Abstract
Circadian dysregulation in sleep pattern, mood, and hypothalamic-pituitary-adrenal (HPA) axis activity, often occurring in a sexually dimorphic manner, are characteristics of depression. However, the inter-relationships among circadian phase, HPA function, and depressive-like behaviors are not well understood. We investigated behavioral and neuroendocrine correlates of depressive/anxiety-like responses during diurnal (‘light’) and nocturnal (‘dark’) phases of the circadian rhythm in the open field (OF), elevated plus maze (EPM), forced swim (FST), and sucrose contrast (SC) tests. Plasma corticosterone (CORT) was measured after a) acute restraint and OF testing and b) FST. Both phase and sex significantly influenced behavioral responses to stress. Males were more anxious than females on the EPM in the light but not the dark phase. Further, the open:closed arm ratio was lower in the dark for females, but not males. By contrast, in the FST, females showed more “despair” (immobility) when tested in the dark, while phase did not affect males. Acute restraint stress increased OF activity in the light, but not the dark, phase. CORT levels were increased in both sexes following the FST, and in males and light phase females post-OF. As expected, females had higher CORT levels than males, even at rest, and this effect was more pronounced in the Dark phase. Together, our data highlight the sexually dimorphic influences of circadian phase and stress on behavioral and hormonal responsiveness.
Keywords: open field, elevated plus maze, sucrose contrast, Porsolt forced swim, HPA axis, circadian phase, diurnal variation, corticosterone, depression, anxiety, rat
1. Introduction
It is estimated that globally, one in five individuals has experienced depression in his/her lifetime. Indeed, depression is the most common psychiatric disorder (1). Despite its pervasiveness, the neurobiological mechanisms underlying depression are still being elucidated. Although the monoamine hypothesis of depression has dominated the field for decades (2), recent evidence suggests a role for other neurobiological systems, most notably the hypothalamic-pituitary-adrenal (HPA) axis (reviewed by (3–5)). Increasing evidence suggests that normal HPA circadian rhythmicity is disturbed in depression, which may mediate the chronobiological symptoms characteristic of depression: alterations in REM sleep, increased sleep fragmentation (6–8), and ‘positive mood variation’(9), i.e., dysphoric mood is more severe in the morning than in the evening (10). Similar alterations have also been reported in a multitude of rodent and primate depression models(11–20).
The significance of studying chronobiological alterations is highly relevant to treatment. Evidence suggests that anti-depressants not only alleviate depressive symptoms, but also restore the regularity of the HPA circadian rhythm (reviewed in (21)). In fact, normalization of HPA disturbances may be a prerequisite for successful treatment; the risk of relapse or resistance to treatment is much higher in patients where a neuroendocrine abnormality persists (22, 23). Thus, altered regulation of circadian neuroendocrine rhythms could also affect treatment responses in patients with mood disorders. The inter-relationships among circadian phase, HPA function, and depressive-like behaviors are not well understood. Perhaps more significantly, there is limited pre-clinical research that has investigated sex differences in relation to circadian rhythm disturbances in depression, despite strong evidence that depression is 1.5 – 3 times more prevalent among women (24).
One of the barriers to investigations of circadian influences on depressive-like behaviors may relate to methodological issues. The peak of behavioral activity occurs during the dark phase of the circadian rhythm for nocturnal rodents, posing a difficulty for experimenters who work during daytime hours. While this is typically addressed by reversing the light cycle in the animal room, behavioral testing is still often conducted under dim white light (25). As light is a potent zeitgeber, even dim white light can alter the circadian rhythm, and thus lighting conditions can create a significant confound in data interpretation (26, 27). In albino rats, this can be controlled by testing animals under red light conditions, because this wavelength is not perceived by albino animals (28).
An emerging area of study is the use of diurnal animal species such as the Degu rat, which circumvents many of these methodological issues. Diurnal animals have been used in a variety of behavioral studies (29, 30), and more recently in behavioral (31, 32) and neural (33) assessment in animal models of depression. The ability to control for the effects of phase and/or lighting conditions in nocturnal species may allow for comparisons between nocturnal and diurnal rodent species in the growing literature on depression and anxiety.
Another methodological concern is that ceiling effects may occur in studies of stress responses during the behavioral and HPA circadian peak. Basal corticosteroid hormone levels normally peak at the beginning of the active period, which is early morning in humans (34, 35) and diurnal rodents (36) and early evening in nocturnal rodents (37–39). Thus differences in CORT levels between control and experimental animals may not be readily detected if behavioral testing is conducted in the dark phase, particularly with tests that involve stress. Furthermore, differences in the HPA response to stress may occur depending on phase of testing (40, 41).
A final complication in both clinical studies and animal models of depression is that anxiety disorders are highly co-morbid with depression. Current estimates for lifetime co-morbidity range from 50% (42) to 85% (43). There is ambiguity in both the clinical manifestations of depressive disorders and their underlying neural correlates (44–47). Current evidence suggests that the HPA axis may be a common link between comorbid depressive and anxiety symptoms, as it is altered under both conditions (48–50). To date, it is not well understood how circadian phase may be involved in the comorbidity of these pathologies.
The objectives of the present study were twofold: 1) to investigate the effects of circadian rhythm and sex on validated tasks that measure depressive- and anxiety-like behaviors; and 2) to determine whether effects of an acute stressor on both behavioral and HPA reactivity can be detected in animals tested in the dark phase. To fulfill these objectives, male and female rats were tested during both light and dark circadian phases in a battery of behavioral tests that measure anxiety- and depressive-like behaviors including the open field, elevated plus maze, forced swim test, and sucrose contrast test. Corticosterone (CORT) levels were measured immediately following the FST and following acute restraint stress followed by OF testing. We hypothesized that: 1) circadian phase and sex would significantly influence outcomes on these tests; 2) the response to and recovery from restraint stress would be influenced by circadian phase and sex; and 3) that CORT levels would significantly vary depending on behavioral task, circadian phase, and sex.
2. Materials and Methods
2.1. Subjects
Male (322–424 g, n=14) and female (192–257 g, n=14) Sprague-Dawley rats (approx. 65 days old) were obtained from Charles River Laboratories (St. Constant, PQ, Canada). Rats were group-housed by sex in polycarbonate cages (24 × 16 × 46 cm) with pine shaving bedding. Colony rooms were maintained with controlled temperature (21– 22°C). Throughout the study, animals were given ad libitum access to standard laboratory chow (Jamieson's Pet Food Distributors Ltd., Delta, BC, Canada) and water. Following a one week habituation period, animals were randomly assigned to colony rooms maintained on either a normal light cycle (“Light phase”; lights on at 1200h and off at 2400h; n=12) or a reversed light cycle (“Dark phase”; lights on at 2400h and off 1200h; n=16), for a further one week habituation period before behavioral testing commenced.
Animal use and care procedures were in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (51) and the Canadian Council on Animal Care Guidelines, and were approved by the University of British Columbia Animal Care Committee (Certificate #: A06-0017).
2.2. Behavioral testing
As noted, we utilized a battery of behavioral tests that measure anxiety- and depressive-like behaviors including the open field (OF, locomotor activity, exploratory behavior), elevated plus maze (EPM, locomotor activity, exploratory behavior, anxiety), Porsolt forced swim test (FST, behavioral ‘despair’), and sucrose contrast test (SC, anhedonia) (See (2)). Testing occurred in the order above, with an attempt to present tests in order of increasing psychological stress. Although we recognize that the sucrose contrast test is less stressful than the forced swim test, this order was selected based on evidence that socially isolating the animals, which is necessary for measuring intake in the sucrose contrast test, is considered a significant stressor.
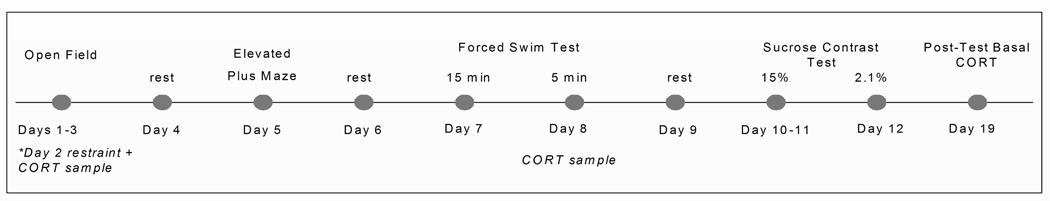
For the OF, EPM and FST, behavior was recorded by a digital video camera (Panasonic CCTV Camera, Model No. WV-BP 334) mounted above (OF, EPM) or in front (FST) of the test apparatus. White noise (30 dB) (“The Masker”, Model AM1100, Soundolier Inc., St. Louis, MO) was used to mask extraneous background noise. Animals tested in the Light phase were tested under soft white light (one 40 W and one 65 W bulb), whereas animals tested in the Dark phase were tested under red lights of the same wattage. For all treatment groups, behavioral testing took place between 1300 and 1600 h. The order of testing was the same for all animals (OF, EPM, FST, SC), with 24 hr between each task for recovery (Fig. 1). We opted not to take pre-test basal CORT samples to minimize disturbance prior to behavioral testing. To investigate how repeated testing might alter resting HPA tone, blood samples were collected via saphenous vein under resting conditions 7 days after the completion of behavioral testing.
Fig. 1.
Experimental design.
2.2.1. Open field
The OF is a classical test of exploratory locomotor behavior and emotionality. Locomotor hyperactivity is thought to reflect environmental neophobia, or increased fear of novel environments. In larger open fields, animals that spend more time in the periphery of the field are said to be more anxious than those that freely explore the center of the field (52). Animals were tested for a 15 min trial on 3 consecutive days. On each test day, immediately prior to testing, animals were marked on their backs with black non-toxic markers for detection by the video tracking software. The OF consisted of a square chamber (40 × 40 × 30 cm), with walls made of opaque, corrugated plastic; the center was defined as a 20 × 20 cm square in the center of the maze. Between trials, the field was wiped clean with 5% dilute acetic acid solution. After exposure to the field on Day 1, the thigh region was shaved in preparation for blood sampling the following day.
Testing on Days 1 and 3 occurred immediately upon removal from the home cage. In contrast, on Day 2, animals were first subjected to an acute restraint stress by confinement in PVC tubes (males: 20.5 × 6.5 cm diameter; females: 15 × 5.5 cm diameter) for 15 min, and then placed directly into the OF. Blood samples were collected from the saphenous vein immediately following exposure to the field. On all 3 test days the total distance traveled (cm), time spent in the center of the field (%), and the distance traveled in the center (cm) were assessed.
2.2.2. Elevated plus maze
Another well-known test to measure anxiety-like behaviors and locomotor activity in rodents is the EPM, a maze in the shape of a +sign, raised ~75 cm above the ground, with two arms enclosed by walls and two arms open to the environment, and (53). The EPM apparatus in the present study (Pathfinder, Model No. 89001B, Lafayette Instruments, Lafayette, IN) had arms 69 × 10.5 × 20 cm and a central platform (diameter 35 cm). it has been shown that animals confined to the open arms have elevated CORT levels (54), and measures such as the number of open arm entries, time spent in the open arms, and full open arm entries are inversely related to anxiety (55). Locomotor activity can also be quantified in this test, by the total distance traveled and the total number of entries into all arms. This measure is distinct from anxiety, as sedative treatments reduce total arm entries without changing the amount of time spent on the open arms (56).
On the test day, animals were placed in the center of the maze, with head position counterbalanced among rats, and behavior was recorded for 5 min. The maze was cleaned with 5% acetic acid between tests. Frequency of partial (two paws on the open arms) and full (all four paws) open arm entries, full closed arm entries, and rearing and grooming behaviors were hand-scored from the videotapes. The percent time on the open arms and the ratio of time on open/time on closed arms were determined by the behavioral analysis software (see 2.4.).
2.2.3. Porsolt forced swim test
The FST is considered to be a measure of ‘behavioral despair’. Animals were exposed to a cylindrical tank of water for 15 min on day 1 and re-exposed for 5 min on day 2. Time spent swimming and ‘immobility’ (minimal movements to keep the head above water) were analyzed. Increased time immobile is thought to reflect increased ‘behavioral despair’ or ‘giving up’, in that antidepressants reverse immobility (57). The forced swim tanks consisted of transparent Plexiglas cylinders (19.5 cm diameter, 43 cm height) which were filled with tap water at 25°C ± 1°C (58) to a height of 25 cm (females) and 30 cm (males). With these parameters, animals’ tails were able to touch the bottom of the tank. Activity was recorded by a video camera placed directly facing both cylinders, which provided an unobstructed view of both animals and allowed for scoring of both swimming and immobility behavior. Following testing, animals were towel-dried and returned to their home cages with their cage mate. Water was changed between animals to prevent any pheromonal influence on behavior (59). On Day 2, blood samples were collected from all animals via saphenous vein sampling 15 min following behavioral testing. Swimming activity was analyzed in one-minute time bins across the 5 minute test period.
2.2.4. Sucrose negative contrast test
Anhedonia, or the inability to experience pleasure from rewarding stimuli, is one of the core symptoms of depression, and can be easily studied in animals using a variety of sucrose intake tasks (60). In the sucrose negative contrast test, animals are presented with several consecutive days of a relatively high sucrose concentration (e.g., 15%) followed by one presentation of a lower concentration (e.g., 2.1%). A hedonic response is measured by a decrease in sucrose consumption in response to the lower concentration. During the sucrose contrast test, all animals were singly-housed. Animals were habituated to a 15% sucrose solution for two consecutive days. Sucrose was offered for 24 hr after lights on (light phase rats) or lights off (dark phase rats), and consumption for each 24 hr period was measured by weighing bottles prior to, and following, the 24 hr exposure period. Fresh sucrose solution was offered daily. On the third day, animals received a 2.1% sucrose solution. Throughout testing, animals had ad libitum access to standard laboratory chow and water.
2.3. Corticosterone radioimmunoassay
Blood samples were taken from each animal at three times during the experiment: post-OF testing on day 2, post-FST testing on day 2, and 7 days post-completion of the SC test (i.e., at the end of all behavioral testing). Samples were taken from Light phase rats between 13:15 and 16:15 hr, within 4 hr of lights-on, and from Dark phase rats within 4 hr of lights-off. Blood samples were centrifuged at 3200 rpm for 10 min at 4°C. Plasma was transferred into 600 µl Eppendorf tubes and stored at −80°C until assayed. Total corticosterone (bound plus free) levels were measured by RIA using a commercially available assay kit (MP Biomedicals, Orangeburg, NY, Catalogue No. 07-120103) with sample and reagent volumes halved. The antiserum cross-reacts 100% for corticosterone. The minimum detectable corticosterone concentration was 7.7 ng/ml and the intra- and inter-assay coefficients of variation were 7.1% and 7.2%, respectively.
2.4. Automated behavioral analysis
Video files from the OF, EPM, and the FST were analyzed using Noldus Ethovision 3.1 software. The sampling rate used to acquire data from the video files was 9.98 samples/sec. Mobility in the FST was tabulated by the behavioral software as the change in pixels of the detected object between video image sample intervals. The immobility threshold was defined at 5% or less.
2.5. Statistical analysis
Data were analyzed using Statistical Package for the Social Sciences (SPSS) v.15.0 software. In each of the data sets, between-subjects factors were Sex (Male or Female) and Phase (Dark or Light). Data from the OF, FST, and SC tests, as well as CORT values, were analyzed using a mixed ANOVA with repeated measures for the factors of Day (1, 2, or 3) for the OF; Time (min) (1, 2, 3, 4, or 5) for the FST; Sucrose concentration (Pre- vs. Post-contrast) for the SC test; and Sampling time (Post-OF, Post-FST, or Post-test resting sample) for CORT levels. For the EPM, frequency of full and partial open entries, closed arm entries, ratio of time on open arms/time on closed arms, percent time on the open arms, rearing, and grooming were analyzed separately. For the SC test, consumption was corrected for body weight. Significant main effects or interactions were further explored for simple main effects; a Fisher’s LSD post-hoc was used for comparisons of three groups or less, and a Šidák correction for > 3 groups. In analyses that included a within-subjects factor, an estimated marginal means procedure with a Šidák correction was employed. Because the Šidák correction accurately controls for familywise α, an overall F test is not required to be significant in order to explore a priori differences between factors, i.e., planned comparisons (61). For repeated measures analyses, the degrees of freedom (df) were corrected to more conservative values using the Huynh-Feldt ε (62) to correct for any violations of the sphericity assumption (61). Alpha was set at 0.05 for all analyses. All results are shown as means ± SEM.
3. Results
3.1. Open field (OF)
3.1. Total distance traveled
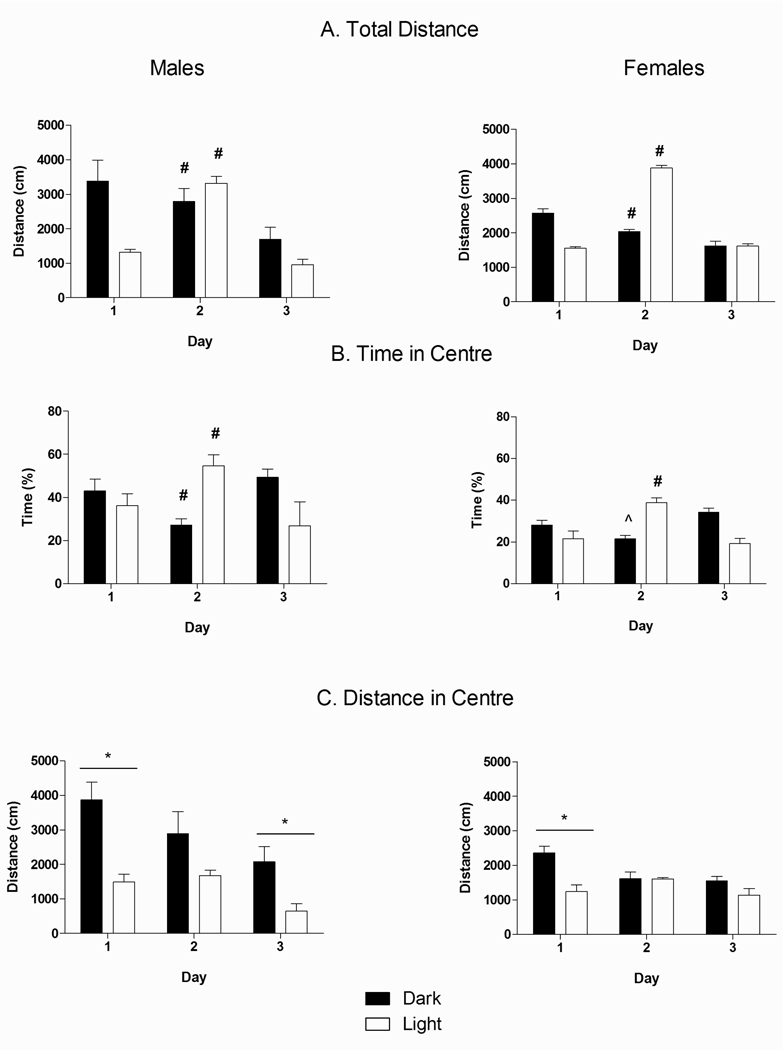
ANOVA revealed a significant main effect of Day [F(2,48) = 35.92, p<0.05] as well as Day × Phase [F(2,48) = 28.55, p<0.05] and Sex × Phase [F(1, 24) = 7.27, p<0.05] interactions for total distance traveled in the OF (Fig. 2A). Acute restraint stress on Day 2 of testing differentially affected distance traveled between Phases: animals tested in the Dark phase generally showed a decrease in distance traveled over days (Day 1 > Day 2 > Day 3 = Day 4, p’s<0.05), whereas rats tested in the Light showed a significant increase in distance traveled on Day 2 compared to Days 1 and 3 (p’s<0.05). Furthermore, while animals tested in the Dark were more active than those in the Light on Day 1 (p<0.05), restraint reversed this effect such that on day 2, Light phase animals were more active than Dark phase animals (p<0.05). Moreover, the Sex × Phase interaction indicated that Dark phase males were significantly more active than Dark phase females (p<0.05), while there were no differences between sexes in the Light phase.
Fig. 2.
Effect of Circadian Phase, Day, and Sex on (A) total distance traveled on the open field; (B) percentage of time in the center of the open field (amount of time in the center/total amount of time in the open field) × 100%); and (C) distance traveled in the center of the open field across the 3 days of testing. Dark bars represent animals tested in their Dark phase (n=8) and transparent bars represent animals tested in their Light phase (n=6). (*) denotes significantly different between Phases, p<0.05. (#) denotes significantly different from other days in Dark animals (p’s<0.05). (##) denotes significantly different from other days in Light animals (p’s<0.05).
3.1.2. Time in center
Fig. 2B shows that females spent less time in the center of the OF than males, as indicated by a significant main effect of Sex [F(1, 24) = 13.65, p<0.05]. ANOVA also revealed a significant Day × Phase interaction [F(2, 48) = 35.27, p<0.05]. Restraint stress prior to testing differentially affected the percent of time spent in the center depending on Phase. While there was no difference between Phases on Day 1, restraint stress prior to testing on Day 2 reduced the time in center for Dark phase tested animals, while it increased the time in center for Light phase tested animals, when compared to Days 1 and 3 (p’s<0.05).
3.1.3. Distance traveled in center
A mixed Factors ANOVA across the testing period for distance traveled in the center of the OF (Fig. 2C) indicated significant main effects of Day [F(2, 48) = 11.16, p<0.05], Sex [F(1, 24) = 5.12, p<0.05], and Phase [F(1, 24) = 17.74, p<0.05], as well as a Day × Phase interaction [F(2, 48) = 3.76, p<0.05] and a trend for a Sex × Phase interaction [F(1, 24) = 3.68, p=0.06]. Thus, Phase of testing significantly influenced the effects of acute stress and had differential effects on males and females. Overall, Dark phase rats showed a decrease in activity in the center from Day 1 to Day 2 (p’s<0.05), whereas Light phase rats showed a decrease in center activity from Day 2 to Day 3 (p’s<0.05). In addition, Dark phase rats overall, traveled more in the center on Days 1 and 3 (p<0.05) compared to Light phase rats, but exposure to acute stress on Day 2 eliminated this difference. Furthermore, planned comparisons on the Sex × Phase trend revealed that males traveled more in the center than females in the Dark phase (p<0.05), but there was no difference between sexes in the Light phase.
3.2. Elevated plus maze (EPM)
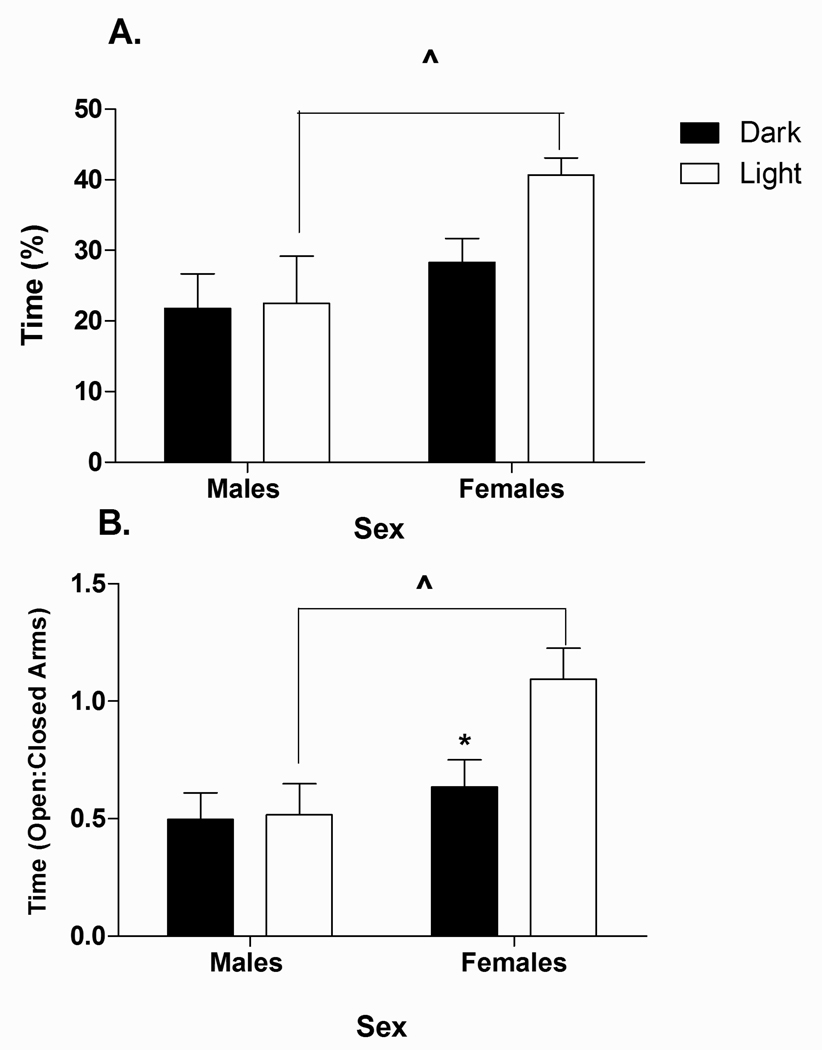
Analysis of percent time on the open arms (Fig. 3A) revealed a significant main effect of Sex [F(1, 24) = 7.22, p<0.05]. Overall, males spent significantly less time on the open arms than females (p<0.05). However, planned comparisons revealed that this effect was only observed for animals tested in the Light phase of their cycle (p<0.05). Further, females tested in the Dark spent significantly less time on the open arms than females tested in the Light (p<0.05).
Fig. 3.
Effect of Circadian phase and Sex on A) percentage of time on the open arms (time on OA/(time on elevated plus maze) × 100%) and B) ratio of time on open arms to closed arms (time on open arm/time on closed arms) on the elevated plus maze in male and female rats. Data represent values for animals tested in either their Dark (n=8) or Light (n=6) phase. (*) indicates significantly different from Light phase females (p<0.05). (^) denotes significantly different between sexes, p<0.05.
Analysis of the ratio of time on open arms/time on closed arms (Fig. 3B) revealed a significant main effect of Sex [F(1,24) = 8.42, p < 0.05], and a trend for a Sex × Phase interaction [F(1,24) = 3.16, p=0.08]. Planned comparisons revealed that whereas Phase did not affect the open/closed arms ratio for males, females tested in the Dark phase had a significantly lower open/closed arms ratio than females tested in the Light phase (p<0.05). In addition, during the Light phase, the open/closed arms ratio was lower in males than in females (p<0.05).
Table 1 indicates that there was no significant influence of Sex or Phase of cycle on % time spent on the closed arms, number of rears, grooms, full open arm entries, or total arm entries. However, males made more partial open arm entries [F(1, 24) = 4.95, p<0.05], and showed a trend for fewer full open arm entries (p=0.09) than females.
Table 1.
Effect of Circadian phase and Sex on elevated plus maze behaviors in male and female rats.*
| Males | Females | |||
|---|---|---|---|---|
| Dark | Light | Dark | Light | |
| Full open arm entrances | 3.5 ± 0.8 | 2.5 ± 0.8 | 3.5 ± 0.8 | 5.3 ± 0.6 |
| Partial open arm entrances | 2.4 ± 0.5^ | 2.3 ± 0.8^ | 1.8 ± 0.5 | 0.5 ± 0.2 |
| Closed arm entrances | 6.6 ± 0.4 | 6.17 ± 0.8 | 7.9 ± 0.5 | 6.17 ± 0.7 |
| Rears | 11.6 ± 1.5 | 13.7 ± 1.9 | 15.4 ± 1.7 | 12.5 ± 1.1 |
| Grooming bouts | 0.8 ± 0.3 | 0.5 ± 0.3 | 1.3 ± 0.6 | 0.5 ± 0.3 |
Data represent behavior scored during EPM testing in animals tested during their Dark (n=8) or Light (n=6) phase.
denotes significantly different from females, p’s<0.05.
3.3. Porsolt forced swim test (FST)
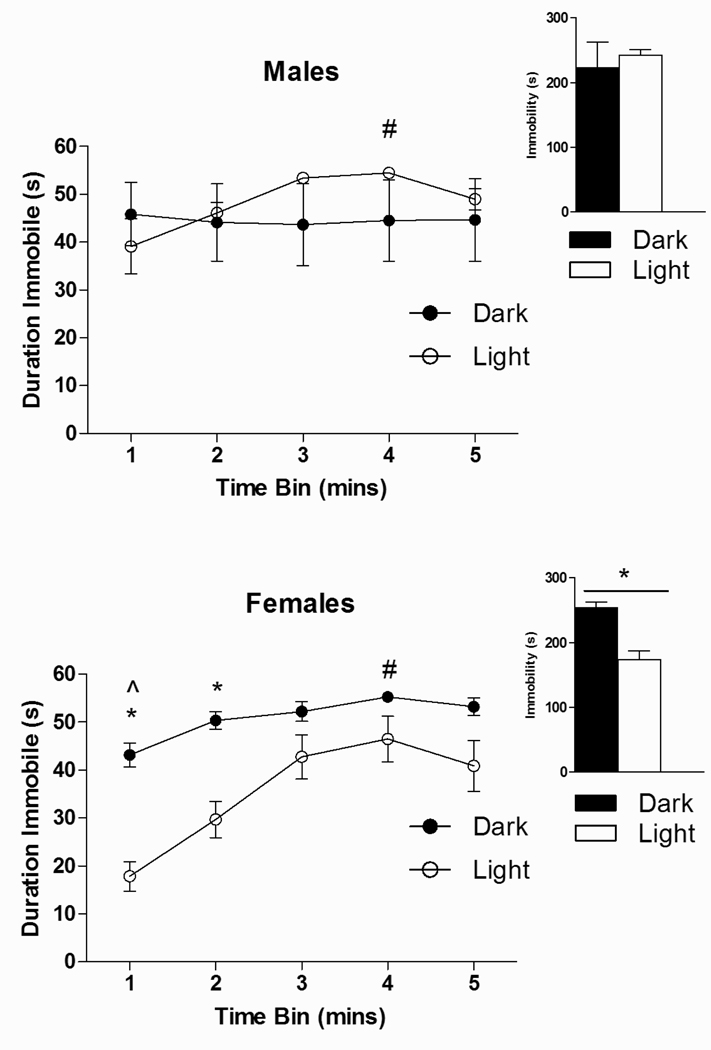
Fig. 4 illustrates that duration of immobility in the FST changed significantly across the testing period [main effect of Time; F(4, 96) = 25.98, p<0.05], and that there were significant Time × Sex [F(4, 96) = 6.47, p<0.05] and Time × Phase [F(4, 96) = 10.80, p<0.05] interactions, as well as a Sex × Phase interaction that approached significance [F(1, 24) = 3.66, p=0.07]. Planned comparisons revealed that Dark phase females were more immobile than Light phase females (p<0.05), with the greatest differences in the first two min of testing (p<0.05). Furthermore, the pattern of immobility for females was similar between phases, such that peak immobility occurred in min 4 (p<0.05). By contrast, for males, peak immobility in the Light phase occurred in min 3 (p<0.05), while there was no significant change in immobility across time in the Dark phase. For total immobility across the entire test period (Fig. 4-inset graph), ANOVA revealed a trend for a Sex × Phase interaction [F(1, 24) = 3.66, p=0.07]. Planned comparisons indicated that, consistent with the data reported by time bin, Dark phase females were more immobile overall than Light phase females (p<0.05).
Fig. 4.
Effect of Circadian Phase, Sex, and Time on immobility in the forced swim test in male and female rats. Data represent the amount of time immobile (sec) on the forced swim test across the 5 minutes of behavioral testing. (*) denotes significantly different from animals tested in their Light phase, p’s<0.05. (#) indicates significantly different from min 1 in Dark phase rats. (##) denotes significantly different compared to mins 1 and 2 in Light phase rats, p’s<0.05. n’s=6–8 for each condition.
3.4. Sucrose negative contrast test
The ANOVA revealed that all animals showed a negative contrast effect, as evidenced by the significant main effect of Concentration [F(1, 10) = 26.24, p<0.05]. There were no significant effects of Sex or Phase on this behavior (data not shown).
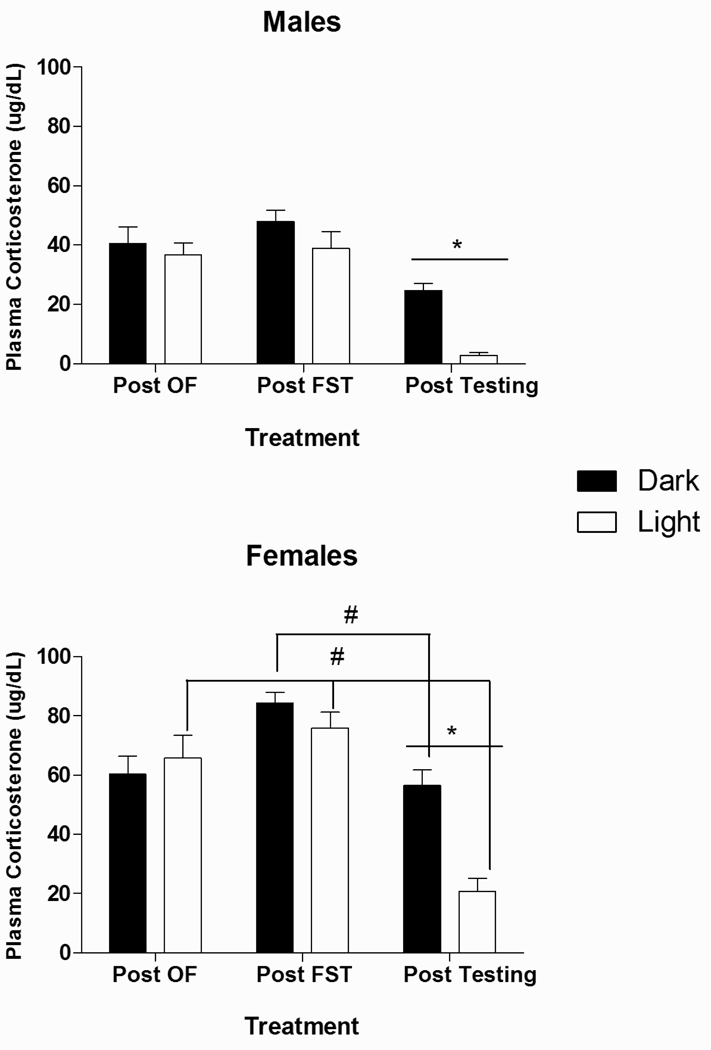
3.5. Plasma Corticosterone (CORT) levels
Fig. 5 illustrates plasma CORT levels measured: 1) after exposure to restraint stress followed by open field testing (Post-OF); 2) following the Day 2 forced swim test (Post-FST); and 3) under resting conditions, one week after completion of behavioral testing (Post-test resting sample). Mixed ANOVA revealed, as expected, a significant main effect of Sex [F(1, 24) = 124.25, p<0.05] such that females had consistently higher CORT levels than males. Simple main effects were then explored within each sex. For both males [F(1, 12) = 13.62, p<0.05] and females [F(1, 12) = 14.45, p<0.05], significant main effects of phase indicated that, as expected, CORT levels were higher in the Dark than in the Light phase (p<0.05). In males, a significant main effect of Sampling time [F(2, 24) = 22.84, p<0.05] indicated that under both Dark and Light conditions, CORT levels were elevated following exposure to both the FST and the OF, relative to Post-test resting values (p’s<0.05). For females, by contrast, there was a significant main effect of Sampling time [F(2, 24) = 14.52, p<0.05] and a Sampling time × Phase interaction [F(2, 24) = 8.15, p<0.05]. Whereas females tested in the Light phase displayed CORT elevations following both the FST and OF compared to resting levels (p<0.05), Dark phase tested females showed CORT elevations only after the FST.
Fig. 5.
Effect of Circadian phase, Sex, and Sampling Time on plasma Corticosterone concentration (µg/dL) in male and female rats. (*) denotes that Dark phase significantly different from Light phase, p<0.05. (#) signifies significantly different from Post-test basal value, p’s<0.05. n’s=6–8 for each condition.
4. Discussion
Our study reveals significant effects of circadian phase and sex on multiple measures of depressive/anxiety-like behaviors and HPA reactivity. Males were more anxious than females on the EPM in the light but not the dark phase. Further, the open:closed arm ratio was lower in the dark than the light for females, but not males. By contrast, in the FST, females showed more “despair” (immobility) when tested in the dark compared to the light, while there was no effect of phase on immobility for males. There were no effects of sex or phase of testing for the sucrose contrast test. In the OF, as expected, overall activity was greater in the dark than the light. However, acute restraint stress increased OF activity in the light, but not the dark, phase, and thus reversed the effect of phase observed under non-stressed conditions. Moreover, stress decreased time in center in the dark but increased time in center in the light. Finally, CORT levels were increased in both males and females following the FST, and in males and light phase-tested females following OF exposure. As expected, females had higher CORT levels than males, even under resting conditions, and this effect was more pronounced in the Dark phase. Together, our data highlight the sexually dimorphic influences of circadian phase and stress on behavioral and hormonal responsiveness, and the importance of considering these factors in animal tests of depression/anxiety behaviors.
4.1. Circadian phase alters stress reactivity on the open field
Our data support previous work indicating that nocturnal rodents are more active in the dark compared to the light phase e.g., (63–65). One of the most intriguing findings from this study was that circadian phase differentially influenced the response to acute restraint stress on multiple OF measures. While overall OF activity was greater in the dark than in the light under non-stressed conditions, the reverse was true following restraint stress. Moreover, stress decreased time in center in the dark, but increased time in center in the light. These results support previous research indicating that prior HPA stimulation (corticotrophin releasing hormone administration) increases locomotor activity in a familiar environment in the light phase, but has no effect on animals tested in the dark phase (66). Together, these data indicate that dark phase testing, when basal activity levels are high, may mask locomotor responses to stress on the OF.
In control animals, it is typical for locomotor activity to attenuate with repeated exposure to the open field. Indeed, “habituation” is a well-documented phenomenon (67, 68). We predicted that acute stress would disrupt habituation to the open field. While this held true for light phase animals, whose activity increased markedly in response to restraint stress, dark phase animals maintained a pattern of habituation, with activity levels declining over days. Thus, as noted, dark phase testing appears to mask the effects of acute stress on OF behaviors in both males and females. These data are in contrast to Dubovicky et al. (69), who showed that both acute and chronic shaker stress did not alter habituation patterns of overall OF motor activity in mice across 21 days of testing. This discrepancy is unlikely to be mediated by differences in lighting conditions, as Dubovicky et al. also used red light for dark-phase testing. Furthermore, in contrast to our data, baseline activity levels did not differ between dark and light phase animals in the Dubovicky et al. study. It is possible that the type of stressor (shaker vs. restraint), species differences (mice vs rats), housing or handling conditions, or the size of the open field apparatus all contributed to the discrepancies between studies. Nevertheless, taken together, the data suggest that when typical baseline activity levels (i.e. dark more active than light) exist, the disruptive effects of stress on habituation and OF activity are specific to the light phase of testing in both males and females.
Thigmotaxis, another commonly reported OF behavior, characterizes the natural tendency of rodents to prefer the periphery over the center space of an open field. Thigmotaxis is considered an index of anxiety since many anxiolytic drugs reduce thigmotaxis, and increase time spent in the central part of the field (reviewed by (52). We found that regardless of phase, females spent less time in the centre than males.
However, as females did not have lower activity overall than males, the reduction of time in centre suggests that females had greater activity in the periphery of the maze, indicating that females were more anxious than males in the OF, regardless of phase and total activity levels. Phase markedly influenced the effects of acute stress on time in centre for both males and females: stress increased time in centre for light phase animals, but decreased time in centre for dark phase animals. This is an interesting finding, given that activity is generally reduced in response to stress for animals tested in a large OF arena. Considering that light phase animals did not increase their distance travelled in the centre, it is possible that the increased time spent in the centre reflects freezing behavior. Similarly, while dark phase animals did not alter their total activity in response to stress, their time in centre was significantly reduced, which may be interpreted as an anxiogenic response.
Together, these findings show that utilizing multiple OF behavioral measures in tandem allows for a clearer resolution of the effects of stress. An important caveat to our findings, however, relates to the size of the field used. Fear and anxiety are typically assessed using a large brightly lit open field; whereas a smaller dimly lit OF can more accurately assess locomotor or exploratory activity (reviewed by (70). In the present study, we opted to use a relatively small field in order to obtain a good measure of locomotor activity, and were surprised to find such marked effects on emotionality measures, i.e., time and distance travelled in the center. Of note, there are previous reports of anxiety measures from comparably sized fields, e.g., Shumake et al. (71) who used a smaller field (43.2cm2 × 30.5cm), and Sanberg et al. (72) who used a similar sized field (40 × 40 × 30.5 cm). However, further validation of these findings is needed through testing in a larger field and an investigation of the effects of anxiogenic and anxiolytic drugs on behavior.
4.2. Sex differences in anxiety observed in light, but not dark phase on the elevated plus maze
Consistent with previous work (73), we found that behavioral testing during the light, but not dark phase, revealed sex differences in anxiety measures. Measures of total time on the open arms (OA) and the open:closed arm ratio indicated that females tested in the dark phase were more anxious than females tested in the light, while phase had no effect on males. Interestingly, however, males were more anxious than females when tested in the light phase. Moreover, while light phase males spent less time on the OA than females, they also made more partial OA entries and tended to make fewer full OA entries. These data suggest that males approached the OA more often than females, whereas females entered the OA less frequently, but explored longer at each entry than males. Approach of the OA has been interpreted as a measure of ‘risk assessment’, defined as “an attempt at entry into open arms followed by avoidance responses” (74). Risk assessment is considered to be an ethologically valid measure of fear/anxiety that is highly sensitive to anxiolytic drugs (75). Overall, our findings demonstrate that sex significantly modulates the presentation of anxiety behaviors on the EPM. Males may show anxiety more in the form of risk-assessment behavior than females, suggesting that different drugs may be more efficacious in alleviating anxiety symptoms in one sex compared to the other.
Our results also highlight the influence of lighting conditions on EPM behavior. Our finding that circadian phase had no significant effect on EPM behavior in males is consistent with previous work by Jones & Nicholas (76), but contrasts with data of Bertoglio and Carobrez (25). Our study and the Jones & Nicholas (76) study utilized red light for dark phase testing, whereas Bertoglio and Carobrez (25) used dim white light. Thus, testing males in the dark phase may yield relatively elevated anxiety scores under white light compared to red light conditions, suggesting that introducing white light during the animal’s normal dark phase may alter behavior in itself. Furthermore, our data demonstrate that testing in the light phase under dim light may reveal innate sex differences that may be inappropriately attributed to treatment effects.
Our data support previous findings that measures of locomotor activity on the EPM are not significantly influenced by circadian phase (25, 77), and extend them by demonstrating that this effect is conserved in females. An important future direction is to explore how circadian phase of testing modulates EPM behavioral responses to prior stress exposure, as was revealed on the OF. Previous work by Chadda et al. (2005) has shown that repeated, but not acute, restraint stress increases locomotor activity in females, but reduces activity in males (78). However, as testing was conducted only during the early part of the light phase in the Chadda et al. study, the role of circadian phase in these results is unresolved.
4.3. Circadian phase influences immobility in females, but not males in the forced swim test
Dark phase females showed a dramatic increase (325%) in overall immobility compared to their light phase counterparts. As animals are more active during their dark phase, one would predict an increase in swimming, and thus a reduction in immobility in dark phase testing. However, our data revealed the opposite, supporting the finding that the FST does not measure physiological activity, but rather is indicative of psychological state (79). Conversely, among males, there was no overall effect of circadian phase on immobility. These data are in contrast with a previous study by Kelliher et al. (80) where males tested in the dark were more immobile than those tested in the light. It is possible that differences in housing conditions (4/cage in Kelliher et al, 2/cage in our study) contributed to these findings. Support for this possibility comes from a previous finding that social enrichment (group-housing) increases immobility (81).
Interestingly, all but light tested males demonstrated an elevation in immobility across the test period. In the paradigm used for this study, animals were able to touch their tails to the bottom of the tank, and this may have influenced our findings. Previous work has shown that at greater water depths, male animals demonstrate significantly less immobility, specifically on Day 2 of this testing procedure (reviewed by (82). As such, immobility levels in the current study are likely lower, and may have contributed to the lack of phase effects in males. By contrast, light- and dark-tested females did differ in immobility levels. This indicates the importance of considering multiple FST behavioral measures, testing conditions, and circadian phase in pre-clinical drug testing, as behavior in the FST represents the prototypical anti-depressant screen: while a drug may not influence overall immobility, it may impact the pattern of immobility across time.
4.4. Contrast effects are observed in both Light and Dark tested Animals in a 24-hr sucrose contrast test
Pre-clinical studies often use sucrose preference or consumption tests as a measure of anhedonia, a core symptom of depression (83). We found that all rats, regardless of sex or phase of testing, showed a significant negative contrast effect. This supports previous work indicating that contrast-based tests are a sensitive measure of reward value (84), and extend these findings to show that the sucrose contrast test is not significantly influenced by phase or sex. Significantly, we demonstrate that our findings hold after controlling for body weight, which has been shown to affect sucrose consumption results (85–87).
While there were no statistically significant effects of phase, we recognize that light phase males did not demonstrate a robust contrast effect. This is possibly due to limited sample size, or to the testing conditions utilized, as other studies from our lab have shown that utilizing this protocol, but testing under dim light conditions, results in high sensitivity to the effects of prenatal ethanol exposure and subsequent chronic mild stress in adulthood (88). Measuring consumption for more limited periods rather than over 24 hr may also increase sensitivity of the SC test to detecting treatment effects (unpublished data).
4.5. Plasma CORT levels are significantly elevated by phase and stress
As expected, CORT levels were higher for females at all sampling times compared to males (89), and resting CORT levels were higher in the dark than the light phase (90–92). While both light and dark phase males had significantly elevated CORT levels following restraint and OF testing, this was not observed in dark phase females. However, comparisons of the current post-test resting CORT levels to basal or pre-test CORT levels from previous work in our lab (36), at both light and dark circadian phases, indicates that post-test resting CORT levels in the present study approached stress levels, suggesting that repeated behavioral testing increased resting HPA tone among females. Notably, there may have been a ceiling effect such that stress-induced elevations were no longer detectable. This finding supports literature indicating that the female HPA axis is more sensitive to the potentially stressful effects of repeated behavioral testing (93, 94). This sensitivity may be one of the mediating factors resulting in elevated levels of depressive disorders reported in women. Further investigations of the roles of both the adrenal and gonadal axes in mediating depressive- and anxiety- like behaviors are underway in our laboratory in order to elucidate further the possible mechanisms underlying the effects observed (88).
4.6. Summary, significance and implications for future studies
Taken together, our data highlight the importance of considering circadian phase effects when investigating both behavioral and HPA axis measures, particularly in models of depression and anxiety. Furthermore, the data reveal differential effects of acute stressors by circadian phase, as well as differences in anxiety and/or emotionality behaviors within and across tasks (Table 2). Restraint stress increased locomotor activity in the OF in the light, but not dark phase of testing. Females generally showed greater anxiety than males in the OF as measured by overall time in centre and activity in center in the dark phase. Similarly, dark phase testing elevated anxiety in females on the EPM, whereas males showed higher anxiety scores in the light phase. Dark phase testing also increased behavioral despair in females, but not males. Hedonic behaviors (SC) across 24 hr appeared to be the most consistent measure, unaffected by phase of testing or sex. Our data also highlight the fact that testing exclusively at the behavioral peak (i.e., during the dark phase) may mask differences in CORT and possibly in activity measured under stressed vs. non-stressed conditions, due to ceiling effects. This consideration is particularly important when testing females, who normally have higher resting and stress-induced CORT levels, as well as locomotor activity, compared to males (e.g., (95–100). In support of this, previous findings show that the effects of stress exposure on CORT are detected more readily during the light rather than the dark phase in males (101, 102). However, dark phase testing may be more sensitive in revealing other aspects of responsiveness, and therefore testing conditions must be chosen according to outcomes being tested. Importantly, our findings demonstrate the power of using a multidimensional behavioral test battery in animal models of depression/anxiety, as different tasks can reveal distinct aspects of depressive- and anxiety-like behaviors. Consideration of effects such as these is critical to verify whether the presence/absence of a specific behavior is due to experimental treatments (i.e. drug administration, prenatal stress etc.) and not experimental conditions. As such, both circadian and stress effects must be sufficiently controlled for in order to isolate the effects of experimental treatments.
Table 2. Data Summary.
I like this table!! Maybe we should add one more column called Open Field – and subheading is “anxiety/fear” and measures are time and distance in center.
| Task | Open Field | Elevated Plus Maze | Forced Swim Test | Sucrose Contrast |
|---|---|---|---|---|
| Behavior Measured | Locomotor Activity | Anxiety | Behavioral Despair | Anhedonia |
| Measure | Total Distance Traveled(cm) |
Time on Open Arms (%) | Overall Time Immobile (s) |
Sucrose consumed per body mass (g/g) |
| Phase-Dark | M >F* | M=F | M=F | M=F |
| Phase-Light | M= F | M<F* | M=F | M=F |
| Sex-Male | D >L* | D=L | D=L | D=L |
| Sex-Female | D =L | D<L* | D>L* | D=L |
M-male, F-female, D-dark, L-light
p< 0.05.
Depression is a widespread disorder exemplified by circadian fluctuation in symptomatology and a presentation that is sexually dimorphic. We have demonstrated that circadian phase and sex are major variables to consider in pre-clinical experiments. The impact of these factors is likely to be even greater in studies designed to assess effects of early or prior adverse experiences such as prenatal stress, prenatal alcohol exposure, maternal deprivation, and/or chronic mild stress. Also, appropriate considerations must be made to avoid ‘ceiling effects’ in order to differentiate between control and experimental treatment groups on the behavioral tasks and endocrine correlates. One possible limitation to the interpretation of the behavioral data in this study is the influence of repeated behavioral testing. However recent research has suggested that this is of negligible consequence when animals are given 24hrs between tasks, as in this study (103). Future work is required to further delineate the correlates of HPA responses and behavior in a repeated test battery commonly employed to assess depressive-like behaviors in rodents. Consideration of these variables in future investigations utilizing pre-clinical models of depressive- and anxiety- like behaviors in rodents will reveal another layer of validity to the current models, and provide a basis for the development of relevant therapeutic targets.
Acknowledgements
The authors would like to thank Peter Chen (Noldus) for his technical expertise and assistance with the Noldus tracking system, and Debbie Sasges for her assistance in this study. This research was supported by NIH/NIAAA AA007789, and grants from the BC Ministry of Children and Family Development through the UBC Human Early Learning Partnership and the Canadian Institute for Advanced Research to JW; a MSFHR award to KGCH, a CFRI Summer Student Award to PV, and IMPART (CIHR STIHR) awards to KGCH and FYC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wong ML, Licinio J. Research and Treatment Approaches to Depression. Nat.Rev.Neurosci. 2001;2:343–351. doi: 10.1038/35072566. [DOI] [PubMed] [Google Scholar]
- 2.Berton O, Nestler EJ. New Approaches to Antidepressant Drug Discovery: Beyond Monoamines. Nat.Rev.Neurosci. 2006;7:137–151. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- 3.Carroll BJ, Cassidy F, Naftolowitz D, Tatham NE, Wilson WH, Iranmanesh A, Liu PY, Veldhuis JD. Pathophysiology of Hypercortisolism in Depression. Acta Psychiatr.Scand.Suppl. 2007;433:90–103. doi: 10.1111/j.1600-0447.2007.00967.x. [DOI] [PubMed] [Google Scholar]
- 4.Mason BL, Pariante CM. The Effects of Antidepressants on the Hypothalamic-Pituitary-Adrenal Axis. Drug News.Perspect. 2006;19:603–608. doi: 10.1358/dnp.2006.19.10.1068007. [DOI] [PubMed] [Google Scholar]
- 5.Barden N. Implication of the Hypothalamic-Pituitary-Adrenal Axis in the Physiopathology of Depression. J.Psychiatry Neurosci. 2004;29:185–193. [PMC free article] [PubMed] [Google Scholar]
- 6.Baldwin DS, Papakostas GI. Symptoms of Fatigue and Sleepiness in Major Depressive Disorder. J.Clin.Psychiatry. 2006;67 Suppl 6:9–15. [PubMed] [Google Scholar]
- 7.Benca RM. Sleep in Psychiatric Disorders. Neurol.Clin. 1996;14:739–764. doi: 10.1016/s0733-8619(05)70283-8. [DOI] [PubMed] [Google Scholar]
- 8.Kupfer DJ. Sleep Research in Depressive Illness: Clinical Implications-a Tasting Menu. Biol.Psychiatry. 1995;38:391–403. doi: 10.1016/0006-3223(94)00295-E. [DOI] [PubMed] [Google Scholar]
- 9.Ushijima K, Morikawa T, To H, Higuchi S, Ohdo S. Chronobiological Disturbances with Hyperthermia and Hypercortisolism Induced by Chronic Mild Stress in Rats. Behav.Brain Res. 2006;173:326–330. doi: 10.1016/j.bbr.2006.06.038. [DOI] [PubMed] [Google Scholar]
- 10.Bouhuys AL. Towards a Model of Mood Responses to Sleep Deprivation in Depressed Patients. Biol.Psychiatry. 1991;29:600–612. doi: 10.1016/0006-3223(91)90095-4. [DOI] [PubMed] [Google Scholar]
- 11.Araki H, Yamamoto T, Watanabe S, Ueki S. Changes in Sleep-Wakefulness Pattern Following Bilateral Olfactory Bulbectomy in Rats. Physiol.Behav. 1980;24:73–78. doi: 10.1016/0031-9384(80)90016-5. [DOI] [PubMed] [Google Scholar]
- 12.Marcilhac A, Maurel D, Anglade G, Ixart G, Mekaouche M, Hery F, Siaud P. Effects of Bilateral Olfactory Bulbectomy on Circadian Rhythms of ACTH, Corticosterone, Motor Activity and Body Temperature in Male Rats. Arch.Physiol.Biochem. 1997;105:552–559. doi: 10.1076/apab.105.6.552.3273. [DOI] [PubMed] [Google Scholar]
- 13.Perret M, Aujard F, Seguy M, Schilling A. Olfactory Bulbectomy Modifies Photic Entrainment and Circadian Rhythms of Body Temperature and Locomotor Activity in a Nocturnal Primate. J.Biol.Rhythms. 2003;18:392–401. doi: 10.1177/0748730403254248. [DOI] [PubMed] [Google Scholar]
- 14.Pieper DR, Lobocki CA. Olfactory Bulbectomy Lengthens Circadian Period of Locomotor Activity in Golden Hamsters. Am.J.Physiol. 1991;261:R973–R978. doi: 10.1152/ajpregu.1991.261.4.R973. [DOI] [PubMed] [Google Scholar]
- 15.Yamazaki A, Ohtsuki Y, Yoshihara T, Honma S, Honma K. Maternal Deprivation in Neonatal Rats of Different Conditions Affects Growth Rate, Circadian Clock, and Stress Responsiveness Differentially. Physiol.Behav. 2005;86:136–144. doi: 10.1016/j.physbeh.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Yoshihara T, Otsuki Y, Yamazaki A, Honma S, Yamasaki Y, Honma K. Maternal Deprivation in Neonatal Rats Alters the Expression of Circadian System Under Light-Dark Cycles and Restricted Daily Feeding in Adulthood. Physiol.Behav. 2005;85:646–654. doi: 10.1016/j.physbeh.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez MM, Noble PM, Lyon CK, Plotsky PM, Davis M, Nemeroff CB, Winslow JT. Alterations in Diurnal Cortisol Rhythm and Acoustic Startle Response in Nonhuman Primates with Adverse Rearing. Biol.Psychiatry. 2005;57:373–381. doi: 10.1016/j.biopsych.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 18.Cheeta S, Ruigt G, van Proosdij J, Willner P. Changes in Sleep Architecture Following Chronic Mild Stress. Biol.Psychiatry. 1997;41:419–427. doi: 10.1016/S0006-3223(96)00058-3. [DOI] [PubMed] [Google Scholar]
- 19.Moreau JL, Scherschlicht R, Jenck F, Martin JR. Chronic Mild Stress-Induced Anhedonia Model of Depression: Sleep Abnormalities and Curative Effects of Electroshock Treatment. Behav.Pharmacol. 1995;6:682–687. [PubMed] [Google Scholar]
- 20.Overstreet DH, Friedman E, Mathe AA, Yadid G. The Flinders Sensitive Line Rat: A Selectively Bred Putative Animal Model of Depression. Neurosci.Biobehav.Rev. 2005;29:739–759. doi: 10.1016/j.neubiorev.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 21.Duncan WC., Jr Circadian Rhythms and the Pharmacology of Affective Illness. Pharmacol.Ther. 1996;71:253–312. doi: 10.1016/s0163-7258(96)00092-7. [DOI] [PubMed] [Google Scholar]
- 22.de Kloet ER, Joels M, Holsboer F. Stress and the Brain: From Adaptation to Disease. Nat.Rev.Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 23.Holsboer F. The Corticosteroid Receptor Hypothesis of Depression. Neuropsychopharmacology. 2000;23:477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- 24.Kessler RC. Epidemiology of Women and Depression. J.Affect.Disord. 2003;74:5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- 25.Bertoglio LJ, Carobrez AP. Behavioral Profile of Rats Submitted to Session 1-Session 2 in the Elevated Plus-Maze during diurnal/nocturnal Phases and Under Different Illumination Conditions. Behav.Brain Res. 2002;132:135–143. doi: 10.1016/s0166-4328(01)00396-5. [DOI] [PubMed] [Google Scholar]
- 26.Garcia AM, Cardenas FP, Morato S. Effect of Different Illumination Levels on Rat Behavior in the Elevated Plus-Maze. Physiol.Behav. 2005;85:265–270. doi: 10.1016/j.physbeh.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 27.Yilmaz A, Aksoy A, Canbeyli R. A Single Day of Constant Light (L/L) Provides Immunity to Behavioral Despair in Female Rats Maintained on an L/D Cycle. Prog.Neuropsychopharmacol.Biol.Psychiatry. 2004;28:1261–1265. doi: 10.1016/j.pnpbp.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 28.Reiter RJ, Tan DX, Poeggeler B, Kavet R. Inconsistent Suppression of Nocturnal Pineal Melatonin Synthesis and Serum Melatonin Levels in Rats Exposed to Pulsed DC Magnetic Fields. Bioelectromagnetics. 1998;19:318–329. doi: 10.1002/(sici)1521-186x(1998)19:5<318::aid-bem6>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 29.Schumann DM, Cooper HM, Hofmeyr MD, Bennett NC. Circadian Rhythm of Locomotor Activity in the Four-Striped Field Mouse, Rhabdomys Pumilio: A Diurnal African Rodent. Physiol.Behav. 2005;85:231–239. doi: 10.1016/j.physbeh.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 30.Vasicek CA, Oosthuizen MK, Cooper HM, Bennett NC. Circadian Rhythms of Locomotor Activity in the Subterranean Mashona Mole Rat, Cryptomys Darlingi. Physiol.Behav. 2005;84:181–191. doi: 10.1016/j.physbeh.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Ashkenazy T, Einat H, Kronfeld-Schor N. We are in the Dark here: Induction of Depression- and Anxiety-Like Behaviours in the Diurnal Fat Sand Rat, by Short Daylight Or Melatonin Injections. Int.J.Neuropsychopharmacol. 2009;12:83–93. doi: 10.1017/S1461145708009115. [DOI] [PubMed] [Google Scholar]
- 32.Einat H, Kronfeld-Schor N, Eilam D. Sand Rats See the Light: Short Photoperiod Induces a Depression-Like Response in a Diurnal Rodent. Behav.Brain Res. 2006;173:153–157. doi: 10.1016/j.bbr.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Helmeke C, Ovtscharoff W, Jr, Poeggel G, Braun K. Imbalance of Immunohistochemically Characterized Interneuron Populations in the Adolescent and Adult Rodent Medial Prefrontal Cortex After Repeated Exposure to Neonatal Separation Stress. Neuroscience. 2008;152:18–28. doi: 10.1016/j.neuroscience.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 34.Horrocks PM, Jones AF, Ratcliffe WA, Holder G, White A, Holder R, Ratcliffe JG, London DR. Patterns of ACTH and Cortisol Pulsatility Over Twenty-Four Hours in Normal Males and Females. Clin. Endocrinol. 1990;32:127–134. doi: 10.1111/j.1365-2265.1990.tb03758.x. [DOI] [PubMed] [Google Scholar]
- 35.Kage A, Fenner A, Weber B, Schoneshofer M. Diurnal and Ultradian Variations of Plasma Concentrations of Eleven Adrenal Steroid Hormones in Human Males. Klin.Wochenschr. 1982;60:659–666. doi: 10.1007/BF01716798. [DOI] [PubMed] [Google Scholar]
- 36.Osborn JA, Kim CK, Yu W, Herbert L, Weinberg J. Fetal Ethanol Exposure Alters Pituitary-Adrenal Sensitivity to Dexamethasone Suppression. Psychoneuroendocrinology. 1996;21:127–143. doi: 10.1016/0306-4530(95)00037-2. [DOI] [PubMed] [Google Scholar]
- 37.Allen-Rowlands CF, Allen JP, Greer MA, Wilson M. Circadian Rhythmicity of ACTH and Corticosterone in the Rat. J.Endocrinol.Invest. 1980;3:371–377. doi: 10.1007/BF03349373. [DOI] [PubMed] [Google Scholar]
- 38.Ishikawa M, Ohdo S, Watanabe H, Hara C, Ogawa N. Alteration in Circadian Rhythm of Plasma Corticosterone in Rats Following Sociopsychological Stress Induced by Communication Box. Physiol.Behav. 1995;57:41–47. doi: 10.1016/0031-9384(94)00192-8. [DOI] [PubMed] [Google Scholar]
- 39.Ishikawa M, Hara C, Ohdo S, Ogawa N. Plasma Corticosterone Response of Rats with Sociopsychological Stress in the Communication Box. Physiol.Behav. 1992;52:475–480. doi: 10.1016/0031-9384(92)90333-w. [DOI] [PubMed] [Google Scholar]
- 40.Atkinson HC, Wood SA, Kershaw YM, Bate E, Lightman SL. Diurnal Variation in the Responsiveness of the Hypothalamic-Pituitary-Adrenal Axis of the Male Rat to Noise Stress. J.Neuroendocrinol. 2006;18:526–533. doi: 10.1111/j.1365-2826.2006.01444.x. [DOI] [PubMed] [Google Scholar]
- 41.Seale JV, Wood SA, Atkinson HC, Bate E, Lightman SL, Ingram CD, Jessop DS, Harbuz MS. Gonadectomy Reverses the Sexually Diergic Patterns of Circadian and Stress-Induced Hypothalamic-Pituitary-Adrenal Axis Activity in Male and Female Rats. J.Neuroendocrinol. 2004;16:516–524. doi: 10.1111/j.1365-2826.2004.01195.x. [DOI] [PubMed] [Google Scholar]
- 42.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. National Comorbidity Survey Replication. The Epidemiology of Major Depressive Disorder: Results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 43.Gorman JM. Comorbid Depression and Anxiety Spectrum Disorders. Depress.Anxiety. 1996;4:160–168. doi: 10.1002/(SICI)1520-6394(1996)4:4<160::AID-DA2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 44.Levine J, Cole DP, Chengappa KN, Gershon S. Anxiety Disorders and Major Depression, Together Or Apart. Depress.Anxiety. 2001;14:94–104. doi: 10.1002/da.1051. [DOI] [PubMed] [Google Scholar]
- 45.Ninan PT, Berger J. Symptomatic and Syndromal Anxiety and Depression. Depress.Anxiety. 2001;14:79–85. doi: 10.1002/da.1049. [DOI] [PubMed] [Google Scholar]
- 46.Belzer K, Schneier FR. Comorbidity of Anxiety and Depressive Disorders: Issues in Conceptualization, Assessment, and Treatment. J.Psychiatr.Pract. 2004;10:296–306. doi: 10.1097/00131746-200409000-00003. [DOI] [PubMed] [Google Scholar]
- 47.Cameron OG, Abelson JL, Young EA. Anxious and Depressive Disorders and their Comorbidity: Effect on Central Nervous System Noradrenergic Function. Biol.Psychiatry. 2004;56:875–883. doi: 10.1016/j.biopsych.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 48.Cameron OG. Anxious-Depressive Comorbidity: Effects on HPA Axis and CNS Noradrenergic Functions. Essent.Psychopharmacol. 2006;7:24–34. [PubMed] [Google Scholar]
- 49.Keck ME. Corticotropin-Releasing Factor, Vasopressin and Receptor Systems in Depression and Anxiety. Amino Acids. 2006;31:241–250. doi: 10.1007/s00726-006-0333-y. [DOI] [PubMed] [Google Scholar]
- 50.Young EA, Abelson JL, Cameron OG. Effect of Comorbid Anxiety Disorders on the Hypothalamic-Pituitary-Adrenal Axis Response to a Social Stressor in Major Depression. Biol.Psychiatry. 2004;56:113–120. doi: 10.1016/j.biopsych.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 51.National Research Council. guide for the care and use of laboratory animals 19961
- 52.Prut L, Belzung C. The Open Field as a Paradigm to Measure the Effects of Drugs on Anxiety-Like Behaviors: A Review. Eur.J.Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- 53.Osborn JA, Kim CK, Steiger J, Weinberg J. Prenatal Ethanol Exposure Differentially Alters Behavior in Males and Females on the Elevated Plus Maze. Alcohol.Clin.Exp.Res. 1998;22:685–696. [PubMed] [Google Scholar]
- 54.Pellow S, Chopin P, File SE, Briley M. Validation of Open:Closed Arm Entries in an Elevated Plus-Maze as a Measure of Anxiety in the Rat. J.Neurosci.Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 55.Cruz AP, Frei F, Graeff FG. Ethopharmacological Analysis of Rat Behavior on the Elevated Plus-Maze. Pharmacol.Biochem.Behav. 1994;49:171–176. doi: 10.1016/0091-3057(94)90472-3. [DOI] [PubMed] [Google Scholar]
- 56.Pellow S, File SE. Anxiolytic and Anxiogenic Drug Effects on Exploratory Activity in an Elevated Plus-Maze: A Novel Test of Anxiety in the Rat. Pharmacol.Biochem.Behav. 1986;24:525–529. doi: 10.1016/0091-3057(86)90552-6. [DOI] [PubMed] [Google Scholar]
- 57.Cryan JF, Valentino RJ, Lucki I. Assessing Substrates Underlying the Behavioral Effects of Antidepressants using the Modified Rat Forced Swimming Test. Neurosci.Biobehav.Rev. 2005;29:547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 58.Johnson SA, Fournier NM, Kalynchuk LE. Effect of Different Doses of Corticosterone on Depression-Like Behavior and HPA Axis Responses to a Novel Stressor. Behav.Brain Res. 2006;168:280–288. doi: 10.1016/j.bbr.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 59.Abel EL. Gradient of Alarm Substance in the Forced Swimming Test. Physiol.Behav. 1991;49:321–323. doi: 10.1016/0031-9384(91)90050-x. [DOI] [PubMed] [Google Scholar]
- 60.Willner P, Benton D, Brown E, Cheeta S, Davies G, Morgan J, Morgan M. "Depression" Increases "Craving" for Sweet Rewards in Animal and Human Models of Depression and Craving. Psychopharmacology (Berl) 1998;136:272–283. doi: 10.1007/s002130050566. [DOI] [PubMed] [Google Scholar]
- 61.Cardinal RN, Aitken MRF. ANOVA for the Behavioural Sciences. New Jersey: Lawrence Erlbaum Associates; 2005. p. 448. [Google Scholar]
- 62.Huynh H, Feldt LS. Conditions Under which Mean Square Ratios in Repeated Measurements Designs have Exact F-Distributions. 1970;65:1582–1589. [Google Scholar]
- 63.Valentinuzzi VS, Buxton OM, Chang AM, Scarbrough K, Ferrari EA, Takahashi JS, Turek FW. Locomotor Response to an Open Field during C57BL/6J Active and Inactive Phases: Differences Dependent on Conditions of Illumination. Physiol.Behav. 2000;69:269–275. doi: 10.1016/s0031-9384(00)00219-5. [DOI] [PubMed] [Google Scholar]
- 64.van den Buuse M. Circadian Rhythms of Blood Pressure, Heart Rate, and Locomotor Activity in Spontaneously Hypertensive Rats as Measured with Radio-Telemetry. Physiol.Behav. 1994;55:783–787. doi: 10.1016/0031-9384(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 65.Valle FP. Effects of Strain, Sex, and Illumination on Open-Field Behavior of Rats. Am.J.Psychol. 1970;83:103–111. [PubMed] [Google Scholar]
- 66.Britton DR, Indyk E. Central Effects of Corticotropin Releasing Factor (CRF): Evidence for Similar Interactions with Environmental Novelty and with Caffeine. Psychopharmacology (Berl) 1990;101:366–370. doi: 10.1007/BF02244055. [DOI] [PubMed] [Google Scholar]
- 67.Einon D, Morgan MJ, Sahakian BJ. The Development of Intersession Habituation and Emergence in Socially Reared and Isolated Rats. Dev.Psychobiol. 1975;8:553–559. doi: 10.1002/dev.420080613. [DOI] [PubMed] [Google Scholar]
- 68.Kasahara M, Groenink L, Breuer M, Olivier B, Sarnyai Z. Altered Behavioural Adaptation in Mice with Neural Corticotrophin-Releasing Factor Overexpression. Genes Brain Behav. 2007;6:598–607. doi: 10.1111/j.1601-183X.2006.00286.x. [DOI] [PubMed] [Google Scholar]
- 69.Dubovicky M, Mach M, Key M, Morris M, Paton S, Lucot JB. Diurnal Behavioral and Endocrine Effects of Chronic Shaker Stress in Mice. Neuro Endocrinol.Lett. 2007;28:846–853. [PubMed] [Google Scholar]
- 70.Walsh RN, Cummins RA. The Open-Field Test: A Critical Review. Psychol.Bull. 1976;83:482–504. [PubMed] [Google Scholar]
- 71.Shumake J, Barrett D, Gonzalez-Lima F. Behavioral Characteristics of Rats Predisposed to Learned Helplessness: Reduced Reward Sensitivity, Increased Novelty Seeking, and Persistent Fear Memories. Behav.Brain Res. 2005;164:222–230. doi: 10.1016/j.bbr.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 72.Sanberg PR, Zoloty SA, Willis R, Ticarich CD, Rhoads K, Nagy RP, Mitchell SG, Laforest AR, Jenks JA, Harkabus LJ. Digiscan Activity: Automated Measurement of Thigmotactic and Stereotypic Behavior in Rats. Pharmacol.Biochem.Behav. 1987;27:569–572. doi: 10.1016/0091-3057(87)90369-8. [DOI] [PubMed] [Google Scholar]
- 73.Rodgers RJ, Cole JC. Influence of Social Isolation, Gender, Strain, and Prior Novelty on Plus-Maze Behaviour in Mice. Physiol.Behav. 1993;54:729–736. doi: 10.1016/0031-9384(93)90084-s. [DOI] [PubMed] [Google Scholar]
- 74.Griebel G, Rodgers RJ, Perrault G, Sanger DJ. Risk Assessment Behaviour: Evaluation of Utility in the Study of 5-HT-Related Drugs in the Rat Elevated Plus-Maze Test. Pharmacol.Biochem.Behav. 1997;57:817–827. doi: 10.1016/s0091-3057(96)00402-9. [DOI] [PubMed] [Google Scholar]
- 75.Carobrez AP, Bertoglio LJ. Ethological and Temporal Analyses of Anxiety-Like Behavior: The Elevated Plus-Maze Model 20 Years on. Neurosci.Biobehav.Rev. 2005;29:1193–1205. doi: 10.1016/j.neubiorev.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 76.Jones N, King SM. Influence of Circadian Phase and Test Illumination on Pre-Clinical Models of Anxiety. Physiol.Behav. 2001;72:99–106. doi: 10.1016/s0031-9384(00)00388-7. [DOI] [PubMed] [Google Scholar]
- 77.Beeler JA, Prendergast B, Zhuang X. Low Amplitude Entrainment of Mice and the Impact of Circadian Phase on Behavior Tests. Physiol.Behav. 2006;87:870–880. doi: 10.1016/j.physbeh.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 78.Chadda R, Devaud LL. Differential Effects of Mild Repeated Restraint Stress on Behaviors and GABA(A) Receptors in Male and Female Rats. Pharmacol.Biochem.Behav. 2005;81:854–863. doi: 10.1016/j.pbb.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 79.Abel EL. Physical Activity does Not Account for the Physiological Response to Forced Swim Testing. Physiol.Behav. 1994;56:677–681. doi: 10.1016/0031-9384(94)90226-7. [DOI] [PubMed] [Google Scholar]
- 80.Kelliher P, Connor TJ, Harkin A, Sanchez C, Kelly JP, Leonard BE. Varying Responses to the Rat Forced-Swim Test Under Diurnal and Nocturnal Conditions. Physiol.Behav. 2000;69:531–539. doi: 10.1016/s0031-9384(00)00213-4. [DOI] [PubMed] [Google Scholar]
- 81.Karolewicz B, Paul IA. Group Housing of Mice Increases Immobility and Antidepressant Sensitivity in the Forced Swim and Tail Suspension Tests. Eur.J.Pharmacol. 2001;415:197–201. doi: 10.1016/s0014-2999(01)00830-5. [DOI] [PubMed] [Google Scholar]
- 82.Detke MJ, Lucki I. Detection of Serotonergic and Noradrenergic Antidepressants in the Rat Forced Swimming Test: The Effects of Water Depth. Behav.Brain Res. 1996;73:43–46. doi: 10.1016/0166-4328(96)00067-8. [DOI] [PubMed] [Google Scholar]
- 83.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. DSM-IV Washington, DC: American Psychiatric Association; 1994. p. 886. [Google Scholar]
- 84.Matthews K, Robbins TW. Early Experience as a Determinant of Adult Behavioural Responses to Reward: The Effects of Repeated Maternal Separation in the Rat. Neurosci.Biobehav.Rev. 2003;27:45–55. doi: 10.1016/s0149-7634(03)00008-3. [DOI] [PubMed] [Google Scholar]
- 85.Willner P. Validity, Reliability and Utility of the Chronic Mild Stress Model of Depression: A 10-Year Review and Evaluation. Psychopharmacology. 1997;134:319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- 86.Forbes NF, Stewart CA, Matthews K, Reid IC. Chronic Mild Stress and Sucrose Consumption: Validity as a Model of Depression. Physiol.Behav. 1996;60:1481–1484. doi: 10.1016/s0031-9384(96)00305-8. [DOI] [PubMed] [Google Scholar]
- 87.Matthews K, Forbes N, Reid IC. Sucrose Consumption as an Hedonic Measure Following Chronic Unpredictable Mild Stress. Physiol.Behav. 1995;57:241–248. doi: 10.1016/0031-9384(94)00286-e. [DOI] [PubMed] [Google Scholar]
- 88.Hellemans KG, Verma P, Yoon E, Yu W, Weinberg J. Prenatal Alcohol Exposure Increases Vulnerability to Stress and Anxiety-Like Disorders in Adulthood. Ann.N.Y.Acad.Sci. 2008;1144:154–175. doi: 10.1196/annals.1418.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kitay JI. Sex Differences in Adrenal Cortical Secretion in the Rat. Endocrinology. 1961;68:818–824. doi: 10.1210/endo-68-5-818. [DOI] [PubMed] [Google Scholar]
- 90.Krieger DT. Circadian Pituitary Adrenal Rhythms. Adv.Exp.Med.Biol. 1975;54:169–189. doi: 10.1007/978-1-4684-8715-2_8. [DOI] [PubMed] [Google Scholar]
- 91.Krieger DT, Allen W, Rizzo F, Krieger HP. Characterization of the Normal Temporal Pattern of Plasma Corticosteroid Levels. J.Clin.Endocrinol.Metab. 1971;32:266–284. doi: 10.1210/jcem-32-2-266. [DOI] [PubMed] [Google Scholar]
- 92.Krieger DT. Factors Influencing the Circadian Periodicity of Adrenal Steroid Levels. Trans.N.Y.Acad.Sci. 1970;32:316–329. doi: 10.1111/j.2164-0947.1970.tb02063.x. [DOI] [PubMed] [Google Scholar]
- 93.Dalla C, Antoniou K, Drossopoulou G, Xagoraris M, Kokras N, Sfikakis A, Papadopoulou-Daifoti Z. Chronic Mild Stress Impact: Are Females More Vulnerable? Neuroscience. 2005;135:703–714. doi: 10.1016/j.neuroscience.2005.06.068. [DOI] [PubMed] [Google Scholar]
- 94.Kim CK, Yu W, Edin G, Ellis L, Osborn JA, Weinberg J. Chronic Intermittent Stress does Not Differentially Alter Brain Corticosteroid Receptor Densities in Rats Prenatally Exposed to Ethanol. Psychoneuroendocrinology. 1999;24:585–611. doi: 10.1016/s0306-4530(99)00015-3. [DOI] [PubMed] [Google Scholar]
- 95.Carroll ME, Anderson MM, Morgan AD. Higher Locomotor Response to Cocaine in Female (Vs. Male) Rats Selectively Bred for High (HiS) and Low (LoS) Saccharin Intake. Pharmacol.Biochem.Behav. 2007;88:94–104. doi: 10.1016/j.pbb.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kawakami SE, Quadros IM, Takahashi S, Suchecki D. Long Maternal Separation Accelerates Behavioural Sensitization to Ethanol in Female, but Not in Male Mice. Behav.Brain Res. 2007;184:109–116. doi: 10.1016/j.bbr.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 97.Takase K, Mitsushima D, Funabashi T, Kimura F. Sex Difference in the 24-h Acetylcholine Release Profile in the premotor/supplementary Motor Area of Behaving Rats. Brain Res. 2007;1154:105–115. doi: 10.1016/j.brainres.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 98.Gozen O, Balkan B, Yararbas G, Koylu EO, Kuhar MJ, Pogun S. Sex Differences in the Regulation of Cocaine and Amphetamine-Regulated Transcript Expression in Hypothalamic Nuclei of Rats by Forced Swim Stress. Synapse. 2007;61:561–568. doi: 10.1002/syn.20395. [DOI] [PubMed] [Google Scholar]
- 99.Milesi-Halle A, McMillan DE, Laurenzana EM;, Byrnes-Blake KA, Owens SM. Sex Differences in (+)−Amphetamine- and (+)−Methamphetamine-Induced Behavioral Response in Male and Female Sprague-Dawley Rats. Pharmacol.Biochem.Behav. 2007;86:140–149. doi: 10.1016/j.pbb.2006.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Craft RM, Clark JL, Hart SP, Pinckney MK. Sex Differences in Locomotor Effects of Morphine in the Rat. Pharmacol.Biochem.Behav. 2006;85:850–858. doi: 10.1016/j.pbb.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Retana-Marquez S, Bonilla-Jaime H, Vazquez-Palacios G, Dominguez-Salazar E, Martinez-Garcia R, Velazquez-Moctezuma J. Body Weight Gain and Diurnal Differences of Corticosterone Changes in Response to Acute and Chronic Stress in Rats. Psychoneuroendocrinology. 2003;28:207–227. doi: 10.1016/s0306-4530(02)00017-3. [DOI] [PubMed] [Google Scholar]
- 102.Krauchi K, Wirz-Justice A, Willener R, Campbell IC, Feer H. Spontaneous Hypertensive Rats: Behavioral and Corticosterone Response Depend on Circadian Phase. Physiol.Behav. 1983;30:35–40. doi: 10.1016/0031-9384(83)90035-5. [DOI] [PubMed] [Google Scholar]
- 103.Paylor R, Spencer CM, Yuva-Paylor LA, Pieke-Dahl S. The use of Behavioral Test Batteries, II: Effect of Test Interval. Physiol.Behav. 2006;87:95–102. doi: 10.1016/j.physbeh.2005.09.002. [DOI] [PubMed] [Google Scholar]