Abstract
Background
Epidemiologic data on serum melatonin, a marker of circadian rhythms, and cancer are sparse due largely to the lack of reliable assays with high sensitivity to detect relatively low melatonin levels in serum collected during daylight, as commonly available in most epidemiologic studies.
Methods
To help expand epidemiologic research on melatonin, we assessed the reproducibility and refined a currently available melatonin radioimmunoassay, and evaluated its application to epidemiologic investigations by characterizing melatonin levels in serum, urine, and/or plasma in 135 men from several ethnic groups.
Results
Reproducibility was high for the standard 1.0 ml serum- (mean coefficient of variation (CV)=6.9%, intraclass correlation coefficient (ICC)=97.4%, n=2 serum pools in triplicate) and urine-based (mean CV=3.5%, ICC=99.9%) assays. Reproducibility for the 0.5 ml refined-serum assay was equally good (mean CV=6.6%%;ICC=99.0%). There was a positive correlation between morning serum melatonin and 6-sulfatoxymelatonin in 24-hour urine(r=0.46, P=0.008; n=49 subjects). Melatonin levels in serum-plasma pairs had a high correlation (r=0.97, P<1 × 10−4; n=20) Morning serum melatonin levels were five times higher than those from the afternoon (before 9 AM mean = 11.0 pg/mL versus after 11 AM mean=2.0 pg/mL). Chinese men had lower melatonin levels (mean=3.4 pg/mL), while Caucasian, African American, and Ghanaian men had similar levels (mean=6.7–8.6 pg/mL).
Conclusions
These results suggest that melatonin can be detected reliably in serum samples collected in epidemiologic studies in various racial groups.
Impact
With improved assays, it may be possible to investigate the role of melatonin and the emerging circadian rhythm hypothesis in cancer etiology in epidemiologic studies.
Keywords: melatonin, radioimmunoassay, assay variation, reproducibility
INTRODUCTION
Circadian rhythm disruption has recently been classified as a probable carcinogen to humans (1) and has been associated with an increased risk for cancers of the prostate (2–7), breast (8–11), and colon (12), and for non-Hodgkin lymphoma (13). Although the underlying mechanism explaining these observations is unknown, it has been suggested that melatonin, a hormone synthesized and secreted by the pineal gland in response to low-light conditions, may mediate the circadian rhythm-cancer relationship (1). Circulating melatonin exhibits a circadian rhythm with highest levels at night, moderate levels in the morning, and substantially lower levels during the afternoon (14). Light exposure can reduce the duration of melatonin secretion and subsequent circulating melatonin levels (15). As such, melatonin may be a key biological intermediary of chronodisruption (16).
Traditionally, in sleep disorder studies, melatonin is measured in urine (as 6-sulfatoxymelatonin (aMT6s)) because overnight or morning first-void urinary aMT6s reflects melatonin levels during the night when melatonin levels are at their highest (17). However, in most large-scale prospective epidemiologic studies, overnight void urine is not routinely collected. In addition, most cohort studies collect blood samples during the day when melatonin levels are quite low. Therefore, reliable assays with high sensitivity are needed to quantify accurately the circulating levels of melatonin. For these reasons, few epidemiologic studies have measured serum melatonin levels and tested the hypothesis that altered melatonin levels are associated with increased cancer risk.
To extend the investigation of the circadian rhythm/melatonin hypothesis to epidemiologic studies, we conducted a methodological study to measure melatonin levels in serum, plasma, and urine samples and to assess the reproducibility of a currently available melatonin radioimmunoassay and the feasibility of applying this assay to epidemiologic studies. Further, we measured serum levels in Caucasian, African American, African, and Chinese men to assess ethnic differences in melatonin levels.
MATERIALS AND METHODS
Serum melatonin assays
To choose an acceptable assay for the study, we evaluated several commercial melatonin kits and concluded that the Buhlmann radioimmunoassay kit (ALPCO, Salem, NH) with a preceding extraction step is acceptable for measuring serum melatonin. This method was preferred over the direct assay because the extraction step can minimize interference from melatonin metabolites as well as remove lipids and other components that may be present in serum, which would result in overestimating melatonin levels. The Buhlmann RIA kit also employs the antibody raised by Kennaway and colleagues (18, 19), that has been validated by gas chromatography mass spectrometry (20). We used 60 samples to optimize the assay for both the standard (1.0 ml) and reduced (0.5 ml) serum volumes. Briefly, melatonin is purified from serum samples using reversed-phase columns, dried, and reconstituted in assay buffer; melatonin standards for the standard curve were processed similarly. Samples were then incubated with α-melatonin antibody and iodinated melatonin for 20 hours, precipitated for the antibody-bound fraction using a solid-phase second antibody for 15 minutes, and centrifuged. The unbound fraction was aspirated and the antibody-bound fraction of iodinated melatonin was counted using a gamma counter.
Urinary aMT6s assays
Urinary aMT6s (expressed as ng/mg Creatinine (Cr)) were measured using the standard (1 ml) protocol by a competitive enzyme-linked immunosorbent assay kit with a capture antibody technique (Buhlmann, Basel, Switzerland) as described previously (21). The assay was performed on a microtiter plate coated with polyclonal antibodies specific for rabbit immunoglobulin. Urinary Cr was measured colorimetrically using a standard procedure.
Study Samples
For the study, only blood and urine samples collected from healthy men without a history of cancer were included. All samples were collected for our other epidemiologic studies and processed following standard procedures; serum samples were derived from blood that was processed within 4 hours of collection and stored at −70°C and 24-hour urine was collected, processed, and stored similarly.
Serum and urine reproducibility assays
Initially, reproducibility of the serum melatonin assay using the standard 1.0 ml volume was assessed with triplicate samples from two pooled serum samples; pooled serum samples were created by combining serum collected before 12 AM from multiple Chinese men. Subsequently, reproducibility of the refined (0.5 ml) assay was assessed using replicate morning serum samples from 14 Caucasian men (2–4 replicates per sample). Reproducibility of urinary aMT6s assays using the standard protocol was assessed with triplicate urine samples from two Chinese men.
Serum-urine correlation
For comparison of urinary aMT6s and serum melatonin levels, urine-serum paired samples collected from 49 Chinese men were used for this purpose, applying the standard protocols (1-ml sample volumes). All serum samples were derived from blood collected before noon. Urine samples were collected for the same participants during a 24-hour period after blood collection.
Serum-plasma correlation
For comparison of melatonin levels in serum and plasma, serum-plasma paired samples from the same blood draw from 20 Caucasian men (13 morning blood draws and 6 afternoon blood draws) were measured using the refined (0.5 ml) assay.
Descriptive studies
To determine the serum melatonin profile by blood collection time, we measured serum melatonin levels in 43 Caucasian American men with blood drawn from one of four time blocks: 1) before 9:00 AM (n=18), 2) between 9:00 AM and 10:59 AM (n=15), 3) between 11:00 AM and 12:59 PM (n=5), and 4) 1:00 PM and later (n=5). To evaluate melatonin levels in various racial/ethnic groups, we measured morning serum melatonin in 50 Chinese, 35 Caucasian American, 11 African American, and 30 Ghanaian men. For subjects with replicate measurements, the mean of the replicate measurement was used for analysis; all replicate samples were derived from the same blood draw.
Statistical Analysis
We used two measures of reproducibility to determine the precision of the melatonin assays: the coefficient of variation (CV) and the intraclass correlation coefficient (ICC). The CV, expressed as a percent, is calculated as 100 times the ratio of the standard deviation to the mean: CV=100 × σ/μ. Small CVs mean less dispersion from the mean and thus better reproducibility. The ICC, expressed as a percent, is calculated as 100 times the ratio of the variance associated with subjects (σα2) to the sum of all variances (associated with subjects and other factors such as measurement error; σα2+σε2): ICC=100 × σα2/(σα2+σε2). In our experience, the useful assays have CVs that are less than 20% and ICCs greater than 80%; better assays have ICCs greater than 90%. Pearson’s correlation was used to measure the correlation between the paired serum and urine samples as well as between the paired serum and plasma samples.
RESULTS
Table 1 shows the means, standard errors, and medians of urine aMT6s, serum melatonin, and plasma melatonin levels in assays using either 1.0 or 0.5 ml of serum. The sensitivity of the melatonin radioimmunoassay using both 1.0 and 0.5 ml serum volumes was 0.5 pg/ml. Urinary aMT6s levels among 49 men ranged from 0.49 to 12.36 ng/mg Cr, with a mean of 3.89 ng/mg Cr (SE=2.27 ng/mg Cr). In the same 49 men, melatonin concentrations measured by the assay using 1.0 ml of serum ranged from 0.50 to 10.44 pg/ml, with a mean of 3.43 pg/ml (SD=2.41 pg/ml). Reproducibility for both urinary aMT6s and serum melatonin assays appears to be good, with mean CVs of 3.5% for the urine assays and 6.9% for serum assays. Reproducibility for serum melatonin assays using 0.5 ml of serum was similar to that using 1.0 ml of serum (mean CV=6.6%)and ICCs were greater than 97% for all assays. Serum and plasma levels were similar (plasma: mean=3.44 pg/mL, range=1.10–13.22 pg/mL; serum: 3.46 pg/mL, range=1.25–12.42 pg/mL).
Table 1.
Melatonin measurement in serum, urine, and plasma samples
| Assay using 1.0 ml of serum/urine* |
Assay using 0.5 ml of serum/plasma ‡ |
|||
|---|---|---|---|---|
| Urine (ng/mg Cr)† | Serum (pg/ml) | Plasma (pg/ml) | Serum (pg/ml) | |
| N | 49 | 49 | 20 | 20 |
| Mean (SE) | 3.89 (0.32) | 3.43 (0.34) | 3.44 (0.66) | 3.46 (0.66) |
| Minimum | 0.49 | 0.50 | 1.10 | 1.25 |
| Maximum | 12.36 | 10.44 | 13.22 | 12.42 |
| Median | 3.72 | 2.61 | 2.41 | 2.11 |
|
Replicate samples | ||||
| N | 2 subjects | 2 pooled samples | - | 14subjects |
| Replicates/sample | 3 | 3 | - | 2–4 |
| CV range (mean CV) | 2.8–4.1% (3.5%) | 5.7–8.1% (6.9%) | - | 1.7–14.2% (6.6%) |
| ICC | 99.9% | 97.4% | - | 99.0% |
Note: Cr Creatinine; CV coefficient of variation; ICC intraclass correlation coefficient
Paired urine-serum samples from the same subject; all subjects were Chinese men.
Melatonin in urine is measured in the form of 6-sulfatoxymelatonin and is adjusted for Cr
Paired plasma-serum samples from the same subject; all subjects were Caucasian American men.
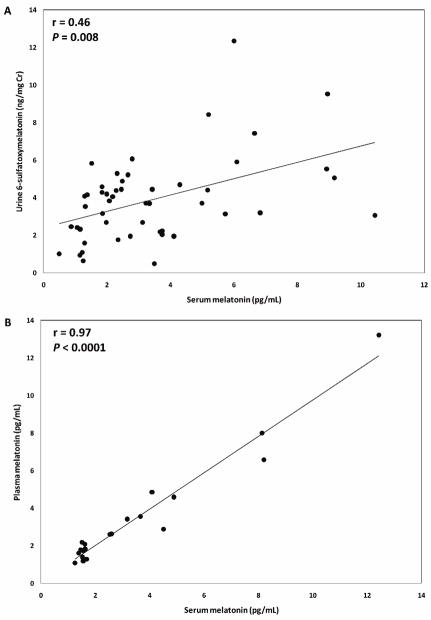
Although direct comparisons between absolute concentrations of urinary aMT6s and serum melatonin are difficult, due to differences in units of measure (ng/mg Cr vs. pg/mL, respectively), there was a positive and significant correlation between urine aMT6s and serum melatonin levels (r=0.46, P=0.008; Figure 1A). There was also a strong correlation between plasma and serum melatonin levels (r=0.97, P<1×10−4; Figure 1B).
Figure 1.
Correlations between urinary aMT6s and serum melatonin among 49 male subjects (A) and between plasma and serum melatonin among 20 male subjects (B)
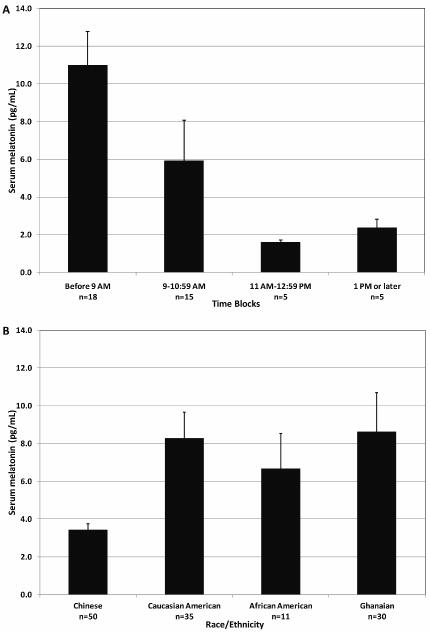
Melatonin levels were detectable in serum throughout the day (Figure 2A), although samples collected before 9 AM had levels that are five times higher than those collected after 11 am (mean=10.98 pg/mL, range: 1.61–26.67 pg/ml and mean=1.96 pg/ml, range: 1.25–4.08 pg/ml, respectively); these differences remained significant after adjusting for age (data not shown In addition, serum melatonin was detectable in all four racial groups, with Chinese men (mean=3.42 pg/mL) having lower serum levels, while Caucasian American (8.26 pg/mL), African American (6.67 pg/mL), and Ghanaian (8.61) men had similar levels (Figure 2B).
Figure 2.
Mean serum melatonin levels, by four time blocks in 35 Caucasian American men (A) and by race/ethnicity for measurements from serum collected before 12 PM (B). Error bars represent the standard errors. The refined 0.5 ml serum melatonin assay was used for all subjects except for Chinese subjects for whom melatonin was assayed using 1.0 ml of serum.
DISSCUSSION
In this methodological study, we showed that melatonin can be detected reliably in serum, plasma, and urine samples collected in the morning from healthy men by radioimmunoassays with preceding extraction. In addition, there is good correlation between urine and serum levels as well as plasma and serum melatonin levels, suggesting that these biological specimens may be used for epidemiologic studies to quantify melatonin levels. We further showed that serum melatonin is detectable throughout the day, although at much lower levels in the afternoon, and in men of different ethnic backgrounds. These data suggest that it is possible to measure melatonin in biological samples, in particular in serum samples, to test the emerging hypothesis that circadian rhythm may play a role in cancer etiology.
In our study, there producibility of the serum assay was good but is considerably lower than that of the urine assay, due largely to the lower concentration of melatonin in serum. Although the absolute melatonin levels measured in urine and serum differ (ng/mg Cr vs. pg/mL, respectively), the strong correlation between these two specimen types and between serum and plasma (r>0.46) suggests that when ranks or quartiles are used to categorize melatonin levels, measurements from each of these sample types are likely to distribute subjects into virtually identical classes, thereby yielding similar risk estimates for assessing the relationship between melatonin and cancer. Although we showed that the assay using 0.5 ml of serum has similar reproducibility as the 1.0 ml assay, for large-scale prospective studies, an even smaller amount is desirable.
Similar to data from previous studies that measured melatonin in the same subjects throughout the day (22–26), in our study, higher melatonin levels were found in subjects with blood collected in the morning hours as compared with those collected in the afternoon (up to a 5-fold difference). Although larger studies are needed to confirm these findings and to characterize further the determinants of circulating melatonin (e.g., age, gender, body size, lifestyle factors, metabolic factors, and hormones, etc.), these data underscore the importance of using morning samples for the measurement of melatonin and the need to control for differences in time of blood collection in epidemiologic studies.
Emerging data suggest that circadian rhythm disruption may play a role in the etiology of several cancers. Recently, the International Agency for Research on Cancer (IARC) concluded that “shift-work that involves circadian disruption is probably carcinogenic to humans” (1). For example, data from observational studies suggest that increased risks for prostate cancer are seen for men with exposure to light at night, occupations that can disrupt their circadian rhythms, such work on a rotating shift or as an airline pilot, and shorter sleep duration (2–7). Similar findings are also reported for breast cancer (8–11), colorectal cancer (12), and non-Hodgkin lymphoma (13). Genetic studies have shown that variants of circadian genes, which dictate the endogenous clock, may be associated with increased risk for prostate cancer (27, 28), breast cancer (29, 30), and non-Hodgkin lymphoma (31, 32). Together, these data suggest a potential role of circadian rhythms in several cancers, warranting further investigation.
It has been proposed that melatonin may be a key biological intermediary of chronodisruption (16). It is possible that cancer risk associated with chronodisruption may be due to low serum melatonin levels. Currently no epidemiologic study has addressed this hypothesis, due largely to the lack of reliable assays with high sensitivity to detect relatively low melatonin levels in serum collected during daylight. Although future studies are needed to determine if our findings are generalizable to women, our data suggest that with improved assays, it may be possible to investigate the role of serum melatonin and the emerging circadian rhythm hypothesis in cancer etiology in epidemiologic studies.
Acknowledgments
This research was supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department of Health and Human Services.
We thank Dr. Frank Z. Stanczyk and Ms. Lilly Cheng of the University of Southern California for melatonin assays; Dr. Karen Stewart and Ms. Ann Truelove of Westat for help with study management; and Dr. B.J. Stone for expert editorial assistance. Most importantly, we acknowledge the study participants for their contributions to making this study possible. This research was supported by the Intramural Research Program of the NIH, National Cancer Institute.
Abbreviation
- aMT6s
6-sulfatoxymelatonin
- Cr
creatinine
- CV
coefficient of variation
- ICC
intraclass correlation coefficient
References
- 1.Straif K, Baan R, Grosse Y, et al. Carcinogenicity of shift-work, painting, and fire-fighting. The Lancet Oncology. 2007;8:1065–6. doi: 10.1016/S1470-2045(07)70373-X. [DOI] [PubMed] [Google Scholar]
- 2.Kubo T, Ozasa K, Mikami K, et al. Prospective cohort study of the risk of prostate cancer among rotating-shift workers: findings from the Japan Collaborative Cohort Study. Am J Epidemiol. 2006;164:549–55. doi: 10.1093/aje/kwj232. [DOI] [PubMed] [Google Scholar]
- 3.Conlon M, Lightfoot N, Kreiger N. Rotating shift work and risk of prostate cancer. Epidemiology. 2007;18:182–3. doi: 10.1097/01.ede.0000249519.33978.31. [DOI] [PubMed] [Google Scholar]
- 4.Band PR, Le ND, Fang R, et al. Cohort study of Air Canada pilots: mortality, cancer incidence, and leukemia risk. Am J Epidemiol. 1996;143:137–43. doi: 10.1093/oxfordjournals.aje.a008722. [DOI] [PubMed] [Google Scholar]
- 5.Irvine D, Davies DM. British Airways flight deck mortality study, 1950–1992. Aviat Space Environ Med. 1999;70:548–55. [PubMed] [Google Scholar]
- 6.Pukkala E, Aspholm R, Auvinen A, et al. Cancer incidence among 10,211 airline pilots: a Nordic study. Aviation, space, and environmental medicine. 2003;74:699–706. [PubMed] [Google Scholar]
- 7.Kakizaki M, Inoue K, Kuriyama S, et al. Sleep duration and the risk of prostate cancer: the Ohsaki Cohort Study. Br J Cancer. 2008;99:176–8. doi: 10.1038/sj.bjc.6604425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schernhammer ES, Laden F, Speizer FE, et al. Rotating night shifts and risk of breast cancer in women participating in the Nurses’ Health Study. J Natl Cancer Inst. 2001;93:1563–8. doi: 10.1093/jnci/93.20.1563. [DOI] [PubMed] [Google Scholar]
- 9.Schernhammer ES, Kroenke CH, Laden F, Hankinson SE. Night work and risk of breast cancer. Epidemiology. 2006;17:108–11. doi: 10.1097/01.ede.0000190539.03500.c1. [DOI] [PubMed] [Google Scholar]
- 10.Kakizaki M, Kuriyama S, Sone T, et al. Sleep duration and the risk of breast cancer: the Ohsaki Cohort Study. Br J Cancer. 2008;99:1502–5. doi: 10.1038/sj.bjc.6604684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Megdal SP, Kroenke CH, Laden F, Pukkala E, Schernhammer ES. Night work and breast cancer risk: a systematic review and meta-analysis. Eur J Cancer. 2005;41:2023–32. doi: 10.1016/j.ejca.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Schernhammer ES, Laden F, Speizer FE, et al. Night-shift work and risk of colorectal cancer in the Nurses’ Health Study. J Natl Cancer Inst. 2003;95:825–8. doi: 10.1093/jnci/95.11.825. [DOI] [PubMed] [Google Scholar]
- 13.Lahti TA, Partonen T, Kyyronen P, Kauppinen T, Pukkala E. Night-time work predisposes to non-Hodgkin lymphoma. Int J Cancer. 2008;123:2148–51. doi: 10.1002/ijc.23566. [DOI] [PubMed] [Google Scholar]
- 14.Arendt J, Skene DJ. Melatonin as a chronobiotic. Sleep Med Rev. 2005;9:25–39. doi: 10.1016/j.smrv.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Cardinali DP, Pevet P. Basic aspects of melatonin action. Sleep Med Rev. 1998;2:175–90. doi: 10.1016/s1087-0792(98)90020-x. [DOI] [PubMed] [Google Scholar]
- 16.Erren TC, Reiter RJ. Defining chronodisruption. J Pineal Res. 2009;46:245–7. doi: 10.1111/j.1600-079X.2009.00665.x. [DOI] [PubMed] [Google Scholar]
- 17.Arendt J, Bojkowski C, Franey C, Wright J, Marks V. Immunoassay of 6-hydroxymelatonin sulfate in human plasma and urine: abolition of the urinary 24-hour rhythm with atenolol. J Clin Endocrinol Metab. 1985;60:1166–73. doi: 10.1210/jcem-60-6-1166. [DOI] [PubMed] [Google Scholar]
- 18.Earl CR, D’Occhio MJ, Kennaway DJ, Seamark RF. Serum Melatonin Profiles and Endocrine Responses of Ewes Exposed to a Pulse of Light Late in the Dark Phase. Endocrinology. 1985;117:226–30. doi: 10.1210/endo-117-1-226. [DOI] [PubMed] [Google Scholar]
- 19.Voultsios A, Kennaway DJ, Dawson D. Salivary melatonin as a circadian phase marker: validation and comparison to plasma melatonin. J Biol Rhythms. 1997;12:457–66. doi: 10.1177/074873049701200507. [DOI] [PubMed] [Google Scholar]
- 20.Lewy AJ, Markey SP. Analysis of melatonin in human plasma by gas chromatography negative chemical ionization mass spectrometry. Science. 1978;201:741–3. doi: 10.1126/science.675255. [DOI] [PubMed] [Google Scholar]
- 21.Wu AH, Wang R, Koh W-P, Stanczyk FZ, Lee H-P, Yu MC. Sleep duration, melatonin and breast cancer among Chinese women in Singapore. Carcinogenesis. 2008;29:1244–8. doi: 10.1093/carcin/bgn100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson C, Franey C, Arendt J, Checkley SA. A comparison of melatonin secretion in depressed patients and normal subjects. Br J Psychiatry. 1988;152:260–5. doi: 10.1192/bjp.152.2.260. [DOI] [PubMed] [Google Scholar]
- 23.Shochat T, Luboshitzky R, Lavie P. Nocturnal melatonin onset is phase locked to the primary sleep gate. Am J Physiol. 1997;273:R364–70. doi: 10.1152/ajpregu.1997.273.1.R364. [DOI] [PubMed] [Google Scholar]
- 24.Benloucif S, Guico MJ, Reid KJ, Wolfe LF, L’Hermite-Baleriaux M, Zee PC. Stability of melatonin and temperature as circadian phase markers and their relation to sleep times in humans. J Biol Rhythms. 2005;20:178–88. doi: 10.1177/0748730404273983. [DOI] [PubMed] [Google Scholar]
- 25.Kumanov P, Tomova A, Isidori A, Nordio M. Altered melatonin secretion in hypogonadal men: clinical evidence. Int J Androl. 2005;28:234–40. doi: 10.1111/j.1365-2605.2005.00534.x. [DOI] [PubMed] [Google Scholar]
- 26.Robeva R, Kirilov G, Tomova A, Kumanov P. Low testosterone levels and unimpaired melatonin secretion in young males with metabolic syndrome. Andrologia. 2006;38:216–20. doi: 10.1111/j.1439-0272.2006.00743.x. [DOI] [PubMed] [Google Scholar]
- 27.Chu LW, Zhu Y, Yu K, et al. Variants in circadian genes and prostate cancer risk: a population-based study in China. Prostate Cancer Prostatic Dis. 2007;11:342–8. doi: 10.1038/sj.pcan.4501024. [DOI] [PubMed] [Google Scholar]
- 28.Chu LW, Li Q, Yu K, et al. Joint effects of circadian genes and serum androgens on prostate cancer risk in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Annual Meeting of the American Society of Human Genetics; 2008 November 11–15; Philadelphia, PA. 2008. [Google Scholar]
- 29.Zhu Y, Brown HN, Zhang Y, Stevens RG, Zheng T. Period3 structural variation: a circadian biomarker associated with breast cancer in young women. Cancer Epidemiol Biomarkers Prev. 2005;14:268–70. [PubMed] [Google Scholar]
- 30.Zhu Y, Stevens R, Leaderer D, et al. Non-synonymous polymorphisms in the circadian gene NPAS2 and breast cancer risk. Breast Cancer Res Treat. 2008;107:421–5. doi: 10.1007/s10549-007-9565-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu Y, Leaderer D, Guss C, et al. Ala394Thr polymorphism in the clock gene NPAS2: A circadian modifier for the risk of non-Hodgkin’s lymphoma. Int J Cancer. 2007;120:432–5. doi: 10.1002/ijc.22321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoffman AE, Zheng T, Stevens RG, et al. Clock-cancer connection in non-Hodgkin’s lymphoma: a genetic association study and pathway analysis of the circadian gene cryptochrome 2. Cancer Res. 2009;69:3605–13. doi: 10.1158/0008-5472.CAN-08-4572. [DOI] [PMC free article] [PubMed] [Google Scholar]