Abstract
This review of the scientific literature examines the widely observed relationship between sleep duration and mortality. As early as 1964, data have shown that 7-hour sleepers experience the lowest risks for all-cause mortality, whereas those at the shortest and longest sleep durations have significantly higher mortality risks. Numerous follow-up studies from around the world (e.g., Japan, Israel, Sweden, Finland, the United Kingdom) show similar relationships. We discuss possible mechanisms, including cardiovascular disease, obesity, physiologic stress, immunity, and socioeconomic status. We put forth a social-ecological framework to explore five possible pathways for the relationship between sleep duration and mortality, and we conclude with a four-point agenda for future research.
SLEEP DURATION AND MORTALITY: THE EVIDENCE
Over 40 years of evidence indicate a strong association between nightly sleep duration and mortality risk1–2. In general, sleep duration is associated with mortality in a U-shaped fashion, such that the lowest risk is most often found in the group who report sleep durations of 7–8 hours (See Tables 1 and 2 for a summary of their findings). Nearly uniformly, mortality risk increases with further deviation from the 7–8hr range.
Table 1.
Studies Investigating Mortality Associated with Sleep Duration
| First Author | Year | N | % Female | Age Range | Years Studied | Covariates besides age | Sleep Duration of Reference Group |
|---|---|---|---|---|---|---|---|
| Amagai | 200418 | 11325 | 61% | 19–93 | 9 | 8 | 7–7.9 hours |
| Ayas | 200332 | 71617 | 100% | 45–65 | 10 | 15 | 8 hours |
| Belloc | 19739 | 6928 | 39% | >18 | 5.5 | 0 | None |
| Branch | 198410 | 1235 | 62% | 66–98 | 4.75 | 6 | 7–8 hours |
| Breslow | 19807 | 6928 | 39% | >18 | 9.5 | 0 | None |
| Burazeri | 200323 | 1842 | 54% | ≥50 | 9–11 | 24 | 6 hours |
| Chen* | 199436 | 10287 | Not reported | 32–86 | 4 | 4 | 6–9 hours |
| Dew | 200333 | 184 | 54% | ≥55 | 4.1–19.5 | 0 | ≥6 hours |
| Ferrie | 200728 | 10308 | 33% | 35–55 | 11.8 and 17.1 | 13 | 7 hours |
| Gale | 199813 | 1299 | Not reported | ≥65 | 23 | 9 | 9 hours |
| Gangwisch | 200835 | 9789 | 63% | 32–86 | 8–10 | 16 | 7 hours |
| Goto | 200317 | 724 | 65% | ≥65 | 12 | 12 | 6–7 hours |
| Gottlieb* | 200230 | 4541 | 56% | nr | 14 | 5 | 7–8 hours |
| Hammond | 19643 | 1064004 | 57% | <30–≥80 | 3 | 0 | None |
| Heslop | 200227 | 3030 | 15% | Men <65 Women <60 | 3.25 | 10 | Always 7–8 hours |
| Hublin | 200725 | 21268 | 52% | 24–101 | 22 | 10 | 7–8 hours |
| Huppert | 199526 | 6096 | 56% | ≥18 | 7 | nr | 6–9 hours |
| Kaplan | 19878 | 6928 | 36% | 38–≥70 | 17 | 6 | 7–8 hours |
| Kojima | 200016 | 5322 | 54% | 20–67 | 12 | 10 | 7–8 hours |
| Kripke | 19794 | 823065 | 56% | 30–>90 | 3.6 | 5 | 7–7.9 hours |
| Kripke | 200231 | 1116936 | 57% | 30–102 | 6 | 32 | 6.5–7.4 hours |
| Lan | 200721 | 3079 | 43% | ≥64 | 10 | 11 | 7–7.9 hours |
| Mallon | 200224 | 1870 | 52% | 45–65 | 12 | 17 | 6–8 hours |
| Patel | 200434 | 82969 | 100% | 40–65 | 10 | 11 | 7 hours |
| Pollak | 199011 | 1855 | 81% | 65–98 | 3.5 | 10 | None |
| Qureshi | 199729 | 7844 | 64% | 32–>72 | 10 | 9 | 6–8 hours |
| Ruigomez | 199514 | 1219 | 61% | ≥65 | 5 | 7 | 7–9 hours |
| Rumble | 199212 | 1020 | Not reported | ≥65 | 5 | 2 | 4–9.9 hours |
| Shankar | 200822 | 58044 | 55.5% | 45–74 | 5 | 17 | 7 hours |
| Suzuki | 200820 | 109778 | 60% | 40–79 | 12.5(m) 12.9(w) | 2 | 7–8 hours |
| Tamakoshi | 200419 | 104298 | 58% | 40–79 | 10 | 14 | 7–7.9 hours |
| Tsubono | 199315 | 4318 | 60% | ≥40 | 4 | 1 | 7–8 hours |
| Wingard | 19826 | 6928 | 36% | 30–69 | 9 | 14 | 7–8 hours |
| Wingard | 19835 | 4713 | 53% | 30–69 | 9 | 7 | 7–8 hours |
=not included in meta-analysis37
Table 2.
Odds Ratios and Mortality Rates in Studies of Sleep Duration and Mortality Across the World
| First Author | Stratum | Sleep Duration (hrs) | Odds Ratio (least adjusted) | 95% CI | Odds Ratio (fully adjusted) | 95% CI | Mortality rate (%) |
|---|---|---|---|---|---|---|---|
| Amagai 2004 (Asia) | Men: | <6 | 2.3 | 1.3– 3.9 | 2.4 | 1.3–4.2 | 1.56 |
| 6 | 1.1 | 0.7– 1.8 | 1.1 | 0.7–1.8 | 0.60 | ||
| 7 | 1.0 | Reference | 1.0 | Reference | 0.59 | ||
| 8 | 1.0 | 0.7–1.3 | 0.9 | 0.6–1.2 | 0.68 | ||
| ≥9 | 1.3 | 0.9–1.8 | 1.1 | 0.8–1.6 | 1.27 | ||
| Women: | <6 | 0.8 | 0.3–2.2 | 0.7 | 0.2–2.3 | 0.19 | |
| 6 | 1.3 | 0.8–2.0 | 1.3 | 0.8–2.1 | 0.31 | ||
| 7 | 1.0 | Reference | 1.0 | Reference | 0.26 | ||
| 8 | 1.0 | 0.7–1.5 | 1.1 | 0.8–1.6 | 0.37 | ||
| ≥9 | 1.5 | 1.0–2.3 | 1.5 | 1.0–2.4 | 0.79 | ||
| Ayas 2003 (North America) | Men & Women: | ≤5 | 1.67 | 1.02–2.74 | 1.12 | 0.68–1.84 | |
| 6 | 1.05 | 0.75–1.46 | 0.91 | 0.65–1.28 | |||
| 7 | 0.80 | 0.58–1.09 | 0.83 | 0.60–1.14 | |||
| 8 | 1.0 | Reference | 1.0 | Reference | |||
| ≥9 | 1.71 | 1.05–2.77 | 1.45 | 0.89–2.36 | |||
| Belloc 1973 (North America) | Men: | ≤6 | 0.09 | ||||
| 7 | 0.63 | ||||||
| 8 | 0.57 | ||||||
| ≥9 | 0.66 | ||||||
| Women: | ≤6 | 0.04 | |||||
| 7 | 0.36 | ||||||
| 8 | 0.46 | ||||||
| ≥9 | 0.66 | ||||||
| Branch 1984 (North America) | Men: | 7–8 | 1.0 | Reference | 0.22 | ||
| <7 or >8 | 1.60 | 0.94–2.28 | 0.31 | ||||
| Women: | 7–8 | 1.0 | Reference | 0.17 | |||
| <7 or >8 | 1.34 | 0.85–1.83 | 0.22 | ||||
| Breslow 1980 (North America) | Men: | ≤6 | 0.16 | ||||
| 7 | 0.12 | ||||||
| 8 | 0.11 | ||||||
| ≥9 | 0.14 | ||||||
| Women: | ≤6 | 1.0 | |||||
| 7 | 0.7 | ||||||
| 8 | 0.8 | ||||||
| ≥9 | 1.0 | ||||||
| Burazeria 2003 (Asia and Africa) | Men (night): | <6 | 1.0 | Reference | 1.0 | Reference | |
| 6–8 | 1.21 | 0.83–1.76 | 1.25 | 0.83–1.87 | |||
| >8 | 1.86 | 1.19–2.91 | 1.91 | 1.16–3.13 | |||
| Men (24h): | <6 | 1.0 | Reference | 1.0 | Reference | ||
| 6–8 | 1.03 | 0.65–1.64 | 1.41 | 0.83–2.39 | |||
| >8 | 1.81 | 1.12–2.93 | 2.13 | 1.23–3.71 | |||
| Women (night): | <6 | 1.0 | Reference | 1.0 | Reference | ||
| 6–8 | 0.82 | 0.57–1.17 | 0.80 | 0.54–1.17 | |||
| >8 | 0.95 | 0.63–1.42 | 1.08 | 0.70–1.66 | |||
| Women (24h): | <6 | 1.0 | Reference | 1.0 | Reference | ||
| 6–8 | 0.68 | 0.46–1.01 | 0.64 | 0.42–0.97 | |||
| >8 | 0.84 | 0.56–1.27 | 0.80 | 0.51–1.24 | |||
| Chen 1994 (North America) | Age ≥60: | <6 or >9 | 0.20 | ||||
| 6–9 | 0.15 | ||||||
| Age <60: | <6 or >9 | .02 | |||||
| 6–9 | .02 | ||||||
| Ferrieb 2007 (Europe) | Time 1: | ≤ 5 | 1.61 | 1.20–2.15 | 1.24 | 0.92–1.67 | 9.54 |
| 6 | 1.11 | 0.91–1.35 | 1.00 | 0.82–1.22 | 6.05 | ||
| 7 | 1.0 | Reference | 1.0 | Reference | 5.24 | ||
| 8 | 1.08 | 0.85–1.38 | 1.07 | 0.84–1.36 | 5.51 | ||
| ≥9 | 1.77 | 0.84–3.76 | 1.54 | 0.72–3.28 | 7.87 | ||
| Time 2: | ≤ 5 | 2.07 | 1.38–3.13 | 1.78 | 1.17–2.71 | 6.90 | |
| 6 | 1.21 | 0.89–1.66 | 1.13 | 0.83–1.55 | 3.75 | ||
| 7 | 1.0 | Reference | 1.0 | Reference | 3.20 | ||
| 8 | 1.13 | 0.85–1.52 | 1.11 | 0.82–1.48 | 3.79 | ||
| ≥9 | 2.00 | 1.18–3.38 | 1.95 | 1.15–3.31 | 6.90 | ||
| Change from baseline: | No Chg | 1.0 | Reference | 1.0 | Reference | ||
| Incr. 5–6 | 0.88 | 0.60–1.28 | 0.92 | 0.63–1.5 | 4.14 | ||
| Incr. 7–8 | 1.84 | 1.31–2.58 | 1.75 | 1.24–2.47 | 5.09 | ||
| Decr. 6–8 | 1.72 | 1.25–2.38 | 1.62 | 1.17–2.25 | 4.65 | ||
| Gale 1998 (Europe) | Men & Women: | ≤7 | 1.0 | 0.7–1.4 | |||
| 8 | 0.8 | 0.7–1.0 | |||||
| 9 | 1.0 | Reference | |||||
| 10 | 1.2 | 1.0–1.4 | |||||
| 11 | 1.3 | 1.0–1.7 | |||||
| ≥12 | 1.7 | 1.2–2.5 | |||||
| Gangwischc 2008 (North America) | All Ages: | ≤ 5 | 1.17 | 0.99–1.39 | |||
| 6 | 0.95 | 0.81–1.11 | |||||
| 7 | 1.0 | Reference | |||||
| 8 | 1.23 | 1.08–1.39 | |||||
| ≥9 | 1.34 | 1.15–1.56 | |||||
| Age <60: | ≤ 5 | 0.67 | 0.43–1.05 | ||||
| 6 | 0.75 | 0.53–1.08 | |||||
| 7 | 1.0 | Reference | |||||
| 8 | 1.02 | 0.75–1.38 | |||||
| ≥9 | 1.04 | 0.66–1.65 | |||||
| Age >60: | ≤ 5 | 1.26 | 1.06–1.53 | ||||
| 6 | 0.98 | 0.83–1.17 | |||||
| 7 | 1.0 | Reference | |||||
| 8 | 1.25 | 1.09–1.44 | |||||
| ≥9 | 1.36 | 1.15–1.60 | |||||
| Goto 2003 (Asia) | Men & Women: | <6 | 1.42 | 0.61–3.27 | 1.29 | 0.50–3.24 | |
| 6–7 | Ref | Reference | 1.0 | Reference | |||
| >7 | 1.62 | 0.99–3.26 | 1.54 | 0.97–2.58 | |||
| Gottlieb 2002 (North America) | Men: | <6 | 1.4 | None reported | |||
| 6 | 0.8 | ||||||
| 7–8 | 1.0 | ||||||
| 9 | 1.3 | ||||||
| >9 | 1.5 | ||||||
| Women: | <6 | 1.7 | None reported | ||||
| 6 | 1.1 | ||||||
| 7–8 | 1.0 | ||||||
| 9 | 0.9 | ||||||
| >9 | 1.8 | ||||||
| Hammond 1964d (North America) | Men, Time 1: | <4 | 6.70 | ||||
| 4 | 3.13 | ||||||
| 5 | 2.08 | ||||||
| 6 | 1.27 | ||||||
| 7 | 0.96 | ||||||
| 8 | 1.18 | ||||||
| 9 | 1.54 | ||||||
| ≥10 | 3.15 | ||||||
| Men, Time 2: | <4 | 5.17 | |||||
| 4 | 2.91 | ||||||
| 5 | 1.99 | ||||||
| 6 | 1.67 | ||||||
| 7 | 1.44 | ||||||
| 8 | 1.59 | ||||||
| 9 | 1.91 | ||||||
| ≥10 | 2.87 | ||||||
| Heslop 2002 (Europe) | Men: | <7 | 1.30 | 1.06–1.61 | 1.15 | 0.93–1.42 | |
| 7–8 | 1.0 | Reference | 1.0 | Reference | |||
| >8 | 1.04 | 0.65–1.66 | 0.91 | 0.57–1.46 | |||
| Women: | <7 | 1.99 | 1.16–3.41 | 1.73 | 0.99–3.03 | ||
| 7–8 | 1.0 | Reference | 1.0 | Reference | |||
| >8 | 0.60 | 0.08–4.37 | 0.58 | 0.08–4.22 | |||
| Hublin 2007 (Europe) | Men: | <7 | 1.34 | 1.19–1.51 | 1.26 | 1.11–1.43 | |
| 7–8 | 1.0 | Reference | 1.0 | Reference | |||
| >8 | 1.32 | 1.17–1.48 | 1.24 | 1.09–1.41 | |||
| Women: | <7 | 1.12 | 0.98–1.28 | 1.21 | 1.05–1.40 | ||
| 7–8 | 1.0 | Reference | 1.0 | Reference | |||
| >8 | 1.20 | 1.06–1.35 | 1.17 | 1.03–1.34 | |||
| Huppert 1995 (Europe) | Men & Women: | <6 | 0.95 | ||||
| 6–9 | 1.0 | ||||||
| >9 | 2.25 | ||||||
| Kaplan 1987 (North America) | Age 38–49: | 7–8 | 1.0 | Reference | |||
| <7 or >8 | 1.23 | 0.88–1.70 | |||||
| Age 50–59: | 7–8 | 1.0 | Reference | ||||
| <7 or >8 | 1.44 | 1.08–1.91 | |||||
| Age 60–60: | 7–8 | 1.0 | Reference | ||||
| <7 or >8 | 0.95 | 0.73–1.24 | |||||
| Age ≥70: | 7–8 | 1.0 | Reference | ||||
| <7 or >8 | 1.05 | 0.85–1.29 | |||||
| Kojima 2000 (Asia) | Men: | <7 | 1.9 | 1.1–3.29 | 1.93 | 1.12–3.35 | |
| 7–8.9 | 1.0 | Reference | 1.0 | Reference | |||
| 9–9.9 | 1.13 | 0.73–1.74 | 1.15 | 0.74–1.77 | |||
| ≥10 | 1.94 | 1.01–3.76 | 1.77 | 0.88–3.54 | |||
| Women: | <7 | 0.92 | 0.53–1.62 | 0.90 | 0.50–1.61 | ||
| 7–8.9 | 1.0 | Reference | 1.0 | Reference | |||
| 9–9.9 | 1.10 | 0.61–2.00 | 1.07 | 0.58–1.95 | |||
| ≥10 | 0.42 | 0.06–3.02 | 0.40 | 0.06–2.92 | |||
| Kripke 1979 (North America) Mortality Ratios | Men: | <4 | 2.8 | None reported | |||
| 4–4.9 | 1.59 | ||||||
| 5–5.9 | 1.38 | ||||||
| 6–6.9 | 1.11 | ||||||
| 7–7.9 | 1.0 | ||||||
| 8–8.9 | 1.10 | ||||||
| 9–9.9 | 1.29 | ||||||
| ≥10 | 1.77 | ||||||
| Women: | <4 | 1.48 | None reported | ||||
| 4–4.9 | 1.40 | ||||||
| 5–5.9 | 1.20 | ||||||
| 6–6.9 | 1.13 | ||||||
| 7–7.9 | 1.0 | ||||||
| 8–8.9 | 1.13 | ||||||
| 9–9.9 | 1.27 | ||||||
| ≥10 | 1.82 | ||||||
| Kripke 2002 (North America) (Mortality Hazard Ratios) | Men: | 3 | 1.19 | 0.96–1.47 | |||
| 4 | 1.17 | 1.06–1.28 | |||||
| 5 | 1.11 | 1.05–1.18 | |||||
| 6 | 1.08 | 1.04–1.11 | |||||
| 7 | 1.0 | Reference | |||||
| 8 | 1.12 | 1.09–1.15 | |||||
| 9 | 1.17 | 1.13–1.21 | |||||
| ≥10 | 1.34 | 1.28–1.40 | |||||
| Women: | 3 | 1.33 | 1.08–1.64 | ||||
| 4 | 1.11 | 1.01–1.22 | |||||
| 5 | 1.07 | 1.01–1.13 | |||||
| 6 | 1.07 | 1.03–1.11 | |||||
| 7 | 1.0 | Reference | |||||
| 8 | 1.13 | 1.09–1.16 | |||||
| 9 | 1.23 | 1.17–1.28 | |||||
| ≥10 | 1.41 | 1.34–1.50 | |||||
| Lan 2007 (Asia) | Men: | <7 | 0.97 | 0.76–1.23 | 0.98 | 0.76–1.25 | |
| 7–7.9 | 1.0 | Reference | 1.0 | Reference | |||
| 8–8.9 | 1.11 | 0.91–1.36 | 1.09 | 0.89–1.33 | |||
| 9–9.9 | 1.33 | 1.08–1.65 | 1.14 | 0.91–1.42 | |||
| ≥10 | 1.86 | 1.48–2.34 | 1.51 | 1.19–1.92 | |||
| Women: | <7 | ||||||
| 7–7.9 | |||||||
| 8–8.9 | |||||||
| 9–9.9 | |||||||
| ≥10 | |||||||
| Mallon 2002 (Europe) | Men: | <6 | 1.1 | 0.6–7.0 | |||
| 7–8 | 1.0 | Reference | |||||
| >8 | 2.0 | 1.2–3.2 | |||||
| Women: | <6 | 1.0 | 0.6–1.8 | ||||
| 7–8 | 1.0 | Reference | |||||
| >8 | 1.3 | 0.6–2.6 | |||||
| Patel 2004 (North America) | Women: | ≤5 | 1.41 | 1.25–1.58 | 1.08 | 0.96–1.22 | |
| 6 | 1.07 | 1.0–1.15 | 0.99 | 0.92–1.06 | |||
| 7 | 1.0 | Reference | 1.0 | Reference | |||
| 8 | 1.18 | 1.1–1.26 | 1.11 | 1.03–1.19 | |||
| ≥9 | 1.72 | 1.55–1.91 | 1.40 | 1.25–1.55 | |||
| Pollak 1990 (North America) | Men: | ≤4 | 0.24 | ||||
| 5 | 0.25 | ||||||
| 6 | 0.16 | ||||||
| 7 | 0.17 | ||||||
| 8 | 0.22 | ||||||
| ≥9 | 0.30 | ||||||
| Women: | ≤4 | 0.19 | |||||
| 5 | 0.07 | ||||||
| 6 | 0.14 | ||||||
| 7 | 0.08 | ||||||
| 8 | 0.13 | ||||||
| ≥9 | 0.18 | ||||||
| Qureshi 1997 (North America) | Stroke: | 6–8 | 1.0 | Reference | 1.0 | Reference | |
| <6 | 1.0 | 0.7–1.5 | 1.0 | 0.70–1.5 | |||
| >8 | 1.5 | 1.1–2.1 | 1.5 | 1.1–2.0 | |||
| CV Disease: | 6–8 | 1.0 | Reference | 1.0 | Reference | ||
| <6 | 1.3 | 0.9–1.7 | 1.3 | 1.0–1.8 | |||
| >8 | 1.2 | 0.9–1.6 | 1.2 | 0.8–1.5 | |||
| Ruigomez 1995 (Europe) | Men: | <7 | 1.06 | 0.61–1.83 | |||
| 7–9 | 1.0 | Reference | |||||
| >9 | 1.30 | 0.71–2.38 | |||||
| Women: | <7 | 0.66 | 0.37–1.16 | ||||
| 7–9 | 1.0 | Reference | |||||
| >9 | 1.46 | 0.79–2.70 | |||||
| Rumble 1992 (Europe) | Men & Women: | <4 | 1.12 | 0.47–2.69 | |||
| ≥10 | 1.60 | 0.74–3.47 | |||||
| Shankar 2008 (Asia) | Men: | ≤5 | 1.7 | 1.35–2.15 | |||
| 6 | 1.20 | 0.99–1.45 | |||||
| 7 | 1.0 | Reference | |||||
| 8 | 1.1 | 0.92–1.32 | |||||
| ≥9 | 1.88 | 1.48–2.40 | |||||
| Women: | <5 | 1.43 | 1.09–1.88 | ||||
| 6 | 1.04 | 0.82–1.31 | |||||
| 7 | 1.0 | Reference | |||||
| 8 | 1.15 | 0.92–1.44 | |||||
| ≥9 | 1.67 | 1.24–2.27 | |||||
| Suzuki 2008 (Asia) | Men: | <7 | 1.03 | 0.97–1.09 | |||
| 7–8 | 1.0 | Reference | |||||
| ≥9 | 1.32 | 1.26–1.40 | |||||
| Women: | <7 | 0.99 | 0.94–1.05 | ||||
| 7–8 | 1.0 | Reference | |||||
| ≥9 | 1.42 | 1.22–1.52 | |||||
| Tamakoshi 2004 (Asia) | Men: | ≤4 | 1.62 | 1.26–2.09 | 0.88 | 0.44–1.78 | |
| 5 | 1.16 | 1.01–1.33 | 1.07 | 0.83–1.38 | |||
| 6 | 1.09 | 1.0–1.19 | 1.11 | 0.95–1.28 | |||
| 7 | 1.0 | Reference | 1.0 | Reference | |||
| 8 | 1.11 | 1.05–1.19 | 1.19 | 1.07–1.32 | |||
| 9 | 1.26 | 1.15–1.37 | 1.27 | 1.08–1.48 | |||
| ≥10 | 1.73 | 1.58–1.90 | 1.75 | 1.46–2.09 | |||
| Women: | ≤4 | 1.60 | 1.28–2.02 | 1.83 | 1.20–2.81 | ||
| 5 | 1.14 | 0.99–1.31 | 1.18 | 0.90–1.53 | |||
| 6 | 1.05 | 0.96–1.15 | 1.17 | 0.99–1.39 | |||
| 7 | 1.0 | Reference | 1.0 | Reference | |||
| 8 | 1.23 | 1.14–1.33 | 1.35 | 1.17–1.56 | |||
| 9 | 1.35 | 1.20–1.51 | 1.57 | 1.26–1.96 | |||
| ≥10 | 1.92 | 1.70–2.17 | 2.12 | 1.67–2.68 | |||
| Tsubono 1993 (Asia) | Men & Women: | ≤6 | 1.26 | 0.81–1.97 | |||
| 7–8 | 1.0 | Reference | |||||
| >9 | 1.58 | 1.16–2.15 | |||||
| Wingard 1982 (North America) | Men & Women: | ≤6 or ≥9 | 1.13 | 1.1–1.7 | |||
| 7–8 | 1.0 | Reference | |||||
| Men: | ≤6 or ≥9 | 0.14 | |||||
| 7–8 | 0.08 | ||||||
| Women: | ≤6 or ≥9 | 0.09 | |||||
| 7–8 | 0.06 | ||||||
| Wingard 1983 (North America) | Men: | ≤6 | RR=1.3e | 14.8 | |||
| 7–8 | 8.2 | ||||||
| ≥9 | 11.1 | ||||||
| Women: | ≤6 | 9.0 | |||||
| 7–8 | 5.6 | ||||||
| ≥9 | 8.5 | ||||||
risk was separately calculated for nighttime sleep (ngt) and total sleep across 24 hours (24h)
risk reported at two time points (Time 1) and (Time 2) followed by risk associated with change in sleep duration from baseline (Change): no change (No chg), increase from 5–6 hours (Incr. 5–6), increase from 7–8h (Incr. 7–8), or decrease from 6, 7 or 8 hours (Decr. 6–8)
risk was calculated for the total sample (total), as well as separately for those aged <60 years (Age <60) and those 60 or older (Age ≥60)
rates were separately calculated for 9 age groups; those presented are means weighted based on number in each age group
relative risk for males & females not sleeping 7–8 hrs= 1.3 (p=0.04)
Note: risks reported in bold font represent statistically significant risks
Early Studies
The first study to examine the relationship between sleep duration and mortality risk in the population was reported in 19643. Those reporting 7 hours of sleep demonstrated the lowest mortality rate. A follow-up analysis of the same sample4 found that 7-hour sleepers were at the lowest risk, and those at the extreme ends (≤4 and ≥10 hours) were at the highest for both men and women.
A series of subsequent investigations analyzed data from adults from the Alameda County Study5–9. Mortality rates were calculated for a 9-year follow-up period adjusted for up to 14 covariates in addition to age. These studies reported that short (≤6 hours) and long (≥9 hours) sleepers had higher mortality rates than 7–8hr sleepers, even after adjustment for covariates5–9. In a later analysis, Kaplan and colleagues8 analyzed age groups separately with a 17 year follow-up. In this analysis, a significant increase in mortality for the <7hr and >8hr sleepers was only present for those aged 50–59 at the time of follow-up.
Studies in the Elderly
In general, studies that have focused on elderly populations have supported findings from earlier research. Branch and Jette10 observed findings similar to the Alameda County Study. Among the elderly, both short (<7 hours) and long (>8 hours) sleepers were at increased mortality risk. Another study in a sample of nursing home residents found that age-adjusted mortality rates were highest in women sleeping ≤4 hours and ≥9 hours and men sleeping ≤5 hours and ≥9 hours11. However, these risks became non-significant after adjusting for covariates. In a study of the elderly in the UK, significant increased mortality risk was found for those reporting ≥10 hours of sleep, and for those who reported “insomnia often.12” However, this study did not account for gender and the reference group had a very broad range of sleep duration (4.0–9.9 hours). Gale and Martyn13 examined data from adults aged ≥65 years in the UK and found that, compared to 9-hour sleepers, those sleeping ≥10 hours were at increased risk of mortality. Conversely, analyses of health survey data from Spain (Barcelona) did not find significantly increased risk in elderly short or long sleepers, compared to the reference group (7–9 hours), though a non-significant increase for long sleep was observed14.
While there is some variation, taken together, these results reveal that in the elderly, increased mortality is associated with both short and long sleep.
Sleep and Mortality: A Global Issue
Following earlier studies, scientists have explored this association around the world. Several of these studies occurred in Japan15–19. One study found increased mortality risk in those reporting sleep ≥9 hours, as compared to 7–8 hours in the farming town of Wakuya15. An analysis of the general population of Gifu prefecture aged 20–67 found that in age-adjusted analyses, increased mortality risk was associated with short (<7 hours) and long ( 10 hours) sleep compared to the reference group (7.0–8.9 hours), but after adjusting for covariates, only the short sleep association remained significant16. In a study of elderly residents from the village of Ohgimi, increased mortality was seen among those sleeping <6 and >7 hours in a model adjusted for age and health behaviors, but when physiological measures of health and functional status were entered into the model, only the relationship with short sleep remained statistically significant17.
More recently, two large-scale studies have examined these relationships in samples representative of the general population of Japan. In one study18, compared to the reference group (7.0–7.9 hours), men were at increased risk of mortality if they reported <6 hours of sleep, and women were at increased risk if they reported ≥9 hours. The largest sleep duration and mortality study in Japan investigated over 100,000 adults from 45 areas of Japan19. Compared to the reference group (7.0–7.9 hours), both men and women were at increased risk of mortality of they slept 8.0–8.9 hours or ≥9 hours on average. Further analysis of this dataset20 reported that in men, ≥9 hours of sleep was associated with increased risks of all-cause mortality, cardiovascular mortality, esophageal cancer, and pancreatic cancer, but decreased risk of prostate cancer; <7 hours of sleep was associated with a decreased risk of stomach cancer. In women, ≥9 hours of sleep was associated with increased all-cause mortality, cardiovascular mortality, urothelial cancer and non-Hodgkins lymphoma; <7 hours of sleep was associated with a decreased risk of lung cancer and cardiovascular disease.
A 10-year study of adults over 60 in Taiwan21 found increased mortality risk (relative to 7 hours) associated with ≥10 hours of sleep in men, and all categories ≥8.0 hours of sleep in women. Analysis of the Singapore Chinese Health Study of ethnic Cantonese and Hokkien adults aged 45–74 found significant increases in mortality risk (relative to 7 hours) associated with ≤5, 6, 8, and ≥9 hours, though after adjusting for covariates, only the relationships in the most extreme categories remained significant22.
In Israel, Burazeri and colleagues23 examined data from adults ≥50 years old. Adjusted analyses found that in men, increased mortality risk was associated with sleep >8 hours, compared to a 6hr reference group. For women, mortality risk was lower for those reporting 6–8 hours of 24-hour sleep, compared to those sleeping <6 hours.
In Sweden, an analysis of survey data from adults aged 45–65 years found that age-adjusted mortality risk was increased in male long sleepers (>8 hours) compared to the reference group (6–8 hours), though this result became non-significant after adjusting for all covariates24. No relationships were seen for women.
In Finland, sleep duration data for adults (age 24–101) comprising all twins born before 1958 was analyzed25. When compared to the reference group (7–8 hours), increased mortality risk was found for men and women who reported <7 or >8 hours of sleep.
In the United Kingdom, three studies have investigated non-elderly, general population samples26–28. Huppert and Whittington26 found that sleep duration was not related to mortality in women, but long sleep duration (>8 hours) predicted mortality in men. Heslop and colleagues27 examined a sample representing the general population of Glasgow, Scotland. This study investigated sleep duration at multiple times. Women reporting <7 hours at second screening were at increased risk of all-cause and cardiovascular mortality when adjusting for covariates, but this relationship became non-significant when stress level was introduced in the model. For men, age-adjusted analyses showed increased risk in those who were <7hr sleepers at both time points compared to those 7–8hr sleepers at both time points, but this became non-significant when marital status and social class were included. Men and women who were always <7hr sleepers demonstrated increased risk for all-cause mortality in models adjusting for age, marital status, social class and health risk factors. Inclusion of stress level significantly attenuated the short sleep-mortality association to the null.
Other studies have also measured sleep duration at multiple points in time. Ferrie and colleagues28 longitudinally examined mortality risk for sleep durations measured during two time periods. The data came from the ongoing Whitehall II cohort. For the first time period, only ≤5 hours of sleep was associated with increased all-cause and cardiovascular mortality risk in adjusted analyses, and only in models that adjusted only for age. For the second time period, increased all-cause mortality was associated with ≤5 or ≥9 hours of sleep and increased cardiovascular mortality was associated with ≤5 hours of sleep. Those reporting having slept 7–8 hours at baseline were protected if they remained in that category at the second time period. Interestingly, mortality risk increased if they deviated above or below that duration category. Those reporting 6 hours of sleep benefitted from an increase to 7 hours, but not 8 hours. Subjects reporting ≤5 hours were subject to decreased mortality risk if they reported 6 hours at the second time point.
Finally, there have been several studies in U.S. population samples29–35. Qureshi and colleagues29 examined data from the National Health and Nutrition Examination Survey (NHANES) and found an increased risk of mortality in long sleepers (>8 hours). Chen and Foley found that mortality was associated with <6 or >9 hours of sleep, but only in those over 60 years of age36. A recent reanalysis of the NHANES data35 found that even after controlling for a variety of covariates, short sleep of ≤5 hours, as well as longer sleep of 8 and ≥9 hours, was associated with increased mortality, relative to 7 hours. However, analysis of age sub-groups (<60 versus >60 years), revealed that only subjects aged >60 demonstrated showed a significant mortality relationship with short and long sleep duration.
Data from the well-known cohorts of the Framingham Study30 and Nurses’ Health Study32, 34 also support the elevated mortality risks associated with short and long sleep. The former study found the highest mortality risk in subjects reporting <6 hours and >9 hours. The latter reported increased all-cause age-adjusted mortality risk (relative to the 7 hours) in all short (≤5 and 6 hours) and long (8 and ≥9) groups.34 Ayas and colleagues32 also examined this dataset in another publication and found similar results.
Dew and colleagues33 reviewed data from several studies to conduct the first electroencephalographic study of sleep and mortality risk. In their sample of 184 individuals, short sleepers (<6 hours) did not have significantly higher mortality risks than the rest of the sample. However, increased mortality risk was associated with sleep latency >30 minutes, sleep efficiency <80%, and slow wave sleep <1%.
Kripke and colleagues conducted the largest study of sleep duration and mortality using over 1 million records from the Cancer Prevention Study II31. After adjustment for demographic risk factors, health habits, status, history, and medications, they found distinct U-shaped curves across sleep duration categories, demonstrating increased mortality risk the further the deviation from 7 hours. Thus, those who reported sleep of 7 hours (6.5–7.4 hours before coding) were at the lowest risk of mortality and those sleeping <6.5 or >7.4 hours were at increasingly greater risk, the further from the nadir of the U curve. Depression and stress were not included in these analyses.
Meta-Analysis
Gallicchio and Kalesan37 present the first meta-analysis of data from the studies described above (see Table 1 for included studies). Using random-effects meta-analysis, the authors report that the pooled RR for all-cause mortality for short sleep was 1.10 (95%CI=[1.06, 1.15]), with cardiovascular-related RR at 1.06 (95%CI=[0.94,1.30]) and cancer-related RR at 0.99 (95%CI=[0.88,1.13]). For long sleep, RR for all-cause mortality was 1.23 (95%CI=[1.16,1.30]), with cardiovascular RR at 1.38 (95%CI=[1.13,1.69]) and cancer RR at 1.21 (95%CI=[1.11,1.32]). Thus, while short sleep carries an increased mortality risk, this is not explained by cardiovascular disease or cancer. For long sleep, the increased risk was significant for all-cause, cancer, and cardiovascular mortality. The methodology of this study has been reviewed elsewhere38. Although there are a number of limitations to the included studies (described below), the meta-analysis meets many of the guidelines proposed by the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) Working Group39. The pooled relative risks reported in this paper are small, but even small shifts in mean effects are of significant population value40.
Limitations of previous epidemiological studies
Despite the valuable contributions of prior research, there are several limitations of this literature. One important limitation of prior epidemiological studies is that definitions of “short” and “long” sleep vary across studies, thus preventing adequate comparisons. For example, while the study by Kripke and colleagues31 contained groups representing every hour of duration within the sample, the study by Rumble and Morgan12 measured only three groups, with the reference group reporting 4–9.9 hours of sleep. Additionally, while the various survey questions used in these studies may have face validity, it is unclear that they are reliable and valid measures of sleep duration. These surveys employed questions which have not been validated against objective sleep recordings; thus, it is unclear what precisely they are measuring. It may be the case that factors such as time in bed, demand characteristics, social desirability, paradoxical insomnia (i.e., sleep state misperception), and other factors covary with these self reports of sleep duration.
Another important source of heterogeneity is the inclusion of covariates. Many studies found relationships only when adjusting for age and in most cases, gender. This suggests that there is an age (or cohort) effect, and a difference between men and women. Other covariates, including indicators of health status and history, sociodemographic and socioeconomic factors, functioning, medication use, and psychological morbidity, varied between studies, with a range of 03, 7-3231 covariates besides age. Both under adjustment and over adjustment present concerns. Not adjusting for enough variables could mean that psome relationships are driven by third factors, and not by sleep duration. Over adjusting may remove some of the causal effects of sleep, if the covariate is on the causal pathway between sleep and mortality.
Also, the stability of sleep as a trait in relation to health outcomes has not been established. The studies that only measure sleep at one time point assume that self-reported sleep duration at one time represents a stable exposure.
Finally, the majority of studies reviewed in this paper use datasets that were not designed to evaluate relationships with sleep. Frequently, analyses are constrained to use the only sleep question available in the survey (usually an estimate of habitual sleep duration), which limits the reliability and validity of the findings.
SLEEP DURATION AND MORTALITY: THE POSSIBLE MECHANISMS
While previous literature clearly describes an association between mortality and both short and long sleep, the remainder of this review will focus on mechanisms and pathways primarily associated with only short sleep, as short and long sleepers seem to represent distinct groups41. Effects for long sleep may be larger, but we chose to focus on short sleep for two reasons. First, shorter sleep appears to be a more salient issue in our society where insufficient sleep is a major public health concern42. Furthermore, large numbers of laboratory studies support mechanisms that may explain a direct link between short sleep and mortality. Thus, short sleep is a more wide-reaching problem with a larger base of research from which to draw conclusions. Second, a fairly comprehensive review of mortality associated with long sleep, and possible mechanisms, was recently published1. We wished to avoid redundancy with that publication, taking advantage of limited space by only addressing short sleep..
While prior research has broadly investigated the relationship between short sleep duration and mortality risk, any association is unlikely to be exclusive or singularly present. Furthermore, it is possible that some or all conditions may be antecedent (but undetected) to the development of short sleep duration. Finally, as described earlier, the link between sleep duration and various conditions may be a result of a common underlying mechanism, leading to an inflated association. Notwithstanding, short sleep duration has been linked with 7 of the 15 leading causes of death in the US (cardiovascular disease, malignant neoplasm, cerebrovascular disease, accidents, diabetes, septicemia, and hypertension)43.
Short Sleep & Cardiovascular Disease
Cardiovascular disease leads as the top cause of death in the United States43. Several18–19, 22, 28, 32, 44–45, but by no means all21, 23–24, 27, 29, 31, 34 population-based studies report increased risk of cardiovascular disease or mortality for short sleep duration in men and/or women from various continents (e.g., North America, Asia, and Europe). Most adjusted for potential confounders including socio-demographic, socio-economic and health factors. All employed a self-reported measure of sleep duration. More recent studies have linked short sleep duration with carotid artery intima-media thickness (a marker of atherosclerotic disease)46 and coronary artery calcification (a risk factor for coronary events)47. Sleep was measured using actigraphy in the latter.
Two prospective studies failed to show consistent independent associations between short sleep duration and stroke29, 48. Other important cardiovascular factors that have been investigated for their associations with short sleep duration include hyperlipidemia and hypertension. Data are suggestive of a correlation between unfavorable triglyceride and high-density lipoprotein profiles in female short sleepers49 (<6 hours) and people with diabetes50.
Two cross-sectional studies suggest ≥60% increased likelihood of hypertension in short sleepers51–52. Longitudinal data support this relationship, but it is unknown if this will be consistently independent of confounders52–53 and whether there are gender-specific associations52. Laboratory data support shorter-term effects of sleep deprivation on blood pressure and sympathetic activity in normotensive54 and hypertensive subjects55. Short sleep may act as a stressor in the acute and chronic setting. The downstream consequences may lead to elevated blood pressure equilibrium.
Emerging work investigating the mechanisms linking sleep deprivation and cardiovascular outcomes has included biomarkers. C-reactive protein (CRP), an acute phase reactant, is a marker of cardiovascular risk56. Elevated CRP concentrations have been reported in healthy subjects exposed to total and partial short-term sleep deprivation57–58. Cytokines (especially interleukin-6 and tumor necrosis factor alpha) levels are also elevated in response to acute total and partial sleep restriction59. It appears, therefore, that sleep deprivation, at least in the acute setting, initiates a response akin to inflammation as evidenced by biomarkers such as CRP and cytokines. It has been suggested that CRP may be a link between sleep restriction and cardiovascular disease60; however, it remains unclear whether CRP is a risk factor or predictor for cardiovascular disease. Further, Taheri and colleagues61 recently reported that there was no relationship between CRP levels and sleep duration in the Wisconsin Sleep Cohort Study. Therefore, it is challenging on several counts to deduce that CRP accounts for the link between chronic short sleep and cardiovascular disease.
It is important to note that the meta-analysis found no significant risk of cardiovascular mortality associated with short sleep37. While we feel that the meta-analysis represents a significant contribution to this literature, there are many reasons why there may have been no significant effect. For example, the measurement of short sleep is imprecise. Second, many of the proposed mechanisms for cardiovascular pathways were not directly assessed in mortality studies. Third, the meta-analysis did not include enough studies, with appropriate covariates, to definitively rule out cardiovascular causes of death.
In summary, while a variety of cardiovascular outcomes have been investigated, results showing independently increased cardiovascular risks for subjects reporting short sleep are inconsistent.
Short Sleep & Obesity
Significant public, media, and scientific attention has focused on the link between short sleep and obesity62. Recent reviews and meta-analyses63–64 have assessed this link in great detail. Cross-sectional (19 adult, 11 pediatric) and longitudinal (5 adult, 4 pediatric) studies have investigated the short sleep-obesity association (references in cited reviews). In adults, most studies report relationships between short sleep and obesity. The association appears to more uniform in the pediatric population. Interestingly, the nature of the relationship may differ between adults and children, with a U-shaped association in adults compared with a negative linear relationship in children. It may be challenging to expose children to sleep in excess of need on account of their increased need for longer sleep times during development and less variability in individual sleep need during childhood and adolescence. Additionally, societal constraints (e.g. school) curtail sleep opportunity.
The mechanisms supportive of the obesogenic effects of short sleep are several, including upregulation of appetite, increased time to eat, lower energy expenditure, and altered glucose metabolism. The relationship between short sleep and reduced leptin and elevated ghrelin has been observed in both epidemiological and laboratory studies65–66. Such perturbations in leptin and ghrelin profiles may culminate in a powerful stimulus to food intake which can ultimately lead to obesity66. Other metabolic hormones have also been implicated in the short sleep-weight gain association including cortisol, insulin, and growth hormone (see detailed review67).
Short Sleep and Obstructive Sleep Apnea
Sleep apnea may partially explain the relationship between short sleep duration and mortality. This disorder is associated with sleep disruption and is associated with increased mortality risk68–70. However, it is largely unaddressed by the current studies. Several researchers have suggested that the relationship between long sleep duration and mortality risk may be the result of patients with sleep apnea compensating for the large degree of fragmentation associated with the disorder by spending more time asleep or excessive amounts of time in bed1. Conversely, the fragmentation associated with sleep apnea may lead individuals to report less overall sleep71. Thus, it may be that mortality is related to variables associated with sleep apnea, rather than long or short sleep per se. However, there is not sufficient evidence to suggest that those with sleep apnea sleep more or less than the average72. Additionally, many predictors of sleep apnea, including age, gender, and body mass index, were controlled for in some of the epidemiological studies. Thus, it is possible that the relationship between sleep duration and mortality may overlap somewhat with that of sleep apnea and mortality, but it is unlikely that these relationships are collinear.
Short Sleep & Diabetes
Nearly all of the epidemiologic (5 longitudinal73–77 and 2 cross-sectional78–79) and laboratory studies80 to date have reported associations between habitual/imposed short sleep and risk of diabetes. Furthermore, glycemic control appears to be worse in people with diabetes who report poorer subjective sleep quality and short sleep duration79. Formal intervention studies are required to assess sleep’s role in the genesis and course of diabetes in the longer term. Of note, significant attenuation of the relationship has been observed upon including BMI and/or hypertension in the multi-variable models, indicating that a significant proportion of the link between short sleep and diabetes risk could be explained by such comorbid risks or conditions.
Short Sleep & Physiologic Stress
Stress affects our sleep and, conversely, sleep deprivation is itself a stressor. Investigators have demonstrated that sleep deprivation or restriction can increase the activity of the neuroendocrine stress systems (hypothalamic-pituitary-adrenal axis and autonomic sympatho-adrenal axis) and also the reactivity of these systems to downstream stressors80. Chronic activation of these systems, and the consequent perturbation in the regulation of serum glucocorticoid levels, may lead to disease, neuronal damage, and earlier aging81.
Short Sleep & Immunity
The impact of sleep deprivation on the immune system has been challenging to study. It is exceedingly difficult to isolate effects of sleep as an independent variable as physiologic processes change relative to wakefulness82. Parameters of cellular and humoral immune systems have been investigated for their associations with differing durations of sleep deprivation. Results have been inconsistent and at times contradictory. A recent analysis of 153 adults found that those who slept <7 hours/night trended towards increased likelihood to develop a cold after rhinovirus exposure compared to those who slept 8 hours (OR=2.94[95%CI=1.18–7.30])83. Interestingly, immune incompetence has been reported with sub-optimal antibody titer responses following vaccination amidst periods of sleep deprivation82. Finally, correlative evidence supports a significant association between NREM sleep and recovery from infection82.
Short Sleep & Socioeconomic Status
A growing interest in the socioeconomic disparity in sleep has emerged with investigators examining the influence of factors such as income, poverty, education, and employment upon sleep duration and/or sleep quality84–86. Findings have been broadly similar: disadvantaged groups have higher likelihood of less/poorer sleep. The challenges of interpreting these associations are similar to other dilemmas in the SES-health literature: First, the influence of socioeconomic status upon health occurs throughout the life-course. Second, this influence depends on the life stage. Third, SES is challenging to measure. Fourth, cross-sectional analyses hinder the ability to discern the direction of the association. It is conceivable that sleep curtailment may lead to socioeconomic disparity which, in and of itself, confers increased risk for mortality87. However, it may be more plausible that socioeconomic status is a proximal cause of short sleep. This would suggest that sleep could be an innocent bystander-- a mediator, moderator or confounder, or it could be in the causal pathway of the SES-mortality relationship. Thus, investigators have begun to examine the influence of sleep quality and sleep duration on the SES-health gradient88–89.
SLEEP DURATION AND MORTALITY: THE POTENTIAL PATHWAYS
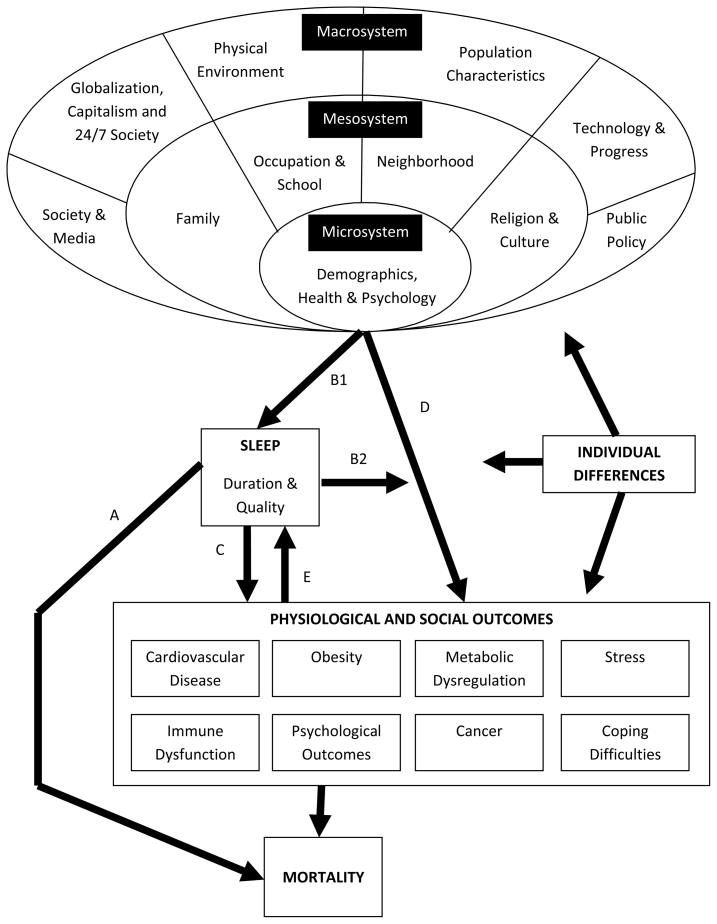
In Figure 1, we present a social-ecological model90 of the determinants of sleep. This model allows for multiple levels of effects, namely the microsystem, the mesosystem, and macrosystem. Specific components of each layer are listed in detail in the diagram, and range from individual characteristics and health behaviors in the microsystem, school and work environments in the mesosystem, and policy and physical environments in the macrosystem. This theoretical pathway has considerable implications for policies and interventions. It suggests that in order to reduce sleep-related mortality, interventions should occur at the individual, social, environmental, and policy levels. Here we introduce the social-ecological model and explore five theoretical pathways through which the relationships between sleep and mortality may be observed. The five pathways are outlined on Figure 1 with letters A-E:
Figure 1. Model for socioecological influences upon sleep duration/behavior and associated outcomes.
A – Direct causal relation between sleep duration and mortality
B1 & B2 – Intermediary role of sleep (mediator and moderator roles)
C – Cause of outcomes that, in turn, confer increased mortality
D – Linked with phenomena that are associated with increased mortality
A: Short Sleep Causes Mortality Directly
The pathway (A) in which short sleep causes mortality directly provides the simplest model – a direct relationship between short sleep and mortality. Evidence from animal models that support this pathway show that sleep deprivation in rats leads to death within 2 weeks91. As described in many of the studies above, epidemiologic data support this pathway with large-scale analyses that adjust for a variety of confounders and still finding that short sleep duration is associated with increased mortality19, 31. This crude depiction suggests that merely altering (e.g., extending) sleep is sufficient to alter mortality risk, yet it oversimplifies the problem. Four more realistic and complex pathways are described below, all of which need to be considered when translating the widely observed relationship between short sleep and mortality into clinical and population-based interventions.
B: Short Sleep is a Mediating or Moderating Factor
In the second potential pathway (B1 and 2), short sleep duration is a mediating (B1) and/or moderating (B2) factor. This perspective provides a more contextual picture of the observed relationship between short sleep duration and mortality. That is, short sleep may result from variety of social, environmental, and physiological changes that lead to increased mortality risk. This pathway is supported by literature examining social determinants of health92–93. The same populations that are at risk for poor health are also at risk for poor sleep93. Those who live in urban environments, are racial minorities, or have low levels of education are generally more likely to be short sleepers93–94. In Pathways B1 and B2, short sleep duration is a behavioral intermediary along the pathway of increased morbidity and mortality88–89, 93.
C: Short Sleep is Mediated or Moderated by Social and Physiologic Factor(s)
Pathway (C) indicates short sleep itself causes physiological and social outcomes that may lead to increased mortality. Again, the challenge with this pathway is that the array of physiological and social outcomes is large, and it is not clear which are the most relevant mechanisms to understanding the sleep/mortality relationship. Some of these potential pathways (e.g. cardiovascular, immune, obesity) are discussed above. For example, short sleep may lead to hormonal and behavioral patterns that are conducive to weight gain or reduced productivity that then lead individuals on a pathway toward heightened mortality risk. These examples show how the effects of short sleep on health may be mediated or moderated by the social determinants of health95.
D: Short Sleep is Associated with Other Characteristics Causally Linked to Mortality
The fourth potential pathway (D) is one in which short sleep is associated with other characteristics causally linked to mortality but is not itself related to mortality. An example here is age, which is independently associated with mortality (i.e., after surviving early life, the risk of mortality increases with age). Also, sleep duration decreases as one ages96, yet the role of short sleep in explaining the high mortality rates among the elderly compared to younger people is a very minor one at best. Statistically, a common strategy to accommodate these types of pathways is to use multivariate models to adjust for confounding factors such as age when looking for a correlation between sleep and mortality. This effectively removes the effect of the D type pathways. Unfortunately, not all characteristics that follow along the D pathway are as easily observable as age, and consequently it is difficult to adjust for their effects. Thus, when discussing the relationship between sleep and mortality, we must consider that other factors may be driving both outcomes.
E: Characteristics that Lead to Mortality Lead to Short Sleep
The final pathway (E), sometimes referred to as reverse causality, is shown by the line labeled E. In this pathway, the same characteristics that eventually lead to increased mortality may also be causally associated with shortened sleep. While it is important to discern the roles of intermediate variables, this can be very challenging statistically. Reverse causality is best studied in very controlled experimental environments or prospective studies where causal sequencing can be readily observed.
SLEEP DURATION AND MORTALITY: THE FUTURE
Many questions remain regarding the sleep-mortality relationship. A better understanding the physiological/psychosocial connections between amount of sleep and shortened lifespan is critical. Simply documenting this relationship is no longer sufficient – we must explore possible mechanisms and pathways as well develop targeted interventions that can positively alter this relationship. We propose several directions for the future:
First, research programs should investigate long and short sleep separately, as both are associated with increased mortality risk but the pathways may vary41. Thus, increased research needs to phenotype habitual long and short sleepers, to better characterize these groups in terms of prospective and/or objective sleep, psychological functioning, performance, and health status, including obesity, cardiovascular functioning, glucose tolerance, etc. Further, sleep quality should be considered more carefully in addition to simply sleep duration. Sleep duration alone is an insufficient measure for characterizing sleep. For example, short sleep is not the same as sleep insufficiency. Studying sleep disturbance, alone and with sleep duration, will better elucidate the public health implications of sleep.
Second, potential mechanisms and pathways to mortality should be explored and clarified. We present a social-ecological model of sleep and mortality, which focuses on a number of potential factors that influence sleep duration at the micro-, meso- and macrosystem level, as well as ways in which the resultant sleep duration (and quality) may impact physiological and social outcomes which may be related to mortality. While this paper presents some evidence for some of these pathways, they need to be explored in greater detail. For example, studies exploring social determinants of sleep are necessary. Studies of attitudes and beliefs about sleep, passed through society, culture and family, will help clarify the components of a social-ecological model of sleep. Additionally, studies of health outcomes such as obesity, cardiovascular disease, and metabolic dysregulation, associated with habitual sleep parameters (verified with objective methods) will clarify which health outcomes are truly associated with habitual sleep.
Third, individual variation in sleep duration needs must be better understood. For some, 7 hours is insufficient, and for some, 7 hours is excessive. There has been little consideration of individual differences regarding sleep duration preferences and how these preferences are related to health outcomes. Current investigations into individual differences associated with susceptibility to sleep loss97 are beginning to clarify this issue.
Fourth, community-based intervention studies are needed to better understand mechanisms that underlie this relationship and reduce mortality risk. For example, one intervention could include increasing sleep time to prevent obesity. We are not aware of any intervention studies that show weight loss in response to changes in sleep behavior, although current evidence has fueled lively discussions40.
PRACTICE POINTS
Sleep duration and mortality are related in a U-shaped fashion with the lowest risk being about 7–8 hours and increasing risk associated with more or less sleep. This relationship holds true across the adult lifespan, various geographic regions, and with the inclusion of a variety of covariates.
Short sleep is related to cardiovascular disease, cancer, cerebrovascular accidents, diabetes, and hypertension. These associations may occur via metabolic or inflammatory processes, physiologic stress, or socioeconomic factors.
There are several pathways by which short sleep may be related to mortality: sleep may directly cause mortality, it may mediate/moderate a relationship, or may be unrelated to mortality but related to a variable that is responsible for the correlation.
A number of factors likely influence sleep duration at the micro-, meso-, and macro-level of the social-ecological model.
RESEARCH AGENDA
Examine short sleep and long sleep as separate pathways toward mortality risk. Also, include poor sleep as a factor that may influence this relationship.
Explore possible mechanisms and pathways of the sleep/mortality relationship. Cardiovascular disease, obesity, metabolic dysregulation, stress, immune dysfunction, psychological outcomes, cancer and coping difficulties may play a role. Also, determinants of sleep duration may include aspects of the social-ecological model, including micro-, meso- and macro-system.
Better understand the role of individual differences in the relationship between sleep duration and health outcomes in general, and mortality specifically.
Develop and evaluate community-based interventions that target aspects of these pathways and may reduce population-level mortality risk.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grandner MA, Drummond SP. Who are the long sleepers? Towards an understanding of the mortality relationship. Sleep Med Rev. 2007 Oct;11(5):341–360. doi: 10.1016/j.smrv.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Youngstedt SD, Kripke DF. Long sleep and mortality: rationale for sleep restriction. Sleep Med Rev. 2004 Jun;8(3):159–174. doi: 10.1016/j.smrv.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Hammond EC. Some Preliminary Findings on Physical Complaints from a Prospective Study of 1,064,004 Men and Women. Am J Public Health Nations Health. 1964 Jan;54:11–23. doi: 10.2105/ajph.54.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4*.Kripke DF, Simons RN, Garfinkel L, Hammond EC. Short and long sleep and sleeping pills. Is increased mortality associated? Arch Gen Psychiatry. 1979 Jan;36(1):103–116. doi: 10.1001/archpsyc.1979.01780010109014. [DOI] [PubMed] [Google Scholar]
- 5.Wingard DL, Berkman LF. Mortality risk associated with sleeping patterns among adults. Sleep. 1983;6(2):102–107. doi: 10.1093/sleep/6.2.102. [DOI] [PubMed] [Google Scholar]
- 6.Wingard DL, Berkman LF, Brand RJ. A multivariate analysis of health-related practices: a nine-year mortality follow-up of the Alameda County Study. Am J Epidemiol. 1982 Nov;116(5):765–775. doi: 10.1093/oxfordjournals.aje.a113466. [DOI] [PubMed] [Google Scholar]
- 7.Breslow L, Enstrom JE. Persistence of health habits and their relationship to mortality. Preventice Medicine. 1980;9:469–483. doi: 10.1016/0091-7435(80)90042-0. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan GA, Seeman TE, Cohen RD, Knudsen LP, Guralnik J. Mortality among the elderly in the Alameda County Study: behavioral and demographic risk factors. Am J Public Health. 1987 Mar;77(3):307–312. doi: 10.2105/ajph.77.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belloc NB. Relationship of health practices and mortality. Prev Med. 1973 Mar;2(1):67–81. doi: 10.1016/0091-7435(73)90009-1. [DOI] [PubMed] [Google Scholar]
- 10.Branch LG, Jette AM. Personal health practices and mortality among the elderly. Am J Public Health. 1984 Oct;74(10):1126–1129. doi: 10.2105/ajph.74.10.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pollak CP, Perlick D, Linsner JP, Wenston J, Hsieh F. Sleep problems in the community elderly as predictors of death and nursing home placement. J Community Health. 1990 Apr;15(2):123–135. doi: 10.1007/BF01321316. [DOI] [PubMed] [Google Scholar]
- 12.Rumble R, Morgan K. Hypnotics, sleep, and mortality in elderly people. J Am Geriatr Soc. 1992 Aug;40(8):787–791. doi: 10.1111/j.1532-5415.1992.tb01850.x. [DOI] [PubMed] [Google Scholar]
- 13.Gale C, Martyn C. Larks and owls and health, wealth, and wisdom. BMJ. 1998 Dec 19–26;317(7174):1675–1677. doi: 10.1136/bmj.317.7174.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruigomez A, Alonso J, Anto JM. Relationship of health behaviours to five-year mortality in an elderly cohort. Age Ageing. 1995 Mar;24(2):113–119. doi: 10.1093/ageing/24.2.113. [DOI] [PubMed] [Google Scholar]
- 15.Tsubono Y, Fukao A, Hisamichi S. Health practices and mortality in a rural Japanese population. Tohoku J Exp Med. 1993 Dec;171(4):339–348. doi: 10.1620/tjem.171.339. [DOI] [PubMed] [Google Scholar]
- 16.Kojima M, Wakai K, Kawamura T, et al. Sleep patterns and total mortality: a 12–year follow-up study in Japan. J Epidemiol. 2000 Mar;10(2):87–93. doi: 10.2188/jea.10.87. [DOI] [PubMed] [Google Scholar]
- 17.Goto A, Yasumura S, Nishise Y, Sakihara S. Association of health behavior and social role with total mortality among Japanese elders in Okinawa, Japan. Aging Clin Exp Res. 2003 Dec;15(6):443–450. doi: 10.1007/BF03327366. [DOI] [PubMed] [Google Scholar]
- 18.Amagai Y, Ishikawa S, Gotoh T, et al. Sleep duration and mortality in Japan: the Jichi Medical School Cohort Study. Journal of epidemiology/Japan Epidemiological Association. 2004 Jul;14(4):124–128. doi: 10.2188/jea.14.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19*.Tamakoshi A, Ohno Y. Self-reported sleep duration as a predictor of all-cause mortality: results from the JACC study, Japan. Sleep. 2004 Feb 1;27(1):51–54. [PubMed] [Google Scholar]
- 20*.Suzuki K. Health conditions and mortality in the Japan Collaborative Cohort Study for Evaluation of Cancer (JACC) Asian Pac J Cancer Prev. 2008;8(Suppl):25–34. [PubMed] [Google Scholar]
- 21.Lan TY, Lan TH, Wen CP, Lin YH, Chuang YL. Nighttime sleep, Chinese afternoon nap, and mortality in the elderly. Sleep. 2007 Sep 1;30(9):1105–1110. doi: 10.1093/sleep/30.9.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shankar A, Koh WP, Yuan JM, Lee HP, Yu MC. Sleep duration and coronary heart disease mortality among Chinese adults in Singapore: a population-based cohort study. Am J Epidemiol. 2008 Dec 15;168(12):1367–1373. doi: 10.1093/aje/kwn281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burazeri G, Gofin J, Kark JD. Over 8 hours of sleep--marker of increased mortality in Mediterranean population: follow-up population study. Croat Med J. 2003 Apr;44(2):193–198. [PubMed] [Google Scholar]
- 24.Mallon L, Broman JE, Hetta J. Sleep complaints predict coronary artery disease mortality in males: a 12-year follow-up study of a middle-aged Swedish population. J Intern Med. 2002 Mar;251(3):207–216. doi: 10.1046/j.1365-2796.2002.00941.x. [DOI] [PubMed] [Google Scholar]
- 25.Hublin C, Partinen M, Koskenvuo M, Kaprio J. Sleep and mortality: a population-based 22-year follow-up study. Sleep. 2007 Oct 1;30(10):1245–1253. doi: 10.1093/sleep/30.10.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huppert FA, Whittington JE. Symptoms of psychological distress predict 7-year mortality. Psychol Med. 1995 Sep;25(5):1073–1086. doi: 10.1017/s0033291700037569. [DOI] [PubMed] [Google Scholar]
- 27.Heslop P, Smith GD, Metcalfe C, Macleod J, Hart C. Sleep duration and mortality: The effect of short or long sleep duration on cardiovascular and all-cause mortality in working men and women. Sleep Med. 2002 Jul;3(4):305–314. doi: 10.1016/s1389-9457(02)00016-3. [DOI] [PubMed] [Google Scholar]
- 28*.Ferrie JE, Shipley MJ, Cappuccio FP, et al. A prospective study of change in sleep duration: associations with mortality in the Whitehall II cohort. Sleep. 2007 Dec 1;30(12):1659–1666. doi: 10.1093/sleep/30.12.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qureshi AI, Giles WH, Croft JB, Bliwise DL. Habitual sleep patterns and risk for stroke and coronary heart disease: a 10-year follow-up from NHANES I. Neurology. 1997 Apr;48(4):904–911. doi: 10.1212/wnl.48.4.904. [DOI] [PubMed] [Google Scholar]
- 30.Gottlieb DJ, Schulman DA, Nam BH, D’Agostino RA, Kannel WA. Sleep Duration Predicts Mortality: The Framingham Study. Sleep. 2002;25(Supp):A108. [Google Scholar]
- 31.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002 Feb;59(2):131–136. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 32.Ayas NT, White DP, Manson JE, et al. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003 Jan 27;163(2):205–209. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 33.Dew MA, Hoch CC, Buysse DJ, et al. Healthy older adults’ sleep predicts all-cause mortality at 4 to 19 years of follow-up. Psychosom Med. 2003 Jan–Feb;65(1):63–73. doi: 10.1097/01.psy.0000039756.23250.7c. [DOI] [PubMed] [Google Scholar]
- 34.Patel SR, Ayas NT, Malhotra MR, et al. A prospective study of sleep duration and mortality risk in women. Sleep. 2004 May 1;27(3):440–444. doi: 10.1093/sleep/27.3.440. [DOI] [PubMed] [Google Scholar]
- 35.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Sleep duration as a risk factor for diabetes incidence in a large U.S. sample. Sleep. 2008 Dec 1;30(12):1667–1673. doi: 10.1093/sleep/30.12.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen D, Foley D. Prevalence of sleep disturbance and mortality in the US population. Sleep Research. 1994;23:116. [Google Scholar]
- 37*.Gallicchio L, Kalesan B. Sleep Duration and Mortality: A Systematic Review and Meta-analysis. J Sleep Res. 2009;18(2):148–158. doi: 10.1111/j.1365-2869.2008.00732.x. [DOI] [PubMed] [Google Scholar]
- 38*.Grandner MA, Patel NP. From sleep duration to mortality: implications of meta-analysis and future directions. J Sleep Res. 2009;18(2):145–147. doi: 10.1111/j.1365-2869.2009.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000 Apr 19;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 40.Young T. Increasing sleep duration for a healthier (and less obese?) population tomorrow. Sleep. 2008 May 1;31(5):593–594. doi: 10.1093/sleep/31.5.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Foley DJ. An epidemiological perspective on one tale of a two-tailed hypothesis. Sleep Med Rev. 2004 Jun;8(3):155–157. doi: 10.1016/j.smrv.2004.02.002. discussion 175–156. [DOI] [PubMed] [Google Scholar]
- 42.Colten HR, Altevogt BM. Sleep disorders and sleep deprivation: an unmet public health problem. Washington, DC: Institute of Medicine: National Academies Press; 2006. Institute of Medicine Committee on Sleep Medicine and Research. [PubMed] [Google Scholar]
- 43.Kung HC, Hoyert DL, Xu J, Murphy SL. Deaths: final data for 2005. Natl Vital Stat Rep. 2008 Apr 24;56(10):1–120. [PubMed] [Google Scholar]
- 44*.Eguchi K, Pickering TG, Schwartz JE, et al. Short sleep duration as an independent predictor of cardiovascular events in Japanese patients with hypertension. Archives of internal medicine. 2008 Nov 10;168(20):2225–2231. doi: 10.1001/archinte.168.20.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meisinger C, Heier M, Lowel H, Schneider A, Doring A. Sleep duration and sleep complaints and risk of myocardial infarction in middle-aged men and women from the general population: the MONICA/KORA Augsburg cohort study. Sleep. 2007 Sep 1;30(9):1121–1127. doi: 10.1093/sleep/30.9.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolff B, Volzke H, Schwahn C, Robinson D, Kessler C, John U. Relation of self-reported sleep duration with carotid intima-media thickness in a general population sample. Atherosclerosis. 2008 Feb;196(2):727–732. doi: 10.1016/j.atherosclerosis.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 47.King CR, Knutson KL, Rathouz PJ, Sidney S, Liu K, Lauderdale DS. Short sleep duration and incident coronary artery calcification. Jama. 2008 Dec 24;300(24):2859–2866. doi: 10.1001/jama.2008.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen JC, Brunner RL, Ren H, et al. Sleep duration and risk of ischemic stroke in postmenopausal women. Stroke. 2008 Jul 17; doi: 10.1161/STROKEAHA.108.521773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaneita Y, Uchiyama M, Yoshiike N, Ohida T. Associations of usual sleep duration with serum lipid and lipoprotein levels. Sleep. 2008 May 1;31(5):645–652. doi: 10.1093/sleep/31.5.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Williams CJ, Hu FB, Patel SR, Mantzoros CS. Sleep duration and snoring in relation to biomarkers of cardiovascular disease risk among women with type 2 diabetes. Diabetes Care. 2007 May;30(5):1233–1240. doi: 10.2337/dc06-2107. [DOI] [PubMed] [Google Scholar]
- 51.Gottlieb DJ, Redline S, Nieto FJ, et al. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep. 2006 Aug 1;29(8):1009–1014. doi: 10.1093/sleep/29.8.1009. [DOI] [PubMed] [Google Scholar]
- 52.Cappuccio FP, Stranges S, Kandala NB, et al. Gender-specific associations of short sleep duration with prevalent and incident hypertension: the Whitehall II Study. Hypertension. 2007 Oct;50(4):693–700. doi: 10.1161/HYPERTENSIONAHA.107.095471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006 May;47(5):833–839. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 54.Lusardi P, Vanasia A, Mugellini A, Zoppi A, Preti P, Fogari R. Evaluation of nocturnal blood pressure by the Multi-P Analysis of 24-hour ambulatory monitoring. Z Kardiol. 1996;85 (Suppl 3):121–123. [PubMed] [Google Scholar]
- 55.Lusardi P, Zoppi A, Preti P, Pesce RM, Piazza E, Fogari R. Effects of insufficient sleep on blood pressure in hypertensive patients: a 24-h study. Am J Hypertens. 1999 Jan;12(1 Pt 1):63–68. doi: 10.1016/s0895-7061(98)00200-3. [DOI] [PubMed] [Google Scholar]
- 56.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003 Jan 28;107(3):499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 57.Meier-Ewert HK, Ridker PM, Rifai N, Price N, Dinges DF, Mullington JM. Absence of diurnal variation of C-reactive protein concentrations in healthy human subjects. Clinical chemistry. 2001 Mar;47(3):426–430. [PubMed] [Google Scholar]
- 58.Meier-Ewert HK, Ridker PM, Rifai N, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. Journal of the American College of Cardiology. 2004 Feb 18;43(4):678–683. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- 59.Vgontzas AN, Zoumakis E, Bixler EO, et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. The Journal of clinical endocrinology and metabolism. 2004 May;89(5):2119–2126. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- 60.Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med. 2007 Aug 15;3(5):519–528. [PMC free article] [PubMed] [Google Scholar]
- 61.Taheri S, Austin D, Lin L, Nieto FJ, Young T, Mignot E. Correlates of serum C-reactive protein (CRP)--no association with sleep duration or sleep disordered breathing. Sleep. 2007 Aug 1;30(8):991–996. doi: 10.1093/sleep/30.8.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pearson H. Medicine: sleep it off. Nature. 2006 Sep 21;443(7109):261–263. doi: 10.1038/443261a. [DOI] [PubMed] [Google Scholar]
- 63*.Cappuccio FP, Taggart FM, Kandala NB, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008 May 1;31(5):619–626. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64*.Taheri S. The link between short sleep duration and obesity: we should recommend more sleep to prevent obesity. Archives of disease in childhood. 2006 Nov;91(11):881–884. doi: 10.1136/adc.2005.093013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004 Dec 7;141(11):846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 66.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004 Dec;1(3):e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep medicine reviews. 2007 Jun;11(3):163–178. doi: 10.1016/j.smrv.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martinez-Garcia MA, Soler-Cataluna JJ, Ejarque-Martinez L, et al. CPAP Treatment Reduces Mortality in Ischemic Stroke Patients with Obstructive Sleep Apnea. Am J Respir Crit Care Med. 2009 Apr 30; doi: 10.1164/rccm.200808-1341OC. [DOI] [PubMed] [Google Scholar]
- 69.Marshall NS, Wong KK, Liu PY, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep. 2008 Aug 1;31(8):1079–1085. [PMC free article] [PubMed] [Google Scholar]
- 70.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008 Aug 1;31(8):1071–1078. [PMC free article] [PubMed] [Google Scholar]
- 71.Komada Y, Inoue Y, Hayashida K, Nakajima T, Honda M, Takahashi K. Clinical significance and correlates of behaviorally induced insufficient sleep syndrome. Sleep Med. 2008 Dec;9(8):851–856. doi: 10.1016/j.sleep.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 72.Jean-Louis G, Kripke DF, Ancoli-Israel S. Sleep and quality of well-being. Sleep. 2000 Dec 15;23(8):1115–1121. [PubMed] [Google Scholar]
- 73.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Sleep duration as a risk factor for diabetes incidence in a large U.S. sample. Sleep. 2007 Dec 1;30(12):1667–1673. doi: 10.1093/sleep/30.12.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ayas NT, White DP, Al-Delaimy WK, et al. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes care. 2003 Feb;26(2):380–384. doi: 10.2337/diacare.26.2.380. [DOI] [PubMed] [Google Scholar]
- 75.Yaggi HK, Araujo AB, McKinlay JB. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes care. 2006 Mar;29(3):657–661. doi: 10.2337/diacare.29.03.06.dc05-0879. [DOI] [PubMed] [Google Scholar]
- 76.Bjorkelund C, Bondyr-Carlsson D, Lapidus L, et al. Sleep disturbances in midlife unrelated to 32-year diabetes incidence: the prospective population study of women in Gothenburg. Diabetes care. 2005 Nov;28(11):2739–2744. doi: 10.2337/diacare.28.11.2739. [DOI] [PubMed] [Google Scholar]
- 77.Mallon L, Broman JE, Hetta J. High incidence of diabetes in men with sleep complaints or short sleep duration: a 12-year follow-up study of a middle-aged population. Diabetes care. 2005 Nov;28(11):2762–2767. doi: 10.2337/diacare.28.11.2762. [DOI] [PubMed] [Google Scholar]
- 78.Gottlieb DJ, Punjabi NM, Newman AB, et al. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Archives of internal medicine. 2005 Apr 25;165(8):863–867. doi: 10.1001/archinte.165.8.863. [DOI] [PubMed] [Google Scholar]
- 79.Knutson KL, Ryden AM, Mander BA, Van Cauter E. Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Archives of internal medicine. 2006 Sep 18;166(16):1768–1774. doi: 10.1001/archinte.166.16.1768. [DOI] [PubMed] [Google Scholar]
- 80.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999 Oct 23;354(9188):1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 81.Meerlo P, Sgoifo A, Suchecki D. Restricted and disrupted sleep: effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep medicine reviews. 2008 Jun;12(3):197–210. doi: 10.1016/j.smrv.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 82.Majde JA, Krueger JM. Links between the innate immune system and sleep. The Journal of allergy and clinical immunology. 2005 Dec;116(6):1188–1198. doi: 10.1016/j.jaci.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 83.Cohen S, Doyle WJ, Alper CM, Janicki-Deverts D, Turner RB. Sleep habits and susceptibility to the common cold. Arch Intern Med. 2009 Jan 12;169(1):62–67. doi: 10.1001/archinternmed.2008.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hale L, Do P. Racial differences in self-reports of sleep duration in a population-based study. Sleep. 2007;30(9):1096–1103. doi: 10.1093/sleep/30.9.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nunes J, Jean-Louis G, Zizi F, et al. Sleep duration among black and white Americans: results of the National Health Interview Survey. Journal of the National Medical Association. 2008 Mar;100(3):317–322. doi: 10.1016/s0027-9684(15)31244-x. [DOI] [PubMed] [Google Scholar]
- 86.Patel SR. Social and demographic factors related to sleep duration. Sleep. 2007 Sep 1;30(9):1077–1078. doi: 10.1093/sleep/30.9.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mackenbach JP, Kunst AE, Cavelaars AE, Groenhof F, Geurts JJ. Socioeconomic inequalities in morbidity and mortality in western Europe. The EU Working Group on Socioeconomic Inequalities in Health. Lancet. 1997 Jun 7;349(9066):1655–1659. doi: 10.1016/s0140-6736(96)07226-1. [DOI] [PubMed] [Google Scholar]
- 88.Moore PJ, Adler NE, Williams DR, Jackson JS. Socioeconomic status and health: the role of sleep. Psychosom Med. 2002 Mar–Apr;64(2):337–344. doi: 10.1097/00006842-200203000-00018. [DOI] [PubMed] [Google Scholar]
- 89*.Sekine M, Chandola T, Martikainen P, McGeoghegan D, Marmot M, Kagamimori S. Explaining social inequalities in health by sleep: the Japanese civil servants study. J Public Health (Oxf) 2006 Mar;28(1):63–70. doi: 10.1093/pubmed/fdi067. [DOI] [PubMed] [Google Scholar]
- 90.Bronfenbrenner U. Toward an experimental ecology of human development. American Psychologist. 1977;32:513–531. [Google Scholar]
- 91.Rechtschaffen A, Bergmann BM. Sleep deprivation in the rat: an update of the 1989 paper. Sleep. 2002 Feb 1;25(1):18–24. doi: 10.1093/sleep/25.1.18. [DOI] [PubMed] [Google Scholar]
- 92.Adler NE, Marmot M, McEwen BS, Stewart J. Socioeconomic status and health in industrial nations: social, psychological, and biological pathways. Vol. 896. New York: Annals of the New York Academy of Sciences; 1999. [PubMed] [Google Scholar]
- 93.Hale L, Peppard PE, Young T. Does the demography of sleep contribute to health disparities? In: Leger D, Prandi-Perumal SR, editors. Sleep Disorders: Their Impact on Public Health. NY: Informa Healthcare; 2007. [Google Scholar]
- 94.Hale L. Who has time to sleep? J Public Health (Oxf) 2005 Jun;27(2):205–211. doi: 10.1093/pubmed/fdi004. [DOI] [PubMed] [Google Scholar]
- 95.Berkman LF, Kawachi Io. Social epidemiology. New York: Oxford University Press; 2000. [Google Scholar]
- 96.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004 Nov 1;27(7):1255–1273. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 97.Balkin TJ, Rupp T, Picchioni D, Wesensten NJ. Sleep loss and sleepiness: current issues. Chest. 2008 Sep;134(3):653–660. doi: 10.1378/chest.08-1064. [DOI] [PubMed] [Google Scholar]