Abstract
Ovariectomy (OVX) leads to hyperphagia and weight gain in rats, which can be prevented by estradiol (E2) replacement; however, the role of endogenous E2 on feeding and energy homeostasis in female mice has not been well characterized. The primary goal of this study was to assess the relative contribution of increased energy intake and decreased energy expenditure to OVX-induced weight gain in female rats and mice. OVX led to hyperphagia in rats, but did not produce daily, nor cumulative, hyperphagia in mice. OVX decreased mass-specific metabolic rate in mice, but not in rats. OVX decreased home cage locomotor activity in both species. Pair-feeding attenuated OVX-induced weight gain in rats and produced both short- and long-term changes in expression of key hypothalamic genes involved in food intake and energy homeostasis, i.e., the anorexigenic neuropeptide pro-opiomelanocortin (POMC) and the orexigenic neuropeptides: melanin-concentrating hormone (MCH) and agouti-related peptide (AgRP). No differences in hypothalamic gene expression were observed between OVX’d and sham mice. The results suggest that OVX-induced weight gain is mediated by hyperphagia and reduced locomotor activity in rats, but that in mice, it is primarily mediated by reduced locomotor activity and metabolic rate.
Keywords: energy expenditure, locomotor activity, indirect calorimetry, food intake, RT-PCR, hypothalamic neuropeptides
1. Introduction
There is uncertainty regarding the evidence that estradiol (E2) influences energy homeostasis by regulating energy expenditure. Pair-feeding of ovariectomized (OVX’d) rats generally does not prevent weight gain (Roy and Wade, 1977); however, this finding is not universal (Liang et al., 2002). If OVX-induced weight gain is not prevented by restricting daily food intake of OVX’d rats, then a role for E2 in the regulation of energy expenditure is strongly suggested. Thus, the primary goal of this study was to assess the relative contribution of increased energy intake and decreased energy expenditure to OVX-induced weight gain in female mice and rats.
In addition, it is well established that E2 exerts both a tonic and a phasic inhibitory effect on food intake in female rats (reviewed in Eckel, 2004). While the hypophagia and hyperactivity that occur on estrus have been well characterized in the female rat (Wade, 1972) they have not been well characterized in the female mouse. In fact, exogenous E2 treatment has been found to increase locomotor activity in OVX’d female mice (Ogawa et al., 2003), yet surprisingly few studies have investigated the role of endogenous E2 on food intake and locomotor activity in the ovarian-intact female mouse. Of these studies, the following conclusive statements can be made: 1.) an estrous rhythm of locomotor activity has not been established in ovarian-intact female mice and 2.) an estrous-related decrease in food intake has not been observed in ovarian-intact female mice (in fact, the absence of these effects has been reported (Barnett and McEwan, 1973; Petersen, 1976)). In light of the increasing use of mice as the pre-clinical vertebrate model of choice, it is crucial to know how exactly endogenous E2 affects food intake and energy homeostasis in the normal ovarian-intact mouse. Experience tells us that we cannot simply extrapolate from what is already known about E2 in the ovarian-intact rat to the mouse. Furthermore, evidence involving exogenous, pharmacological doses of E2 does not necessarily indicate how endogenous, physiological levels of E2 affect the physiology of the ovarian-intact mouse. Therefore, the secondary goal of this study was to characterize the daily food intake and locomotor activity patterns of ovarian-intact mice. After observing hyperphagia in OVX’d rats (an effect that was clearly absent in OVX’d mice), we sought to measure changes in gene expression of key hypothalamic neuropeptides involved in feeding and energy homeostasis.
2. Materials and Methods
2.1 Animals and housing
Female C57BL/6J mice (14 wk old) and Long-Evans rats (14 wk old) were obtained from Charles River Laboratories (Charlotte, NC). Upon arrival, animals were housed individually in polycarbonate cages containing wood chip bedding and a red, translucent igloo niche (Bio-Serv, Frenchtown, NJ; niches were in mouse cages only). Animals were given 2 wk to fully recover from shipment stress and to acclimate to a reversed circadian cycle (11a-11p dark:11p-11a light). Pellet chow (4.5% fat, physiological caloric value = 3.3 kcal/g; Purina 5001; Purina Ralston, Richmond, IN) and deionized water were made freely available. The room was maintained at 23 ± 1.0°C. Animal usage and all procedures were in strict compliance with the guidelines of the National Institutes of Health and approved by the Florida State University Institutional Animal Care and Use Committee.
2.2 Indirect calorimetry and behavioral monitoring
Measurements of gas exchange and monitoring of animal behavior were performed using previously published approaches (Rashotte et al., 1995). At ~ 4 mos of age, animals were housed in individual cages fitted with custom-made polycarbonate lids providing a near air-tight seal for continuous determination of 23-h oxygen consumption (VO2; ml/min) and carbon dioxide production (VCO2; ml/min). Respiratory quotient (RQ; VCO2/ VO2), an index of substrate utilization, was calculated from these measures. Total energy expenditure was estimated using the Weir equation: energy expenditure = (kcal/min) = 3.91(VO2) + 1.1(VCO2)/1,000 (Simonson and DeFronzo, 1990). VO2 was normalized to the recorded daily body weight of each animal. Energy balance was calculated using the value for total energy expenditure and subtracting it from the recorded daily caloric intake. Gas-exchange data were obtained for 23 h. During the excluded 1 h (10a-11a) of the full 24 h day, animals were alert and handled (see below); thus the equivalent of an additional 1 h of average dark-phase energy expenditure was added to estimate total daily energy expenditure.
The cage calorimeters were housed inside environmental chambers to provide more precise control of cage ambient temperature (± 0.1°C). The shoebox cage was positioned on a custom-designed force platform sensitive to the transfer of the animal’s mass throughout the cage in order to obtain quantification of locomotor activity. While housed in the cage calorimeters, animals were given free access to a powdered form of the same pellet chow described above. Food intake, water intake and body weight of each animal were determined during a daily maintenance period that occurred 1 h before the onset of dark. At this time, daily behavioral and metabolic data were compiled and transferred for offline processing.
2.3 Acclimation, baseline, and ovariectomy (OVX) or sham surgery
Animals were allowed to acclimate to the metabolic housing units for ~ 6-7 d. Cycle phase was determined daily during the last hour of the light phase by cellular profile analysis of vaginal mucosa smear samples (taken by lavage). Standard criteria (Becker et al., 2005) were used to assign cycle phase, according to the types of cells present in the samples as determined by observing them under a light microscope. Once acclimated, 3 d of baseline data were collected for later comparison to data collected post-surgery. On the last day of baseline, animals were anesthetized with halothane and were either ovariectomized (OVX’d, n = 10/mice; n = 12/rat) or they underwent sham surgery (n = 10/mice; n = 8/rat). The intra-abdominal OVX surgery consisted of exposing the ovaries via a single midline skin incision. Each ovary was cauterized and excised at the tip of the uterine horn. The retroperitoneal muscle was then re-closed with silk suture and skin was re-closed with wound clips.
To compare responses to OVX, mice (n = 10 sham, n = 10 OVX) and rats (n = 8 sham, n = 12 OVX) were returned to their home cage calorimeters after surgery and observed daily for 3 wk. After 3 wk, all animals were euthanized (shams were terminated during estrus) with an overdose of pentobarbital sodium (60 mg/kg i.p.; Nembutal Sodium Solution, Henry Schein, Inc., Melville, NY). Animals were decapitated and hypothalami were immediately removed and placed in liquid nitrogen, and then stored at -80°C. Fat pads were removed and weighed.
2.4 Locomotor activity and food intake in ovarian-intact female mice
In order to determine the role of endogenous estradiol in regulating locomotor activity and food intake in the ovarian-intact mouse, an additional subset of ovarian-intact female C57/B6 mice (4 mos old, n = 8) were obtained and housed in home cage calorimeters for ~ 30 d. Cycle phase was determined daily (as described above in 2.3.).
2.5 Effect of pair-feeding on OVX-induced weight gain
Two additional cohorts of Long-Evans rats (14 wks of age) were obtained. The use of the home cage calorimeters was not employed for this additional experiment. To determine the role of food intake on OVX-induced weight gain, these two subsets of rats were part of either a “short-term” (3 wk) or a “long-term” (13 wk) pair-feeding study. In both pair-feeding studies, OVX’d rats were given either ad libitum access to powdered chow (OVX-AL, n = 4/per study) or they were individually pair-fed each day to the amount of food consumed daily on non-estrous days prior to OVX (OVX-PF, n = 5/per study). By preventing the OVX’d rats from over-eating, pair-feeding allows for the factor of increased food intake to be excluded when observing the effect of OVX on body weight. Sham rats (n = 4/per study) were given ad libitum access to powdered chow and were terminated on estrus at the end of the study.
2.6 Measurement of hypothalamic gene expression using reverse transcriptase polymerase chain reaction (RT-PCR)
Total RNA was extracted from whole hypothalamic tissue using TRIzol reagent (Invitrogen) in conjunction with phase lock gels (Eppendorf). Each sample received chloroform, was shaken vigorously for 15 sec, and then left to stand at room temperature for 3 min. Total RNA was precipitated from all samples. Concentrations of each RNA sample was obtained using a NanoDrop Spectrophotometer (ND-1000, Thermo Fisher Scientific) and its integrity was determined by denaturing gel electrophoresis as previously described (Goda and Minton, 1995). cDNA synthesis was performed by using iScript cDNA Synthesis Kit (Bio-Rad). RT-PCR was performed using the iCycler iQ Real-Time PCR Detection System (BioRad) in conjunction with iQ SYBR Green Supermix (BioRad). All data were normalized to the expression of the housekeeping gene RP27, as previously described (Resuehr et al., 2006). The PCR cycling conditions were as follows: HotStart 5 min at 95°C, cycling 40 times at 95°C denaturation for 15 s, annealing and fluorescent data collection at 55°C for 30 sec, extension at 72°C for 30 sec. A melt curve was performed to assess the stringency of the PCR after the cycling. A negative control was performed by omission of template cDNA. The primers used in this study (Table 1) were for some of the key hypothalamic neuropeptides involved in food intake and energy homeostasis, i.e., the anorexigenic neuropeptides: pro-opiomelanocortin (POMC) and leptin (ObRb) and the orexigenic neuropeptides: melanin-concentrating hormone (prepro-MCH), MCH receptor-1 (MCHR-1), neuropeptide-Y (NPY), agouti-related peptide (AgRP), and orexin (ORX).
Table 1.
Oligonucleotide primer sequences used for quantitative RT-PCR amplification.
| Target | Primer sequence |
|---|---|
| AgRP | |
| Sense | 5’- GCA GAC CGA GCA GAA GAT GT -3’ |
| Antisense | 5’ CTT GAA GAA GCG GCA GTA GC -3’ |
| Prepro-MCH | |
| Sense | 5’- TCG GTT GTT GCT CCT TCT CT -3 |
| Antisense | 5’- TTC CCT CTT TTC CTG TGT GG -3’ |
| MCH-R1 | |
| Sense | 5’- TCA GCT TGG GCT ATG CTA ACA G -3 |
| Antisense | 5’- CAA CAC CAA GCG TTT TCG AA-3’ |
| NPY | |
| Sense | 5’- AGA GAT CCA GCC CTG AGA CA -3’ |
| Antisense | 5’- AAC GAC AAC AAG GGA AAT GG -3’ |
| ObRb | |
| Sense | 5’- CTG GGT TTG CGT ATG GAA GT -3’ |
| Antisense | 5’- CCA GTC TCT TGC TCC TCA CC -3’ |
| ORX | |
| Sense | 5’- ACC ACT GCA CCG AAG ATA CC -3’ |
| Antisense | 5’- AGT TCG TAG AGA CGG CAG GA -3’ |
| POMC | |
| Sense | 5’- GGT GTA CCC CAA TGT CG -3 |
| Antisense | 5’- CTT CTC GGA GGT CAT GAA GC -3’ |
| RP27 | |
| Sense | 5’- TGG CGC TAA GAA AAG GAA GA -3’ |
| Antisense | 5’- ACC CAT GAA AAC TCC AGC AC -3’ |
AgRP: agouti-related peptide, prepro-MCH: prepro-melanin-concentrating hormone, MCH-R1: melanin-concentrating hormone receptor-1, NPY: neuropeptide-Y, ObRb: long isoform of the leptin receptor, ORX: orexin (a.k.a. hypocretin), POMC: proopiomelanocortin, and RP27: generic housekeeping gene.
2.8 Statistical analyses
Using SigmaPlot 12.0 statistical software, mean differences were analyzed using two-way repeated measures ANOVA (repeated measure = time (days) x treatment group) to evaluate effect of treatment (e.g., SHAM vs. OVX) over time for dependent variables (i.e., body weight, food intake, locomotor activity, VO2). Tukey’s post-hoc tests were used to determine specific days on which treatment groups were significantly different from one another. Single-value measurements, e.g., effect of OVX or pair-feeding on hypothalamic gene expression, were statistically assessed by one-way ANOVA. Tukey’s post hoc test was used to investigate differences between means after significant (p < 0.05) main or interactive ANOVA effects were found.
3. Results
3.1 Energy homeostasis and ingestive behavior
Mice
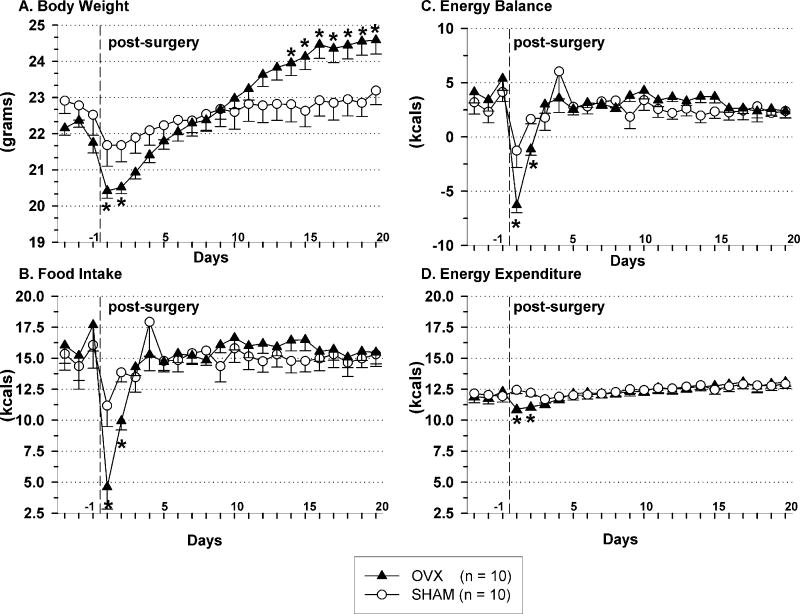
OVX’d mice gained weight (Fig.1A) with no accompanying increase in food intake; neither daily (Fig.1B), nor cumulative (shams = 93.4 +/- 6.6 g, OVX = 96.6 +/- 4.9 g; post-surgical days 1-21) food intake increases were observed in OVX’d mice. Other than on post-surgical days 1 and 2, daily energy expenditure and daily energy balance did not differ between OVX and sham mice (Fig.1C and D. respectively, p > 0.05).
Fig. 1.
Body weight (A), daily caloric intake (B), energy balance (C) and energy expenditure (D) during 3 baseline and 20 post-surgical days in ovariectomized (triangles) and sham (circles) mice. The vertical dashed line indicates the end of the baseline period and the day when surgery was performed. Data represent mean ± SEM, n = 10/per group; (*) indicates p < 0.05 for OVX vs. SHAM mice and for pre-OVX baseline vs. post-OVX data. Abbreviation: OVX, ovariectomized.
Ovarian-intact mice (n = 8) exhibited no difference in daily food intake on estrous- vs. non-estrous days (5.5 ± 0.4 g vs. 5.6 ± 0.3 g) over a period of 21 d.
Rats
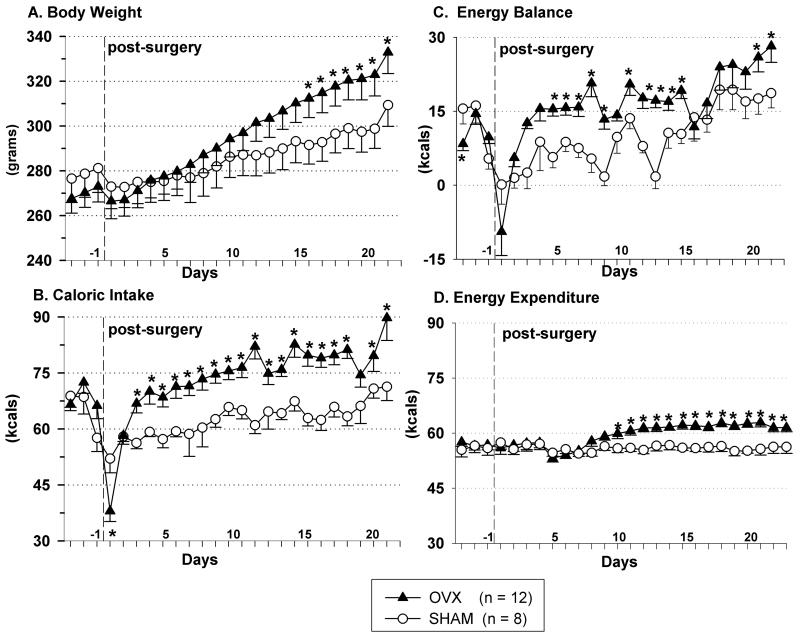
OVX’d rats gained weight (Fig. 2A) with an accompanying increase in daily food intake (Fig. 2B). Daily energy expenditure was greater in OVX’d rats, compared to their sham counterparts (Fig. 2C, p < 0.05). Daily energy balance was greater in OVX’d rats, compared to their sham counterparts (Fig. 2D, p < 0.05).
Fig. 2.
Body weight (A), daily caloric intake (B), energy balance (C) and energy expenditure (D) during 3 baseline days and 22 post-surgical days in ovariectomized (triangles) and sham (circles) rats. The vertical dashed line indicates the end of the baseline period and the day when surgery was performed. Data represent mean ± SEM, n = 12/OVX, n = 8/SHAM; (*) indicates p < 0.05 for OVX vs. SHAM rats. Abbreviation: OVX, ovariectomized.
3.2 Oxygen consumption (VO2), respiratory quotient (RQ), and locomotor activity
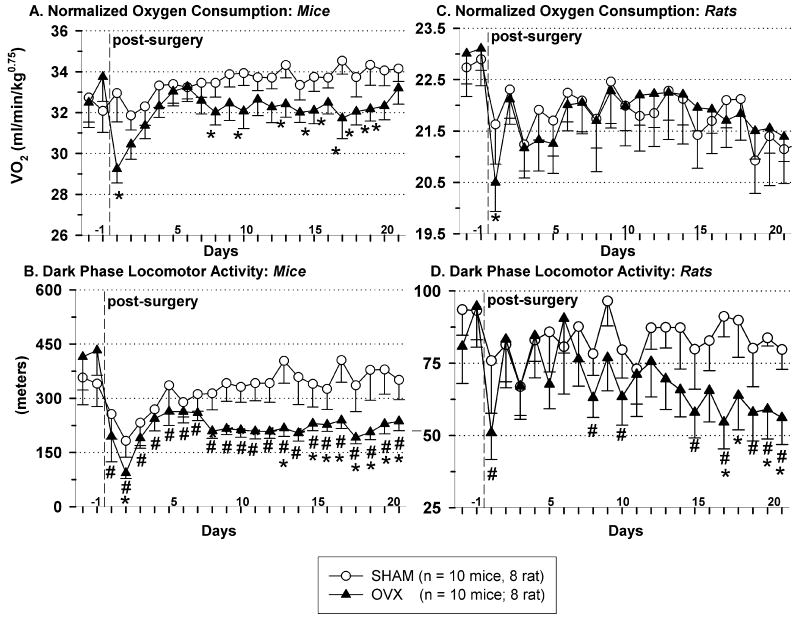
Compared to baseline, OVX’d mice (Fig. 3A, p < 0.05), but not rats (Fig. 3C, p > 0.05), exhibited decreased dark-phase mass specific oxygen consumption (VO2). Light-phase mass specific metabolic rate was not different between groups in either species (data not shown). Indicating a shift in substrate utilization toward fat storage, both OVX’d rats and mice had greater respiratory quotient (RQ) values compared to their sham counterparts (p < 0.05, both dark- and light-phase RQ were different in rats: OVX dark RQ = 0.94 ± 0.01, sham dark RQ = 0.91 ± 0.01, OVX light RQ = 0.93 ± 0.01, sham light RQ = 0.90 ± 0.01; light-phase, but not dark-phase, RQ was different in mice, OVX light RQ = 0.89 ± 0.01 sham light RQ = 0.86 ± 0.01).
Fig. 3.
Dark-phase oxygen consumption (VO2), normalized to body weight, in mice (A) and rats (C). Dark-phase locomotor activity in mice (B) and rats (D). The vertical dashed line indicates the end of the baseline period and the day when surgery was performed. Data represent mean ± SEM; mice: n = 10/per group, rats: n = 8/per group; (*) indicates p < 0.05 for OVX vs. sham animals; (#) indicates p < 0.05 for pre-OVX baseline vs. post-OVX data. Abbreviation: OVX, ovariectomized. *Note: different scales are used for mouse vs. rat data.
OVX decreased dark-phase locomotor activity in both mice (Fig.3B) and rats (Fig.3D), compared to their respective baselines and compared to the dark-phase locomotor activity of their sham counterparts (in mice only). Light-phase locomotor activity was not different between groups in either species (p > 0.05, data not shown).
Ovarian-intact mice exhibited no difference in dark-phase locomotor activity on estrous- vs. non-estrous days (361 ± 16 m vs. 379 ± 14 m) over a period of 21 d (post-acclimation to the home cage calorimeters).
3.3 Fat pads
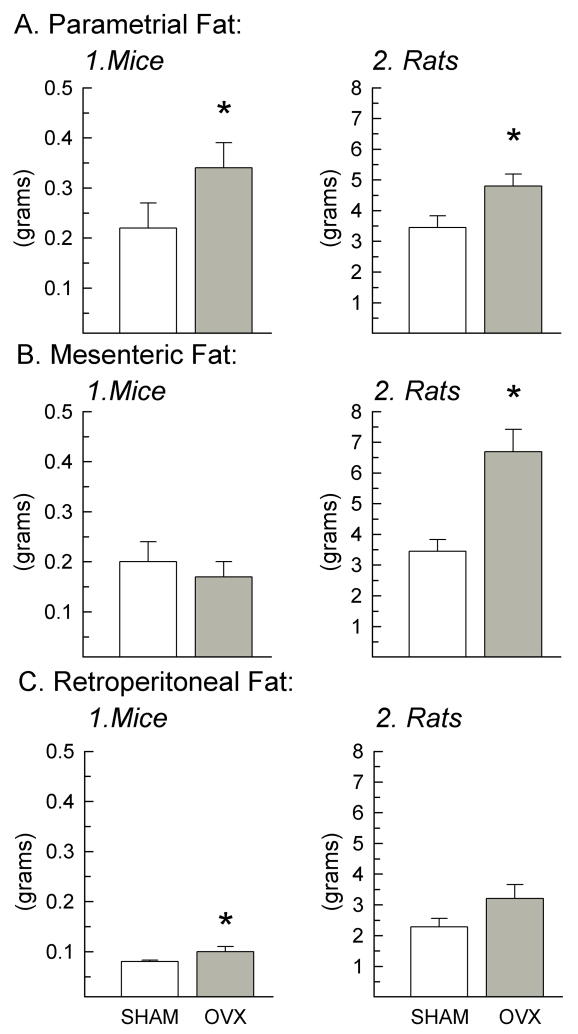
OVX increased parametrial fat in both species (Fig.4A, Mice: degrees of freedom (d.f.) = 19, F = 6.94, p = 0.017; Rats: d.f. = 23, F = 6.12, p = 0.022), compared to shams. OVX increased mesenteric fat in rats (Fig.4B.2., F = 15.8, p < 0.001), but not in mice (Fig.4B.1., p > 0.05). OVX increased retroperitoneal fat in mice (Fig.4C.1, F = 5.43, p < 0.05), but not in rats (Fig.4C.2., F = 4.23, p = 0.053).
Fig. 4.
Parametrial, mesenteric, and retroperitoneal fat pad weights excised at the termination of the study of OVX (gray bars) and sham (white bars) mice (A1, B1, C1; n = 10/group) and rats (A2, B2, C2; n = 12/group). Data represent mean ± SEM; (*) indicates p < 0.05 in OVX vs. sham animals. Abbreviation: OVX, ovariectomized.
3.4 Pair-feeding
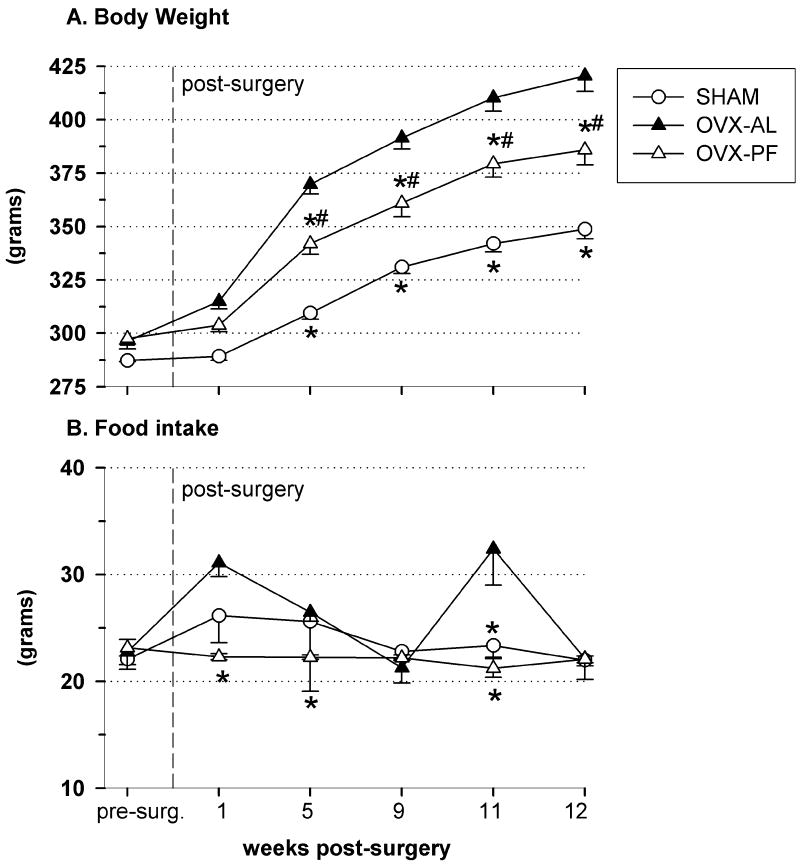
Pair-feeding attenuated, but did not prevent, OVX-induced weight gain (short-term pair-feeding cohort: 3 wk weight gain: SHAM = 10.2 +/- 8.5, OVX-pair-fed = 28.5 +/- 8.5, OVX-ad libitum-fed = 51 +/- 10.5 g). By post-surgical week 5, the pair-fed group had developed a clear intermediate body weight that was greater than that of shams (Fig.5A, d.f. = 12, F = 23.6, p = 0.007), yet less than that of OVX ad libitum-fed rats (Fig.5A, p = 0.022). This spread of body weights (OVX-AL > OVX-PF > SHAM) remained consistent throughout the remainder of the 13-wk study, despite the variability in food intake for the OVX-AL and sham rats from week to week (Fig.5B).
Fig. 5.
Body weight (A) and food intake (B) of OVX-AL (n = 4; closed triangles), OVX-PF (n = 5; open triangles), and SHAM (n = 4; circles) rats in the long-term pair-feeding study. Food intake values are 4-day averages; sham values represent food intake data obtained on non-estrous days only. Data represent mean ± SEM; (*) indicates p < 0.05 compared to OVX-AL, (#) indicates p < 0.05 compared to SHAM. Abbreviations: OVX, ovariectomized; AL, ad libitum-fed; PF, pair-fed.
3.5 Hypothalamic mRNA expression
Rats
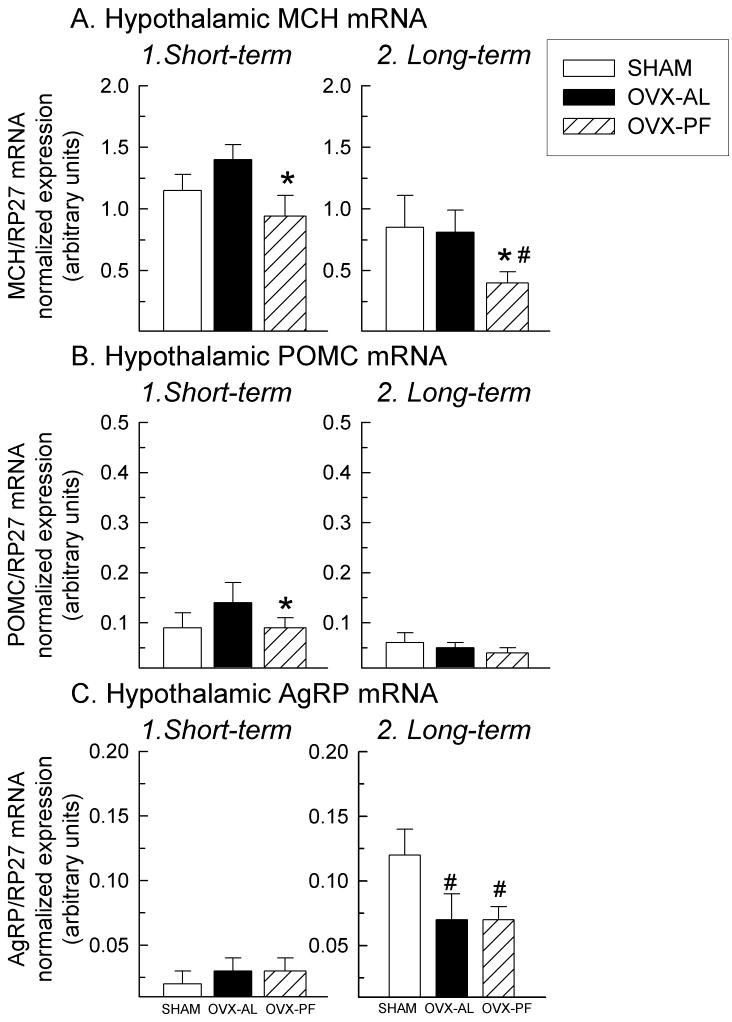
Compared to ad libitum-fed OVX’d rats, both short-term (3.5 wk) and long-term (13 wk) pair-feeding of OVX’d rats led to a reduction in mRNA of the orexigenic neuropeptide, prepro-MCH (Fig.6A, p < 0.05, d.f. = 12, short term: F = 3.55, p = 0.048; Fig.6B, long-term: F = 8.64, p = 0.009 OVX-AL vs. OVX-PF; p = 0.02 OVX-AL vs. SHAM). Short-term pair-feeding led to a reduction in the anorexigenic neuropeptide, POMC mRNA in OVX rats (Fig.6B, d.f. = 12, F = 5.23, p = 0.01 OVX-AL vs. OVX-PF). Correspondingly, sham rats had less POMC mRNA expression than OVX rats (Fig.6B, d.f. = 12, F = 5.23, p = 0.02 OVX-AL vs. SHAM). OVX’d rats (both PF and AL) in the long-term study had reduced AgRP levels, compared to shams (Fig.6C, d.f. = 12, F = 6.89, p = 0.01 SHAM vs. OVX-AL; p = 0.01 SHAM vs. OVX-PF). By 13 wk, all rats (OVX-PF, OVX-AL, and shams) had enhanced AgRP mRNA expression, compared to the rats in the short-term PF cohort (terminated at 3.5 wk post-surgery; Fig.6C, p = 0.009). There were no differences found in NPY, ORX, or ObRb mRNA expression (data not shown, p > 0.05) in the short-term study; these genes were not quantified in the long-term study.
Fig. 6.
Hypothalamic MCH (A; note: different scale), POMC (B), and AgRP (C) mRNA, as measured by quantitative RT-PCR and normalized to expression of the housekeeping gene, RP27. Data represent mean ± SEM; (*) indicates p < 0.05 compared to OVX-AL, (#) indicates p < 0.05 compared to SHAM. Abbreviations: OVX, ovariectomy; AL, ad libitum; PF, pair-fed; MCH, melanin-concentrating hormone; POMC, pro-opiomelanocortin; AgRP, agouti-related peptide.
Mice
In an additional cohort of mice (N=4/group, we determined the effect of OVX on expression of three key hypothalamic genes involved in feeding (prepro-MCH, MCHR-1, and POMC mRNA). Because no changes in food intake were observed between OVX’d and sham mice (Fig.1B, p > 0.05), no changes were expected in orexigenic and/or anorexigenic tone. Consistent with the behavioral data indicating a lack of hyperphagia in OVX’d mice, no changes in hypothalamic gene expression was found between OVX’d and sham mice in any of these genes (data not shown; prepro-MCH: d.f. = 7, F = 0.22, p = 0.66; MCHR-1: F = 1.513, p = 0.24; POMC: F = 1.23, p = 0.29).
4. Discussion
The primary goal of this study was to assess the relative contribution of energy intake and expenditure to OVX-induced weight gain in female mice and rats. Three new findings were obtained. First, OVX-induced weight gain in rats is mediated by hyperphagia and reduced locomotor activity; while in mice, it is mediated by reduced locomotor activity and reduced metabolic rate. Second, pair-feeding of OVX’d rats attenuated, but did not prevent, OVX-induced weight gain. Lastly, ovarian-intact mice did not demonstrate estrous-related hypophagia, nor home cage hyperactivity.
To date, the role of endogenous E2 on food intake and locomotor activity in the ovarian-intact mouse has not been well characterized. Of the few studies that have measured these parameters in ovarian-intact mice, the absence of both an estrous-related increase in locomotor activity and decrease in food intake have been reported (Barnett and McEwan, 1973; Petersen, 1976; Meziane et al., 2007). The results of the present study are consistent with these findings, suggesting that the phasic effect of E2, while very well established in the rat, is notably absent in the mouse. To our knowledge, no other studies have measured food intake and/or home cage activity across the estrous cycle in ovarian-intact mice. This may be due, at least in part, to the inherent difficulty in assessing the murine estrous cycle, as mice tend to have longer cycles than rats (~ 7 d vs. ~ 4 d) and are prone to atypical cycling patterns (vom Saal et al., 1994).
With regard to the tonic effect of E2 on food intake in mice, the evidence is conflicting. One study reports a cumulative increase over time in OVX’d mice (Geary et al., 2001), an effect that was unexpectedly absent in the present study. The lack of corroboration could be due to: different ages and strains of mice and different length of time of post-OVX observation. In contrast, another study reports that OVX’d vehicle-treated mice do not exhibit hyperphagia, but do exhibit increased post-OVX weight gain (Blaustein et al., 1976), which is consistent with the present study. Additionally, a third group reports that at 4 mos post-OVX, there was no difference in food intake between sham and OVX’d mice, but that by 5 mos, OVX’d mice ate slightly more (Gomori et al., 2007). These findings support the notion that, unlike rats, 3 wk post-OVX is not a sufficient amount of time to observe a food intake effect in mice. Finally, exogenous E2 injections in OVX’d mice have been shown to produce a decrease in food intake (Blaustein et al., 1976), yet they were unable to decrease body weight, suggesting that OVX-induced body weight and food intake changes may be dissociable. Taken together, it is possible that endogenous E2 may not be a potent regulator of food intake in the female mouse. However, the possibility that endogenous E2 modulates micro-meals and that the post-OVX observation period must exceed 4 wk in order to observe a food intake effect cannot be excluded.
In contrast to mice, it is well established that E2 has a phasic (Eckel et al., 2000) and tonic (Geary and Asarian, 1999) inhibitory effect on feeding in female rats. The present study confirms that OVX’d rats are hyperphagic, have greater energy expenditure, and are in greater positive energy balance than their sham counterparts. Furthermore, female rats are hyperactive on estrus (Finger, 1969) and E2 replacement in OVX’d rats increases activity (Gentry and Wade, 1976), while OVX’d rats with no E2 replacement are hypoactive (Wade, 1972). OVX-induced hypoactivity has also been reported in mice (Heine et al., 2000; Gorzek et al., 2007). Consistent with these findings, both mass-specific metabolic rate and locomotor activity were suppressed in OVX’d mice in the present study, while only locomotor activity was suppressed in OVX’d rats. It is reasonable to suggest that instead of suppressing food intake, endogenous E2 may act mainly by modulating these components of energy homeostasis (metabolic rate and locomotor activity) in female mice since OVX’d mice were not hyperphagic, yet still gained weight rapidly.
The observation that energy expenditure was not suppressed in OVX’d animals of either species, is perplexing, yet it is not a new finding. Some have sought to understand this by looking at fuel utilization (Ainslie et al., 2001; Chen and Heiman, 2001), intrascapular brown adipose tissue (iBAT) weight (Ainslie et al., 2001), iBAT protein content (Richard, 1986), and the amount of bound guanosine diphosphate (GDP) (Richard, 1986). While no differences have been reported between OVX’d and sham rats, to our knowledge, these parameters have not been reported in mice. Such information may provide additional insight into why OVX’d mice get obese, despite normophagia and unchanged energy expenditure. Nonetheless, it is important to acknowledge the possibility that our indirect calorimetry system may not be sensitive enough to measure the small changes in oxygen consumption that likely occur in the relatively small mouse.
Because of conflicting reports regarding the contribution of increased food intake to OVX-induced weight gain in the rat ((Roy and Wade, 1977; Shimomura et al., 1989) vs. (Liang et al., 2002)), we performed short- and long-term pair-feeding experiments. The data show that pair-feeding attenuated, but did not prevent, OVX-induced weight gain. This pivotal finding strongly suggests a role for E2 in regulating energy expenditure, as decreased food intake alone did not prevent OVX-induced weight gain.
To investigate whether key hypothalamic neuropeptides involved in energy homeostasis are altered by lack of ovarian hormones, we measured total hypothalamic mRNA levels of a number of genes that have been linked to having a role in E2-induced hypophagia. E2 decreases the orexigenic effect of MCH (Messina et al., 2006). Compared to ad libitum-fed OVX’d rats, pair-fed OVX’d rats had reduced total hypothalamic prepro-MCH mRNA. The lack of an increase in prepro-MCH mRNA in pair-fed rats was at first perplexing, but seems reasonable when considering the fact that these rats were not fasted, but were merely pair-fed. It is also possible that the metabolic adaptations associated with obesity have overridden the normal signals that would have otherwise increased prepro-MCH in response to mild caloric restriction.
Given that MCH neurons are likely targets of E2 action (Murray et al., 2000; Mystkowski et al., 2000), it was predicted that endogenous E2 would exert an inhibitory effect on prepro-MCH mRNA expression. Thus, it was surprising that prepro-MCH mRNA levels of shams (terminated on estrus) were not decreased. That an effect was not observed could be due to temporal compensation by other neuropeptides involved in energy homeostasis, or because an optimal inhibitory effect of endogenous E2 on prepro-MCH mRNA would be best uncovered at a different time point, i.e., at proestrus, when circulating E2 levels are highest. Such critical factors require further experimental investigation.
Consistent with previous reports (Brady et al., 1990; Kim et al., 1996), short-term pair-feeding led to a reduction in POMC mRNA, supporting the notion of POMC being a critical catabolic effector (Schwartz et al., 2003). That shams did not exhibit any long-term alterations in POMC mRNA compared to OVX’d rats is consistent with previous reports finding no phasic changes in POMC mRNA in ovarian-intact rats (Rocha et al., 2004), in OVX’d rats treated with E2 (Treiser and Wardlaw, 1992), nor in OVX’d vs. ovarian-intact rats (Wise et al., 1990). Also consistent with previous reports (Korner et al., 2001; Rocha et al., 2004; Santollo and Eckel, 2008), there were no differences in AgRP mRNA between any of the groups at the 3.5 wk time point. However, the presence of endogenous E2 (in shams) was associated with increased AgRP mRNA at the 13.5 wk time point. This is consistent with the observed increase in AgRP mRNA reported in a model of chronic hyperestrogenemia in male rats (Mystkowski et al., 2000), suggesting the possibility that increased AgRP mRNA occurs in response to estrous-related hypophagia.
Consistent with the behavioral data indicating a lack of hyperphagia in OVX’d mice, no changes in hypothalamic gene expression were observed between OVX’d and sham mice, suggesting there were no alterations in orexigenic nor anorexigenic tone, as measured by these genes. Whether or not other genes involved in energy homeostasis are implicated in the physiology of the OVX’d mouse remains to be elucidated.
In conclusion, the results indicate that clear species-specific differences exist between rats and mice in response to OVX. Namely, OVX-induced weight gain is mediated by hyperphagia and hypoactivity in rats, but it is largely mediated by hypoactivity and reduced metabolic rate in mice. The possibility that altered substrate utilization plays a role in OVX-induced obesity and the possibility of nuclei-specific changes in hypothalamic mRNA expression warrant further investigation. Lastly, pair-feeding of OVX’d rats attenuates, but does not prevent OVX-induced weight gain, strongly suggesting a metabolic role for E2-modulation of energy homeostasis.
Acknowledgments
This work was supported by a Ruth L. Kirschstein National Research Service Award (NRSA) Predoctoral Training Grant, National Institute for Neurological Disorders and Stroke, NIH F31 NS057856-01 (M. M. Messina).
We gratefully acknowledge Dr. James Olcese, Holly E. Sikes, Gelson J. Taube, Jr., and James Sharkey for their generous assistance with molecular techniques and Dr. Lisa A. Eckel for her always helpful assistance and feedback. We would also like to thank the Florida State University Program in Neuroscience’s Technical Support Group for providing expertise in instrumentation (Ross Henderson, Paul Hendricks, Ron Thompson) and computer programming (Chris Baker).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ainslie DA, Morris MJ, Wittert G, Turnbull H, Proietto J, Thorburn AW. Estrogen deficiency causes central leptin insensitivity and increased hypothalamic neuropeptide Y. Int J Obes Relat Metab Disord. 2001;25(11):1680–8. doi: 10.1038/sj.ijo.0801806. [DOI] [PubMed] [Google Scholar]
- Barnett SA, McEwan IM. Movements of virgin, pregnant and lactating mice in a residential maze. Physiol Behav. 1973;10(4):741–6. doi: 10.1016/0031-9384(73)90155-8. [DOI] [PubMed] [Google Scholar]
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146(4):1650–73. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- Blaustein JD, Gentry RT, Roy EJ, Wade GN. Effects of ovariectomy and estradiol on body weight and food intake in gold thioglucose-treated mice. Physiol Behav. 1976;17(6):1027–30. doi: 10.1016/0031-9384(76)90028-7. [DOI] [PubMed] [Google Scholar]
- Brady LS, Smith MA, Gold PW, Herkenham M. Altered expression of hypothalamic neuropeptide mRNAs in food-restricted and food-deprived rats. Neuroendocrinology. 1990;52(5):441–7. doi: 10.1159/000125626. [DOI] [PubMed] [Google Scholar]
- Chen Y, Heiman ML. Increased weight gain after ovariectomy is not a consequence of leptin resistance. Am J Physiol Endocrinol Metab. 2001;280(2):E315–22. doi: 10.1152/ajpendo.2001.280.2.E315. [DOI] [PubMed] [Google Scholar]
- Clegg DJ, Brown LM, Zigman JM, Kemp CJ, Strader AD, Benoit SC, Woods SC, Mangiaracina M, Geary N. Estradiol-dependent decrease in the orexigenic potency of ghrelin in female rats. Diabetes. 2007;56(4):1051–8. doi: 10.2337/db06-0015. [DOI] [PubMed] [Google Scholar]
- Della-Zuana O, Presse F, Ortola C, Duhault J, Nahon JL, Levens N. Acute and chronic administration of melanin-concentrating hormone enhances food intake and body weight in Wistar and Sprague-Dawley rats. Int J Obes Relat Metab Disord. 2002;26(10):1289–95. doi: 10.1038/sj.ijo.0802079. [DOI] [PubMed] [Google Scholar]
- Eckel LA. Estradiol: a rhythmic, inhibitory, indirect control of meal size. Physiol Behav. 2004;82(1):35–41. doi: 10.1016/j.physbeh.2004.04.023. [DOI] [PubMed] [Google Scholar]
- Eckel LA, Houpt TA, Geary N. Spontaneous meal patterns in female rats with and without access to running wheels. Physiol Behav. 2000;70(3-4):397–405. doi: 10.1016/s0031-9384(00)00278-x. [DOI] [PubMed] [Google Scholar]
- Finger FW. Estrus and general activity in the rat. J Comp Physiol Psychol. 1969;68(3):461–6. doi: 10.1037/h0027490. [DOI] [PubMed] [Google Scholar]
- Geary N, Asarian L. Cyclic estradiol treatment normalizes body weight and test meal size in ovariectomized rats. Physiol Behav. 1999;67(1):141–7. doi: 10.1016/s0031-9384(99)00060-8. [DOI] [PubMed] [Google Scholar]
- Geary N, Asarian L, Korach KS, Pfaff DW, Ogawa S. Deficits in E2-dependent control of feeding, weight gain, and cholecystokinin satiation in ER-alpha null mice. Endocrinology. 2001;142(11):4751–7. doi: 10.1210/endo.142.11.8504. [DOI] [PubMed] [Google Scholar]
- Gentry RT, Wade GN. Sex differences in sensitivity of food intake, body weight, and running-wheel activity to ovarian steroids in rats. J Comp Physiol Psychol. 1976;90(8):747–54. doi: 10.1037/h0077246. [DOI] [PubMed] [Google Scholar]
- Goda SK, Minton NP. A simple procedure for gel electrophoresis and northern blotting of RNA. Nucleic Acids Res. 1995;23(16):3357–8. doi: 10.1093/nar/23.16.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomori A, Ishihara A, Ito M, Matsushita H, Ito M, Mashiko S, Iwaasa H, Matsuda M, Bednarek MA, Qian S, Macneil DJ, Kanatani A. Blockade of MCH1 receptor signalling ameliorates obesity and related hepatic steatosis in ovariectomized mice. Br J Pharmacol. 2007;151(6):900–8. doi: 10.1038/sj.bjp.0707292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorzek JF, Hendrickson KC, Forstner JP, Rixen JL, Moran AL, Lowe DA. Estradiol and tamoxifen reverse ovariectomy-induced physical inactivity in mice. Med Sci Sports Exerc. 2007;39(2):248–56. doi: 10.1249/01.mss.0000241649.15006.b8. [DOI] [PubMed] [Google Scholar]
- Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci U S A. 2000;97(23):12729–34. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EM, Welch CC, Grace MK, Billington CJ, Levine AS. Chronic food restriction and acute food deprivation decrease mRNA levels of opioid peptides in arcuate nucleus. Am J Physiol. 1996;270(5 Pt 2):R1019–24. doi: 10.1152/ajpregu.1996.270.5.R1019. [DOI] [PubMed] [Google Scholar]
- Korner J, Savontaus E, Chua SC, Jr, Leibel RL, Wardlaw SL. Leptin regulation of Agrp and Npy mRNA in the rat hypothalamus. J Neuroendocrinol. 2001;13(11):959–66. doi: 10.1046/j.1365-2826.2001.00716.x. [DOI] [PubMed] [Google Scholar]
- Liang YQ, Akishita M, Kim S, Ako J, Hashimoto M, Iijima K, Ohike Y, Watanabe T, Sudoh N, Toba K, Yoshizumi M, Ouchi Y. Estrogen receptor beta is involved in the anorectic action of estrogen. Int J Obes Relat Metab Disord. 2002;26(8):1103–9. doi: 10.1038/sj.ijo.0802054. [DOI] [PubMed] [Google Scholar]
- Messina MM, Boersma G, Overton JM, Eckel LA. Estradiol decreases the orexigenic effect of melanin-concentrating hormone in ovariectomized rats. Physiol Behav. 2006;88(4-5):523–8. doi: 10.1016/j.physbeh.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Messina MM, Overton JM. Cardiovascular effects of melanin-concentrating hormone. Regul Pept. 2007;139(1-3):23–30. doi: 10.1016/j.regpep.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Meziane H, Ouagazzal AM, Aubert L, Wietrzych M, Krezel W. Estrous cycle effects on behavior of C57BL/6J and BALB/cByJ female mice: implications for phenotyping strategies. Genes Brain Behav. 2007;6(2):192–200. doi: 10.1111/j.1601-183X.2006.00249.x. [DOI] [PubMed] [Google Scholar]
- Murray JF, Baker BI, Walker R, Baker BI, Thody AJ, Nijenhuis WA, Yukitake J, Wilson CA. The influence of gonadal steroids on pre-pro melanin-concentrating hormone mRNA in female rats. J Neuroendocrinol. 2000;12(1):53–9. doi: 10.1046/j.1365-2826.2000.00425.x. [DOI] [PubMed] [Google Scholar]
- Mystkowski P, Seeley RJ, Hahn TM, Baskin DG, Havel PJ, Matsumoto AM, Wilkinson CW, Peacock-Kinzig K, Blake KA, Schwartz MW. Hypothalamic melanin-concentrating hormone and estrogen-induced weight loss. J Neurosci. 2000;20(22):8637–42. doi: 10.1523/JNEUROSCI.20-22-08637.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Chan J, Gustafsson JA, Korach KS, Pfaff DW. Estrogen increases locomotor activity in mice through estrogen receptor alpha: specificity for the type of activity. Endocrinology. 2003;144(1):230–9. doi: 10.1210/en.2002-220519. [DOI] [PubMed] [Google Scholar]
- Petersen S. The temporal pattern of feeding over the oestrous cycle of the mouse. Anim Behav. 1976;24(4):939–55. doi: 10.1016/s0003-3472(76)80023-1. [DOI] [PubMed] [Google Scholar]
- Qu D, Ludwig DS, Gammeltoft S, Piper M, Pelleymounter MA, Cullen MJ, Mathes WF, Przypek R, Kanarek R, Maratos-Flier E. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380(6571):243–7. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- Rashotte ME, Basco PS, Henderson RP. Daily cycles in body temperature, metabolic rate, and substrate utilization in pigeons: influence of amount and timing of food consumption. Physiol Behav. 1995;57(4):731–46. doi: 10.1016/0031-9384(94)00315-7. [DOI] [PubMed] [Google Scholar]
- Resuehr D, Sikes HE, Olcese J. Exploratory investigation of the effect of melatonin and caloric restriction on the temporal expression of murine hypothalamic transcripts. J Neuroendocrinol. 2006;18(4):279–89. doi: 10.1111/j.1365-2826.2006.01414.x. [DOI] [PubMed] [Google Scholar]
- Richard D. Effects of ovarian hormones on energy balance and brown adipose tissue thermogenesis. Am J Physiol. 1986;250(2 Pt 2):R245–9. doi: 10.1152/ajpregu.1986.250.2.R245. [DOI] [PubMed] [Google Scholar]
- Rocha M, Bing C, Williams G, Puerta M. Physiologic estradiol levels enhance hypothalamic expression of the long form of the leptin receptor in intact rats. J Nutr Biochem. 2004;15(6):328–34. doi: 10.1016/j.jnutbio.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Roy EJ, Wade GN. Role of food intake in estradiol-induced body weight changes in female rats. Horm Behav. 1977;8(3):265–74. doi: 10.1016/0018-506x(77)90001-0. [DOI] [PubMed] [Google Scholar]
- Santollo J, Eckel LA. Estradiol decreases the orexigenic effect of neuropeptide Y, but not agouti-related protein, in ovariectomized rats. Behav Brain Res. 2008;191(2):173–7. doi: 10.1016/j.bbr.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Seeley RJ, Barsh GS, Baskin DG, Leibel RL. Is the energy homeostasis system inherently biased toward weight gain? Diabetes. 2003;52(2):232–8. doi: 10.2337/diabetes.52.2.232. [DOI] [PubMed] [Google Scholar]
- Shearman LP, Camacho RE, Sloan Stribling D, Zhou D, Bednarek MA, Hreniuk DL, Feighner SD, Tan CP, Howard AD, Van der Ploeg LH, MacIntyre DE, Hickey GJ, Strack AM. Chronic MCH-1 receptor modulation alters appetite, body weight and adiposity in rats. Eur J Pharmacol. 2003;475(1-3):37–47. doi: 10.1016/s0014-2999(03)02146-0. [DOI] [PubMed] [Google Scholar]
- Shimomura Y, Shimizu H, Kobayashi I, Kobayashi S. Importance of feeding time in pair-fed, ovariectomized rats. Physiol Behav. 1989;45(6):1197–200. doi: 10.1016/0031-9384(89)90109-1. [DOI] [PubMed] [Google Scholar]
- Simonson DC, DeFronzo RA. Indirect calorimetry: methodological and interpretative problems. Am J Physiol. 1990;258(3 Pt 1):E399–412. doi: 10.1152/ajpendo.1990.258.3.E399. [DOI] [PubMed] [Google Scholar]
- Treiser SL, Wardlaw SL. Estradiol regulation of proopiomelanocortin gene expression and peptide content in the hypothalamus. Neuroendocrinology. 1992;55(2):167–73. doi: 10.1159/000126111. [DOI] [PubMed] [Google Scholar]
- vom Saal FS, Finch CE, Nelson JF. Natural history and mechanisms of reproductive aging in humans, laboratory rodents, and other selected vertebrates. In: Knobil E, Neill J, editors. The Physiology of Reproduction. 2. 1994. pp. 861–1010. [Google Scholar]
- Wade GN. Gonadal hormones and behavioral regulation of body weight. Physiol Behav. 1972;8(3):523–34. doi: 10.1016/0031-9384(72)90340-x. [DOI] [PubMed] [Google Scholar]
- Wise PM, Scarbrough K, Weiland NG, Larson GH. Diurnal pattern of proopiomelanocortin gene expression in the arcuate nucleus of proestrous, ovariectomized, and steroid-treated rats: a possible role in cyclic luteinizing hormone secretion. Mol Endocrinol. 1990;4(6):886–92. doi: 10.1210/mend-4-6-886. [DOI] [PubMed] [Google Scholar]