Abstract
Inflammation-mediated hypochlorous acid (HOCl) can damage DNA, DNA precursors, and other biological molecules, thereby producing an array of damage products such as 5-chlorouracil (ClU). In this study, we prepared and studied 5-chloro-2’-deoxyuridine (CldU) and ClU-containing oligonucleotide templates. We demonstrate that human K-562 cells grown in culture with 10 µM CldU incorporate substantial amounts of CldU without significant toxicity. When in the template, ClU residues pair with dATP, but also with dGTP, in a pH-dependent manner with incorporation by human polymerase β, Avian Myeloblastosis Virus reverse transcriptase (AMV-RT), and Escherichia coli Klenow fragment (exo−) polymerase. The enhanced miscoding of ClU is attributed to the electron-withdrawing 5-chlorine substituent that promotes formation of an ionized ClU-G mispair. When mispaired with G, ClU is targeted for removal by human glycosylases. The formation, incorporation and repair of ClU could promote transition mutations and other forms of heritable DNA damage.
Introduction
Inflammation and cancer have been linked in numerous studies (1–3), yet the mechanistic basis for this relationship remains unknown. Components of innate immunity generate highly reactive molecules that have potent antimicrobial activity; however, these chemically reactive species can cause collateral damage to host molecules including proteins, lipids and nucleic acids. Among these reactive species are superoxide, hydrogen peroxide and HOCl— the latter generated by myeloperoxidase from activated neutrophils (4–9). Among the known nucleic acid reaction products resulting from inflammation-mediated reactive species are oxidized and halogenated DNA bases, including 5-chlorouracil (ClU) (5–7). Of the halogenated uracils, ClU is arguably the most likely product of inflammation-mediated damage. 5-fluorouracil (FU) and 5-iodouracil (IU) have yet to be reported under physiological conditions. 5-bromouracil (BrU) is a potential by-product of inflammation from eosinophil peroxidase-derived HOBr (5); however, since the number of neutrophils in human blood is substantially larger than that of eosinophils, we anticipate that physiological ClU formation exceeds that of BrU. Although ClU is perhaps the most likely halogenated base to form in mammalian tissues, little is currently known about the biological impact of this damaged base.
Substantial work has been published previously on the similar halogenated analogs 5-fluorouracil (FU) and 5-bromouracil (BrU) (10–18). FU is a chemotherapy agent used in the treatment of several human malignancies whereas BrU is a thymine mimic and mutagenic base analog. The distinct differences in the biological properties of FU and BrU can be attributed in part to the different sizes of the 5-fluoro and 5-bromo substituents. The smaller size of the 5-substituent of the corresponding 2’-deoxynucleotide analog of FU (FdUMP) inhibits thymidylate synthase (TS), diminishing TMP needed for DNA replication (19). Further, both uracil and FU incorporated into DNA as a consequence of TS inhibition result in DNA glycosylase-mediated DNA fragmentation (19–20). Glycosylases are known to distinguish uracil and FU from BrU and thymine upon the basis of the size of the 5-substituent (21–27).
Since the 5-chloro group of ClU is intermediate in size between fluoro and bromo, the biological properties of ClU could overlap with both FU and BrU. While it is known that 5-chloro-2’-deoxyuridine (CldU) added to culture media is metabolized and incorporated into DNA as shown by antibody binding (28–32), CldU induces senescence, toxicity, and sister-chromatid exchanges by as yet unknown mechanisms (33–36).
The electronic-inductive properties of all three halogens, F, Cl and Br, are similar in that they withdraw electron density from the pyrimidine ring, increasing the acidity of both the N1 and N3 protons (37, 38). An electron-withdrawing substituent in the 5-position of a pyrimidine deoxynucleoside also weakens the glycosidic bond and renders the corresponding deoxynucleoside more susceptible to cleavage by DNA repair glycosylases (22–27). While ClU residues in DNA can be cleaved by glycosylases, the efficiency of repair appears to be highly context-dependent (25). All three halogenated bases form base pairs with adenine in duplex DNA in a configuration similar to that of a normal T–A base pair (13–15, 39). When mispaired with guanine, all three halogenated base pairs undergo a pH-dependent transition from a neutral wobble to ionized base pair in a configuration approaching that of a Watson-Crick base pair (16–18, 40). Previous studies have examined the polymerase coding properties of FU and BrU and have established that the mispairing of both analogs increases with pH, consistent with the role of base ionization in miscoding (41, 42).
In this study, we have examined the polymerase-directed coding properties of the ClU analog as a template base in an oligodeoxyribonucleotide with three polymerases: human polymerase β, Avian Myeloblastosis Virus reverse transcriptase and Escherichia coli Klenow fragment (exo-). These three polymerases comprise a group of model polymerases from eukaryotic, viral, and prokaryotic origin. Using mass spectrometry we have also examined the incorporation of 5-chloro-2’-deoxyuridine (CldU) into the DNA of replicating mammalian cells and the repair of ClU by the human glycosylase, SMUG1, when paired with adenine or mispaired with guanine. The results reported here may, in part, explain some of the biological properties of ClU incorporation into DNA.
Materials and Methods
Synthesis and characterization of CldU and ClU phosphoramidite and oligonucleotides containing ClU
Synthesis of CldU
Commercially available 2’-deoxyuridine, or dU (Chem-Impex International, Wood Dale, IL), was converted to 5-chloro-2’-deoxyuridine (CldU), as diagramed in Figure 1, according to the method published by Kumar et al. (43). The product CldU was characterized by NMR and mass spectrometry.
Figure 1. Schematic diagram of CldU and CldU-phosphoramidite.
Synthesis of ClU-phosphoramidite
The phosphoramidite of CldU was prepared by standard methods, as previously described. CldU was dried with anhydrous pyridine and converted to the 5-dimethyoxytrityl derivative. The corresponding 3’-cyanoethyl-diisopropylaminophosphoramidite was prepared using established procedures (44).
Synthesis of oligonucleotides containing ClU
Oligonucleotide resins and phosphoramidites of the normal DNA bases were obtained from Glen Research (Sterling, VA). Oligonucleotide synthesis was conducted with either a Pharmacia gene assembler (GE Healthcare Bio-Sciences, Piscataway, NJ) or an Expedite oligonucleotide synthesizer from Applied Biosystems (Foster City, CA). Oligonucleotides containing ClU were deprotected with concentrated aqueous ammonia at room temperature for 24 h and purified by HPLC. Phosphoramidites and precursors as well as oligonucleotides containing ClU were characterized by NMR and mass spectrometry as previously described (9,39,40). Oligonucleotide sequences utilized in this study are shown in Figure 2.
Figure 2. Template and primer sequences.
Sequences for studies of (A) Incorporation of dATP or dGTP opposite template ClU or T residue. (B) Repair by hSMUG1 glycosylase.
Incorporation of CldU into the DNA of K-562 cells
Growth of cells in CldU-containing media
Human erythroleukemia K-562 cells obtained from ATCC (CLL-243) were grown at 37°C in a humidified incubator at 5% CO2 in RPMI-1640 media (Cellgro, Manassas, VA). Media was supplemented with 10% fetal bovine serum (Gibco-Invitrogen, Carlsbad, CA) and 1% L-glutamine-penicillin-streptomycin solution.
Cells were initially seeded at 1.0 × 105 cells/mL and treated with either 10 µM CldU or 10 µM thymidine (negative control) for 63 h, or two cell doublings (45). Cells were counted by trypan blue exclusion, pelleted by centrifugation (500 × g) and washed with 10 mL sterile PBS.
DNA Extraction and GC/MS analysis
DNA was extracted from pelleted cells using DNeasy Blood and Tissue 1 µL Kit (Qiagen, Valencia, CA). The amount of DNA in samples was determined by measuring the ratio of UV absorbance at 260 and 280 nm. Samples containing approximately 1 µg of DNA were dried under reduced pressure and resuspended in 88% formic acid in sealed vials and heated at 140°C for 40 min. Formic acid was removed under reduced pressure, and the residue containing purine and pyrimidine free bases was derivatized in 20 µL anhydrous acetonitrile and 20 µL MTBSTFA with 1% TBDMCS (Pierce, Rockford, IL) in sealed vials at 140°C for 40 min. Samples were injected into the GC/MS (Hewlett Packard, Palo Alto, CA) in splitless mode. Mass spectra were collected in the selected ion mode, monitoring the M-57 fragment ion for each pyrimidine and purine examined (45). The retention times (min) for the silylated DNA bases were thymine (12.01), 5-chlorouracil (12.33), cytosine (12.57), 5-methylcytosine (12.68), adenine (14.41) and guanine (15.03). A standard curve was established using authentic standards between ClU and T comparing relative concentrations with relative peak areas (Figure S1).
Polymerase kinetic studies for ClU miscoding as a function of pH derived from gel electrophoretic studies
Polymerase incorporation assays
Human polymerase β (pol β was obtained from Enzymax (Lexington, KY), and AMV-RT and exonuclease-deficient Klenow fragment (exo−) polymerase were obtained from New England Biolabs (Ipswich, MA). For the polymerase incorporation assays, the primers were 5’-32P-end labeled by T4 polynucleotide kinase (New England Biolabs) with [γ-32P]adenosine triphosphate (MP Biomedicals, Costa Mesa, CA) under conditions recommended by the enzyme supplier. The labeled oligonucleotides were purified using G25 Sephadex columns (Roche Applied Science, Indianapolis, IN). A 2-fold excess of the complementary template strand was then added to the labeled primer mixture, incubated at 95°C for 5 min and allowed to cool to room temperature gradually to create the oligo-primer duplex.
Optimal buffer conditions were used for each polymerase examined. For polymerase incorporation assays with pol β, 50 nM of labeled substrate was incubated with 63 nM pol β, 15 µM of the complementary TTP (running start) and increasing concentrations of the target site dNTP in 1 × buffer (20 mM NaCl, 20 mM KCl, 50 mM Tris-HCl, 10 mM MgCl2, 2 mM dithiothreitol, 20 µg/mL BSA, 1% glycerol, pH 6.5, 7.0, 7.5, 8.0, 8.5, 9.0, or 9.5 at 25°C) in a total volume of 20 µL at 37°C for 30 min. For assays with AMV-RT, 33.05 nM of the labeled substrate was incubated with 2.83 nM AMV-RT, 100 µM of the complementary running start dNTP and increasing concentrations of the target site dNTP in 1 X buffer (75 mM NaCl, 60 mM Tris-HCl, 8 mM MgCl2, 0.5 mM dithiothreitol, pH 6.5, 7.0, 7.5, 8.0, 8.5, or 9.0 at 25°C) in a total volume of 20 µL at 37°C for 30 min. For assays with Klenow polymerase, 50 nM of labeled substrate was incubated with 10 nM Klenow, 500 nM of the complementary running start dNTP and increasing concentrations of the target site dNTP in 1 X buffer (50 mM NaCl, 10 mM Tris-HCl, 10 mM MgCl2, 1 mM dithiothreitol, pH 6.5, 7.0, 7.5, 8.0, 8.5, or 9.0 at 25°C) in a total volume of 20 µL at 37°C for 30 min. Reactions were stopped using equal volumes of Maxam-Gilbert loading buffer (98% formamide, 0.01 M EDTA, 1 mg/mL xylene cyanole and 1mg/mL bromophenol blue). The reaction products were electrophoresed on 20% denaturing polyacrylamide gels containing 8 M urea and quantified using a phosphorimager and ImageQuant 5.2 software (Molecular Dynamics, GE Healthcare Bio-Sciences).
The incorporation of two complementary dNTPs in this “running start model” confirms the viability of the 3’ end of the primer for extension. The third position of incorporation, the target site, is the site of interest for kinetic measurements. The relative nucleotide insertion at the template target site was determined by calculating the ratio of band intensity of the primer extended at the target site compared with the band intensity of primer extended to a site just prior to the target. When using high concentrations of the correct “running start” nucleotide, TTP, the primer is rapidly extended to the target site, and incorporation at the target site becomes rate-limiting.
Determination of kcat and Km at pH 7.5
The values of kcat and Km for the incorporation of dNTP against a template ClU residue were determined using the polymerase incorporation assays described above at pH 7.5. A series of reaction mixtures containing increasing concentrations of dNTP were incubated for 0, 0.5, 2, 5, 15, 30, or 120 min. The concentration of extended product formed by nucleotide insertion at the template target was determined by calculating the ratio of band intensity of the primer extended at the target site with the band intensity of the primer extended to a site just prior to the target. Initial velocities were evaluated by plotting product concentration versus time. The kcat and Km values were obtained by a nonlinear least squares fit of velocity versus dNTP concentration.
Determination of Kinetic Parameters of Nucleotide Incorporation as a function of pH
The relationship between enzyme velocity and the kinetic parameters Km and Vmax can be written as v0 = (Vmax * [S])/(Km + [S]). As presented by Goodman and colleagues (46,47), velocity can be used to estimate Vmax/Km when Km > [S]. The values of Vmax/Km were determined at fixed substrate concentration, [S], and the substrate concentration used in these measurements was less that 15% of the Km value for each polymerase shown in Table 1. For assays with pol β examining the incorporation of dATP or dGTP opposite ClU and T, the nucleotide concentrations used were 25 nM and 30 µM, respectively. For assays with AMV-RT examining the incorporation of dATP opposite ClU and T, the dATP concentrations used were 10 nM and 30 nM, respectively; for the incorporation of dGTP opposite ClU and T, the dGTP concentration was 60 µM. For assays with Klenow examining the incorporation of dATP or dGTP opposite ClU and T, the nucleotide concentrations used were 1 nM and 4 µM, respectively.
Table 1.
Kinetic parameter values (kcat, Km) of incorporation of dATP and dGTP against a template ClU residue with pol β, AMV-RT, and Klenow at pH 7.5.
| pol β | AMV-RT | Klenow | ||
|---|---|---|---|---|
| dATP:ClU | kcat (s−1) | 1.20 × 10−04 ± 1.57 × 10−05 | 3.75 × 10−02 ± 4.81 × 10−03 | 1.06 × 10−03 ± 4.38 × 10−05 |
| Km (M) | 1.65 × 10−07 ± 8.76 × 10−08 | 3.17 × 10−07 ± 1.57 × 10−07 | 6.39 × 10−09 ± 1.44 × 10−09 | |
| dGTP:ClU | kcat (s−1) | 4.95 × 10−05 ± 8.21 × 10−06 | 8.30 × 10−03 ± 1.38 × 10−03 | 2.21 × 10−03 ± 2.05 × 10−04 |
| Km (M) | 2.20 × 10−04 ± 1.31 × 10−04 | 3.90 × 10−04 ± 2.17 × 10−04 | 2.67 × 10−05 ± 9.79 × 10−06 |
Using the polymerase incorporation assays described above, the primer was extended by the addition of the complementary nucleotide up to the T or ClU target site. The incorporation opposite the target base was measured as a function of the complementary or non-complementary nucleotide. The relative velocity of nucleotide insertion at the template target site was determined by calculating the ratio of band intensity of the primer extended to the target site compared with the band intensity of primer extended to a site just prior to the target. The average Vmax/Km value and standard error for each polymerase was determined from three independent data sets.
Examination of hSMUG1 removal of ClU
The glycosylase studies were performed using human single-stranded selective monofunctional uracil-DNA glycosylase (hSMUG1). hSMUG1 was cloned and purified by our lab (27). A 100 pmol of each self-complementary DNA substrate shown in Figure 2B (T-G, ClU-A, ClU-G) was annealed separately in 20 mM Tris-HCl pH 8.0, 1 mM EDTA, 1 mM DTT, 50 mM NaCl, 0.1 mg/mL BSA. A typical reaction was done by incubating with 1.4 nmol recombinant hSMUG1 at 37°C for 24h. The sample was then desalted for MALDI-TOF analysis with a Bio-spin 6 column and a cation exchange column containing AG50W–X8 beads on the H+ form (Bio-Rad, Hercules, CA). MALDI-TOF experiments were performed with a Bruker Autoflex time of flight mass spectrometer operated in positive ion and reflectron modes (48). Kinetic studies ClU-A cleavage by hSMUG1 were also performed with 5’-32P-end labeled substrates and gel electrophoresis, as described previously (27).
Results
Preparation and characterization of CldU and oligonucleotides containing a ClU residue
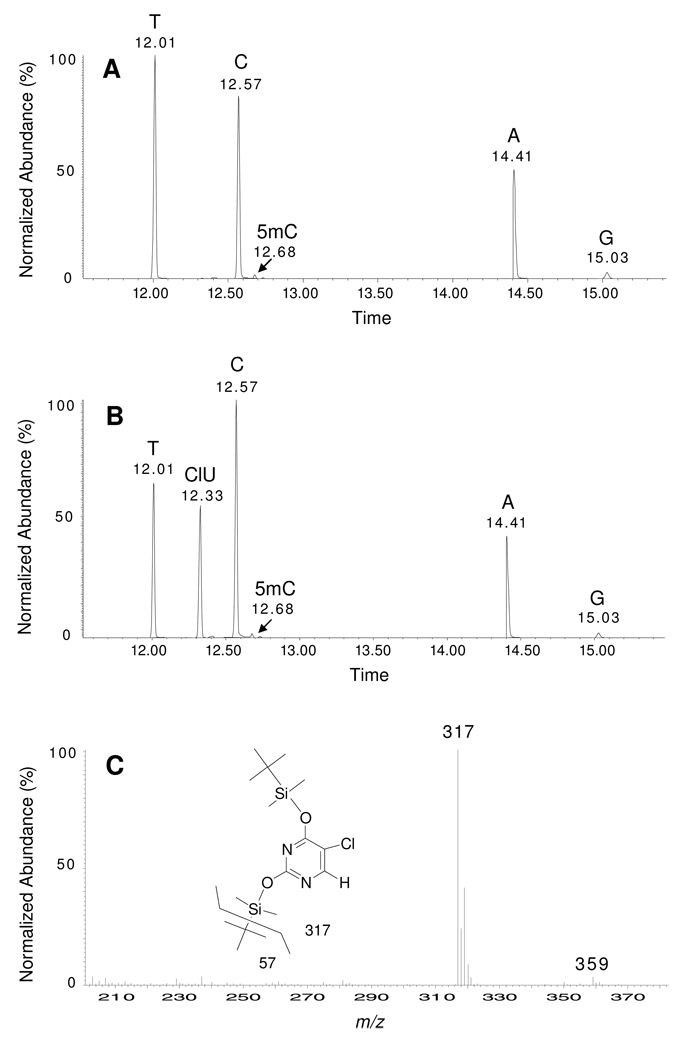
In this study, commercially available dU was converted to CldU (Figure 1) and characterized by NMR spectroscopy and mass spectrometry as previously described (9,39,40). CldU was also converted to the corresponding 5’-dimethoxytrityl-3’-phosphoramidite derivative and inserted into synthetic oligonucleotides (Figure 2). The composition of ClU-containing oligonucleotides was verified by GC/MS analysis following formic acid hydrolysis. The mass spectrum of a chlorine-containing molecule is easily discerned by the characteristic appearance of the M and M+2 isotopes (Figure 3C).
Figure 3. GC/MS analysis of DNA bases from DNA extracted from K-562 cells following growth in T or CldU containing media.
Total ion chromatogram of DNA bases in K-562 cells exposed to (A) 10 µM T or (B) 10 µM CldU. (C) Mass spectrum of the TBDMS derivative of ClU.
Incorporation of CldU into the DNA of replicating mammalian cells
We next examined the incorporation of CldU into the DNA of human K-562 cells grown in CldU containing culture media. The concentration of CldU in the media was 10 µM, and cells were grown for two cell doublings. Cells were harvested, and the DNA was extracted and separated from low molecular weight metabolites. The extracted DNA was hydrolyzed in formic acid and the liberated free bases were converted to their TBDMS derivatives and analyzed by GC/MS. Peaks corresponding to the normal bases C, T, A, G and 5-methylcytosine (5mC) were observed in the total ion chromatograms shown in Figures 3A and 3B. An additional peak was observed in the chromatogram of CldU treated cells (Figure 3B) at 12.33 min, the mass spectrum of which (Figure 3C) corresponds to 5-chlorouracil. The T content in the DNA from CldU treated cells (Figure 3B) is decreased in comparison to that of the control cells (Figure 3A), whereas the ClU content increases proportionately. The similar size of the T and ClU peaks (Figure 3B) indicates that the DNA of the cultured cells contain similar amounts of T and ClU. Upon the basis of a standard curve comparing peak volumes with relative concentration of ClU and T (Figure S1), the DNA extracted from the cells contained a mole ratio of ClU to T of 0.89 ± 0.02. Under the culture conditions used here, the percent viable cells in the CldU-treated culture was approximately 95%, as compared to thymidine-treated controls. These data reveal that a substantial amount of CldU is incorporated into the DNA of growing mammalian cells with minimal apparent toxicity over the time period examined here.
Incorporation of dATP opposite a template ClU residue
The capacity of ClU in synthetic oligonucleotides to serve as a template for polymerase-directed incorporation of dATP was then investigated. The template-primer complexes used in this study, containing ClU or T, are shown in Figure 2A. The incorporation of the normal triphosphate, dATP, opposite template ClU and T residues is demonstrated using the same gel assay described above. The Km and kcat for the insertion of dATP opposite template ClU at pH 7.5 is given in Table 1.
In order to estimate the impact of increasing pH on the incorporation of dATP opposite template ClU and T residues, Vmax/Km was estimated at selected values of pH from 6.5 to 9 or 9.5 by measuring vo/[S] at a fixed [S] where [S] was no more than 15% of Km for each enzyme as described in Materials and Methods. These data are presented in Table 2 and plotted in Figures S2. These data show that the polymerase-mediated incorporation of dATP opposite template ClU and T does vary with increasing pH; however, the observed incorporation of dATP opposite template ClU does not differ substantially from incorporation opposite template T at any value of pH for any of the three polymerases examined. These data confirm that the template properties of ClU and T are similar with respect to dATP incorporation.
Table 2.
Kinetic parameter values (Vmax/Km) of misincorporation of dATP against a template ClU or T residue and incorporation of dGTP against a template ClU and T residue with pol β, AMV-RT, and Klenow determined from three independent data sets.
| pol β | AMV-RT | Klenow | ||
|---|---|---|---|---|
| pH | Vmax/Km(s−1) | |||
| dATP:ClU | 6.5 | 1.18 × 10−05 ± 6.75 × 10−06 | 2.42 × 10−04 ± 2.74 × 10−05 | 8.38 × 10−05 ± 1.42 × 10−05 |
| 7.0 | 2.01 × 10−05 ± 5.99 × 10−06 | 2.83 × 10−04 ± 9.02 × 10−06 | 9.60 × 10−05 ± 1.59 × 10−06 | |
| 7.5 | 3.15 × 10−05 ± 3.55 × 10−06 | 2.80 × 10−04 ± 3.25 × 10−05 | 1.33 × 10−04 ± 1.82 × 10−05 | |
| 8.0 | 5.09 × 10−05 ± 2.63 × 10−06 | 1.97 × 10−04 ± 2.63 × 10−05 | 1.70 × 10−04 ± 1.27 × 10−05 | |
| 8.5 | 5.61 × 10−05 ± 5.78 × 10−06 | 1.03 × 10−05 ± 1.55 × 10−05 | 1.23 × 10−04 ± 2.05 × 10−05 | |
| 9.0 | 4.06 × 10−05 ± 3.92 × 10−06 | 7.59 × 10−05 ± 7.73 × 10−06 | 9.62 × 10−05 ± 1.24 × 10−05 | |
| 9.5 | 3.57 × 10−05 ± 1.04 × 10−06 | N/A | N/A | |
| dATP:T | 6.5 | 1.33 × 10−05 ± 4.72 × 10−06 | 1.82 × 10−04 ± 1.11 × 10−05 | 5.98 × 10−05 ± 4.75 × 10−06 |
| 7.0 | 2.78 × 10−05 ± 4.29 × 10−06 | 2.00 × 10−04 ± 1.05 × 10−05 | 1.29 × 10−04 ± 3.08 × 10−05 | |
| 7.5 | 3.50 × 10−05 ± 8.55 × 10−06 | 2.17 × 10−04 ± 4.17 × 10−06 | 1.76 × 10−04 ± 1.68 × 10−05 | |
| 8.0 | 5.76 × 10−05 ± 1.04 × 10−05 | 1.49 × 10−04 ± 1.46 × 10−05 | 2.14 × 10−04 ± 2.58 × 10−05 | |
| 8.5 | 5.27 × 10−05 ± 1.09 × 10−05 | 8.77 × 10−05 ± 5.83 × 10−06 | 1.53 × 10−04 ± 2.36 × 10−05 | |
| 9.0 | 4.11 × 10−05 ± 1.56 × 10−05 | 5.50 × 10−05 ± 7.48 × 10−06 | 1.05 × 10−04 ± 1.55 × 10−05 | |
| 9.5 | 2.94 × 10−05 ± 1.02 × 10−05 | N/A | N/A | |
| dGTP:ClU | 6.5 | 1.11 × 10−08 ± 4.57 × 10−10 | 1.10 × 10−08 ± 2.07 × 10−09 | 5.93 × 10−08 ± 3.12 × 10−08 |
| 7.0 | 2.01 × 10−08 ± 1.80 × 10−09 | 1.73 × 10−08 ± 2.93 × 10−09 | 7.39 × 10−08 ± 3.85 × 10−09 | |
| 7.5 | 3.56 × 10−08 ± 4.87 × 10−09 | 4.72 × 10−08 ± 1.34 × 10−09 | 1.21 × 10−07 ± 2.32 × 10−08 | |
| 8.0 | 1.01 × 10−07 ± 1.16 × 10−08 | 8.60 × 10−08 ± 3.89 × 10−09 | 2.86 × 10−07 ± 2.69 × 10−08 | |
| 8.5 | 3.21 × 10−07 ± 1.69 × 10−08 | 1.04 × 10−07 ± 1.49 × 10−09 | 3.90 × 10−07 ± 2.68 × 10−08 | |
| 9.0 | 4.65 × 10−07 ± 1.12 × 10−08 | 1.08 × 10−07 ± 4.90 × 10−09 | 4.61 × 10−07 ± 4.45 × 10−08 | |
| 9.5 | 5.56 × 10−07 ± 1.56 × 10−08 | N/A | N/A | |
| dGTP:T | 6.5 | 4.91 × 10−09 ± 1.06 × 10−09 | 1.33 × 10−08 ± 1.21 × 10−09 | 2.64 × 10−08 ± 1.65 × 10−08 |
| 7.0 | 7.84 × 10−09 ± 9.51 × 10−10 | 1.72 × 10−08 ± 1.14 × 10−09 | 4.72 × 10−08 ± 1.42 × 10−09 | |
| 7.5 | 9.70 × 10−09 ± 2.23 × 10−09 | 2.79 × 10−08 ± 4.82 × 10−09 | 8.00 × 10−08 ± 1.41 × 10−08 | |
| 8.0 | 1.52 × 10−08 ± 2.62 × 10−09 | 4.48 × 10−08 ± 2.77 × 10−09 | 1.77 × 10−07 ± 1.10 × 10−08 | |
| 8.5 | 1.77 × 10−08 ± 1.81 × 10−09 | 5.91 × 10−08 ± 2.47 × 10−09 | 2.22 × 10−07 ± 1.43 × 10−08 | |
| 9.0 | 5.20 × 10−08 ± 6.47 × 10−09 | 2.06 × 10−08 ± 6.23 × 10−09 | 2.59 × 10−07 ± 9.27 × 10−09 | |
| 9.5 | 1.08 × 10−07 ± 1.20 × 10−08 | N/A | N/A | |
Misincorporation of dGTP opposite template ClU by DNA polymerases
The kinetic parameters for the incorporation of dGTP opposite template ClU at pH 7.5 were determined as shown in Table 1. When comparing the “correct” incorporation of dATP opposite template ClU versus the “incorrect” incorporation of dGTP, the observed Km for “incorrect” incorporation is three orders of magnitude higher than for “correct” incorporation. The value of kcat for incorrect base pair formation declines by approximately one order of magnitude. Similar results were obtained with the three model polymerases examined.
The misincorporation of dGTP opposite template ClU and T was then investigated as functions of both dGTP concentration and solution pH. Gel electrophoretic data obtained with pol β is shown in Figure 4. Corresponding gels for AMV reverse transcriptase and Klenow polymerase are shown in supplementary figures (Figure S3 and Figure S4). These data visually illustrate the difference between the misincorporation of dGTP opposite template ClU and T with increasing pH. With all three polymerases, the misincorporation of dGTP opposite both ClU and T increases with increasing dGTP concentration and increasing pH; however, the impact of increasing pH is much more apparent with dGTP incorporation opposite ClU that with template T.
Figure 4. Gel electrophoretic analysis of the incorporation by pol β of dGTP against template ClU and T residues as a function of solution pH.
(A) Template ClU. (B) Template T. A labeled primer is extended by the incorporation of two T deoxynucleotides (TTP is present at a saturating concentration of 15 µM) to reach the template target site ClU or T. Lane 1 includes the primer only while dGTP is present in the following concentrations: Lane 2: 0 µM; Lane 3: 15 µM; Lane 4: 30 µM; Lane 5: 60 µM; Lane 6: 125 µM; Lane 7: 250 µM; Lane 8: 1000 µM; Lane 9: 3000 µM. Template sequences corresponding to Figure 2A and the incorporated bases are shown on the right side of the figure.
The misinsertion efficiency of dGTP opposite template T and ClU residues, Vmax/Km, was measured as described above for dATP as a function of pH and the results are recorded in Table 2 and plotted in Figure 5. For pol β and Klenow, the misincorporation opposite both ClU and T increases continuously with increasing pH, whereas with AMV-RT the misinsertion of dGTP opposite T shows an apparent optimum of pH 8.5.
Figure 5. Kinetic parameters of misincorporation as a function of solution pH corresponding to Table 2.
(A) Pol β misincorporation. (B) AMV-RT misincorporation. (C) Klenow misincorporation. T:dGTP (▼), ClU:dGTP (■).
Both the visual presentation of the gel data shown in Figure 4, Figure S3, and Figure S4 and the graphical presentation of the measured Vmax/Km values show that the misincorporation of dGTP opposite template ClU increases with increasing pH more rapidly than that with template T. The increased misinsertion of dGTP opposite ClU with increasing pH is consistent with the formation of an ionized base pair as shown in Figure 6. The pH-dependent insertion frequency of dGTP opposite template ClU (Table 2) was normalized to the pH-dependence of dATP insertion opposite template ClU (Table 2) to generate the misinsertion parameter, fins:
| (Equation 1) |
Figure 6. Proposed base pairing schemes for ClU.
(A) Neutral wobble configuration. (B) Ionized pseudo-Watson-Crick configuration.
To determine if a relationship exists between the misinsertion of dGTP and the fraction of ionized ClU base pairs, fins was plotted versus the percentage of ionized base pairs at a given pH, as shown in Figure 7, where the percentage of ionized base pairs is given by the following equation:
| (Equation 2) |
Figure 7. Relationship between polymerase kinetic parameters and base ionization.
Pol β (■): The apparent pKa is 8.9; the slope of the line is 1.89 × 10−4 with an intercept of 4.89 × 10−4 and r2 is 0.99. AMV-RT (●): The apparent pKa is 8.5; the slope of the line is 1.90 × 10−5 with an intercept of 8.50 × 10−6 and r2 is 0.99. Klenow (▼): The apparent pKa is 8.7; the slope of the line is 6.32 × 10−5 with an intercept of 6.32 × 10−4 and r2 is 0.99.
The pKa in Equation 2 corresponds to the “apparent” pKa for the formation of the ionized ClU-G base pair in the active site of the polymerase. The value of the pKa was obtained by varying the pKa between 7 and 12 with each polymerase independently until the best fit was obtained for the plots shown in Figure 7. The values obtained were 8.9, 8.5, and 8.7 for pol β, AMV-RT, and Klenow, respectively. The linear plot in Figure 7, relating fins to the percentage of ionized base pairs for pol β, has a slope 1.89 × 10−4 with an intercept of 4.89 × 10−4 and a r2 value of 0.99. For AMV-RT, the plot has a slope of 1.90 × 10−5 with an intercept of 8.50 × 10−6 and a r2 value of 0.99. For Klenow, the plot has a slope of 6.32 × 10−5 with an intercept of 6.32 × 10−4 and a r2 value of 0.99.
Repair of ClU in synthetic oligonucleotides by human SMUG1
The repair of ClU by the human glycosylase, hSMUG1, was examined by two separate assays. In the first assay, we examined the cleavage rate constant of ClU by hSMUG1 using 32P-5’-end-labeled oligonucleotides with the same sequence and reaction conditions described previously (26). We observed that the cleavage rate of the ClU-G by hSMUG1 is at least two orders of magnitude faster than that of ClU-A; no cleavage of ClU-A was observed where the limit of detection of the method was 1 × 10−6 s−1 (data not shown).
In the second assay, we examined the cleavage rate of ClU by hSMUG1 using MALDI-TOF-MS within the oligonucleotide sequence shown in Figure 2B. The thermodynamic stability and structure of this sequence containing ClU have been studied previously (39, 40). ClU mispaired with G was selectively cleaved from the duplex, generating an oligonucleotide containing an abasic site. The relative peak heights of cleaved and intact oligonucleotides indicate that 47% of ClU-G mispair-containing oligonucleotides were cleaved. In contrast, no cleavage activity was observed for the oligonucleotide duplexes containing T–G (Figure 8B) or ClU-A (Figure 8C). The theoretical mass of the abasic site containing oligonucleotide is 3552.59 amu, and the observed mass is 3552.52 amu. The reduction in oligonucleotide mass was observed to be 128 amu, which reflects the displacement of ClU (-145 amu) by a hydroxyl group (17 amu).
Figure 8. Activity of hSMUG1 on synthetic oligonucleotides containing T-G, ClU-A or ClU-G.
MALDI spectra of oligonucleotides. (A) Mixture of T-G, ClU-A and ClU-G oligonucleotide without enzyme reaction. (B) T–G oligonucleotide reacted with hSMUG1. (C) ClU-A oligonucleotide reacted with hSMUG1. (D) ClU-G oligonucleotide reacted with hSMUG1, generating an oligonucleotide with an abasic site. The cleaved ClU residue is too small to be seen with this method.
Discussion
Inflammation-mediated reactive molecules can generate ClU
Multiple reactive species are generated during an inflammatory response, and these chemically reactive species are not selective in damaging pathogens (4–9). Among the multiple reaction products is ClU in DNA, as well as the corresponding nucleosides and nucleotides in the precursor pool (4–8). The purpose of this work was to investigate the capacity of CldU to be incorporated into the DNA of replicating mammalian cells. Further, we wished to examine the potential pH-dependent miscoding properties of ClU and to examine its context-dependent repair by the human repair glycoyslase, SMUG1. The three polymerases examined here, pol β, AMV-RT, and Klenow (exo-), represent commercially available model polymerases. The polymerases examined here, as well as the oligonucleotide templates utilized here, are intended to represent model systems and are not intended to be of special significance to the potential mutagenicity or toxicity of ClU and its analogs.
CldU is incorporated into replicating human cells
When CldU is placed into tissue culture medium, mammalian cells incorporate the analog into DNA. We observe that 10 µM concentration of CldU in the media does not alter cell division kinetics; however, the mole ratio of ClU to T in the DNA is 0.89 ± 0.02 when cells are grown for two cell doublings. Previously it has been shown that CldU is metabolized and incorporated into DNA using antibodies that bind selectively to DNA containing halogenated bases (28–32). We have confirmed and extended these finding using a mass spectral method as reported here. In the studies reported here, CldU is more similar to BrdU in acting as a T analog. The chloro substituent of T is sufficiently large that ClU metabolites are not efficient poisons of thymidylate synthase, although a previous study suggested that the toxicity of CldU could in part be attributed to inhibition of thymidylate synthase (32). Further, the larger size of the chloro substituent, relative to the hydrogen of uracil, allows ClU to accumulate in the DNA without removal by UNG (22). The above results suggest that ClU is a good substitute for T in cell culture. However, the T-methyl group is an important recognition point for sequence-specific DNA binding proteins (49), and it is yet to be determined whether ClU can substitute for T in all sequence-specific DNA-protein interactions.
When in template DNA, ClU codes as a T analog
When in an oligonucleotide template, ClU directs the incorporation of dATP. The magnitude of the Km for the incorporation of dATP opposite ClU is 1000-fold lower than for dGTP opposite ClU measured at pH 7.5, and the kcat for incorporation of dATP is 10-fold higher than for dGTP for each of the three polymerases examined (Table 1). These data are in accord with the cell incorporation studies described above demonstrating that CldU functions as a thymidine analog.
The efficiency of polymerase insertion opposite template ClU is pH dependent
In this study, a previously described method (42) was used to approximate Vmax/Km with increasing pH. For all three polymerases, Vmax/Km for the incorporation of dATP was observed to increase and then decrease with increasing pH with an apparent pH optimum between pH 7 and 8, depending upon the polymerase. Indistinguishable behavior was seen for the incorporation of dATP opposite template T. The observed pH-dependent behavior likely reflects pH-dependent changes on the polymerase and dNTP phosphate. Although more acidic than the imino proton of T, the imino proton of ClU is an integral part of the ClU-A base pair, as seen in a recent NMR structural study (39).
The misincorporation of dGTP opposite template ClU and T was also investigated by two independent methods. In the first method, the concentration dependence for dGTP was measured at three values of pH, as shown in Figure 4, Figure S3 and Figure S4. Although the incorporation of dGTP opposite ClU and T appears low at pH 6.5, the incorporation of dGTP opposite ClU is clearly stimulated by increasing the pH to 8.5. The observed increased misincorporation of dGTP opposite template ClU is consistent with previous data from Goodman and coworkers with FU and BrU (42) and with the formation of an ionized base pair approximating Watson-Crick genometry (40) as shown in Figure 6. In a second series of experiments, Vmax/Km was measured as a function of pH at a selected concentration of dNTP which was less than 15% of the Km (Table 2).
Changing solution pH alters both polymerase/substrate properties, as demonstrated with dATP opposite ClU, and the template miscoding property of ClU, as demonstrated with dGTP. In order to estimate the impact of pH on the base pairing of ClU, the misinsertion frequency, fins, was determined as described in Equation 1. The magnitude of fins was observed to increase continuously with increasing pH, suggesting that the ionized base pair was contributing to the increased miscoding.
If the ionized form of ClU does explain the increased formation of the dGTP-ClU mispair with increasing pH, a linear relationship should exist between the fraction of the ionized form of ClU and the pH-dependent fins. The fraction of the ionized form can be determined from the pH and the apparent pKa value for the ionizing species as indicated in Equation 2. In this study, values for the apparent pKa were iterated between 7 and 12, and the value that provided the best fit between the percent ionized and fins was determined. The complex or apparent pKa values measured for the three polymerases are 8.9, 8.5 and 8.7 for pol β, AMV-RT, and Klenow, respectively. The pKa for CldU monomer is approximately 7.9 (37); however, for the ClU-G base pair in a duplex oligonucleotide, the apparent pKa was recently measured to be 8.7 (40). Our values appear to correlate with the previously estimated pKa value 8.6 of the BrU-G base pair for AMV-RT (42). We believe this data is consistent with base ionization contributing substantially to the miscoding of ClU, however, our data cannot be used to eliminate the contribution of other potential forms to ClU miscoding at lower pH. Similar data was observed here with the three polymerases selected for study. It is possible that divergent results might be obtained with other polymerases on other templates.
ClU is repaired when mispaired with G much faster than when paired with A
Although ClU ionization could enhance miscoding relative to T, the mutagenic potential of ClU might be amplified by DNA repair glycosylases. While misincorporated uracil residues are rapidly repaired from U–A base pairs, U–G mispairs or from single-stranded substrates by uracil DNA glycosylase (UNG), the larger 5-chloro substituent of ClU prevents UNG removal of ClU (22).
Previous studies have established that ClU is repaired by two other members of the uracil glycosylase family, TDG and hSMUG1; however, ClU is removed much more efficiently, or exclusively, when mispaired with G rather than paired with A. ClU is removed by TDG when mispaired with G three to five orders of magnitude faster than when paired with A (25). In a previous study from this laboratory, hSMUG1 was shown to exploit reduced duplex stability when selecting pyrimidines for removal (27); however, the repair of ClU-A was not investigated in that study. In this study, we have extended the previous results to include ClU-A. We observe here that when examined in the same sequence, under the same conditions that led to substantial removal of ClU mispaired with G, no cleavage of ClU paired with A is observed. The limit of detection with that method is 1 × 10−6 s−1, and therefore, the selectivity of hSMUG1 for ClU-G over ClU-A is at least two orders of magnitude in the C(ClU)G sequence.
In the current study we also examined the relative efficiency of repair of ClU when paired with A or mispaired with G in a sequence that has been investigated in both thermodynamic and structural studies (39, 40). ClU was paired with G or A in the duplex oligonucleotide sequence shown in Figure 2B. Upon incubation with hSMUG1, cleavage of ClU can be measured directly by MALDI-TOF analysis as shown in Figure 8. The removal of ClU results in the formation of an abasic-site containing oligonucleotide with a mass reduced by 128 amu. hSMUG1 cleaves the ClU-G duplex, as shown in Figure 8D. In contrast, no cleavage of the ClU-A duplex (Figure 8C) is observed. The free energy difference between the ClU-A and ClU-G duplexes examined in this repair assay is 2.7 kcal/mol (40). Upon the basis of the parameters described previously for hSMUG1 (27), a free energy difference of 2.7 kcal/mol would predict a discrimination between ClU-G and ClU-A of approximately 30. Although we do not wish to over-interpret the MALDI-TOF data presented here, the data presented in Figure 8 indicates that ClU-G is cleaved by hSMUG1 in the G(ClU)G sequence at a rate at least 30 times that of ClU-A.
ClU-G is repaired much faster than T–G by both TDG and hSMUG1
The primary physiological role of TDG is believed to be for removing T residues mispaired with G that arise from the deamination of 5mC. While TDG does remove T mispaired with G, it removes ClU mispaired with G between two and three orders of magnitude faster, depending upon the sequence context (25). The primary characteristic that allows discrimination between T–G and ClU-G by TDG is likely due to the differences in the electronic inductive properties of a methyl group and a chlorine substituent as seen for E. coli mismatch uracil-DNA glycosylase (22). The discrimination of hSMUG1 for ClU-G versus T–G is approximately two orders of magnitude and derives primarily from differences in the free energy of solvation of the chlorine substituent versus the T methyl group (27).
Mutagenic potential of ClU
ClU residues in DNA and ClU metabolites can be formed at sites of inflammation (4–8). Once formed, CldU in the precursor pool can be converted to CldUTP and incorporated into DNA (29–31). When in DNA, the ClU-A base pair is thermodynamically stable and is not subject to glycosylase removal by the predominant glycosylases found in mammalian cells (24, 25). The data reported here on in vitro polymerase incorporation, the lack of absence of repair of ClU when paired with A, and the growth of cells in the presences of CldU with substantial incorporation into DNA are consistent with previous observations on the incorporation of CldU into cellular DNA and suggest that ClU could, therefore, be a persistent lesion in DNA. The amount of ClU in DNA would be expected to increase with increased cellular exposure to inflammation.
Occasionally, the ClU residue could miscode with G. The increased miscoding potential of ClU relative to T can be attributed in part to an increased tendency to ionize at physiological pH, resulting from the electron-withdrawing property of the chloro substituent. Although the miscoding potential of ClU might be modest at physiological pH, mechanisms for repair could substantially amplify the mutagenic potential of ClU.
Polymerase directed misinsertion of dGTP opposite template T could potentially be repaired by the mismatch repair (MMR) pathway. The MMR pathway recognizes structural perturbations associated with mispairs and preferentially removes the mispaired base in the newly replicated strand (51). A ClU-G mispair could potentially be repaired by MMR, although this has not yet been demonstrated.
The primary pathway for the repair of a G–T mispair, particularly outside a CpG dinucleotide, would be MMR. In contrast, the ClU-G mispair is also recognized by members of the BER pathway and, in particular, hSMUG1 and TDG. In this context, MMR and BER could compete with distinctly different biological outcomes (Figure 9). If identified first by MMR, the G of a ClU-G mispair generated by polymerase misincorporation would be removed, allowing for template-mediated repair. In contrast, if the ClU-G mispair were first encountered by TDG or hSMUG1, the ClU residues would be removed, allowing repair synthesis on the incorrect G-containing template, generating a transition mutation. Due to the large number of potential damage products derived from inflammation-mediated reactive chemical species, a multitude of transitions, transversions, insertions and deletions might result from inflammation-mediated DNA damage, and therefore, the T–A to C–G mutation resulting from ClU pairing with G might not be apparent. However, the mutagenic potential of ClU formation should be considered in future studies of inflammation-mediated mutation.
Figure 9. Repair schemes for T and ClU mispaired with guanine.
The T–G and ClU-G mispairs can be repaired by either the mismatch repair (MMR) or the base-excision repair (BER) pathways. ClU is rapidly repaired by BER, potentially promoting a transition mutation. The asterisk (*) denotes the gap in the opposing strand generated as a repair intermediate.
Supplementary Material
Acknowledgments
Funding
This work is supported by grant GM41336 from the National Institutes of Health.
References
- 1.Srikrishna G, Freeze HH. Endogenous damage-associated molecular pattern molecules at the crossroads of inflammation and cancer. Neoplasia. 2009;11:615–628. doi: 10.1593/neo.09284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeNardo DG, Coussens LM. Inflammation and breast cancer. Balancing immune response: crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res. 2007;9:212–221. doi: 10.1186/bcr1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valinluck V, Sowers LC. Inflammation-mediated cytosine damage: A mechanistic link between inflammation and the epigenetic alterations in human cancers. Cancer Res. 2007;67:5583–5586. doi: 10.1158/0008-5472.CAN-07-0846. [DOI] [PubMed] [Google Scholar]
- 4.Dale DC, Boxer L, Liles WC. The phagocytes: neutrophils and monocytes. Blood. 2008;112:935–945. doi: 10.1182/blood-2007-12-077917. [DOI] [PubMed] [Google Scholar]
- 5.Henderson JP, Byun J, Takeshita J, Heineke JW. Phagocytes produce 5-chlorouracil and 5-bromouracil, two mutagenic products of myeloperoxidase, in human inflammatory tissue. J. Biol. Chem. 2003;278:23522–23528. doi: 10.1074/jbc.M303928200. [DOI] [PubMed] [Google Scholar]
- 6.Jiang Q, Blount BC, Ames BN. 5-Chlorouracil, a marker of DNA damage from hypochlorous acid during inflammation. J. Biol. Chem. 2003;278:32834–32840. doi: 10.1074/jbc.M304021200. [DOI] [PubMed] [Google Scholar]
- 7.Takeshita J, Byun J, Nhan TQ, Pritchard DK, Pennathur S, Schwartz SM, Chait A, Heineke JW. Myeloperoxidase generates 5-chlorouracil in human atherosclerotic tissue. J. Biol. Chem. 2006;281:3096–3104. doi: 10.1074/jbc.M509236200. [DOI] [PubMed] [Google Scholar]
- 8.Knaapen AM, Güngör N, Schins RP, Borm PJ, Van Schooten FJ. Neutrophils and respiratory tract DNA damage and mutagenesis: a review. Mutagenesis. 2006;21:225–236. doi: 10.1093/mutage/gel032. [DOI] [PubMed] [Google Scholar]
- 9.Kang JI, Sowers LC. Examination of hypochlorous acid-induced damage to cytosine residues in a CpG dinucleotide in DNA. Chem. Res. Toxicol. 2008;21:1211–1218. doi: 10.1021/tx800037h. [DOI] [PubMed] [Google Scholar]
- 10.Litman RM, Pardee AB. The induction of mutants of bacteriophage T2 by 5-bromouracil. III. Nutritional and structural evidence regarding mutagenic action. Biochim. Biophys. Acta. 1960;42:117–130. doi: 10.1016/0006-3002(60)90758-7. [DOI] [PubMed] [Google Scholar]
- 11.Sternglanz H, Bugg CE. Relationship between the mutagenic and base-stacking properties of halogenated uracil derivatives. The crystal structure of 5-chloro- and 5-bromouracil. Biochim. Biophys. Acta. 1975;378:1–11. doi: 10.1016/0005-2787(75)90130-6. [DOI] [PubMed] [Google Scholar]
- 12.Noskov V, Negishi K, Ono A, Matsuda A, Ono B, Hayatsu H. Mutagenicity of 5-bromouracil and N6-hydroxyadenine studied by yeast oligonucleotide transformation assay. Mutat. Res. 1994;308:43–51. doi: 10.1016/0027-5107(94)90197-x. [DOI] [PubMed] [Google Scholar]
- 13.Sowers LC, Eritja R, Kaplan BE, Goodman MF, Fazakerley GV. Structural and dynamic properties of a fluorouracil-adenine base pair in DNA studied by proton NMR. J. Biol. Chem. 1987;262:15436–15442. [PubMed] [Google Scholar]
- 14.Kremer AB, Mikita T, Beardsley GP. Chemical consequences of the incorporation of 5-fluorouracil into DNA as studied by NMR. Biochemistry. 1987;26:391–397. doi: 10.1021/bi00376a009. [DOI] [PubMed] [Google Scholar]
- 15.Fazakerley GV, Sowers LC, Eritja R, Kaplan BE, Goodman MF. Structural and dynamic properties of a bromouracil-adenine base pair in DNA studied by proton NMR. J. Biomol. Struct. Dyn. 1987;5:639–650. doi: 10.1080/07391102.1987.10506417. [DOI] [PubMed] [Google Scholar]
- 16.Sowers LC, Eritja R, Kaplan B, Goodman MF, Fazakerley GV. Equilibrium between a wobble and ionized base pair formed between fluorouracil and guanine in DNA as studied by proton and fluorine NMR. J. Biol. Chem. 1988;263:14794–14801. [PubMed] [Google Scholar]
- 17.Sowers LC, Goodman MF, Eritja R, Kaplan B, Fazakerley GV. Ionized and wobble base-pairing for bromouracil-guanine equilibrium under physiological conditions. A nuclear magnetic resonance study on an oligonucleotide containing a bromouracil-guanine base-pair as a function of pH. J. Mol. Biol. 1989;205:437–447. doi: 10.1016/0022-2836(89)90353-7. [DOI] [PubMed] [Google Scholar]
- 18.Patel DJ, Kozlowski SA, Marky LA, Rice JA, Broka C, Dallas J, Itakura K, Breslauer KJ. Structure, dynamics and energetics of deoxyguanosine·thymidine wobble base pair formation in the self-complementary d(CGTGAATTCGCG) duplex in solution. Biochemistry. 1982;21:437–444. doi: 10.1021/bi00532a003. [DOI] [PubMed] [Google Scholar]
- 19.Parker WB, Cheng YC. Metabolism and mechanism of action of 5-fluorouracil. Pharmacol. Ther. 1990;48:381–395. doi: 10.1016/0163-7258(90)90056-8. [DOI] [PubMed] [Google Scholar]
- 20.Wyatt MD, Wilson DM., III Participation of DNA repair in the response to 5-fluorouracil. Cell Mol. Life Sci. 2009;66:788–799. doi: 10.1007/s00018-008-8557-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wibley JEA, Waters TR, Haushalter K, Verdine GL, Pearl LH. Structure and specificity of the vertebrate anti-mutator uracil-DNA glycosylase SMUG1. Mol. Cell. 2003;11:1647–1659. doi: 10.1016/s1097-2765(03)00235-1. [DOI] [PubMed] [Google Scholar]
- 22.Liu P, Burdzy A, Sowers LC. Substrate recognition by a family of uracil-DNA glycosylases: UNG, MUG and TDG. Chem. Res. Toxicol. 2002;15:1001–1009. doi: 10.1021/tx020030a. [DOI] [PubMed] [Google Scholar]
- 23.Liu P, Burdzy A, Sowers LC. Repair of the mutagenic DNA oxidation product, 5-formyluracil. DNA Repair. 2003;2:199–210. doi: 10.1016/s1568-7864(02)00198-2. [DOI] [PubMed] [Google Scholar]
- 24.Bennett MT, Rodgers MT, Hebert AS, Ruslander LE, Eisele L, Drohat AC. Specificity of human thymine DNA glycosylase depends on N-glycosidic bond stability. J. Am. Chem. Soc. 2006;128:12510–12519. doi: 10.1021/ja0634829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morgan MT, Bennett MT, Drohat AC. Excision of 5-halogenated uracils by human thymine DNA glycosylase. Robust activity for DNA contexts other than CpG. J. Biol. Chem. 2007;282:27578–27586. doi: 10.1074/jbc.M704253200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu P, Theruvathu JA, Darwanto A, Lao VV, Pascal T, Goddard WA, III, Sowers LC. Mechanisms of base selection by the Escherichia coli mispaired uracil glycosylase. J. Biol. Chem. 2008;283:8829–8836. doi: 10.1074/jbc.M707174200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Darwanto A, Theruvathu JA, Sowers JL, Rogstad DK, Pascal T, Goddard WA, III, Sowers LC. Mechanism of base selection by human single-stranded selective monofunctional uracil-DNA-glycosylase. J. Biol. Chem. 2009;284:15835–15846. doi: 10.1074/jbc.M807846200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Visser DW, Frisch DM, Huang B. Synthesis of 5-chlorodeoxyuridine and a comparative study of 5-halodeoxyuridines E. coli. Biochem. Pharmacol. 1960;5:157–164. doi: 10.1016/0006-2952(60)90017-4. [DOI] [PubMed] [Google Scholar]
- 29.Jaunin F, Visser AE, Cmarko D, Aten JA, Fakan S. A new immunocytochemical technique for ultrastructural analysis of DNA replication in proliferating cells after application of two halogenated deoxyuridines. J. Histochem. Cytochem. 1998;46:1203–1209. doi: 10.1177/002215549804601014. [DOI] [PubMed] [Google Scholar]
- 30.Svetlova M, Solovjeva L, Blasius MM, Shevelev I, Hubscher UU, Hanawalt P, Tomilin N. Differential incorporation of halogenated deoxyuridines during UV-induced DNA repair synthesis in human cells. DNA Repair. 2005;4:359–366. doi: 10.1016/j.dnarep.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Yamada K, Semba R, Ding X, Ma N, Nagahama M. Discrimination of cell nuclei in early S-phase, mid-to-late S-phase, and G2/M-phase by sequential administration of 5-bromo-2’-deoxyuridine and 5-chloro-2’-deoxyuridine. J. Histochem. Cytochem. 2005;53:1365–1370. doi: 10.1369/jhc.4A6601.2005. [DOI] [PubMed] [Google Scholar]
- 32.Brandon ML, Mi L-J, Chaung W, Teebor G, Boorstein RJ. 5-chloro-2’-deoxyuridine cytotoxicity results from base excision repair of uracil subsequent to thymidylate synthase inhibition. Mutat. Res. 2000;459:161–169. doi: 10.1016/s0921-8777(99)00061-0. [DOI] [PubMed] [Google Scholar]
- 33.Heartlein MW, O’Neill JP, Pal BC, Preston RJ. The induction of specific-locus mutations and sister-chromatid exchanges by 5-bromo and 5-chloro-deoxyuridine. Mutat. Res. 1982;92:411–416. doi: 10.1016/0027-5107(82)90239-1. [DOI] [PubMed] [Google Scholar]
- 34.DuFrain RJ. Probing sister chromatid exchange formation with halogenated pyrimidines. Basic Life Sci. 1984;29:41–58. doi: 10.1007/978-1-4684-4889-4_3. [DOI] [PubMed] [Google Scholar]
- 35.Zwanenburg TS, Hansson K, Darroudi F, Van Zeeland AA, Natarajan AT. Effects of 3-aminobenzamide on Chinese hamster cells treated with thymidine analogues and DNA-damaging agents. Chromosomal aberrations, mutations and cell-cycle progression. Mutat. Res. 1985;151:251–262. doi: 10.1016/0027-5107(85)90077-6. [DOI] [PubMed] [Google Scholar]
- 36.Michishita E, Kurahashi T, Suzuki T, Fukuda M, Fujii M, Hirano H, Ayusawa D. Changes in nuclear matrix proteins during the senescencelike phenomenon induced by 5-chlorodeoxyuridine in HeLa cells. Exp. Gerontol. 2002;37:885–890. doi: 10.1016/s0531-5565(02)00033-5. [DOI] [PubMed] [Google Scholar]
- 37.Privat EJ, Sowers LC. A proposed mechanism for the mutagenicity of 5-formyluracil. Mutat. Res. 1996;354:151–156. doi: 10.1016/0027-5107(96)00005-x. [DOI] [PubMed] [Google Scholar]
- 38.La Francois CJ, Jang YH, Cagin T, Goddard WA, III, Sowers LC. Conformation and proton configuration of pyrimidine deoxynucleoside oxidation damage products in water. Chem. Res. Toxicol. 2000;13:462–470. doi: 10.1021/tx990209u. [DOI] [PubMed] [Google Scholar]
- 39.Theruvathu JA, Kim CH, Rogstad DK, Neidigh JW, Sowers LC. Base-pairing configuration and stability of an oligonucleotide duplex containing a 5-chlorouracil-adenine base pair. Biochemistry. 2009;48:7539–7546. doi: 10.1021/bi9007947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Theruvathu JA, Kim CH, Darwanto A, Neidigh J, Sowers LC. pH-dependent configurations of a 5-chlorouracil-guanine base pair. Biochemistry. 2009;48 doi: 10.1021/bi901154t. 11312-11218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Driggers PH, Beattie KL. Effect of pH on the base-mispairing properties of 5-bromouracil during DNA synthesis. Biochemistry. 1988;27:1729–1735. doi: 10.1021/bi00405a052. [DOI] [PubMed] [Google Scholar]
- 42.Yu H, Eritja R, Bloom LB, Goodman MF. Ionization of bromouracil and fluorouracil stimulates base mispairing frequencies with guanine. J. Biol. Chem. 1993;268:15935–15943. [PubMed] [Google Scholar]
- 43.Kumar R, Wiebe LI, Knaus EE. A mild and efficient methodology for the synthesis of 5-halogeno uracil nucleosides that occurs via a 5-halogeno-6-azido-5,6-dihydro intermediate. Can. J. Chem. 1994;72:2005–2010. [Google Scholar]
- 44.Nielsen J, Taagaard M, Marugg JE, van Boom JH, Dahl O. Application of 2-cyanoethyl N,N,N’,N’-tetraisopropylphosphorodiamidite for in situ preparation of deoxyribonucleoside phosphoramidites and their use in polymer-supported synthesis of oligodeoxyribonucleotides. Nucleic Acids Res. 1986;14:7391–7403. doi: 10.1093/nar/14.18.7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herring JL, Rogstad DK, Sowers LC. Enzymatic methylation of DNA in cultured human cells studied by stable isotope incorporation and mass spectrometry. Chem. Res. Toxicol. 2009;22:1060–1068. doi: 10.1021/tx900027w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Randall SK, Eritja R, Kaplan BE, Petruska J, Goodman MF. Nucleotide insertion kinetics opposite abasic lesions in DNA. J. Biol. Chem. 1987;262:6864–6870. [PubMed] [Google Scholar]
- 47.Boosalis MS, Petruska J, Goodman MF. DNA polymerase insertion fidelity. J. Biol. Chem. 1987;262:14689–14696. [PubMed] [Google Scholar]
- 48.Darwanto A, Farrel A, Rogstad DK, Sowers LC. Characterization of DNA glycosylase activity by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Anal. Biochem. 2009;394:13–23. doi: 10.1016/j.ab.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.lvarie R. Thymine methyls and DNA-protein interactions. Nucleic Acids Res. 1987;15:9975–9983. doi: 10.1093/nar/15.23.9975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petruska J, Goodman MF, Boosalis MS, Sowers LC, Cheong C, Tinoco I., Jr Comparison between DNA melting thermodynamics and DNA polymerase fidelity. Proc. Natl. Acad. Sci. U.S.A. 1988;85:6252–6256. doi: 10.1073/pnas.85.17.6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Modrich P. Mechanisms in eukaryotic mismatch repair. J. Biol. Chem. 2006;281:30305–30309. doi: 10.1074/jbc.R600022200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.