Abstract
The primary aim of this review is to examine evidence for a functional role of gamma and theta oscillations in human episodic memory. It is proposed here that gamma and theta oscillations allow for the transient interaction between cortical structures and the hippocampus for the encoding and retrieval of episodic memories as described by the hippocampal memory indexing theory (Teyler, T. J., & DiScenna, P. (1986). The hippocampal memory indexing theory. Behavioral Neuroscience, 100, 147–154). Gamma rhythms can act in the cortex to bind perceptual features and in the hippocampus to bind the rich perceptual and contextual information from diverse brain regions into episodic representations. Theta oscillations act to temporally order these individual episodic memory representations. Through feedback projections from the hippocampus to the cortex these gamma and theta patterns could cause the reinstatement of the entire episodic memory representation in the cortex. In addition, theta oscillations could allow for top-down control from the frontal cortex to the hippocampus modulating the encoding and retrieval of episodic memories.
1. Introduction
Research in cognitive neuroscience thus far has focused on the functional role of particular brain regions. However, to perform the myriad of cognitive tasks that make us human it is recognized that particular brain regions do not work alone, but interact through complex and dynamic neural networks. A major question currently faced by cognitive neuroscientists is: How do the functionally specialized brain areas interact to perform rich cognitive abilities (Başar & Schürmann, 2001; Miller & Wilson, 2008; Varela, Lachaux, Rodriguez, & Martinerie, 2001)? Neural assemblies involve neurons with reciprocal connections that interact at different spatial scales. Neurons in local populations process information whereas larger neural assemblies, including neurons connected across separate brain areas, combine multiple processes that are important for higher-level cognitive tasks. Because cognitive demands change over time, we must not only look at slowly changing anatomical connections but also at quickly changing (transient) interactions between neural assemblies. Both local and global neural assemblies can transiently interact with each other to perform immediate cognitive tasks. Neural oscillations could allow for transient neural network interactions responsible for complex cognitive tasks.
Fluctuations in postsynaptic potentials produce local oscillations. In addition, oscillators in one brain region can phase synchronize with oscillators in another region through long-range connections. A mechanism for interaction for both local populations of neurons and large neural assemblies is through phase synchronization of oscillations. As neurons oscillate, they effectively open and close their window to both send and receive information (Buzsáki & Draguhn, 2004; Womelsdorf et al., 2007). For information to be transferred from one neuronal group to another, the sending neuron must be excitable at the same time that the receiving group is excitable. This requires the coupling of oscillations between sending and receiving neurons through phase synchronization (Fries, 2005). This pattern of neural interaction allows for efficient neural communication through the transient coupling of neurons firing synchronously forming functional neural networks. It has been shown that the temporal coincidence of postsynaptic potentials has a greater impact on postsynaptic firing than non-coincident firing (Salinas & Sejnowski, 2001). In addition, this pattern of interaction causes precise timing of input and output from sending and receiving neurons (Mainen & Sejnowski, 1995). Therefore, neurons must bundle their signal by firing together synchronously to effectively send their signal to a target neuron. Neural assemblies interact at different temporal scales. In local neural assemblies, with close connections between neurons, communication can happen during faster, higher frequencies. In large-scale brain assemblies, with long-range connections between neurons, communication happens during slower, lower frequencies (Fries, 2005). There is also evidence that different bands of activity can multiplex such that slower oscillations provide a framework for other faster oscillations to operate such that fast oscillations communicate content while slow oscillations mediate transient connectivity (Buzsáki & Draguhn, 2004; Fries, 2005; Varela, Lachaux, Rodriguez, & Martinerie, 2001; Ward, Doesburg, Kitajo, MacLean, & Roggeveen, 2006).
Research has shown that synchronized neural firing can allow for the transient interaction between visual areas for visual perception. It is proposed here that neural oscillations not only allow for synchronized neural firing for the transient interaction between brain areas for perceptual tasks but also for complex cognitive tasks. The following provides a general model of how neural oscillations can allow for synchronized neural firing for the transient interactions important for complex cognitive tasks. During a cognitive task oscillations can allow for local neurons to fire synchronously at high frequencies to process information. Multiple processes can then be combined through large-scale brain networks whose oscillations become phase synchronized causing neurons within these large-scale brain networks to fire synchronously. Transient linking through synchronous firing provides a mechanism to amplify relevant processing (synchronously firing neurons) and filter out irrelevant neural firing (asynchronously firing noise). When processing is complete, phase dysynchronization of oscillations can cause neurons to stop firing synchronously. New brain networks whose oscillations become phase synchronized can then cause the same neurons to synchronously fire with other neurons to form a different neural assembly to perform a different cognitive task. Therefore, oscillations provide a robust mechanism for neurons to effectively form transient neural networks depending on the demands of the present cognitive task.
Episodic memory is a complex cognitive function that involves many interacting brain regions and allows us to store the content of experience that can be retrieved later. Episodic memory is unique from semantic memory in that it stores contextual (spatial and temporal) information about individual events that can be later retrieved. In addition, episodic memories are robust to interference allowing us to store and retrieve separate but similar experiences. Although episodic and semantic memories are distinct, semantic memories may be originally encoded through episodic processes and the encoding of episodic memories may rely on semantic elaboration. By this definition all of the studies reviewed employ episodic memory tasks.
Neural oscillations have been linked to various cognitive phenomena in humans. There is not an exact mapping of oscillatory rhythms to specific cognitive processes. Instead neural oscillations in a specific frequency range in one brain region may function in one cognitive process and in another cognitive process in another brain region. In addition, different neural oscillations can each function in a specific cognitive phenomenon (Başar, Başar-Eroglu, Karakas, & Schürmann, 1999; Kahana, 2006; Klimesch, Freunberger, Sauseng, & Gruber, 2008). The primary aim of this review will be to examine evidence for a functional role of gamma and theta oscillations in human episodic memory systems.
The review will focus on gamma and theta rhythms because recent evidence has suggested a functional role of gamma (25–100 Hz) and theta (4–8 Hz) oscillations in episodic memory. Other rhythms such as delta (1–4 Hz), alpha (8–12 Hz), and beta rhythms (15 Hz) will not be discussed because they have not been consistently reported as being related to episodic memory and the cortical-hippocampal network. Delta and beta rhythms have been reported in only a few studies (Düzel et al., 2003; Düzel, Neufang, & Heinze, 2005; Hanslmayr, Spitzer, & Bauml, 2009; Klimesch, Doppelmayr, Schwaiger, Winkler, & Gruber, 2000; Mormann et al., 2005; Sederberg, Schulze-Bonhage, Madsen, Bromfield, Litt et al., 2007; Sederberg, Schulze-Bonhage, Madsen, Bromfield, McCarthy et al., 2007; Weiss & Rappelsberger, 2000), but do not always correlate with episodic memory (Doppelmayr, Klimesch, Schwaiger, Auinger, & Winkler, 1998; Hanslmayr, Spitzer, & Bauml, 2009; Klimesch, Doppelmayr, Yonelinas et al., 2001; Klimesch, Freunberger, Sauseng, & Gruber, 2008; Osipova et al., 2006).
Separating the neural rhythms involved in semantic and episodic memory is often difficult as episodic tasks often include semantically meaningful items. There is convincing evidence that different neural rhythms contribute to semantic and episodic memory. The alpha rhythm has been related to semantic memory because it consistently phase desynchronizes with the presentation of semantically related items (Klimesch, 1996, 1999; Klimesch, Doppelmayr, Schimke, & Ripper, 1997; Klimesch, Freunberger, Sauseng, & Gruber, 2008; Klimesch et al., 2004; Klimesch, Schimke, & Schwaiger, 1994; Klimesch, Vogt, & Doppelmayr, 2000; Mölle, Marshall, Fehm, & Born, 2002). Importantly, neural rhythms have been shown to dissociate when comparing semantic and episodic memory tasks. For example, Klimesch (1999) reviews a series of studies comparing alpha and theta modulations while subjects performed a semantic or episodic task. In the semantic task subjects were given a concept prime (e.g. eagle) and then a target feature (e.g. claws) and they had to judge whether the target is semantically congruent with the prime. In the episodic task subjects were given a concept and feature and they had to judge whether the concept/feature paired was previously shown at study. Comparing these two designs several studies found a consistent post-target decrease in alpha power for the semantic task and a post-target increase in theta power for the episodic task. Klimesch (1999) posits that the theta rhythm likely involves the hippocampal-cortical network, whereas the alpha rhythm likely involves the thalamo-cortical network. These results are consistent with other results indicating that alpha is related to semantic memory whereas theta is related to episodic memory.
First, the methods used to measure rhythmic neural activity will be discussed. Second, the basis of neural oscillations will be discussed. Third, a review of the animal literature will provide the anatomical and physiological basis for understanding the role of gamma and theta oscillations in cortical-hippocampal episodic memory networks. Finally, a review of recent evidence from recording the electrical activity of the human brain will explore the functional role of gamma and theta oscillations in human episodic memory. To date, little work has been done to synthesize the findings from these studies. This review will aim to synthesize these findings by connecting them to what is known about gamma and theta oscillations at a neural and systems level in the animal brain. Integrating these levels of analysis will elucidate the functional role of gamma and theta oscillations in episodic memory.
2. Measurement Methods
The following review will include studies from animals and humans showing rhythmic neural activity. The review of the animal literature includes studies using single or multi unit recording and local field potentials (LFPs). The review of the human literature includes studies using intracranial electroencephalography (iEEG) in patients with epilepsy and scalp electroencephopholgraphy (EEG) and magnetoencephalography (MEG) in normal subjects. Single and multi unit recording intracellularly represents the firing of action potentials whereas LFPs recorded extracellularly represents fluctuations in postsynaptic potentials. Like LFPs, iEEG recorded extracellularly represents fluctuations in postsynaptic potentials. EEG and MEG measured externally represent the summed postsynaptic potentials of synchronously active regions of cortex and hippocampus that has spread through the brain, skull, and scalp. Although they often coincide, it should be noted that firing of action potentials are not necessarily related to oscillations of postsynaptic potentials. In addition, oscillations of postsynaptic potentials do not always result in the firing of postsynaptic action potentials. To maintain the distinction between the firing of action potentials and fluctuations in postsynaptic potentials we will refer to rhythmic and synchronous neural firing when referring to patterns of action potentials and we will refer to oscillations and phase synchronization when referring to fluctuations in poststynaptic potentials.
3. Neural mechanism of rhythmic activity
To understand how gamma and theta oscillations contribute to episodic memory, it is essential to first consider their basic underlying neural mechanisms of rhythmic activity. Studies using single unit and multi unit recording show that rhythmic firing can occur because of intrinsic firing patterns of excitatory principle cells or common input from a pacemaker like the thalamus. More commonly in the cortex and the hippocampus rhythmic firing is an emergent property of interactions between excitatory principle cells and inhibitory interneurons (Whittington, Traub, Kopell, Ermentrout, & Buhl, 2000). Rhythmic firing can happen at different spatial scales depending on the length of inhibitory interneuron to excitatory principle cell connections (Fries, 2005; Soltesz & Deschênes, 1993; Whittington, Traub, Kopell, Ermentrout, & Buhl, 2000) and at different temporal scales depending on the duration of inhibition (Buzsáki & Chrobak, 1995). In addition, rhythmic firing at different frequencies can multiplex such that faster rhythms are modulated by and nested within slower rhythms (Bragin et al., 1995; Chrobak & Buzsáki, 1998; Csicsvari, Jamieson, Wise, & Buzsáki, 2003; Penttonen, Kamondi, Acsády, & Buzsáki, 1998; Soltesz & Deschênes, 1993).
In the cortex and hippocampus the collective activity of excitatory principle cells and inhibitory interneurons leads to neurons firing together and being inhibited together — creating rhythmic firing patterns (Buzsáki, 2006). Activation of excitatory principle cells leads to excitation of inhibitory interneurons. Inhibitory interneurons then act to inhibit further excitation. As inhibition wears off excitatory principle cells are free to fire again. Excitatory principle cells and inhibitory interneurons are both important for the stable rhythmic firing patterns (Whittington, Traub, Kopell, Ermentrout, & Buhl, 2000). Excitatory principle cells receive variable excitation from presynaptic neurons. This variability in excitatory drive results in variability in the firing rate of postsynaptic excitatory principle cells. Excitatory principle cells and inhibitory interneurons act together to stabilize a network with variable firing rates. Stable rhythmic firing occurs when the firing rate of excitatory principle cells is averaged by inhibitory neurons firing synchronously and effectively defining a window in which the excitatory principle cells can fire. The firing rate of excitatory principle cells must be fast enough that they fire every time inhibition wears off and inhibition must wear off fast so that excitatory principle cells are ready to fire again when mutual inhibition among inhibitory interneurons fades.
3.1 Neural mechanism of gamma and theta rhythms
The neural mechanism responsible for gamma and theta rhythmic firing in the cortex and the hippocampus are similar, but primarily differ in the speed of inhibitory neurons. Whereas gamma rhythmic firing occur through the interaction of excitatory principle cells and fast basket cell inhibitory interneurons (Banks, White, & Pearce, 2000; Bartos, Vida, & Jonas, 2007; Buhl, Halasy, & Somogyi, 1994; Mann & Paulsen, 2005; Penttonen, Kamondi, Acsády, & Buzsáki, 1998; Whittington, Traub, Kopell, Ermentrout, & Buhl, 2000) acting on fast Gamma-aminobutyric acid (GABAa) receptors (Banks, White, & Pearce, 2000; Soltesz & Deschênes, 1993; Traub, Whittington, Colling, Buzsáki, & Jefferys, 1996; Whittington, Traub, Kopell, Ermentrout, & Buhl, 2000), theta rhythmic firing occur through the interaction of excitatory principle cells and slow stellate cell inhibitory interneurons (Dickson et al., 2000; Rotstein et al., 2005) acting on slow GABAa receptors (Banks, White, & Pearce, 2000; reviewed in Buzsáki, 2002; Cobb, Buhl, Halasy, Paulsen, & Somogyi, 1995; Soltesz & Deschênes, 1993). It has also been shown in the hippocampus that gamma rhythms are more prevalent in the short-range connections through the activity of local interneuron networks whereas theta rhythms are more prevalent in further reaching areas through the activity of long-range interneuron to principle cell connections (Gloveli et al., 2005).
Although there is little evidence of the mechanism responsible for nesting of gamma rhythms within theta rhythms, some researchers have speculated that theta rhythms modulate gamma rhythms through the action of inhibitory interneurons (Bragin et al., 1995). The rhythmic firing of inhibitory interneurons firing at theta frequency provide a depolarizing and hyperpolarizing current that activates and deactivates inhibitory interneurons firing at gamma frequency. Supporting this idea, it has been shown that inhibitory interneurons acting on slow GABAa receptors can also inhibit the activity of inhibitory interneurons acting on fast GABAa receptors (Banks, White, & Pearce, 2000). This results in the activity of slow inhibitory interneurons inhibiting fast inhibitory interneurons that cause gamma rhythms. Therefore, theta rhythms can modulate gamma rhythms providing a possible mechanism for the nesting of gamma rhythms within theta rhythms.
4. Unified model of gamma and theta oscillations in episodic memory
Neural oscillations in the gamma and theta frequencies have been found in cognitive tasks such as those used to investigate episodic memory (reviewed in Başar, Başar-Eroglu, Karakas, & Schürmann, 1999; Bastiaansen & Hagoort, 2003; Herrmann, Munk, & Engel, 2004; Jensen, Kaiser, & Lachaux, 2007; Kahana, 2006; Kahana, Seelig, & Madsen, 2001; Klimesch, 1996, 1999; Klimesch, Freunberger, & Sauseng, in press; Klimesch, Freunberger, Sauseng, & Gruber, 2008; Sauseng, Griesmayr, Freunberger, & Klimesch, in press). The following section will provide a comprehensive review of studies measuring gamma and theta oscillations using iEEG in patients with epilepsy and scalp EEG and MEG in normal subjects that have shown gamma and theta power modulations, phase synchronization between brain regions, and gamma/theta coupling during the encoding and retrieval of episodic memories. Table 1 provides details of the episodic memory paradigm, recording and analysis methods, results, and conclusions for each study reviewed below.
Table 1.
Episodic Memory-related Oscillations in Humans
| Authors and Year | Memory Paradigm | Sample size (N) | Recording and Analysis Methods | Results | Conclusions |
|---|---|---|---|---|---|
| Burgess & Ali, 2002 | Word recognition Remember/Know responses | (10) | EEG, wavelet power and coherence | Greater frontal and parietal gamma power (300–500 ms) and coherence for “remember” than “know” responses. Theta modulated gamma coherence. |
Gamma is related to feature binding or consciousness and modulation by theta may be related to binding. |
| Burgess & Gruzelier, 1997 | Word recognition | (15) | EEG, ERD power | Greater frontal theta power 125ms and (500–750ms) for hits than correct rejections. | Theta phase synchronization is associated with word recognition. |
| Doppelmayr, Klimesch, Schwaiger, Auinger, & Winkler, 1998 | Word recognition | (26) | EEG, ERD power | Greater right lateralized parietal theta power (500–1000 ms) for good memory performers. Greater bilateral parietal theta power (500–1000 ms) for bad memory performers. |
Right hemisphere is more involved in episodic memory. |
| Doppelmayr, Klimesch, Schwaiger, Stadler, & Röhm, 2000 | Word recognition | (26) | EEG, IBP, ERBP | Greater evoked theta power (<400 ms) for good than bad memory performers. Induced theta power (>400 ms) for good and bad memory performers. |
For good memory, early coordination of sensory and episodic areas interact (evoked) and later represent memory (induced). |
| Düzel et al., 2003 | Word recognition | (14) | MEG, wavelet power and phase locking factor | Greater theta power (200–700 ms) for hits than correct rejections. Greater theta/gamma coupling for hits than correct rejections. |
Medial temporal lobe theta modulates gamma. |
| Düzel, Neufang, & Heinze, 2005 | Word recognition | (8) | MEG, wavelet power and phase locking factor | Greater frontal midline induced theta/gamma coupling for hits than correct rejections. | Induced theta represents large-scale integration of distributed information by the medial temporal lobe. |
| Fell et al., 2003 | SM word recognition | Epilepsy patients (9) | iEEG, wavelet power and coherence | Greater rhinal-hippocampal theta coherence for subsequently recalled than not recalled words. Greater theta/gamma coupling for subsequently recalled than not recalled words. |
Gamma holds memory representation and theta escorts memory to medial temporal lobe structures. |
| Fell et al., 2001 | SM word recognition | Epilepsy patients (9) | iEEG, wavelet power and circular variance phase synchronization | Greater MTL-hippocampal gamma phase synchronization (100–600 ms) for subsequently recalled than not recalled words. | Gamma provides a mechanism to transiently couple neural populations such as hippocampus and cortex for memory tasks. |
| Gruber, Keil, & Müller, 2001 | PAL and CR control task | (10) | EEG, wavelet power, PLV, and PLS | Greater frontal and centro-parietal induced gamma power (400–500 ms) for paired associate than control task. Anterior-posterior gamma phase synchrony (140–240 ms) for paired associate than control task. |
Induced gamma over form widespread cell assemblies for the storage of associative information. |
| Gruber, Tsivilis, Giabbiconi, & Müller, 2008 | Recognition of pictures with old/new + source judgment (encoding task) | (14) | EEG, wavelet power | Greater widespread parieto-occipital induced gamma power (210–330 ms) for hits than correct rejections. Greater fronto-central induced theta (600–1200 ms) for correct than incorrect source judgments. |
Induced gamma is related to familiarity or the binding of cortical representations and induced theta is related to recollection. |
| Gruber, Tsivilis, Montaldi, & Müller, 2004 | SM paradigm for word recognition | (19) | EEG, wavelet power | Greater widespread induced gamma power (200–300 ms) for subsequently recognized than not recognized words and for hits than correct rejections. | Gamma at encoding and retrieval indicates establishment and retrieval of cortical object representations. |
| Guderian & Düzel, 2005 | Face recognition Old/New + source judgment background) | (9) | MEG, wavelet power and phase locking factor | Greater prefrontal, mediotemporal, visual cortex induced theta for correct than incorrect source judgments. | Theta oscillations are related to the binding of distributed cortical representations during recollection. |
| Hanslmayr, Spitzer, & Bauml, 2009 | SM word recognition | (25) | EEG, ERD power | Greater gamma power for the deep and shallow encoding task. Greater theta power for the shallow encoding task. |
Gamma relates to both semantic and non-semantic episodic encoding whereas theta only relates to non-semantic episodic encoding. |
| Klimesch, Doppelmayr, Schimke, & Ripper, 1997 | SM word recognition | (18) | EEG, ERD power | Greater left frontal (0–250 ms and 750–100 ms) theta power for subsequently recalled than not recalled words and for hits than correct rejections and misses. | Theta phase synchronization is important for successful episodic encoding and retrieval. |
| Klimesch, Doppelmayr, Schwaiger, Winkler, & Gruber, 2000 | Word recognition | (25) | EEG, IBP | Greater induced theta power (375–750 ms) for hits than correct rejections. | Theta from hippocampus is feedback to cortex where it acts to suppress neural firing to favor the retrieval of relevant information and suppression of irrelevant information. |
| Klimesch, Doppelmayr, Stadler et al., 2001 | Picture recognition | (13) | EEG, ERD power | Greater theta power during retrieval than encoding. Numerically greater theta power for hits than correct rejections. |
Theta relates to retrieval mode rather than memory trace or retrieval success. |
| Klimesch, Doppelmayr, Yonelinas et al., 2001 | Word recognition Remember/Know responses | (13) | EEG, ERBP | Greater widespread theta power (300–450 ms) for “know” than “remember” responses. Greater widespread theta power (450–625 ms) for “remember” than “know” responses. |
Theta relates to conscious retrieval related to both remembering and knowing. |
| Klimesch, Schimke, & Schwaiger, 1994 | Semantic congruency and word recognition | (12) | EEG, ERD power | Frontal and occipital theta power (~400 ms) for word recognition. | Theta related to word recognition. |
| Klimesch, Vogt, & Doppelmayr, 2000 | Word recall | (60) | EEG, power | Less theta during rest for good than bad memory performers. | Less baseline theta and increased event related theta is related to good memory performance. |
| Mölle, Marshall, Fehm, & Born, 2002 | SM word and face pair cued recall | (17) | EEG, FFT power | Greater left frontal and temporal theta power for subsequently recalled than not recalled words. Greater right parietal theta power for subsequently recalled than not recalled faces. |
Theta interactions in cortical-hippocampal circuit indicate intentional learning. |
| Mormann et al., 2005 | Word recognition | Epilepsy patients (12) | iEEG, wavelet power and phase (entropy) | Theta phase reset and greater theta power (>190 ms) for hits and correct rejections. Theta modulated gamma power in perirhinal cortex for correct rejections and hippocampus for hits. |
Gamma represents memory encoding and retrieval in the perirhinal cortex and hippocampus, respectively. Theta serves a general facilitating function during memory tasks. |
| Osipova et al., 2006 | SM picture recognition | (13) | MEG, multitaper power | Greater occipital gamma power for subsequently recognized than not recognized pictures and for hits than correct rejections and misses. Greater right temporal-parietal theta power for subsequently recognized than not recognized pictures and for hits than rejections and misses. |
Gamma is phase synchronized activity in perceptual areas that are then sent to memory system where theta can then act to encode those items. |
| Sederberg, Kahana, Howard, Donner, & Madsen, 2003 | SM word recall | Epilepsy patients (10) | iEEG, wavelet power | Greater widespread gamma power (600–1300 ms) for subsequently recalled than not recalled words. Greater right temporal and frontal theta power (600–1300 ms) for subsequently recalled than not recalled words. |
Gamma is related to attention to ítems to improve encoding. Theta is involved in successful memory encoding. |
| Sederberg, Schulze-Bonhage, Madsen, Bromfield, McCarthy et al., 2007 | SM word recall | Epilepsy patients (35) | iEEG, wavelet power | Greater frontal gamma power and greater gamma power in left temporal and hippocampal later for subsequently recalled than not recalled words. Greater left frontal theta power early for for subsequently recalled than not recalled words. |
Suggest that early gamma in frontal areas is related to attention, then hippocampal gamma related to encoding, and temporal gamma related to semantic elaboration leading to more hippocampal gamma. |
| Sederberg, Schulze-Bonhage, Madsen, Bromfield, Litt et al., 2007 | SM word recall | Epilepsy patients (52) | iEEG, wavelet power | Greater hippocampus, left temporal, inferior prefrontal, and occipital cortex gamma power for subsequently recalled than not recalled words. Greater hippocampal (500–400 ms before response), left temporal, and inferior prefrontal cortex (250 ms before response) gamma power for hits than false alarms. |
Gamma activity at encoding is reinstated at retrieval when the hippocampus activates and reinstates cortical representations. |
| Summerfield & Mangels, 2005a | SM word recognition Old/New + source judgment (font color) | (19) | EEG, ICA, wavelet coherence | Greater left frontal theta power for subsequent hits with incorrect source judgments. Greater left frontal and posterior theta coherence for subsequent hits with incorrect source judgments. Greater right and bilateral frontal theta power (400 ms) for subsequent hits with correct source judgment than incorrect source judgments. Greater right frontal and posterior theta coherence (800 ms) for subsequent hits with correct source judgment than incorrect source judgments. |
Theta coherence may be important for brain regions to exchange information during learning, with left hemisphere processing item information and right hemisphere processing context. |
| Summerfield & Mangels, 2005b | DRM word recognition | (19) | EEG, ICA, wavelet coherence | Greater frontal gamma power (>100 ms) for hits than correct rejections. Greater parieto-occipital gamma power (>100 ms) for hits than false alarms. Greater parieto-occipital and right frontal gamma coherence (600 ms) for hits than correct rejections and false alarms. |
Gamma phase synchronization important for frontal areas to provide top-down control to recapitulate sesnsory representations in posterior regions. |
| Weiss & Rappelsberger, 2000 | SM word recall | (23) | EEG, FFT power and coherence | Greater temporal and parietal theta coherence for subsequently recalled than not recalled words. | Theta phase synchronization between distant brain regions enables efficient encoding of verbal memory. |
| Weiss, Müller, & Rappelsberger, 2000 | SM word (concrete and abstract) recall | (23) | EEG, FFT power and coherence | Greater left frontal, temporal, parietal, and occipital theta coherence for subsequently recalled than not recalled words. | Theta coherence represents the difference between successfully and unsuccessfully encoded words. |
SM: Subsequent memory, DRM: Deese-Roediger-McDermott, PAL: Paired associate learning, CR: Choice reaction, iEEG: Intracranial encephalography, EEG: electroencephalography, MEG: Magnetoencephalography, FFT: Fast Fourier Transform, ICA: Independent components analysis, ERD: Event-related desynchronization, ERBP: Event-related band power, IBP: Induced band power, PLV: Phase locking value, PLS: Phase locking statistic, MTL: Medial temporal lobe
A unified model will be proposed here that describes a functional role for gamma and theta oscillations in human episodic memory by integrating what is known about gamma and theta oscillations at the neural and behavioral level in animals into the framework of the influential hippocampal memory indexing theory (Teyler & DiScenna, 1986; Teyler & Rudy, 2007). It is proposed here that gamma and theta oscillations allow for the interaction between cortical structures and the hippocampus for the encoding and retrieval of episodic memories. This model incorporates and extends previous models of gamma and theta oscillations in episodic memory. The proposed model incorporates previous theories of the functional role for gamma (Herrmann, Lenz, Junge, Busch, & Maess, 2004; Jensen, Kaiser, & Lachaux, 2007; Klimesch, Freunberger, Sauseng, & Gruber, 2008) and theta oscillations (Bastiaansen & Hagoort, 2003; Klimesch, 1996, 1999; Klimesch, Freunberger, & Sauseng, in press; Klimesch, Freunberger, Sauseng, & Gruber, 2008; Sauseng, Griesmayr, Freunberger, & Klimesch, in press) and gamma/theta interactions (Jensen & Colgin, 2007; Jensen & Lisman, 2005; Lisman, 1999, 2005) in episodic memory. It extends these previous models by providing a unified model of gamma and theta oscillations that allow for the cortical-hippocampal interactions described by the hippocampal indexing memory theory.
4.1 Unified model for encoding
For encoding, the hippocampal memory indexing theory (Teyler & DiScenna, 1986) states that when presented a stimulus, spatio-temporal patterns of cortical activity project to the hippocampus. The hippocampus binds these representations from diverse cortical regions into a sparse, unified episodic memory representation. It does this by quickly modifying synaptic strength between input neurons and hippocampal neurons and between hippocampal neurons activated by the cortical inputs through LTP.
4.1.1 Gamma rhythmic firing modulates perceptual feature binding
Just as visual perception consists of several visual features that must be integrated into a perceptual whole, episodic memory consists of several different individual memory representations that include several features that must be integrated into an individual memory representation. The animal and human literature is consistent with the proposal that gamma and theta oscillations in the cortex and the hippocampus lead to the encoding of episodic memories.
Research on the animal visual system has provided a plausible functional role for gamma oscillations in the cortex. In order to perceive objects in a visual image, the distributed parallel processing of features in a single object must be combined. To solve the “binding problem” the “binding by synchrony” (BBS) theory (von der Malsburg & Schneider, 1986) proposes that the neurons representing features of an object are transiently coupled through synchronous firing. The BBS theory is supported by multi unit recording studies showing that gamma rhythmic firing is synchronized with contiguous visual input in primary visual cortex (V1), secondary visual cortex (V2), posteromedial lateral suprasylvian area (Engel, König, Kreiter, & Singer, 1991; Engel, Kreiter, König, & Singer, 1991; Frien, Eckhorn, Bauer, Woelbern, & Kehr, 1994; Gray, König, Engel, & Singer, 1989). Although these results indicate that the binding of visual features for coherent visual representations is supported by synchronous firing between neurons in areas of visual cortex, the BBS theory remains controversial (Shadlen & Movshon, 1999). The BBS theory has not been supported by studies showing that synchronous firing was not related to contour grouping in V1 (Roelfsema, Lamme, & Spekreijse, 2004), was not greater for non-coherent than coherent motion (Thiele & Stoner, 2003) or for bound objects in MT (Palanca & DeAngelis, 2005). One carefully controlled study (Dong, Mihalas, Qiu, von der Heydt, & Niebur, 2008) showed that synchronous firing was not greater for one versus two objects in V1 and V2 but was greater for border selective neurons, which could indicate that synchronous firing is a marker of higher-level visual binding. These results suggests that cortical gamma rhythmic firing could provide a mechanism to bind relevant stimulus features for perceptual representations and provide a coherent pattern of activity as input to the hippocampus.
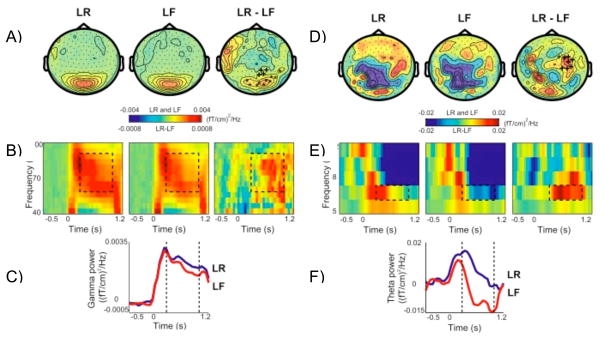
In humans there should be gamma power increases in modality specific perceptual areas during the encoding of new memories. Many studies have shown greater gamma power in posterior cortex for subsequently remembered than forgotten items (Gruber, Tsivilis, Montaldi, & Müller, 2004; Hanslmayr, Spitzer, & Bauml, 2009; Osipova et al., 2006; Sederberg, Kahana, Howard, Donner, & Madsen, 2003; Sederberg, Schulze-Bonhage, Madsen, Bromfield, Litt et al., 2007; Sederberg, Schulze-Bonhage, Madsen, Bromfield, McCarthy et al., 2007) (see Figure 1A–C).
Figure 1.
Gamma and theta power during encoding. (A) Grand average of the topography of gamma power for later remembered (LR) and later forgotten (LF) and their difference (LR-LF). Plus signs represent right temporal sensors that showed a significant power increase for LR compared to LF. (B) Grand average time-frequency plots of power from one significant right temporal sensor showing the time course of gamma oscillations for LR, LF, and their difference. (C) Grand average gamma power averaged between 60 and 90 Hz for LR and LF for the same sensor as B. (D) Grand average of the topography of theta power for later remembered (LR) and later forgotten (LF) and their difference (LR-LF). (E) Grand average time-frequency plots of power from one significant sensor showing the time course of theta oscillations for LR, LF, and their difference. (F) Grand average theta power averaged between 4.5 and 8.5 Hz for LR and LF for the same sensor as E (reprinted with permission from Osipova et al., 2006).
4.1.2 Gamma oscillations lead to spike-timing-dependent plasticity
The animal literature has also shown that gamma oscillations provide the precise timing necessary for spike-timing-dependent plasticity. LTP results when calcium enters the postsynaptic neuron initiating a cascade of molecular processes for synaptic modification and memory formation. Spike-timing-dependent plasticity is a form of LTP that depends on the timing of presynaptic neuron firing relative to postsynaptic neuron firing (Skaggs, McNaughton, Wilson, & Barnes, 1996). Spike-timing-dependent plasticity occurs when two circumstances are met. First, the presynaptic neuron must release glutamate that binds to N-methyl-D-aspartic acid (NMDA) receptors. Second, the postsynaptic neuron must be depolarized in order to remove a magnesium plug that blocks the NMDA receptor. The binding of glutamate occurs slowly (~20 ms) but removal of the magnesium plug occurs very rapidly (<1 ms). Based on these constraints, spike-timing-dependent plasticity occurs when the presynaptic neuron fires and releases glutamate prior to postsynaptic depolarization. It has recently been proposed that LFP gamma oscillations are important for spike-timing-dependent plasticity to occur (Axmacher, Mormann, Fernández, Elger, & Fell, 2006). Neural oscillations modulate when excitatory principle cells can fire. Excitatory principle cells are more likely to fire on the positive peak of neural oscillations. Because gamma oscillations occur at 25–100 Hz, the pattern of inputs has the exact timing parameters necessary for spike-timing-dependent plasticity. Slower oscillations would not provide the tight timing parameters necessary for coincident activation of presynaptic and postsynaptic neurons. And faster oscillations would have multiple cycles within the time-period of spike-timing-dependent plasticity. This would cause ambiguity as the window for LTP would include presynaptic neuron spikes before and after postsynaptic spikes. Although gamma oscillations have been implicated in spike-timing-dependent plasticity, it has not yet been shown that gamma oscillations are linked to memory in animals. Therefore, gamma phase synchronization between cortical and hippocampal neurons and between hippocampal neurons could provide the mechanism by which individual memory representations get encoded.
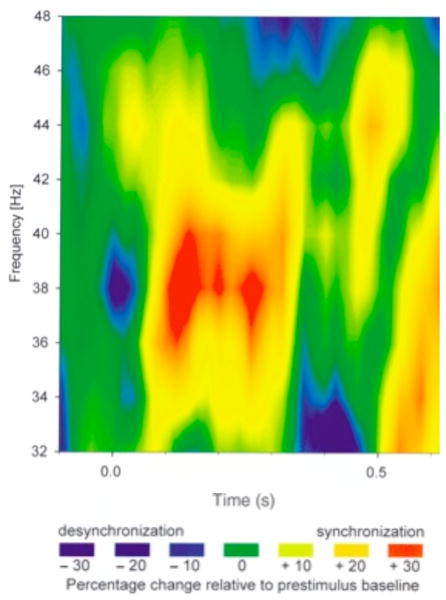
In humans this would result in gamma phase synchronization between these structures during the encoding of new episodic memories. This idea is supported by an iEEG study showing gamma phase synchronization between medial temporal (rhinal) cortex electrodes and hippocampal electrodes during successful encoding of words (see Figure 2, Fell et al., 2001).
Figure 2.
Gamma phase synchronization for encoding. Difference of phase synchronization between rhinal cortex and hippocampus (%) relative to prestimulus baseline for subsequently recalled minus not recalled words. Blue indicates more phase desynchronization, red indicates more phase synchronization, ranging from −30% to +30% change from prestimulus baseline. Phase synchronization differences for successfully recalled versus not recalled were greatest for 36–40Hz from 100–300 ms and 500–600 ms (reprinted with permission from Fell et al., 2001).
4.1.3 Theta oscillations modulate long-term potentiation
The animal literature has also shown that theta oscillations influence the encoding of new episodic memories. The positive peak of the LFP theta oscillation causes depolarization which leads to the opening of NMDA channels causing calcium influx initiating the cascade of molecular processes for synaptic modification and memory formation (Huerta & Lisman, 1995). A number of studies have shown that stimulating at theta frequency is optimal for the induction of LTP in dentate gyrus (Greenstein, Pavlides, & Winson, 1988) and CA1 neurons (Larson & Lynch, 1986; Larson, Wong, & Lynch, 1986; Rose & Dunwiddie, 1986) in the hippocampus. In addition, studies have found LTP in dentate gyrus (Pavlides, Greenstein, Grudman, & Winson, 1988) and CA1 neurons (Hölscher, Anwyl, & Rowan, 1997; Huerta & Lisman, 1995; Hyman, Wyble, Goyal, Rossi, & Hasselmo, 2003) in the hippocampus when stimulating at the positive peak of the theta oscillation and no effect or depotentiation of previously potentiated synapses when stimulating at the trough of the theta oscillation. These results indicate that the firing of neurons at the peak of the theta oscillation is optimal for synaptic plasticity.
The following studies used in vivo hippocampal preparations and behavioral measures to examine whether theta oscillations affect learning and performance on commonly used memory tasks. The level of theta activity prior to training and during training positively correlated with the rate of acquisition on classical conditioning paradigms (reviewed in Berry & Seager, 2001). In addition, lesioning the septum as well as pharmacologically blocking acetylcholine has been shown to decrease theta oscillations in the hippocampus and decrease the rate of acquisition on classical conditioning paradigms (reviewed in Berry & Seager, 2001). Blocking theta oscillations by lesioning the septum has also been shown to decrease the rate of learning on a spatial maze task (Winson, 1978) whereas enhancing theta oscillations by pharmacologically blocking seratonin increased performance on a radial maze task (Stäubli & Xu, 1995). In addition to affecting the rate of learning, hippocampal theta activity positively correlated with rats performance on a Morris maze task (Olvera-Cortés, Cervantes, & Gonzalez-Burgos, 2002) and theta oscillations were greater for correct non-matched compared to incorrect matched odors in a delay non-match to sample task recognition memory tasks (Wiebe & Stäubli, 2001). Combined, these studies show the presence of the theta oscillations in the cortical-hippocampal network and indicate that theta oscillations are important for synaptic plasticity, learning, and performance on memory tasks.
In humans there should be theta power increases in posterior regions during the encoding of new memories. Some studies have used the subsequent memory paradigm and found greater theta power for subsequently remembered than forgotten items (Hanslmayr, Spitzer, & Bauml, 2009; Osipova et al., 2006; Sederberg, Kahana, Howard, Donner, & Madsen, 2003) (see Figure 1D–F).
4.1.4 The theta/gamma code model and episodic memory
Although the hippocampal memory indexing theory (Teyler & DiScenna, 1986) does not specify how multiple memories make up coherent episodes, individual memory representations must be combined and temporally ordered in episodic memory. In rats, pyramidal neurons in the hippocampus fire for a particular location in the environment. These “place cells” fire strong bursts of spikes when the rat runs through a location in the environment, the “place field”, and less strongly on the periphery of the place field. Pyramidal neurons in the hippocampus fire at the peak of theta oscillations as the rat enters the place field and then fire progressively earlier on progressive theta cycles as the rat runs from location to location (reviewed in Buzsáki, 2002; O’Keefe & Recce, 1993; Skaggs, McNaughton, Wilson, & Barnes, 1996; reviewed in Yamaguchi et al., 2007). For example, if a rat is moving along a path from position A to position C the neurons coding position A will originally fire late in the theta cycle and then will fire progressively earlier in the theta cycle as the rat approaches position C (Lisman, 1999). This modulation of pyramidal cell firing in relation to the phase of theta oscillations is called phase precession. Phase precession has been shown in the CA1 region of the hippocampus (O’Keefe & Recce, 1993) and more broadly across the entire hippocampus (Skaggs, McNaughton, Wilson, & Barnes, 1996).
The following explains how LTP leads to phase precession (Axmacher, Mormann, Fernández, Elger, & Fell, 2006) (see Figure 3). When the animal enters a place field, theta oscillations reset so that the cells firing to the place field will be activated at the peak of the theta cycle. As described above, inputs arriving at the peak of theta oscillations induce LTP (reviewed in Buzsáki, 2002; Hölscher, Anwyl, & Rowan, 1997; Huerta & Lisman, 1995; Pavlides, Greenstein, Grudman, & Winson, 1988). The activated place cells are then strengthened by LTP so that the same environmental cues will evoke firing at earlier phases of theta oscillations on subsequent cycles. After learning, when the animal enters a familiar place field, the same input arrives at potentiated synapses, leading to activation of the postsynaptic neuron even when it is not in its depolarized state at the peak of theta oscillations. Therefore, experience can cause LTP that allows neurons to fire at progressively earlier phases of the theta cycle leading to phase precession.
Figure 3.

Phase precession. When the animal enters a place field, the theta rhythm resets so that the cells firing to the place field will be activated at the peak of the theta cycle. Inputs arriving at the peak of the theta rhythm induce LTP. The activated place cells are then strengthened by LTP so that the same environmental cues will evoke firing at earlier phases of the theta rhythm on subsequent cycles (reprinted with permission from Axmacher, Mormann, Fernández, Elger, & Fell, 2006).
It has been proposed that the interaction between gamma and theta oscillations encodes and temporally orders working memory representations (Lisman & Idiart, 1995). Extending this theory, it has been suggested that the theta/gamma code model can work as a working memory buffer for encoding episodic memories (Jensen & Lisman, 2005) and for ordering place and episodic memories (Lisman, 1999). The theta/gamma code may also be a more domain general coding scheme for representing order in the cortex (Jensen & Colgin, 2007; Lisman, 2005). For episodic memory temporal ordering, the theta/gamma code model posits that in each gamma cycle a set of neurons fires for a given episodic memory representation. Different assemblies of neurons representing different episodic memory representations fire in different gamma cycles. Gamma cycles are superimposed onto different phases of the theta cycle. The coupling between gamma and theta oscillations is not static but changes over time with learning. Through phase precession, as episodes are presented, each gamma cycle representing a specific episodic memory representation fires at an earlier phase of the theta cycle. The theta/gamma code suggests that populations of neurons do not fire continuously within the theta cycle, but within gamma cycles superimposed on the theta rhythm. Consistent with this suggestion, research has shown that populations of neurons fire 5–7 times within the theta cycle (Bragin et al., 1995; Csicsvari, Jamieson, Wise, & Buzsáki, 2003; Soltesz & Deschênes, 1993). For example, episodic memories include a series of specific memory representations such as “Jerry dropped candy”, “Monkey in cage grabbed it”, “Jerry sad” (Lisman, 1999). Analogous to the rat moving along a path from position A–C the neurons coding “Jerry dropped candy”, “Monkey in cage grabbed it”, and “Jerry sad” will originally fire late in the theta cycle and then will fire progressively earlier in the theta cycle. Therefore, neurons coding for each memory representation will be active in a different phase of the theta cycle and will maintain the temporal order of the sequence of events making up the episodic memory. Therefore, the interaction between gamma and theta oscillations provides a mechanism for the binding and temporal ordering of individual episodic memory representations from dispersed cortical regions for encoding episodic memories.
In humans this should be represented by gamma/theta coupling during encoding of episodic memories. Although there is no direct evidence of temporal ordering of gamma oscillations superimposed on theta oscillations during episodic encoding, consistent with this idea, one iEEG study has shown coherence between rhinal and hippocampal areas and gamma/theta coupling for successfully encoded words (Fell et al., 2003). Therefore, there is growing evidence in animals and humans that gamma power increases in posterior cortical regions and theta modulation of gamma oscillations in medial temporal lobe regions support the encoding of new episodic memories.
4.2 Unified model for retrieval
For retrieval, the hippocampal memory indexing theory (Teyler & DiScenna, 1986) states that, when part of the memory representation is re-presented, cortical activity spreads to the hippocampus, which in turn activates the entire unified memory representation in the hippocampus. The hippocampus then reactivates the spatio-temporal pattern of activity in the cortex for the entire unified episodic memory representation.
The model proposed here suggests that at retrieval cortical gamma oscillations provide input to the hippocampus causing activation of the hippocampal gamma modulated representations of each individual memory representation temporally ordered by theta oscillations. Through feedback projections from the hippocampus to the cortex these gamma and theta patterns should cause the reinstatement of the entire memory representation in the cortex. Although the animal literature provides strong evidence for gamma and theta oscillations modulating encoding through LTP (Axmacher, Mormann, Fernández, Elger, & Fell, 2006; reviewed in Berry & Seager, 2001; reviewed in Buzsáki, 2002; Greenstein, Pavlides, & Winson, 1988; Hölscher, Anwyl, & Rowan, 1997; Huerta & Lisman, 1995; Larson & Lynch, 1986; Pavlides, Greenstein, Grudman, & Winson, 1988; Rose & Dunwiddie, 1986; Stäubli & Xu, 1995; Winson, 1978), there is little evidence supporting the role of gamma and theta oscillations at retrieval. A few studies show that hippocampal theta rhythms during testing positively correlate with performance on spatial (Olvera-Cortés, Cervantes, & Gonzalez-Burgos, 2002) and recognition memory tasks (Wiebe & Stäubli, 2001).
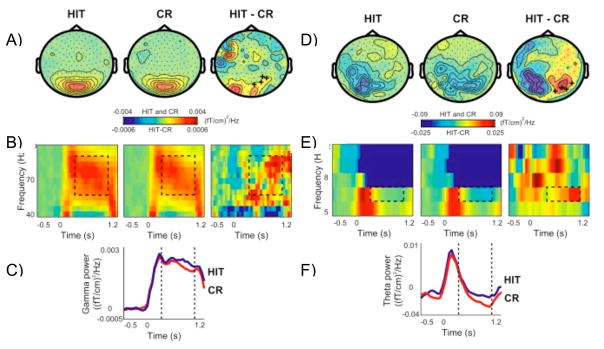
Although there is little support from the animal literature, the human literature is consistent with the proposal that at retrieval, gamma and theta oscillations cause the hippocampal reinstatement of memory representations in posterior cortex. In humans there is greater gamma (Gruber, Keil, & Müller, 2001; Gruber, Tsivilis, Giabbiconi, & Müller, 2008; Gruber, Tsivilis, Montaldi, & Müller, 2004; Osipova et al., 2006; Summerfield & Mangels, 2005b) (see Figure 4A–C) and theta power (Düzel et al., 2003; Klimesch, Doppelmayr, Schwaiger, Winkler, & Gruber, 2000; Klimesch, Doppelmayr, Stadler et al., 2001; Mormann et al., 2005; Osipova et al., 2006) (see Figure 4D–F) in hippocampal and posterior cortical regions for correctly remembered items than new items. Again, although there is no direct evidence of temporal ordering of gamma oscillations superimposed on theta oscillations during episodic retrieval, theta oscillations have been shown to modulate gamma oscillations in anterior temporal scalp locations (Düzel et al., 2003) and the hippocampus (Mormann et al., 2005) during successful retrieval. When given a partial cue, the cortical-hippocampal network is needed to retrieve the rich details of episodic memory. In order to retrieve details of the study episode such as associated source information or the feeling of “remembering”, requires the reactivation of the entire cortical-hippocampal network. It has been shown that theta power increases in frontal, mediotemporal, and visual cortex with the retrieval of source information (background details) (Guderian & Düzel, 2005). Some research has shown greater gamma power over parietal scalp locations (Burgess & Ali, 2002) and theta power in widespread scalp locations (Klimesch, Doppelmayr, Yonelinas et al., 2001) for words given a “remember” than a “know” response. Therefore, there is some evidence in animals and humans consistent with the idea that the retrieval of episodic memory involves the reinstatement of encoded memory representations in posterior cortical regions through the activity of gamma and theta oscillations.
Figure 4.
Gamma and theta power during retrieval. (A) Grand average of the topography of gamma power for hits (HIT) and correct rejections (CR) and their difference (HIT-CR). P lus signs represent right occipital sensors that showed a significant power increase for HIT compared to CR. (B) Grand average time-frequency plots of power from one significant right occipital sensor showing the time course of gamma oscillations for HIT and CR and their difference. (C) Grand average gamma power averaged between 60 and 90 Hz for HIT and CR for the same sensor as B. (D) Grand average of the topography of theta power for hits (HIT) and correct rejections (CR) and their difference (HIT-CR). (E) Grand average time-frequency plots of power from one significant sensor showing the time course of theta oscillations for HIT and CR and their difference. (F) Grand average theta power averaged between 60 and 90 Hz for HIT and CR for the same sensor as E (reprinted with permission from Osipova et al., 2006).
4.3 Frontal-hippocampal oscillations
Another important aspect of episodic memory to consider is how top-down control can guide encoding and retrieval. It has been proposed that theta oscillations allow for top-down control in episodic memory (Kahana, Seelig, & Madsen, 2001; Klimesch, 1996, 1999; Klimesch, Freunberger, & Sauseng, in press; Klimesch, Freunberger, Sauseng, & Gruber, 2008; Sauseng, Griesmayr, Freunberger, & Klimesch, in press). In rats it has been shown that neural firing in the medial prefrontal cortex, an analogue to the human dorsolateral prefrontal cortex, phase locked to the hippocampal theta rhythm (Hyman, Zilli, Paley, & Hasselmo, 2005) and that medial prefrontal phase locking to the hippocampal theta rhythm positively correlated with behavior on spatial maze tasks (Jones & Wilson, 2005; Siapas, Lubenov, & Wilson, 2005).
4.3.1 Human frontal-hippocampal oscillations during encoding
In humans, many studies have implicated the ventrolateral prefrontal cortex in selecting goal-relevant information and inhibiting distracting information whereas the dorsolateral prefrontal cortex aids in ordering information for episodic encoding and retrieval (reviewed in Blumenfeld & Ranganath, 2007). The human studies presented are consistent with the idea that theta phase synchronization between frontal and posterior regions provides top-down control to modulate the encoding of episodic memories. Some studies have shown theta power increases in frontal areas (Klimesch, Doppelmayr, Schimke, & Ripper, 1997; Sederberg, Schulze-Bonhage, Madsen, Bromfield, McCarthy et al., 2007; Summerfield & Mangels, 2005a) and frontal and posterior scalp locations (Mölle, Marshall, Fehm, & Born, 2002; Sederberg, Kahana, Howard, Donner, & Madsen, 2003) during encoding of successfully remembered items. Importantly, theta phase synchronization between frontal and posterior regions (including parietal and temporal cortex) has been shown for successful memory encoding (Weiss, Müller, & Rappelsberger, 2000; Weiss & Rappelsberger, 2000) including source information (Summerfield & Mangels, 2005a).
4.3.2 Human frontal-hippocampal oscillations during retrieval
It has been proposed that the dorsolateral prefrontal cortex maintains temporal context and, when a partial cue is presented at retrieval, can provide top-down control to the hippocampus and posterior cortex to retrieve associated memory representations (Polyn & Kahana, 2008). The frontal cortex has also been implicated in post-retrieval processing or the monitoring and evaluation of the content of retrieved information in relation to the goals of the retrieval attempt (reviewed in Blumenfeld & Ranganath, 2007). The human studies presented here are consistent with the idea that theta phase synchronization between frontal and posterior regions provides top-down control to modulate the retrieval of episodic memories. Some studies have reported theta power increases in frontal scalp locations (Burgess & Gruzelier, 1997; Düzel, Neufang, & Heinze, 2005; Klimesch, Doppelmayr, Schimke, & Ripper, 1997) during successful retrieval and frontal and posterior scalp locations for retrieval of specific details of the study episode (Gruber, Tsivilis, Giabbiconi, & Müller, 2008; Guderian & Düzel, 2005). Some of these studies report theta power increases over frontal scalp locations early (before 200 ms) (Burgess & Gruzelier, 1997; Klimesch, Doppelmayr, Schimke, & Ripper, 1997), supporting the role for theta oscillations in frontal top-down control to the hippocampus and posterior cortex to retrieve associated memory representations. Some studies report theta power increases over frontal scalp locations (Burgess & Gruzelier, 1997; Klimesch, Doppelmayr, Schimke, & Ripper, 1997) and fronto-central scalp locations late (after 500 ms) (Gruber, Tsivilis, Giabbiconi, & Müller, 2008), supporting the role for theta oscillations in frontal top-down control to the hippocampus and posterior cortex for post-retrieval processing. Therefore, in addition to the direct influences on encoding and retrieval of episodic memory representations, theta rhythms can allow for top-down control from frontal cortex for the encoding and retrieval of episodic memories.
Combined the studies described above suggest that modulation of gamma and theta oscillations and phase synchronization between brain regions play a role in episodic memory processes. Future research should be careful to distinguish between movement and cognitive correlates of oscillations. Some studies have shown a strong relationship between micro-saccades and the onset of induced gamma band responses (iGBR) (Jerbi et al., 2009; Trujillo, Peterson, Kaszniak, & Allen, 2005; Yuval-Greenberg & Deouell, 2009; Yuval-Greenberg, Tomer, Keren, Nelken, & Deouell, 2008). Although micro-saccades are particularly problematic for scalp recorded EEG (Trujillo, Peterson, Kaszniak, & Allen, 2005; Yuval-Greenberg & Deouell, 2009; Yuval-Greenberg, Tomer, Keren, Nelken, & Deouell, 2008), even iEEG can become contaminated by micro-saccades when recording electrodes are close to extraocular muscles (Jerbi et al., 2009). It is difficult to discriminate eye movement-related from cognitive-related iGBR because both vary depending on perceptual and cognitive processes. Future research is necessary to determine whether eye movements and/or cognitive processes lead to greater iGBRs for subsequently remembered than not subsequently remembered items and old than new items.
Performing an episodic memory task does not involve a unitary process but involves multiple processes distributed throughout the brain. For example, perceptual processes, attentional processes, working memory processes, memory retrieval processes such as familiarity and recollection, and decisional factors all contribute to performance on memory tasks. In order to clarify the role that gamma and theta oscillations play in encoding and retrieval of episodic memories, it is important to differentiate neural oscillatory patterns related to the different cognitive operations involved in episodic memory tasks, but few studies address the exact role that gamma and theta oscillations play in episodic memory tasks. One promising approach in this direction is using multivariate analysis which can indicate whether neural oscillations in different brain regions correlate with different task variables (Kahana, 2006). Using a multivariate linear model on a working memory task Jacobs, Hwang, Curran, and Kahana (2006) were able to find that EEG theta oscillations in different brain regions correlate with different task variables on a the Sternberg task. Theta power increases in left parietal scalp locations correlated with memory: Theta power was greater for recognized than correctly rejected words. Theta power increases in central scalp locations correlated with decision-making: Theta power was greater for slower reaction times than faster reaction times. And theta power at widespread scalp locations correlated with memory load: Theta power was greater for short than long study lists. Although these results are promising in showing that theta oscillations, possibly originating from different cortical regions, play a distinct role in contributing to working memory, further investigation is required to gain a clearer understanding of the relationship between neural oscillations in different frequency bands across different brain regions and the cognitive components of episodic memory. It is also important to specify how gamma and theta oscillations contribute to the interaction of these different processes. Further research is also needed to clarify how synchronization between brain regions leads to the complex network dynamics underlying episodic memory.
4.4 Predictions
The model proposed here not only helps guide understanding of gamma and theta oscillations in studies of episodic memory, but can direct future research. The model proposed here suggests that cortical gamma oscillations represent perceptual features that provide coherent input to the hippocampus for LTP during encoding. The gamma/theta patterns at encoding are reinstated at retrieval. These ideas could be tested with experiments requiring the encoding and retrieval of specific information such as the judgment of modality specific sources (e.g. visual, auditory). It would be expected that different modality specific sources would elicit different gamma oscillation patterns. In addition, the model proposed here suggests that gamma oscillations represent individual memory representations and theta oscillations act to temporally order memory representations. It would be expected that tasks requiring the encoding and retrieval of the temporal order of items would elicit greater phase locking of gamma and theta oscillations. Finally, it has been suggested that theta oscillations arising from frontal-hippocampal interactions provide top-down control for encoding and retrieval of episodic memories. It would be expected that unconstrained memory tasks requiring the use of strategic encoding and retrieval would elicit theta power increases in frontal cortex and theta phase synchronization between frontal and hippocampal areas.
5. Conclusions
Many current models of brain function involve the interaction of different brain systems, but little research has addressed how the functionally specialized areas of the brain interact to perform cognitive tasks. The brain is made up of neural assemblies with reciprocal connections that connect local populations to combine multiple processes that are important for higher-level cognitive tasks. Since cognitive demands change over time there must be transient interactions between neurons to perform immediate cognitive tasks. Neural oscillations could allow for transient network interactions in the brain responsible for complex cognitive tasks.
It was proposed here that gamma and theta oscillations allow for the interaction between cortical structures and the hippocampus for the encoding and retrieval of episodic memories. The animal literature and the human literature provided evidence for general principles of gamma and theta oscillations in episodic memory. These principles were integrated into a model of the cortical-hippocampal network leading to the encoding and retrieval of episodic memories. According to this model, cortical gamma oscillations bind relevant stimulus features for perceptual representations and provide a coherent pattern of activity as input to the hippocampus. Gamma phase synchronization between cortical and hippocampal neurons and between hippocampal neurons provide the mechanism by which individual episodic memory representations from diverse cortical regions get encoded into hippocampal representations. Therefore, gamma oscillations can act in the cortex to bind modality specific perceptual representations and in the hippocampus to bind the rich perceptual and contextual information from diverse brain regions into episodic representations. Theta oscillations act to temporally order these individual episodic memory representations. Through feedback projections from the hippocampus to the cortex, these gamma and theta patterns could cause the reinstatement of the entire memory representation in the cortex. In addition, theta oscillations could provide top-down control from frontal cortex to the hippocampus for selective encoding and retrieval of episodic memories. Therefore, this unified model demonstrates how neural oscillations allow for transient interactions between brain regions to perform complex cognitive tasks. Understanding the role of oscillations in the network dynamics involved in the encoding and retrieval of information is not only important for understanding episodic memory, but can also serve as a model for understanding large-scale brain network dynamics and their relation to other cognitive phenomena.
Acknowledgments
This work was supported by NIH grant MH64812 and NSF grant #SBE-0542013 to the Temporal Dynamics of Learning Center, an NSF Science of Learning Center. We thank Lewis O. Harvey and Albert Kim for comments on an earlier draft.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Axmacher N, Mormann F, Fernández G, Elger CE, Fell J. Memory formation by neuronal synchronization. Brain Research Reviews. 2006;52:170–182. doi: 10.1016/j.brainresrev.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Banks MI, White JA, Pearce RA. Interactions between distinct GABA(A) circuits in hippocampus. Neuron. 2000;25:449–457. doi: 10.1016/s0896-6273(00)80907-1. [DOI] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nature Reviews Neuroscience. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Başar E, Başar-Eroglu C, Karakas S, Schürmann M. Are cognitive processes manifested in event-related gamma, alpha, theta and delta oscillations in the EEG? Neuroscience Letters. 1999;259:165–168. doi: 10.1016/s0304-3940(98)00934-3. [DOI] [PubMed] [Google Scholar]
- Başar E, Schürmann M. Toward new theories of brain function and brain dynamics. International Journal of Psychophysiology. 2001;39:87–89. doi: 10.1016/s0167-8760(00)00134-3. [DOI] [PubMed] [Google Scholar]
- Bastiaansen M, Hagoort P. Event-induced theta responses as a window on the dynamics of memory. Cortex. 2003;39:967–992. doi: 10.1016/s0010-9452(08)70873-6. [DOI] [PubMed] [Google Scholar]
- Berry SD, Seager MA. Hippocampal theta oscillations and classical conditioning. Neurobiology of Learning and Memory. 2001;76:298–313. doi: 10.1006/nlme.2001.4025. [DOI] [PubMed] [Google Scholar]
- Blumenfeld RS, Ranganath C. Prefrontal cortex and long-term memory encoding: an integrative review of findings from neuropsychology and neuroimaging. The Neuroscientist. 2007;13:280–291. doi: 10.1177/1073858407299290. [DOI] [PubMed] [Google Scholar]
- Bragin A, Jandó G, Nádasdy Z, Hetke J, Wise K, Buzsáki G. Gamma (40–100 Hz) oscillation in the hippocampus of the behaving rat. The Journal of Neuroscience. 1995;15:47–60. doi: 10.1523/JNEUROSCI.15-01-00047.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhl EH, Halasy K, Somogyi P. Diverse sources of hippocampal unitary inhibitory postsynaptic potentials and the number of synaptic release sites. Nature. 1994;368:823–828. doi: 10.1038/368823a0. [DOI] [PubMed] [Google Scholar]
- Burgess AP, Ali L. Functional connectivity of gamma EEG activity is modulated at low frequency during conscious recollection. International Journal of Psychophysiology. 2002;46:91–100. doi: 10.1016/s0167-8760(02)00108-3. [DOI] [PubMed] [Google Scholar]
- Burgess AP, Gruzelier JH. Short duration synchronization of human theta rhythm during recognition memory. Neuroreport. 1997;8:1039–1042. doi: 10.1097/00001756-199703030-00044. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Rhythms of the Brain. New York: Oxford University Press; 2006. [Google Scholar]
- Buzsáki G, Chrobak JJ. Temporal structure in spatially organized neuronal ensembles: a role for interneuronal networks. Current Opinion in Neurobiology. 1995;5:504–510. doi: 10.1016/0959-4388(95)80012-3. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Chrobak JJ, Buzsáki G. Gamma oscillations in the entorhinal cortex of the freely behaving rat. The Journal of Neuroscience. 1998;18:388–398. doi: 10.1523/JNEUROSCI.18-01-00388.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature. 1995;378:75–78. doi: 10.1038/378075a0. [DOI] [PubMed] [Google Scholar]
- Csicsvari J, Jamieson B, Wise KD, Buzsáki G. Mechanisms of gamma oscillations in the hippocampus of the behaving rat. Neuron. 2003;37:311–322. doi: 10.1016/s0896-6273(02)01169-8. [DOI] [PubMed] [Google Scholar]
- Dickson CT, Magistretti J, Shalinsky MH, Fransén E, Hasselmo ME, Alonso A. Properties and role of I(h) in the pacing of subthreshold oscillations in entorhinal cortex layer II neurons. Journal of Neurophysiology. 2000;83:2562–2579. doi: 10.1152/jn.2000.83.5.2562. [DOI] [PubMed] [Google Scholar]
- Dong Y, Mihalas S, Qiu F, von der Heydt R, Niebur E. Synchrony and the binding problem in macaque visual cortex. Journal of Vision. 2008;8:30, 31–16. doi: 10.1167/8.7.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doppelmayr M, Klimesch W, Schwaiger J, Auinger P, Winkler T. Theta synchronization in the human EEG and episodic retrieval. Neuroscience Letters. 1998;257:41–44. doi: 10.1016/s0304-3940(98)00805-2. [DOI] [PubMed] [Google Scholar]
- Doppelmayr M, Klimesch W, Schwaiger J, Stadler W, Röhm D. The time locked theta response reflects interindividual differences in human memory performance. Neuroscience Letters. 2000;278:141–144. doi: 10.1016/s0304-3940(99)00925-8. [DOI] [PubMed] [Google Scholar]
- Düzel E, Habib R, Schott B, Schoenfeld A, Lobaugh N, McIntosh AR, et al. A multivariate, spatiotemporal analysis of electromagnetic time-frequency data of recognition memory. Neuroimage. 2003;18:185–197. doi: 10.1016/s1053-8119(02)00031-9. [DOI] [PubMed] [Google Scholar]
- Düzel E, Neufang M, Heinze HJ. The oscillatory dynamics of recognition memory and its relationship to event-related responses. Cerebral Cortex. 2005;15:1992–2002. doi: 10.1093/cercor/bhi074. [DOI] [PubMed] [Google Scholar]
- Engel AK, König P, Kreiter A, Singer W. Interhemispheric synchronization of oscillatory neuronal responses in cat visual cortex. Science. 1991;252:1177–1179. doi: 10.1126/science.252.5009.1177. [DOI] [PubMed] [Google Scholar]
- Engel AK, Kreiter AK, König P, Singer W. Synchronization of oscillatory neuronal responses between striate and extrastriate visual cortical areas of the cat. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:6048–6052. doi: 10.1073/pnas.88.14.6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell J, Klaver P, Elfadil H, Schaller C, Elger CE, Fernández G. Rhinal-hippocampal theta coherence during declarative memory formation: interaction with gamma synchronization? European Journal of Neuroscience. 2003;17:1082–1088. doi: 10.1046/j.1460-9568.2003.02522.x. [DOI] [PubMed] [Google Scholar]
- Fell J, Klaver P, Lehnertz K, Grunwald T, Schaller C, Elger CE, et al. Human memory formation is accompanied by rhinal-hippocampal coupling and decoupling. Nature Neuroscience. 2001;4:1259–1264. doi: 10.1038/nn759. [DOI] [PubMed] [Google Scholar]
- Frien A, Eckhorn R, Bauer R, Woelbern T, Kehr H. Stimulus-specific fast oscillations at zero phase between visual areas V1 and V2 of awake monkey. Neuroreport. 1994;5:2273–2277. doi: 10.1097/00001756-199411000-00017. [DOI] [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends in Cognitive Sciences. 2005;9:474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Gloveli T, Dugladze T, Rotstein HG, Traub RD, Monyer H, Heinemann U, et al. Orthogonal arrangement of rhythm-generating microcircuits in the hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:13295–13300. doi: 10.1073/pnas.0506259102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray CM, König P, Engel AK, Singer W. Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature. 1989;338:334–337. doi: 10.1038/338334a0. [DOI] [PubMed] [Google Scholar]
- Greenstein YJ, Pavlides C, Winson J. Long-term potentiation in the dentate gyrus is preferentially induced at theta rhythm periodicity. Brain Research. 1988;438:331–334. doi: 10.1016/0006-8993(88)91358-3. [DOI] [PubMed] [Google Scholar]
- Gruber T, Keil A, Müller MM. Modulation of induced gamma band responses and phase synchrony in a paired associate learning task in the human EEG. Neuroscience Letters. 2001;316:29–32. doi: 10.1016/s0304-3940(01)02361-8. [DOI] [PubMed] [Google Scholar]
- Gruber T, Tsivilis D, Giabbiconi CM, Müller MM. Induced Electroencephalogram Oscillations during Source Memory: Familiarity is Reflected in the Gamma Band, Recollection in the Theta Band. Journal of Cognitive Neuroscience. 2008;20:1043–1053. doi: 10.1162/jocn.2008.20068. [DOI] [PubMed] [Google Scholar]
- Gruber T, Tsivilis D, Montaldi D, Müller MM. Induced gamma band responses: an early marker of memory encoding and retrieval. Neuroreport. 2004;15:1837–1841. doi: 10.1097/01.wnr.0000137077.26010.12. [DOI] [PubMed] [Google Scholar]
- Guderian S, Düzel E. Induced theta oscillations mediate large-scale synchrony with mediotemporal areas during recollection in humans. Hippocampus. 2005;15:901–912. doi: 10.1002/hipo.20125. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Spitzer B, Bauml KH. Brain oscillations dissociate between semantic and nonsemantic encoding of episodic memories. Cerebral Cortex. 2009;19:1631–1640. doi: 10.1093/cercor/bhn197. [DOI] [PubMed] [Google Scholar]
- Herrmann CS, Lenz D, Junge S, Busch NA, Maess B. Memory-matches evoke human gamma-responses. BMC Neuroscience. 2004;5:13. doi: 10.1186/1471-2202-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann CS, Munk MH, Engel AK. Cognitive functions of gamma-band activity: memory match and utilization. Trends in Cognitive Sciences. 2004;8:347–355. doi: 10.1016/j.tics.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Hölscher C, Anwyl R, Rowan MJ. Stimulation on the positive phase of hippocampal theta rhythm induces long-term potentiation that can Be depotentiated by stimulation on the negative phase in area CA1 in vivo. The Journal of Neuroscience. 1997;17:6470–6477. doi: 10.1523/JNEUROSCI.17-16-06470.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta PT, Lisman JE. Bidirectional synaptic plasticity induced by a single burst during cholinergic theta oscillation in CA1 in vitro. Neuron. 1995;15:1053–1063. doi: 10.1016/0896-6273(95)90094-2. [DOI] [PubMed] [Google Scholar]
- Hyman JM, Wyble BP, Goyal V, Rossi CA, Hasselmo ME. Stimulation in hippocampal region CA1 in behaving rats yields long-term potentiation when delivered to the peak of theta and long-term depression when delivered to the trough. The Journal of Neuroscience. 2003;23:11725–11731. doi: 10.1523/JNEUROSCI.23-37-11725.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman JM, Zilli EA, Paley AM, Hasselmo ME. Medial prefrontal cortex cells show dynamic modulation with the hippocampal theta rhythm dependent on behavior. Hippocampus. 2005;15:739–749. doi: 10.1002/hipo.20106. [DOI] [PubMed] [Google Scholar]
- Jacobs J, Hwang G, Curran T, Kahana MJ. EEG oscillations and recognition memory: theta correlates of memory retrieval and decision making. Neuroimage. 2006;32:978–987. doi: 10.1016/j.neuroimage.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Jensen O, Colgin LL. Cross-frequency coupling between neuronal oscillations. Trends in Cognitive Sciences. 2007;11:267–269. doi: 10.1016/j.tics.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Jensen O, Kaiser J, Lachaux JP. Human gamma-frequency oscillations associated with attention and memory. Trends in Neurosciences. 2007;30:317–324. doi: 10.1016/j.tins.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Jensen O, Lisman JE. Hippocampal sequence-encoding driven by a cortical multi-item working memory buffer. Trends in Neurosciences. 2005;28:67–72. doi: 10.1016/j.tins.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Jerbi K, Freyermuth S, Dalal S, Kahane P, Bertrand O, Berthoz A, et al. Saccade related gamma-band activity in intracerebral EEG: dissociating neural from ocular muscle activity. Brain Topography. 2009;22:18–23. doi: 10.1007/s10548-009-0078-5. [DOI] [PubMed] [Google Scholar]
- Jones MW, Wilson MA. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biology. 2005;3:e402. doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahana MJ. The cognitive correlates of human brain oscillations. The Journal of Neuroscience. 2006;26:1669–1672. doi: 10.1523/JNEUROSCI.3737-05c.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahana MJ, Seelig D, Madsen JR. Theta returns. Current Opinion in Neurobiology. 2001;11:739–744. doi: 10.1016/s0959-4388(01)00278-1. [DOI] [PubMed] [Google Scholar]
- Klimesch W. Memory processes, brain oscillations and EEG synchronization. International Journal of Psychophysiology. 1996;24:61–100. doi: 10.1016/s0167-8760(96)00057-8. [DOI] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Research Reviews. 1999;29:169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Schimke H, Ripper B. Theta synchronization and alpha desynchronization in a memory task. Psychophysiology. 1997;34:169–176. doi: 10.1111/j.1469-8986.1997.tb02128.x. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Schwaiger J, Winkler T, Gruber W. Theta oscillations and the ERP old/new effect: independent phenomena? Clinical Neurophysiology. 2000;111:781–793. doi: 10.1016/s1388-2457(00)00254-6. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Stadler W, Pöllhuber D, Sauseng P, Röhm D. Episodic retrieval is reflected by a process specific increase in human electroencephalographic theta activity. Neuroscience Letters. 2001;302:49–52. doi: 10.1016/s0304-3940(01)01656-1. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Yonelinas A, Kroll NE, Lazzara M, Röhm D, et al. Theta synchronization during episodic retrieval: neural correlates of conscious awareness. Cognitive Brain Research. 2001;12:33–38. doi: 10.1016/s0926-6410(01)00024-6. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Freunberger R, Sauseng P. Oscillatory mechanisms of process binding in memory. Neuroscience and Biobehavioral Reviews. doi: 10.1016/j.neubiorev.2009.10.004. (in press) [DOI] [PubMed] [Google Scholar]
- Klimesch W, Freunberger R, Sauseng P, Gruber W. A short review of slow phase synchronization and memory: Evidence for control processes in different memory systems? Brain Research. 2008:31–44. doi: 10.1016/j.brainres.2008.06.049. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Schack B, Schabus M, Doppelmayr M, Gruber W, Sauseng P. Phase-locked alpha and theta oscillations generate the P1-N1 complex and are related to memory performance. Cognitive Brain Research. 2004;19:302–316. doi: 10.1016/j.cogbrainres.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Schimke H, Schwaiger J. Episodic and semantic memory: an analysis in the EEG theta and alpha band. Electroencephalography and Clinical Neurophysiology. 1994;91:428–441. doi: 10.1016/0013-4694(94)90164-3. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Vogt F, Doppelmayr M. Interindividual differences in alpha and theta power reflect memory performance. Intelligence. 2000;27:347–362. [Google Scholar]
- Larson J, Lynch G. Induction of synaptic potentiation in hippocampus by patterned stimulation involves two events. Science. 1986;232:985–988. doi: 10.1126/science.3704635. [DOI] [PubMed] [Google Scholar]
- Larson J, Wong D, Lynch G. Patterned stimulation at the theta frequency is optimal for the induction of hippocampal long-term potentiation. Brain Research. 1986;368:347–350. doi: 10.1016/0006-8993(86)90579-2. [DOI] [PubMed] [Google Scholar]
- Lisman JE. Relating hippocampal circuitry to function: recall of memory sequences by reciprocal dentate-CA3 interactions. Neuron. 1999;22:233–242. doi: 10.1016/s0896-6273(00)81085-5. [DOI] [PubMed] [Google Scholar]
- Lisman JE. The theta/gamma discrete phase code occuring during the hippocampal phase precession may be a more general brain coding scheme. Hippocampus. 2005;15:913–922. doi: 10.1002/hipo.20121. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Idiart MA. Storage of 7 +/− 2 short-term memories in oscillatory subcycles. Science. 1995;267:1512–1515. doi: 10.1126/science.7878473. [DOI] [PubMed] [Google Scholar]
- Mainen ZF, Sejnowski TJ. Reliability of spike timing in neocortical neurons. Science. 1995;268:1503–1506. doi: 10.1126/science.7770778. [DOI] [PubMed] [Google Scholar]
- Mann EO, Paulsen O. Mechanisms underlying gamma (‘40 Hz’) network oscillations in the hippocampus--a mini-review. Progress in Biophysics and Molecular Biology. 2005;87:67–76. doi: 10.1016/j.pbiomolbio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Miller EK, Wilson MA. All my circuits: using multiple electrodes to understand functioning neural networks. Neuron. 2008;60:483–488. doi: 10.1016/j.neuron.2008.10.033. [DOI] [PubMed] [Google Scholar]
- Mölle M, Marshall L, Fehm HL, Born J. EEG theta synchronization conjoined with alpha desynchronization indicate intentional encoding. European Journal of Neuroscience. 2002;15:923–928. doi: 10.1046/j.1460-9568.2002.01921.x. [DOI] [PubMed] [Google Scholar]
- Mormann F, Fell J, Axmacher N, Weber B, Lehnertz K, Elger CE, et al. Phase/amplitude reset and theta-gamma interaction in the human medial temporal lobe during a continuous word recognition memory task. Hippocampus. 2005;15:890–900. doi: 10.1002/hipo.20117. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Recce ML. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus. 1993;3:317–330. doi: 10.1002/hipo.450030307. [DOI] [PubMed] [Google Scholar]
- Olvera-Cortés E, Cervantes M, Gonzalez-Burgos I. Place-learning, but not cue-learning training, modifies the hippocampal theta rhythm in rats. Brain Research Bulletin. 2002;58:261–270. doi: 10.1016/s0361-9230(02)00769-4. [DOI] [PubMed] [Google Scholar]
- Osipova D, Takashima A, Oostenveld R, Fernández G, Maris E, Jensen O. Theta and gamma oscillations predict encoding and retrieval of declarative memory. The Journal of Neuroscience. 2006;26:7523–7531. doi: 10.1523/JNEUROSCI.1948-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanca BJ, DeAngelis GC. Does neuronal synchrony underlie visual feature grouping? Neuron. 2005;46:333–346. doi: 10.1016/j.neuron.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Pavlides C, Greenstein YJ, Grudman M, Winson J. Long-term potentiation in the dentate gyrus is induced preferentially on the positive phase of theta-rhythm. Brain Research. 1988;439:383–387. doi: 10.1016/0006-8993(88)91499-0. [DOI] [PubMed] [Google Scholar]
- Penttonen M, Kamondi A, Acsády L, Buzsáki G. Gamma frequency oscillation in the hippocampus of the rat: intracellular analysis in vivo. European Journal of Neuroscience. 1998;10:718–728. doi: 10.1046/j.1460-9568.1998.00096.x. [DOI] [PubMed] [Google Scholar]
- Polyn SM, Kahana MJ. Memory search and the neural representation of context. Trends in Cognitive Sciences. 2008;12:24–30. doi: 10.1016/j.tics.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelfsema PR, Lamme VA, Spekreijse H. Synchrony and covariation of firing rates in the primary visual cortex during contour grouping. Nature Neuroscience. 2004;7:982–991. doi: 10.1038/nn1304. [DOI] [PubMed] [Google Scholar]
- Rose GM, Dunwiddie TV. Induction of hippocampal long-term potentiation using physiologically patterned stimulation. Neuroscience Letters. 1986;69:244–248. doi: 10.1016/0304-3940(86)90487-8. [DOI] [PubMed] [Google Scholar]
- Rotstein HG, Pervouchine DD, Acker CD, Gillies MJ, White JA, Buhl EH, et al. Slow and fast inhibition and an H-current interact to create a theta rhythm in a model of CA1 interneuron network. Journal of Neurophysiology. 2005;94:1509–1518. doi: 10.1152/jn.00957.2004. [DOI] [PubMed] [Google Scholar]
- Salinas E, Sejnowski TJ. Correlated neuronal activity and the flow of neural information. Nature Reviews Neuroscience. 2001;2:539–550. doi: 10.1038/35086012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauseng P, Griesmayr B, Freunberger R, Klimesch W. Control mechanisms in working memory: a possible function of EEG theta oscillations. Neuroscience and Biobehavioral Reviews. doi: 10.1016/j.neubiorev.2009.12.006. (in press) [DOI] [PubMed] [Google Scholar]
- Sederberg PB, Kahana MJ, Howard MW, Donner EJ, Madsen JR. Theta and gamma oscillations during encoding predict subsequent recall. The Journal of Neuroscience. 2003;23:10809–10814. doi: 10.1523/JNEUROSCI.23-34-10809.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sederberg PB, Schulze-Bonhage A, Madsen JR, Bromfield EB, Litt B, Brandt A, et al. Gamma oscillations distinguish true from false memories. Psychological Science. 2007;18:927–932. doi: 10.1111/j.1467-9280.2007.02003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sederberg PB, Schulze-Bonhage A, Madsen JR, Bromfield EB, McCarthy DC, Brandt A, et al. Hippocampal and neocortical gamma oscillations predict memory formation in humans. Cerebral Cortex. 2007;17:1190–1196. doi: 10.1093/cercor/bhl030. [DOI] [PubMed] [Google Scholar]
- Shadlen MN, Movshon JA. Synchrony unbound: a critical evaluation of the temporal binding hypothesis. Neuron. 1999;24:67–77. 111–125. doi: 10.1016/s0896-6273(00)80822-3. [DOI] [PubMed] [Google Scholar]
- Siapas AG, Lubenov EV, Wilson MA. Prefrontal phase locking to hippocampal theta oscillations. Neuron. 2005;46:141–151. doi: 10.1016/j.neuron.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL, Wilson MA, Barnes CA. Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus. 1996;6:149–172. doi: 10.1002/(SICI)1098-1063(1996)6:2<149::AID-HIPO6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Soltesz I, Deschênes M. Low- and high-frequency membrane potential oscillations during theta activity in CA1 and CA3 pyramidal neurons of the rat hippocampus under ketamine-xylazine anesthesia. Journal of Neurophysiology. 1993;70:97–116. doi: 10.1152/jn.1993.70.1.97. [DOI] [PubMed] [Google Scholar]
- Stäubli U, Xu FB. Effects of 5-HT3 receptor antagonism on hippocampal theta rhythm, memory, and LTP induction in the freely moving rat. The Journal of Neuroscience. 1995;15:2445–2452. doi: 10.1523/JNEUROSCI.15-03-02445.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield C, Mangels JA. Coherent theta-band EEG activity predicts item-context binding during encoding. Neuroimage. 2005a;24:692–703. doi: 10.1016/j.neuroimage.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Summerfield C, Mangels JA. Functional coupling between frontal and parietal lobes during recognition memory. Neuroreport. 2005b;16:117–122. doi: 10.1097/00001756-200502080-00008. [DOI] [PubMed] [Google Scholar]
- Teyler TJ, DiScenna P. The hippocampal memory indexing theory. Behavioral Neuroscience. 1986;100:147–154. doi: 10.1037//0735-7044.100.2.147. [DOI] [PubMed] [Google Scholar]
- Teyler TJ, Rudy JW. The hippocampal indexing theory and episodic memory: updating the index. Hippocampus. 2007;17:1158–1169. doi: 10.1002/hipo.20350. [DOI] [PubMed] [Google Scholar]
- Thiele A, Stoner G. Neuronal synchrony does not correlate with motion coherence in cortical area MT. Nature. 2003;421:366–370. doi: 10.1038/nature01285. [DOI] [PubMed] [Google Scholar]
- Traub RD, Whittington MA, Colling SB, Buzsáki G, Jefferys JG. Analysis of gamma rhythms in the rat hippocampus in vitro and in vivo. Journal of Physiology. 1996;493(Pt 2):471–484. doi: 10.1113/jphysiol.1996.sp021397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo LT, Peterson MA, Kaszniak AW, Allen JJ. EEG phase synchrony differences across visual perception conditions may depend on recording and analysis methods. Clinical Neurophysiology. 2005;116:172–189. doi: 10.1016/j.clinph.2004.07.025. [DOI] [PubMed] [Google Scholar]
- Varela F, Lachaux JP, Rodriguez E, Martinerie J. The brainweb: phase synchronization and large-scale integration. Nature Reviews Neuroscience. 2001;2:229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- von der Malsburg C, Schneider W. A neural cocktail-party processor. Biological Cybernetics. 1986;54:29–40. doi: 10.1007/BF00337113. [DOI] [PubMed] [Google Scholar]
- Ward LM, Doesburg SM, Kitajo K, MacLean SE, Roggeveen AB. Neural synchrony in stochastic resonance, attention, and consciousness. Canadian Journal of Experimental Psychology. 2006;60:319–326. doi: 10.1037/cjep2006029. [DOI] [PubMed] [Google Scholar]
- Weiss S, Müller HM, Rappelsberger P. Theta synchronization predicts efficient memory encoding of concrete and abstract nouns. Neuroreport. 2000;11:2357–2361. doi: 10.1097/00001756-200008030-00005. [DOI] [PubMed] [Google Scholar]
- Weiss S, Rappelsberger P. Long-range EEG synchronization during word encoding correlates with successful memory performance. Cognitive Brain Research. 2000;9:299–312. doi: 10.1016/s0926-6410(00)00011-2. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Kopell N, Ermentrout B, Buhl EH. Inhibition-based rhythms: experimental and mathematical observations on network dynamics. International Journal of Psychophysiology. 2000;38:315–336. doi: 10.1016/s0167-8760(00)00173-2. [DOI] [PubMed] [Google Scholar]
- Wiebe SP, Stäubli UV. Recognition memory correlates of hippocampal theta cells. The Journal of Neuroscience. 2001;21:3955–3967. doi: 10.1523/JNEUROSCI.21-11-03955.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winson J. Loss of hippocampal theta rhythm results in spatial memory deficit in the rat. Science. 1978;201:160–163. doi: 10.1126/science.663646. [DOI] [PubMed] [Google Scholar]
- Womelsdorf T, Schoffelen JM, Oostenveld R, Singer W, Desimone R, Engel AK, et al. Modulation of neuronal interactions through neuronal synchronization. Science. 2007;316:1609–1612. doi: 10.1126/science.1139597. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Sato N, Wagatsuma H, Wu Z, Molter C, Aota Y. A unified view of theta-phase coding in the entorhinal-hippocampal system. Current Opinion in Neurobiology. 2007 doi: 10.1016/j.conb.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Yuval-Greenberg S, Deouell LY. The broadband-transient induced gamma-band response in scalp EEG reflects the execution of saccades. Brain Topography. 2009;22:3–6. doi: 10.1007/s10548-009-0077-6. [DOI] [PubMed] [Google Scholar]
- Yuval-Greenberg S, Tomer O, Keren AS, Nelken I, Deouell LY. Transient induced gamma-band response in EEG as a manifestation of miniature saccades. Neuron. 2008;58:429–441. doi: 10.1016/j.neuron.2008.03.027. [DOI] [PubMed] [Google Scholar]