a very important alternative. It is hoped that researchers, who report such multistep synthetic pathways, revisit their work again and again developing second and third generation syntheses in pursuit of practicality and scalability. Synthesis and testing of structurally simplified analogues of natural compounds, which may be more amenable to large-scale preparation, is another important research direction. Rather surprisingly, so far such efforts have not resulted in compounds with simpler structures but comparable medicinal potential. This work, however, is quite empirical in character due to our poor understanding of the biological mechanism(s) of action associated with the Amaryllidaceae anticancer isocarbostyrils. The mechanistic studies, revealing inhibition of protein biosynthesis by narciclasine and some of its natural and synthetic congeners, as discussed in this review, are thirty years old. There has been very little new research done in this area since then. Undoubtedly, the insights that may be gained from the structural studies of these isocarbostyrils and their ribosomal binding site(s) will have a tremendous impact on our understanding of the bioactive pharmacophore(s) associated with these compounds. Such knowledge will guide more successful analogue generation efforts.
Lastly, natural products are often referred to as evolutionarily selected privileged medicinal structures.228–230 In the process of their biosynthesis they interact with many biological macromolecules and, thus, they are likely to bind to a variety of structurally and functionally discreet receptors. It follows that natural products may manifest multiple biological activities, or exhibit the same activity through a number of different mechanisms. Examples of such observations abound in the literature. In this respect, preliminary accounts of the preclinical study of pancratistatin phosphate prodrugs reported by Pettit and coworkers are intriguing and indicate that the mechanism of action of pancratistatin against in vivo antineoplastic disease involves at least in part angiogenesis/antivascular targeting.179,180 Whether this effect is related to the inhibition of protein biosynthesis in the vascular endothelial cells or is due to an entirely different mode of action is one of the many stimulating questions that will draw interest and incite further research efforts in this area. We are looking forward to updating this review in a few years time, hopefully, with the results of the clinical trials of the Amaryllidaceae anticancer constituents or their synthetic analogues.
Figure 19.
Ring C analogues.
Acknowledgements
We thank the US National Institutes of Health (CA-99957 and RR-16480) and Italian Ministry University and Research (MIUR) for financial support (contribution DISSPAPA N. 166). Kristen A. Kornienko is gratefully acknowledged for designing the cover art. This review is dedicated to the memory of Dr. Mosi Cicala.
References
- 1.Hartwell JL. Lloydia. 1967;30:379. [Google Scholar]
- 2.Gardeil JB, translator. Traduction des Oeuvres Médicales d’Hippocrate. 4 vols. Toulouse: Fages, Meilhec et Cie; 1801. [Google Scholar]
- 3.Aetios of Amida. The gynaecology and obsterics of the VI century, A.D. Philadelphia: Blakiston Co.; 1950. 215 pp. (transl. by J. V. Ricci) [Google Scholar]
- 4.Leclerc L Ibn al-Baitar. Traité des Simples par Ibn el-Beïthar. I. Vol. 23. Paris: Notices et Extraits des Manuscrits de la Bibl. Nat.; 1877. 476 pp. 1881, 25(I), 489 pp.; 1883, 26(I), 483 pp. [Google Scholar]
- 5.Martin SF. The Alkaloids. New York: Academic Press; 1987. p. 251. [Google Scholar]
- 6.Gibbs RD. Chemotaxonomy of Flowering Plants. Vol. III. Montreal: McGill-Queen’s University Press; 1974. p. 1924. [Google Scholar]
- 7.Tojo E. J. Nat. Prod. 1991;54:1387. [Google Scholar]
- 8.Noumbissie BE, Kapnang H, Fomum ZT, Martin M-T, Bodo B. J. Nat. Prod. 1992;55:137. [Google Scholar]
- 9.Bastida J, Codina C, Viladomat F, Rubiralta M, Quirion J-C, Weniger B. J. Nat. Prod. 1992;55:122. [Google Scholar]
- 10.Plinius Secundus C. In: Natural History. Bostock J, Riley HT, translators. 6 vols. London: Bohn; 1855. (Pliny the Elder) [Google Scholar]
- 11.Duke JA, Duke PK. Medicinal Plants of the Bible. New York: Trad-Medic Books; 1983. p. 98. [Google Scholar]
- 12.Hartwell JL. Plants Used Aagainst Cancer. Lawrence, MA: Quarterman Publication; 1982. [Google Scholar]
- 13.Kosteletzky BF. Allgemeine medizinisch-pharmazeutische Flora. 6 vols. Prague: Borrosch and André; 1831–1836. [Google Scholar]
- 14.Cook JW, Loudon JD. The Alkaloids. New York: Academic Press; 1952. p. 331. [Google Scholar]
- 15.Piozzi F, Marino ML, Fuganti C, Di Martino A. Phytochemistry. 1969;8:1745. [Google Scholar]
- 16.Rinner U, Hudlicky T. Synlett. 2005:365. [Google Scholar]
- 17.Chapleur Y, Chrétien F, Ibn-Ahmed S, Khaldi M. Curr. Org. Synth. 2006;3:341. [Google Scholar]
- 18.Louw CAM, Regnier TJC, Korsten L. J. Ethnopharmacol. 2002;82:147. doi: 10.1016/s0378-8741(02)00184-8. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 19.Nakagawa Y, Uyeo S, Yayima H. Chem. Ind. 1956:1238. and references cited therein. [Google Scholar]
- 20.Arrigoni O, Arrigoni Liso R, Calabrese G. Nature. 1975;256:513. [Google Scholar]
- 21.Evidente A, Motta A. Studies in Natural Products Chemistry. Amsterdam: Elsevier; 2002. p. 581. 2001. [Google Scholar]
- 22.Ghosal S, Saini KS, Razdan S. Phytochemistry. 1985;24:2141. [Google Scholar]
- 23.Wildman WC. The Alkaloids. New York: Academic Press; 1960. p. 289. [Google Scholar]
- 24.Wildman WC. The Alkaloids. New York: Academic Press; 1968. p. 307. [Google Scholar]
- 25.Fuganti C. The Alkaloids. New York: Academic Press; 1975. p. 83. [Google Scholar]
- 26.Suffness M, Cordell GA. The Alkaloids. New York: Academic Press; 1985. p. 3. [Google Scholar]
- 27.Bastida J, Viladomat F, Codina C. Studies in Natural Products Chemistry. Amsterdam: Elsevier; 1998. p. 323. [Google Scholar]
- 28.Evidente A. Flavour and Fragrance. Dordrecht: Kluver; 2000. p. 109. [Google Scholar]
- 29.Bezhenova ED, Aliev KV, Zairov VB. Farmakol. Alkaloid Ser. Glik. 1972:100. [Google Scholar]
- 30.Zakirov UB, Umarova SS. Farmakol. Alkaloid Ser. Glik. 1971:96. [Google Scholar]
- 31.Ieven M, VandenBerghe DA, Mertens I, Vlietinck AJ, Lammens E. Planta Med. 1979;36:311. doi: 10.1055/s-0028-1097277. [DOI] [PubMed] [Google Scholar]
- 32.Pettit GR, Gaddamidi V, Goswami A, Cragg GM. J. Nat. Prod. 1984;47:796. doi: 10.1021/np50035a007. [DOI] [PubMed] [Google Scholar]
- 33.Evidente A, Andolfi A, Abou-Donia AH, Touema SM, Hammoda HM, Shawky E, Motta A. Phytochemistry. 2004;65:2113. doi: 10.1016/j.phytochem.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 34.Ali AA, Ross SA, El-Moghazy A, El-Moghazy SA. J. Nat. Prod. 1983;46:350. and references cited therein. [Google Scholar]
- 35.Jeffs PW, Mueller L, Abou-Donia AH, Seif El-Din AA, Campau D. J. Nat. Prod. 1988;51:549. [Google Scholar]
- 36.Evidente A, Andolfi A, Abou-Donia AH, Darwish FA, Hammoda HAM, Motta A. Alex. J. Pharm. Sci. 2005;19:49. [Google Scholar]
- 37.Evidente A, Abou-Donia AH, Darwish FA, Amer ME, Kassem FF, Hammoda HAM, Motta A. Phytochemistry. 1999;51:1151. [Google Scholar]
- 38.Fennell CW, van Staden J. J. Ethopharmacol. 2001;78:15. doi: 10.1016/s0378-8741(01)00305-1. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 39.Ghosal S, Saini KS, Frahm AW. Phytochemistry. 1983;22:2305. [Google Scholar]
- 40.Houghton PJ, Agbedahunsi JM, Adegbulugbe A. Phytochemistry. 2004;65:2893. doi: 10.1016/j.phytochem.2004.08.052. [DOI] [PubMed] [Google Scholar]
- 41.Elgorashi EE, van Staden J. J. Ethnopharmacol. 2004;90:27. doi: 10.1016/j.jep.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 42.Ramanathan S, Furusawa E, Kroposki M, Furusawa S, Cutting W. Chemotherapy. 1968;13:121. doi: 10.1159/000220538. [DOI] [PubMed] [Google Scholar]
- 43.Abou-Donia AH, Darwish FA, Ghazy NM. Alex J. Pharm. Sci. 1989;3:122. [Google Scholar]
- 44.Evidente A, Lanzetta R, Abou-Donia AH, Amer ME, Kassem FF, Harraz F. Arch. Pharm. 1994;327:595. [Google Scholar]
- 45.Sener B, Orhan I, Satayavivad J. Phytother. Res. 2003;17:1220. doi: 10.1002/ptr.1346. [DOI] [PubMed] [Google Scholar]
- 46.Ghosal S, Saini KS, Rao PH, Kumar Y, Jaiswal DK, Chattopadhyay S. Proc.6th IndoSoviet Symp., Chemistry of Natural Products; Pune, India: NCL; 1981. p. 71. [Google Scholar]
- 47.Ali AA, Mesbah MK, Mohamed MH. Bull. Pharm. Sci Assiut University. 1984;7:351. [Google Scholar]
- 48.Sandberg F, Michel KH. Acta Pharm. Suecica. 1968;5:61. [PubMed] [Google Scholar]
- 49.Vasquez Tato MP, Castedo L, Riguera R. Heterocycles. 1988;27:2833. [Google Scholar]
- 50.Abou-Donia AH, Abib A-A, Seif El Din A, Evidente A, Gaber M, Scopa A. Phytochemistry. 1992;31:2139. [Google Scholar]
- 51.Ghosal S, Singh SK, Srivastava RS. Phytochemistry. 1986;25:1975. [Google Scholar]
- 52.Ghosal S, Singh SK, Kumar Y, Unnikrishnan S, Chattopadhyay S. Planta Med. 1988;54:114. doi: 10.1055/s-2006-962364. [DOI] [PubMed] [Google Scholar]
- 53.Evidente A, Randazzo G, Surico G, Lavermicocca P, Arrigoni O. J. Nat. Prod. 1985;48:564. [Google Scholar]
- 54.Evidente A, Iasiello I, Randazzo G. J. Nat. Prod. 1984;47:1003. [Google Scholar]
- 55.Normatov M, Abduazimov KhA, Yunusov SYu. Uzbeksk. Khim. Zh. 1965;9:25. [Google Scholar]
- 56.Ali AA, El-Sayed HM, Abdallah OM, Steglich W. Phytochemistry. 1986;25:2399. [Google Scholar]
- 57.Ghosal S, Kumar Y, Singh SK, Kumar A. J. Chem. Res. Synop. 1986:112. [Google Scholar]
- 58.Watt JW, Breyer-Brandwijk MG. Medicinal and Poisonous Plants of Southern and Eastern Africa. London: Livingstone, E. and S. Ltd.; 1962. p. 42. [Google Scholar]
- 59.Ghosal S, Singh SK, Srivastava RS. Phytochemistry. 1985;24:151. [Google Scholar]
- 60.Ghosal S, Ashutosh, Radzan S. Phytochemistry. 1985;24:635. [Google Scholar]
- 61.Scott G. Veld and Flora. 1993;79:84. [Google Scholar]
- 62.Hutchings A, Scott AH, Lewis G, Cunningham AB. Zulu Medicinal Plants: An Inventory. Pietermaritzburg: University Natal Press; 1996. [Google Scholar]
- 63.Piozzi F, Fuganti C, Mondelli R, Cerotti G. Tetrahedron. 1968;24:1119. [Google Scholar]
- 64.Ceriotti G. Nature. 1967;213:595. doi: 10.1038/213595a0. [DOI] [PubMed] [Google Scholar]
- 65.Savona G, Piozzi F, Marino ML. J. Chem. Soc., Chem. Commun. 1970:1006. [Google Scholar]
- 66.Immirzi A, Fuganti C. J. Chem. Soc., Chem. Commun. 1972:240. [Google Scholar]
- 67.Okamoto T, Torii Y, Isogai Y. Chem. Pharm. Bull. 1968;16:1860. doi: 10.1248/cpb.16.1860. [DOI] [PubMed] [Google Scholar]
- 68.Iraida S, Zaida T. Revista Cubana de Farmacia. 1989;23:151. Journal written in Spanish; CAN 112:115819 AN 1990:115819. [Google Scholar]
- 69.Shuzo T, Masae Y. Yakugaku Zasshi. 1974;94:617. Journal written in Japanese; CAN: 81:74924 AN 1974: 74924. [Google Scholar]
- 70.Pettit GR, Gaddamidi V, Herald DL, Singh SB, Gragg GM, Schimdt JM, Boettner FE, William M, Sagawa Y. J. Nat. Prod. 1986;49:995. doi: 10.1021/np50048a005. [DOI] [PubMed] [Google Scholar]
- 71.Ghosal S, Singh S, Kumar Y, Srivastava RS. Phytochemistry. 1989;28:611. [Google Scholar]
- 72.Pettit GR, Pettit GR, III, Backhaus RA, Boyd MR, Meerow AW. J. Nat. Prod. 1993;56:1682. doi: 10.1021/np50100a004. [DOI] [PubMed] [Google Scholar]
- 73.Pettit GR, Gaddamidi V, Cragg GM, Herald DL, Sagawa Y. J. Chem. Soc., Chem. Commun. 1984:1693. [Google Scholar]
- 74.Pettit GR, Cragg GM, Singh SB, Duke JA, Doubek DL. J. Nat. Prod. 1990;53:176. doi: 10.1021/np50067a026. [DOI] [PubMed] [Google Scholar]
- 75.Pettit GR, Meng Y, Herald DL, Knight JC, Day JF. J. Nat. Prod. 2005;68:1256. doi: 10.1021/np0402367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ghosal S, Lochan R, Ashutosh, Kumar Y, Srivastava RS. Phytochemistry. 1985;24:1825. [Google Scholar]
- 77.Abou-Donia AH, De Giulio A, Evidente A, Gaber M, Habib A-A, Lanzetta R, Seif El Din AA. Phytochemistry. 1991;30:3445. [Google Scholar]
- 78.Ghosal S, Datta K, Singh SK, Kumar Y. J. Chem. Res. 1990:334. [Google Scholar]
- 79.Kojima K, Mutsuga M, Inoue M, Ogihara Y. Phytochemistry. 1998;48:1199. [Google Scholar]
- 80.Pettit GR, Gaddamidi V, Gragg GM. J. Nat. Prod. 1984;47:1018. doi: 10.1021/np50036a020. [DOI] [PubMed] [Google Scholar]
- 81.Bi Y-R, Yung K-H, Wong Y-S. Plant Sci. 1998;135:103. [Google Scholar]
- 82.Pettit GR, Pettit GR, III, Groszek G, Backhaus RA, Doubek DL, Barr RJ. J. Nat. Prod. 1995;58:756. doi: 10.1021/np50119a017. [DOI] [PubMed] [Google Scholar]
- 83.Pettit GR, Pettit GR, III, Backhaus RA, Boettner FE. J. Nat. Prod. 1995;58:37. doi: 10.1021/np50115a004. [DOI] [PubMed] [Google Scholar]
- 84.Pettit GR, Eastham SA, Melody N, Orr B, Herald DL, McGregor J, Knight JC, Doubek DL, Pettit GR, III, Garner LC, Bell JA. J. Nat. Prod. 2006;69:7. doi: 10.1021/np058068l. [DOI] [PubMed] [Google Scholar]
- 85.Evidente A. Planta Med. 1991;57:293. doi: 10.1055/s-2006-960098. [DOI] [PubMed] [Google Scholar]
- 86.Bernfeld P. Biogenesis of Natural Compounds. Oxford: Pergamon Press; 1963. p. 985. [Google Scholar]
- 87.Battersby AR, Herbert RB, McDonald E, Ramage R, Clements JH. Chem. Commun. 1966:603. doi: 10.1039/p19720001741. [DOI] [PubMed] [Google Scholar]
- 88.Battersby AR, Binks R, Breuer SW, Fales HM, Wildman WC, Highet RJ. J. Chem. Soc. 1964:1595. [Google Scholar]
- 89.Battersby AR, Binks R, Breuer SW, Fales HM, Wildman WC. Proc. Chem. Soc. 1961:243. [Google Scholar]
- 90.Kirby GW, Tiwari HP. J. Chem. Soc. (C) 1966:676. [Google Scholar]
- 91.Barton DHR, Kirby GW, Taylor JB, Thomas GM. Proc. Chem. Soc. 1962:179. [Google Scholar]
- 92.Barton DHR, Kirby GW, Taylor JB, Thomas GM. J. Chem. Soc. 1963:4545. [Google Scholar]
- 93.Battersby AR, Fales HM, Wildman WC. J. Am. Chem. Soc. 1961;83:4098. [Google Scholar]
- 94.Jeffs PW. Proc. Chem Soc. 1962:80. [Google Scholar]
- 95.Fuganti C. Tetrahedron Lett. 1973;14:1785. [Google Scholar]
- 96.Murphy CF, Wildman WC. Tetrahedron Lett. 1964;5:3863. [Google Scholar]
- 97.Barton DHR. Proc. Chem. Soc. 1963:293. [Google Scholar]
- 98.Barton DHR. Pure Appl. Chem. 1964;9:35. [Google Scholar]
- 99.Barton DHR, Kirby GW. Proc. Chem. Soc. 1960:392. [Google Scholar]
- 100.Fuganti C, Staunton J, Battersby AR. Chem. Commun. 1971:1154. [Google Scholar]
- 101.Fuganti C, Mazza M. Chem. Commun. 1971:1388. [Google Scholar]
- 102.Battersby AR, Hebert RB, McDonald E, Ramage R, Clements JH. Chem. Commun. 1966:603. doi: 10.1039/p19720001741. [DOI] [PubMed] [Google Scholar]
- 103.Fuganti C. Gazz. Chim. Ital. 1973;103:1255. [Google Scholar]
- 104.Bakhaus RA, Pettit GR, III, Huang DS, Pettit GR, Groszek G, Odgers JC, Ho J, Meerow A. Acta Hort. 1992;364:366. [Google Scholar]
- 105.Mondon A, Krohn K. Chem. Ber. 1975;108:445. [Google Scholar]
- 106.Pettit GR, Melody N, O’Sullivan M, Thompson MA, Herald DL, Coates B. J. Chem. Soc., Chem. Commun. 1994:2725. [Google Scholar]
- 107.Pettit GR, Melody N, Herald DL, Schmidt JM, Pettit RK, Chapuis J-C. Heterocycles. 2002;56:139. [Google Scholar]
- 108.Pettit GR, Melody N, Herald DL, Knight JC, Chapuis J-C. J. Nat. Prod. 2007;70:417. doi: 10.1021/np068046e. [DOI] [PubMed] [Google Scholar]
- 109.Pettit GR, Melody N, Herald DL. J. Org. Chem. 2001;66:2583. doi: 10.1021/jo000710n. [DOI] [PubMed] [Google Scholar]
- 110.Carrasco L, Fresno M, Vazquez D. FEBS Lett. 1975;52:236. doi: 10.1016/0014-5793(75)80813-1. [DOI] [PubMed] [Google Scholar]
- 111.Jimenez A, Sanchez L, Vazquez D. FEBS Lett. 1975;55:53. doi: 10.1016/0014-5793(75)80955-0. [DOI] [PubMed] [Google Scholar]
- 112.Jimenez A, Sanchez L, Vazquez D. FEBS Lett. 1975;60:66. doi: 10.1016/0014-5793(75)80420-0. [DOI] [PubMed] [Google Scholar]
- 113.Jimenez A, Santos A, Alonso G, Vazquez D. Biochim. Biophys. Acta. 1976;425:342. doi: 10.1016/0005-2787(76)90261-6. [DOI] [PubMed] [Google Scholar]
- 114.Baez A, Angoso M, Alonso G, Vazquez D. Biochimie. 1977;59:751. doi: 10.1016/s0300-9084(77)80258-7. [DOI] [PubMed] [Google Scholar]
- 115.Baez A, Vazquez D. Biochim. Biophys. Acta. 1978;518:95. doi: 10.1016/0005-2787(78)90119-3. [DOI] [PubMed] [Google Scholar]
- 116.Barendse LVJ. Vakblad voor de Bloemisterij. 1974;29:12. [Google Scholar]
- 117.van Doorn WG. J. Am. Hort. Sci. 1998;123:146. [Google Scholar]
- 118.van Doorn WG, Sinz A, Tomassen MM. Phytochemistry. 2004;65:571. doi: 10.1016/j.phytochem.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 119.Sasse JM, Wardrop JJ, Rowan KS, Aspinall D, Coombe BG, Paleg LG, Buta JG. Physiol. Plantarum. 1982;55:51. [Google Scholar]
- 120.Sasse JM, Galbraith MN, Horn DHS, Adamson DA. Plant Growth Substances. Berlin: Springer-Verlag; 1972. pp. 299–305. [Google Scholar]
- 121.Katekar GF, Geissler AE. Plant Physiol. 1980;66:1190. doi: 10.1104/pp.66.6.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Isogai Y, Nomoto S, Noma T, Okamoto T. Plant Growth Substances. Tokyo: Hirokawa Publishing Co.; 1974. pp. 9–14. [Google Scholar]
- 123.Edelbluth E, Kaldewey H. Planta. 1976;131:285. doi: 10.1007/BF00385428. [DOI] [PubMed] [Google Scholar]
- 124.Kang BG, Lee JS, Oh SE, Horiuchi Y, Imaseki H. Plant Cell Physiol. 1984;25:249. [Google Scholar]
- 125.Sasse JM, Cerana R, Colombo R. Physiol. Plantarum. 1984;62:303. [Google Scholar]
- 126.Kaldeway H, Ginkel U. Plant Growth Substances. Tokyo: Hirokawa Publishing Co.; 1974. pp. 1079–1089. [Google Scholar]
- 127.Bi YR, Zhang LX, Guo JK, Yung KH, Wong YS. N. Z. J. Crop. Hortic. Sci. 2003;31:335. [Google Scholar]
- 128.Numata A, Takemura T, Ohbayashi H, Katsuno T, Yamamoto K, Sato K, Kobayashi S. Chem. Pharm. Bull. 1983;31:2146. [Google Scholar]
- 129.Evidente A, Arrigoni O, Liso R, Calabrese G, Randazzo G. Phytochemistry. 1986;25:2739. [Google Scholar]
- 130.Ghosal S, Shanthy A, Kumar A, Kumar Y. Phytochemistry. 1985;24:2703. [Google Scholar]
- 131.Ghosal S, Kumar Y, Singh S. Phytochemistry. 1984;23:1167. [Google Scholar]
- 132.Green RI, Greenberg NH, MacDonald MM, Schumacher AM, Abbott BJ. Cancer Chemother. Rep. 1972;3:1. [Google Scholar]
- 133.Nakayama M, Horie T, Tsukayama M, Masumura M, Hayashi S. Z. Naturforsch., C: Biosci. 1978;33:587. [Google Scholar]
- 134.Pettit GR, Fujii Y, Hasler JA, Schmidt JM, Michel C. J. Nat. Prod. 1982;45:263. doi: 10.1021/np50021a007. [DOI] [PubMed] [Google Scholar]
- 135.Paull KD, Shoemaker RH, Hodes L, Monks A, Scudiero DA, Rubinstein L, Plowman J, Boyd MR. J. Natl. Cancer Inst. 1989;81:1088. doi: 10.1093/jnci/81.14.1088. [DOI] [PubMed] [Google Scholar]
- 136.Mutsuga M, Kojima K, Yamashita M, Ohno T, Ogihara Y, Inoue M. Biol. Pharm. Bull. 2002;25:223. doi: 10.1248/bpb.25.223. [DOI] [PubMed] [Google Scholar]
- 137.Hudlicky T, Moser M, Banfield SC, Rinner U, Chapuis J-C, Pettit GR. Can. J. Chem. 2006;84:1313. [Google Scholar]
- 138.Kekre N, Griffin C, McNulty J, Pandey S. Cancer Chemother. Pharmacol. 2005;56:29. doi: 10.1007/s00280-004-0941-8. [DOI] [PubMed] [Google Scholar]
- 139.Pandey S, Kekre N, Naderi J, McNulty J. Artif. Cells, Blood Substitutes, Biotechnol. 2005;33:279. doi: 10.1081/bio-200066621. [DOI] [PubMed] [Google Scholar]
- 140.McLachlan A, Kekre N, McNulty J, Pandey S. Apoptosis. 2005;10:619. doi: 10.1007/s10495-005-1896-x. [DOI] [PubMed] [Google Scholar]
- 141.Rinner U, Hillebrenner HL, Adams DR, Hudlicky T, Pettit GR. Bioorg. Med. Chem. Lett. 2004;14:2911. doi: 10.1016/j.bmcl.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 142.Blaskó G, Major E, Blaskó G, Rósza I, Szántay C. Eur. J. Med. Chem. 1986;21:91. [Google Scholar]
- 143.Rinner U, Hudlicky T, Gordon H, Pettit GR. Angew. Chem. Int. Ed. 2004;43:5342. doi: 10.1002/anie.200460218. [DOI] [PubMed] [Google Scholar]
- 144.Hudlicky T, Rinner U, Finn KJ, Ghiviriga I. J. Org. Chem. 2004;70:3490. doi: 10.1021/jo040292c. [DOI] [PubMed] [Google Scholar]
- 145.Moser M, Sun X, Hudlicky T. Org. Lett. 2005;7:5669. doi: 10.1021/ol052372o. [DOI] [PubMed] [Google Scholar]
- 146.Hudlicky T, Rinner U, Gonzales D, Akgun H, Schilling S, Siengalewicz P, Martinot TA, Pettit GR. J. Org. Chem. 2002;67:8726. doi: 10.1021/jo020129m. [DOI] [PubMed] [Google Scholar]
- 147.Ibn-Ahmed S, Khaldi M, Chrétien F, Chapleur Y. J. Org. Chem. 2004;69:6722. doi: 10.1021/jo049153l. [DOI] [PubMed] [Google Scholar]
- 148.Chrétien F, Ibn-Ahmed S, Masion A, Chapleur Y. Tetrahedron. 1993;49:7463. [Google Scholar]
- 149.Phung AN, Zannetti MT, Whited G, Fessner W-D. Angew. Chem. Int. Ed. 2003;42:4821. doi: 10.1002/anie.200352023. [DOI] [PubMed] [Google Scholar]
- 150.Nadein ON, Kornienko A. Org. Lett. 2004;6:831. doi: 10.1021/ol049942p. [DOI] [PubMed] [Google Scholar]
- 151.Kireev AS, Nadein ON, Agustin VJ, Bush NE, Evidente A, Manpadi M, Ogasawara MA, Rastogi SK, Rogelj S, Shors ST, Kornienko A. J. Org. Chem. 2006;71:5694. doi: 10.1021/jo0607562. [DOI] [PubMed] [Google Scholar]
- 152.Lopes RS, Lopes CC, Heathcock CH. Tetrahedron Lett. 1992;33:6775. [Google Scholar]
- 153.Rigby JH, Gupta V. Synlett. 1995:547. [Google Scholar]
- 154.Banwell MG, Cowden CJ, Mackay MF. J. Chem. Soc., Chem. Commun. 1994:61. [Google Scholar]
- 155.Banwell MG, Cowden CJ, Gable RW. J. Chem. Soc., Perkin Trans. I. 1994:3515. [Google Scholar]
- 156.McNulty J, Mao J, Gibe R, Mo R, Wolf S, Pettit GR, Herald DL, Boyd MR. Bioorg. Med. Chem. Lett. 2001;11:169. doi: 10.1016/s0960-894x(00)00614-4. [DOI] [PubMed] [Google Scholar]
- 157.McNulty J, Larichev V, Pandey S. Bioorg. Med. Chem. Lett. 2005;15:5315. doi: 10.1016/j.bmcl.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 158.Pandey G, Murugan A, Balakrishnan M. J. Chem. Soc., Chem. Commun. 2002:624. doi: 10.1039/b200624c. [DOI] [PubMed] [Google Scholar]
- 159.Shukla KH, Boehmler DJ, Bogacyzk S, Duvall BR, Peterson WA, McElroy WT, DeShong P. Org. Lett. 2006;8:4183. doi: 10.1021/ol061070z. [DOI] [PubMed] [Google Scholar]
- 160.Ortiz JC, Ozores L, Cagide-Fagín F, Alonso R. J. Chem. Soc., Chem. Commun. 2006:4239. doi: 10.1039/b606277f. [DOI] [PubMed] [Google Scholar]
- 161.Keck GE, Wager TT. J. Org. Chem. 1996;61:8366. [Google Scholar]
- 162.Keck GE, Wager TT, Rodriquez JFD. J. Am. Chem. Soc. 1999;121:5176. [Google Scholar]
- 163.Akgün H, Hudlicky T. Tetrahedron Lett. 1999;40:3081. [Google Scholar]
- 164.Gabrielsen B, Monath TP, Huggins JW, Kefauver DF, Pettit GR, Groszek G, Hollingshead M, Kirsi JJ, Shannon WM, Shubert EM, Dare J, Ugarkar B, Ussery MA, Phelan MJ. J. Nat. Prod. 1992;55:1569. doi: 10.1021/np50089a003. [DOI] [PubMed] [Google Scholar]
- 165.Gabrielsen B, Monath TP, Huggins JW, Kirsi JJ, Hollingshead M, Shannon WM, Pettit GR. Natural Products as Antiviral Agents. New York: Plenum Press; 1992. p. 121. [DOI] [PubMed] [Google Scholar]
- 166.Sidwell RW, Huffman JH, Barnard DL, Smee DF, Warren RP, Chirigos MA, Kende M, Huggins J. Antiviral Res. 1994;25:105. doi: 10.1016/0166-3542(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 167.Furusawa E, Irie H, Combs D, Wildman WC. Chemotherapy. 1980;26:36. doi: 10.1159/000237881. [DOI] [PubMed] [Google Scholar]
- 168.Quarzane-Amara M, Franetich J-F, Mazier D, Pettit GR, Meijer L, Doerig C, Desportes-Livage I. Antimicrob. Agents Chemother. 2001;45:3409. doi: 10.1128/AAC.45.12.3409-3415.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Fox BW. Trans. Roy. Soc. Trop. Med. Hyg. 1991;85:22. doi: 10.1016/0035-9203(91)90140-t. [DOI] [PubMed] [Google Scholar]
- 170.Veronese FM, Ceriotti G, Keller G, Lora S, Carenza M. Radiat. Phys. Chem. 1990;35:88. [Google Scholar]
- 171.Veronese FM, Ceriotti G, Caliceti P, Lora S, Carenza M. J. Control. Release. 1991;16:291. [Google Scholar]
- 172.Beijnen JH, Flora KP, Halbert GW, Henrar REC, Slack JA. Br. J. Cancer. 1995;72:210. doi: 10.1038/bjc.1995.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Torres-Labandeira JJ, Davignon P, Pitha J. J. Pharm. Sci. 1991;80:384. doi: 10.1002/jps.2600800421. [DOI] [PubMed] [Google Scholar]
- 174.Pettit GR, Freeman S, Simpson MJ, Thompson MA, Boyd MR, Williams MD, Pettit GR, III, Doubek DL. Anti-Cancer Drug Des. 1995;10:243. [PubMed] [Google Scholar]
- 175.Pettit GR, Orr B, Ducki S. Anti-Cancer Drug Des. 2000;15:389. [PubMed] [Google Scholar]
- 176.Pettit GR, Melody N, Simpson M, Thompson M, Herald DL, Knight JC. J. Nat. Prod. 2003;66:92. doi: 10.1021/np020225i. [DOI] [PubMed] [Google Scholar]
- 177.Pettit GR, Melody N, Herald DL. J. Nat. Prod. 2004;67:322. doi: 10.1021/np030299+. [DOI] [PubMed] [Google Scholar]
- 178.Pettit GR, Melody N. J. Nat. Prod. 2005;68:207. doi: 10.1021/np0304518. [DOI] [PubMed] [Google Scholar]
- 179.Bibby MC, Holwell SE, Pettit GR. Anti-Vascular and Anti-Tumor Effects of the Novel Agent Pancratistatin Phosphate; Biological Basis for Antiangiogenic Therapy Conference; November 8–10; Milan, Italy. 1999. [Google Scholar]
- 180.Shnyder SD, Cooper PA, Millington NJ, Gill JH, Pettit GR, Bibby MC. Clin. Cancer Res. 2005;11(24 Suppl):8971s. [Google Scholar]
- 181.Polt R. Organic Synthesis: Theory and Applications. Vol. 3. Greenwich, CT: Jai Press Inc.; 1997. Amaryllidaceae Alkaloids with Antitumor Activity; pp. 109–148. [Google Scholar]
- 182.Hudlicky T. Chem. Rev. 1996;96:3. doi: 10.1021/cr950012g. [DOI] [PubMed] [Google Scholar]
- 183.Rigby JH, Mateo ME. J. Am. Chem. Soc. 1997;119:12655. [Google Scholar]
- 184.Rigby JH, Maharoof USM, Mateo ME. J. Am. Chem. Soc. 2000;122:6624. [Google Scholar]
- 185.Gonzalez D, Martinot T, Hudlicky T. Tetrahedron Lett. 1999;40:3077. [Google Scholar]
- 186.Elango S, Yan T-H. J. Org. Chem. 2002;67:6954. doi: 10.1021/jo020155k. [DOI] [PubMed] [Google Scholar]
- 187.Danishefsky S, Lee JY. J. Am. Chem. Soc. 1989;111:4829. [Google Scholar]
- 188.Tian X, Hudlicky T, Königsberger K. J. Am. Chem. Soc. 1995;117:3643. [Google Scholar]
- 189.Hudlicky T, Tian X, Königsberger K, Maurya R, Rouden J, Fan B. J. Am. Chem. Soc. 1996;118:10752. [Google Scholar]
- 190.Trost BM, Pulley SR. J. Am. Chem. Soc. 1995;117:10143. [Google Scholar]
- 191.Doyle TJ, Hendrix M, VanDerveer D, Javanmard S, Haseltine J. Tetrahedron. 1997;53:11153. [Google Scholar]
- 192.Magnus P, Sebhat IK. J. Am. Chem. Soc. 1998;120:5341. [Google Scholar]
- 193.Magnus P, Sebhat IK. Tetrahedron. 1998;54:15509. [Google Scholar]
- 194.Kim S, Ko H, Kim E, Kim D. Org. Lett. 2002;44:1343. doi: 10.1021/ol0256419. [DOI] [PubMed] [Google Scholar]
- 195.Ko H, Kim E, Park JE, Kim D, Kim S. J. Org. Chem. 2004;69:112. doi: 10.1021/jo035371n. [DOI] [PubMed] [Google Scholar]
- 196.Li M, Wu A, Zhou P. Tetrahedron Lett. 2006;47:3707. [Google Scholar]
- 197.Ohta S, Kimoto S. Tetrahedron Lett. 1975:2279. [Google Scholar]
- 198.Ohta S, Kimoto S. Chem. Pharm. Bull. 1976;24:2969. [Google Scholar]
- 199.Ohta S, Kimoto S. Chem. Pharm. Bull. 1976;24:2977. [Google Scholar]
- 200.Paulsen H, Stubbe M. Tetrahedron Lett. 1982;23:3171. [Google Scholar]
- 201.Paulsen H, Stubbe M. Liebigs Ann. Chem. 1983:535. [Google Scholar]
- 202.Ugarkar BG, DaRe J, Schubert EM. Synthesis. 1987:715. [Google Scholar]
- 203.Chida N, Ohtsuka M, Ogawa S. Tetrahedron Lett. 1991;32:4525. [Google Scholar]
- 204.Chida N, Ohtsuka M, Ogawa S. J. Org. Chem. 1993;58:4441. [Google Scholar]
- 205.Hudlicky T, Olivo HF. J. Am. Chem. Soc. 1992;114:9694. [Google Scholar]
- 206.Hudlicky T, Olivo HF, McKibben B. J. Am. Chem. Soc. 1994;116:5108. [Google Scholar]
- 207.Martin SF, Tso H-H. Heterocycles. 1993;35:85. [Google Scholar]
- 208.Elango S, Yan T-H. Tetrahedron. 2002;58:7335. [Google Scholar]
- 209.Zhang H, Padwa A. Org. Lett. 2006;8:247. doi: 10.1021/ol052524f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Padwa A, Zhang H. J. Org. Chem. 2007;72:2570. doi: 10.1021/jo0626111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Keck GE, McHardy SF, Murry JA. J. Am. Chem. Soc. 1995;117:7289. [Google Scholar]
- 212.Keck GE, McHardy SF, Murry JA. J. Org. Chem. 1999;64:4465. [Google Scholar]
- 213.Tian X, Maurya R, Königsberger K, Hudlicky T. Synlett. 1995:1125. [Google Scholar]
- 214.Chida N, Jitsuoka M, Yamamoto Y, Ohtsuka M, Ogawa S. Heterocycles. 1996;43:1385. [Google Scholar]
- 215.Keck GE, Wager TT, McHardy SF. J. Org. Chem. 1998;63:9164. [Google Scholar]
- 216.Aceña JL, Arjona O, León ML, Plumet J. Org. Lett. 2000;2:3683. doi: 10.1021/ol000268v. [DOI] [PubMed] [Google Scholar]
- 217.Håkansson AE, Palmelund A, Holm H, Madsen R. Chem. Eur. J. 2006;12:3243. doi: 10.1002/chem.200501429. [DOI] [PubMed] [Google Scholar]
- 218.Zhang H, Padwa A. Tetrahedron Lett. 2006;47:3905. [Google Scholar]
- 219.Shin I-J, Choi E-S, Cho C-G. Angew. Chem. Int. Ed. 2007;46:2303. doi: 10.1002/anie.200604612. [DOI] [PubMed] [Google Scholar]
- 220.Isobe K, Taga J-I, Tsuda Y. Heterocycles. 1978;9:625. [Google Scholar]
- 221.Fujimura T, Shibuya M, Ogasawara K, Iwabuchi Y. Heterocycles. 2005;66:167. [Google Scholar]
- 222.Hudlicky T, Boros EE, Boros CH. Synthesis. 1992:174. [Google Scholar]
- 223.Ikeda M, El-Bialy SAA, Yakura T. Heterocycles. 1999;51:1957. [Google Scholar]
- 224.Lautens M, Fang Y-Q. Org. Lett. 2003;5:3679. doi: 10.1021/ol035354k. [DOI] [PubMed] [Google Scholar]
- 225.Rinner U, Siengalewicz P, Hudlicky T. Org. Lett. 2002;4:115. doi: 10.1021/ol0169877. [DOI] [PubMed] [Google Scholar]
- 226.Bright KL, Bernays EA, Moran VC. J. Insect Behav. 1994;7:779. [Google Scholar]
- 227.Bernays EA, Bright KL. Comp. Biochem. Physiol. 1993;104A:125. [Google Scholar]
- 228.Tan DS. Comb. Chem. High Throughput Screen. 2004;7:631. doi: 10.2174/1386207043328418. [DOI] [PubMed] [Google Scholar]
- 229.Boldi AM. Curr. Opin. Chem. Biol. 2004;8:281. doi: 10.1016/j.cbpa.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 230.Liao Y, Hu Y, Wu J, Zhu Q, Donovan M, Fathi R, Yang Z. Curr. Med. Chem. 2003;10:2285. doi: 10.2174/0929867033456738. [DOI] [PubMed] [Google Scholar]