Abstract
Acute inflammation is a common feature of many life-threatening pathologies, including septic shock. One hallmark of acute inflammation is the peroxidation of polyunsaturated fatty acids forming bioactive products, which regulate inflammation. Myeloperoxidase (MPO) is an abundant phagocyte-derived hemoprotein released during phagocyte activation. Here, we investigated the role of MPO in modulating biologically active arachidonic acid (AA) and linoleic acid (LA) metabolites during acute inflammation. Wild-type and MPO-knockout (KO) mice were exposed to intraperitoneally injected endotoxin for 24 h, and plasma LA and AA oxidation products were comprehensively analyzed using a liquid chromatography-mass spectrometry method. Compared to wild-type mice, MPO-KO mice had significantly lower plasma levels of LA epoxides and corresponding LA- and AA-derived fatty acid diols. AA and LA hydroxy intermediates (hydroxyeicosatetraenoic and hydroxyoctadecadienoic acids) were also significantly lower in MPO-KO mice. Conversely, MPO-deficient mice had significantly higher plasma levels of cysteinyl-leukotrienes with well-known pro-inflammatory properties. In vitro experiments revealed significantly lower amounts of AA and LA epoxides, LA- and AA-derived fatty acid diols, and AA and LA hydroxy intermediates in stimulated polymorphonuclear neutrophils isolated from MPO-KO mice. Our results demonstrate that MPO modulates the balance of pro- and anti-inflammatory lipid mediators during acute inflammation. In this way, may control acute inflammatory diseases.
Keywords: Myeloperoxidase, Lipoxygenase, Epoxygenase, Pro-inflammatory mediators, Sepsis
Introduction
Acute inflammation is a common sign of many acute life-threatening pathologies including septic shock and multiple organ failure [1]. Polymorphonuclear neutrophils (PMNs) play an important role in the pathogenesis of sepsis, producing a wide range of inflammatory mediators. However, the exact mechanisms by which these cells participate in sepsis remain incompletely characterized. Activated PMNs release myeloperoxidase (MPO), an abundant hemoprotein that represents up to 5% of total PMN proteins. MPO is thought to mediate primarily host defense reactions [2-4]. Recent evidence suggests that, aside from its role in host defense, reactive intermediates formed by MPO-catalyzed reactions may modify signaling mediators, leading to alterations in cellular signaling [3, 5, 6]. During acute inflammation, MPO contributes to vascular dysfunction by nitric oxide catalytic consumption and suppressing nitric oxide bioavailability, an effect that disrupts nitric oxide-dependent signaling pathways [7]. Further, MPO catalyzes posttranslational modification of proteins (e.g. chlorination, nitration, and dityrosine bridges formation), in a way that may affect their structure and function [2, 3, 8-10]. The ability of MPO to modulate redox-sensitive signaling pathways may point to a role for this protein in regulating the inflammatory process [5, 7].
Lipid peroxidation is a characteristic feature of acute inflammation, including inflammation associated with septic shock [11]. Oxidative metabolites of arachidonic acid (AA) and linoleic acid (LA) are potent inflammatory mediators, and an increase in their synthesis generally correlates with the severity of sepsis or trauma [11]. Epoxides of AA and LA are epoxyeicosatrienoic acids (EETs) and epoxyoctadecenoic acids (EpOMEs). EETs have the ability to decrease inflammatory responses, including fever [12, 13]. EETs and EpOMEs are further metabolized by soluble epoxide hydrolase to their corresponding diols, dihydroxyeicosatrienoic acids (DHETs) and dihydroxyoctadecenoic acids (DHOMEs) [12-16]. In contrast to epoxides, DHETs posses less anti-inflammatory properties and DHOMEs are mostly pro-inflammatory [13, 17]. Other initial products of AA and LA metabolism are hydroperoxy-eicosatrienoic acids (HPETEs) and hydroperoxy-octadecadienoic acids (HPODEs), respectively, and their corresponding reduced forms, hydroxyeicosatetraenoic acids (HETEs) and hydroxyoctadecadienoic acids (HODEs) [13, 18]. H(P)ETEs and H(P)ODEs stimulate both pro- and anti-inflammatory pathways depending on the positional isomer of the metabolite, cell type, and bioactivity levels [18]. Hydroperoxy fatty acids can be converted to specific epoxy leukotrienes (LTs). Primarily, 5-HPETE is metabolized to the highly unstable epoxide intermediate LTA4. Further, LTB4 is formed from LTA4 or LTA4 conjugates with glutathione to yield LTC4, which is additionally metabolized to LTD4 and LTE4 [18, 19]. LTs have long been recognized as potent inducers of the inflammatory response [19].
Cytochrome P450 mono-oxygenases, cyclooxygenases, and lipoxygenases (LOXs) are widely recognized as the primary enzymes participating in the formation of biologically active lipid mediators [11, 13, 18]. However, various heme peroxidases that are structurally similar to the family of cytochrome P450 enzymes may also participate in the metabolism of biologically active lipids [20]. Among these is MPO. The MPO/hydrogen peroxide system or hypochlorous acid (a MPO-derived oxidant) promotes the formation of epoxides, peroxides, and chlorohydrins from polyunsaturated fatty acids and cholesterol in isolated PMNs, high-density lipoprotein particles, or chemical systems [21-33]. Recent studies of MPO-KO mice also show that MPO catalyzes the initiation of lipid peroxidation and lipoprotein oxidation in vivo [8, 9, 34, 35]. However, whether MPO participates in the formation of AA- and LA-derived lipid mediators that are involved in the regulation of the inflammatory response is unclear.
Here, we investigated MPO-dependent modulation of selected biologically active AA and LA metabolites in mice with acute inflammation induced by i.p. application of lipopolysaccharide (LPS), a non-infectious model of systemic sepsis in mice. We focused on AA and LA metabolites that are generally considered as products of Cytochrome P450 mono-oxygenase and lipoxygenase pathways. Alterations in a wide spectra of AA- and LA-derived lipid oxidation products were simultaneously monitored in the plasma of MPO-KO and wild-type mice and isolated PMNs using a unique liquid chromatography-mass spectrometry method [14, 15]. Our findings show that MPO modulates the formation of pro- and anti-inflammatory lipid mediators and suggest that this enzyme is a direct systemic regulator of the acute inflammatory response.
Material and methods
Materials
The following AA and LA metabolites were purchased from Cayman Chemicals (Ann Arbor, MI): (±)9(10)epoxy-12Z-octadecenoic acid [9(10)-EpOME]; (±)12(13)-epoxy-9Z-octadecenoic acid [12(13)-EpOME]; (±)5(6)-epoxy-8Z,11Z,14Z-eicosatrienoic acid [5(6)-EET); (±)8(9)-epoxy-5Z,11Z,14Zeicosatrienoic acid [8(9)-EET]; (±)14(15)-epoxy-5Z,8Z,11Z- eicosatrienoic acid [14(15)-EET]; (±)11(12)-epoxy-5Z,8Z,14Z-eicosatrienoic acid [11(12)-EET]; (±)13-hydroxy-9Z,11E-octadecadienoic acid (13-HODE); (±)9-hydroxy-10E,12Z- octadecadienoic acid (9-HODE); 5-HETE; 8-HETE; 9-HETE; 11-HETE; 12-HETE; 15-HETE; 19-HETE, 20-HETE; 5,6-DHET; 8,9-DHET; 11,12-DHET; 14,15-DHET, 9-oxo-10E,12Z-octadecadienoic acid (9-oxo-ODE); 13-keto-9Z,11E-octadecadienoic acid (13-oxo-ODE); 5-oxo-6E,8Z,11Z,14Z-eicosatetraenoic acid (5-oxo-ETE); 15-oxo-5Z,8Z,11Z,13E-eicosatetraenoic acid (15-oxo-ETE); leukotriene B4 (LTB4); LTC4; LTD4; LTE4; N-acetyl-leukotriene E4 (Na-LTE4); and 6-oxo-9α,11α,15S-trihydroxy-prost-13E-en-1-oic-3,3,4,4-d4 acid (6-keto prostaglandin F1α–d4). Lordan Fine Lipids provided (±)9,10-Dihydroxy-12(Z)-octadecenoic Acid (9,10-DHOME) and (±)12,13-Dihydroxy-9(Z)-octadecenoic Acid (12,13-DHOME). The remainder of the metabolites were synthesized in house [12-(3-cyclohexylureido)-dodecanoic acid; 10(11)-epoxyheptadecanoic acid; and 10(11)-dihydroxynonadecanoic acid] as described previously [36]. Omni-Solv™ acetonitrile and methanol were from EM Science (Gibbstown, NJ). All other chemical reagents of a high performance liquid chromatography grade or better were purchased from Sigma-Aldrich (St. Louis, MO) or Fisher Scientific (Pittsburgh, PA).
Animal experiment
All animal protocols were approved by the Animal Research Committee of the University of California at Davis. MPO-KO mice backcrossed on a C57BL/6J background have been comprehensively characterized elsewhere [37-39]. Age- and sex-matched wild-type C57BL/J6 mice and 4-month-old male MPO-KO mice weighing 22 – 28 g were given a single i.p. injection of E. coli lipopolysaccharide (LPS) (10 mg/kg), which was freshly prepared in sterile phosphate buffer saline (pH 7.4). Control mice received an equivalent amount of sterile phosphate buffer saline. The LPS murine model of acute systemic inflammation was selected since we have previously observed extensive activation of the P450-epoxygenase/soluble epoxide hydrolase and LOXs lipid metabolism pathways in this model [14, 15]. Twenty-four hours after LPS administration, the mice received an overdose of pentobarbital (i.p.), and blood was collected via cardiac puncture with an ethylenediaminetetraacetic acid-rinsed syringe. The plasma was immediately separated, and a combination of triphenylphosphine, ethylenediaminetetraacetic acid (1 mM), and butylated hydroxytoluene (0.2% w/w) was added to stabilize the samples. All samples were stored at < -70°C until analysis.
In vitro experiments with isolated PMNs
Isolation of mouse neutrophils was performed, as described previously, with modifications [40, 41]. A sterile solution of casein (2% w/v in phosphate buffer saline) was injected into the peritoneal cavity of the mice (1 ml per mouse) to enrich the exudates of PMNs. The mice were euthanized by carbon dioxide 24 h later, and peritoneal lavage was performed using repeated applications of phosphate buffer saline. The resulting cells were washed twice with ice-cold phosphate buffer saline (200 × g for 10 min at 4°C), and contaminating erythrocytes were lysed in 0.45% NaCl. PMNs were purified using Histopaque-1077 and -1119 (800 × g for 30 min at room temperature), washed three times with ice-cold phosphate buffer saline (200 × g for 10 min at 4°C), and resuspended in Hank's balanced salt solution containing 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid. All samples were > 93% pure as determined by Diff-Quik staining and had > 96% viable PMNs as determined by trypan blue dye exclusion. For determination of AA and LA metabolites, PMN samples were pooled from four or five wild-type or MPO-KO mice to obtain a sufficient amount of PMNs. PMNs (2 × 106) in 1 ml of Hank's balanced salt solution were pre-incubated with or without AA (50 μM) and LA (50 μM) at 37°C for 5 min. Stock solutions of AA and LA were in ethanol and final concentration of ethanol in cell suspension was < 0.05%. A subset of samples was incubated for an additional 30 min (control PMNs). Another subset (activated PMNs) was stimulated first with PMA (phorbol 12-myristate 13-acetate) (100 nM) for 5 min and then with the calcium ionophore A23187 (2 μM) for 25 min. Stimulation of samples was terminated with 0.5 ml ice-cold methanol. A combination of triphenylphosphine, ethylenediaminetetraacetic acid, and butylated hydroxytoluene (0.2% w/w) was then immediately added to stabilize the samples. All samples were stored at < -70°C until analysis.
Oxylipin profile analysis
The analysis was performed as described previously [42-44]. Immediately before solid phase extraction, 250 μl serum or 1.5 ml PMN suspension was diluted 1:1 (v/v) with 2.5 mM phosphoric acid. Each sample was then spiked with surrogates 26.7 nM 6-keto prostaglandin F1α–d4, 26.7 nM 10(11)-epoxyheptadecanoic acid, and 26.7 nM 10(11)-dihydroxynonadecanoic acid. Oasis HLB 60 mg cartridges (Waters Milford, MA) were preconditioned with 2 ml of methanol and 2 ml of solution containing 2.5 mM phosphoric acid and 10% methanol (pH 3.8). After the samples were applied, the cartridges were washed with 2 ml of the 2.5 mM phosphoric acid-10% methanol mixture. The analytes were eluted with 2 ml of ethyl acetate. The ethyl acetate residue was evaporated under nitrogen gas, and the samples were resuspended in 100 μl of methanol containing 26.7 nM of the internal standard 12-(3-cyclohexylureido)-dodecanoic acid. The samples were then vortexed for 5 min, transferred to autosample vials, and stored at -80°C until analysis. A Waters 2790 separation module equipped with a Luna C18 column (2.0 × 150 mm, 5-μm; Phenomenex Torrance, CA) and a Quattro Ultima tandem-quadrupole mass spectrometer (Micromass, Manchester, UK) equipped with an electrospray ionization source were used for high performance liquid chromatography separation and electrospray tandem mass spectrometry, respectively. Mobile phase A was water with 0.1% glacial acetic acid. Mobile phase B consisted of acetonitrile: methanol (84:16) with 0.1% glacial acetic acid. Gradient elution was performed at a flow rate of 400 μL /min. Chromatography was optimized to separate all analytes in 21 min. The Quattro Ultima tandem-quadrupole mass spectrometer was operated in multiple reaction monitoring modes, and both negative and positive ion electrospray was used as the means of ionization.
Data collection and processing were performed using MassLynx software v 4.0, as described previously [14, 15, 43, 44]. Surrogates were added to samples before extraction to mimic the extraction of AA and LA metabolites. Internal standard was used to verify surrogate recovery and to monitor instrument response. The analytes were linked to their corresponding surrogates for the purpose of quantification. In addition, if the surrogate recoveries, as monitored by internal standards, were not between 80-120%, the sample was considered invalid and not included in the dataset. Calibration curves were prepared to generate a series of six calibration points spanning a concentration range from 0.1– 100 nM of all analytes, with the surrogates and internal standards (at a constant concentration of 26.7 nM).
Statistical analysis
Data are expressed as mean ± standard error of the mean (SEM). Groups were compared using an unpaired Student's t test. Values of p less than 0.05 were considered significant.
Results
MPO deficiency suppresses endotoxemia-induced formation of LA epoxides and vicinal dihydroxy metabolites of AA and LA
To model acute inflammation associated with systemic endotoxemia/sepsis, we injected (i.p.) mice with LPS and measured AA and LA metabolites 24 h later. In wild-type animals, LPS-induced endotoxemia greatly increased the plasma levels of LA epoxides (EpOMEs) as well as vicinal dihydroxy metabolites of AA and LA (DHETs and DHOMEs, respectively), confirming that the P450-epoxygenase/soluble epoxide hydrolase lipid metabolism pathways are activated in our non-infectious model of systemic sepsis (Table 1). Interestingly, following induction of septic MPO-KO mice had significantly lower plasma levels of 12(13)-EpOME; 9,10-DHOME; and 12,13-DHOME. Septic MPO-KO mice also had significantly lower levels of 5,6-DHETE; 8,9-DHETE; 11,12-DHETE; and 14,15-DHETE. The plasma concentration of another LA epoxide, 9(10)-EpOME, was not significantly different in WT and MPO-KO mice. The legend to Table 1. lists other metabolites belonging to these metabolic pathways and that fall below the detection limit in both wild-type and MPO-KO mice.
Table 1. MPO deficiency suppresses endotoxemia-induced increases in EpOMEs, DHOMEs, and DHETs.
Plasma levels of EpOMEs (epoxides of LA), DHOMEs (dihydroxy metabolites of LA), and DHETs (dihydroxy metabolites of AA) at 24 h after induction of endotoxemia. Results represent mean ± SEM derived from at least four animals per group. *p < 0.05 MPO-KO vs. wild-type, +p < 0.05 control wild-type vs. LPS treated wild-type, #p < 0.05 control MPO-KO vs. LPS treated MPO-KO. Plasma levels of other determined metabolites including epoxides of AA (EETs) and degradation products 9-oxo-ODE, 13- oxo-ODE, 5-oxo-ETE, and 15-oxo-ETE were under detection limits in both wild-type and MPO-KO mice. Limits of quantification are in Supplement Table 1.
| Plasma concentration (nM) | Control WT | Control MPO-KO | LPS WT | LPS MPO-KO |
|---|---|---|---|---|
| (±)9(10)epoxy-12Z-octadecenoic acid (9(10)-EpOME) | 3.5 ± 1.0 | 4.3 ± 0.7 | 5.5 ± 1.1 | 3.0 ± 0.5 |
| (±)12(13)-epoxy-9Z-octadecenoic acid (12(13)-EpOME) | 6.0 ± 0.9 | 6.9 ± 1.1 | 13.0 ± 2.13 + | 7.4 ± 0.8 * |
| (±)9,10-dihydroxy-12(Z)-octadecenoic acid (9,10-DHOME) | 2.7 ± 0.4 | 4.0 ± 1.0 | 19.4 ± 4.4 + | 11.0 ± 1.4 # |
| (±)12,13-dihydroxy-9(Z)-octadecenoic acid (12,13-DHOME) | 9.1 ± 2.1 | 12.1 ± 3.8 | 50.6 ± 9.4 + | 30.4 ± 3.1 *# |
| (±)5,6-dihydroxy-8Z, 11Z, 14Z-eicosatrienoic acid (5,6-DHET) | 2.3 ± 0.5 | 1.6 ± 0.5 | 6.4 ± 1.7 + | 3.0 ± 0.6 * |
| (±)8,9-dihydroxy-5Z, 11Z, 14Z-eicosatrienoic acid (8,9-DHET) | 5.0 ± 1.3 | 4.9 ± 0.3 | 12.2 ± 1.2 + | 7.7 ± 1.1 *# |
| (±)11,12-dihydroxy-5Z, 8Z, 14Z-eicosatrienoic acid (11,12-DHET) | 3.2 ± 0.5 | 3.5 ± 0.3 | 10.6 ± 1.8 + | 6.0 ± 0.6 *# |
| (±)14,15-dihydroxy-5Z,8Z, 11Z-eicosatrienoic acid (14,15-DHET) | 5.4 ± 0.7 | 4.5 ± 0.3 | 15.8 ± 2.1 + | 7.9 ± 0.8 *# |
MPO deficiency suppresses endotoxemia-induced increases in HETEs and HODE
In wild-type mice, endotoxin challenge also significantly increased the formation of lipid metabolites from LOX-catalyzed pathways (Table 2). However, induction of these AA (5-HETE, 11-HETE, 12-HETE, and 15-HETE) and LA (9-HODE and 13-HODE) metabolites was significantly suppressed in MPO-KO mice. The 12-HETE plasma concentrations were approximately two orders higher compared to other metabolites and associated with high variability. This was most likely due to contamination from platelets in the isolated plasma samples. The legend to Table 2. lists other metabolites belonging to these metabolic pathways and that fall below the detection limit in both wild-type and MPO-KO mice.
Table 2. MPO deficiency suppresses endotoxemia-induced increases in HETEs and HODEs.
Plasma levels of HETEs (AA-derived lipid metabolites) and HODEs (LA-derived lipid metabolites) generated by LOX-catalyzed pathways. HETEs and HODEs were analyzed 24 h after induction of endotoxemia. Results represent mean ± SEM derived from at least four animals per group. *p < 0.05 MPO-KO vs. wild-type, +p < 0.05 control wild-type vs. LPS treated wild-type, #p < 0.05 control MPO-KO vs. LPS treated MPO-KO. Plasma levels of other determined metabolites including 8-HETE, 9-HETE, 19-HETE, and 20-HETE were under detection limits in both wild-type and MPO-KO mice. Limits of quantification are in Supplement Table 1.
| Plasma concentration (nM) | Control WT | Control MPO-KO | LPS WT | LPS MPO-KO |
|---|---|---|---|---|
| (±)5-hydroxy-6E, 8Z,11Z, 14Z-eicosatetraenoic acid (5-HETE) | 3.1 ± 0.4 | 2.2 ± 0.2 * | 18.4 ± 2.8 + | 8.0 ± 1.3 *# |
| (±)11-hydroxy-5Z, 8Z,12E, 14Z-eicosatetraenoic acid (11-HETE) | 1.38 ± 0.34 | 1.01 ± 0.27 | 2.39 ± 0.21 + | 0.89 ± 0.25 * |
| (±)12-hydroxy-5E, 8Z,10Z, 14Z-eicosatetraenoic acid (12-HETE) | 3485 ± 786 | 3202 ± 374 | 4555 ± 665 | 3869 ± 746 * |
| (±)12-hydroxy-5E, 8Z,10Z, 14Z-eicosatetraenoic acid (15-HETE) | 1.24 ± 0.18 | 1.19 ± 0.08 | 2.03 ± 0.31 + | 0.97 ± 0.08 *# |
| (±)9-hydroxy-10E,12Z-octadecadienoic acid (9-HODE) | 5.5 ± 1.2 | 5.2 ± 0.9 | 10.6 ± 0.7 + | 5.8 ± 1.0 * |
| (±)13-hydroxy-9Z,11E-octadecadienoic acid (13-HODE) | 8.4 ± 1.3 | 7.6 ± 1.3 | 22.3 ± 2.2 + | 11.8 ± 1.2 *# |
MPO deficiency increases endotoxemia-induced formation of LTs
The involvement of the enzyme 5-LOX in AA and LA metabolism is complex, since its primary metabolite 5-hydroperoxy arachidonate (5-HPETE) feeds multiple biosynthetic pathways, including pathways for the synthesis of LTs and 5-HETE. In an alternate metabolic pathway, 5-HPETE can undergo stereospecific dehydration to LTA4 by a second 5-LOX-catalyzed step. LTA4 can then be converted to LTB4 or by LTC4 synthase into the cysteinyl-LTs LTC4, LTD4, and LTE4. In contrast to other AA and LA mediators, cysteinyl-LTs revealed to be higher in plasma of MPO-KO mice than in plasma of wild-type mice (Table 3). Levels of LTA4 were not detectable in this study and LTB4 did not differ significantly among WT and MPO-KO mice. However, particularly interesting were significantly higher levels of LTD4, LTE4, and LTE4-NA in plasma of septic MPO-KO mice. LTC4 and LTE4 levels were also higher in control MPO-KO mice than in wild-type mice, suggesting that these cysteinyl-LTs accumulate in MPO-KO mice even in the absence of an acute inflammatory response. This could due to a disturbance of peritoneum by i.p. application of sterile saline in control mice.
Table 3. MPO deficiency enhances endotoxemia-induced increases in cysteinyl LTs.
LT plasma levels were analyzed 24 h after endotoxemia induction. Results represent mean ± SEM derived from at least four animals per group. *p < 0.05 MPO-KO vs. wild-type, +p < 0.05 control wild-type vs. LPS treated wild-type, #p < 0.05 control MPO-KO vs. LPS treated MPO-KO. Plasma levels of LTA4 were under detection limits in both wild-type and MPO-KO mice. Limits of quantification are in Supplement Table 1.
| Plasma concentration (nM) | Control WT | Control MPO-KO | LPS WT | LPS MPO-KO |
|---|---|---|---|---|
| Leukotriene B4 (LTB4) | 0.4 ± 0.5 | 0.5 ± 0.7 | 16.2 ± 6.8 | 20.6 ± 4.3 |
| Leukotriene C4 (LTC4) | 2.1 ± 1.4 | 9.9 ± 0.6 * | 9.2 ± 1.4 + | 16.3 ± 1.8 *# |
| Leukotriene D4 (LTD4) | 3.3 ± 2.1 | 4.1 ± 1.9 | 6.6 ± 1.6 | 24.9 ± 4.9 *# |
| Leukotriene E4 (LTE4) | 0.9 ± 0.3 | 4.2 ± 0.8 * | 3.1 ± 0.6 + | 14.3 ± 1.5 *# |
| N-acetyl-leukotriene E4 (LTE4-NA) | 0.1 ± 0.0 | 0.1 ± 0.0 | 6.4 ± 0.5 + | 14.6 ± 4.2 *# |
Activated PMNs from wild-type mice contain significantly higher levels of AA and LA epoxides, vicinal dihydroxy metabolites, HETEs, and HODEs than those from MPO-KO mice
Knowing that the MPO deficiency affects systemic fatty acid metabolism in septic mice, we evaluated AA and LA mediator profiles in PMNs isolated from MPO-KO and wild-type mice. In these experiments, purified AA (50 μM) and LA (50 μM) were added to a suspension of isolated PMNs, which were then activated by successive exposure to PMA and a calcium ionophore. Samples containing non-activated PMNs and lacking exogenous AA and LA served as a control. The need of the AA and LA concentrations selected, as well as the use of the combination of PMA and calcium ionophore, were intended to maximize production of AA and LA mediators by isolated PMNs, and were based on preliminary data and literature [41, 45, 46].
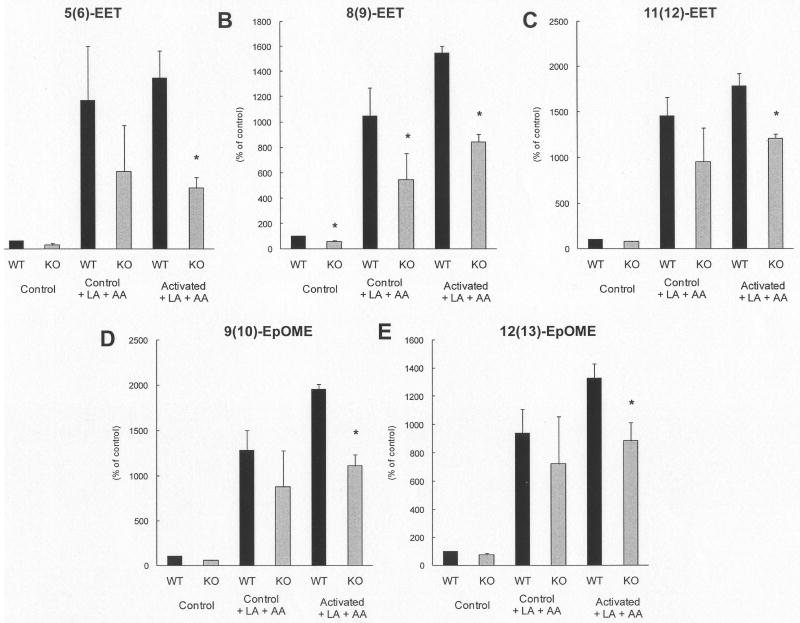
Consistent with our in vivo findings, PMNs isolated from MPO-KO mice formed, in the presence of exogenous AA and LA, significantly lower levels of AA epoxides [5(6)-EET, 8(9)-EET, 11(12)-EET, and 14(15)-EET] and LA epoxides [9(10)EpOME and 12(13)-EpOME)] than wild-type PMNs (Fig. 1). Correspondingly, levels of vicinal diols of AA and LA were detected to be significantly lower in MPO-KO PMNs in the presence of exogenous AA and LA (Fig. 2). Concentrations of AA metabolites (5-HETE, 8-HETE, 9-HETE) and LA metabolites (9-HODE and 13-HODE) were significantly lower in PMNs isolated from MPO-KO mice (Fig. 3). Oxo-octadeca-dienoic acids (9-oxo-ODE and 13-oxo-ODE), which are formed by degradation of unstable LA-derived hydroperoxides (HPODE), were significantly decreased in MPO-KO PMNs in the presence of exogenous AA and LA (Fig. 4A, B). The same was true of the AA metabolites, oxo-epoxyeicosatrienoic acids (5-oxo-EET and 15-oxo-EET) (Fig. 4C, D). The complex profile of LTs was not analyzed in the homogenates of PMNs. However, determination of cysteinyl-LTs in supernatants of PMNs isolated from wild-type and MPO-KO mice incubated in absence and presence of PMA for 10 min, 30 min, and 120 min showed no significant differences (data not shown).
Fig. 1. Increased production of EET and EpOME by PMNs isolated from wild-type compared to PMNs isolated from MPO-KO mice.
Relative levels of EETs (AA epoxides) and EpOMEs (LA epoxides) in control and PMA/calcium ionophore activated PMNs incubated in the absence or the presence of AA and LA. Results are expressed as the percentage (mean ± SEM) of wild-type controls incubated in the absence of AA and LA and are derived from at least three samples (pooled from four or five animals). p < 0.05 MPO-KO vs. wild-type, #p < 0.05 control MPO-KO vs. LPS treated MPO-KO. Average values in control samples of WT mice 5(6)-EET - 4.72 nM; 8(9)-EET - 2.33 nM; 11(12)-EET - 2.02 nM; 9(10)-EpOME - 2.22 nM; and 12(13)-EpOME - 2.97 nM. Concentrations of 14(15)-EET were under detection limits in samples from both wild-type and MPO-KO mice. Limits of quantification are in Supplement Table 1.
Fig. 2. Increased production of DHET and DHOME by PMNs isolated from wild-type compared to PMNs isolated from MPO-KO mice.
Relative levels of DHETs (AA dihydroxy metabolites) and DHOMEs (LA dihydroxy metabolites) in control and PMA/calcium ionophore activated PMNs incubated in the absence or the presence of AA and LA. Results are expressed as the percentage (mean ± SEM) of wild-type controls incubated in the absence of AA and LA. All results are derived from at least three samples (pooled from four or five animals). p < 0.05 MPO-KO vs. wild-type, +p < 0.05 control wild-type vs. LPS treated wild-type, #p < 0.05 control MPO-KO vs. LPS treated MPO-KO. Average values in control samples of WT mice 11,12-DHET - 0.10 nM;14,15-DHET - 0.09 nM; 9,10-DHOME - 0.10 nM; 12,13-DHOME - 0.22 nM. Concentrations of 5,6-DHET and 8,9-DHET were under detection limits in samples from both wild-type and MPO-KO mice. Limits of quantification are in Supplement Table 1.
Fig. 3. Increased production of HETE and HODE by PMNs isolated from wild-type compared to PMNs isolated from MPO-KO mice.
Relative levels of AA- (HETEs) and LA-derived (HODEs) lipid metabolites in control and PMA/calcium ionophore activated PMNs incubated in the absence or the presence of AA and LA. Results are expressed as the percentage (mean ± SEM) of wild-type controls incubated in the absence of AA and LA and are derived from at least three samples (pooled from four or five animals). p < 0.05 MPO-KO vs. wild-type, +p < 0.05 control wild-type vs. LPS treated wild-type, #p < 0.05 control MPO-KO vs. LPS treated MPO-KO. Average values in control samples of WT mice 5-HETE - 17.72 nM, 8-HETE - 0.1 nM, 9-HETE - 0.1 nM, 9 HODE - 73.07 nM, and 13 HODE - 65.76 nM. Concentrations of 11-HETE, 12-HETE, 15-HETE, 19-HETE, and 20-HETE were under detection limits in samples from both wild-type and MPO-KO mice. Limits of quantification are in Supplement Table 1.
Fig. 4. Increased production of oxo ODEs and oxo EETs by PMNs isolated from wild-type compared to PMNs isolated from MPO-KO mice.
Relative levels of oxo ODEs (LA degradation products) and oxo-EETs (AA degradation products) in control and PMA/calcium ionophore activated PMNs incubated in the absence or the presence of AA and LA. Results are expressed as the percentage (mean ± SEM) of wild-type controls incubated in the absence of AA and LA and are derived from at least three samples (pooled from four or five mice). p < 0.05 MPO-KO vs. wild-type, +p < 0.05 control wild-type vs. LPS treated wild-type, #p < 0.05 control MPO-KO vs. LPS treated MPO-KO. Average values in control samples of WT mice 9-oxo-ODE - 10.27 nM, 13-oxo-ODE - 20.62 nM, 5-oxo-EET - 2.11 nM, 15-oxo-EET - 5.75 nM. Limits of quantification are in Supplement Table 1.
Wild-type and MPO-KO mice have comparable numbers of blood leukocytes and differentiation counts
The number of peripheral blood leukocytes and PMNs was determined in wild-type and MPO-KO mice before sepsis and 24 h after induction of sepsis. Both wild-type and MPO-KO mice exhibited a drop in peripheral blood PMNs after endotoxin challenge. However, no significant differences in total leukocyte blood count and differentials were observed (control wild type 4.31 ± 0.54 × 109/L with 22 ± 9 % neutrophil granulocytes; control MPO-KO 4.05 ± 0.62 × 109/L with 25 ± 11 % neutrophil granulocytes; LPS treated wild types 3.22 ± 0.32 × 109/L with 35 ± 10 % neutrophil granulocytes; and LPS treated MPO-KO 3.48 ± 0.46 × 109/L with 34 ± 12 % neutrophil granulocytes). Eosinophilic polymorphonuclear granulocytes were under 2 % and basophilic polymorphonuclear granulocytes were not found. Similarly, other authors have not found any significant differences in the number of leukocytes and PMNs between wild-type and MPO-KO mice in various inflammatory models [35, 39, 47].
Discussion
Our results illustrate the significance of MPO in the formation of biologically active metabolites of AA and LA. Data suggests that during acute inflammation, MPO plays an important role in the formation of AA and LA epoxides and hydroxy intermediates together with the catabolism of cysteinyl-LTs. Accordingly, the formation of AA and LA lipid mediators (HETEs, HODEs and H(P)ODEs) was suppressed in MPO-KO mice and PMNs isolated from these animals. Interestingly, the difference between AA and LA metabolites formed in PMNs from wild-type and MPO-KO mice was also observed in the absence of any direct oxidative burst stimulation. However, PMNs elicited into the peritoneum by casein injection are partially activated although some responsiveness to pro-inflammatory stimuli remains [40, 41]. Thus the activation of PMNs with casein together with the activation of PMNs by the isolation procedure can partly trigger PMNs to produce the reactive oxygen species including hydrogen peroxide. However, this observation suggests that metabolic pathways not directly related to the activation of MPO can also be involved in the observed phenomenon.
Several mechanisms may underlie initiation of lipid peroxidation by MPO-catalyzed reactions. A wide range of oxidized lipid intermediates were shown to be formed in reactions of cholesterol and unsaturated fatty acids with MPO in the presence of low molecular weight intermediates (such as chloride, nitrite, or tyrosine) through MPO-generated diffusible radical species [2, 3, 8-10]. An alternative diffusible radical species formed by MPO that may participate in lipid peroxidation is nitrogen dioxide [28, 29, 48, 49]. Epoxides can be formed from chlorohydrins that undergo HCl elimination [21, 22, 50]. Further, MPO as a member of the family of heme peroxidases can also be suggested to directly function as a fatty acid epoxygenase and lipoxygenase [20, 51]. However, the epoxidation mechanisms catalyzed by heme proteins are not well understood. The putative mechanism for the epoxidation of alkenes by chloroperoxidases, also known as the ferryl-oxygen transfer mechanism [20, 52], is comparable to the oxygen transfer mechanism of cytochrome P450.
The nearly uniform distribution of various isomers of AA and LA lipid peroxides formed during endotoxemia is consistent with a free radical, rather than a regioselective (e.g., involving LOXs and cyclooxygenases) mechanism of AA and LA oxidation. However, the employed methodological approach did not allow analysis of stereoisomer specificity in our study. It was not possible to compare activity of all enzymes involved in AA and LA in MPO-KO and wild-type mice. Moreover, other studies show that the cellular and enzymatic components capable of affecting lipid peroxidation are comparable between MPO-KO and wild-type mice under acute inflammatory conditions. Zhang et al. showed that cyclooxygenase -1, cyclooxygenase -2, and 12-LOX levels are similar in isolated peritoneal leukocytes from MPO-KO and wild-type mice [35]. Likewise, another two in vivo studies of MPO-KO mice have suggested that initiation of lipid peroxidation is dependent upon MPO [34, 35]. Interestingly, MPO-KO mice under septic conditions induced by Candida revealed significantly reduced plasma levels of isolevuglandins, a family of ketoaldehydes generated by free radical-induced peroxidation of arachidonate-containing lipids [34]. Similarly, activated PMNs isolated from MPO-deficient human subjects form 9-HETE and 9-HODE only after addition of exogenous MPO [30]. Nevertheless, the possibility that MPO-KO alters the activities of various AA and LA metabolic enzymes by changing their tissue-specific expression, posttranslational modification, or cellular localization cannot be excluded.
Our data demonstrate for the first time, that the MPO deficiency leads to the accumulation of cysteinyl LTs in systemic circulation during the course of acute inflammation. The finding that these important pro-inflammatory mediators are deactivated by MPO in an in vivo model of acute inflammation is supported by several in vitro studies [53-55]. MPO is thought to degrade cysteinyl LTs by their oxidation [53-55]. Previously, the MPO-dependent inactivation of LTs and cysteinyl LTs in particular has been demonstrated in cell-free systems and in activated human phagocytes. In the latter case, human MPO-deficient PMNs and monocytes were unable to degrade LTs unless MPO was added back into the system [53-55]. However, in our experiments we were not able to confirm these findings using PMNs isolated from wild-type and MPO-KO mice.
Interestingly, metabolites that were under the detection limit in plasma of both wild-type and MPO-KO mice and in PMNs isolated from wild-type and MPO-KO mice differ (legends to Table 1., 2 and Fig. 1., 2., and 3.). For example, epoxides of AA were under the detection limits in the plasma of mice, similar to our other studies [14-15], which is probably related to their low half-life in vivo. Similarly, undetectable levels of various degradation products of unstable LA- and AA-derived hydroperoxides in vivo in contrast to in vitro could also be coupled with their short half-life in the blood circulation. On the other hand, the presence of detectable levels of some dihydroxy metabolites and hydroperoxides of AA and LA only in vivo can be connected with a limited range of enzymes involved in AA and LA metabolic pathways presented in PMNs in contrast to the complex metabolism of AA and LA by various cell types in vivo. This raises the question of an importance of various cell types and their enzymatic accessories in the formation of biologically active AA and LA metabolites during the course of acute inflammation. It is not possible to exclude differences in the activity of various enzymes in cells of different origin between wild-type and MPO-KO mice as discussed above; however, our data strongly support the importance of MPO in this process.
The MPO-dependent modulation of AA and LA mediators could significantly affect the physiological functions of the organism. It is interesting to speculate about the role of MPO-dependent formation of polyunsaturated fatty acid epoxides with mostly anti-inflammatory effects during acute inflammation which could theoretically compensate for the decreased activity of cytochrome p450 enzymes due to inflammation [56]. Simultaneously, MPO can catalyze degradation of the cysteinyl-LTs LTC4, LTD4, and LTE4 which stimulate various leukocyte functions, including chemotactic movement and tissue infiltration [13, 18, 19].
Nevertheless, the MPO-dependent formation of lipid mediators with anti-inflammatory properties and the catabolism of pro-inflammatory LTs may have positive effects on the inflammatory process. In this study, no significant effects of MPO deficiency on mortality or physiological functions such as blood pressure (data not shown) were observed up to time interval of blood collection. However, several studies using various types of inflammatory models recently showed an increased level of the inflammatory process in MPO-deficient mice [37, 47, 57-59]. Thus the specific modulation of AA and LA metabolites by MPO can be included among other previously suggested anti-inflammatory effects, such as clearance of noxious stimuli, oxidative inactivation of pro-inflammatory mediators, inactivation of proteases, catabolism of NO, and inhibition of inducible nitric oxide synthase expression [60].
The present study provides evidence that MPO plays an important role in the formation of both pro-and anti-inflammatory AA- and LA-derived lipid mediators in our a non-infectious model of systemic sepsis in mice. Our findings invite a reappraisal of the enzymatic participants in lipid peroxidation in vivo, with MPO added to the ranks of enzymes like LOXs, cyclooxygenases, and cytochrome P450 complexes.
Supplementary Material
Acknowledgments
This work was supported by a postdoctoral fellowship from Philip Morris USA Inc. and Philip Morris International (to L.K.), by grants from The Academy of Sciences of the Czech Republic M200040908, AV0Z50040507, and AV0Z50040702 (to L.K.), University of California, Davis, Health Systems Research Award (to J.P.E.), and the Paul F. Gulyassy Endowed Professorship (to J.P.E.). K.S. received the John Kinsella Dissertation Award and was supported by a NIEHS Training Grant in Environmental Toxicology. Partial support was received from NIEHS grants R37 ES02710 and R01 ES013933 analytical support was from NIEHS Superfund Basic Research Program P42 ES004699. Partial support was received from the Deutsche Forschungsgemeinschaft (to S.B.) We thank Denise Lau for critical comments on the manuscript. The authors have no conflicting financial interests.
List of abbreviations
- AA
arachidonic acid
- DHETE
dihydroxyeicosatrienoic acids
- DHOME
dihydroxyoctadecenoic acid
- EET
epoxyeicosatrienoic acid
- EpOME
epoxyoctadecenoic acid
- H(P)ETEs
hydroxy-eicosatrienoic acid and hydroperoxy-eicosatrienoic acids
- H(P)ODEs
hydroxy-octadecadienoic acid and hydroperoxy-octadecadienoic acids
- LA
linoleic acid
- LOX
lipoxygenase
- LPS
lipopolysaccharide
- MPO
myeloperoxidase
- MPO-KO
MPO-deficient mice
- oxo-EET
oxo-epoxyeicosatrienoic acids
- oxo-ODE
oxo-octadeca-dienoic acids
- PMA
phorbol 12-myristate 13-acetate
- PMNs
polymorphonuclear neutrophils
- SEM
standard error of the mean
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aldridge AJ. Role of the neutrophil in septic shock and the adult respiratory distress syndrome. Eur J Surg. 2002;168:204–214. doi: 10.1080/11024150260102807. [DOI] [PubMed] [Google Scholar]
- 2.Winterbourn CC, Vissers MC, Kettle AJ. Myeloperoxidase. Curr Opin Hematol. 2000;7:53–58. doi: 10.1097/00062752-200001000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 4.Winterbourn CC, Hampton MB, Livesey JH, Kettle AJ. Modeling the reactions of superoxide and myeloperoxidase in the neutrophil phagosome: implications for microbial killing. J Biol Chem. 2006;281:39860–39869. doi: 10.1074/jbc.M605898200. [DOI] [PubMed] [Google Scholar]
- 5.Nauseef WM. Contributions of myeloperoxidase to proinflammatory events: more than an antimicrobial system. Int J Hematol. 2001;74:125–133. doi: 10.1007/BF02981994. [DOI] [PubMed] [Google Scholar]
- 6.Lau D, Baldus S. Myeloperoxidase and its contributory role in inflammatory vascular disease. Pharmacol Ther. 2006;111:16–26. doi: 10.1016/j.pharmthera.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 7.Eiserich JP, Baldus S, Brennan ML, Ma W, Zhang C, Tousson A, Castro L, Lusis AJ, Nauseef WM, White CR, Freeman BA. Myeloperoxidase, a leukocyte-derived vascular NO oxidase. Science. 2002;296:2391–2394. doi: 10.1126/science.1106830. [DOI] [PubMed] [Google Scholar]
- 8.Shao B, Oda MN, Oram JF, Heinecke JW. Myeloperoxidase: an inflammatory enzyme for generating dysfunctional high density lipoprotein. Curr Opin Cardiol. 2006;21:322–328. doi: 10.1097/01.hco.0000231402.87232.aa. [DOI] [PubMed] [Google Scholar]
- 9.Nicholls SJ, Hazen SL. Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2005;25:1102–1111. doi: 10.1161/01.ATV.0000163262.83456.6d. [DOI] [PubMed] [Google Scholar]
- 10.Heinecke JW. Tyrosyl radical production by myeloperoxidase: a phagocyte pathway for lipid peroxidation and dityrosine cross-linking of proteins. Toxicology. 2002;177:11–22. doi: 10.1016/s0300-483x(02)00192-0. [DOI] [PubMed] [Google Scholar]
- 11.Cook JA. Eicosanoids. Crit Care Med. 2005;33:S488–491. doi: 10.1097/01.ccm.0000196028.19746.42. [DOI] [PubMed] [Google Scholar]
- 12.Kroetz DL, Zeldin DC. Cytochrome P450 pathways of arachidonic acid metabolism. Curr Opin Lipidol. 2002;13:273–283. doi: 10.1097/00041433-200206000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Newman JW, Morisseau C, Hammock BD. Epoxide hydrolases: their roles and interactions with lipid metabolism. Prog Lipid Res. 2005;44:1–51. doi: 10.1016/j.plipres.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Schmelzer KR, Inceoglu B, Kubala L, Kim IH, Jinks SL, Eiserich JP, Hammock BD. Enhancement of antinociception by coadministration of nonsteroidal anti-inflammatory drugs and soluble epoxide hydrolase inhibitors. Proc Natl Acad Sci U S A. 2006;103:13646–13651. doi: 10.1073/pnas.0605908103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmelzer KR, Kubala L, Newman JW, Kim IH, Eiserich JP, Hammock BD. Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proc Natl Acad Sci U S A. 2005;102:9772–9777. doi: 10.1073/pnas.0503279102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu Z, Xu F, Huse LM, Morisseau C, Draper AJ, Newman JW, Parker C, Graham L, Engler MM, Hammock BD, Zeldin DC, Kroetz DL. Soluble epoxide hydrolase regulates hydrolysis of vasoactive epoxyeicosatrienoic acids. Circ Res. 2000;87:992–998. doi: 10.1161/01.res.87.11.992. [DOI] [PubMed] [Google Scholar]
- 17.Moghaddam MF, Grant DF, Cheek JM, Greene JF, Williamson KC, Hammock BD. Bioactivation of leukotoxins to their toxic diols by epoxide hydrolase. Nat Med. 1997;3:562–566. doi: 10.1038/nm0597-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuhn H, O'Donnell VB. Inflammation and immune regulation by 12/15-lipoxygenases. Prog Lipid Res. 2006;45:334–356. doi: 10.1016/j.plipres.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Lewis RA, Lee CW, Levine L, Morgan RA, Weiss JW, Drazen JM, Oh H, Hoover D, Corey EJ, Austen KF. Biology of the C-6-sulfidopeptide leukotrienes. Adv Prostaglandin Thromboxane Leukot Res. 1983;11:15–26. [PubMed] [Google Scholar]
- 20.Ortiz de Montellano PR. Catalytic sites of hemoprotein peroxidases. Annu Rev Pharmacol Toxicol. 1992;32:89–107. doi: 10.1146/annurev.pa.32.040192.000513. [DOI] [PubMed] [Google Scholar]
- 21.Winterbourn CC, van den Berg JJ, Roitman E, Kuypers FA. Chlorohydrin formation from unsaturated fatty acids reacted with hypochlorous acid. Arch Biochem Biophys. 1992;296:547–555. doi: 10.1016/0003-9861(92)90609-z. [DOI] [PubMed] [Google Scholar]
- 22.Heinecke JW, Li W, Mueller DM, Bohrer A, Turk J. Cholesterol chlorohydrin synthesis by the myeloperoxidase-hydrogen peroxide-chloride system: potential markers for lipoproteins oxidatively damaged by phagocytes. Biochemistry. 1994;33:10127–10136. doi: 10.1021/bi00199a041. [DOI] [PubMed] [Google Scholar]
- 23.Spickett CM, Jerlich A, Panasenko OM, Arnhold J, Pitt AR, Stelmaszynska T, Schaur RJ. The reactions of hypochlorous acid, the reactive oxygen species produced by myeloperoxidase, with lipids. Acta Biochim Pol. 2000;47:889–899. [PubMed] [Google Scholar]
- 24.Arnhold J, Osipov AN, Spalteholz H, Panasenko OM, Schiller J. Effects of hypochlorous acid on unsaturated phosphatidylcholines. Free Radic Biol Med. 2001;31:1111–1119. doi: 10.1016/s0891-5849(01)00695-5. [DOI] [PubMed] [Google Scholar]
- 25.Panasenko OM, Spalteholz H, Schiller J, Arnhold J. Myeloperoxidase-induced formation of chlorohydrins and lysophospholipids from unsaturated phosphatidylcholines. Free Radic Biol Med. 2003;34:553–562. doi: 10.1016/s0891-5849(02)01358-8. [DOI] [PubMed] [Google Scholar]
- 26.Iwase H, Takahashi T, Takatori T, Shimizu T, Aono K, Yamada Y, Iwadate K, Nagao M. pH dependent alterations of monoepoxides and monochlorohydrins of linoleic acid, and their existence in vivo. Biochem Biophys Res Commun. 1995;215:945–951. doi: 10.1006/bbrc.1995.2555. [DOI] [PubMed] [Google Scholar]
- 27.Hazen SL, Hsu FF, Duffin K, Heinecke JW. Molecular chlorine generated by the myeloperoxidase-hydrogen peroxide-chloride system of phagocytes converts low density lipoprotein cholesterol into a family of chlorinated sterols. J Biol Chem. 1996;271:23080–23088. doi: 10.1074/jbc.271.38.23080. [DOI] [PubMed] [Google Scholar]
- 28.Podrez EA, Poliakov E, Shen Z, Zhang R, Deng Y, Sun M, Finton PJ, Shan L, Gugiu B, Fox PL, Hoff HF, Salomon RG, Hazen SL. Identification of a novel family of oxidized phospholipids that serve as ligands for the macrophage scavenger receptor CD36. J Biol Chem. 2002;277:38503–38516. doi: 10.1074/jbc.M203318200. [DOI] [PubMed] [Google Scholar]
- 29.Schmitt D, Shen Z, Zhang R, Colles SM, Wu W, Salomon RG, Chen Y, Chisolm GM, Hazen SL. Leukocytes utilize myeloperoxidase-generated nitrating intermediates as physiological catalysts for the generation of biologically active oxidized lipids and sterols in serum. Biochemistry. 1999;38:16904–16915. doi: 10.1021/bi991623w. [DOI] [PubMed] [Google Scholar]
- 30.Zhang R, Shen Z, Nauseef WM, Hazen SL. Defects in leukocyte-mediated initiation of lipid peroxidation in plasma as studied in myeloperoxidase-deficient subjects: systematic identification of multiple endogenous diffusible substrates for myeloperoxidase in plasma. Blood. 2002;99:1802–1810. [PubMed] [Google Scholar]
- 31.Savenkova ML, Mueller DM, Heinecke JW. Tyrosyl radical generated by myeloperoxidase is a physiological catalyst for the initiation of lipid peroxidation in low density lipoprotein. J Biol Chem. 1994;269:20394–20400. [PubMed] [Google Scholar]
- 32.Kapiotis S, Sengoelge G, Hermann M, Held I, Seelos C, Gmeiner BM. Paracetamol catalyzes myeloperoxidase-initiated lipid oxidation in LDL. Arterioscler Thromb Vasc Biol. 1997;17:2855–2860. doi: 10.1161/01.atv.17.11.2855. [DOI] [PubMed] [Google Scholar]
- 33.Kapiotis S, Hermann M, Exner M, Laggner H, Gmeiner BM. Copper- and magnesium protoporphyrin complexes inhibit oxidative modification of LDL induced by hemin, transition metal ions and tyrosyl radicals. Free Radic Res. 2005;39:1193–1202. doi: 10.1080/10715760500138981. [DOI] [PubMed] [Google Scholar]
- 34.Poliakov E, Brennan ML, Macpherson J, Zhang R, Sha W, Narine L, Salomon RG, Hazen SL. Isolevuglandins, a novel class of isoprostenoid derivatives, function as integrated sensors of oxidant stress and are generated by myeloperoxidase in vivo. Faseb J. 2003;17:2209–2220. doi: 10.1096/fj.03-0086com. [DOI] [PubMed] [Google Scholar]
- 35.Zhang R, Brennan ML, Shen Z, MacPherson JC, Schmitt D, Molenda CE, Hazen SL. Myeloperoxidase functions as a major enzymatic catalyst for initiation of lipid peroxidation at sites of inflammation. J Biol Chem. 2002;277:46116–46122. doi: 10.1074/jbc.M209124200. [DOI] [PubMed] [Google Scholar]
- 36.Kim IH, Morisseau C, Watanabe T, Hammock BD. Design, synthesis, and biological activity of 1,3-disubstituted ureas as potent inhibitors of the soluble epoxide hydrolase of increased water solubility. J Med Chem. 2004;47:2110–2122. doi: 10.1021/jm030514j. [DOI] [PubMed] [Google Scholar]
- 37.Brennan M, Gaur A, Pahuja A, Lusis AJ, Reynolds WF. Mice lacking myeloperoxidase are more susceptible to experimental autoimmune encephalomyelitis. J Neuroimmunol. 2001;112:97–105. doi: 10.1016/s0165-5728(00)00392-1. [DOI] [PubMed] [Google Scholar]
- 38.Gaut JP, Yeh GC, Tran HD, Byun J, Henderson JP, Richter GM, Brennan ML, Lusis AJ, Belaaouaj A, Hotchkiss RS, Heinecke JW. Neutrophils employ the myeloperoxidase system to generate antimicrobial brominating and chlorinating oxidants during sepsis. Proc Natl Acad Sci U S A. 2001;98:11961–11966. doi: 10.1073/pnas.211190298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brennan ML, Wu W, Fu X, Shen Z, Song W, Frost H, Vadseth C, Narine L, Lenkiewicz E, Borchers MT, Lusis AJ, Lee JJ, Lee NA, Abu-Soud HM, Ischiropoulos H, Hazen SL. A tale of two controversies: defining both the role of peroxidases in nitrotyrosine formation in vivo using eosinophil peroxidase and myeloperoxidase-deficient mice, and the nature of peroxidase-generated reactive nitrogen species. J Biol Chem. 2002;277:17415–17427. doi: 10.1074/jbc.M112400200. [DOI] [PubMed] [Google Scholar]
- 40.Luo Y, Dorf M. Isolation of mouse neutrophils. In: Coligan J, Bierer B, Margulies D, Shevach E, Strober W, editors. Current Protocols in Immunology. Wiley InterScience; 1997. pp. 3.20.21–23.20.26. [Google Scholar]
- 41.Hayakawa M, Sugiyama S, Takamura T, Yokoo K, Iwata M, Suzuki K, Taki F, Takahashi S, Ozawa T. Neutrophils biosynthesize leukotoxin, 9, 10-epoxy-12-octadecenoate. Biochem Biophys Res Commun. 1986;137:424–430. doi: 10.1016/0006-291x(86)91227-1. [DOI] [PubMed] [Google Scholar]
- 42.Yang J, Schmelzer K, Georgi K, Hammock BD. Quantitative profiling method for oxylipin metabolome by liquid chromatography electrospray ionization tandem mass spectrometry. Anal Chem. 2009;81:8085–8093. doi: 10.1021/ac901282n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fife KL, Liu Y, Schmelzer KR, Tsai HJ, Kim IH, Morisseau C, Hammock BD, Kroetz DL. Inhibition of soluble epoxide hydrolase does not protect against endotoxin-mediated hepatic inflammation. J Pharmacol Exp Ther. 2008;327:707–715. doi: 10.1124/jpet.108.142398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Inceoglu B, Jinks SL, Ulu A, Hegedus CM, Georgi K, Schmelzer KR, Wagner K, Jones PD, Morisseau C, Hammock BD. Soluble epoxide hydrolase and epoxyeicosatrienoic acids modulate two distinct analgesic pathways. Proc Natl Acad Sci U S A. 2008;105:18901–18906. doi: 10.1073/pnas.0809765105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Werz O, Klemm J, Samuelsson B, Radmark O. Phorbol ester up-regulates capacities for nuclear translocation and phosphorylation of 5-lipoxygenase in Mono Mac 6 cells and human polymorphonuclear leukocytes. Blood. 2001;97:2487–2495. doi: 10.1182/blood.v97.8.2487. [DOI] [PubMed] [Google Scholar]
- 46.Ozawa T, Sugiyama S, Hayakawa M, Taki F, Hanaki Y. Neutrophil microsomes biosynthesize linoleate epoxide (9,10-epoxy-12-octadecenoate), a biological active substance. Biochem Biophys Res Commun. 1988;152:1310–1318. doi: 10.1016/s0006-291x(88)80428-5. [DOI] [PubMed] [Google Scholar]
- 47.Brennan ML, Anderson MM, Shih DM, Qu XD, Wang X, Mehta AC, Lim LL, Shi W, Hazen SL, Jacob JS, Crowley JR, Heinecke JW, Lusis AJ. Increased atherosclerosis in myeloperoxidase-deficient mice. J Clin Invest. 2001;107:419–430. doi: 10.1172/JCI8797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Podrez EA, Schmitt D, Hoff HF, Hazen SL. Myeloperoxidase-generated reactive nitrogen species convert LDL into an atherogenic form in vitro. J Clin Invest. 1999;103:1547–1560. doi: 10.1172/JCI5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O'Donnell VB, Eiserich JP, Chumley PH, Jablonsky MJ, Krishna NR, Kirk M, Barnes S, Darley-Usmar VM, Freeman BA. Nitration of unsaturated fatty acids by nitric oxide-derived reactive nitrogen species peroxynitrite, nitrous acid, nitrogen dioxide, and nitronium ion. Chem Res Toxicol. 1999;12:83–92. doi: 10.1021/tx980207u. [DOI] [PubMed] [Google Scholar]
- 50.Carr AC, van den Berg JJ, Winterbourn CC. Chlorination of cholesterol in cell membranes by hypochlorous acid. Arch Biochem Biophys. 1996;332:63–69. doi: 10.1006/abbi.1996.0317. [DOI] [PubMed] [Google Scholar]
- 51.Duescher RJ, Elfarra AA. 1,3-Butadiene oxidation by human myeloperoxidase. Role of chloride ion in catalysis of divergent pathways. J Biol Chem. 1992;267:19859–19865. [PubMed] [Google Scholar]
- 52.Mason RP, Chignell CF. Free radicals in pharmacology and toxicology--selected topics. Pharmacol Rev. 1981;33:189–211. [PubMed] [Google Scholar]
- 53.Lee CW, Lewis RA, Tauber AI, Mehrotra M, Corey EJ, Austen KF. The myeloperoxidase-dependent metabolism of leukotrienes C4, D4, and E4 to 6-trans-leukotriene B4 diastereoisomers and the subclass-specific S-diastereoisomeric sulfoxides. J Biol Chem. 1983;258:15004–15010. [PubMed] [Google Scholar]
- 54.Henderson WR, Klebanoff SJ. Leukotriene production and inactivation by normal, chronic granulomatous disease and myeloperoxidase-deficient neutrophils. J Biol Chem. 1983;258:13522–13527. [PubMed] [Google Scholar]
- 55.Neill MA, Henderson WR, Klebanoff SJ. Oxidative degradation of leukotriene C4 by human monocytes and monocyte-derived macrophages. J Exp Med. 1985;162:1634–1644. doi: 10.1084/jem.162.5.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morgan ET. Regulation of cytochrome p450 by inflammatory mediators: why and how? Drug Metab Dispos. 2001;29:207–212. [PubMed] [Google Scholar]
- 57.Milla C, Yang S, Cornfield DN, Brennan ML, Hazen SL, Panoskaltsis-Mortari A, Blazar BR, Haddad IY. Myeloperoxidase deficiency enhances inflammation after allogeneic marrow transplantation. Am J Physiol Lung Cell Mol Physiol. 2004;287:L706–714. doi: 10.1152/ajplung.00015.2004. [DOI] [PubMed] [Google Scholar]
- 58.Ichimori K, Fukuyama N, Nakazawa H, Aratani Y, Koyama H, Takizawa S, Kameoka Y, Ishida-Okawara A, Kohi F, Suzuki K. Myeloperoxidase has directly-opposed effects on nitration reaction--study on myeloperoxidase-deficient patient and myeloperoxidase-knockout mice. Free Radic Res. 2003;37:481–489. doi: 10.1080/1071576031000099830. [DOI] [PubMed] [Google Scholar]
- 59.Takizawa S, Aratani Y, Fukuyama N, Maeda N, Hirabayashi H, Koyama H, Shinohara Y, Nakazawa H. Deficiency of myeloperoxidase increases infarct volume and nitrotyrosine formation in mouse brain. J Cereb Blood Flow Metab. 2002;22:50–54. doi: 10.1097/00004647-200201000-00006. [DOI] [PubMed] [Google Scholar]
- 60.Kumar AP, Ryan C, Cordy V, Reynolds WF. Inducible nitric oxide synthase expression is inhibited by myeloperoxidase. Nitric Oxide. 2005;13:42–53. doi: 10.1016/j.niox.2005.04.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.