Abstract
Background
PRO 140 is a humanized CCR5 monoclonal antibody that has demonstrated potent antiviral activity when administered intravenously to adults infected with CCR5-tropic (R5) human immunodeficiency virus type 1 (HIV-1). This study is the first to evaluate subcutaneous (SC) administration.
Methods
A randomized, double-blind, placebo-controlled study was conducted in 44 subjects with HIV-1 RNA > 5,000 copies/mL, CD4+ cells > 300/μL, no antiretroviral therapy for ≥12 weeks, and only R5 HIV-1 detectable. Subjects received placebo, 162mg PRO 140, or 324mg PRO 140 weekly for three weeks or 324mg PRO 140 every other week for two doses by SC infusion. Subjects were monitored for 58 days for safety, antiviral effects and PRO 140 serum concentrations.
Results
SC PRO 140 demonstrated potent and prolonged antiretroviral activity. Mean log10 reductions in HIV-1 RNA were 0.23, 0.99 (p=0.0093), 1.37 (p=0.0001), and 1.65 (p<0.0001) for the placebo, 162mg weekly, 324mg biweekly and 324mg weekly dose groups, respectively. Viral loads remained suppressed between successive doses. Treatment was generally well tolerated.
Conclusions
This trial is the first to demonstrate proof of concept for a mAb administered subcutaneously in HIV-1 infected subjects. SC PRO 140 offers the potential for significant dose-dependent HIV-1 RNA suppression and infrequent patient self-administration.
Trial registration
Clinicaltrials.gov register NCT00642707
Keywords: PRO 140, CCR5, HIV-1 co-receptor, monoclonal antibody, subcutaneous
INTRODUCTION
Highly active antiretroviral therapy (HAART) has transformed management of HIV-1 infection and offers the potential for a normal life expectancy for many individuals with access to care (1;2). However, even when effective in reducing plasma viremia to levels that are undetectable in standard assays, HAART does not eradicate HIV-1, and long-term morbidities still occur (3–6). In addition, treatment fails to achieve or maintain optimal viral suppression in many individuals. Further improvement in care may be realized with the development of new antiretroviral agents that inhibit viruses resistant to existing therapies, exhibit minimal drug or food interactions, reduce chronic toxicities, and permit dosing to be infrequent and flexible.
CCR5 co-receptor antagonists represent an emerging antiretroviral treatment class and the first to target a host molecule. CCR5 is a chemokine receptor that mediates activation and migration of T cells and other leukocytes. CCR5 also binds the HIV-1 envelope glycoprotein gp120 and serves as a portal for HIV-1 entry into CD4+ cells (7). CCR5-using (R5) viruses typically mediate transmission and then predominate through the progression to symptomatic disease. Viruses can use an alternative chemokine receptor, CXCR4, either exclusively or in addition to CCR5. CXCR4-using viruses may be present early on but tend to become apparent in an increasing percentage of subjects in later phases of disease (8–11). One small-molecule CCR5 antagonist (maraviroc; Pfizer, Inc.) has been approved for use in antiretroviral treatment-experienced patients (12), and phase 3 clinical studies of a second small-molecule compound (vicriviroc; Merck) are ongoing (13).
PRO 140 is a humanized CCR5 monoclonal antibody (mAb) that potently inhibits R5 viruses and synergizes with small-molecule CCR5 antagonists in laboratory studies (14;15). PRO 140 does not inhibit CXCR4-using viruses. Previously, an intravenous (IV) form of PRO 140 was tested as monotherapy in HIV-1 subjects with only R5 virus detectable (16). Single doses, ranging up to 5 mg/kg, were generally well tolerated relative to placebo and demonstrated potent and prolonged antiviral activity, with a 1.83 log10 mean reduction in HIV-1 RNA observed at 5 mg/kg. These findings supported development of subcutaneous (SC) formulations with the potential for patient self-administration. The present study examined the antiviral effects, safety and pharmacokinetics (PK) of SC PRO 140 administered weekly or every other week to HIV-infected individuals.
SUBJECTS AND METHODS
Study design
A phase 2a study was conducted to evaluate the antiviral activity, tolerability and PK of weekly or biweekly SC doses of PRO 140 in adults with asymptomatic HIV-1 infection. The protocol was approved by the institutional review board at each site. All subjects provided written informed consent. Entry criteria included age ≥ 18 years, plasma HIV-1 RNA ≥ 5,000 copies/mL, CD4+ lymphocytes ≥ 300/μL with no documented count ≤ 250/μL, no antiretroviral therapy for ≥ 12 weeks, no history of acquired immunodeficiency syndrome-defining illness and only R5 HIV-1 detectable. PRO 140 was provided as a sterile 135mg/mL solution in phosphate buffer, pH 6.8. Placebo was a matched, sterile, buffer solution without PRO 140. Subjects were dosed on an outpatient basis with [1] placebo on Days 1, 8, 15; [2] 162mg PRO 140 on Days 1, 8, 15 (162mg weekly); [3] 324mg PRO 140 on Days 1, 15 and placebo on Day 8 (324mg biweekly); or 324mg PRO 140 on Days 1, 8, 15 (324mg weekly). Study drug was administered by site personnel via SC infusion into the arm over approximately 5 minutes using a syringe pump and Cleo 90 infusion set (Smiths Medical).
Virological evaluations
Plasma HIV-1 RNA was determined with the Cobas Amplicor HIV-1 Monitor Test (version 1.5; Roche Diagnostics) at screening, baseline (Day 1), and Days 3, 5, 8 (pre-dose), 10, 12, 15 (pre-dose), 22, 29, 43 and 59. Samples <400 copies/mL were re-analyzed with the Ultrasensitive method. Co-receptor tropism was determined at screening for all subjects and after viral rebound for PRO 140-treated subjects using the first-generation Trofile assay (Monogram Biosciences). The Enhanced Sensitivity (ES) Trofile assay, which was introduced after the study was initiated, was used in post-hoc analyses. Viral susceptibility to PRO 140 was determined for all subjects at baseline (Day 1) and after viral rebound for PRO 140-treated subjects using Phenosense Entry (Monogram Biosciences) as previously described (16).
Safety evaluations
Vital signs, concomitant medications, and adverse events were recorded during screening and on Days 1, 3, 5, 8, 10, 12, 15, 22, 29, 43 and 59. Physical examinations and clinical laboratory tests for serum chemistries, hematology and urinalysis were performed during screening and on Days 1, 8, 15, 29 and 59. 12-lead electrocardiograms were obtained during screening and on Days 1, 3, 8, 10 and 59.
Bioanalytical methods
Serum concentrations of PRO 140 and of antibodies to PRO 140 were determined by ELISA as previously described (16). Serum for PK analysis was taken at 0h (pre-dose), 0.5h, 1h, 3h, 6h, 24h, 32h, 48h, 56h and 96h post-treatment during the first week and then on Days 8, 12, 15, 22, 29 and 59. PK metrics were estimated after non-compartmental analysis using WinNonlin (Version 5.2, Pharsight). Sera for anti-PRO 140 antibodies were taken on Days 1, 8, 15, 29 and 59. To assess the presence of PRO 140 neutralizing antibodies, human CCR5-expressing CEM-NKR cells were incubated with phycoerythrin- (PE-) labeled PRO 140 in the presence of test serum. The resulting fluorescent signal was measured by flow cytometry and compared with the signal obtained in control serum. CD4+ and CCR5+ lymphocytes were determined essentially as described (16). Lymphocyte counts were determined at screening (CD4+ cells only) and on Days 1, 3, 8, 15, 22, 29, 43 and 59. CCR5 receptor occupancy was assessed by comparing the numbers of lymphocytes that stained positive for binding of PE-labeled PRO 140 pre- and post-treatment. On treatment days, bioanalytical samples were taken pre-dose unless otherwise indicated.
Statistical methods
All subjects who received at least one dose of study drug were included in the safety evaluations. Efficacy analyses were performed on log10 transformed HIV-1 RNA data, and changes were calculated relative to baseline (Day 1, pre-dose). Treatment and placebo groups were compared using an analysis of variance model. If the overall F test was found to be statistically significant, each treatment group was compared to placebo using pairwise t-tests. Fisher’s exact tests were used to compare treatment groups with placebo for the percentage of subjects with a ≥ 1 log10 reduction in HIV-1 RNA from baseline at any time post-treatment and with <400 copies HIV-1 RNA at any time post-treatment. Mann-Whitney U tests were used to compare CCR5 receptor occupancy data between placebo and treatment groups. Two-sided tests were used for all analyses.
RESULTS
Subject characteristics and disposition
Of the 138 subjects screened, 46 were randomized and 44 received at least one dose of study drug. The most frequent reasons for screen failure were non-reportable co-receptor tropism (23 subjects), CD4+ cells < 300/μL (21 subjects), dual/mixed co-receptor tropism (13 subjects) and HIV-1 RNA < 5000 copies/mL (12 subjects). Demographic and baseline characteristics for treated subjects are summarized in Table 1. For all subjects, the median age, CD4+ cell count and plasma HIV-1 RNA value at baseline were 42 years, 410 cells/μL and 25,100 copies/mL, respectively. Baseline characteristics were similar for the different treatment groups; however, median weights in the 324mg weekly and biweekly groups differed by 28%. Fifteen subjects reported prior antiretroviral therapy. Forty-one of 44 treated subjects completed the study. Two placebo subjects discontinued due to adverse events and one subject treated with 162mg withdrew for reasons unrelated to safety.
Table 1.
Baseline characteristics of treated subjects
| PRO 140 |
|||||
|---|---|---|---|---|---|
| Characteristic | Placebo (n=10) | 162mg Weekly (n=11) | 324mg Biweekly (n=12) | 324mg Weekly (n=11) | All Subjects (n=44) |
| Age, years | 44.9 (32.3–51.6) | 40.0 (29.1–44.6) | 45.9 (31.0–59.6) | 41.1 (34.8–53.6) | 42.4 (29.1–59.6) |
| Sex, male/female (n) | 9/1 | 10/1 | 11/1 | 10/1 | 40/4 |
| Race, black/white (n) | 3/7 | 5/6 | 5/7 | 4/7 | 17/27 |
| Weight, kg | 82.3 (59.4–107) | 77.0 (59.3–94.4) | 88.3 (58.9–102) | 69.0 (60.8–83.6) | 79.1 (58.9–107) |
| CD4+ cell count, cells/μL | 410 (312–878) | 352 (307–611) | 493 (357–911) | 389 (341–638) | 410 (307–911) |
| HIV-1 RNA, log10 copies/mL | 4.09 (3.94–5.13) | 4.43 (3.92–4.97) | 4.60 (4.03–6.68) | 4.19 (3.61–4.77) | 4.40 (3.61–6.68) |
NOTE: Data are median (range) values unless otherwise indicated.
Antiviral effects
Significant, dose-dependent antiviral effects were observed for each PRO 140 group. Mean log10 reductions in HIV-1 RNA at virologic nadir were 0.23, 0.99, 1.37 and 1.65 for the placebo, 162mg weekly, 324mg biweekly and 324mg weekly groups, respectively. The reduction for each PRO 140 group was highly statistically significant relative to placebo (Table 2). Individual viral nadirs typically were observed on Day 22 for subjects in the 324mg groups, with a range of Day 15 to Day 29.
Table 2.
Change in HIV-1 RNA and CD4+ T cells
| PRO 140 |
||||
|---|---|---|---|---|
| Effect | Placebo | 162mg Weekly | 324mg Biweekly | 324mg Weekly |
| Maximum log10 change in HIV-1 RNA | −0.23±0.29 | −0.99±0.56 (p=0.0093) | −1.37±0.89 (p=0.0001) | −1.65±0.57 (p<0.0001) |
| Day 22 log10 change in HIV-1 RNA | −0.15±020 | −0.75±0.41 (p=0.0072) | −1.20±1.00 (p=0.0001) | −1.51±0.65 (p<0.0001) |
| Number of subjects with ≥1 log10 decrease in HIV-1 RNA at any time post-treatment, (%)† | 0/10 (0%) | 6/11 (55%) (p=0.012) | 9/12 (75%) (p=0.0005) | 10/11 (89%) (p<0.0001) |
| Number of subjects with <400 copies HIV-1 RNA/mL at any time post-treatment, (%) | 0/10 (0%) | 1/11 (11%) | 2/12 (17%) | 8/11 (73%) (p=0.001) |
| Median (range) change in CD4+ cell count at Days 8, 15 and 22, cells/μL | 4 (−70–+71) | 29 (−169–+198) | 104 (−97–+317) | 76 (−52–+219) |
| 12 (−101–+104) | 50 (−204–+278) | 44 (−46–+178) | 76 (−84–+ 246) | |
| 14 (−184–+179) | 55 (−140–+153) | 99 (−21–+243) | 84 (−110–+296) | |
NOTE: Data are mean ± SD values unless otherwise indicated
Includes one subject each in the 324mg biweekly and 324mg weekly groups with a 0.99 log10 reduction in HIV-1 RNA
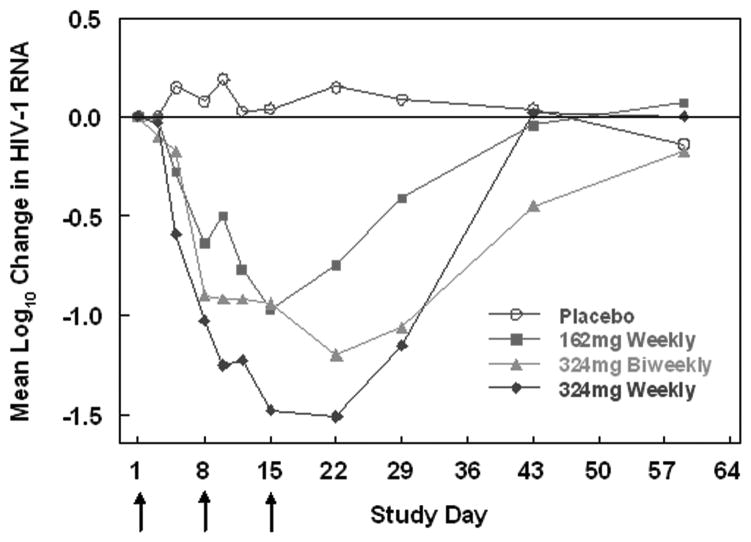
The mean log10 change in HIV-1 RNA over time is depicted in Fig. 1. Significant antiviral effects were observed following the first dose of PRO 140 (P=0.0066 to P<0.0001 for the different treatment groups at Day 8), and viral loads decreased with continued dosing. At Day 22, the mean log10 decreases in viral load were 0.15, 0.75, 1.20 and 1.51 for the placebo and ascending PRO 140 dose groups (Fig. 1 and Table 2). Viral loads remained suppressed between successive weekly and biweekly 324mg doses. For the 324mg weekly and biweekly groups, mean viral load reductions exceeded 1 log10 (P≤0.0012) through Day 29.
Figure 1. Mean log10 change from baseline in HIV-1 RNA over time for the different treatment groups.
The arrows indicate days that study drug was administered (Days 1, 8 and 15).
Antiviral response was defined as a ≥ 1 log10 decrease in HIV-1 RNA at any time post-treatment. With inclusion of one subject each with a 0.99 log10 reduction, antiviral response rates were 89% (10 of 11 subjects, P≤0.0001) and 75% (9 of 12 subjects, P=0.0005) for the 324mg weekly and biweekly groups, respectively. Six of 11 subjects treated with 162 mg (55%, P=0.012) had a ≥ 1 log10 decrease in viral load; no placebo subject did (Table 2). The 324mg weekly subject who did not experience a ≥ 1 log10 decrease in HIV-1 RNA had only R5 virus post-treatment. Pre- and post-treatment viral susceptibility to PRO 140, PRO 140 serum levels, and receptor occupancy data for this subject were similar to those of others treated with 324mg weekly. Seventy-three percent of subjects in the 324mg weekly group had a viral load of < 400 copies/mL while no placebo subject did (P =0.001; Table 2).
Safety
SC PRO 140 was generally well tolerated. Forty of 44 subjects overall and ten of ten placebo subjects reported at least one adverse event (AE). Of these, approximately half were considered to be unrelated to study drug. There were no drug-related serious adverse events or dose-limiting toxicities. The most frequently reported systemic AEs were diarrhea (14%), headache (14%), lymphadenopathy (11%), and hypertension (9%). No obvious dose-proportional trend in the frequency of AEs was observed. There was no clinically relevant drug-related effect on QTc intervals or other electrocardiogram parameters. There were no notable findings in laboratory safety tests
Administration-site reactions were infrequent, mild, transient (1 or 2 days), and self-resolving. Rates were similar for placebo and PRO 140 groups. Adverse events reported in >5% of subjects were induration (20%), pain (9%), and irritation (7%). No SC infusions were paused or discontinued for any reason.
Pharmacokinetics
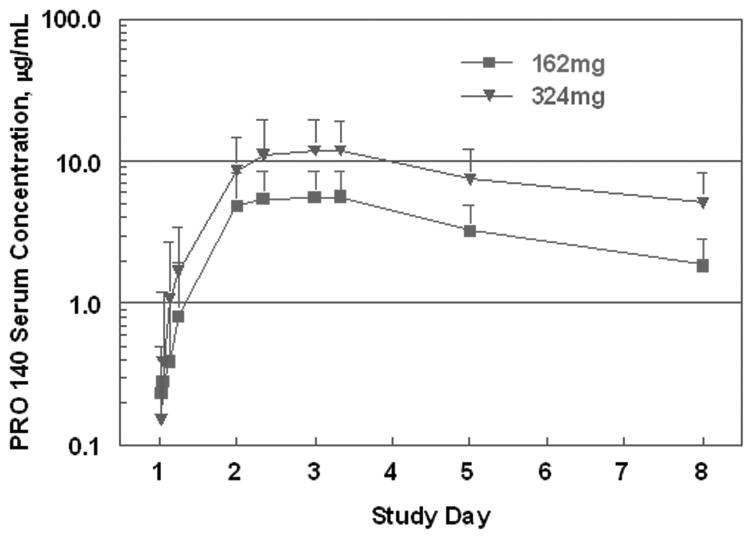
Serum concentrations observed during the first week of treatment are depicted in Fig. 2 and were used to calculate PK metrics. Data for the 324mg weekly and biweekly groups were pooled for this analysis. Peak concentrations typically were observed between 32 to 56 hours post-dose and averaged 6.1 and 13.8 mg/L for subjects treated with 162mg and 324mg, respectively. The corresponding mean terminal half-lives were 3.4 and 3.7 days. During the first week after treatment, the mean area under the PRO 140 concentration-time curve (AUC) values were 24.4 and 58.8 mg × day/L for 162mg and 324mg doses, respectively. Mean AUC values from time zero to infinity were approximately 36% higher. Mean trough concentrations for the 162mg weekly group increased from 1.86 μg/mL on Day 8 to 2.89 and 3.55 μg/mL on Days 15 and 22, respectively. The corresponding values for the 324mg weekly group were 5.45, 8.50, and 8.75 μg/mL.
Figure 2. Mean serum concentrations of PRO 140 following a 162mg or 324mg SC dose.
The error bars depict standard deviations. The curve for 324mg includes data for the 324mg weekly and biweekly treatment groups.
Low-titered anti-PRO 140 antibodies (1:32 or less) were detected at Days 29 and/or 59 in two subjects treated with 162mg weekly, two subjects treated with 324mg biweekly, and three subjects treated with 324mg weekly. All other subjects were negative for anti-PRO 140 antibodies. Anti-PRO 140 antibodies had no obvious effect on PK or antiviral responses. For example, subjects with positive anti-PRO 140 antibody test results experienced a mean 1.38 log10 decrease in HIV-1 RNA, whereas the mean decrease was 1.32 log10 in PRO 140-treated subjects who tested negative for anti-PRO 140 antibodies. All sera that tested positive for anti-PRO 140 antibodies were non-neutralizing; i.e., the sera did not block binding of PRO 140 to CCR5+ cells in vitro.
Co-receptor tropism and viral susceptibility to PRO 140
Eligible subjects had only R5 virus detected in the first-generation Trofile assay at screening. Co-receptor tropism was determined using the same method after viral rebound in subjects treated with PRO 140. All subjects in the 162mg weekly and 324mg weekly groups maintained R5-only co-receptor tropism following treatment. However, three subjects in the 324mg biweekly group had dual/mixed virus detected during the study. One of these subjects experienced a 1.1 log10 nadir in viral load at Day 8. Minimal antiviral responses (<0.5 log10 nadir reduction) were observed for the other two subjects. When the three subjects were censored, the mean maximum decrease in viral load for the 324mg biweekly group was 1.60 log10.
Screening samples from the three subjects with dual/mixed virus were analyzed post-study using the ES Trofile assay. One subject had detectable levels of dual/mixed virus pre-treatment and would have been excluded from the study if ES Trofile had been available for screening. Dual/mixed virus was first detected on Day 15 or Day 29 in the other two subjects. Studies are ongoing to determine if these subjects had pre-existing dual/mixed virus at levels below the ES Trofile’s limit of detection. Both subjects had only R5 virus detected in ES Trofile at end of study (Day 59).
All viruses were susceptible to inhibition by PRO 140 in the R5 PhenoSense Entry assay. The mean Fold Change at baseline was 1.92 (range 0.83 to 3.94). There was no significant change in Fold Change values post-treatment (P>0.8). All post-treatment values were ≤ 1.7-fold of the pre-treatment values. Maximum percent inhibition of R5 viruses was 98–100% both pre- and post-treatment for all subjects, indicating essentially complete inhibition by PRO 140.
Lymphocyte and receptor occupancy analyses
D4+ lymphocytes ranged from 307 to 911 cells/μL at baseline (Table 1). An increase in CD4+ lymphocyte counts was observed for each PRO 140 treatment group at Days 8, 15 and 22 (Table 2); however, the changes were not statistically significant.
The median baseline CCR5+ lymphocyte count was 35 cells/μL (range 3 to 266 cells/μL). CCR5+ lymphocytes were not depleted following treatment; however, high levels of receptor occupancy were observed. The median number of cells that stained positive with fluorescently labeled PRO 140 ex vivo was reduced to ≤ 7% of pre-treatment values between Days 3 and 43 for all PRO 140 treatment groups (P ≤0.0038 relative to placebo at each timepoint). At Day 59, receptor occupancy levels were not significantly different from placebo for any PRO 140 treatment group (P ≥0.08).
DISCUSSION
In this first study to evaluate SC delivery of a mAb for HIV-1 therapy, PRO 140 demonstrated potent and prolonged antiretroviral activity. The mean maximum reduction in HIV-1 RNA observed for the 324mg weekly dose was 1.65 log10 and is similar in magnitude to that observed in short-term monotherapy studies, both of small-molecule CCR5 antagonists and of IV PRO 140 (16–19). Treatment was generally well tolerated. The findings provide clinical proof of concept for SC PRO 140 as a potent and long-acting antiretroviral agent. The SC dosage form offers the potential for infrequent self-administration by patients, and this issue will be explored in future studies.
Viral load reductions and antiviral response rates increased as the total amount of PRO 140 administered over three weeks was increased from 486mg (162mg weekly) to 648mg (324mg biweekly) to 932mg (324mg weekly). Since trough concentrations increased following repeat SC dosing, a loading dose potentially could be used to increase the initial rate of virologic suppression. Faster initial viral decay rates have been correlated with improved long-term virologic outcomes (20;21). Following an initial loading dose, potent virologic suppression and steady-state trough concentrations of PRO 140 might be attainable with SC maintenance doses similar to those examined here.
Significant antiviral effects were observed for PRO 140 administered both weekly and every other week, and virologic suppression was maintained between successive doses. A weekly regimen may offer appreciable latitude of one or more days in the timing of subsequent dosing. Frequent dosing can provide a barrier to adherence (22–24), such that once-daily regimens are recommended over twice-daily regimens (25). However, there currently are a limited number of once-daily antiretroviral regimens, especially for patients with drug-resistant virus. The availability of a long-acting antiretroviral agent would provide an additional option for constructing alternative treatment regimens. that are more accommodating. The mean reduction observed at virologic nadir following three weekly 324mg SC doses (1.65 log10, Table 2) was similar in magnitude to the 1.83 log10 reduction observed previously for a single 5 mg/kg IV dose (16); however, pharmacodynamic differences were evident. Peak serum concentrations were approximately 10-fold lower following SC dosing (13.8 μg/mL) than IV dosing (173 μg/mL (16)), indicating that the high serum concentrations achieved with IV dosing are not required for potent virologic suppression. Although SC and IV PRO 140 exhibited similar serum half-lives, the apparent overall exposure, as determined by AUC analysis of serum concentrations, was approximately 3.5-fold lower following a single 324mg SC dose as compared with a single 5 mg/kg IV dose (mean 367mg total dose).
Proteins and other macromolecules drain from SC sites into both blood capillaries and the lymphatic system. In animals, proteins with molecular weights of greater than 16,000 daltons have been observed to drain primarily into the lymphatic system following SC administration (26). Such proteins transit through lymph fluid and typically are not absorbed significantly into the blood until they reach the thoracic duct. Since the molecular weight of PRO 140 is approximately 150,000 daltons, a substantial amount of SC PRO 140 can be expected to drain into the lymphatic system and potentially encounter CCR5+ cells in lymphoid tissues prior to reaching the bloodstream. For these reasons, serum concentrations may not provide a full picture of the overall exposure following SC dosing of PRO 140.
In order to evade inhibition by CCR5 drugs, HIV-1 can adapt either to use CCR5 in the presence of drug or to use an alternative co-receptor (27). In clinical trials of CCR5 inhibitors, adaptation to an alternative co-receptor typically has reflected outgrowth of pre-existing CXCR4-using viruses rather than de novo mutation of R5 viruses (28–31). Three PRO 140-treated subjects (9%) had dual/mixed virus detected during the study. Each of these subjects was in the 324mg biweekly group. This imbalance may be due to chance given the small number of subjects in each group. These subjects had minimal or blunted antiviral responses, as expected. One subject had pre-existing dual/mixed virus at levels that would have excluded the subject from the study if the ES Trofile assay had been available for screening. Further analysis of the pre-treatment viruses of the other two subjects is ongoing and will be reported separately. A loading dose may decrease the potential for dual/mixed virus to emerge following initiation of treatment with PRO 140, as may the presence of additional antiretroviral agents in future combination regimens. Lastly, since the potential for outgrowth of CXCR4-using viruses is related to the sensitivity of the tropism test used for screening, this resistance pathway may diminish in clinical importance as tropism assays become increasingly sensitive in detecting CXCR4-using viruses.
There was no change in R5 viral susceptibility to PRO 140 following three weeks of monotherapy, indicating no adaptation of virus to use CCR5 in the presence of drug. Similarly, no development of R5 viral resistance was observed following treatment with single IV doses of PRO 140, which produced significant antiviral effects for two weeks (16). In contrast, phenotypic and/or genotypic resistance has been reported within two weeks of monotherapy with some non-nucleoside reverse transcriptase inhibitors (32–35). The PRO 140 results are especially notable in that monotherapy was followed by slow washout of drug. Such conditions can foster development of antiretroviral drug resistance (36;37). Overall, our findings suggest that PRO 140 presents a high barrier to resistance.
This study was the first to examine SC administration of PRO 140 in humans. An infusion pump was used to control and potentially pause administration, if necessary. SC administration was well tolerated. No infusions were interrupted for any reason. SC infusion currently is used by individuals with primary immunodeficiency to self-administer at home significantly larger amounts (~11 grams) and volumes (~70 mL total, up to 15 mL/site) of SC immunoglobulin weekly (38;39). Self-administration of 324mg SC PRO 140 would be much simpler in comparison. Also, the favorable local tolerability observed in the present study supports further studies to examine self-administration of PRO 140 by SC injection.
In summary, the current study establishes proof of concept for SC infusion of a therapeutic mAb for HIV-1. Subcutaneous PRO 140 offers the potential for significant suppression of HIV-1 replication and infrequent patient self-administration.
Acknowledgments
Financial support: Public Health Service grant AI066329 from the National Institutes of Health.
We thank the subjects for their participation in the study. The authors gratefully acknowledge the assistance of all site personnel and study investigators, including Nicholaos Bellos, Daniel Berger, Gary Blick, Stephen Brown, Cynthia Brinson, Edwin DeJesus, Paul DenOuden, Ralph Liporace, David Prelutsky, Robert Redfield, Peter Ruane, Peter Shalit and Michael Wohlfeller.
Footnotes
Potential conflict of interest:
PD, NS, YR, AJM, PJM, SAM and WCO are current or past employees of Progenics Pharmaceuticals and may hold stock or stock options in the company. Progenics has a proprietary commercial interest in PRO 140.
Presented in part:
16th Conference on Retroviruses and Opportunistic Infections, Abstract 571a, February 8–11, 2009, Montreal, Canada.
References
- 1.Bhaskaran K, Hamouda O, Sannes M, et al. Changes in the risk of death after HIV seroconversion compared with mortality in the general population. JAMA. 2008;300(1):51–9. doi: 10.1001/jama.300.1.51. [DOI] [PubMed] [Google Scholar]
- 2.Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372(9635):293–9. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Effros RB, Fletcher CV, Gebo K, et al. Aging and infectious diseases: workshop on HIV infection and aging: what is known and future research directions. Clin Infect Dis. 2008;47(4):542–53. doi: 10.1086/590150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weber R, Sabin CA, Friis-Moller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166(15):1632–41. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 5.Mondy K, Tebas P. Cardiovascular risks of antiretroviral therapies. Annu Rev Med. 2007;58:141–55. doi: 10.1146/annurev.med.58.072905.180040. [DOI] [PubMed] [Google Scholar]
- 6.Robertson KR, Smurzynski M, Parsons TD, et al. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS. 2007;21(14):1915–21. doi: 10.1097/QAD.0b013e32828e4e27. [DOI] [PubMed] [Google Scholar]
- 7.Lederman MM, Penn-Nicholson A, Cho M, Mosier D. Biology of CCR5 and its role in HIV infection and treatment. JAMA. 2006;296(7):815–26. doi: 10.1001/jama.296.7.815. [DOI] [PubMed] [Google Scholar]
- 8.Dean M, Carrington M, Winkler C, et al. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Hemophilia Growth and Development Study, Multicenter AIDS Cohort Study, Multicenter Hemophilia Cohort Study, San Francisco City Cohort, ALIVE Study. Science. 1996;273(5283):1856–62. doi: 10.1126/science.273.5283.1856. [DOI] [PubMed] [Google Scholar]
- 9.Moyle GJ, Wildfire A, Mandalia S, et al. Epidemiology and predictive factors for chemokine receptor use in HIV-1 infection. J Infect Dis. 2005;191(6):866–72. doi: 10.1086/428096. [DOI] [PubMed] [Google Scholar]
- 10.Wilkin TJ, Su Z, Kuritzkes DR, et al. HIV type 1 chemokine coreceptor use among antiretroviral-experienced patients screened for a clinical trial of a CCR5 inhibitor: AIDS Clinical Trial Group A5211. Clin Infect Dis. 2007;44(4):591–5. doi: 10.1086/511035. [DOI] [PubMed] [Google Scholar]
- 11.Brumme ZL, Goodrich J, Mayer HB, et al. Molecular and clinical epidemiology of CXCR4-using HIV-1 in a large population of antiretroviral-naive individuals. J Infect Dis. 2005;192(3):466–74. doi: 10.1086/431519. [DOI] [PubMed] [Google Scholar]
- 12.Gulick RM, Lalezari J, Goodrich J, et al. Maraviroc for previously treated patients with R5 HIV-1 infection. N Engl J Med. 2008;359(14):1429–41. doi: 10.1056/NEJMoa0803152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. [Accessed 6 August 2009];Clinicaltrials.gov study numbers NCT00474370 and NCT00523211. Available at: http://www.clinicaltrials.gov.
- 14.Murga J, Franti M, Pevear DC, Maddon PJ, Olson WC. Potent antiviral synergy between monoclonal antibody and small-molecule CCR5 inhibitors of human immunodeficiency virus type 1. Antimicrobial Agents and Chemotherapy. 2006;50(10):3289–96. doi: 10.1128/AAC.00699-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trkola A, Ketas TJ, Nagashima KA, et al. Potent, broad-spectrum inhibition of human immunodeficiency virus type 1 by the CCR5 monoclonal antibody PRO 140. J Virol. 2001;75(2):579–88. doi: 10.1128/JVI.75.2.579-588.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobson JM, Saag MS, Thompson MA, et al. Antiviral activity of single-dose PRO 140, a CCR5 monoclonal antibody, in HIV-infected adults. J Infect Dis. 2008;198:1345–52. doi: 10.1086/592169. [DOI] [PubMed] [Google Scholar]
- 17.Fatkenheuer G, Pozniak AL, Johnson MA, et al. Efficacy of short-term monotherapy with maraviroc, a new CCR5 antagonist, in patients infected with HIV-1. Nat Med. 2005;11(11):1170–2. doi: 10.1038/nm1319. [DOI] [PubMed] [Google Scholar]
- 18.Schurmann D, Fatkenheuer G, Reynes J, et al. Antiviral activity, pharmacokinetics and safety of vicriviroc, an oral CCR5 antagonist, during 14-day monotherapy in HIV-infected adults. AIDS. 2007;21(10):1293–9. doi: 10.1097/QAD.0b013e3280f00f9f. [DOI] [PubMed] [Google Scholar]
- 19.Lalezari J, Thompson M, Kumar P, et al. Antiviral activity and safety of 873140, a novel CCR5 antagonist, during short-term monotherapy in HIV-infected adults. AIDS. 2005;19:1443–8. doi: 10.1097/01.aids.0000183633.06580.8a. [DOI] [PubMed] [Google Scholar]
- 20.Polis MA, Sidorov IA, Yoder C, et al. Correlation between reduction in plasma HIV-1 RNA concentration 1 week after start of antiretroviral treatment and longer-term efficacy. Lancet. 2001;358(9295):1760–5. doi: 10.1016/s0140-6736(01)06802-7. [DOI] [PubMed] [Google Scholar]
- 21.Kuritzkes DR, Ribaudo HJ, Squires KE, et al. Plasma HIV-1 RNA dynamics in antiretroviral-naive subjects receiving either triple-nucleoside or efavirenz-containing regimens: ACTG A5166s. J Infect Dis. 2007;195(8):1169–76. doi: 10.1086/512619. [DOI] [PubMed] [Google Scholar]
- 22.Maitland D, Jackson A, Osorio J, Mandalia S, Gazzard BG, Moyle GJ. Switching from twice-daily abacavir and lamivudine to the once-daily fixed-dose combination tablet of abacavir and lamivudine improves patient adherence and satisfaction with therapy. HIV Med. 2008;9(8):667–72. doi: 10.1111/j.1468-1293.2008.00618.x. [DOI] [PubMed] [Google Scholar]
- 23.Boyle BA, Jayaweera D, Witt MD, Grimm K, Maa JF, Seekins DW. Randomization to once-daily stavudine extended release/lamivudine/efavirenz versus a more frequent regimen improves adherence while maintaining viral suppression. HIV Clin Trials. 2008;9(3):164–76. doi: 10.1310/hct0903-164. [DOI] [PubMed] [Google Scholar]
- 24.Molina JM, Podsadecki TJ, Johnson MA, et al. A lopinavir/ritonavir-based once-daily regimen results in better compliance and is non-inferior to a twice-daily regimen through 96 weeks. AIDS Res Hum Retroviruses. 2007;23(12):1505–14. doi: 10.1089/aid.2007.0107. [DOI] [PubMed] [Google Scholar]
- 25.Hammer SM, Eron JJ, Jr, Reiss P, et al. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA. 2008;300(5):555–70. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 26.Porter CJ, Charman WN. Transport and absorption of drugs via the lymphatic system. Adv Drug Deliv Rev. 2001;50(1–2):1–2. doi: 10.1016/s0169-409x(01)00146-6. [DOI] [PubMed] [Google Scholar]
- 27.Moore JP, Kuritzkes DR. A piece de resistance: how HIV-1 escapes small molecule CCR5 inhibitors. Curr Opin HIV AIDS. 2009;4(2):118–24. doi: 10.1097/COH.0b013e3283223d46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsibris AM, Korber B, Arnaout R, et al. Quantitative deep sequencing reveals dynamic HIV-1 escape and large population shifts during CCR5 antagonist therapy in vivo. PLoS ONE. 2009;4(5):e5683. doi: 10.1371/journal.pone.0005683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Westby M, Lewis M, Whitcomb J, et al. Emergence of CXCR4-using human immunodeficiency virus type 1 (HIV-1) variants in a minority of HIV-1-infected patients following treatment with the CCR5 antagonist maraviroc is from a pretreatment CXCR4-using virus reservoir. J Virol. 2006;80(10):4909–20. doi: 10.1128/JVI.80.10.4909-4920.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis M, Simpson P, Fransen S, et al. CXCR4-using virus detected in patients receiving maraviroc in the phase III studies MOTIVATE 1 and 2 originates from a pre-existing minority of CXCR4-using virus. Antiviral Therapy. 2007;12:S65. [Google Scholar]
- 31.Marozsan AJ, Parsons T, Huang W, et al. Clonal analysis of HIV-1 co-receptor tropism change following treatment with PRO 140, a CCR5 monoclonal antibody. 48th Annual ICAAC/IDSA 46th Annual Meeting, Abstract H-1218; October 25–28, 2008; Washington, DC. [Google Scholar]
- 32.Saag MS, Emini EA, Laskin OL, et al. A short-term clinical evaluation of L-697,661, a non-nucleoside inhibitor of HIV-1 reverse transcriptase. L-697,661 Working Group. N Engl J Med. 1993;329(15):1065–72. doi: 10.1056/NEJM199310073291502. [DOI] [PubMed] [Google Scholar]
- 33.Richman DD, Havlir D, Corbeil J, et al. Nevirapine resistance mutations of human immunodeficiency virus type 1 selected during therapy. J Virol. 1994;68(3):1660–6. doi: 10.1128/jvi.68.3.1660-1666.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Havlir DV, Eastman S, Gamst A, Richman DD. Nevirapine-resistant human immunodeficiency virus: kinetics of replication and estimated prevalence in untreated patients. J Virol. 1996;70(11):7894–9. doi: 10.1128/jvi.70.11.7894-7899.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vazquez-Rosales G, Garcia Lerma JG, Yamamoto S, et al. Rapid screening of phenotypic resistance to nevirapine by direct analysis of HIV type 1 reverse transcriptase activity in plasma. AIDS Res Hum Retroviruses. 1999;15(13):1191–200. doi: 10.1089/088922299310287. [DOI] [PubMed] [Google Scholar]
- 36.Chi BH, Sinkala M, Mbewe F, et al. Single-dose tenofovir and emtricitabine for reduction of viral resistance to non-nucleoside reverse transcriptase inhibitor drugs in women given intrapartum nevirapine for perinatal HIV prevention: an open-label randomised trial. Lancet. 2007;370(9600):1698–705. doi: 10.1016/S0140-6736(07)61605-5. [DOI] [PubMed] [Google Scholar]
- 37.Darwich L, Esteve A, Ruiz L, Bellido R, Clotet B, Martinez-Picado J. Variability in the plasma concentration of efavirenz and nevirapine is associated with genotypic resistance after treatment interruption. Antivir Ther. 2008;13(7):945–51. [PubMed] [Google Scholar]
- 38.Ochs HD, Gupta S, Kiessling P, Nicolay U, Berger M. Safety and efficacy of self-administered subcutaneous immunoglobulin in patients with primary immunodeficiency diseases. J Clin Immunol. 2006;26(3):265–73. doi: 10.1007/s10875-006-9021-7. [DOI] [PubMed] [Google Scholar]
- 39.Gardulf A, Andersen V, Bjorkander J, et al. Subcutaneous immunoglobulin replacement in patients with primary antibody deficiencies: safety and costs. Lancet. 1995;345(8946):365–9. doi: 10.1016/s0140-6736(95)90346-1. [DOI] [PubMed] [Google Scholar]