Abstract
Kisspeptin is a potent regulator of the hypothalamo-pituitary-gonadal axis. The activation of several vernal and pubertal behaviors involves the action of locally synthesized estradiol by hypothalamic aromatase-expressing neurons. Little is known about kisspeptin in non-mammalian systems, and its interaction with aromatase remains unexamined. The Mallard drake is a seasonal breeder and an excellent model for studying the neural mechanisms that regulate the HPG. The goals of these studies were to determine (a) if and how kisspeptin regulates the drake HPG, (b) if kisspeptin and aromatase are expressed in the Mallard brain, and (c) if kisspeptin is co-localized or in apposition with, aromatase- and gonadotropin hormone releasing hormone (GnRH) positive neurons. Central kisspeptin administration increased plasma luteinizing hormone, an effect blocked by pretreatment with the GnRH antagonist, acyline, suggesting a conservation of kisspeptin function and mechanism of action in birds and mammals. The distribution of kisspeptin in the mallard brain was examined with immunocytochemistry (ICC). Neurons that express kisspeptin-like immunoreactive (ir) protein were observed in the medial preoptic nucleus (POM) and in ir-fibers throughout the drake brain. Virtually all POM kisspeptin-ir soma also expressed aromatase-ir, suggesting that autocrine mechanisms may predominate in the interaction between steroid provision and kisspeptin expression. No colocalization was observed between KP-ir and GnRH-ir, although both were easily detected in close proximity in the tuberoinfundibular area. Taken together, these data suggest that in the drake, estradiol synthesized by aromatase and kisspeptin co-expressing POM neurons may regulate the HPG via an effect on GnRH secretion.
Keywords: seasonal breeder, metastin, hypothalamus, GnRH
Introduction
The GnRH secretagogue, kisspeptin, plays a critical role in the physiology of puberty in mammals and in the activation of the hypothalamo-pituitary-gonadal axis (HPG) in seasonal breeders (Clarkson and Herbison, 2006; Estrada, 2006; Han, 2005; Messager, 2005a; Plant, 2006a; Pompolo, 2006). The expression of kisspeptin, a hypothalamic neuropeptide encoded by the KiSS-1 gene, is tightly regulated by gonadal steroids in both male and female mammals (Irwig, 2004; Matsui, 2004; Navarro, 2004). Indeed, kiss-1 positive hypothalamic neurons express estrogen, androgen, and progesterone receptors, supporting the hypothesis that these neurons may underlie the action of steroids and perhaps steroidal feedback on the HPG (Dhillo, 2005; Messager, 2005b; Smith, 2005b; Thompson, 2004).
While great attention has been paid to the expression, regulation, and physiological effects of kisspeptin neurons in mammals, much less is known about kisspeptin in other vertebrate species. To date, a kiss-1–like gene sequence remains to be identified in birds; however, orthologous genes (designated kiss-2) have been identified in mammalian, reptilian, amphibian and teleost species (Lee, 2009). Although there is a dearth of evidence for a kiss-like gene in avian species, kisspeptin-like immunoreactivity (-lir) has been described in other non-mammalian species without an identified gene (Dunham, 2009).
The interactions among steroids, kisspeptin, and GnRH are quite well studied in rodent and primate models (Dhillo et al., 2005; Irwig et al., 2004; Masui, 2004; Messager et al., 2005b; Navarro et al., 2004; Smith et al., 2005b; Thompson et al., 2004). However, in non-mammalian species, relatively little information is available about how steroids mediate the actions of kisspeptin on GnRH release. Notably, both the onset of puberty and the vernal activation of the avian HPG are times of dramatic changes in the peripheral and central synthesis of steroids, particularly estrogens (Nicholls, 1988). Specifically, aromatase (estrogen synthase) is expressed not only in the ovary, but in hypothalamic neurons of several species of vertebrates including mammals and birds (Hutchison and Steimer, 1984; Naftolin, 2001; Saldanha, 1999; Schlinger and Callard, 1989; Schumacher and Balthazart, 1987). In several avian species, the hypothalamic aromatization of circulating androgens is both necessary and sufficient for the expression of masculine copulatory and aggressive behaviors (Balthazart, 1990; Balthazart and Surlemont, 1990). Thus, seasonally breeding birds may provide excellent models for studies of the interactions among steroid provision, kisspeptin, and activation and regulation of the HPG.
The Mallard duck is a seasonal breeder that has long been utilized as a model for studies on HPG activation (Benoit, 1936). During the non-breeding season the drake's testicles regress to less than 10% of their reproductive size and to a near pre-pubertal state of seminiferous tubule differentiation (Johnson, 1961). In response to an increase in day length, the drake's HPG is activated and an increase in luteinizing hormone (LH) and follicle stimulating hormone (FSH) elicit testicular hypertrophy, spermatogenesis, and testosterone secretion. During the active breeding season, the drake's HPG is regulated via gonadal steroid feedback, similar to that of mammals (Haase, 1983). Thus, it is reasonable to hypothesize an interaction between kisspeptins and the drake HPG.
The purpose of these studies was to determine the central action of kisspeptin on the drake HPG, then to determine the pattern of kisspeptin expression using immunocytochemistry. Finally, we used double-label immunocytochemistry to identify the overlap and co-expression of kisspeptin with aromatase and GnRH in the drake brain. Our results indicate that, as in mammals, kisspeptin is a potent activator of the avian HPG and is abundantly expressed in the drake hypothalamus. We also report that almost all kisspeptin-expressing hypothalamic neurons are aromatase positive, suggesting a tight coupling of hypothalamic estrogen synthesis and kisspeptin expression by autocrine pathways. Finally, KP-ir fibers appose GnRH fibers in the tuberal hypothalamus. To the best of our knowledge, this is the first demonstration of a mechanism by which central steroid provision may regulate the HPG via actions on kisspeptin.
Methods
Mallard drakes from a long-day season were obtained from Swanhaven Waterfowl, Inc. (Wills Point, TX) and housed in the Hope College aviary on LD 12:12. Birds arrived at Hope College in early May and were housed in floor pens (5–6 drakes and 2–3 hens per pen) and allowed ad lib access to food and water. Birds were housed for one week prior to the start of the experiment. Drakes were considered gonadally active based upon plumage and observations of the drakes mounting hens. Gonadal status was confirmed at the end of the experiment based upon testis size (> 5 grams per testis, data not shown). All housing and experimental procedures were approved by Hope College Animal Care and Use Committee.
Experiment 1: Effects of ICV Kisspeptin on LH secretion
Mallards (Anas platyrhynchos) were anesthetized with isofluorane. A surgical plane of anesthesia was induced in a tank (3.5% isofluorane, 4L/min flow rate) then maintained via endotracheal intubation (1.5–2.0% isofluorane, 2.0L.min flow rate). Birds were monitored regularly for respiration, cardiac rate, and level of anesthesia. Just prior to surgery, analgesics (Ketofen, 1.5 mg/kg subcutaneously) were administered to the drakes. Birds were then placed into a dual-rail stereotaxic instrument (Kopf, Inc.) their head feathers removed, scalp cleaned and incised. A trephine hole was drilled 1.0 mm off midline and 4.5 mm posterior to the caudal border of the optical orbit. A 24 Ga stainless steel cannula (Plastics One, Inc.) was placed 2.5 mm ventral to the level of the dura mater. These coordinates were determined previously to allow for injections into the lateral ventricle.
After surgery, animals were monitored during recovery from anesthesia, and subsequently, placed back in their original floor pens and handled daily. During handling, cannulae were checked to insure that they remained securely attached to the head and that no signs of distress and or injury were apparent. One week after surgery, drakes were injected intracerebroventricularly (ICV) with either 0.5 nmol kisspeptin-52 (KP; Phoenix Pharmaceuticals) in 3 μl PB or PB alone (vehicle). Birds were pretreated (30 minutes prior to ICV injection) with either saline or 10mg/kg acyline, a selective GnRH receptor antagonist. Blood (~1.0 ml) was collected via the brachial vein 30 minutes after ICV injection, plasma collected and stored at −20°C until processed for LH levels (Sharp, 1987). The doses of KP and acyline, as well as the time course of blood collection were determined from previously reported experiments (Irwig et al., 2004; Plant, 2006b). LH data were log-transformed (see Results) and analyzed by ANOVA. Significant sources of variation were subsequently detected with post-hoc Fisher Least Square Means tests.
Experiment 2: Immunocytochemistry for Localization of Kisspeptin
Antibodies
Two primary antibodies were utilized for this experiment. The first is a commercially available rabbit anti-kisspeptin (Phoenix Pharmaceuticals) polyclonal antibody raised against the human kisspeptin-10 peptide. This antibody has been utilized in numerous mammalian and several non-mammalian species (Felip, 2008; Mechaly, 2009; van Aerle, 2008). The second antibody was a rabbit anti-aromatase (Bethyl Labs, Montgomery,TX) raised against the hydrophilic peptide (RRNANENQGDGMDQH) representing the predicted amino acids 495–509. The sequence identity between zebra finch and Mallard duck aromatase is not known; however, this antibody has been utilized in numerous avian species (for review, see Saldanha and Schlinger, 2008).
Tissue preparation
Immunocytochemical procedures were carried out as described by the Fraley lab previously (Fraley and Kuenzel, 1993; Fraley, 2003; Horowitz, 2001; Irwig et al., 2004; Johnson, 2007). Briefly, brains (n = 5 gonadally active drakes) were collected and static-fixed in 4% paraformaldehyde. Four parallel series of 40μm coronal sections of brain tissue were cut on a sliding microtome (American Optical Company, Buffalo, NY, USA) from the diagonal band of Broca (DBB) through the mammillary bodies, and stored in cryopreservative (0.9% NaCl, 30% sucrose, 1% polyvinylpyrolidine mw 40,000, 30% ethylene glycol in 0.05M PB) solution at −20°C until processed. Immunocytochemistry for KP was performed on one set of hypothalamic sections by a standard ABC (avidin/biotin complex) reaction, as previously described (Dhillo et al., 2005). Briefly, sections were washed in PB, incubated in 10 mM sodium citrate (pH = 4.3, 80°C for 30 min) followed by 0.3% H2O2 in PB (30 min @ room temp.) and pre-incubated in blocking solution (PB, 1.0% non-fat dried milk (NFM), 0.05% Triton X-100) for 3 h at room temperature. Sections were then transferred to blocking solution containing rabbit anti-KP polyclonal antibody (Phoenix Pharmaceuticals, 1:1,000) or anti-AZAC (aromatase polyclonal, 1:5,000) and incubated for 48 h at 4°C with agitation. After three PB washes, sections were incubated for 3 h at room temperature in blocking solution with secondary antibody (1:500, biotinylated-anti-rabbit, Vector Laboratories, Burlingame, CA USA). KP immunoreactivity was visualized with the standard ABC reaction with Ni-3,3'5,5' diaminobenzidine (DAB) as the chromagen to produce a blue-black reaction product (DAB Chromagen Kit No. PK6100, Vector Laboratories). Sections were mounted on Superfrost Plus slides (VWR Scientific, West Chester, PA, USA), air-dried, dehydrated in graded ethanol, and cleared with Citrosolv, after which glass coverslips were applied.
For KP double-labeling with either aromatase or GnRH, sections were processed using the described tyramide-amplification procedure to minimize the probability of cross-reactivity between the two rabbit polyclonal primary antibodies (Berghorn, 1994; Buki, 2000; Wang, 1999). The sections were incubated in KP primary antibody (1:1000 in KPBS with 0.4% Triton-X) for 48 hrs at 4°C. After washing, sections were incubated with a goat anti-rabbit, biotinylated secondary antibody (1:5000 in KPBS, 0.4% Triton-X, Vector Labs) for 2 hrs at 4°C, then washed in KPBS 8 × 10'. Sections were then incubated in ABC solution (Vector Laboratories, in KPBS with 0.4% Triton-X) for 1 hour at room temperature and again washed in KPBS. Sections were then incubated in biotinylated tyramine (5 μl/ml KPBS with 0.005% H2O2) for 20 min at room temp. After washing, sections were incubated in streptavidin-texas red fluorophore (5 μl/ml KPBS with 0.04% Triton-X) for 3 hrs at 37°C. After 8 × 10' washes in KPBS, sections were either incubated in anti-AZAC (1:100 in KPBS with 0.04% Triton-X) or rabbit anti-GnRH (HU6H, generous gift of Dr. Henryk Urbanski, 1:100 in KPBS with 0.04% Trition-X) for 48 hrs at 4°C. After washing in KPBS, sections were incubated in a donkey, anti-rabbit FITC chromophore for 2 hrs at room temperature. Sections were again washed, mounted on slides, air-dried and coverslipped. Concentrations and incubation times were determined in preliminary experiments to saturate binding sites and thus prevent nonspecific cross reactivity between the polyclonal antibodies.
Immunocytochemical controls
For both the single and double label staining, elimination of either the primary or secondary antibody prevented cell body or fiber staining for that peptide. Rotation of fluorophores had no effect on the pattern of staining for any of the 3 primary antibodies. Single label KP immunoreactivity was eliminated if the sections were pretreated in solution containing an excess of KP-54, however, staining was not affected by pretreatment with avian gonadotropin inhibitory hormone (GnIH) or galanin-like peptide (GALP).
Analysis of Immunocytochemistry
All sections that contained KP immunoreactive neurons were analyzed under bright-field illumination (Leica Microsystems DM5100, Wetzlar, Germany). All KP neurons were counted bilaterally at 20x magnification. Single-labeled KP neurons were counted if brown bipolar neurons were observed with clear nuclei lacking dark staining. Fiber distribution was mapped and compared qualitatively.
Results
Experiment 1: Effects of ICV Kisspeptin on LH secretion
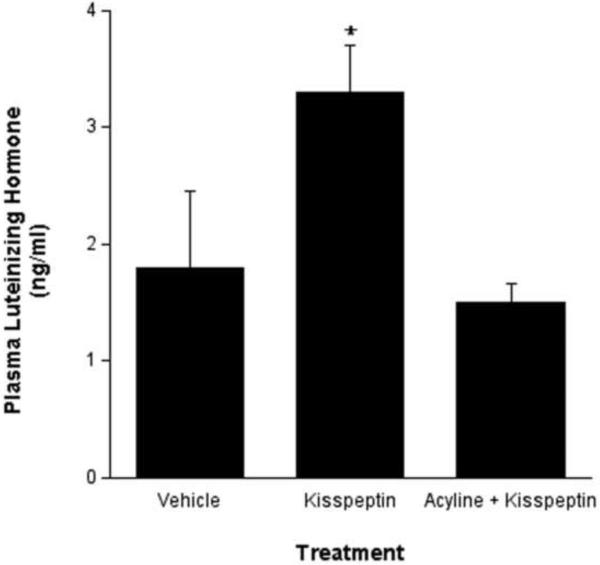
Mallard drakes were group housed (5–6 per pen) in the Hope College aviary. These conditions were recently described to elicit hierarchical social conditions that may affect hormone levels (Poisbleau, 2005) and as such, we noted different base-line levels of circulating LH in these birds. Since variation was unequal across groups, LH data were log transformed prior to statistical analyses. ICV injection of KP into the drake brain elicited a robust increase in circulating LH levels compared to vehicle injections. Pretreatment with the GnRH receptor antagonist, acyline, prevented the relative KP-induced increase in LH levels (Figure 1).
Fig. 1.
Intracerebroventricular KP significantly increased plasma LH levels compared to control injection (p < 0.01). The KP-induced increase in LH secretion was blocked with a prior administration of the GnRH receptor antagonist, acyline. Statistical analyses were conducted on logarithmic concatenations of the actual data depicted here.
Experiments 2: ICC for kisspeptin and aromatase
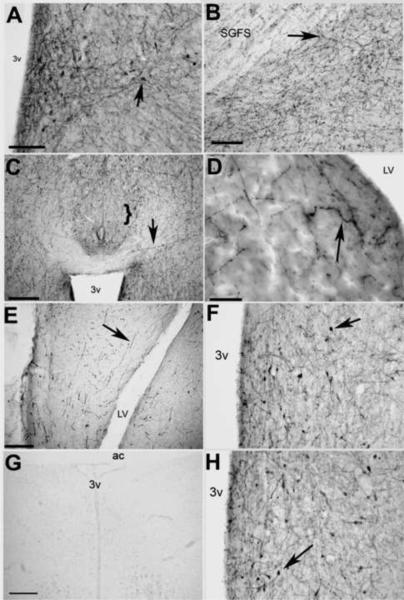
Elimination of the primary antibody or preadsorption with 50-fold concentration of kisspeptin eliminated all immunostaining. Preadsorption with GALP, GnIH or its mammalian ortholog, RFRP-3, had no effect on KP-lir. KP-lir cell bodies were only observed in the POM. No KP-lir cell bodies were observed in the paraventricular nucleus as would be the case if the antibody cross-reacted with avian GnIH (Kriegsfeld, 2006). KP-lir fibers were observed throughout the brain. The densest fiber distribution was observed in the diencephalon, particularly in areas that contain GnRH I cell bodies, such as the diagonal band and bed nucleus of the stria terminalis (BnST) and areas that contain GnRH I fibers, such as the tuberoinfundibular region and the median eminence (ME). Of particular note is that KP-lir fibers were virtually absent from the bed nucleus of the pallial commisure (nCPa), and only a few, sparse fibers were observed in the telencephalon. An exception to the telencephalic sparsity of KP-lir fibers was the ventral hippocampus. Table 1 illustrates the distribution of KP-lir fibers in the male duck brain. Figure 2 shows representative photomicrographs of KP-lir cell bodies and fibers.
Table 1.
Distribution of KP-ir fibers in the Brain of the Mallard Duck
| Structure | KP-ir Fiber Density |
|---|---|
| Diencephalon | |
| medial preoptic nucleus | ++++ |
| paraventricular nucleus | +++++ |
| ventromedial nucleus | +++ |
| lateral hypothalamus | +++ |
| median eminence | +++++ |
| periventricular hypothalamic nucleus | +++ |
| infundibular nucleus | +++++ |
| periventricular organ | ++ |
| dorsomedial nucleus | ++ |
| medial mammillary nucleus | + |
| bed nucleus of the pallial commisure | - |
| medial septal nucleus | + |
| lateral septal nucleus | +++ |
| Lateral Septal Organ | + |
| Subseptal Organ | - |
| Extra-Diencephalic Structures | |
| Neostriatum | + |
| paleostriatum | + |
| archistriatum | + |
| Dorsolateral Cortical Area | + |
| ventral hippocampus | +++ |
| dorsal hippocampus | ++ |
| parahippocampal area | + |
| globus pallidus | - |
| optic tectum | +++ |
| cerebellum | - |
Fig. 2.
Representative photomicrographs of KP-lir. A) KP-lir cell bodies were only observed in the POM (bar = 30 μm). B) KP-lir fibers were observed in the optic tectum (bar = 30 μm). C) Dense KP-lir fibers were observed throughout the diencephalon, however, few--if any--fibers were observed in the bed nucleus of the pallial commisure (nCPa, bracket, bar = 50 μm.). D) KP-lir fibers were also observed in the septal area (bar = 20 μm), and E) in the hippocampus (bar = 50 μm). F) Preadsorption of sections with an unrelated peptide, galanin-like peptide, had no effect on KP-lir. G) Preadsorption of sections with excess kisspeptin eliminated KP-lir staining in the POM. Bar = 50 μm. H) Preadsorption of sections with a related RF-amide peptide, gonadotropin inhibitory peptide, also had no effect on KP-lir. Bar = 50 μm. 3v = third ventricle, LV = lateral ventricle, SGFS = striatum griseum et fibrosum superficiale, arrows designate positive KP-lir.
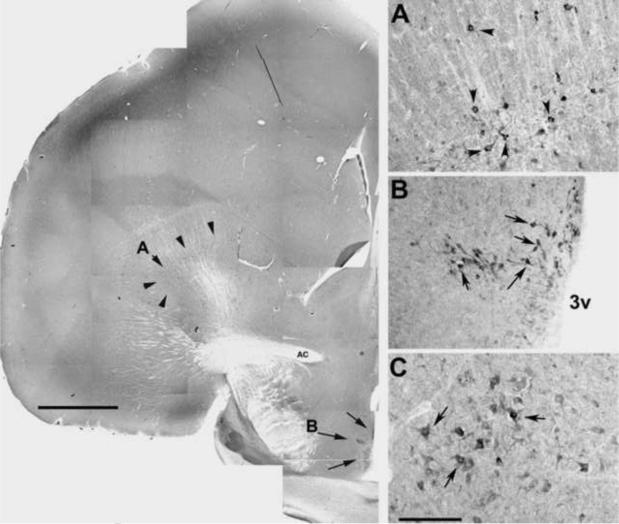
Aromatase-ir cell bodies were observed in the diencephalon (i.e. the POM) as described in other avian species (Balthazart, 1990; Balthazart and Surlemont, 1990; Naftolin et al., 2001; Saldanha et al., 1999; Schlinger and Callard, 1989; Schumacher and Balthazart, 1987). However, in the duck we also made the novel observation of densely stained cell bodies in the hippocampus, globus pallidus and red nuclei, areas known to be involved in memory and motor functions (Figure 3).
Fig. 3.
Representative photomicrographs of aromatase-ir. The left panel (bar = 150 μm) is a composite photomicrograph showing the rostral diencephalon at the level of the anterior commissure (AC). Arrow heads indicate aromatase-ir cell bodies in the globus pallidum (panel A is higher power), arrows indicate aromatase-ir cell bodies in the POM and periventriuclar region (panel B shows higher power). C) Aromatase-ir cell bodies were also observed in nucleus ruber (not observed in composite photomicrograph; bar = 50 μm). 3v = third ventricle.
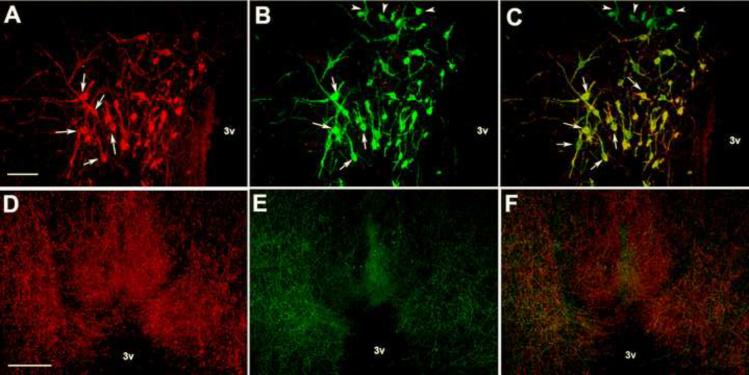
Double staining for kisspeptin- and aromatase- like immunreactivity demonstrated that nearly 100% of the KP-lir cell bodies in the POM were also immunoreactive for aromatase (Figure 4a,b,c). The KP-lir cell bodies in the POM were only approximately 70% of the aromatase immunoreactive cell bodies. Double-labeling of aromatase and KP-lir was not observed in other brain areas. Table 2 illustrates the cell body distribution for KP-lir and aromatase-ir. We utilized a rabbit polyclonal antibody against GnRH to demonstrate that the tyramide amplification prevented cross-reactivity between two rabbit polyclonal primary antibodies. Indeed, we observed no colocalization of GnRH-ir and KP-lir; however, there was substantial overlap between these two phenotypic fibers. The greatest density of KP-lir and GnRH-ir fiber close contacts were observed in the tuberoinfundibular region as they approach the median eminence (Figure 4d,e,f).
Fig. 4.
Representative photomicrographs of fluorescent immunocytochemistry for KP-lir (A, arrows), aromatase-ir (B, arrows). C) An overlay of kisspeptin- and aromatse-ir indicating colocalization of the two peptides (arrows). Bar = 50 μm. Representative photomicrographs of KP-lir fibers (D), GnRH-immunoreactive fibers (E) in the tuberoinfundibular region. F illustrates an overlay of panels D & E. Bar = 100 μm, 3v = third ventricle.
Table 2.
Distribution of KP-ir and Aromatase-ir Cell Bodies
| Structure | KP-ir Cell Bodies | Aromatase-ir Cell Bodies |
|---|---|---|
| Diencephalic | ||
| medial preoptic nucleus | ++++1 | ++++ |
| Extra-diencephalic | ||
| Globus Pallidus | - | ++ |
| Red Nucleus | - | ++ |
| ventral hippocampus | - | +++ |
Virtually all KP-ir cell bodies colocalized aromatase-ir. Colocalization of the two peptides was not observed in any other brain structure.
Discussion
The purpose of these studies was to understand if, and how, locally synthesized estradiol may interact with kisspeptin to regulate the avian HPG. In the mallard drake, we find that, as in mammals, the KiSS-1 gene product, kisspeptin, increases LH secretion via a GnRH-dependant mechanism. We also report that the drake POM appears to be the central locus for kisspeptin expression. More specifically, immunocytochemical techniques revealed kisspeptin expression in neuronal perikarya within the POM. However, the KP-lir is detectable in fibers that may emanate from this locus into other diencephalic and telencephalic areas including the BnST, POA, VMN, and hippocampus. Finally, we report that virtually all kisspeptin-expressing neurons co-express the estrogen-synthetic enzyme aromatase, providing evidence for an autocrine mechanism whereby locally synthesized steroids may regulate kisspeptin expression in the avian brain. We also demonstrate that KP-lir and GnRH-ir fibers are in close proximity lending anatomical evidence to our hypothesis that kisspetin regulates the HPG in the drake. To the best of our knowledge, this is the first report of kisspeptin expression in the brain of an avian species and the first to demonstrate co-expression of aromatase and kisspeptin in any vertebrate. Taken together, these data suggest that locally synthesized estradiol may permit and/or potentiate the action of kisspeptin on GnRH in birds.
In the duck, kisspeptin appears to modulate the HPG in a manner very similar to that reported in mammals (Kauffman, 2007; Smith, 2006). The present results show that ICV administration of low doses of kisspeptin (Experiment 1) was sufficient to significantly increase plasma LH levels. Moreover, the mechanism of this action appears to involve higher GnRH neurons, since concomitant administration of the GnRH receptor antagonist, acycline, was sufficient to block kisspeptin-induced LH secretion. It would be valuable to measure the release of GnRH in portal blood in response to kisspeptin administration; however, this technique (neither portal blood collection or RIA for GnRH) has been established in the Mallard duck. Regardless, in addition to kisspeptin-induced LH secretion, we also noted a dense, close-proximity of KP-lir fibers with GnRH- fibers as they approach the median eminence—a site for regulation of GnRH release into the pituitary portal blood. Thus, as in mammals, kisspeptin appears to affect the avian HPG via a GnRH dependent mechanism.
Neither preadsorption with GnIH nor GALP compromised the detection of kisspeptin-ir in the drake brain. We are particularly confident in the lack of cross-reactivity between the kisspeptin-ir and GnIH expression since no immunoproduct was detected in the PVN the sole diencephalic locus of GnIH-ir in birds (Bentley, 2006). Our staining protocol and findings are similar to that reported in other non-mammalian models, namely the teleost (Felip, 2008; Mechaly, 2009; van Aerle, 2008). Taken together, these data point to the authenticity of the data obtained from the ICC data on kisspeptin expression in the drake brain.
In murine rodents, recent data strongly suggests that estradiol plays a role in the autocrine and trans-synaptic regulation of kisspeptin. Specifically, Peilecka-Fortuna et al. (2008), report that estradiol potentiates kisspeptin-dependent GnRH neuronal firing. Further, this same report suggests that this potentiation may occur via a trans-synaptic mechanism, invoking local aromatization by hypothalamic neurons in this phenomenon. The present data support this hypothesis by demonstrating that all kisspeptin-expressing neurons in the drake are indeed aromatase positive and may readily aromatize circulating androgen into estrogens. While we are aware of the potential confounds of cross-reactivity in the co-detection of two antigens using antibodies generated in the same species (here, polyclonal antibodies raised in rabbit), we are confident that this does not explain the present results. Firstly, while every kisspeptin positive neuron was aromatase-positive, the converse was not true. That is, many aromatase expressing neurons at diencephalic and telencephalic brain areas were devoid of kisspeptin-expression suggesting a low (if any) level of cross reactivity. Secondly, while aromatase-ir was detected with a secondary antibody directly conjugated to the fluorophore, that for kisspeptin was detected using streptavidin bound to a fluorophore, thereby further decreasing the possibility of cross-reactivity across primary and secondary antibodies. Lastly, no co-localization was observed between the kisspeptin and GnRH polyclonal antibodies using this tyramide amplification protocol. Given these protocols and observations we are confident in our inference that all detectable kisspeptin neurons in the mallard brain are indeed aromatase positive. This suggests an autocrine mechanism whereby locally synthesized estrogen may affect kisspeptin expression in the Mallard. Given that these were gonadally active drakes, it is conceivable that estrogen up-regulates kisspeptin expression, as is well described in the mammalian literature (Adachi, 2007; Kauffman, 2008; Smith, 2007; Smith, 2008; Smith, 2005a). However, our studies focus on the anatomical colocalization of aromatase and kisspeptin in the POM. Although this strongly suggests estrogenic regulation of the kiss-1 gene, our data cannot state either an up- or down-regulatory effects of steroids on kisspeptin.
Kisspeptin and aromatase co-expressing neurons may affect GnRH in several ways. De novo synthesized intracellular estrogen may potentiate kisspeptin release, increase the expression of the kisspeptin receptor GPR54 in GnRH neurons, or affect GnRH release independent of kisspeptin-GPR54 signaling. In rodents, steroids modify kisspeptin in a site-specific manner. Specifically, while kisspeptin appears to be negatively regulated by estradiol in the arcuate nucleus, it is positively regulated by estradiol in the AVPV (Smith et al., 2006; Smith et al., 2005b). Kisspeptin neurons in the AVPV are believed to affect GnRH activity (Kauffman et al., 2007). We do not yet know precisely how administered or endogenous estradiol may affect kisspeptin in birds, however, a positive effect of estradiol on kisspeptin is suggested by the current pattern of results and what is known about steroid receptor expression in the avian diencephalon.
The expression of steroid receptors in GnRH neurons has been difficult to demonstrate in birds, suggesting the possibility that, like mammals, steroid effects on GnRH may occur via an intermediary such as one of the kisspeptins. The present data strongly suggest the possibility that kisspeptin positive neurons may synthesize estrogen. However, whether these neurons express steroid receptors or not, is unknown. The expression of steroid receptors, while reasonably well described in several avian species, unfortunately does not include anseriformes. Nonetheless, the POM is a site of abundant estrogen receptor (ER) and androgen receptor (AR) expression in many avian species based upon studies using in situ hybridization for steroid receptor transcripts and immunocytochemistry (Balthazart, 1991; Jacobs, 1996; Lakaye, 1998; Saldanha and Coomaralingam, 2005). More specifically, ERs are co-expressed in a subpopulation of aromatase positive neurons in the avian POA (Balthazart, 1992; Saldanha and Coomaralingam, 2005). Whether (or not) these cells also express kisspeptin remains to be tested. The hypothesis emerging from these data is that aromatase, ER, and kisspeptin-expressing neurons may be potent effectors of GnRH release in the duck. This hypothesis is currently being examined in our laboratories.
Alternatively (or in addition), locally synthesized estradiol by aromatase-positive neurons in the drake diencephalon may directly affect GnRH neurons by increasing GPR54 activity. Although plausible, this mode of action seems improbable given the incongruities regarding ER expression in GnRH neurons in several vertebrates. However, ER beta has been reported in mammalian GnRH neurons (Hrabovszky, 2007; Hrabovszky, 2001) but this possibility remains to be tested in the drake. The presence of steroid receptors on GnRH neurons in the drake may suggest that locally synthesized extracellular estradiol may enhance GPR54 activity and hence alter GnRH secretion in the drake.
To our knowledge, this is the first report of aromatase expression in an anseriforme brain. In general terms, central aromatase expression in birds demonstrates two patterns of expression. While passerine songbirds demonstrate abundant aromatase at numerous telencephalic and diencephalic areas, the distribution in galliformes and columbiformes of this protein is limited to diencephalic and some limbic areas (Hutchison and Steimer, 1984; Saldanha, 1998; Schumacher and Balthazart, 1987). The duck appears to demonstrate a pattern of aromatase expression that resembles the passerine brain. Specifically, in addition to the diencephalon, telencephalic loci such as the hippocampus, the red nucleus and globus pallidus contain considerable aromatase. More research is necessary to decipher the physiological roles of telencephalic aromatase expression in the duck brain.
In summary, the present data suggest a novel and exciting mode of communication between estrogen synthesizing (aromatase expressing) and kisspeptin-positive neurons in the avian brain. We hypothesize that estrogen synthesized within kisspeptin neurons may increase kisspeptin release and/or amplify the response to kisspeptin in GnRH neurons via an effect on GPR54 via a trans-synaptic mechanism. Given the rich history of autocrine and paracrine effects of steroids in the vertebrate diencephalon, this hypothesis seems viable. In addition, the recent documentation of presynaptic aromatization in several vertebrates including birds (Naftolin, 1996; Peterson, 2005; Rohmann, 2006; Schlinger and Callard, 1989) strongly supports the possibility that estrogen synthesis via synaptocrine pathways may affect postsynaptic GPR54 expression in GnRH neurons postsynaptic to kisspeptin cells.
Acknowledgements
We are particularly indebted to Dr. Peter Sharp for his assistance with the LH assays. We also thank Dr. Wayne Kuenzel and Dr. Gloria Hoffman for their advice on the neuroanatomy and immunolocalization protocols respectively, and Angela Stoyanovitch, Marlie Johnson, and Dr. Susan M. Fraley for their assistance with surgeries, and critical comments on an earlier version of this manuscript. This work was supported by NIH (RO1 042767 to CJS, K01 DK066238-01A1 to GSF), and a NSF-REU grant to the Hope Biology Department.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, Inoue K, Maeda K. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev. 2007;53:367–78. doi: 10.1262/jrd.18146. [DOI] [PubMed] [Google Scholar]
- Balthazart J. Brain aromatization of testosterone regulates male reproductive behavior in birds. Prog Clin Biol Res. 1990;342:92–8. [PubMed] [Google Scholar]
- Balthazart J, Foidart A, Surlemont C, Harada N, Naftolin F. Neuroanatomical specificity in the autoregulation of aromatase-immunoreactive neurons by androgens and estrogens: an immunocytochemical study. Brain Res. 1992;574:280–90. doi: 10.1016/0006-8993(92)90828-w. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Sante P, Ball GF. Testosterone effects on staining density and autoradiographic investigations of the alpha 2-adrenergic receptor in the medial preoptic nucleus of the Japanese quail: relationship to the activation of reproductive behavior. Arch Int Physiol Biochim Biophys. 1991;99:385–92. [PubMed] [Google Scholar]
- Balthazart J, Surlemont C. Androgen and estrogen action in the preoptic area and activation of copulatory behavior in quail. Physiol Behav. 1990;48:599–609. doi: 10.1016/0031-9384(90)90198-d. [DOI] [PubMed] [Google Scholar]
- Benoit J. Le role des yeux dans l'action stimulante de la lumiere sur le development testiculaire chez le carnard. Canadian Royal Soc. Biol. 1936;120:133–136. [Google Scholar]
- Bentley GE, Kriegsfeld LJ, Osugi T, Ukena K, O'Brien S, Perfito N, Moore IT, Tsutsui K, Wingfield JC. Interactions of gonadotropin-releasing hormone (GnRH) and gonadotropin-inhibitory hormone (GnIH) in birds and mammals. J Exp Zoolog A Comp Exp Biol. 2006;305:807–14. doi: 10.1002/jez.a.306. [DOI] [PubMed] [Google Scholar]
- Berghorn KA, Bonnett JH, Hoffman GE. cFos immunoreactivity is enhanced with biotin amplification. J Histochem Cytochem. 1994;42:1635–42. doi: 10.1177/42.12.7983364. [DOI] [PubMed] [Google Scholar]
- Buki A, Walker SA, Stone JR, Povlishock JT. Novel application of tyramide signal amplification (TSA): ultrastructural visualization of double-labeled immunofluorescent axonal profiles. J Histochem Cytochem. 2000;48:153–61. doi: 10.1177/002215540004800116. [DOI] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147:5817–25. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillo WS, Chaudhri OB, Patterson M, Thompson EL, Murphy KG, Badman MK, McGowan BM, Amber V, Patel S, Ghatei MA, Bloom SR. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J Clin Endocrinol Metab. 2005;90:6609–15. doi: 10.1210/jc.2005-1468. [DOI] [PubMed] [Google Scholar]
- Dunham LA, Lutterschmidt DI, Wilczynski W. Kisspeptin-like immunoreactive neuron distribution in the green anole (Anolis carolinensis) Brain Behav Evol. 2009;73:129–37. doi: 10.1159/000215930. [DOI] [PubMed] [Google Scholar]
- Estrada KM, Clay CM, Pompolo S, Smith JT, Clarke IJ. Elevated KiSS-1 expression in the arcuate nucleus prior to the cyclic preovulatory gonadotrophin-releasing hormone/lutenising hormone surge in the ewe suggests a stimulatory role for kisspeptin in oestrogen-positive feedback. J Neuroendocrinol. 2006;18:806–9. doi: 10.1111/j.1365-2826.2006.01485.x. [DOI] [PubMed] [Google Scholar]
- Felip A, Zanuy S, Pineda R, Pinilla L, Carrillo M, Tena-Sempere M, Gomez A. Evidence for two distinct KiSS genes in non-placental vertebrates that encode kisspeptins with different gonadotropin-releasing activities in fish and mammals. Mol Cell Endocrinol. 2008 doi: 10.1016/j.mce.2008.11.017. [DOI] [PubMed] [Google Scholar]
- Fraley GS, Kuenzel WJ. Immunocytochemical and histochemical analyses of gonadotrophin releasing hormone, tyrosine hydroxylase, and cytochrome oxidase reactivity within the hypothalamus of chicks showing early sexual maturation. Histochemistry. 1993;99:221–9. doi: 10.1007/BF00269140. [DOI] [PubMed] [Google Scholar]
- Fraley GS, Shimada I, Baumgartner JW, Clifton DK, Steiner RA. Differential patterns of Fos induction in the hypothalamus of the rat following central injections of galanin-like peptide and galanin. Endocrinology. 2003;144:1143–6. doi: 10.1210/en.2002-0114. [DOI] [PubMed] [Google Scholar]
- Haase E. The annual reproductive cycle in mallards. J Steroid Biochem. 1983;19:731–7. doi: 10.1016/0022-4731(83)90004-3. [DOI] [PubMed] [Google Scholar]
- Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–56. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz JM, Vernace VA, Myers J, Stachowiak MK, Hanlon DW, Fraley GS, Torres G. Immunodetection of Parkin protein in vertebrate and invertebrate brains: a comparative study using specific antibodies. J Chem Neuroanat. 2001;21:75–93. doi: 10.1016/s0891-0618(00)00111-3. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Kallo I, Szlavik N, Keller E, Merchenthaler I, Liposits Z. Gonadotropin-releasing hormone neurons express estrogen receptor-beta. J Clin Endocrinol Metab. 2007;92:2827–30. doi: 10.1210/jc.2006-2819. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Steinhauser A, Barabas K, Shughrue PJ, Petersen SL, Merchenthaler I, Liposits Z. Estrogen receptor-beta immunoreactivity in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology. 2001;142:3261–4. doi: 10.1210/endo.142.7.8176. [DOI] [PubMed] [Google Scholar]
- Hutchison JB, Steimer T. Androgen metabolism in the brain: behavioural correlates. Prog Brain Res. 1984;61:23–51. doi: 10.1016/S0079-6123(08)64427-1. [DOI] [PubMed] [Google Scholar]
- Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80:264–72. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- Jacobs EC, Arnold AP, Campagnoni AT. Zebra finch estrogen receptor cDNA: cloning and mRNA expression. J Steroid Biochem Mol Biol. 1996;59:135–45. doi: 10.1016/s0960-0760(96)00096-9. [DOI] [PubMed] [Google Scholar]
- Johnson MA, Tsutsui K, Fraley GS. Rat RFamide-related peptide-3 stimulates GH secretion, inhibits LH secretion, and has variable effects on sex behavior in the adult male rat. Horm Behav. 2007;51:171–80. doi: 10.1016/j.yhbeh.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson OW. Reproductive Cycle of the Mallard Duck. The Condor. 1961;63:351–364. [Google Scholar]
- Kauffman AS. Sexual differentiation and the Kiss1 system: Hormonal and developmental considerations. Peptides. 2008 doi: 10.1016/j.peptides.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Hoffman GE, Steiner RA, Tena-Sempere M. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology. 2007;148:1774–83. doi: 10.1210/en.2006-1540. [DOI] [PubMed] [Google Scholar]
- Kriegsfeld LJ. Driving reproduction: RFamide peptides behind the wheel. Horm Behav. 2006;50:655–66. doi: 10.1016/j.yhbeh.2006.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakaye B, Foidart A, Grisar T, Balthazart J. Partial cloning and distribution of estrogen receptor beta in the avian brain. Neuroreport. 1998;9:2743–8. doi: 10.1097/00001756-199808240-00011. [DOI] [PubMed] [Google Scholar]
- Lee YR, Tsunekawa K, Moon MJ, Um HN, Hwang JI, Osugi T, Otaki N, Sunakawa Y, Kim K, Vaudry H, Kwon HB, Seong JY, Tsutsui K. Molecular evolution of multiple forms of kisspeptins and GPR54 receptors in vertebrates. Endocrinology. 2009;150:2837–46. doi: 10.1210/en.2008-1679. [DOI] [PubMed] [Google Scholar]
- Masui T, Doi R, Mori T, Toyoda E, Koizumi M, Kami K, Ito D, Peiper SC, Broach JR, Oishi S, Niida A, Fujii N, Imamura M. Metastin and its variant forms suppress migration of pancreatic cancer cells. Biochem Biophys Res Commun. 2004;315:85–92. doi: 10.1016/j.bbrc.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Matsui H, Takatsu Y, Kumano S, Matsumoto H, Ohtaki T. Peripheral administration of metastin induces marked gonadotropin release and ovulation in the rat. Biochem Biophys Res Commun. 2004;320:383–8. doi: 10.1016/j.bbrc.2004.05.185. [DOI] [PubMed] [Google Scholar]
- Mechaly AS, Vinas J, Piferrer F. Identification of two isoforms of the Kisspeptin-1 receptor (kiss1r) generated by alternative splicing in a modern teleost, the Senegalese sole (Solea senegalensis) Biol Reprod. 2009;80:60–9. doi: 10.1095/biolreprod.108.072173. [DOI] [PubMed] [Google Scholar]
- Messager S. Kisspeptin and its receptor: new gatekeepers of puberty. J Neuroendocrinol. 2005a;17:687–8. doi: 10.1111/j.1365-2826.2005.01357.x. [DOI] [PubMed] [Google Scholar]
- Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci U S A. 2005b;102:1761–6. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naftolin F, Horvath TL, Balthazart J. Estrogen synthetase (aromatase) immunohistochemistry reveals concordance between avian and rodent limbic systems and hypothalami. Exp Biol Med (Maywood) 2001;226:717–25. doi: 10.1177/153537020222600802. [DOI] [PubMed] [Google Scholar]
- Naftolin F, Leranth C, Horvath TL, Garcia-Segura LM. Potential neuronal mechanisms of estrogen actions in synaptogenesis and synaptic plasticity. Cell Mol Neurobiol. 1996;16:213–23. doi: 10.1007/BF02088177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, Fernandez-Fernandez R, Barreiro ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology. 2004;145:4565–74. doi: 10.1210/en.2004-0413. [DOI] [PubMed] [Google Scholar]
- Nicholls TJ, Goldsmith AR, Dawson A. Photorefractoriness in birds and comparison with mammals. Physiol Rev. 1988;68:133–76. doi: 10.1152/physrev.1988.68.1.133. [DOI] [PubMed] [Google Scholar]
- Peterson RS, Yarram L, Schlinger BA, Saldanha CJ. Aromatase is pre-synaptic and sexually dimorphic in the adult zebra finch brain. Proc Biol Sci. 2005;272:2089–96. doi: 10.1098/rspb.2005.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pielecka-Fortuna J, Chu Z, Moenter SM. Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology. 2008;149:1979–86. doi: 10.1210/en.2007-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant TM. The role of KiSS-1 in the regulation of puberty in higher primates. Eur J Endocrinol. 2006a;155(Suppl 1):S11–6. doi: 10.1530/eje.1.02232. [DOI] [PubMed] [Google Scholar]
- Plant TM, Ramaswamy S, Dipietro MJ. Repetitive activation of hypothalamic G protein-coupled receptor 54 with intravenous pulses of kisspeptin in the juvenile monkey (Macaca mulatta) elicits a sustained train of gonadotropin-releasing hormone discharges. Endocrinology. 2006b;147:1007–13. doi: 10.1210/en.2005-1261. [DOI] [PubMed] [Google Scholar]
- Poisbleau M, Fritz H, Guillon N, Chastel O. Linear social dominance hierarchy and corticosterone responses in male mallards and pintails. Horm Behav. 2005;47:485–92. doi: 10.1016/j.yhbeh.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Pompolo S, Pereira A, Estrada KM, Clarke IJ. Colocalization of kisspeptin and gonadotropin-releasing hormone in the ovine brain. Endocrinology. 2006;147:804–10. doi: 10.1210/en.2005-1123. [DOI] [PubMed] [Google Scholar]
- Rohmann KN, Schlinger BA, Saldanha CJ. Subcellular compartmentalization of aromatase is sexually dimorphic in the adult zebra finch brain. J Neurobiol. 2006;67:1–9. doi: 10.1002/dneu.20303. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Clayton NS, Schlinger BA. Androgen metabolism in the juvenile oscine forebrain: a cross-species analysis at neural sites implicated in memory function. J Neurobiol. 1999;40:397–406. doi: 10.1002/(sici)1097-4695(19990905)40:3<397::aid-neu11>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Coomaralingam L. Overlap and co-expression of estrogen synthetic responsive neurons in the songbird brain--a doble label immunocytochemical study. General and Comparative Endocrinology. 2005;141:66–75. doi: 10.1016/j.ygcen.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Popper P, Micevych PE, Schlinger BA. The passerine hippocampus is a site of high aromatase: inter- and intraspecies comparisons. Horm Behav. 1998;34:85–97. doi: 10.1006/hbeh.1998.1447. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Schlinger BA. Steroidogenesis and Neuroplasticity in the songbird brain. In: Ritsner MS, Weizman A, editors. Neuroactive Steroids in Brain Function Behavioral and Neuropsychiatric Disorders. Novel Strategies for Research and Treatment. Springer; 2008. [Google Scholar]
- Schlinger BA, Callard GV. Localization of aromatase in synaptosomal and microsomal subfractions of quail (Coturnix coturnix japonica) brain. Neuroendocrinology. 1989;49:434–41. doi: 10.1159/000125149. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Balthazart J. Neuroanatomical distribution of testosterone-metabolizing enzymes in the Japanese quail. Brain Res. 1987;422:137–48. doi: 10.1016/0006-8993(87)90548-8. [DOI] [PubMed] [Google Scholar]
- Sharp PJ, Dunn IC, Talbot RT. Sex differences in the LH responses to chicken LHRH-I and -II in the domestic fowl. J Endocrinol. 1987;115:323–31. doi: 10.1677/joe.0.1150323. [DOI] [PubMed] [Google Scholar]
- Smith JT. Kisspeptin signalling in the brain: Steroid regulation in the rodent and ewe. Brain Res Rev. 2007 doi: 10.1016/j.brainresrev.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Smith JT. Sex steroid control of hypothalamic Kiss1 expression in sheep and rodents: Comparative aspects. Peptides. 2008 doi: 10.1016/j.peptides.2008.08.013. [DOI] [PubMed] [Google Scholar]
- Smith JT, Clifton DK, Steiner RA. Regulation of the neuroendocrine reproductive axis by kisspeptin-GPR54 signaling. Reproduction. 2006;131:623–30. doi: 10.1530/rep.1.00368. [DOI] [PubMed] [Google Scholar]
- Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005a;146:3686–92. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005b;146:2976–84. doi: 10.1210/en.2005-0323. [DOI] [PubMed] [Google Scholar]
- Thompson EL, Patterson M, Murphy KG, Smith KL, Dhillo WS, Todd JF, Ghatei MA, Bloom SR. Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. J Neuroendocrinol. 2004;16:850–8. doi: 10.1111/j.1365-2826.2004.01240.x. [DOI] [PubMed] [Google Scholar]
- van Aerle R, Kille P, Lange A, Tyler CR. Evidence for the existence of a functional Kiss1/Kiss1 receptor pathway in fish. Peptides. 2008;29:57–64. doi: 10.1016/j.peptides.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Wang G, Achim CL, Hamilton RL, Wiley CA, Soontornniyomkij V. Tyramide signal amplification method in multiple-label immunofluorescence confocal microscopy. Methods. 1999;18:459–64. doi: 10.1006/meth.1999.0813. [DOI] [PubMed] [Google Scholar]