Abstract
Background
Left ventricular (LV) mass is a strong predictor of cardiovascular disease (CVD), and magnetic resonance imaging (MRI) of the heart is a standard of reference for LV mass measurement. Ethnicity is believed to affect ECG performance. We evaluated the diagnostic and prognostic performance of ECG for left ventricular hypertrophy (LVH) as defined by MRI in relationship to ethnicity.
Methods and Results
Data were analyzed from 4967 participants (48% males, mean age 62 ± 10 years; 39% Caucasian, 13% Chinese, 26% African American, 22% Hispanic) enrolled in the Multi-Ethic Study of Atherosclerosis (MESA) who were followed for a median of 4.8 yearsfor incident CVD. Thirteen traditional ECG-LVH criteria were assessed and showed overall and ethnicity-specific low sensitivity (10–26%) and high specificity (88–99%) in diagnosing MRI-defined LVH. 10 out of 13 ECG-LVH criteria showed superior sensitivity and diagnostic performance in African Americans as compared to Caucasians (p=0.02–0.001). The sum of amplitudes of S wave in V1, S wave in V2 and R wave in V5 (a MESA specific ECG-LVH criterion) offered higher sensitivity (40.4%) compared to prior ECG-LVH criteria while maintaining good specificity (90%) and diagnostic performance (ROC area=0.65). In fully adjusted models, only the MESA-specific ECG-LVH criterion, Romhilt-Estes score, Framingham score, Cornell voltage, Cornell duration product and Framingham-adjusted Cornell voltage predicted increased CVD risk (p<0.05).
Conclusions
ECG has low sensitivity but high specificity for detecting MRI-defined LVH. The performance of ECG for LVH detection varies by ethnicity, with African Americans showing higher sensitivity and overall performance compared to other ethnic groups.
Introduction
Left ventricular hypertrophy (LVH) is a strong independent predictor of future cardiovascular events 1–3. Electrocardiography (ECG) is widely used to detect LVH because it is easy to acquire, readily available and inexpensive. Previous ECG-LVH correlation studies have applied autopsy 4 or more commonly M-mode and 2-D echocardiography for the measurement of LV mass 5–10. Echocardiographic quantification of ventricular mass has been perceived as less reliable due to limited standardization of echocardiography, and its reliance on LV wall thickness measurements and geometric assumptions about the shape of the LV 11. Cardiac MRI has emerged as the current standard of reference for an accurate and reproducible in-vivo measurement of LV mass due to its 3D multi-planar visualization of the heart, excellent blood-myocardial contrast and high spatial-temporal resolution. Despite the advantages of MRI, no large epidemiologic studies have previously used MRI for the purpose of ECG-LVH validation 12, 13. Further, ethnicity-specific variations in the ECG criteria for LVH have not been systematically explored.
The Multi-Ethnic Study of Atherosclerosis (MESA) offers a large multi-ethnic cohort study sample in which both ECG and cardiac MRI were performed. In this study, we evaluated the diagnostic utility of the established ECG-LVH criteria for detecting MRI-defined LVH, and explored alternative ECG measures that may be more sensitive for LVH detection. We also evaluated these ECG measures in relationship to cardiovascular event prediction.
Methods
Study sample and traditional risk factor measurements
MESA is a prospective longitudinal study initiated in July 2000 to explore the prevalence, correlates and progression of subclinical cardiovascular disease (CVD) in a population-based multi-ethnic cohort free of clinically recognized CVD at enrollment, and selected from six U.S. participating field centers. The study objectives, design and methods have been previously reported 14. Hypertension was defined according to the JNC VI (1997) criteria, as diastolic blood pressure ≥ 90 mmHg or systolic blood pressure ≥ 140 mm Hg or self reported history of hypertension and use of any anti-hypertensive medication. Diabetes was labeled as presence of either treated diabetes, defined as current use of insulin or oral hypoglycemic agents; untreated diabetes, defined as fasting glucose ≥ 126 mg/dl or impaired glucose tolerance, defined as fasting glucose 100 – 125 mg/dl. Physical activity was analyzed as daily hours spent in light, moderate and vigorous physical activities combined.
Cardiovascular magnetic resonance imaging
The MESA cardiac MRI protocol, image analysis, and inter- and intra-reader reproducibility have been previously reported 15. LV mass was measured as the sum of the myocardial area (the difference between endocardial and epicardial contours) times slice thickness plus image gap in the end-diastolic phase multiplied by the specific gravity of the myocardium (1.05 g/ml) 15.
Determination of LVH
Observed LV mass (oLVM) was determined from MRI. Individual LV mass was predicted using the following allometric height and weight indexation equations previously derived from a separate reference MESA subpopulation of 822 men and women (47% Caucasians, 22% Chinese, 18% African American, 13% Hispanics) without LVH risk factors 2.
The 95th percentile cut-off value of (oLVM/pLVM) was calculated as 1.31. This indicates that all subjects with observed LV mass more than 1.31 times of that predicted on the basis of height, weight and gender had LV mass greater than 95% of the reference population and constituted LVH for the purposes of this study.
Electrocardiography
Standard 12-lead ECGs were digitally acquired using a Marquette MAC-PC electrocardiograph (Marquette Electronics, Milwaukee, Wisconsin) at 10mm/mV calibration and speed of 25 mm/sec. All ECGs were centrally read, and visually inspected for technical errors and inadequate quality. Participants with pacemakers and ECG-diagnosed atrial fibrillation/flutter were excluded from the study.
The following traditional ECG criteria for LVH were tested: Sokolow-Lyon voltage (SV1 + RV5/V6 ≥ 3.5 mV and/or RaVL ≥ 1.1 mV) 16; gender-specific Cornell voltage [SV3 + RaVL > 2.8 mV (for men) and > 2.0 mV (for women)] 6; Romhilt-Estes point score (partition values ≥ 5 points and ≥ 4 points were examined) 17; Framingham ECG score (presence of a strain pattern and at-least one of the following voltage criteria – RI + SIII ≥ 2.5 mV, SV1/V2 + RV5/V6 ≥ 3.5 mV, the S wave on the right precordial lead ≥ 2.5 mV and the R wave on the left precordial lead ≥ 2.5 mV) 7; Left ventricular strain (presence of isolated ST-T wave ischemic abnormalities as per Novacode 5.5 or 5.6) 18; Perguia score [requires positivity of at-least one of the following three criteria: SV3 + RaVL > 2.4 mV (men) or > 2.0 mV (women), left ventricular strain or Romhilt-Estes score of ≥ 5] 5; Minnesota code 3.1 (RV5/V6 > 2.6 mV or RI/II/III/aVF > 2 mV or RaVL > 1.2 mV) 19; Lewis index [(RI + SIII) – (RIII + SI) > 1.7 mV] 9; Framingham-adjusted Cornell voltage (Men: [RaVL + SV3 + 0.0174*(age – 49) + 0.191*(BMI – 26.5)] ≥ 2.8 mV; Women: [RaVL + SV3 + 0.0387*(age – 50) + 0.212*(BMI – 24.9)] ≥ 2.0 mV) 10; Cornell voltage product [(RaVL + SV3)*QRS duration ≥ 243600 μVms] 20; Sokolow-Lyon voltage product [(SV1 + RV5/RV6)*QRS duration ≥ 371000 μVms] 20 and Gubner and Ungerleider voltage (RI + SIII ≥ 2.2 mV) 21.
Cardiovascular disease events
A detailed description has been published on adjudication of events as well as follow-up procedures in MESA 2. CVD events considered included myocardial infarction, resuscitated cardiac arrest, definite angina, probable angina (if followed by revascularization), stroke, transient ischemic attack, percutaneous transluminal coronary angioplasty, coronary stent, coronary atherectomy, coronary bypass graft, coronary or other revascularization, congestive heart failure, peripheral vascular disease, coronary heart disease death, stroke death, other atherosclerotic death or other CVD death.
Statistical methods
All baseline continuous variables were reported as mean ± SD and categorical variables as frequency (%). Student’s unpaired t-test/one-way analysis of variance (ANOVA) were used to compare continuous variables. Categorical values were compared using Pearson’s chi-square test. Diagnostic characteristics of various ECG-LVH criteria were computed and compared against the reference standard MRI diagnosis of LVH using receiver operating characteristic (ROC) analysis. Besides traditional ECG diagnostic criteria, ECG variables showing significant differences between participants without and with MRI-LVH were further assessed to determine the combination that maximized the sensitivity of LVH detection at specificity of 90%. Ethnicity-specific comparisons between sensitivities and specificities of ECG-LVH criteria were done using the Pearson’s chi-square test or Fisher’s exact test.
Survival function curves were compared using Mantel (log-rank) test across all ECG-LVH measures. Univariate and multivariate Cox proportional hazards models were used to estimate the prognostic effect of ECG-LVH measures on occurrence of CVD events during follow-up. The following covariates were adjusted in the multivariate Cox models: age, gender, BMI, hypertension, diabetes, plasma total and HDL cholesterol, pack-years of smoking, ethnicity, any lipid/hypertension lowering medication, number of alcohol drinks per week and physical activity. A p-value < 0.05 was considered statistically significant. Statistical analyses were performed using STATA statistical software (Version 9.0, College Station, TX).
This research was supported by contracts N01-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute (NHLBI). The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper, and its final contents.
Results
Study sample and baseline characteristics
The study sample consisted of 4967 participants [2365 men (47.6%), mean age 61.5 ± 10.1 years] (Table 1). The ethnicity of the study sample was 38.8% Caucasians, 13.2 % Chinese, 25.7% African Americans and 22.3% Hispanics. 384/4967 participants (7.7%) had LVH on MRI.
Table 1.
Baseline characteristics of the study population according to MRI-defined LVH
| Variable | No MRI-LVH (N= 4583) | MRI-LVH (N= 384) | P Value |
|---|---|---|---|
| Age (yr) | 61.3 (10.1) | 64.2 (10.2) | < 0.0001 |
| Men, % | 47.4 | 50.3 | 0.28 |
| Ethnicity, % | < 0.001 | ||
| Caucasians | 40 | 25.8 | |
| Chinese | 13.5 | 8.6 | |
| African American | 24.6 | 39.1 | |
| Hispanics | 21.9 | 26.6 | |
| BMI (kg/m2) | 27.74 (4.9) | 27.72 (4.9) | 0.96 |
| Hypertension, % | 39.9 | 71.9 | < 0.001 |
| Diabetes, % | 23.7 | 34.9 | < 0.001 |
| Total cholesterol (mg/dl) | 194.5 (35.3) | 191.4 (36.5) | 0.09 |
| HDL cholesterol (mg/dl) | 51.1 (14.9) | 52.6 (16.1) | 0.07 |
| Pack years of smoking | 10.64 (22.3) | 12.4 (19.7) | 0.09 |
| Alcohol drinks per week | 4.0 (9.8) | 4.1 (8.7) | 0.85 |
| Physical activity (hours/day) | 12.7 (5.8) | 12.4 (5.9) | 0.43 |
| Cardiovascular events, % | 5.4 | 16.7 | < 0.001 |
LVH, left ventricular hypertrophy; BMI, body mass index
Overall diagnostic performance of ECG-LVH measures against MRI-LVH
Sensitivity, specificity and AUC of traditional ECG-LVH measures to detect MRI-LVH ranged from 5.7% to 26.0%, 88.7% to 99.2% and 0.52 to 0.59 respectively. All criteria showed a specificity > 90% except Lewis index (88.7%). Romhilt-Estes ≥ 5 and Framingham score were the most specific criteria, with specificity > 99% for both. They also were the least sensitive criteria, with sensitivity of 5.7% and 7.0% respectively. Sokolow-Lyon voltage was the most sensitive traditional ECG-LVH marker with the best overall diagnostic performance (sensitivity= 26.0%, AUC= 0.59), followed by Perguia score (sensitivity= 24.7%, AUC= 0.59). Only 3 of 13 criteria considered had a sensitivity of > 20% (Table 2).
Table 2.
Diagnostic characteristics of traditional ECG criteria for detecting MRI-defined LVH in the overall MESA cohort (n= 4967 participants)
| ECG criterion | Sensitivity (95% CI) | Specificity (95% CI) | ROC area (95% CI) |
|---|---|---|---|
| Sokolow-Lyon voltage | 26.0 (21.7, 30.7) | 92.6 (91.8, 93.3) | 0.59 (0.57, 0.62) |
| Romhilt-Estes ≥ 4 | 15.9 (12.4, 19.9) | 97.0 (96.5, 97.5) | 0.56 (0.55, 0.58) |
| Romhilt-Estes ≥ 5 | 5.7 (3.6, 8.6) | 99.1 (98.8, 99.4) | 0.52 (0.51, 0.54) |
| Cornell voltage | 15.1 (11.7,19.1) | 97.3 (96.7, 97.7) | 0.56 (0.54, 0.58) |
| Perguia score | 24.7 (20.5, 29.4) | 93.2 (92.4, 93.9) | 0.59 (0.56, 0.61) |
| Minnesota code 3.1 | 16.9 (13.3, 21.1) | 95.5 (94.9, 96.1) | 0.56 (0.54, 0.58) |
| Framingham score | 7.0 (4.7, 10.1) | 99.2 (98.9, 99.4) | 0.53 (0.52, 0.54) |
| Lewis index | 23.2 (19.0, 27.7) | 88.7 (87.8, 89.6) | 0.56 (0.54, 0.58) |
| Adjusted Cornell voltage* | 15.1 (11.7, 19.1) | 97.2 (96.7, 97.7) | 0.56 (0.54, 0.58) |
| LV strain pattern | 10.7 (7.8,14.2) | 97.0 (96.5, 97.5) | 0.54 (0.52, 0.55) |
| Cornell duration product | 14.8 (11.4, 18.8) | 97.3 (96.8, 97.7) | 0.56 (0.54, 0.58) |
| Sokolow-Lyon duration product | 12.5 (9.4, 16.2) | 98.4 (98, 98.8) | 0.56 (0.54, 0.57) |
| Gubner and Ungerleider | 13.8 (10.5, 17.7) | 94.5 (93.8, 95.2) | 0.54 (0.52, 0.56) |
Framingham-adjusted
Ethnicity-specific diagnostic performance of ECG-LVH measures against MRI-LVH
Table 3 shows the utility of ECG in detecting MRI-LVH across different ethnic groups. Sensitivity in African Americans was significantly higher than Caucasians on 10/13 (76.9%) criteria. AUC was significantly higher in African Americans than Caucasians on 7/13 (53.8%) criteria. Sensitivity in Chinese was significantly higher than Caucasians for Framingham-adjusted Cornell voltage, Perguia score, Cornell voltage, and Framingham score criteria. Sensitivity in Hispanics was significantly higher than Caucasians for Framingham-adjusted Cornell voltage, and Cornell voltage criteria. Chinese and Hispanics were similar to Caucasians with respect to AUC on all of the ECG Criteria except Framingham score (Hispanics > Caucasians).
Table 3.
Ethnicity-specific diagnostic characteristics of traditional ECG criteria for detecting MRI-defined LVH
| Sensitivity | Specificity | ROC area | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ECG Criteria | Cn. | Ch. | AA. | H. | Cn. | Ch. | AA. | H. | Cn. | Ch. | AA. | H. |
| Sokolow-Lyon | 19.2 | 18.2 | 36.7* | 19.6 | 95.0 | 92.9 | 86.8* | 94.4 | 0.57 | 0.56 | 0.62 | 0.57 |
| Romhilt-Estes ≥ 4 | 11.1 | 18.2 | 22.7† | 9.8 | 97.1 | 97.7 | 95.6 | 98.0 | 0.54 | 0.58 | 0.59† | 0.54 |
| Romhilt-Estes ≥ 5 | 5.0 | 9.1 | 7.3 | 2.9 | 99.1 | 99.2 | 98.8 | 99.7 | 0.52 | 0.54 | 0.53 | 0.51 |
| Cornell voltage | 6.1 | 24.2* | 18.7* | 15.7† | 98.6 | 95.3 | 96.6 | 96.7 | 0.52 | 0.59 | 0.58† | 0.56 |
| Perguia score | 13.1 | 33.3† | 31.3* | 23.5 | 95.0 | 91.8 | 91.1 | 93.1 | 0.54 | 0.63 | 0.61* | 0.58 |
| Minnesota code 3.1 | 11.1 | 21.2 | 22.7† | 12.7 | 96.7 | 95.8 | 92.1* | 97.0 | 0.54 | 0.59 | 0.57 | 0.55 |
| Framingham score | 1.0 | 9.1† | 11.3* | 5.9 | 99.3 | 99.2 | 98.7 | 99.7 | 0.50 | 0.54 | 0.55* | 0.53† |
| Lewis index | 17.2 | 6.1 | 30.7† | 23.5 | 90.9 | 95.3 | 82.8* | 87.3 | 0.54 | 0.51 | 0.57 | 0.55 |
| Adjusted Cornell voltage‡ | 6.1 | 24.2* | 18.7* | 15.7† | 98.6 | 95.3 | 96.5 | 96.7 | 0.52 | 0.59 | 0.58† | 0.56 |
| LV strain pattern | 4.0 | 12.1 | 14.7† | 10.8 | 97.3 | 97.6 | 95.7 | 97.6 | 0.51 | 0.55 | 0.55† | 0.54 |
| Cornell duration product | 11.1 | 18.2 | 14.7 | 17.6 | 97.6 | 96.6 | 96.8 | 97.7 | 0.54 | 0.57 | 0.56 | 0.58 |
| Sokolow-Lyon duration product | 8.1 | 12.1 | 18.7† | 7.8 | 98.8 | 97.7 | 97.2 | 99.6 | 0.53 | 0.55 | 0.58† | 0.54 |
| Gubner and Ungerleider | 11.1 | 3.0 | 16.7 | 15.7 | 96.0 | 97.6 | 90.3* | 94.7 | 0.54 | 0.50 | 0.54 | 0.55 |
Cn., Caucasians; Ch., Chinese; AA., African Americans; H., Hispanics;
p≤ 0.005;
p< 0.05; p-values stand for pair-wise comparisons between other ethnic groups and Caucasians taken as the reference; highlighted values stand for significant differences with respect to Caucasians;
Framingham-adjusted
In contrast, specificity was ≥ 90% for Caucasians, Chinese and Hispanics and ≥ 82% for African Americans across all criteria. Large significant differences in specificity were seen between Caucasians and African Americans on Sokolow-Lyon (95% vs. 86.8%), Lewis index (90.9% vs. 82.8%), Minnesota code 3.1 (96.7% vs. 92.1%), and Gubner and Ungerleider (96.0% vs. 90.3%) criteria.
MESA-specific ECG-LVH criterion and its ethnicity- and gender-specific cut-off values
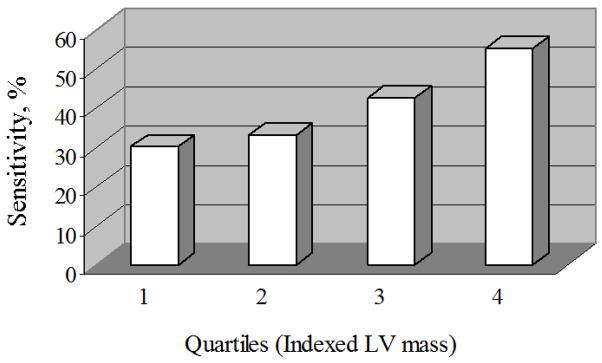
We found that the sum of amplitudes of S waves in V1 and V2 and R wave in V5 revealed a sensitivity of 40.4% (95% CI 35.4%, 45.5%) and AUC of 0.65 (0.63, 0.68) at a cut-off ≥ 4.2 mV corresponding to a specificity of 90% (Table 4). This MESA ECG correlate of LVH (SV1 + SV2 + RV5 ≥ 4.2 mV) showed significantly greater sensitivity and AUC values than Sokolow-Lyon and Perguia score that were the best traditional ECG-LVH measures as discussed above (p< 0.001, p< 0.0001 respectively). Its sensitivity to detect LVH showed an increase in proportion to severity levels of LVH (Figure 1).
Table 4.
Ethnicity- and gender-specific cut-offs and corresponding diagnostic performance of the MESA ECG-LVH criterion*
| Cut-off (mV) | Sensitivity (95% CI) | Specificity (95% CI) | ROC area (95% CI) | |
|---|---|---|---|---|
| Overall | ≥ 4.2 | 40.4 (35.4, 45.5) | 90.0 (89.1, 90.9) | 0.65 (0.63, 0.68) |
| Caucasians | ||||
| Men | ≥ 4.1 | 41.7 (27.6, 56.8) | 90.0 (87.8, 91.9) | 0.66 (0.59, 0.73) |
| Women | ≥ 3.7 | 35.3 (22.4, 49.9) | 90.1 (88.0, 91.9) | 0.63 (0.56, 0.69) |
| Chinese | ||||
| Men | ≥ 4.9 | 18.8 (40.1, 45.6) | 90.1 (86.2, 93.2) | 0.54 (0.44, 0.64) |
| Women | ≥ 4.2 | 64.7 (38.3, 85.8) | 89.9 (86.0, 93.0) | 0.77 (0.66, 0.89) |
| African American | ||||
| Men | ≥ 4.8 | 37.2 (26.5, 48.9) | 90.1 (87.2, 92.6) | 0.64 (0.58, 0.69) |
| Women | ≥ 4.3 | 38.9 (27.6, 51.1) | 90.1 (87.4, 92.3) | 0.65 (0.59, 0.70) |
| Hispanics | ||||
| Men | ≥ 4.3 | 37.3 (24.1, 51.9) | 90.0 (87.1, 92.5) | 0.64 (0.57, 0.71) |
| Women | ≥ 3.7 | 33.3 (20.8, 47.9) | 90.0 (87.1, 92.5) | 0.62 (0.55, 0.68) |
MESA ECG-LVH criterion: SV1+SV2+RV5
Figure 1.
Sensitivity of the MESA ECG-LVH criterion to detect MRI-defined LVH at increasing quartiles of indexed LV mass in the overall MESA cohort (n=4967 participants).
The ethnicity- and gender-specific partition values and sensitivity and AUC values at a specificity of 90% are shown in Table 4. All ethnic groups showed higher sensitivity and test performance with the MESA ECG-LVH correlate as compared to existing ECG-LVH criteria. Except Chinese men, both men and women of all ethnic groups showed a sensitivity > 33%. Partition values were systematically higher in men than women across all ethnicities.
Ethnic differences in ECG amplitudes
All ethnic groups showed significantly higher MESA ECG-LVH voltage, Sokolow-Lyon voltage, Cornell voltage and RV5 amplitude as compared to Caucasians after adjustment for common traditional risk factors (all p< 0.05, not shown in tables).
Prognostic significance of ECG-LVH measures
Of the 4967 participants, 25 had no follow-up completed and an additional 4 were excluded from follow-up due to pre-baseline physician-diagnosed cardiovascular disease. Total 307 incident cardiovascular events were observed in the remaining 4938 participants over a median follow-up of 4.8 years.
Event rate was three-fold higher in participants with MRI-LVH compared to those without MRI-LVH (10.1 vs. 3.1, p<0.001). MRI-LVH was associated with significant hazard for cardiovascular events both in univariate (HR 3.38, 95% CI 2.57, 4.47) as well as multivariate Cox models [HR 2.31 (1.72, 3.11)] adjusted for baseline risk factors. Participants with LVH defined by the MESA ECG-LVH criterion had nearly two-fold higher events compared with participants without LVH (5.92 vs. 3.34, p< 0.001). The unadjusted hazard ratio was statistically significant for all ECG-LVH criteria. However, most ECG-LVH criteria lost their prognostic significance in a multivariate model that included traditional cardiovascular risk factors. ECG-LVH criteria that predicted increased cardiovascular event risk in the multivariate model included the MESA specific criterion [HR 1.64 (1.21, 2.21)], Romhilt-Estes score ≥ 5 [HR 2.02 (1.15, 3.54), Cornell voltage [HR 1.65 (1.03, 2.63)], Framingham score [HR 2.04 (1.20, 3.49)], Framingham-adjusted Cornell voltage [HR 1.65 (1.03, 2.63)], and Cornell duration product [(HR 1.67 (1.09, 2.54)]. The prognostic power of ECG criteria based on composite measures such as the Framingham ECG score and Romhilt-Estes score ≥ 5 (both with specificity > 99%) was less than MRI but superior to other ECG-LVH criteria (Table 5).
Table 5.
Association of ECG correlates of LVH with incident total cardiovascular disease events (n=307 among 4938 participants followed for a median of 4.8 years)
| CV Event Rate |
||||
|---|---|---|---|---|
| ECG criterion | No LVH | LVH | Unadjusted HR (95% CI) | Adjusted HR (95% CI) |
| SV1 + SV2 + RV5 (≥4.2 mV) | 3.34 | 5.92* | 1.74 (1.32, 2.31)† | 1.64 (1.21, 2.21)† |
| Sokolow-Lyon voltage | 3.45 | 5.83* | 1.77 (1.28, 2.44)† | 1.26 (0.90, 1.76) |
| Romhilt-Estes ≥ 4 | 3.50 | 7.51* | 2.21 (1.47, 3.32)† | 1.05 (0.69, 1.61) |
| Romhilt-Estes ≥ 5 | 3.54 | 13.59* | 4.07 (2.38, 6.95)† | 2.02 (1.15, 3.54)‡ |
| Cornell voltage | 3.55 | 6.55† | 2.01 (1.28, 3.16)† | 1.65 (1.03, 2.63)§ |
| Perguia score | 3.36 | 7.14* | 2.32 (1.71, 3.16)† | 1.36 (0.99, 1.88) |
| Minnesota code 3.1 | 3.54 | 5.42* | 1.62 (1.35, 1.94)† | 1.15 (0.76, 1.74) |
| Framingham score | 3.53 | 14.08* | 3.95 (2.35, 6.64)† | 2.04 (1.20, 3.49)‡ |
| Lewis index | 3.43 | 5.34* | 1.67 (1.24, 2.24)† | 1.19 (0.87, 1.60) |
| Adjusted Cornell voltage¶ | 3.55 | 6.51† | 1.98 (1.26, 3.13)† | 1.65 (1.03, 2.63)§ |
| LV strain pattern | 3.51 | 7.89* | 2.34 (1.53, 3.58)† | 1.14 (0.73, 1.76) |
| Cornell duration product | 3.48 | 8.57* | 2.84 (1.89, 4.24)† | 1.67 (1.09, 2.54)§ |
| Sokolow-Lyon duration product | 3.59 | 6.41§ | 1.78 (1.02, 3.09)§ | 1.26 (0.71, 2.23) |
| Gubner and Ungerleider | 3.55 | 5.32§ | 1.52 (1.02, 2.26)§ | 1.01 (0.67, 1.51) |
p≤ 0.0005,
p< 0.005,
p≤ 0.01,
p< 0.05; measured per 10,000 person days;
Framingham-adjusted; HR = hazard ratio adjusted for age, body mass index, gender, hypertension, diabetes, total and HDL cholesterol, pack-years of smoking, ethnicity, lipid and hypertensive medication, number of alcohol drinks per week and physical activity
Discussion
In this study, we assessed the diagnostic and prognostic utility of standard electrocardiographic measures of LVH in a large multi-ethnic sample, using cardiac MRI to quantify LV mass. We found that commonly used ECG-LVH criteria show a low overall sensitivity that varied by ethnicity, a high specificity, and overall low diagnostic performance in diagnosing LVH as defined by MRI. This finding is consistent with previous studies that have also shown ECG to be typically insensitive and highly specific in detecting LVH 4–9.
Definition of LVH
An appropriate method to assess LV mass in relationship to overall body size is required to define LVH. Most previous ECG-LVH studies, primarily based on echocardiography, have used either body surface area or height to index LV mass but this has been shown not to fully remove the correlation of MRI measured LV mass with weight and/or height. We used an allometric height and weight index derived from MRI measurement of LV mass and appropriately adjusted for ethnic- and gender- variations in association of LV mass to body size and height. This approach has been previously used with MRI data, and demonstrated a similar increased CVD risk in subjects with increased LV mass 2.
Performance of electrocardiographic criteria
Our results indicate ethnic variation to be most prominent between Caucasians and African Americans. ECG-LVH criteria were found to have significantly higher sensitivity and lower specificity in African Americans as compared to Caucasians which is consistent with previous studies 8, 22, 23. In addition, overall diagnostic performance (defined by AUC) is higher in African Americans compared to Caucasians. As compared to Chinese and Hispanics, Caucasians tend to show lower sensitivity but specificity and overall diagnostic performance lie in a clinically similar range.
Reasons for the ethnic variation in the performance of ECG-LVH criteria are not known. Most of the ECG voltages showed higher amplitudes in other ethnic groups than Caucasians that persisted even after adjusting for baseline confounding covariates that would be expected to affect ethnic differences. These differences, attributable at least in part to anthropometric differences in chest size and configuration in different ethnic groups 22, may produce apparent ethnic differences in ECG performance when identical test criteria are used across all ethnicities. Like earlier studies 23, our findings provide further evidence in favor of the need for ethnicity-specific partition values of existing criteria instead of non-ethnicity specific values for the most accurate and optimum ECG characterization of LVH across different ethnic groups.
Previous studies documenting ECG-LVH and cardiovascular morbidity and mortality involved patients with acute Q-wave infarction 24, hypertension 25, 26, elderly subjects 27, predominantly Caucasian 21, 26 and male populations 21, 24, 25, 27. We performed a comparative assessment of the prognostic implications of different ECG-LVH criteria in a multi-ethnic cohort consisting of both men and women free of significant baseline CVD. The results from this study therefore may be more generalizable and applicable in a large population-based setting than the previous studies. Our results indicate that a limited number of ECG-LVH criteria show an independent association with incident CVD, including the MESA specific LVH criterion, Romhilt-Estes ≥ 5, Framingham ECG score, Cornell voltage, Framingham-adjusted Cornell voltage, and Cornell duration product.
Limitations
Limitations are, firstly, that we defined LVH at the 95th percentile of the indexed LV mass as it has commonly been used as an upper limit of “normal” LV mass, and it has showed the highest association with heart failure events in one of our earlier MESA studies 2. However, sensitivities of all ECG-LVH criteria would be higher if we had chosen the cut-off at a lower percentile level. Secondly, we considered a composite end-point consisting of both “hard” and “soft” cardiovascular events to ensure enough statistical power. Thirdly, ECG could not be compared to alternative tests such as echocardiography for their relative utility in detecting MRI-defined LVH due to non-availability of echocardiographic data. Finally, external validation of the MESA optimized criterion in another independent sample, and further studies on ethnicity-specific prognostic significance of ECG-LVH criteria are required.
Conclusions
Although ECG has a low sensitivity to diagnose MRI-defined LVH, the standard ECG is the first line investigation for suspected LVH in view of its simplicity, widespread availability and low cost. The performance of ECG for LVH detection varies substantially by ethnicity, with the African American subgroup showing higher overall performance compared to other ethnic groups. Our results from a multi-ethnic study population suggest alternative ECG criteria may improve detection of LVH. Most ECG models are not predictive of cardiovascular events in fully adjusted models.
Acknowledgments
The authors thank other investigators, staff, and participants of the MESA Study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Footnotes
Disclosures: There are no conflicts of interest and financial disclosures for any of the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gardin JM, McClelland R, Kitzman D, et al. M-mode echocardiographic predictors of six- to seven-year incidence of coronary heart disease, stroke, congestive heart failure, and mortality in an elderly cohort (the Cardiovascular Health Study) Am J Cardiol. 2001;87:1051–7. doi: 10.1016/s0002-9149(01)01460-6. [DOI] [PubMed] [Google Scholar]
- 2.Bluemke DA, Kronmal RA, Lima JA, et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148–55. doi: 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sukhija R, Aronow WS, Kakar P, et al. Prevalence of echocardiographic left ventricular hypertrophy in persons with systemic hypertension, coronary artery disease, and peripheral arterial disease and in persons with systemic hypertension, coronary artery disease, and no peripheral arterial disease. Am J Cardiol. 2005;96:825–6. doi: 10.1016/j.amjcard.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 4.Casale PN, Devereux RB, Alonso DR, et al. Improved sex-specific criteria of left ventricular hypertrophy for clinical and computer interpretation of electrocardiograms: validation with autopsy findings. Circulation. 1987;75:565–72. doi: 10.1161/01.cir.75.3.565. [DOI] [PubMed] [Google Scholar]
- 5.Schillaci G, Verdecchia P, Borgioni C, et al. Improved electrocardiographic diagnosis of left ventricular hypertrophy. Am J Cardiol. 1994;74:714–9. doi: 10.1016/0002-9149(94)90316-6. [DOI] [PubMed] [Google Scholar]
- 6.Devereux RB, Casale PN, Eisenberg RR, et al. Electrocardiographic detection of left ventricular hypertrophy using echocardiographic determination of left ventricular mass as the reference standard. Comparison of standard criteria, computer diagnosis and physician interpretation. J Am Coll Cardiol. 1984;3:82–7. doi: 10.1016/s0735-1097(84)80433-7. [DOI] [PubMed] [Google Scholar]
- 7.Levy D, Labib SB, Anderson KM, et al. Determinants of sensitivity and specificity of electrocardiographic criteria for left ventricular hypertrophy. Circulation. 1990;81:815–20. doi: 10.1161/01.cir.81.3.815. [DOI] [PubMed] [Google Scholar]
- 8.Lee DK, Marantz PR, Devereux RB, et al. Left ventricular hypertrophy in black and white hypertensives. Standard electrocardiographic criteria overestimate racial differences in prevalence. JAMA. 1992;267:3294–9. [PubMed] [Google Scholar]
- 9.Morrison I, Clark E, Macfarlane PW. Evaluation of the electrocardiographic criteria for left ventricular hypertrophy. Anadolu Kardiyol Derg. 2007;7 (Suppl 1):159–63. [PubMed] [Google Scholar]
- 10.Norman JE, Jr, Levy D, Campbell G, et al. Improved detection of echocardiographic left ventricular hypertrophy using a new electrocardiographic algorithm. J Am Coll Cardiol. 1993;21:1680–6. doi: 10.1016/0735-1097(93)90387-g. [DOI] [PubMed] [Google Scholar]
- 11.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Alfakih K, Walters K, Jones T, et al. New gender-specific partition values for ECG criteria of left ventricular hypertrophy: recalibration against cardiac MRI. Hypertension. 2004;44:175–9. doi: 10.1161/01.HYP.0000135249.66192.30. [DOI] [PubMed] [Google Scholar]
- 13.Vottonen P, Husso M, Sipola P, et al. Electrocardiographic left ventricular hypertrophy has low diagnostic accuracy in middle-aged subjects. Blood Press. 2007;16:328–34. doi: 10.1080/08037050701288255. [DOI] [PubMed] [Google Scholar]
- 14.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 15.Natori S, Lai S, Finn JP, et al. Cardiovascular function in multi-ethnic study of atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol. 2006;186(6 Suppl 2):S357–65. doi: 10.2214/AJR.04.1868. [DOI] [PubMed] [Google Scholar]
- 16.Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. 1949. Ann Noninvasive Electrocardiol. 2001;6:343–68. doi: 10.1111/j.1542-474X.2001.tb00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romhilt DW, Estes EH., Jr A point-score system for the ECG diagnosis of left ventricular hypertrophy. Am Heart J. 1968;75:752–8. doi: 10.1016/0002-8703(68)90035-5. [DOI] [PubMed] [Google Scholar]
- 18.Rautaharju PM, Park LP, Chaitman BR, et al. The Novacode criteria for classification of ECG abnormalities and their clinically significant progression and regression. J Electrocardiol. 1998;31:157–87. [PubMed] [Google Scholar]
- 19.Blackburn H. Classification of the electrocardiogram for population studies: Minnesota Code. J Electrocardiol. 1969;2:305–10. doi: 10.1016/s0022-0736(69)80120-2. [DOI] [PubMed] [Google Scholar]
- 20.Okin PM, Roman MJ, Devereux RB, et al. Electrocardiographic identification of increased left ventricular mass by simple voltage-duration products. J Am Coll Cardiol. 1995;25:417–23. doi: 10.1016/0735-1097(94)00371-v. [DOI] [PubMed] [Google Scholar]
- 21.Hsieh BP, Pham MX, Froelicher VF. Prognostic value of electrocardiographic criteria for left ventricular hypertrophy. Am Heart J. 2005;150:161–7. doi: 10.1016/j.ahj.2004.08.041. [DOI] [PubMed] [Google Scholar]
- 22.Rautaharju PM, Park LP, Gottdiener JS, et al. Race- and sex-specific ECG models for left ventricular mass in older populations. Factors influencing overestimation of left ventricular hypertrophy prevalence by ECG criteria in African-Americans. J Electrocardiol. 2000;33:205–18. doi: 10.1054/jelc.2000.7667. [DOI] [PubMed] [Google Scholar]
- 23.Okin PM, Wright JT, Nieminen MS, et al. Ethnic differences in electrocardiographic criteria for left ventricular hypertrophy: the LIFE study. Losartan Intervention For Endpoint. Am J Hypertens. 2002;15:663–71. doi: 10.1016/s0895-7061(02)02945-x. [DOI] [PubMed] [Google Scholar]
- 24.Boden WE, Kleiger RE, Schechtman KB, et al. Clinical significance and prognostic importance of left ventricular hypertrophy in non-Q-wave acute myocardial infarction. Am J Cardiol. 1988;62:1000–4. doi: 10.1016/0002-9149(88)90537-1. [DOI] [PubMed] [Google Scholar]
- 25.Alderman MH, Madhavan S, Cohen H, et al. Low urinary sodium is associated with greater risk of myocardial infarction among treated hypertensive men. Hypertension. 1995;25:1144–52. doi: 10.1161/01.hyp.25.6.1144. [DOI] [PubMed] [Google Scholar]
- 26.Verdecchia P, Schillaci G, Borgioni C, et al. Prognostic value of a new electrocardiographic method for diagnosis of left ventricular hypertrophy in essential hypertension. J Am Coll Cardiol. 1998;31:383–90. doi: 10.1016/s0735-1097(97)00493-2. [DOI] [PubMed] [Google Scholar]
- 27.Kahn S, Frishman WH, Weissman S, et al. Left ventricular hypertrophy on electrocardiogram: prognostic implications from a 10-year cohort study of older subjects: a report from the Bronx Longitudinal Aging Study. J Am Geriatr Soc. 1996;44:524–9. doi: 10.1111/j.1532-5415.1996.tb01437.x. [DOI] [PubMed] [Google Scholar]