Abstract
Merkel Cell Virus (MCV) is a newly discovered polyoma virus recently found in a rare skin cancer, Merkel cell carcinoma. However, MCV has also been detected in some normal tissue samples. We tested and compared the relative quantity of the MCV in a set of diverse human tissue samples with the Merkel cell carcinoma samples. The levels of MCV in Merkel cell carcinomas were over 60 times higher than the highest values in all other tissues. Low quantities of MCV were detected in diverse tissue samples independently of malignant or benign histologic status. Higher levels of the virus were found in the upper aerodigestive tract, digestive system, and saliva compared to the lung and genitourinary system samples. These results confirm that MCV is widespread in the human body and suggest a possible fecal-oral transmission route similar to the Hepatitis A virus. Despite widespread presence of the virus, it appears that only neuroendocrine skin cells are susceptible to transformation by MCV.
INTRODUCTION
Merkel cell carcinomas are rare neuroendocrine skin tumors that develop from neuroendocrine cells responsible for the sense of touch and pressure. The incidence of Merkel cell carcinoma (MCC) has tripled over the past 20 years to about 1,500 cases a year. Increased ascertainment has at least in part contributed to this rise in incidence. People at risk include those with fair skin, excessive sunlight exposure, and a history of numerous non-melanoma skin cancers. Additionally, patients under immunosuppression including solid organ transplant recipients, and patients with altered lymphocytic function such as patients with AIDS, lymphoma, and leukemia appear to be predisposed to MCC (1–3). The existing relationship between immune suppression and Merkel cell carcinoma suggests the possibility of an infectious agent in the pathogenesis of this carcinoma. Through digital transcriptome substraction, a high throughput sequencing technique that detects sequences that are not in the human genome, MCV was originally discovered. The virus was integrated into the Merkel cell carcinoma cells in a monoclonal pattern, suggesting that it might trigger or induce tumor formation (4).
The Polyomaviridae family is a group of non-enveloped, small double-stranded DNA viruses that have been isolated from human and animal species. The polyomaviruses’ genomes encode a large T-antigen which is the major protein involved in neoplastic transformation through deregulation of the p53 and retinoblastoma family member tumor suppressors (5, 6) However, in humans a direct association between the polyomaviruses and cancer has proven elusive (6). There are five human polyoma viruses: BK virus (BKV), JC virus (JCV), KI virus (KIV), WU virus (WUV), and Merkel cell virus (MCV). BKV and JCV are widespread in the human population and cause clinical disease in rare instances. JCV is associated with leukoencephalopathy and BKV with nephropathy among immunocompromised patients (7, 8). These viruses are typically non oncogenic and the evidence for their role in cancer remains controversial (9, 10). Both KIV and WUV are newly discovered polyoma viruses that were detected in the respiratory tract and have not been reported in human cancers (11, 12). MCV may potentially be the first human oncogenic polyoma virus yet described, and the eighth human virus associated with cancer.
Recent studies have confirmed the presence of MCV in Merkel cell carcinoma cohorts through PCR (13–17). MCV has also been detected in non malignant tissues such as skin, gut, and more recently in respiratory secretion samples (4, 15, 18). Using a new quantitative assay we tested a large set of benign and malignant human samples for the presence and level of MCV.
MATERIALS AND METHODS
Human Tissue Samples
The reference Merkel cell carcinoma tumor was collected by the department of Dermatology at the Johns Hopkins Hospital as excess pathological tissue not required for diagnosis. The rest of the human samples were collected from the available tissue bank in our laboratory and anonymous excess pathology tissues from the Johns Hopkins Hospital. The reference Merkel cell carcinoma and the remaining skin samples (other than MCC tumors) were collected under IRB 05-05-05-0e and 04-08-05-03e protocols. All other samples (including Merkel cell carcinomas and saliva samples) were collected and tested under IRB 03-11-12-06e. Prostate, testis, and six of the seven MCC samples were paraffin embedded samples, while all the rest were fresh frozen tissues.
DNA extraction
Microdissected tissues and saliva were extracted using techniques previously described. In brief, DNA was extracted by digestion with 50 µg/mL proteinase K (Boehringer) in the presence of 1% SDS at 48°C followed by phenol/chloroform extraction and ethanol precipitation. Extracted DNA was dissolved in either LoTE (2.5 mM EDTA, 10 mM Tris–HCl [pH 8]) or molecular grade water and stored at −20°C. Samples were diluted to 150 or 90 ng in each reaction.
Real Time PCR
We quantitatively tested diverse human tissues for MCV sequences based on real time PCR of MCV fragments of the large T antigen. Specific primers and fluorescent probes were designed to amplify the VP1 from the late gene region and LT3 from the T antigen region. The β-actin gene was used to normalize levels for DNA input as well as an internal loading control. Table 1 shows primers and probes used for the real time PCR. Molecular grade water was used as a non template control. To prevent potential contamination, the PCR reactions were prepared in a room with no amplified product and the DNA was prepared in a hood with overnight UV-radiation.
Table 1.
Primers and fluorescent probes used.
| Gene | Forward primer (3’–5’) | Probe (3’–5’) | Reverse primer (3’–5’) |
|---|---|---|---|
| VP1 | CCTGATTTTTAGGTGTCATTTT (3790–3811) |
GGGGGAGAACCTCTGGATTTGCA (3901–3924) |
GTAAATTACCATATGTTTGCCA (3926–3950) |
| LT3 | TAAAGCAAAAAAACTGTCTGACG (602–625) |
CTTGGGAAAGTTTTGACTGGTGGCA (680–705) |
TAGAAAAGGTGCAGATGCAGTA (734–756) |
| β-actin | TCACCCACACTGTGCCCATCTACGA (390–414) |
ATGCCCTCCCCCATGCCATCCTGCGT (432–461) |
CAGCGGAACCGCTCATTGCCAATGG (496–522) |
Relative quantification of the virus was performed using the virus signal in a positive Merkel cell carcinoma sample as reference. Standard curves were developed by diluting reference Merkel cell carcinoma DNA to 90ng, 9ng, 0.9 ng, 0.09ng, and 0.009ng. (Taqman HT7900 Applied Biosystem). The amount of input DNA tested was 150ng for all samples, equivalent to testing approximately 22,700 cells.
To confirm specific amplification of the assay for MCV sequences, randomly selected positive PCR products at high, medium, and low levels were run on an agarose gel revealing single band products. Ten randomly selected positive PCR products from both genes, VP1 and LT3, were further sequenced revealing MCV sequences.
Statistical Analysis
Comparisons between subgroups of the sample set were done using Stata data analysis and statistical software version 8. Unpaired t-tests for samples with unequal variance using the Welch correction were performed to evaluate for significant difference between VP1 and LT3 signals within organ systems of malignant and benign tissues. Results were considered significant when the p values were less than 0.05. After exclusion of MCC samples, quartiles were obtained by multiplying the frequency of positivity by the median levels of the virus in the positive samples to account for MCV levels.
RESULTS
MCV by system
We tested DNA from 293 human samples for two MCV genes, VP1 and LT3, with quantitative PCR. The sample set included the skin – Merkel cell carcinoma and non-Merkel cell carcinoma – , the upper aero digestive tract – esophagus and oral cavity –, the digestive system – liver and colon –, the genitourinary system – kidney, bladder, prostate, and testis –, and the respiratory system – lung. Six of seven Merkel cell carcinomas were positive for the MCV. We found low quantities of MCV in the other tissue types, but Merkel cell carcinomas had higher quantitative values (p<0.05). When comparing the values of MCV in the diverse organ systems, the levels in the aerodigestive tract and digestive system were significantly higher than the levels for the respiratory and genitourinary systems (VP1 p<0.01 and LT3 p<0.0001). All other comparisons were not significant. We were unable to detect differences in MCV levels according to fixation technique. Figure 1 shows the box plots for the positive values within the different tissue groups.
Figure 1.
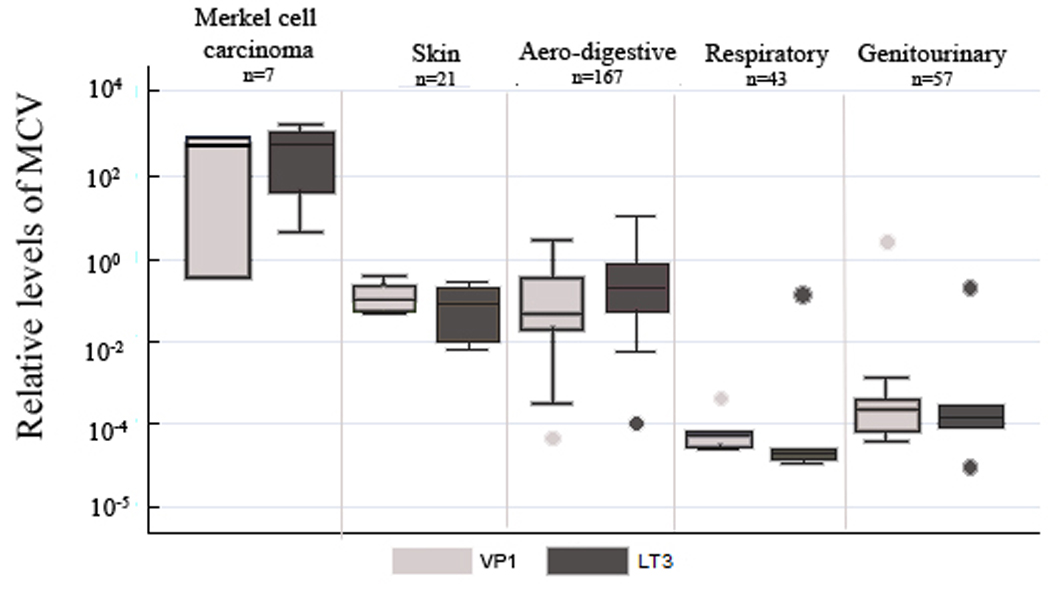
Box plots showing MCV relative levels through the different issue groups. Markel cell carcinoma, skin, areodigestive, respiratory, and genitourinary samples are shown. The values for gene VP1 and LT3 are shown separately. Boxplots show the middle 50% of data, the line is the median, and the bars extend the median by 1.5 times the interquartile range. Saliva samples were not included in the aero-digestive system.
MCV by organ
Oral cavity and saliva show some samples with levels above the levels in the Non-Merkel cell carcinoma skin samples. Esophagus, liver, and colon all have levels comparable to those of Non-Merkel cell carcinoma skin samples. Lung, bladder, prostate, and testis have levels below the rest of the samples. Saliva samples were in the highest quartile. Interestingly, all saliva samples (10) tested positive. The oral cavity and the liver samples were in the next quartile followed by skin, colon, and testis. The final quartile had the lung, esophagus, prostate, bladder, and kidney. Figure 2 shows the levels of MCV by organ using a scatter plot and a human figure labeled by the quantiles. For more detailed information on the samples please refer to Table 2.
Figure 2.
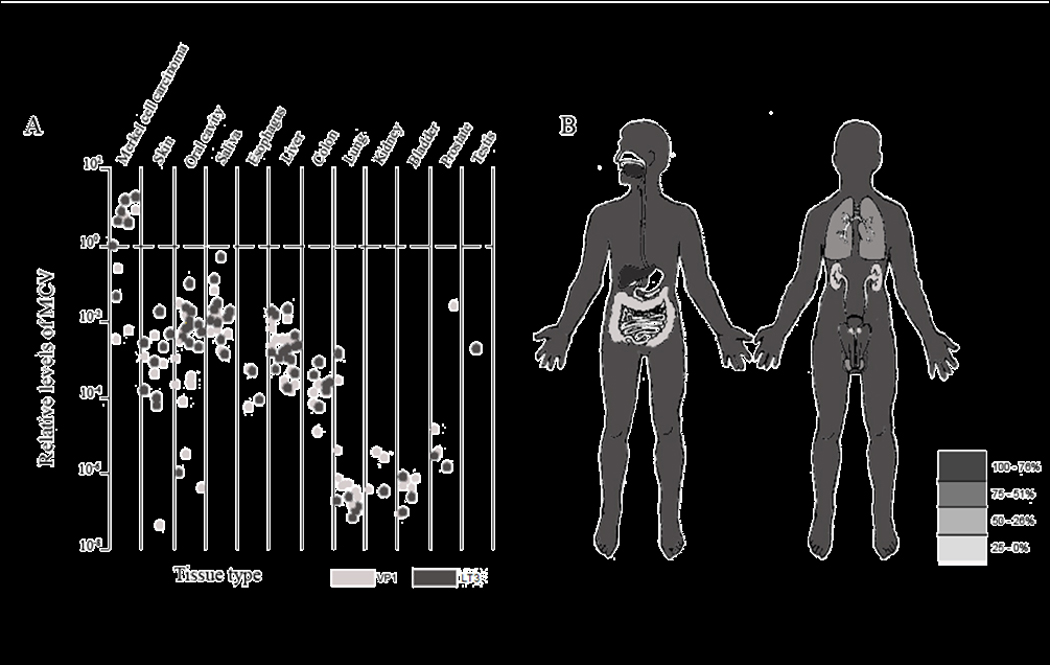
TABLE 2.
Human tissues tested for the presence of MCV by quantitative PCR. Median relative levels for both genes, VP1 and LT3, are shown in the table. The levels are derived in reference to a MCC tumor by dividing the gene of interest by β–actin and multiplying by 1,000. Median relative levels were calculated among the positive samples.
| Tissue type | Total | Either gene positive |
Frequency of positivity (%) |
Median relative level |
|---|---|---|---|---|
| Skin | ||||
| Merkel Cell Carcinoma | 7 | 6 | 86% | 718.876 |
| Non-Merkel Cell Carinoma | 21 | 10 | 48% | 0.545 |
| Squamous cell carcinoma | 12 | 3 | ||
| Normal skin | 9 | 7 | ||
| Aerodigestive tract | ||||
| Oral Cavity | 67 | 51 | 76% | 1.919 |
| Squamous cell carcinoma | 47 | 19 | ||
| Normal mucosa | 10 | 2 | ||
| Saliva from patients without cancer | 10 | 10 | 9.264 | |
| Esophagus | 11 | 4 | 36% | 0.027 |
| Normal esophagus | 6 | 1 | ||
| Esophageal cancer | 5 | 3 | ||
| Liver | 37 | 20 | 54% | 1.099 |
| Normal liver | 15 | 5 | ||
| Biliary cirrhosis | 2 | 2 | ||
| Liver cancer | 16 | 10 | ||
| Metastatic colon adenocarcinoma | 4 | 3 | ||
| Colon | 50 | 9 | 18% | 0.198 |
| Colon cancer | 25 | 4 | ||
| Normal colon | 25 | 5 | ||
| Respiratory | ||||
| Lung | 43 | 11 | 26% | 0.0003 |
| Lung cancer | 28 | 10 | ||
| Normal lung | 15 | 1 | ||
| Genitourinary | ||||
| Renal Clear Cell Carcinoma | 16 | 3 | 19% | 0.001 |
| Bladder | 10 | 6 | 60% | 0.004 |
| Bladder cancer | 8 | 6 | ||
| Normal bladder | 2 | 0 | ||
| Prostate Adenocarcinoma | 22 | 4 | 18% | 0.002 |
| Seminoma | 9 | 1 | 11% | 0.934 |
| Total | 293 | 125 | 43% | |
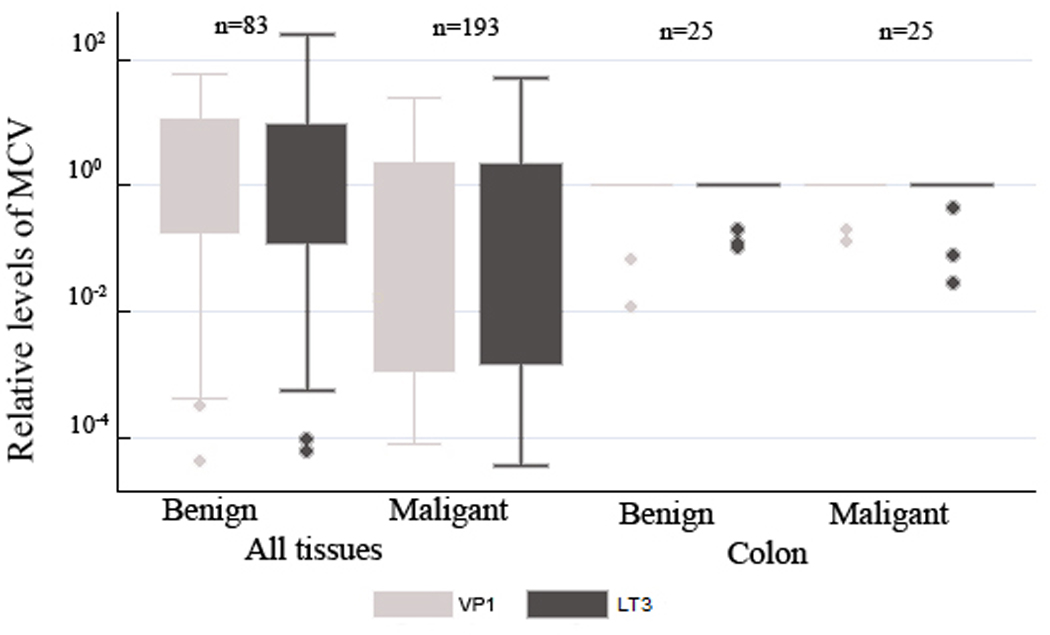
MCV in benign and malignant tissues
There was no difference in the values for VP1 and LT3 between malignant and benign tissues (>0.05). Figure 3 shows box plots comparing the values for benign and malignant tissues in all samples as well as in the colon subset for a tissue specific comparison.
Figure 3.
DISCUSSION
After Feng and colleagues initial report of clonal integration of MCV in Merkel cell carcinoma samples, the interest in the well-known Polyoma viridae family was rekindled. Consistent with previous reports, our results confirm the presence of MCV in 6 of the 7 Merkel cell carcinoma samples (4, 13–17). Our study has tested the largest variety of human tissues for the presence of MCV to date. Through the use of a quantitative assay, we were able to categorize different human tissues based on their MCV levels. In spite of the widespread presence of MCV, Merkel cell carcinomas displayed higher levels of viral VP1 and LT3 DNA than all the other tissues tested. This suggests that neoplastic Merkel cells in the skin are likely to harbor clonal integration of MCV as initially described by Southern blot analysis (4). Lack of access to larger DNA fragments from fresh tissue samples prevented us from directly confirming this observation by Southern blot but attempts to develop a specific in situ hybridization assay are in progress.
The present study has been able to identify tissues that were not previously known to harbor MCV DNA. In the original report of the virus, the majority of the control tissues were negative for MCV while some gut and skin samples were negative by conventional PCR and positive Southern Blotting after PCR amplification (9 of 84 samples) (4, 15). Surprisingly in our study, the skin showed lower levels of MCV than the oral cavity mucosa, the saliva, and the liver. The higher levels of MCV in the aerodigestive tract and digestive system over the respiratory and genitourinary system imply that the first two are more likely involved in harboring and eventually transmitting the virus.
It is possible to approximate the number of viral copies in tissue samples other than the reference tumor through proportionality. Knowing the median relative levels of the virus and arbitrarily assuming the reference tumor has 10 MCV copies per genome, to allow easy comparison, we can illustrate the relative viral load in different tissue samples. In this analysis, MCCs have an average of 10 copies per genome (range from 173 to 0.05 copies per genome), saliva has an average of 0.128 copies per genome (range from 5 to 0.01), while oral cavity, liver, and skin samples have 0.026, 0.015, and 0.007 copies per genome respectively. Lung, kidney, bladder, and prostate would have <0.001 copies per genome. Until the copy number of MCV is available for an established cell line, these calculations will remain rough estimates of the real absolute counts.
The distribution of MCV appears distinct from that of the other human polyomaviruses. A fecal-oral transmission route for MCV similar to the Hepatitis A virus and other viruses that infect humans seems plausible. Colonization of the skin through direct contact and ingestion could result in hepatic infection, excretion, and re-infection. Given the high levels of MCV in saliva, transmission of the virus through this body fluid seems possible. This pattern differs from BKV which primary infects the kidney, and JCV which targets the oligodendrocytes in the central nervous system.
The possibility of contamination is unlikely since the water controls were consistently negative, and the assay was reproducible for individual DNA samples that were obtained at two different time points from our tissue bank (that does not contain MCC). Furthermore, note that some of the samples were positive for only one of the viral genes. This can be attributed to simple PCR failure to detect low levels of MCV (based on fragment size or amplification efficiency) or to disruption of the virus during integration in the host cells (as might be the case for one MCC, which was highly positive for LT3 and negative for VP1). However, both genes showed similar levels of positivity in a tissue specific pattern which would not be expected if the single gene signals were artifacts.
By indentifying lower levels of MCV, we have identified human tissues that are likely to harbor virus at any given time. It is questionable whether the presence of MCV DNA in these tissues represents viral infection or presence of virus shed from another organ. Large T-antigen helicase truncation mutations have been described in clonally integrated MCV but not in other PCR positive tissues (19). With our current sample set we are unable to fully sequence MCV genome for mutations or to determine if the DNA represents intracellular or a viron-associated MCV.
Low levels of MCV are present in a wide variety of human tissues while high levels of MCV are unique to MCC. Antibody evidence from blood tests indicates some 15 to 30 percent of adults are infected with a still undiscovered human relative of the African green monkey virus (20). If MCV turns out to be this long-sought agent, then more than 1 billion people worldwide could already be infected (21). It is obvious that the mere presence of MCV is not enough for malignant transformation. A long period of co-evolution of MCV with its human host might grant protection from disease in a similar manner to other polyoma viruses. While determining what makes Merkel precursor cells particularly susceptible to MCV remains a challenge, our tissue study confirms and extends the initial observations described following discovery of MCV. Further epidemiological and molecular studies that define the distribution of MCV in human tissues will help us understand in which tissues the presence of the virus can collaborate in oncogenic transformation.
Footnotes
Conflict of Interest
Nanette J. Liégeois is the president and founder of the Meridian Skin Care Ltd. There are no products related to the content of this article.
REFERENCES
- 1.Lemos B, Nghiem P. Merkel cell carcinoma: more deaths but still no pathway to blame. J Invest Dermatol. 2007;127:2100–2103. doi: 10.1038/sj.jid.5700925. [DOI] [PubMed] [Google Scholar]
- 2.Engels EA, Frisch M, Goedert JJ, Biggar RJ, Miller RW. Merkel cell carcinoma and HIV infection. Lancet. 2002;359:497–498. doi: 10.1016/S0140-6736(02)07668-7. [DOI] [PubMed] [Google Scholar]
- 3.Bichakjian CK, Lowe L, Lao CD, Sandler HM, Bradford CR, Johnson TM, Wong SL. Merkel cell carcinoma: critical review with guidelines for multidisciplinary management. Cancer. 2007;110:1–12. doi: 10.1002/cncr.22765. [DOI] [PubMed] [Google Scholar]
- 4.Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096–1100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qian W, Wiman KG. Polyoma virus middle T and small t antigens cooperate to antagonize p53-induced cell cycle arrest and apoptosis. Cell Growth Differ. 2000;11:31–39. [PubMed] [Google Scholar]
- 6.Haustein SV, Kolterman AJ, Sundblad JJ, Fechner JH, Knechtle SJ. Nonhuman primate infections after organ transplantation. Ilar J. 2008;49:209–219. doi: 10.1093/ilar.49.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordon J, Khalili K. The human polyomavirus, JCV, and neurological diseases (review) Int J Mol Med. 1998;1:647–655. doi: 10.3892/ijmm.1.4.647. [DOI] [PubMed] [Google Scholar]
- 8.Safak M, Khalili K. An overview: Human polyomavirus JC virus and its associated disorders. J Neurovirol. 2003;9 Suppl 1:3–9. doi: 10.1080/13550280390195360. [DOI] [PubMed] [Google Scholar]
- 9.Newton R, Ribeiro T, Casabonne D, Alvarez E, Touze A, Key T, Coursaget P. Antibody levels against BK virus and prostate, kidney and bladder cancers in the EPIC-Oxford cohort. Br J Cancer. 2005;93:1305–1306. doi: 10.1038/sj.bjc.6602869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirsch HH, Steiger J. Polyomavirus BK. Lancet Infect Dis. 2003;3:611–623. doi: 10.1016/s1473-3099(03)00770-9. [DOI] [PubMed] [Google Scholar]
- 11.Allander T, Andreasson K, Gupta S, Bjerkner A, Bogdanovic G, Persson MA, Dalianis T, Ramqvist T, Andersson B. Identification of a third human polyomavirus. J Virol. 2007;81:4130–4136. doi: 10.1128/JVI.00028-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaynor AM, Nissen MD, Whiley DM, Mackay IM, Lambert SB, Wu G, Brennan DC, Storch GA, Sloots TP, Wang D. Identification of a novel polyomavirus from patients with acute respiratory tract infections. PLoS Pathog. 2007;3:e64. doi: 10.1371/journal.ppat.0030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kassem A, Schopflin A, Diaz C, Weyers W, Stickeler E, Werner M, Zur Hausen A. Frequent detection of Merkel cell polyomavirus in human Merkel cell carcinomas and identification of a unique deletion in the VP1 gene. Cancer Res. 2008;68:5009–5013. doi: 10.1158/0008-5472.CAN-08-0949. [DOI] [PubMed] [Google Scholar]
- 14.Becker JC, Houben R, Ugurel S, Trefzer U, Pfohler C, Schrama D. MC Polyomavirus Is Frequently Present in Merkel Cell Carcinoma of European Patients. J Invest Dermatol. 2008 doi: 10.1038/jid.2008.198. [DOI] [PubMed] [Google Scholar]
- 15.Garneski KM, Warcola AH, Feng Q, Kiviat NB, Leonard JH, Nghiem P. Merkel Cell Polyomavirus Is More Frequently Present in North American than Australian Merkel Cell Carcinoma Tumors. J Invest Dermatol. 2008 doi: 10.1038/jid.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duncavage EJ, Zehnbauer BA, Pfeifer JD. Prevalence of Merkel cell polyomavirus in Merkel cell carcinoma. Mod Pathol. 2009 doi: 10.1038/modpathol.2009.3. [DOI] [PubMed] [Google Scholar]
- 17.Foulongne V, Kluger N, Dereure O, Brieu N, Guillot B, Segondy M. Merkel cell polyomavirus and Merkel cell carcinoma, France. Emerg Infect Dis. 2008;14:1491–1493. doi: 10.3201/eid1409.080651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bialasiewicz S, Lambert SB, Whiley DM, Nissen MD, Sloots TP. Merkel cell polyomavirus DNA in respiratory specimens from children and adults. Emerg Infect Dis. 2009;15:492–494. doi: 10.3201/eid1503.081067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shuda M, Feng H, Kwun HJ, Rosen ST, Gjoerup O, Moore PS, Chang Y. T antigen mutations are a human tumor-specific signature for Merkel cell polyomavirus. Proc Natl Acad Sci U S A. 2008;105:16272–16277. doi: 10.1073/pnas.0806526105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brade L, Muller-Lantzsch N, zur Hausen H. B-lymphotropic papovavirus and possibility of infections in humans. J Med Virol. 1981;6:301–308. doi: 10.1002/jmv.1890060405. [DOI] [PubMed] [Google Scholar]
- 21.Brade L, Mueller-Lantzsch N, Kaiser S, Scharrer M. Biochemical studies on structural and nonstructural proteins of the African green monkey B-lymphotropic papovavirus (LPV) Virology. 1983;127:469–474. doi: 10.1016/0042-6822(83)90160-5. [DOI] [PubMed] [Google Scholar]


