Abstract
Prostate cancer is a significant cause of morbidity and mortality among men worldwide. The cornerstone treatment for metastatic prostate cancer is androgen deprivation, which has known effects on prostate tissue apoptosis and thymic regrowth. These findings, plus interest in developing immune-based treatments for prostate cancer, lead us to question whether androgen deprivation causes changes in the adaptive immune responses of prostate cancer patients, and if the timing of changes has implications for the sequencing of immunotherapies in combination with androgen deprivation. Peripheral blood mononuclear cells (PBMC) were obtained from patients prior to beginning androgen deprivation therapy (ADT) and at several time points thereafter. These cells were analyzed for the frequency of specific lymphocyte populations, and their response to stimulation. The development of prostate antigen-specific immune responses was assessed using SEREX. Patients developed expansion of the naïve T cell compartment persisting over the course of androgen deprivation, together with an increase in effector cell response to stimulation, and the generation of prostate tissue-associated IgG antibody responses, implying a potential benefit to the use of ADT in combination with prostate cancer-directed immunotherapies. The optimal timing and sequence of androgen deprivation with immune-based therapies awaits future experimental evaluation.
Keywords: Prostate cancer, androgen deprivation, T-cell, antibody
INTRODUCTION
Prostate cancer is a significant cause of morbidity and mortality in the United States, and remains the second leading cause of cancer-related death in men [1]. The last decade has observed enormous progress in the treatment of metastatic prostate cancer. Docetaxel was approved by the FDA as the only contemporary chemotherapeutic agent demonstrated to prolong survival in patients with androgen-independent, metastatic prostate cancer [2, 3]. Despite this advance, many patients and physicians believe the small survival benefit provided by chemotherapy may not warrant its use in all individuals due to its potential negative impact on quality of life [4]. Consequently, there is great interest in the development of treatments with fewer side effects. Immunological therapies, and vaccines (also known as “active immunotherapies”) in particular, are one such treatment option being investigated [5, 6]. Recent clinical trials have demonstrated an increase in overall survival of patients treated with prostate cancer vaccines [7, 8], superior to what has been observed with chemotherapeutic agents and FDA approval for at least one prostate cancer vaccine is anticipated in the near future.
ADT is the cornerstone of treatment for patients with metastatic prostate cancer, and the concurrent use of ADT is the standard of care for all other treatments for metastatic prostate cancer. ADT is achieved clinically through orchiectomy or with administration of chemical castrants such as gonadotropin releasing hormone (GnRH) agonists, and/or anti-androgens. GnRH agonists function by continuous stimulation of the GnRH receptor, which ultimately induces desensitization and blockade of the pituitary-gonadal axis achieving castrate levels of testosterone [9]. The non-steroidal anti-androgens compete directly with testosterone and dihydrotestosterone for the ligand-binding domain of the androgen receptor. In addition to competitive inhibition of the androgen receptor, the steroidal anti-androgens have anti-gonadotrophic effects which suppress testosterone synthesis [10]. While the individual therapies function through different mechanisms to inactivate the androgen receptor, the ultimate outcome is atrophy and death of androgen-dependent cells of the prostate [11, 12].
In the case of orchiectomy, prostatic glandular cells undergo apoptosis characterized by DNA fragmentation, cell surface blebbing, and the formation of apoptotic bodies [13]. The apoptotic bodies are recognized by scavenger receptors, phagocytized and digested by macrophages [14]. However, an expansion of dendritic cells have been reported at the prostate following orchiectomy as well [15], implicating a possible dendritic cell role in the phagocytosis and presentation of prostate antigens. Furthermore, prostate cancer patients undergoing androgen deprivation experience a T-cell infiltration of the prostate by one month post treatment, and the infiltrates appear to be of an oligoclonal nature. These findings imply that an adaptive response to the prostate may arise following androgen deprivation [15]. In the case of androgen deprivation achieved by chemical castration, tumor cell vacuolization and glandular atrophy are observed. However, the levels of apoptosis appear unchanged, implying the predominant form of cell death may be achieved through an alternative mechanism [11].
Numerous observations have also demonstrated an effect of androgens on lymphocyte numbers and function. Briefly, thymic involution can be reversed in mouse [16] and rat [17] models following orchiectomy. This regrowth is characterized by an increase in the weight and cellularity of the thymus, and can be reversed when androgens are re-administered to the castrate subject [18]. The observed thymic regrowth has been attributed to an expansion of the thymic epithelia expressing increased levels of CCL25, which promotes immigration of early thymic progenitors to the thymus [19]. A robust expansion of the common lymphoid progenitor cells can also be observed in castrated mice given hematopoeitic stem cell transplants compared to sham-treated animals [20], data suggesting immunosuppressive effects of testosterone. In humans, administration of GnRH agonists in prostate cancer patients have been reported to induce expansion of signal joint recent thymic emigrant cells (sj T rec) [21]. This T cell expansion can occur late in life when sj TRECS are reported to be at their lowest levels [22], and the risk for prostate cancer increases. These observations suggest that androgens have an immunosuppressive effect, and ADT might function to reverse this suppression late in life.
The observations that ADT can affect T lymphocytes, and elicit prostate tissue-associated inflammation, suggest ADT might be combined with active immunotherapies. In a mouse model of prostate cancer, tumor-bearing animals receiving an adoptive transfer of prostate tumor-specific T cells, followed by castration and vaccination, had greater T-cell expansion and development of an effector phenotype in the adoptively transferred cells compared with tumor bearing mice similarly treated but with sham castration [23]. Roden et al. demonstrated splenocytes from castrated mice receiving an ovalbumin-specific vaccine proliferated more robustly in response to ovalbumin stimulation compared with splenocytes of similarly vaccinated controls from non-castrate mice [24]. Koh et al. reported that mice vaccinated with a dendritic cell vaccine, and then surgically castrated, had a greater number of antigen-specific IFN γ-secreting cells compared to vaccinated mice receiving sham surgery [25]. Taken together, these results from multiple investigator groups suggest that it might be clinically beneficial to combine active immunotherapies with androgen deprivation [26]. However, timing the administration of these therapies to gain maximum benefit needs to be experimentally determined, and if the effects of androgen deprivation on the adaptive arm of the immune system are persistent over time.
In order to investigate the effects of ADT on the adaptive immune system, and if the effects are persistent, our analysis focused on the frequency of circulating T cell subsets collected from prostate cancer patients at various time points up to 24 months after beginning androgen deprivation. We then characterized the ability of T-cell subsets to proliferate and express cytokines after receptor or mitogen activation. Finally, given the observations that ADT elicits T-cell infiltration of prostate tissue [15], we asked if antigen-specific responses to proteins expressed in the prostate develop following ADT, which proteins were recognized, and if these responses are persistent over the course of therapy. For these studies we employed the SEREX methodology [27]. We report an alteration to the T-cell repertoire develops following ADT, with an expansion of naïve T cells and RTEs. This T cell expansion is detectable at least by one month after beginning ADT, and the expansion was detectable up to two years later in specific individuals. Similarly, IgG responses were elicited to prostate tissue antigens as early as one month after beginning ADT, as well as after many months of treatment. Together our findings suggest that changes in the adaptive immune system following androgen deprivation may occur early after beginning treatment, and may be persistent for long periods of time. These observations may suggest that active immunotherapies might be used in sequence with androgen deprivation and/or might be affected by the concurrent use of androgen deprivation.
MATERIALS AND METHODS
Subjects
All specimens were obtained as part of a prospective, single-institution clinical trial at the University of Wisconsin Carbone Comprehensive Cancer Center in which patients with biochemically recurrent or newly metastatic prostate cancer were treated with androgen deprivation. Samples remaining from that trial were used for the current studies. All patients gave written informed consent for their blood products to be used for immunological research, and none had ever previously received treatment with androgen deprivation. Blood counts and absolute lymphocyte counts were performed by the University of Wisconsin Hospital and Clinics Clinical Laboratory. Blood was obtained immediately prior to beginning treatment with a GnRH agonist (leuprolide), and at 1, 3, 6, 12 and 24 months following the initiation (and continuation) of therapy. Because of the small volume blood draws, and the use of samples for other analyses, PBMC were not available for all time points for all subjects. Serum was aliquoted and stored at −80°C until use. PBMC were prepared from heparinized blood by Ficol (GE Healthcare, Piscataway, NJ) gradient centrifugation and cryopreserved in liquid nitrogen until use.
Fluorescence Associated Cell Sorting (FACS) staining of surface and intracellular antigens
PBMC were plated at 2 × 105 cells per well of a 96-well microtiter plate, blocked with 1 μg/ml mouse IgG, and stained with fluorochrome-labeled antibodies specific for CD3 (clone SK7, BD Biosciences, San Jose, CA), CD4 (clone RPA-T4, BD Biosciences), CD8 (clone RPAT8, eBioscience, San Diego, CA), CD31 (clone WM59, eBioscience), CD45RO (clone UCHL1, BD Biosciences), and/or CCR7 (clone 3D12, BD Biosciences). For intracellular stains, PBMC were stimulated with phytohemagglutinin (PHA) (Thermo Fisher, Waltham, MA) 10 μg/ml, or phorbol 12-myristate 13-acetate (PMA) (Sigma, St. Louis, MO) 10 ng/ml, and ionomycin (Thermo Fisher) 1 μg/ml, and incubated with GolgiStop (BD Biosciences) 2μM final concentration. After 5 hours the cells were blocked with 1 μg/ml mouse IgG, stained with the described surface antigens, permeabilized (cyotofix/cytoperm, BD Biosciences), and stained fluorochrome-labeled antibodies specific for human IFNγ (clone B27, BD Biosciences), IL-4 (clone 8D4-8, BD Biosciences), or IL-17 (clone eBio64Dec17, eBioscience) for 30 min on ice. Fluorescently-labeled cells were sorted on an LSR II flow cytometer (BD Biosciences), and data analyzed with Flow Jo software (version 8.8.6, Tree Star Inc, Ashland, OR). The percentage of marker positive events was determined among 20,000 live (7AADlow) events. CD3+, CD4+ or CD8+, T-cell subsets were further characterized with CD31+, CD45RO+/−, CCR7+/−, or IFNγ, IL-4, IL-17. Correction was made for background staining using fluorochrome-labeled control IgG stains.
In vitro cell stimulation
96-well flat bottom plates were incubated overnight at 4°C with serial dilutions of anti-human CD3 (clone UCHT1, BD Biosciences) and anti-human CD28 (clone 37407, R&D Systems, Minneapolis, MN) in phosphate-buffered saline (PBS), with a starting concentration of 6 μg/ml. PBMC were then stained with PKH-26 (Sigma Aldrich) following the manufacturer’s protocol, and cultured in individual wells at 2 × 105 cells per well in T-cell medium (RPMI 1640 (Thermo Fisher), 10% human AB serum (Valley Biomedical, Winchester VA), 1 mM sodium pyruvate (Thermo Fisher), 0.045 mM βMeOH (Thermo Fisher), and 2 IU/ml penicillin/2 μg/ml streptomycin (Thermo Fisher)). 120 hrs after stimulation the cells were stained with human anti-CD4 APC (clone RPA-T4, BD Biosciences) and human anti-CD8 FITC (clone RPA-T8, BD Biosciences). Cells were washed with PBS+3% FCS prior to flow analysis (LSR II flow cytometer). Percentage of marker positive events were determined by collecting events on day 5, then by gating PKH26+ events among CD4+ or CD8+ subpopulations. Parent populations were determined with unstimulated PKH26+ PBMC for each sample set and precursor frequency of proliferating cells was calculated with the ModFit LT software (version 3.2, Verity Software House, Topsham, ME).
SEREX
SEREX, serological identification of antigens by recombinant expression, was performed as previously described [27, 28]. Briefly, a λ phage cDNA expression library constructed from normal prostate tissue mRNA (TripleEX, Clontech, Palo Alto, CA) was used to transduce XL-1 Blue E. coli (Stratagene, La Jolla, CA). Approximately 104 pfu in top agarose were plated per 150 mm × 15 mm Petri plates, and multiple plates were evaluated for each screening such that a total of approximately 2 × 105 pfu were screened per patient time point. For the screening, nitrocellulose membranes incubated in 10-mM isopropyl-β-D-thiogalactopyranoside (IPTG) (Thermo Fisher) were laid over phage-transduced bacterial lawns, and incubated overnight at 37°C. The filters were then screened with individual patient sera obtained post-ADT, and diluted 1:100, at 4°C overnight. IgG antibodies were detected with an alkaline phosphatase-conjugated monoclonal anti-human IgG antiserum (Sigma Aldrich), and colorimetrically stained with 0.3 mg/ml nitro blue tetrazolium chloride (NBT) (Research Products International, Mt. Prospect, Illinois) and 0.15 mg/ml 5–bromo 4-chloro 3-indoylphosphate (BCIP) (Thermo Fisher) in 100 mM Tris pH 9.5, 100 mM NaCl, and 5mM MgCl2. Immunoreactive spots were identified visually, and the corresponding plaques extracted. These phage were then spotted directly onto a lawn of XL-1 bacteria for a prioritization screen, similar to that described above, but using sera obtained pre-treatment as well as post-treatment. Plaques demonstrating increased immunoreactivity with the post-treatment sera relative to the pre-treatment sera were purified and sequenced to identify the encoded gene product.
Statistical analyses
Unless otherwise stated, statistical comparisons were performed using the two-tailed, paired T test analysis with the PRISM package (GraphPad Software, version 3, La Jolla, CA).
RESULTS
Increased frequency of CD4+ naïve T-cells are detectable in the peripheral blood of patients treated with ADT by as early as one month
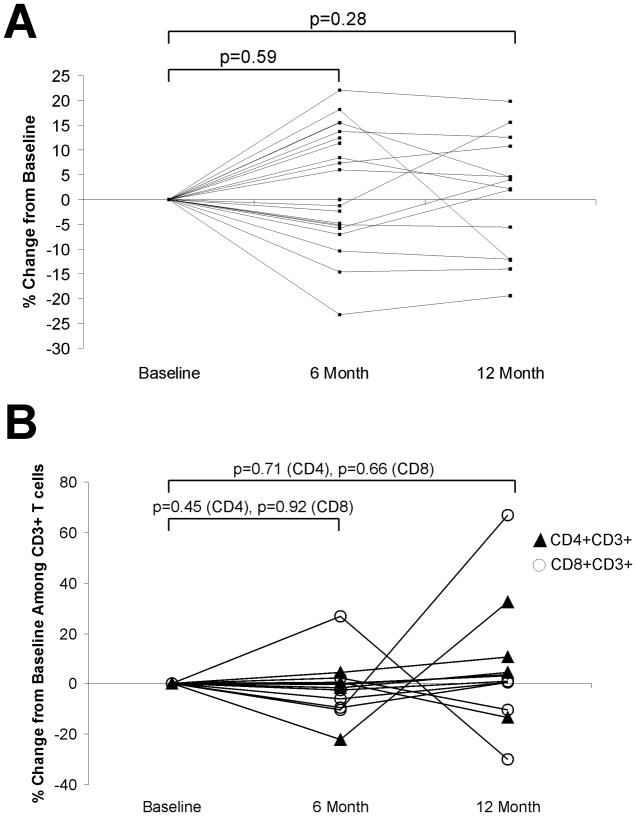
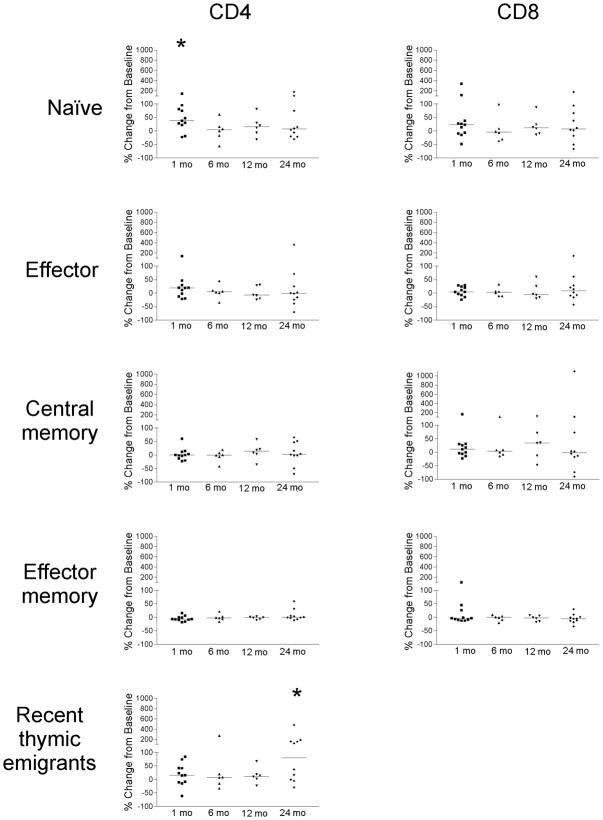
It has been previously reported that there is an increase in the number of circulating T cells, and particularly naïve (TREC+) T-cells, following ADT. Sutherland et al. reported this increase in prostate cancer patients by 4 months of ADT [21]. To determine if changes in T-cell frequencies occur earlier, and/or persist over longer periods of time following ADT, we collected blood from patients with prostate cancer prior to beginning ADT, and at multiple time points up to two years later. As shown in Figure 1A, the absolute numbers of lymphocytes (T- and B-cell) were not significantly altered from baseline to 6 months post treatment in 21 patients analyzed (p = 0.59, paired t test). Similarly, no significant change in absolute numbers of lymphocytes was observed 12 months after ADT in the same individuals for whom samples were available (n=14, p=0.28). Moreover, no significant changes were observed in the frequency of CD4+ or CD8+ populations among CD3+ T cells (Figure 1B). Consequently, we questioned whether changes in individual T-lymphocyte subpopulations occurred. PBMC were collected from 17 individuals prior to beginning ADT and after one month (n=11), 6 months (n=6), 12 months (n=6), or 24 months (n=10) of treatment. CD4+ or CD8+ CD3+ T cells were sub typed as naïve (CD45RO−/CCR7+), effector (CD45RO−/CCR7−), effector memory (CD45RO+/CCR7−) or central memory (CD45RO+/CCR7+). As demonstrated in Figure 2, the frequency of several subpopulations was observed to increase in several individuals, and the median frequency of naïve CD4+ T cells was significantly increased (p=0.003, paired t-test) one month after beginning ADT. No significant changes were observed in the CD4+ and CD8+ effector, central memory, or effector memory populations compared to baseline. A separate analysis of the effector memory (CD45RO+/CCR7−) population subdivided on the basis of CD28 expression, similarly showed no difference at baseline and after 24 months (data not shown). To determine if the expansion of CD4+ naïve T-cells could be attributed to thymic output or potentially due to proliferation of naïve T cells in the periphery, we analyzed the CD4+ RTE population, as defined by CD31+ CD45RO− expression. As further demonstrated in Figure 2, the median CD4+ RTE population was increased by one month after beginning ADT, and significantly so (p=0.04, paired t-test) after 24 months of therapy. These findings suggest that the naïve T-cell expansion is primarily attributed to thymic output, and this output may continue over time.
Figure 1. Lymphocyte counts remain unchanged in prostate cancer patients treated with androgen deprivation.
Blood was collected from patients immediately prior to treatment and after 6 (n=21) and 12 months (n=14 of these 21) of ADT. Shown are the percent change from baseline in absolute lymphocytes per microliter (panel A), and the percent change from baseline in the CD4+ or CD8+ T cells among CD3+ T cells (panel B).
Figure 2. An increase in the number of naïve T cells is observed following androgen deprivation.
PBMC from patients with prostate cancer obtained immediately prior to treatment (n=17), after 1 month (n=11), 6 months (n=6), 12 months (n=6), and 24 months (n=10) of androgen deprivation, were evaluated for the frequency of T-cell subsets by flow cytometry. Shown are the % change in frequency from baseline among the CD3+CD4+ or CD3+CD8+ T cells of naïve cells (CD45RO−CCR7+), effector cells (CD45RO−CCR7−), central memory (CD45RO+CCR7+), effector memory (CD45RO+CCR7−), or recent thymic emigrants (CD45RO−CD31+) at each of these time points. The bars show median change in frequency at each time point, and “*” indicates a significant change in median frequency (p ≤ 0.05) by a two-tailed paired T test.
PBMC from patients treated with ADT have a more robust proliferative response to CD3 and CD28 stimulation
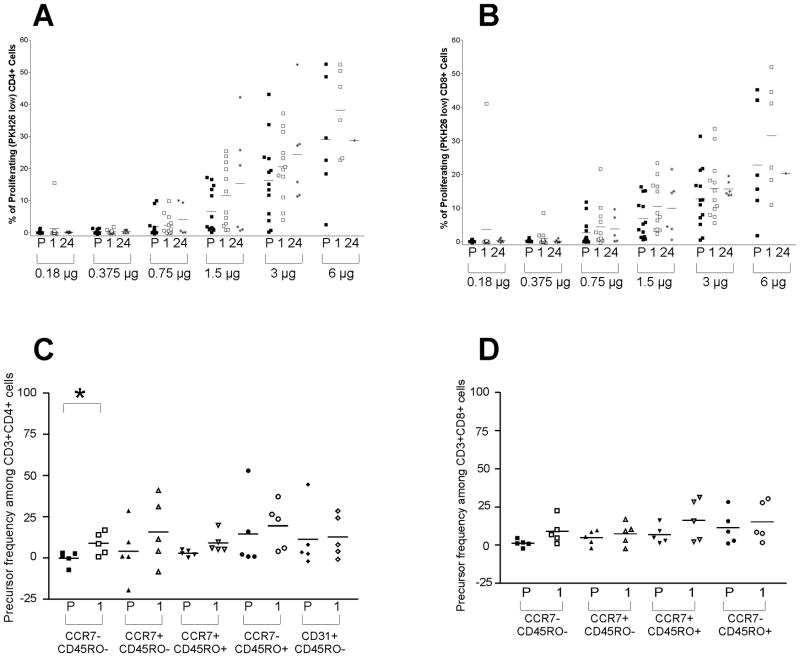
It has been suggested that thymic regrowth elicited by ADT augments not only T-cell numbers, but also responsiveness to T-cell receptor activation in mice [24]. While augmented numbers of proliferating T-cells were not detected in the peripheral blood of patients treated with ADT [21], T-cell numbers from castrated mice have been reported to proliferate more vigorously in response to CD3 and CD28 stimulation compared with sham-treated mice [24]. Given this observation, we asked if similar responses were detectable in prostate cancer patients undergoing ADT, and furthermore, whether these responses might be due to the increased frequency of naïve T-cells and CD4+ RTEs. PBMC from 12 subjects collected at baseline, and 1 and 24 months after beginning ADT, were evaluated for cellular proliferation in response to anti-CD3 and anti-CD28 stimulation. As shown in Figure 3A and 3B, increases in the frequency of CD4+ and CD8+ cells proliferating in response to stimulation were observed after 1 month of ADT. Phenotypic analysis demonstrated the increased proliferation was detectable in nearly all subpopulations (naïve, effector, memory), but was most evident in the effector (CCR7−, CD45RO−) and central memory (CCR7+ CD45RO+) cells (Figure 3C and 3D). These results suggest GnRH agonist induced androgen deprivation not only affects the frequency of T-cell subsets, likely elicited by thymic regrowth, but also affects T-cell function or responsiveness to activation.
Figure 3. PBMC from prostate cancer patients undergoing androgen deprivation demonstrate an increased capacity to proliferate in response to CD3 and CD28 signaling.
PBMC obtained from individual patients pretreatment (P) and after 1 or 24 months of treatment with androgen deprivation were labeled with PKH26, and incubated for 120 hours with titrated doses of plate bound anti-CD3 and anti-CD28. The precursor frequency for both CD4+ (panel A) and CD8+ (panel B) cells, as well as their respective subsets CD4+ (panel C) and CD8+ (panel D) when stimulated with 1.5 μg/ml of anti-CD3 and 1.5 μg/ml of anti-CD28, are shown. The bars show median % of PKH26low cells at each time point (panel A and B), or median precursor frequency of proliferating cells (panel C and D). “*” indicates a significant change from pretreatment (P) to 1-month (1) of treatment (p ≤ 0.05) by a two-tailed paired T test.
Th1 and Th17 effector memory subsets decrease in the periphery by 24 months of ADT
Given our findings that T-cell subset frequency and responsiveness to CD3 and CD28 stimulation are affected by androgen deprivation, we next sought to identify if the Th bias of peripheral T-cells was also altered following ADT. PBMC from six patients, obtained at baseline and after 1 month of androgen deprivation, were stimulated with PMA and ionomycin for 5 hours and the frequency of IFN-γ, IL-4, and IL-17 were determined by intracellular flow cytometric staining. No significant changes were observed in the Th bias of total CD4+ and CD8+ T cells from baseline (data not shown). We then asked if the if the Th bias was altered in the CD4+ and CD8+ T-lymphocyte subpopulations by 24 months of ADT. As shown in Figure 4, decreases were observed in CD8+ effector memory IFNγ-secreting (p=0.05, paired t-test, panel A), and CD4+ effector memory phenotype IL-17-secreting (p=0.04, paired t-test, panel C) T cells after 24 months.
Figure 4. Changes in cytokine-secreting T-cell subpopulations are detectable after 24 months of ADT.
PBMC obtained from 7 individuals at baseline and after 24 months of ADT were stimulated with PMA and ionomycin and assessed for IFNγ (panel A), IL-4 (panel B), or IL-17 (panel C) secretion by intracellular flow cytometry. CD3+CD4+ and CD3+CD8+ subsets were defined as recent thymic emigrants (CD45RO−CD31+), naïve (CD45RO−CCR7+), central memory (CD45RO+CCR7+), effector memory (CD45RO+CCR7−), or effector cells (CD45RO−CCR7−). Shown are the mean and standard deviation of the respective T-cell subsets. “*” indicates a significant change from pretreatment to the 24-month value (p ≤ 0.05) by a two-tailed paired T test.
A diverse repertoire of IgG responses develop to prostate-expressed proteins following ADT
In addition to the effects on T-cells, it has been previously reported that stimulated splenocytes from castrated mice produce higher levels of IgG compared to the splenocytes of sham treated mice [29]. Furthermore it has been reported that ADT leads to prostate tissue destruction and mononuclear cell infiltration [11, 15, 30]. Consequently, we hypothesized that patients treated with ADT might develop adaptive responses to prostate tissue antigens following therapy. To test this, sera were obtained from 20 prostate cancer patients 1–3 months after beginning ADT and were evaluated for prostate protein-associated IgG antibodies by SEREX [27]. As shown in Table 1, IgG responses to prostate-associated proteins were detectable in all patients. Antibody responses elicited following ADT to individual prostate tissue-associated proteins were detectable in ten of the twenty patients (Table 1 and Figure 5A). In total, 31 unique purified gene products were identified (Table 1). To determine if the antigens identified following ADT were recognized in common among multiple patients treated with ADT, phage encoding these antigens were evaluated by immunoblot analysis using sera obtained from 30 patients 1–3 months after beginning ADT. We identified one protein, RNA binding motif protein 25, to which IgG responses were elicited following ADT in two of the patients analyzed (data not shown). Otherwise, however, the recognition of individual antigens appeared to be specific to individual patients.
Table 1. IgG responses to prostate-associated proteins are elicited after short-term ADT.
Sera were collected from 20 patients immediately prior to and after 1 and/or 3 months of treatment with androgen deprivation (as shown). Sera from the post-treatment time point(s) were screened for IgG in SEREX studies and the number of immunoreactive plaques/200,000 screened plaques is shown (primary). These plaques were then screened with sera obtained prior to androgen deprivation, and the number of phage to which IgG responses were elicited or augmented after treatment (Pre −/Post +) is shown, as well as the sequence identification of the encoded proteins.
| Subject | Sera Screened | Immunoreactive Plaques | Sequence Identification | |||
|---|---|---|---|---|---|---|
| Baseline | 1 month | 3 month | Primary | Pre − /Post+ | ||
| 1 | x | x | x | 57 | 1 | Telomere-associated ADP-ribosyl transferase |
| 2 | x | x | 51 | 6 | Actinin, alpha 2 | |
| ElaC homolog 2 | ||||||
| Caldesmon 1 | ||||||
| A kinase anchor protein | ||||||
| Cell division cycle 73, Paf1/RNA pol II complex component | ||||||
| Tankyrase, TRF1-interacting ankyrin-related ADP-ribose pol 2 | ||||||
| 3 | x | x | 21 | 0 | ||
| 4 | x | x | 58 | 0 | ||
| 5 | x | 32 | 0 | |||
| 6 | x | x | 31 | 0 | ||
| 7 | x | x | 16 | 0 | ||
| 8 | x | x | 14 | 0 | ||
| 9 | x | x | 13 | 0 | ||
| 10 | x | x | 38 | 6 | Fms - related tyrosine kinase 3 ligand | |
| Palmitoylated 5 membrane protein | ||||||
| Chromosome 14 gene contig. | ||||||
| Clone RP11-421D6 on chromosome 13 | ||||||
| TSC22 domain family member 1 | ||||||
| Transgelin transcript variant 2 | ||||||
| 11 | x | x | 21 | 0 | ||
| 12 | x | x | 37 | 2 | NSFL1(p97) cofactor (p47) NSFL1C, transcript variant 3 | |
| Chromosome 16 gene contig. | ||||||
| 13 | x | x | 30 | 1 | Paxillin | |
| 14 | x | x | 37 | 4 | Chromosome 6 gene contig. | |
| RNA polymerase II | ||||||
| Amyloid beta precursor protein binding protein 1 | ||||||
| Rho guanine nucleotide exchange factor 12 | ||||||
| 15 | x | x | 36 | 4 | Chromosome 1 gene contig. | |
| Chromosome 5 gene contig. | ||||||
| Regulator of chromosome condensation 2 | ||||||
| Chromosome 16 gene contig. | ||||||
| 16 | x | x | 34 | 0 | ||
| 17 | x | x | 33 | 0 | ||
| 18 | x | x | 84 | 1 | Glucosamine-Phosphate N-acetyltransferase 1 | |
| 19 | x | x | 129 | 1 | Sorbitol dehydrogenase | |
| 20 | x | x | 63 | 5 | Chromosome 4 gene contig. | |
| Similar to laminin receptor 1 | ||||||
| Myosin heavy chain 11 | ||||||
| Metastasis associated lung adenocarcinoma transcript 1 | ||||||
| Sec 61 alpha subunit | ||||||
Figure 5. IgG responses to prostate-associated proteins are elicited after androgen deprivation treatment and detectable by SEREX analysis.
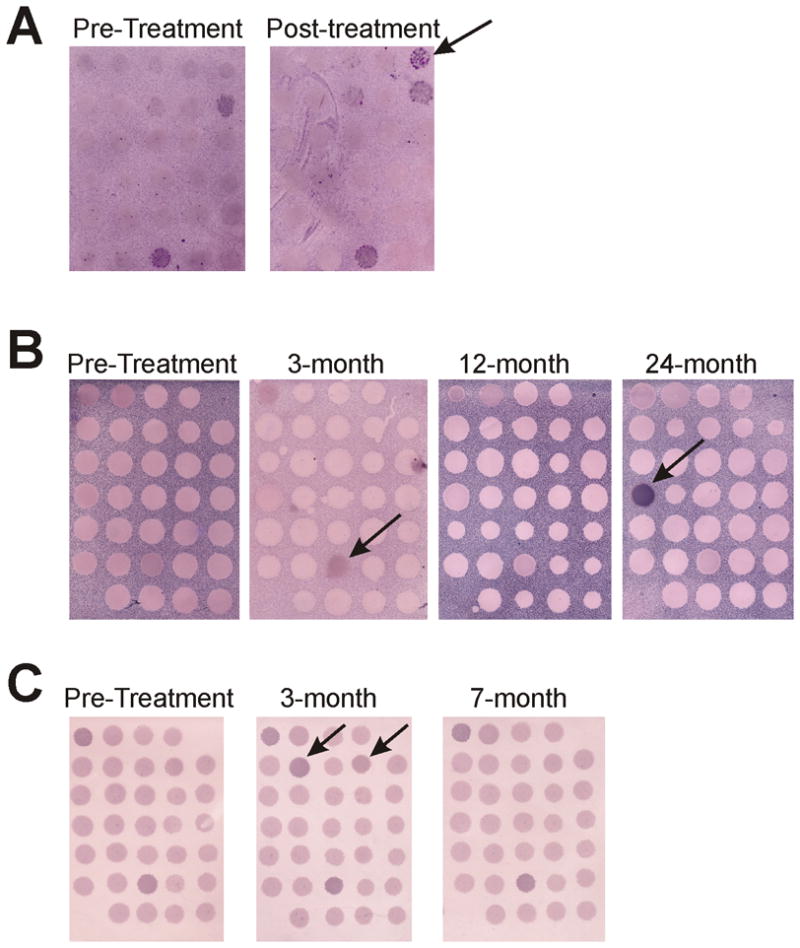
Panel A: Shown are SEREX membranes with individual λ phage plaques encoding proteins expressed by normal prostate tissue, and screened with sera from an individual obtained prior to treatment (baseline) or after 1 month of androgen deprivation. The arrows highlight plaques to which IgG responses were elicited and detectable after treatment. Panels B and C: Examples of replicate membranes encoding the antigens identified in Table 1, and screened with sera from two individuals (panel B or panel C) obtained at baseline and 3, 7, 12, or 24 months after androgen deprivation. The arrows highlight individual immunoreactive plaques to which responses were differentially elicited over time.
To determine if IgG responses developed at later time points, sera were obtained from 11 of the 20 patients after 24 months of ADT and used for detecting antibody responses to proteins from the same panel of 31 antigens. With the exception of one antigen to which IgG responses were clearly augmented after many months of ADT in one patient (Figure 5B), IgG responses were not more frequently detected after 24 months of treatment. In fact, IgG responses that were elicited or augmented shortly after beginning ADT appeared to wane over time in two of the eleven individuals (Figure 5B and 5C).
DISCUSSION
ADT is a standard treatment for patients with recurrent prostate cancer, and a treatment known to elicit prostate tissue destruction, prostate tissue immune cell infiltration, and to have effects on thymic regrowth. While ADT is the primary treatment for metastatic prostate cancer, ultimately prostate cancer grows in a castrate-resistant fashion, and it has been suggested that patients receiving ADT may have a shortened time to progression [31]. These observations, in the context of immune-based therapies being developed for patients with metastatic prostate cancer, make an understanding of the effects of ADT on prostate cancer-associated immune responses of particular importance. In this report we evaluated alterations to the lymphocyte repertoire and adaptive immune responses in prostate cancer patients treated with ADT. While we found no significant change in the numbers of circulating lymphocytes (T- and B-cell), we did identify changes within T-cell subsets following therapy. Specifically, we found the CD4+ naïve T cell population expands by 1 month of therapy, and more specifically the CD4+ RTE population. An increase in the CD4+ RTE population was detectable as long as 24 months after beginning ADT. In addition, we demonstrated CD4+ and CD8+ cells were more sensitive to CD3 and CD28 stimulation following therapy, and effector and memory T-cell subsets were observed to have the most robust responses to the stimulation. Curiously, we observed fewer IFNγ-secreting and IL-17-secreting memory T cells following long-term ADT. In terms of prostate antigen-specific immune responses, our results demonstrated responses to proteins of the prostate are elicited following ADT as early as one month after beginning treatment, but some responses developed months later. Individual antigens recognized were, for the most part, specific to individual patients and not commonly recognized among different individuals. Taken together, these results suggest that ADT with a GnRH analogue: 1) induces an expansion of the naïve T cell compartment with continued thymic output; 2) may decrease the T-cell activation threshold; and 3) may elicit changes and adaptive responses by 1 month after beginning therapy with changes persisting over the course of therapy. These observations suggest androgen deprivation may augment the desired effects of prostate cancer-directed immunotherapies by increasing the pool of naïve T cells which could respond to the immunotherapy, by enhancing T-cell responsiveness, and these effects may persist over the course of androgen deprivation.
Given the limited availability of patient PBMCs we were unable to test each patient’s T cell levels at each time point following androgen deprivation. Moreover, the multiple comparisons and small sample sizes could have introduced bias and/or reduced our ability to detect subtle differences occurring earlier after androgen deprivation. However, in our studies we found no significant change from baseline of total circulating lymphocyte counts, or CD4+ or CD8+ T-cell subsets, in patients by 6 months or 1 year after initiating ADT. The data suggests overall T-cell numbers, and CD4+ and CD8+ T-cell numbers in particular, remain relatively stable over time. We did, however, identify an increase in the CD4+ naïve T cell (CCR7+, CD45RO−) population, as well as the RTE (CD31+, CD45RO−) population, by as early as one month of ADT. Interestingly, we found the CD4+ RTE population was persistently increased even 24 months after beginning ADT. This observation that naïve T cells continue to be produced and thymically educated suggests the pool of T cells with greater TCR diversity may be present after ADT, and potentially useful in the context of active immunotherapies. In this context, the timing of immune therapies, whether beginning immediately after ADT or months-to-years after beginning ADT, may be less important. Animal studies may be warranted to specifically investigate the timing and sequence of combination therapies.
Cell activation studies showed a greater percentage of the CD4+ and CD8+ cells from androgen-deprived patients are activated in response to CD3 and CD28 stimulation compared to baseline cells. This increased responsiveness to TCR and co-receptor stimulation could be due to greater expression of both CD3 and CD28 on T cells following ADT, or potentially due to changes in T-cell signaling. Further studies could elucidate the mechanism for this response. However, taken together these observations imply that there may be greater T-cell reactivity in patients following ADT, another observation that may have importance in the consideration of combination therapies with immune-based treatments. We did not, however, detect a change in cytokine-secreting T-cell phenotypes following mitogen-induced activation, at least shortly after initiation of androgen deprivation. Our results differ from observations in murine systems made by Viselli et al. who showed that supernatants from splenocytes of castrated mice incubated with concanavalin A for 20 – 48 hours have greater levels of IFNγ compared to supernatants of sham-castrated mice [29]. Similarly, Koh et al. demonstrated that castration alone induces IFNγ secretion in mouse splenocytes [25]. These findings may be attributed to species differences or to stimulation of NK cells within the splenocyte populations, whereas our investigation focused on T-cell cytokine expression alone.
It has previously been demonstrated that patients with prostate cancer treated with ADT develop an oligoclonal population of prostate-infiltrating lymphocytes within weeks of beginning treatment [15]. Consequently, we hypothesized patients treated with ADT might develop adaptive IgG responses specifically to proteins of the prostate. Using the SEREX methodology we were able to identify specific proteins to which IgG responses were elicited after beginning ADT [27]. Similar to the changes observed in T cells, IgG responses to proteins of the prostate could be observed as early as 1 month following ADT and remained stable over time. The majority of responses were observed by 3 months of therapy, however there was quite a bit of variability with some detectable responses being lost over time, and other responses appearing only after many months. Interestingly, while specific antigens did not appear to be recognized in common among multiple patients, many of the antigens we identified are involved in the same molecular pathways. For example, paxillin can recruit rho guanine nucleotide exchange factors to cell–extracellular matrix contact sites which leads to downstream cell migration activity [32]. The actinin α2 and transgelin proteins have been reported to regulate AR-mediated transcription [33, 34], whereas TSC22 domain family member 1 has been shown to be regulated by the androgen receptor [35]. The recognition of these particular antigens might have been directly influenced by changes in expression levels following androgen deprivation.
In conclusion, we identified that patients treated with ADT develop persistent changes in adaptive immune responses. In particular some patients developed a continued expansion of naïve T cells through thymic output. In addition, T cells in the periphery of treated patients proliferated more robustly to TCR and co-receptor stimulation, and IgG responses developed to proteins of the prostate which could be detected in the sera by one month after beginning ADT. It is interesting to note that vaccines for the treatment of prostate tumors have to date shown greatest efficacy in patients with late-stage disease when patients are undergoing ADT [7, 8]. Future studies will explore in experimental models whether there may be a benefit to sequencing androgen deprivation with active immunotherapies.
Acknowledgments
The authors would like to thank Brett Maricque, Heath Smith, and Cindy Zuleger for their helpful comments and review of the article. This work was supported for DGM and MDM by the US Army Medical Research and Materiel Command Prostate Cancer Research Program (W81XWH-06-1-0184) and for MDM by NIH T32 CA009135.
ABBREVIATIONS
- ADT
Androgen deprivation therapy
- FACS
Fluorescence associated cell sorter
- GnRH
Gonadotrophin releasing hormone
- IFNγ
Interferon gamma
- Ig
Immunoglobulin
- IL-4
Interleukin – 4
- IL-17
Interleukin – 17
- IPTG
Isopropyl-β-D-thiogalactopyranoside
- PBMC
Peripheral blood mononuclear cell
- PBS
Phosphate-buffered saline
- PHA
Phytohemagglutinin
- PMA
Phorbol 12-myristate 13-acetate
- RTE
Recent thymic emigrant
- sj TREC
Signal-joint T cell receptor excision circle
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics. CA Cancer J Clin. 2009;59(4):225. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Theodore C, James ND, Turesson I, Rosenthal MA, Eisenberger MA. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 3.Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M, Benson MC, Small EJ, Raghavan D, Crawford ED. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351(15):1513. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 4.Kirk TN, Moyad MA. National survey of advanced prostate cancer patients reveals disparity between perceptions and reality of treatment. Proc Amer Soc Clin Oncol. 2006;24:490s. [Google Scholar]
- 5.McNeel DG. Prostate cancer immunotherapy. Curr Opin Urol. 2007;17(3):175. doi: 10.1097/MOU.0b013e3280eb10eb. [DOI] [PubMed] [Google Scholar]
- 6.Kipp RT, McNeel DG. Immunotherapy for prostate cancer - recent progress in clinical trials. Clin Adv Hematol Oncol. 2007;5(6):465. [PubMed] [Google Scholar]
- 7.Small EJ, Schellhammer PF, Higano CS, Redfern CH, Nemunaitis JJ, Valone FH, Verjee SS, Jones LA, Hershberg RM. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24(19):3089. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 8.Madan RA, Gulley JL, Schlom J, Steinberg SM, Liewehr DJ, Dahut WL, Arlen PM. Analysis of overall survival in patients with nonmetastatic castration-resistant prostate cancer treated with vaccine, nilutamide, and combination therapy. Clin Cancer Res. 2008;14(14):4526. doi: 10.1158/1078-0432.CCR-07-5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conn PM, Crowley WF., Jr Gonadotropin-releasing hormone and its analogs. Annu Rev Med. 1994;45:391. doi: 10.1146/annurev.med.45.1.391. [DOI] [PubMed] [Google Scholar]
- 10.Anderson J. The role of antiandrogen monotherapy in the treatment of prostate cancer. BJU Int. 2003;91(5):455. doi: 10.1046/j.1464-410x.2003.04026.x. [DOI] [PubMed] [Google Scholar]
- 11.Mercader M, Sengupta S, Bodner BK, Manecke RG, Cosar EF, Moser MT, Ballman KV, Wojcik EM, Kwon ED. Early effects of pharmacological androgen deprivation in human prostate cancer. BJU Int. 2007;99(1):60. doi: 10.1111/j.1464-410X.2007.06538.x. [DOI] [PubMed] [Google Scholar]
- 12.Staack A, Kassis AP, Olshen A, Wang Y, Wu D, Carroll PR, Grossfeld GD, Cunha GR, Hayward SW. Quantitation of apoptotic activity following castration in human prostatic tissue in vivo. Prostate. 2003;54(3):212. doi: 10.1002/pros.10179. [DOI] [PubMed] [Google Scholar]
- 13.Kyprianou N, Isaacs JT. Activation of programmed cell death in the rat ventral prostate after castration. Endocrinology. 1988;122(2):552. doi: 10.1210/endo-122-2-552. [DOI] [PubMed] [Google Scholar]
- 14.Isaacs JT. Advances and controversies in the study of programmed cell death/apoptosis in the development of and therapy for cancer. Curr Opin Oncol. 1994;6(1):82. doi: 10.1097/00001622-199401000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Mercader M, Bodner BK, Moser MT, Kwon PS, Park ES, Manecke RG, Ellis TM, Wojcik EM, Yang D, Flanigan RC, Waters WB, Kast WM, Kwon ED. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci U S A. 2001;98(25):14565. doi: 10.1073/pnas.251140998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olsen NJ, Watson MB, Henderson GS, Kovacs WJ. Androgen deprivation induces phenotypic and functional changes in the thymus of adult male mice. Endocrinology. 1991;129(5):2471. doi: 10.1210/endo-129-5-2471. [DOI] [PubMed] [Google Scholar]
- 17.Greenstein BD, Fitzpatrick FT, Adcock IM, Kendall MD, Wheeler MJ. Reappearance of the thymus in old rats after orchidectomy: inhibition of regeneration by testosterone. J Endocrinol. 1986;110(3):417. doi: 10.1677/joe.0.1100417. [DOI] [PubMed] [Google Scholar]
- 18.Olsen NJ, Viselli SM, Fan J, Kovacs WJ. Androgens accelerate thymocyte apoptosis. Endocrinology. 1998;139(2):748. doi: 10.1210/endo.139.2.5729. [DOI] [PubMed] [Google Scholar]
- 19.Williams KM, Lucas PJ, Bare CV, Wang J, Chu YW, Tayler E, Kapoor V, Gress RE. CCL25 increases thymopoiesis after androgen withdrawal. Blood. 2008;112(8):3255. doi: 10.1182/blood-2008-04-153627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldberg GL, Sutherland JS, Hammet MV, Milton MK, Heng TS, Chidgey AP, Boyd RL. Sex steroid ablation enhances lymphoid recovery following autologous hematopoietic stem cell transplantation. Transplantation. 2005;80(11):1604. doi: 10.1097/01.tp.0000183962.64777.da. [DOI] [PubMed] [Google Scholar]
- 21.Sutherland JS, Goldberg GL, Hammett MV, Uldrich AP, Berzins SP, Heng TS, Blazar BR, Millar JL, Malin MA, Chidgey AP, Boyd RL. Activation of thymic regeneration in mice and humans following androgen blockade. J Immunol. 2005;175(4):2741. doi: 10.4049/jimmunol.175.4.2741. [DOI] [PubMed] [Google Scholar]
- 22.Haines CJ, Giffon TD, Lu LS, Lu X, Tessier-Lavigne M, Ross DT, Lewis DB. Human CD4+ T cell recent thymic emigrants are identified by protein tyrosine kinase 7 and have reduced immune function. J Exp Med. 2009;206(2):275. doi: 10.1084/jem.20080996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drake CG, Doody AD, Mihalyo MA, Huang CT, Kelleher E, Ravi S, Hipkiss EL, Flies DB, Kennedy EP, Long M, McGary PW, Coryell L, Nelson WG, Pardoll DM, Adler AJ. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell. 2005;7(3):239. doi: 10.1016/j.ccr.2005.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roden AC, Moser MT, Tri SD, Mercader M, Kuntz SM, Dong H, Hurwitz AA, McKean DJ, Celis E, Leibovich BC, Allison JP, Kwon ED. Augmentation of T cell levels and responses induced by androgen deprivation. J Immunol. 2004;173(10):6098. doi: 10.4049/jimmunol.173.10.6098. [DOI] [PubMed] [Google Scholar]
- 25.Koh YT, Gray A, Higgins SA, Hubby B, Kast WM. Androgen ablation augments prostate cancer vaccine immunogenicity only when applied after immunization. Prostate. 2009;69(6):571. doi: 10.1002/pros.20906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aragon-Ching JB, Williams KM, Gulley JL. Impact of androgen-deprivation therapy on the immune system: implications for combination therapy of prostate cancer. Front Biosci. 2007;12:4957. doi: 10.2741/2441. [DOI] [PubMed] [Google Scholar]
- 27.Dunphy EJ, McNeel DG. Antigen-specific IgG elicited in subjects with prostate cancer treated with flt3 ligand. J Immunother (1997) 2005;28(3):268. doi: 10.1097/01.cji.0000158853.15664.0c. [DOI] [PubMed] [Google Scholar]
- 28.Dunphy EJ, Eickhoff JC, Muller CH, Berger RE, McNeel DG. Identification of antigen-specific IgG in sera from patients with chronic prostatitis. J Clin Immunol. 2004;24(5):492. doi: 10.1023/B:JOCI.0000040920.96065.5a. [DOI] [PubMed] [Google Scholar]
- 29.Viselli SM, Stanziale S, Shults K, Kovacs WJ, Olsen NJ. Castration alters peripheral immune function in normal male mice. Immunology. 1995;84(2):337. [PMC free article] [PubMed] [Google Scholar]
- 30.Montironi R, Magi-Galluzzi C, Muzzonigro G, Prete E, Polito M, Fabris G. Effects of combination endocrine treatment on normal prostate, prostatic intraepithelial neoplasia, and prostatic adenocarcinoma. J Clin Pathol. 1994;47(10):906. doi: 10.1136/jcp.47.10.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ross RW, Xie W, Regan MM, Pomerantz M, Nakabayashi M, Daskivich TJ, Sartor O, Taplin ME, Kantoff PW, Oh WK. Efficacy of androgen deprivation therapy (ADT) in patients with advanced prostate cancer: association between Gleason score, prostate-specific antigen level, and prior ADT exposure with duration of ADT effect. Cancer. 2008;112(6):1247. doi: 10.1002/cncr.23304. [DOI] [PubMed] [Google Scholar]
- 32.Deakin NO, Turner CE. Paxillin comes of age. J Cell Sci. 2008;121(Pt 15):2435. doi: 10.1242/jcs.018044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang SM, Huang CJ, Wang WM, Kang JC, Hsu WC. The enhancement of nuclear receptor transcriptional activation by a mouse actin-binding protein, alpha actinin 2. J Mol Endocrinol. 2004;32(2):481. doi: 10.1677/jme.0.0320481. [DOI] [PubMed] [Google Scholar]
- 34.Yang Z, Chang YJ, Miyamoto H, Ni J, Niu Y, Chen Z, Chen YL, Yao JL, di Sant’Agnese PA, Chang C. Transgelin functions as a suppressor via inhibition of ARA54-enhanced androgen receptor transactivation and prostate cancer cell growth. Mol Endocrinol. 2007;21(2):343. doi: 10.1210/me.2006-0104. [DOI] [PubMed] [Google Scholar]
- 35.Yoon HG, Wong J. The corepressors silencing mediator of retinoid and thyroid hormone receptor and nuclear receptor corepressor are involved in agonist- and antagonist-regulated transcription by androgen receptor. Mol Endocrinol. 2006;20(5):1048. doi: 10.1210/me.2005-0324. [DOI] [PubMed] [Google Scholar]