Abstract
Gonadotropin-regulated testicular RNA helicase (GRTH/DDX25), a multifunctional protein and a component of ribonucleoprotein complexes, is essential for the completion of spermatogenesis. We investigated the nuclear/cytoplasmic shuttling of GRTH in germ cells and its impact on the chromatoid body (CB) -a perinuclear organelle viewed as a storage/processing site of mRNAs. GRTH resides in the nucleus, cytoplasm and CB of round spermatids. Treatment of these cells with inhibitors of nuclear export or RNA synthesis caused nuclear retention of GRTH and its absence in the cytoplasm and CB. The nuclear levels of GRTH bound RNA messages were significantly enhanced and major reduction was observed in the cytoplasm. This indicated GRTH main transport function of mRNAs to the cytoplasm and CB. MVH, a germ cell helicase, and MIWI, a component of the RNA-induced-silencing complex (RISC), confined to the CB/cytoplasm, were absent in the CB and accumulated in the cytoplasm upon treatment. This also occurred in spermatids of GRTH-KO mice. The CB changed from lobular-filamentous to a small condensed structure after treatment resembling the CB of GRTH-KO. No interaction of GRTH with MVH or RISC members in both protein and RNA were observed. Besides of participating in the transport of messages of relevant spermatogenic genes, GRTH was found to transport its own message to cytoplasmic sites. Our studies suggest that GRTH through its export/transport function as a component of mRNP is essential to govern the CB structure in spermatids and to maintain systems that may participate in mRNA storage and their processing during spermatogenesis.
Keywords: GRTH/DDX25, DEAD-box RNA helicase, chromatoid body, round spermatids, GRTH-mRNP, nuclear export, spermatogenesis
1. Introduction
Spermatogenesis depends on the integrated expression of an array of testicular genes that operate in a temporal sequence to produce mature spermatozoa. Gene expression in early steps of round spermatids undergoes temporal uncoupling from translation [1]. Several mRNAs that associate with ribonuclear particles (mRNPs) are repressed translationally at cytoplasmic sites and in germ cells presumably in the chromatoid body (CB) of round spermatids, for translation at later stages of spermiogenesis [2, 3]. CBs have a non-membranous, amorphous structure and are present in the cytoplasm of male germ cells during development. Those present in round spermatids are believed to possess functional commonalities to somatic P-bodies that regulate RNAs fate [4, 5]. Some studies have indicated a nuclear or nucleolus origin for the CBs [6, 7]. However, several groups favour their provenance from inter-mitochondrial dense material in pachytene spermatocytes, which condenses into a single distinct filamentous lobular structure that resides at a perinuclear site in round spermatids. In late spermatids the CB moves to a caudal site at the base of the flagellum where it subsequently undergoes fragmentation and disappears from the cytoplasm [8, 9].
RNA helicases members of DEAD (Glu-Asp-Ala-Glu)-box protein family are known to modulate the RNA structure that is a crucial step in many fundamental biological processes. These proteins participate in various aspects of RNA metabolism and translational events [10]. The discovery of GRTH/Ddx25 which is a testis-specific member of the DEAD-box family of RNA helicases present in Leydig cells and germ cells of the male testis [11–13], has provided new insights into the post-transcriptional regulation of meiotic and post-meiotic germ-cell development. This multifunctional enzyme, which is present in cytoplasm, nucleus of spermatocytes and spermatids, and CB of spermatids is essential for the spermatogenic process [14, 15]. GRTH was found to increase translation of messages in vitro [11] and to associate with polyribosomes [15] where it may participate in the translation of specific RNA transcripts at certain stages of development. GRTH null mice are sterile, due to spermatid arrest at step 8 and failure to elongate [14]. Also, marked changes in the structure and diminution in the size of CBs are observed in GRTH null mice [14]. GRTH associates with mRNA in a specific set of testicular gene transcripts, including those of chromatin remodeling proteins (transition protein 1 and 2, protamine 1 & 2), cytoskeleton structural proteins (Fsc1/Odf1), and testicular angiotensin converting enzyme (tACE) but not cyclic response element modulator (CREM) and acrosine [14]. Our previous studies demonstrating reduced cytoplasmic to total ratios of a specific set of mRNAs in round spermatids of GRTH null mice indicated that GRTH as a component of mRNP participates in RNA export from nucleus to cytoplasmic sites [15]. This concept was strengthened by the finding of association of GRTH with chromosome region maintenance-1 protein (CRM1) which is involved in the nuclear export pathway and the observation that COS 1 cells transfected with GRTH-GFP treated with nuclear export inhibitor exhibited the exclusive presence of GRTH in the nucleus due to blockade of its export to cytoplasmic sites [15]. The presence of GRTH in the CB of wild-type mice, and the major structural and size changes observed in the GRTH null mice revealed by EM studies of wild type and null mice, respectively [14] have indicated that the action of GRTH as a translational regulator occurs at least in part in the CB.
This study provides a direct evidence for the nuclear: cytoplasmic shuttling function of GRTH in germ cells. We present data on the cellular localization of GRTH in spermatids during different stages of the spermatogenesis of wild-type mice at nuclear, cytoplasmic sites and specifically in the CB. Further, we determined changes of relevant cytoplasmic proteins observed in GRTH-null mice, which at difference to GRTH, are not found at nuclear sites or involve in transport. Such proteins include the mouse Vasa homolog (MVH), a RNA helicase distantly related to GRTH which concentrates in the CB [16] and interacts with Dicer in vitro [17], and MIWI, a murine PIWI/Argonaut protein [18, 19], that has been proposed to be a member of the RNA induced silencing complex (RISC) with residence in the CB [20]. Our studies also revealed that GRTH associates with and exports its own message as well as other relevant messages. We also address the relevance of the transport function of GRTH and associated messages to the structure of the CB utilizing acute cultures of germ cells. We have demonstrated the essential participation of the GRTH export/transport function in the structure of the CB and for integrity of functional components within this organelle during spermatogenesis.
2. Materials and methods
2.1. Animals
GRTH wild type and GRTH−/− male mice [14] were housed in temperature and light-controlled conditions. Studies were approved by NICHD, Animal and Care and Use Committee. The animals were killed by asphyxiation with CO2. Testes were excised and decapsulated for purification of germ cells and for protein extraction. Seminiferous tubules were used to isolate stage-specific spermatogenic cells.
2.2. Germ cells preparation
Testicular germ cells from wild type or GRTH knockout adult mice [14] were prepared by collagenase/trypsin dispersion and purified by centrifugal elutriation [12, 21]. Briefly, after collagenase dispersion, seminiferous tubules were minced and incubated in medium 199 containing 0.1% bovine serum albumin, 0.1 % trypsin (Sigma), and 10 μg/ml DNAase (Sigma) for 15 min in a rotary water bath (80 rpm, 35 °C). After the addition of soybean 0.04% trypsin inhibitor, the samples were filtered through a 300-. 90- and 40- um mesh screen and glass wool, and the germ cells were pelleted and resuspended either in DMEM:F12 (1:1) for in vitro culture study or in elutriation buffer (medium 199/0.5% Bovine serum albumin, 1 mM EDTA, 50 U/ml Heparin, 50 mg/ml gentamycin) containing 2 μg/ml DNase for germ cells purification. The spermatids were purified by centrifugal elutriation using Beckman Avanti 21B centrifuge with elutriator rotor model J 5.0 as described previously [12, 21]. The fraction collected with flow rate of 41.4 ml/min at 3000 rpm containing round spermatids at purity of 84% was used for Western blot and immuno-precipitation analyses.
2.3. Cellular compartment fraction
Nuclear and cytoplasm extracts of germinal cells were prepared using extraction reagents kit (Pierce Biotechnology) in the presence of a mixture of protease inhibitors (Roche, Applied Sciences), phosphatase inhibitor mixture (Pierce Biotechnology) and RNase inhibitor (Roche, Applied Sciences).
2.4. In vitro study of the GRTH action
Cells released from the tubule segments (stage VII–VIII) or prepared from collagenase/trypsin digestion/elutriation method were incubated in presence of 180 μM DRB (5,6-dichloro-1-β-D-ribofuranosyl-benzimi-dazole, RNA polymerase inhibitor) (Calbiochem, San Diego, CA), or 200 nM Leptomycin B (LMB, nuclear protein export inhibitor) (Calbiochem) for 3–6 h at 37°C. Cells for immuno-fluorescence, electron microscopy, RNA and protein analyses were processed as indicated below.
2.5. Immuno-fluorescence
The stage-specific spermatogenic cells were prepared from segments of seminiferous tubules using transilumination-assisted microdissection technique [3]. Briefly, cells were released from the tubules by squeezing with fine forceps in 20 μl of 100 mM sucrose solution dotted on petri dish, and the cells were subsequently suspended. For immuno-staining, cell suspensions were spread on slides pre-dipped in the fixing solution [1% paraformaldehyde (PFA), 0.15% Triton X-100] and dried overnight in humidified box. The slides were permeabilized with 0.2% Triton X-100 for 5 min. Non-specific signals were blocked by incubating slides in 5% BSA for 60 min at 22 °C. For primary antibody incubation (4 °C for 16h) with either a specific GRTH rabbit polyclonal antibody purified by peptide affinity chromatography (1:200) [12] or MVH (1:200) (ab13840 rabbit, Abcam, Cambridge, MA) or Hiwi (Miwi) (1:200) (sc-22685 goat, Santa Cruz Biotechnology Inc., Santa Cruz, CA) was used. Subsequently, slides were incubated with secondary antibody Alexa Fluor 568 for 1 h at 22 °C. Slides were mounted in ProLong Gold antifade reagent with nuclei stain, 4′, 6-diamidino-2-phenylindole (DAPI) (Invitrogen). Samples were visualized by using Axioplan 2 imaging fluorescence microscope (Carl Zeiss, Thornwood, NY), digitized images were taken and processed with Axiovision v4.5 software (Carl Zeiss).
2.6. Electron microscopy studies
Cells were fixed in 2.5% glutaraldehyde in PBS at 4°C overnight, post-fixed in 1% osmium tetroxide, en bloc-stained with 2% uranyl acetate, dehydrated and embedded in Spurr’s epoxy. Thin sections were poststained with lead citrate and examined under transmission electron microscope.
2.7. Western Blot Analysis
Protein extracts from testis or round spermatids in the presence of protease inhibitors (Roche Diagnostics, Germany) were separated on 8–16% SDS-PAGE gel for Western analysis using rabbit anti-GRTH polyclonal antibody raised against the C-terminal (aa. 466-479) peptide and purified by peptide affinity chromatography [12]. Other antibodies used were obtained from commercial sources including MVH (Abcam), Hiwi (Miwi) and Dicer from Santa Cruz Biotechnology Inc, and anti-Ago2 (Argonaute 2) antibody (C34C6 #2897 rabbit, Cell Signaling Technology, Danvers, MA). Anti-β-actin antiserum (Sigma) was used for normalization. Primary antibodies were used at dilution 1:500 to 1:1000, and 2nd antibody conjugated to horseradish peroxidase (Santa Cruz) was employed at 1:10,000. Immuno-signals were visualized by the super-signal chemiluminescence kit (Pierce).
2.8. Immuno-precipitation Analysis
Immuno-precipitation analysis for study GRTH-association with relevant proteins or RNA complexes (GTRH-RNP) was performed by affinity-purified GRTH antiserum. Protein from mouse testicular extracts (1 mg), 500 μg of purified total round spermatids, or isolated cytoplasmic and nuclear extracts from the germ cells were initially subjected to preclearing by incubation with 40 μl of protein A-agarose (50% of slurry) and 2 μg of normal rabbit IgG in 1X RIPA buffer (Millipore) with gentle agitation. The recovered supernatant was incubated with peptide affinity purified anti-GRTH antiserum (aa 465-477) (2 μg) at 4°C for 16 h in the presence of 1x protease inhibitor mixture, RNase inhibitor (Roche Applied Sciences) and phosphatase inhibitor (Pierce) to co-immuno-precipitate the relevant protein(s) or RNA(s) associated with GRTH. 50 ul of protein A-agrose in 50% slurry was added, and the incubation was continued for 4 hr at 4°C. Protein A-precipitated GRTH-RNP complex was recovered by brief centrifugation, followed by three sequential washes with 1x RIPA buffer. The complex was either eluted for Western blot analysis by adding 2x SDS protein gel loading buffer or process for RNA extraction. Anti-CRM1 antibody (sc-5595 rabbit, Santa Cruz) was used as positive control [15].
2.9. Real time PCR quantification of mRNA
mRNA form whole cells, cytoplasmic and nuclear fractions of germ cells were isolated using RNeasy minikit (Qiagen). The RNA from immuno-precipitated GRTH-RNP complex was extracted by Trizol LS (Invitrogen, Carlsbad, Ca) followed by RNeasy microkit (Qiagen). Total RNA was treated with DNAase I to remove any possible co-purified genomic DNA. 100 ng of RNA was reverse transcribed using a SuperScript III first strand synthesis system (Invitrogen) containing a mixture of oligo (dT)20. The first strand cDNA was used as template in real time PCR with SYBR Green Master Mix and an ABI 7500 sequence detection system (Applied Biosynthesis). The cycling program was set as follows: denaturation at 95 °C for 10 min, followed by 50 cycles of 95 °C for 15 s and 60 °C for 1 min. Specific primers for genes of interest were designed to prevent nonspecific amplification of genomic DNA. GRTH (NM_013932), F: TCC AAA ATC CAA GAG ATG GC (exon 5), R: GAA CTT GCC CAT CCT TTC AA (exon 7)., Protamine 2 (M16456), F: CTC GTA AGA GGC TAC ATA GGA TC (exon 1), R: AGA CAT CGA CAT GGA ATG GTG G (exon 2)., MIWI (NM_021311), F: TGG CCG AGG ACG ACA GAG GG (exon 4), R: CAC TGT GGG CGC GAG GTC AG (exon 6)., MVH (NM_010029), F: AAG ATT GGG AGG CAG AAT (exon 2), R: GCC ACC AGT TGT AGA T (exon 4)., CREM (NM_013498), F: GAT TGA AGA AGA AAA TCA GA (exon 4), R: CAT GCT GTA ATC AGT TCA TAG (exon 7)., β-actin (NM_007393), F: TAA AGA CCT CTA TGC CCA CAC ATG (exon 5), R: CAC GAT GGA GGG GCC GGA CTC ATC (exon 6). The specificity of the PCR products was verified by melting curve and agarose gel analyses. The results presented are from three individual experiments, in which each sample was assayed in triplicate, normalized to the level of β-actin mRNA. In the case of GRTH-RNP complex, results were normalized to the input RNA used for reverse transcription.
2.10. Statistical analysis
The significance of the differences in the message between groups (GRTH-IP versus IgG-IP or LMB versus control) was determined by non-parametric Kruskall Wallis followed by Dunn’s multiple-comparison test using Prism statistical software version 4.
3. Results
3.1. Localization of RNA helicase (GRTH/DDX25 and MVH/DDX4), PIWI/Argonaute protein (MIWI) in cellular compartment of developing germ cells
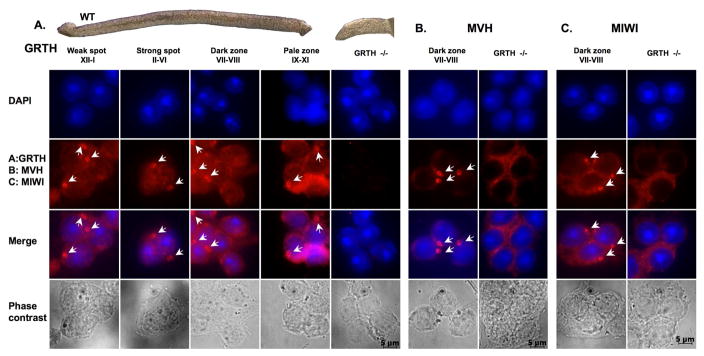
Under the phase-contrast dissection microscopy the light absorption pattern of seminiferous tubules correlates with defined stages of the spermatogenic wave, (Fig. 1, top panel), including weak spot of stages XII-I, strong spot of stages II–VI, dark zone of stages VII–VIII and the pale zone of stages of IX–XI [3]. Unlike the well established absorption variations observed in seminiferous tubules of wild type mice, a homogenous invariant light absorption pattern was observed in seminiferous tubules of GRTH-knockout mice (GRTH−/−). To elucidate the GRTH association with the CB of the germ cells, the GRTH distribution in the cellular compartments of germ cells at different developing stages was examined by immuno-fluorescence (Fig. 1, A). Immuno-staining of MVH, mouse homologue of Drosophila Vasa [16, 22] and MIWI, a PIWI/argonaute protein [22], both known to localize in the CB in other studies, were performed in parallel (Fig. 1, B & C). The dried-down cell preparations showed the presence of GRTH immuno-staining in the nuclear, cytoplasm compartments and the CB of round spermatids derived from different stages of the seminiferous epithelium (Fig. 1A). No GRTH signals were observed in GRTH null mice, used as a negative control for the study. MVH or MIWI signals were only present in the cytoplasm and CB but absent in the nucleus of germ cells of wild type mice (Fig. 1, B & C). In contrast, MVH or MIWI immuno-signals were observed only in the cytoplasm but not CB in GRTH knockout mice. This study demonstrated that GRTH was present in the CB of wild type male germ cells during spermatogenesis. However, in the absence of GRTH in GRTH null mice, (Fig. 1, B & C) MVH and MIWI were completely excluded from the CB.
Fig. 1. A–C. Subcellular localization of RNA helicase (GRTH/DDX25 and MVH/DDX4) and MIWI in spermatids during spermatogenesis.
Top: Specific stages of spermatogenic cells in seminiferous tubules of wild type mice compared to those of GRTH−/− mice were characterized by their trans-illumination properties under phase-contrast microscopy. Dried-down slides of male germ cells from different stages were immuno-stained with antibodies (2nd panel) to GRTH (A), MVH (B), MIWI (C), nuclear staining by DAPI (1st panel), merged image (3rd panel) and phase contrast of the cells (4th panel). Alexa Fluor 568 anti-rabbit IgG was used as a secondary antibody. Arrow-heads indicate chromatoid bodies (CB).
3.2. Disappearance of GRTH, MVH and MIWI signal from the CB after LMB and DRB treatment
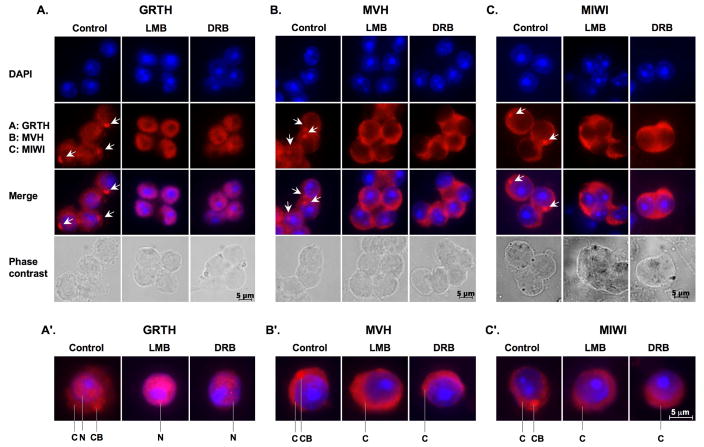
To gain insights into the shuttling function of GRTH from the nucleus to the cytoplasm and/or to CB of germ cells, we investigated its export function via the CRM1 export pathway in the spermatids from stages VII–VIII seminiferous tubules (dark zone). These stages of germ cells contain a relative high expression of GRTH in the three different cellular compartments shown in Fig. 1A and 2A control (nucleus, cytoplasm and CB). Our study showed that GRTH immuno-signals disappeared in both cytoplasm and CB and accumulated solely in the nucleus after the treatment with CRM1/exporting 1 inhibitor (LMB). Similar results were observed after treatment of cells with the RNA polymerase inhibitor (DRB) (Fig. 2, A & A′). These results indicated the participation of a CRM1-dependent export pathway in the transport function of GRTH from nucleus to both cytoplasm and CB in germ cells. This shuttling also required the presence of mRNA. Unlike GRTH, MVH immuno-staining was retained in the cytoplasmic compartment and no signal was detected in either nucleus or the CB (Fig. 2, B & B′) after LMB or DRB treatment. Similar cellular redistribution after drug treatment was also shown for MIWI (Fig. 2, C & C′). All the above findings indicate major mechanistic differences operative in the mobilization between cellular compartments in the case of MVH and MIWI (between cytoplasmic sites and the CB) and GRTH (from nuclear and cytoplasmic sites and the CB). Also, this function of GRTH appears to be required in order for the CB to function as a storage and presumably processing organelle for messages, and to accommodate key proteins that may participate in these functions (i.e. MVH, MIWI and others) in the round spermatids.
Fig. 2. Disappearance of GRTH and MVH and MIWI signal from CBs via a different mechanism after LMB and DRB treatment.
Spermatids isolated from stages VII–VIII were incubated with vehicle (control), nuclear protein export inhibitor (LMB) or RNA polymerase II inhibitor (DRB) for 3 h. Dried-down slides were immuno-stained with GRTH (A), or MVH (B) or MIWI antibodies (C) (2nd panel), nuclear staining by DAPI (1st panel), and merged image (3rd panel) and phase contrast (4th panel). Alexa Fluor 568 anti-IgG was used as a secondary antibody. Arrow-heads indicate the CBs. A′–C′: Single cell magnification of merged images. C: cytoplasm., N: Nucleus., CB: chromatoid body.
3.3. GRTH participates in the nuclear export function of its own message and protamine 2
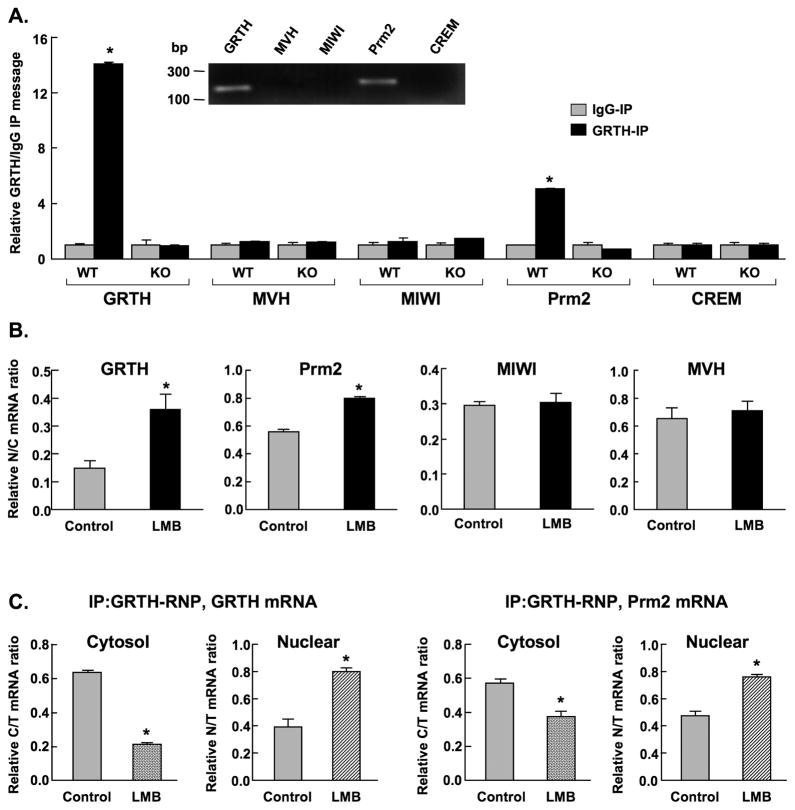
Subsequent studies demonstrated the involvement of GRTH on the export of its own message from nucleus to cytoplasm where significant association of GRTH message with GRTH protein was observed in the testicular extracts of wild type (WT) mice (Fig. 3A). No message was detected in GRTH knockout mice (KO) or IgG-immuno-precipitated WT and KO group (negative controls). This was further confirmed by the presence of protamine 2 (Prm 2) and absence of cAMP response element modulator (CREM) messages in the immuno-precipitated WT testicular extracts reported in this and in our earlier study [14]. Messages for MVH and MIWI were not detected (Fig. 3A). Significant nuclear accumulation of GRTH and Prm 2 messages reflected by increases in the ratio of nuclear to cytoplasmic RNA level were observed upon LMB treatment of cultured germ cells (Fig. 3B). This effect was further revealed in immuno-precipitation studies of mRNA associated with GRTH protein in individual cellular compartments. Significant increases in both GRTH and Prm2 messages were observed in the nucleus and decreases in the cytoplasm of germ cells (Fig. 3C). These results provide concrete evidence for the participation of GRTH protein as GRTH-RNP complex in the export of its own message and of other relevant messages (exemplified in this study by Prm 2) between cellular compartments. LMB did not have any effect on the message distribution of MVH or MIWI between nuclear and cytoplasmic compartments (Fig. 3B).
Fig. 3. Real time-PCR analysis of RNA message associated with GRTH.
A. Evaluation of messages: GRTH, mouse homolog of drosophila VASA (MVH), mouse PIWI Like homolog 1 (MIWI), protamine 2 (Prm2) and cAMP responsive element modulator (CREM) in immuno-precipitated testicular GRTH complexes (GRTH-IP) from wild type (WT) and GRTH knockout (KO) adult mice. Data was expressed as a relative ratio of messages from GRTH-IP to IgG immuno-precipitated negative control group (IgG-IP). Inset: Gel image of messages with the expected fragment size (bp). B. Total message was determined and normalized by β-actin in isolated cytoplasm (C) and nuclear (N) fractions of round spermatids treated with nuclear export inhibitor LMB (200 nM) for 3 hours. C. GRTH and Prm2 messages were determined in immuno-precipitated GRTH complexes from isolated cytoplasm and nuclear fractions of round spermatids treated with LMB. Specific sets of primers for genes of interest were designed accordingly. The values are the means ± S.E. of three independent experiments in triplicate. *, p< 0.05. The ratios of nuclear to cytoplasmic (N/C), nuclear to total (N/T) or cytoplasm to total (C/T) RNA of specific genes were used to represent the effect of LMB on the export of messages from nucleus to cytoplasm.
3.4. EM analysis of chromatoid body after LMB/DRB treatment
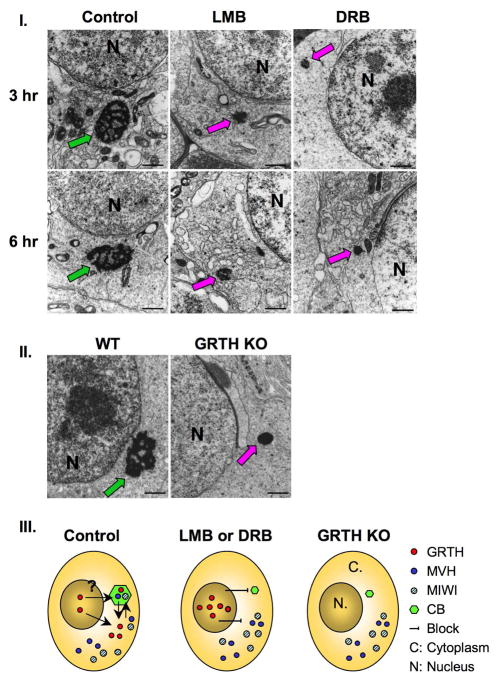
In further studies we investigated the impact of GRTH depletion at cytoplasmic sites and more specifically in the CB of round spermatids induced in culture by the inhibitor of nuclear export transport (LMB) or mRNA transcription (DRB). Round spermatids collected from stages VII–VIII were subjected to EM analysis after treatment of acute cultures with LMB or DRB for 3 and 6 h. The typical filamentous and lobular like structure of nuage appearance associated with normal CB was observed in the control germ cells (Fig. 4-I, left panel-control). In mark contrast, size diminution of the CB and condensed appearance was noted in most round spermatids examined after LMB (Fig. 4-I, middle panel) or DRB (Fig. 4-I, right panel) treatment at 3hr and similarly at 6 hr. Abnormalities of the CB in most cases resemble the marked changes we previously reported in spermatids of GRTH null mice (Fig. 4-II) [14]. The coincidence of CB abnormal structure with the absence of GRTH in the in vitro studies highlights the relevance of GRTH and associated messages for conferring the CB typical structure in spermatids. The distribution of GRTH, MVH and MIWI in cellular compartments of round spermatids derived from this EM and immuno-staining studies (Fig. 1 and 2) are summarized in the diagram of Fig. 4-III. All indicated proteins (GRTH, MVH, MIWI) were present in the CB and cytoplasm of wild type germ cells (control group), and GRTH was also present in the nucleus. After the inhibitor treatment, all these proteins (GRTH, MIWI and MVH) were excluded from the abnormally condensed CB and while MIWI and MVH were confined to cytoplasmic sites GRTH was retained in the nucleus. Similarly, MVH and MIWI were only present in cytoplasm of germ cells in GRTH KO mice. This led us to hypothesize that GRTH mRNA transport function is important to maintain the nuage structure of the CB that appears to be required for its MVH/MIWI localization and presumably function within this organelle. Furthermore, in the case of DRB, inhibitor of the blockade of mRNA formation would lead to a similar reduction of the CB whose nuage structure is likely to be conferred by mRNAs residence in this organelle.
Fig. 4. Panel I. EM analysis of the CB after LMB treatment.
A. Spermatids isolated from stages VII–VIII tubular fragments were incubated with vehicle (control), LMB or DRB for 3 and 6 h. Typical CB structure (red arrow). Abnormal compact CB (pink arrows). N, nuclear. Panel II. CB of spermatids from WT and GRTH−/− previously reported (7) are presented for comparative purposes. Green arrow: CB-wild type, Pink arrow: CB - null mice. Panel III. Diagram illustrates the relevance GRTH for the structural integrity of the CB in the spermatids of mouse testis. Distribution of GRTH/Ddx25 (red circle), MVH/Ddx4 (solid blue circle) and MIWI (blue-stripped circle) in cellular compartments of round spermatids; Nucleus (N), cytoplasm (C) and CB (green, hexagon). Abnormal compact CB noted in GRTH−/− mice and after treatment with inhibitor (LMB or DRB) compared with the CB in spermatids of wild type (stages VII–VIII).
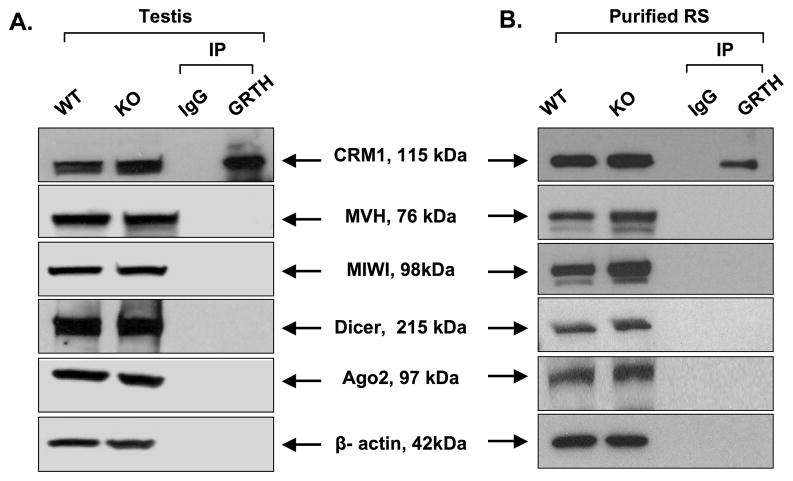
3.5. Assessment of protein levels of components of the miRNA pathway and their lack of interaction with GRTH
Since CB has been proposed to participate in the si/mi/pi RNA pathway associated with MVH and MIWI [19] in the translational regulation of essential messages during spermatogenesis, potential association between GRTH and these factors and RISC components (Dicer and Argonaute protein 2 [Ago2]) were examined in both total testicular extracts and purified round spermatids of mouse testis. No particular change in the protein expression including MVH, MIWI, Dicer, Ago2 was observed between wild type and GRTH KO mice (Fig 5-A and B). Immuno-precipitation of either total testis extract or the purified germ cells using GRTH antibody showed no interaction between GRTH and any of the factors mentioned above by Western blot analysis. The presence of CRM1 in Western blot after GRTH antibody immuno-precipitation known for its association with GRTH [15] was used as the positive control for this procedure. These results indicate that GRTH does not undergo physical interaction with members of the proposed si/mi/pi RNA pathway in the germ cells or influence their protein expression.
Fig. 5. Assessment of expression of selected protein members of the si/mi/pi RNA pathway and their interaction with GRTH.
Protein extracts from whole testis (A) or purified round spermatids (RS) (B) were utilized for Western analysis of wild type (WT) and GRTH knockout samples (KO) for assessment of protein expression with antibodies to the indicated relevant proteins (CRM1, MVH, MIWI, Dicer and Argonaute protein 2 [Ago2]) and β-actin. These preparations were also used for studies of GRTH association with relevant proteins in immuno-precipitation (IP) studies with GRTH antibody followed by Western analysis utilizing specific antibodies for the specified proteins indicated above. CRM1 was used as the control for IP procedure. IgG was used as a negative control.
4. Discussion
Although the CB has long been hypothesized to be a site of post-transcriptional processing and storage of mRNA species during spermatogenesis [2, 3, 8, 9, 23], the dynamic regulation of this cytoplasmic organelle as a site of transport of transcripts as components of ribonuclear particles (RNP) between cellular compartments of spermatids, has not been established. We have now demonstrated the direct participation of GRTH in the shuttling function between nuclear, cytoplasmic and CB compartments of spermatids as GRTH-RNP complexes, and also of its requirement for the structural integrity of the CB, the potential germ cell-specific RNA processing center in spermatids.
The presence of intense GRTH immuno-signals in the CB of round spermatids at different stages of development during spermiogenesis (Fig. 1A) supports the notion that GRTH as a mRNP particle might execute (at least in part) its post-transcriptional regulatory function in this organelle [14, 15]. GRTH is the only known RNA helicase specifically required for the distinct morphology of the CB in the testis, based on the marked changes of its appearance and diminution in size in round spermatids of GRTH null mice [14]. Another member of DEAD box family, MVH, is closely associated with the CB, but its impact on CB formation cannot be derived from current models since germ cells lacking MVH undergo arrest at the zygotene stage of spermatocytes, well before CB formation [16]. On the other hand, MIWI, a PIWI/Argonaute protein, could be involved in the formation of the CB since EM findings show considerable CB distortion (thin threads and granules of non-compacted materials) in surviving spermatids of MIWI null mice [22] where germ cells arrest at step 1 of spermatids [18]. The presence of a positive intense MVH signal adjacent to the nucleus of MIWI null spermatids (presumably at the CB) [22] further rules out MVH direct impact on CB formation. In the case of GRTH null mice, both MVH and MIWI signals (Fig. 1 B & C) were only observed in the cytoplasm and not in the compacted abnormal CB. This indicates the essential direct and or indirect requirement of GRTH in the structural integrity of the CB, and for the association of presumably functional components of the si/mi/pi RNA pathway and other factors such as MIWI and MVH within this organelle structure.
The importance of GRTH for maintaining the normal morphology of the CB could be further extrapolated from findings of the complete disappearance of MVH or MIWI from the CB without changing their accumulation in the cytoplasm of the spermatids after either LMB or DRB treatment (Fig. 2, B & B′, C & C′). Both proteins are normally localized in the cytoplasm [18, 24] and are commonly used as CB markers in spermatids. Theoretically, the distribution status of their signal between different cellular compartments should not be altered after the treatment with either nuclear export or RNA polymerase inhibitors, where the action of both agents specifically occurs at nuclear sites. Since both proteins were also not found in the CB of the GRTH null mice (Fig. 1, B & C) where the CB displays a highly compacted structure (Fig 4-II) [14], the absence of either MVH or MIWI in the CB in this in vitro study might result from altered CB structure triggered by the loss of GRTH-mRNA complexes after either treatment. This is supported by the subsequent EM finding of reduced size and highly compacted CB structure after either LMB or DRB treatment (Fig. 4-I). It is reasonable to assume that the filamentous-lobular structure of the CB is very sensitive to the decrease in GRTH-dependent RNAs transport to this organelle (protamine and others relevant mRNAs in spermiogenesis) [14, 15] which ultimately seems to compromise its structure and the recruitment of MVH and MIWI.
The striking accumulation of GRTH in the nucleus with its complete absence in the CB after LMB treatment in the in vitro study (Fig. 2A) demonstrated the transport function of GRTH from the nucleus to the cytoplasmic sites via a CRM1-dependent export pathway that could involve direct transit or indirect trafficking to the CB. A similar retention of the GRTH signal was found in the nucleus when mRNA synthesis was inhibited by DRB. This is consistent with the functionality of GRTH as a mRNA- dependent shuttle protein previously revealed in our studies in COS-1 cells [13–15]. In the case of the DRB treatment, it cannot be excluded the involvement of a short half-life protein(s) in addition of the absence of mRNAs for the changes noted in the CB. However, this appears not to be the case since similar changes in the structure of CB are observed with the LMB treatment where export of GRTH is abolished and transcription is not altered. Based on the ultra-structural evidence of the CB’s intimate but transient contact with nuclear pores [2], GRTH could directly transport specific transcripts for translational regulation from the nucleus to the CB. However, we cannot rule out an indirect route of GRTH-RNP transport from the nucleus via cytoplasm to the CB. Movement of mRNA in yeast has been demonstrated between P-bodies and polysomes [4]. Therefore direct shuttling from cytoplasm to the CB and the loss of GRTH signal in the CB could result from GRTH-mRNP cytoplasmic depletion caused by blockade of the nuclear export function (this study) or the absence of GRTH in the null mice [14].
GRTH protein binds GRTH mRNA as a component of GRTH-mRNP (Fig. 3A–C) and has the ability to transport between cellular compartments its own message in addition to others from relevant germ cell genes (this study and previous findings). This notion is supported by the significant increases of GRTH mRNA associated with GRTH protein in the nuclear compartment and decrease at cytoplasmic sites. The blockade of transport of relevant messages by GRTH including its own by LMB treatment shown in this in vitro study contributes to the disruption of CB structure integrity. In the case of MVH and MIWI, our study showed that LMB has no effect on their RNA or protein expression, except from the exclusion of MIWI and MVH proteins from the abnormal CB (Fig. 1 and 2). The later is likely due to the disrupted structural integrity of CB resulting from lack of GRTH transport function of mRNAs.
The co-localization of Dicer endonuclease, and components of RISC complex (MIWI/Argonaute proteins/si/mi/pi RNAs) in the CB was demonstrated by immuno-fluorescence analysis [20]. Also, evidence of interactions between MIWI/Dicer [25], MIWI/MVH [19], MIWI/kinesin motor protein, KIF17b [22], MIWI/cap-binding complex members [25] and Dicer/MVH [17] was derived from studies in transfected COS-1 cells and whole testis homogenates. Taken together these studies provided the basis for a proposed model of the CB as the center for microRNA pathways on germ cell specific RNA processing in haploid male germ cells [20]. However, it remains to be determined whether such interactions could occur at the CB since to date isolation and high degree purification of this organelle has not been achieved for the assessment of these interactions/associations.
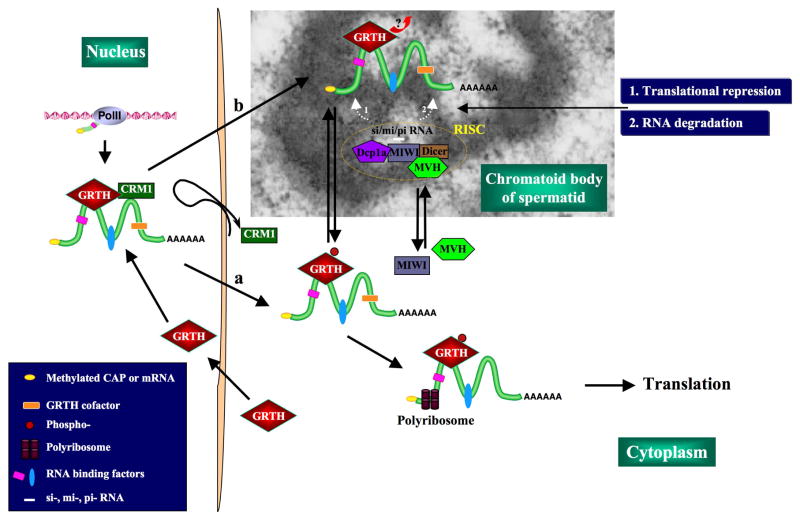
Although GRTH is closely associated with the CB during germ cell development, it does not seem to directly participate in the proposed microRNA regulatory pathway. No apparent interactions between GRTH with any of the factors involved in the pathway were noted in immuno-precipitation studies utilizing GRTH pull-down antibody assay of extracts from total testis and round spermatids (Fig. 5). Thus, rather than participating in translational regulation (either translational repression or RNA degradation) via the si/mi/pi RNA pathway in the CB, the nuclear export of GRTH-RNP complexes could be required to maintain CB structural integrity in spermatids and to deliver essential RNAs to the CB presumably for silencing/storage and/or degradation of genes during germ cell development. Subsequently, GRTH could be involved in the transport of mRNAs from CB to the polysomes for translation during spermatogenesis (Fig. 6).
Fig. 6. Postulated model of GRTH action in the CB of male germ cells.
During spermatogenesis, specific RNA transcripts associated to GRTH in mRNP complexes are found in the nucleus of round spermatids. These GRTH-RNP complexes are transported from nucleus to cytoplasm (a) and the CB (b) via a CRM1-dependent export pathway. GRTH-RNP complexes are required to maintain CB filamentous-lobulated perinuclear structure (CB-EM image). GRTH does not directly interact with protein factors of si/mi/pi RNA pathway that might occur in the CB. We propose that specific messages associated with GRTH are presented to members of this system (RISC: RNA induced silencing complex- [decapping enzyme Dcp1a, MIWI, MVH, Dicer]) for either storage (noted as 1) or degradation (noted as 2). GRTH most probably dissociate from mRNA at the CB site during this process. Messages are transported by GRTH from CB to polyribosomes for translation.
Acknowledgments
This work was supported by the Intramural Research Program of the NICHD, National Institutes of Health
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Steger K. Haploid spermatids exhibit translationally repressed mRNAs. Anat Embryol (Berl) 2001;203:323–334. doi: 10.1007/s004290100176. [DOI] [PubMed] [Google Scholar]
- 2.Parvinen M, Parvinen LM. Active movements of the chromatoid body. A possible transport mechanism for haploid gene products. J Cell Biol. 1979;80:621–628. doi: 10.1083/jcb.80.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toppari J, Kangasniemi M, Kaipia A, Mali P, Huhtaniemi I, Parvinen M. Stage- and cell-specific gene expression and hormone regulation of the seminiferous epithelium. J Electron Microsc Tech. 1991;19:203–214. doi: 10.1002/jemt.1060190207. [DOI] [PubMed] [Google Scholar]
- 4.Brengues M, Teixeira D, Parker R. Movement of eukaryotic mRNAs between polysomes and cytoplasmic processing bodies. Science. 2005;310:486–489. doi: 10.1126/science.1115791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eulalio A, Behm-Ansmant I, Izaurralde E. P bodies: at the crossroads of post-transcriptional pathways. Nat Rev Mol Cell Biol. 2007;8:9–22. doi: 10.1038/nrm2080. [DOI] [PubMed] [Google Scholar]
- 6.Yokota S. Historical survey on chromatoid body research. Acta Histochem Cytochem. 2008;41:65–82. doi: 10.1267/ahc.08010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peruquetti RL, Assis IM, Taboga SR, de Azeredo-Oliveira MT. Meiotic nucleolar cycle and chromatoid body formation during the rat (Rattus novergicus) and mouse (Mus musculus) spermiogenesis. Micron. 2008;39:419–425. doi: 10.1016/j.micron.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Fawcett DW, Eddy EM, Phillips DM. Observations on the fine structure and relationships of the chromatoid body in mammalian spermatogenesis. Biol Reprod. 1970;2:129–153. doi: 10.1095/biolreprod2.1.129. [DOI] [PubMed] [Google Scholar]
- 9.Susi FR, Clermont Y. Fine structural modifications of the rat chromatoid body during spermiogenesis. Am J Anat. 1970;129:177–191. doi: 10.1002/aja.1001290205. [DOI] [PubMed] [Google Scholar]
- 10.Silverman E, Edwalds-Gilbert G, Lin RJ. DExD/H-box proteins and their partners: helping RNA helicases unwind. Gene. 2003;312:1–16. doi: 10.1016/s0378-1119(03)00626-7. [DOI] [PubMed] [Google Scholar]
- 11.Tang PZ, Tsai-Morris CH, Dufau ML. A novel gonadotropin-regulated testicular RNA helicase. A new member of the dead-box family. J Biol Chem. 1999;274:37932–37940. doi: 10.1074/jbc.274.53.37932. [DOI] [PubMed] [Google Scholar]
- 12.Sheng Y, Tsai-Morris CH, Dufau ML. Cell-specific and hormone-regulated expression of gonadotropin-regulated testicular RNA helicase gene (GRTH/Ddx25) resulting from alternative utilization of translation initiation codons in the rat testis. J Biol Chem. 2003;278:27796–27803. doi: 10.1074/jbc.M302411200. Epub 2003 May 6. [DOI] [PubMed] [Google Scholar]
- 13.Dufau ML, Tsai-Morris CH. Gonadotropin-regulated testicular helicase (GRTH/DDX25): an essential regulator of spermatogenesis. Trends Endocrinol Metab. 2007;18:314–320. doi: 10.1016/j.tem.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Tsai-Morris CH, Sheng Y, Lee E, Lei KJ, Dufau ML. Gonadotropin-regulated testicular RNA helicase (GRTH/Ddx25) is essential for spermatid development and completion of spermatogenesis. Proc Natl Acad Sci U S A. 2004;101:6373–6378. doi: 10.1073/pnas.0401855101. Epub 2004 Apr 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheng Y, Tsai-Morris CH, Gutti R, Maeda Y, Dufau ML. Gonadotropin-regulated testicular RNA helicase (GRTH/Ddx25) is a transport protein involved in gene-specific mRNA export and protein translation during spermatogenesis. J Biol Chem. 2006;281:35048–35056. doi: 10.1074/jbc.M605086200. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka SS, Toyooka Y, Akasu R, Katoh-Fukui Y, Nakahara Y, Suzuki R, Yokoyama M, Noce T. The mouse homolog of Drosophila Vasa is required for the development of male germ cells. Genes Dev. 2000;14:841–853. [PMC free article] [PubMed] [Google Scholar]
- 17.Kotaja N, Bhattacharyya SN, Jaskiewicz L, Kimmins S, Parvinen M, Filipowicz W, Sassone-Corsi P. The chromatoid body of male germ cells: similarity with processing bodies and presence of Dicer and microRNA pathway components. Proc Natl Acad Sci U S A. 2006;103:2647–2652. doi: 10.1073/pnas.0509333103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng W, Lin H. miwi, a murine homolog of piwi, encodes a cytoplasmic protein essential for spermatogenesis. Dev Cell. 2002;2:819–830. doi: 10.1016/s1534-5807(02)00165-x. [DOI] [PubMed] [Google Scholar]
- 19.Kuramochi-Miyagawa S, Kimura T, Ijiri TW, Isobe T, Asada N, Fujita Y, Ikawa M, Iwai N, Okabe M, Deng W, Lin H, Matsuda Y, Nakano T. Mili, a mammalian member of piwi family gene, is essential for spermatogenesis. Development. 2004;131:839–849. doi: 10.1242/dev.00973. [DOI] [PubMed] [Google Scholar]
- 20.Kotaja N, Sassone-Corsi P. The chromatoid body: a germ-cell-specific RNA-processing centre. Nat Rev Mol Cell Biol. 2007;8:85–90. doi: 10.1038/nrm2081. [DOI] [PubMed] [Google Scholar]
- 21.Bucci LR, Brock WA, Johnson TS, Meistrich ML. Isolation and biochemical studies of enriched populations of spermatogonia and early primary spermatocytes from rat testes. Biol Reprod. 1986;34:195–206. doi: 10.1095/biolreprod34.1.195. [DOI] [PubMed] [Google Scholar]
- 22.Kotaja N, Lin H, Parvinen M, Sassone-Corsi P. Interplay of PIWI/Argonaute protein MIWI and kinesin KIF17b in chromatoid bodies of male germ cells. J Cell Sci. 2006;119:2819–2825. doi: 10.1242/jcs.03022. [DOI] [PubMed] [Google Scholar]
- 23.Saunders PT, Millar MR, Maguire SM, Sharpe RM. Stage-specific expression of rat transition protein 2 mRNA and possible localization to the chromatoid body of step 7 spermatids by in situ hybridization using a nonradioactive riboprobe. Mol Reprod Dev. 1992;33:385–391. doi: 10.1002/mrd.1080330404. [DOI] [PubMed] [Google Scholar]
- 24.Toyooka Y, Tsunekawa N, Takahashi Y, Matsui Y, Satoh M, Noce T. Expression and intracellular localization of mouse Vasa-homologue protein during germ cell development. Mech Dev. 2000;93:139–149. doi: 10.1016/s0925-4773(00)00283-5. [DOI] [PubMed] [Google Scholar]
- 25.Grivna ST, Pyhtila B, Lin H. MIWI associates with translational machinery and PIWI-interacting RNAs (piRNAs) in regulating spermatogenesis. Proc Natl Acad Sci U S A. 2006;103:13415–13420. doi: 10.1073/pnas.0605506103. [DOI] [PMC free article] [PubMed] [Google Scholar]