Abstract
The myelin sheath allows axons to rapidly conduct action potentials in the vertebrate nervous system. Incompletely understood axonal signals activate specific transcription factors, including Oct6 and Krox20, that initiate myelination in Schwann cells. Elevation of cAMP can mimic axonal contact in vitro, but the mechanisms that regulate cAMP levels in vivo are unknown. Using mutational analysis in zebrafish, we report that Gpr126 is required autonomously in Schwann cells for myelination. In gpr126 mutants, Schwann cells failed to express oct6 and krox20, and were arrested at the promyelinating stage. Elevation of cAMP in gpr126 mutants, but not krox20 mutants, could restore myelination. We propose that Gpr126 drives the differentiation of promyelinating Schwann cells by elevating cAMP levels, thereby triggering Oct6 expression and myelination.
During peripheral nervous system (PNS) development, promyelinating Schwann cells associate with one segment of an axon and differentiate into myelinating Schwann cells that iteratively wrap their membrane around an axonal segment to form the myelin sheath (1). Axonal signals transiently activate the expression of the transcription factor Oct6 in Schwann cells that will form myelin, and cAMP can mimic axonal contact in vitro (2, 3). Oct6 regulates Krox20 expression (4), and both transcription factors are required for Schwann cells to initiate myelination (5–7). Neuregulin signals and their ErbB receptors are involved in regulation of Oct6 and Krox20 (8), but the signaling pathways in Schwann cells that regulate myelination are not well understood.
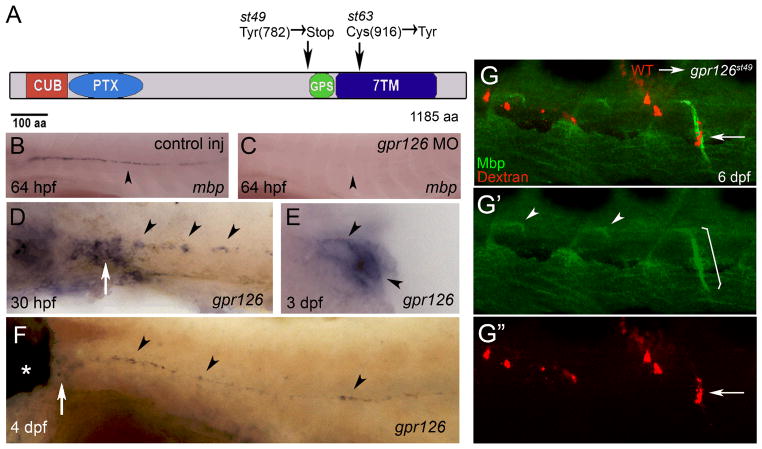
In a genetic screen for zebrafish mutants with abnormalities in myelinated axons, we previously identified two allelic mutations, st49 and st63, in which Myelin basic protein (Mbp) expression was not observed in peripheral nerves (9). Central nervous system (CNS) Mbp expression and PNS axonal marker expression were unaffected (9; Fig. S1). Except for an enlargement of the ear that was evident at 5 days post fertilization (dpf), st49 homozygous mutant larvae were morphologically indistinguishable from wild-type and heterozygous siblings (Fig. S2). High-resolution mapping experiments placed the st49 mutation in a region of Linkage Group 20 (LG20) that contains g-protein coupled receptor 126 (gpr126), which encodes a member of the adhesion G-protein coupled receptor (GPCR) family (10, 11). In addition to a type II seven transmembrane domain (7TM), Gpr126 contains CUB (Complement, Uegf, Bmp1), Pentraxin (PTX), and GPCR proteolytic site (GPS) domains (12; Fig. 1A). By sequencing, we identified lesions in gpr126 in both mutations: st49 introduces a premature stop codon before the GPS domain, and st63 changes a highly conserved cysteine to a tyrosine in the 7TM domain (Fig. 1A). All mutants tested were homozygous for their respective lesions (st49, n=105; st63, n=122). In addition, injection of wild-type embryos with a morpholino that blocks splicing of gpr126 pre-mRNA phenocopied gpr126 mutants (Fig. 1B, C; Fig. S3). These results provide strong evidence that gpr126 is the gene disrupted by the st49 and st63 mutations.
Fig. 1.
The st49 and st63 lesions disrupt zebrafish gpr126, which is required autonomously in Schwann cells for Mbp expression. (A) Schematic representation of Gpr126 showing functional domains and the lesions in the st49 and st63 mutations. (B) WT zebrafish embryo injected with control morpholino (MO) showing mbp mRNA in PLLn Schwann cells at 64 hpf (n=13/13). (C) WT zebrafish embryo injected with gpr126 MO showing lack of mbp mRNA in PLLn Schwann cells at 64 hpf (n=19/21). (D–F) Expression of gpr126 in wild-type (WT) larvae examined by whole-mount in situ hybridization. (D) gpr126 mRNA in PLLn Schwann cells (arrowheads) and PLL ganglion (PLLg; arrow) at 30 hpf. (E) Cross-section of a WT larva at 3 dpf showing gpr126 expression in PLLn Schwann cells (arrowheads). (F) gpr126 expression in the ear (*), PLLg (arrow), and in PLLn Schwann cells (arrowheads) at 4 days postfertilization (dpf). For (B–D and F), lateral views are shown, with anterior left and dorsal top. (G) Lateral views at 6 dpf of a chimeric larva generated by transplantation of Texas red dextran-labeled WT cells (red) into a gpr126st49 mutant. WT Schwann cells (arrow in G, G”) express Mbp (green) when associated with mutant motor axons (bracket in G′). In motor nerves without transplanted WT Schwann cells, Mbp expression is only observed at motor nerve exit points as described in Fig. S1 (arrowheads, G′).
By in situ hybridization from 30 hours post fertilization (hpf) to 4 dpf, we observed expression of gpr126 mRNA in Schwann cells of the posterior lateral line nerve (PLLn), a prominent sensory nerve in zebrafish (Fig. 1D-F), and in other sites (Fig. S4). Additionally, we detected gpr126 expression in adult PLLn by RT-PCR (Fig. S4). Thus, gpr126 is expressed in Schwann cells in the embryo, and this expression persists into adulthood.
To test whether Gpr126 was required autonomously in Schwann cells, we created genetic chimeras by transplanting fluorescently labeled wild-type cells into gpr126st49 mutant animals at blastula stages. In sensory and motor nerves of eleven such chimeras, Schwann cells expressed Mbp; in all of these cases, all Mbp-expressing Schwann cells were wild-type (Fig. 1G). In five of the eleven chimeras, we observed wild-type Schwann cells, but no wild-type neurons, associated with rescued nerves. This indicates that wild-type Schwann cells can activate Mbp expression when associated with gpr126st49 mutant neurons. In addition, we observed >40 cases in which wild-type neurons alone failed to rescue Mbp expression in gpr126st49 mutant Schwann cells (Fig. S6). In accord with the expression of gpr126 in Schwann cells, the analysis of chimeras indicates that Gpr126 functions autonomously in Schwann cells.
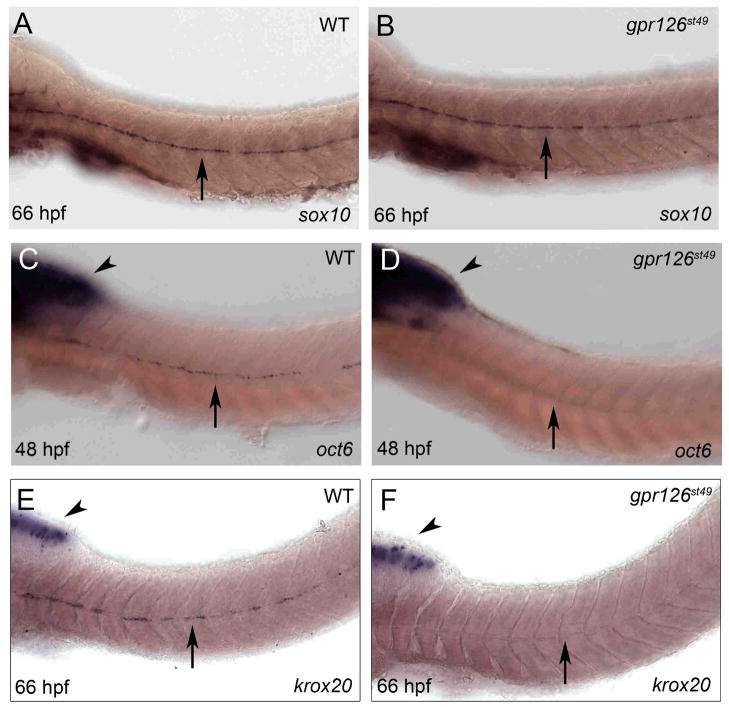
To define the function of Gpr126 in myelination, we analyzed markers for different stages of Schwann cell development in gpr126st49 mutants. Expression of sox10, which marks neural crest-derived cells, including Schwann cells, was not disrupted at any stage (Fig. 2A, B; Fig. S7). The earliest defect we noted was at 42–48 hpf, when PLLn Schwann cells begin to express oct6 in wild-type larvae. We did not observe oct6 expression in Schwann cells in gpr126st49 mutants at any stage (Fig. 2C, D; Fig. S7). Additionally, krox20 expression was first detected in PLLn Schwann cells in wild-type larvae at 54–60 hpf, but was not observed in Schwann cells of gpr126st49 mutants (Fig. 2E, F; Fig. S7). This analysis indicates that gpr126 is required for differentiating Schwann cells to initiate expression of oct6 and krox20.
Fig. 2.
gpr126 is essential for expression of oct6 and krox20 in Schwann cells. Whole mount in situ hybridization of (A) WT and (B) gpr126st49 mutant zebrafish larvae at 66 hpf showing sox10 mRNA expression in PLLn Schwann cells (arrow). (C) WT zebrafish embryo at 48 hpf showing oct6 expression in the brain (arrowhead) and in PLLn Schwann cells (arrow) as previously described (22). (D) gpr126st49 mutant embryo at 48 hpf showing normal oct6 expression in the brain (arrowhead) but not in PLLn Schwann cells (arrow). (E) WT zebrafish larva at 66 hpf showing krox20 mRNA expression in the brain (arrowhead) and in PLLn Schwann cells (arrow). (F) gpr126st49 mutant larva at 66 hpf showing normal krox20 expression in the brain (arrowhead) but not in PLLn Schwann cells (arrow). For (A–F), anterior is left and dorsal is up. Genotypes were determined by PCR after photography; see Fig. S7 for quantification of these experiments.
Neuregulin1 (Nrg) signaling through ErbB2 and ErbB3 receptors is required for many steps of Schwann cell development, including myelination (8; 13–17). We therefore examined erbb3 and nrg1 type III expression in gpr126st49 mutants and found that this expression was not disrupted (Fig. S8). Additionally, gpr126 expression was not disrupted in the ganglia of erbb2st61 zebrafish mutants, in which Schwann cells reach the ganglia but do not co-migrate with growing axons of the PLLn (15; Fig. S8). These data show that Gpr126 does not regulate erbb3 or nrg1 type III expression and that Nrg1/ErbB signals do not regulate gpr126 expression; these signals may therefore function independently to regulate myelination.
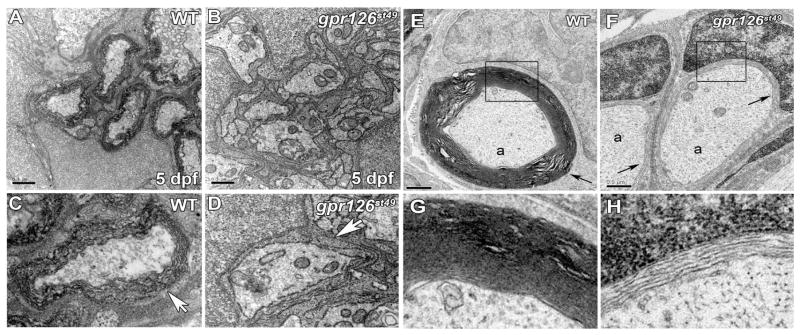
Our marker studies indicated that early steps in Schwann cell development were normal in gpr126st49 mutants and suggested that Schwann cells are arrested at the promyelinating stage. To test this hypothesis, we examined the ultrastructure of gpr126st49 mutant PLL nerves by transmission electron microscopy (TEM). At 5 dpf in wild-type and gpr126st49/+ heterozygotes, many PLLn axons were surrounded by several wraps of myelin (Fig. 3A, C). In contrast, no myelin was observed in the PLLn of gpr126st49 mutants (Fig. 3B, D). In the mutants, Schwann cells associated with axons, but myelination did not progress beyond 1.5 wraps of uncompacted Schwann cell cytoplasm. This phenotype was also evident in motor nerves (Fig. S9). Ultrastructural studies confirmed that early stages of Schwann cell development appeared normal in gpr126st49 mutants (Fig. S10). Additionally, total axon number and the number of large-caliber axons ensheathed by promyelinating Schwann cells were normal at all stages in gpr126st49 mutants (Fig. 3B; Fig. S10). Thus, gpr126 is essential for promyelinating Schwann cells to initiate myelination in both sensory and motor nerves.
Fig. 3.
Schwann cells sort, but do not myelinate axons in gpr126st49 mutants. (A) Transmission electron micrograph showing cross-section through a WT PLLn at 5 dpf. (B) Transmission electron micrograph showing cross-section through a gpr126st49 mutant PLLn at 5 dpf. (C) Magnified view of a WT axon wrapped by several layers of myelin (arrow). (D) Magnified view of a gpr126st49 mutant axon showing that Schwann cell cytoplasm surrounds the axon (arrow), but does not turn more than 1.5 times around the axon. For (A, B), scale bar = 0.5 μm. (E) Transmission electron micrograph showing cross-section through a WT PLLn at 6 months of age. An axon (a) surrounded by compact myelin (arrow) is shown. (F) Transmission electron micrograph showing cross-section through a gpr126st49 mutant PLLn at 6 months of age. Axons (a) surrounded by a few loose wraps of Schwann cell cytoplasm (arrow) are shown. For (E) and (F), scale bar = 0.5 μm. (G) Magnified view of boxed region in (E) showing compact myelin surrounding an axon in WT PLLn. (H) Magnified view of boxed region in (F) showing loose Schwann cell cytoplasm surrounding an axon in gpr126st49 mutant PLLn. See Fig. S10 for quantification of these experiments.
Homozygous gpr126st49 mutants survive to adulthood and are viable and fertile. To determine if a myelin defect persisted in adult gpr126st49 mutants, we examined PLL nerves at four months and six months of age. At both stages, dissected PLL nerves of gpr126st49 mutants were thinner and less opaque than PLL nerves from wild-type and gpr126st49/+ zebrafish (Fig. S9). By TEM analysis, we found that axons in wild-type animals were surrounded by compact myelin at six months of age (Fig. 3E, G), but we found no evidence of compact myelin in gpr126st49 adult zebrafish (Fig. 3F, H) at the same stage. The absence of myelin at six months of age in gpr126st49 mutant PLL nerves indicates that the requirement for gpr126 in Schwann cell myelination persists into adulthood.
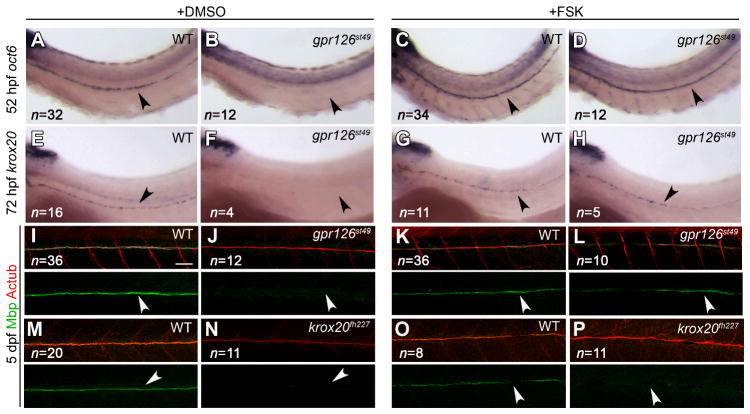
In cultured Schwann cells, intracellular increase of cAMP mimics axonal contact and drives differentiation (18). However, the mechanism by which cAMP is elevated in Schwann cells in vivo is unknown. Many GPCRs signal through cAMP to activate downstream targets; we therefore treated gpr126st49 mutants with forskolin to elevate cAMP. Treatment with forskolin restored oct6, krox20, and Mbp expression (Fig. 4AL) and ultrastructurally normal myelin (Fig. S11) in gpr126st49 mutants, suggesting that Gpr126 functions to drive myelination by elevating cAMP levels in Schwann cells. Because Krox20 is activated downstream of cAMP elevation, we would not expect forskolin treatment to rescue Krox20 mutants. As a control, we therefore generated zebrafish krox20 mutants by TILLING (19). Like murine Krox20 mutants, Schwann cells in krox20fh227 mutants are arrested at the promyelinating stage (7; Fig. S9); as expected, forskolin treatment did not rescue Mbp expression in krox20fh227 mutants (Fig. 4M–P). These data support the hypothesis that Gpr126 functions in Schwann cells to elevate levels of cAMP, thereby activating oct6 and krox20 expression to initiate myelination.
Fig. 4.
Treatment with forskolin rescues gpr126st49 mutant phenotypes. (A–D) oct6 expression in 52 hpf zebrafish larvae treated with DMSO (+DMSO) or forskolin (+FSK) from 45–52 hpf. Schwann cells in gpr126st49 mutants express oct6 after FSK treatment (D), but not after DMSO treatment (B). (E–H) krox20 expression in 72 hpf zebrafish larvae treated with DMSO or FSK from 45–52 hpf. Schwann cells in gpr126st49 mutants express krox20 after FSK treatment (H), but not after DMSO treatment (F). (I–P) Myelin basic protein (Mbp; green) and Acetylated Tubulin (AcTub; red) expression in 5 dpf zebrafish larvae treated with DMSO or FSK from 45–52 hpf. Schwann cells in gpr126st49 mutants express Mbp after FSK treatment (L), but not after DMSO treatment (J). Schwann cells in krox20fh227 mutants do not express Mbp after DMSO (N) or FSK (P) treatment. For (A–P), the PLLn is indicated with an arrowhead, and the number of larvae examined in each experiment is shown in the lower left of all panels. For (I–P), scale bar = 50 μm.
We demonstrate that Gpr126 is essential for Schwann cells to initiate myelination. Like most adhesion GPCRs, Gpr126 is an orphan receptor that has not been shown to interact with G-proteins. Previously, a biochemical study raised the possibility that Gpr126 functions as a diffusible signal (20). Our data, however, suggest that Gpr126 acts as a receptor in Schwann cells that signals through G-proteins to transiently elevate cAMP. In Schwann cells, cAMP has been shown to activate a cascade including PKA, NF-κB, and CREB to induce transcription of oct6 (21; Fig. S12). Our data show that Gpr126 acts autonomously in Schwann cells, and that forskolin treatment is sufficient to restore myelination in gpr126st49 mutants. We show that gpr126 is expressed independently of Nrg1/ErbB signals, and propose that Gpr126 elevates cAMP in Schwann cells following axonal contact to trigger myelination.
Supplementary Material
Acknowledgments
We thank members of the Talbot laboratory for helpful discussions and suggestions. We thank T. Reyes and C. Hill for fish facility support and J. Perrino and J. Buchanan for electron microscopy advice. We thank E. Wolf-Saxon and T. Donn for identifying the krox20fh227 mutation with the support of NIH grant HG002995 to C.B.M. This work was also supported by a postdoctoral award from the NMSS to K.R.M. (FG 1719-A-1), by a Stanford NIH Training Grant to T.D.G., by a fellowship from Telethon Italy to S.M. (GFP03011), by a Stanford Gabilan Fellowship to J.R.P., and by grants from the NIH (R01 NS050223) and the MDA (MDA 92233) to W.S.T. The GenBank accession number for zebrafish gpr126 is GQ202546.
Footnotes
References and Notes
- 1.Jessen KR, Mirksy R. Nat Rev Neurosci. 2005;6:671. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- 2.Monuki ES, Weinmaster G, Kuhn R, Lemke G. Neuron. 1989;3:783. doi: 10.1016/0896-6273(89)90247-x. [DOI] [PubMed] [Google Scholar]
- 3.Scherer SS, et al. J Neurosci. 1994;14:1930. doi: 10.1523/JNEUROSCI.14-04-01930.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zorick T, et al. Development. 1999;126:1397. doi: 10.1242/dev.126.7.1397. [DOI] [PubMed] [Google Scholar]
- 5.Jaegle M, et al. Science. 1996;273:507. doi: 10.1126/science.273.5274.507. [DOI] [PubMed] [Google Scholar]
- 6.Bermingham JR, Jr, et al. Genes Dev. 1996;10:1751. doi: 10.1101/gad.10.14.1751. [DOI] [PubMed] [Google Scholar]
- 7.Topilko P, et al. Nature. 1994;371:796. doi: 10.1038/371796a0. [DOI] [PubMed] [Google Scholar]
- 8.Taveggia C, et al. Neuron. 2005;47:681. doi: 10.1016/j.neuron.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pogoda HM, et al. Dev Biol. 2006;298:118. doi: 10.1016/j.ydbio.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 10.Fredriksson R, et al. Biochem Biophys Res Commun. 2003;301:725. doi: 10.1016/s0006-291x(03)00026-3. [DOI] [PubMed] [Google Scholar]
- 11.Bjarnadottir TK, et al. Genomics. 2004;84:23. doi: 10.1016/j.ygeno.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Stehlik C, et al. FEBS Lett. 2004;569:149. doi: 10.1016/j.febslet.2004.05.038. [DOI] [PubMed] [Google Scholar]
- 13.Garratt AN, et al. J Cell Biol. 2000;148:1035. doi: 10.1083/jcb.148.5.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michailov GV, et al. Science. 2004;304:700. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- 15.Lyons DA, et al. Curr Biol. 2005;15:513. doi: 10.1016/j.cub.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 16.Chen S, et al. J Neurosci. 2006;26:3079. doi: 10.1523/JNEUROSCI.3785-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sherman DL, Brophy PJ. Nat Rev Neurosci. 2005;6:683. doi: 10.1038/nrn1743. [DOI] [PubMed] [Google Scholar]
- 18.Jessen KR, Mirsky R, Morgan L. Ann N Y Acad Sci. 1991;633:78. doi: 10.1111/j.1749-6632.1991.tb15597.x. [DOI] [PubMed] [Google Scholar]
- 19.Moens CB, Donn TM, Wolf-Saxon ER, Ma TP. Brief Funct Genomics Proteomics. 2008;7:454. doi: 10.1093/bfgp/eln046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moriguchi T, et al. Genes Cells. 2004;9:549. doi: 10.1111/j.1356-9597.2004.00743.x. [DOI] [PubMed] [Google Scholar]
- 21.Svaren J, Meijer D. Glia. 2008;56:1541. doi: 10.1002/glia.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levavasseur F, et al. Mech Dev. 1998;74:89. doi: 10.1016/s0925-4773(98)00067-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.