Abstract
The study has been designed to investigate the molecular mechanisms by which benfotiamine, a lipid-soluble analogue of Vitamin B1 effects lipopolysaccharide (LPS) – induced inflammatory signals leading to cytotoxicity in mouse macrophage cell line RAW264.7. Benfotiamine prevented LPS-induced apoptosis, expression of Bcl-2 family of pro-apoptotic proteins, caspase-3 activation and PARP cleavage, altered mitochondrial membrane potential and release of cytochrome-c and apoptosis inducing factor (AIF), phosphorylation and subsequent activation of p38-MAPK, stress activated kinases (SAPK/JNK), Protein kinase C, and cytoplasmic-phospholipase A2 in RAW cells. Further, phosphorylation and degradation of inhibitory kappa B (IκB) and consequent activation and nuclear translocation of redox-sensitive transcription factor NF-κB was significantly prevented by benfotiamine. The LPS-induced increased expression of cytokines and chemokines and other inflammatory marker proteins iNOS and COX-2 and their metabolic products NO and PGE2 were also blocked significantly. Thus, our results elucidate the molecular mechanism of anti-inflammatory action of benfotiamine in LPS-induced inflammation in murine macrophage. Benfotiamine suppresses oxidative stress-induced NF-κB activation and prevents the bacterial endotoxin-induced inflammation indicating that vitamin B1 supplementation could be beneficial in the treatment of inflammatory diseases.
Keywords: Benfotiamine, vitamin B1, Oxidative stress, NF-κB, Macrophages, Inflammation
Introduction
Macrophages play a key role in inflammatory and immune reactions through releasing a variety of inflammatory markers such as cytokines, chemokines, growth factors, iNOS, and COX-2 [1]. Production of these inflammatory markers contributes to the efficient control of growth and dissemination of invading pathogens. However, the excessive levels of inflammatory markers produced by bacterial cell wall components such as LPS leads to amplified inflammatory responses and devastating illnesses characteristic of severe septic shock which causes multi-organ failure and death [2, 3]. The preceding evidences suggest that the increased circulating levels of LPS leads to increased mitochondrial activity and formation of ROS resulting in disturbed redox homeostasis in macrophages which activate redox-sensitive transcription factors, such as NF-κB and AP1 [4, 5]. NF-κB and AP1, in turn, translocate to the nucleus and transcribe various inflammatory genes causing tissue damage and dysfunction leading to apoptotic cell death of macrophages [4, 6-9]. Therefore, regulation of redox signaling during infections could be an opportunity to prevent the mortality associated with sepsis. Indeed, various antioxidants such as n-acetylcysteine (NAC), resveratrol, silymarin, curcumin and vitamins such as Vit C and E have been shown to resolve inflammation associated with bacterial endotoxins [10-19].
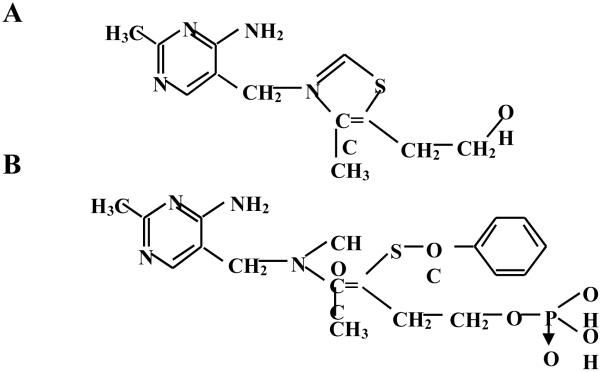
Vitamin B1 has a long history of use as an oral supplement without reported adverse effects. Due to its therapeutic action in some frequently observed clinical syndromes, thiamine hydrochloride has been advised and used over a long period of time [20-23]. There are no reports of adverse effects of oral thiamine, even at dosages of more than a hundred milligrams a day [22]. Benfotiamine, a unique derivative of thiamine, is the most potent of the allithiamines, a group of lipid-soluble forms of thiamine [24]. Chemically known as S-benzoylthiamine-O-monophosphate, it is found in trace in roasted garlic and other herbs of genus Allium. As compared to thiamine, benfotiamine has a unique open thiazole ring structure (Fig 1) which makes it pass directly through cell membranes, readily crossing the intestinal wall and being taken straight into the cell [24]. Compared to water-soluble thiamine salts, benfotiamine is absorbed better in the intestine reaching maximum plasma levels of thiamine about 5 times higher; bioavailability reaches maximum at about 3.6 times as high as that of thiamine hydrochloride and other lipophilic thiamine derivates [25]. Various studies have also suggested that benfotiamine prevents diabetic complications such as diabetic neuropathy and microangiopathy by blocking metabolic pathways such as the hexosamine pathway, the formation of advanced glycation end-product and diacylglycerol-protein kinase C pathway [26-28]. Benfotiamine apparently removes glucose derived glyceraldehyde 3-phosphate and fructose 6-phosphate through the activation of pentose phosphate pathway enzyme, transketolase [26]. Furthermore, benfotiamine has been shown to block the activation of PKC and prevents NF-κB activation thereby prevent experimental diabetic retinopathy [26].
Fig.1.
Chemical structures of A. thiamine and B. benfotiamine. Benfotiamine contains an open thiazole ring which helps benfotiamine readily enter the cell through plasma membrane increasing its bioavailability. Once in cytoplasm the ring closes and gives it a structure of thiamine.
Although relationship between vitamin B1 deficiency and bacterial infections has been noticed in animal and human studies, the mechanism by which benfotiamine supplementation prevents inflammatory response and its involvement in the inflammatory pathologies is not clear. Since vitamin B1 supplementation has been shown to be safe for human use, benfotiamine could be developed as novel therapeutic approach for inflammatory complications. Indeed, our recent report indicate an anti-inflammatory role of benfotiamine in preventing inflammation associated with bacterial endotoxin-induced uveitis in rats [29], suggesting that benfotiamine could be anti-inflammatory. However, the molecular mechanism of anti-inflammatory action of benfotiamine is not known. Therefore, the present study investigates the effect of benfotiamine on the bacterial endotoxin, LPS-induced inflammatory signaling events in murine macrophages, RAW264.7 cell line. Our results indicate that pretreatment of macrophages with benfotiamine significantly prevents LPS-induced expression of inflammatory markers such as iNOS, COX-2, activation of PKC and NF-κB, expression of apoptotic proteins, decreased mitochondrial membrane potential (MMP), and transcription of various inflammatory markers leading to apoptosis. These results suggest possible therapeutic application of benfotiamine supplementation for the prevention of inflammatory complications.
Materials and Methods
Materials and Chemicals
LPS from Escherichia coli (Strain 0111:B4) and benfotiamine were obtained from Sigma-Aldrich (St. Louis, MO). Dulbecco modified Eagle’s medium (DMEM), phosphate-buffered saline (PBS), penicillin and streptomycin solution, trypsin/EDTA solution and fetal bovine serum (FBS) were obtained from Invitrogen Corporation (Carlsbad, CA). PGE2 assay kit was obtained from Cayman Chemical Inc. (Ann Arbor, MI). Nitric oxide assay kit was from OXIS International Inc. (Beverly Hills, CA). Antibodies against phospho-p65 (ser536), phospho-p50 (ser337), p65, p50, iNOS, COX-2, Bcl-2, Bax, Bak, Bad, Bcl-XL, β-actin and GAPDH were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Antibodies against Caspase 3, cytochrome c and PARP, phospho- p38, -JNK/SAPK, -cPLA2 (ser505), -IκBα, -PKCβII (ser660), -PKCβII (Thr641) -PKCδ (ser643) and -PKCμ (ser744/748) and against p38, JNK/SAPK, cPLA2, IκBα, PKCβII, PKCδ and PKCμ were from Cell Signaling Tech. (Danvers, MA). Dihydroethidium (DHE) and Annexin V-FITC apoptosis detection kit was purchased from Calbiochem, EMD chemicals Inc. (Gibbstown, NJ). All other reagents used were of analytical grade.
Cell Culture and LPS treatment
The murine macrophage cell line (RAW264.7) was obtained from American Type Cell Culture (Manassas, VA) were cultured in DMEM supplemented with 10% heat-inactivated fetal bovine serum, 100U/mL penicillin and 100 μg/mL streptomycin and maintained at 37°C in a humidified incubator containing 95% O2 and 5% CO2. The cells were pretreated with either 50 or 100 μM benfotiamine or carrier for overnight in serum-free medium and subsequently stimulated with 1 μg/mL LPS from E. coli for 24 h, unless stated otherwise.
Annexin V Staining and Flow Cytometry
Apoptotic cell death was examined using the annexin V-FITC/PI, (molecular probes, Invitrogen) and analyzed by flow cytometry using the LYSIS II software (FACScan, BD Pharmingen) according to the manufacturer’s instructions as described earlier [30].
In situ detection of superoxide
The cells were rinsed with PBS and incubated with Dihydroethidium (DHE) (2.5 μmol/L) at 37°C for 15 min followed by stimulation with LPS for 1 h. Generation of superoxide in the cells was determined as described previously [30].
TUNEL Assay
Approximately 1×105 cells were seeded on chambered slides and starved in 0.1% serum medium with benfotiamine or carrier for 24 h. The cells were incubated with LPS (1 μg/mL) for additional 24 h, washed in ice cold PBS, fixed in 4% paraformaldehyde, and permeabilized in 0.2% TritonX-100 solution. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) was performed using DeadEnd colorimetric TUNEL system from Promega Corp. (Madison, WI) essentially as described by the manufacturer. The number of TUNEL-positive cells in a given field was counted to assess the apoptosis. The images were obtained using a light microscope (EPI-800 microscope).
Measurement of Mitochondrial Membrane Potential (MMP)
The serum starved RAW cells were washed with PBS and incubated with LPS without or with Benfotamine for 4 h. MMP was determined essentially as described by us earlier [31].
Western Blot Analysis
The cells were lysed in ice-cold RIPA lysis buffer containing 1 mM DTT, 1 mM phenylmethylsulfonylfluoride (PMSF), and 1:100 dilution of protease inhibitor and phosphatase inhibitor cocktails (Sigma-Aldrich, St. Louis, MO) on ice. The cell lysates were cleared by centrifugation at 12,000 g for 10 min at 4° C. In some experiments cytosolic and membrane extract were prepared separately. The amount of protein in the lysates was determined using Bradford reagent (Biorad laboratories, Hercules, CA). Western blot analysis was performed as described earlier [30].
EMSA for NF-κB
The cells were pretreated with benfotiamine (100 μM) for 24 h followed by LPS (1 μg/ml) treatment for 1 h at 37°C. The cytosolic and nuclear extracts were prepared as described by us earlier [30]. Consensus oligonucleotides for NF-κB transcription factors were 5′-end labeled by using T4 polynucleotide kinase and EMSA was performed as described [30, 32].
NF-κB-Dependent Secretory Alkaline Phosphatase (SEAP) Assay
The cells (2.5×105 cells/well in 6-well plate) were transfected with pNF-κB-SEAP2-construct and pTAL-SEAP control plasmid (Clontech, USA) using Lipofectamine™ 2000 (Invitrogen, Carlsbad, CA) following suppliers instructions. The cells were treated with benfotiamine for 24 h and then stimulated with LPS (1 μg/ml) for 48 h. The SEAP assay was performed in the culture media as described previously [28].
Determination of cytokines and chemokines
The levels of cytokines and chemokine in the cell culture media were determined with commercially available mouse-specific cytokine antibody array system according to the manufacturer’s (Ray Biotech, Inc., Norcross, GA) instructions. The Kodak Image Station was used for the densitometry analysis of the array.
Determination of NO and PGE2
The level of nitric oxide in the culture media was measured by using a nitric oxide assay kit according to the manufacturer’s OXIS International Inc (Beverly Hills, CA) instructions. PGE2 production was measured by enzyme immunoassay kit following the manufacturer’s (Cayman Chemical Inc, Ann Arbor, MI) instructions.
Statistical analysis
Data are expressed as the mean ± SD. All the Data were analyzed by student’s t-test using Microsoft Excel software. P<0.05 was considered as statistically significant.
Results
Effect of benfotiamine on LPS-induced macrophage cell death
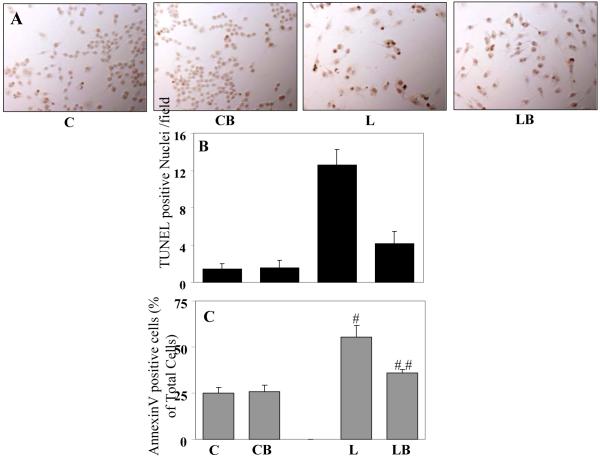
Since, LPS is well known to cause cell death in a variety of cells including macrophages, the protective effect of benfotiamine in LPS-induced cell viability in macrophages was examined at first. As shown in Fig. 2A and B, LPS caused cell death after 24 h of treatment, as assayed by the number of TUNEL positive nuclei and benfotiamine significantly prevented LPS-induced cell death. Benfotiamine alone did not alter the cell survival. Next, LPS-induced macrophage apoptosis was assayed by using annexin V staining followed by FACS analysis. LPS stimulation caused more than 60% cells to undergo apoptosis as determined by annexinV positive cells after 12 h incubation with LPS as compared to approximately 25% in control. In benfotiamine-treated group approximately 30% cells were annexin V positive after LPS-challenge which was a significant protection from LPS-induced apoptosis (Fig. 2C).
Fig. 2. Benfotiamine prevents LPS-induce apoptosis in RAW cells.
A. The RAW cells were treated with LPS (1 μg/mL) for 24h with or withour benfotiamine and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) was performed. The number of TUNEL-positive cells in a given field was counted to assess the apoptosis and presented as bar diagram in B, a total of 6 fields were counted. C. The RAW cells were treated with LPS (1 μg/mL) for 12h with or without benfotiamine. Apoptotic cell death was examined using the annexin V-FITC/PI and analyzed by flow-cytometry. Twenty thousand events were acquired for each sample. Bars represent percentage of total cells (n=3). *p<0.0001 Vs Control; **p<0.002 Vs LPS; #p<0.002 Vs Control; ##p< 0.006 Vs LPS. C, control; CB, control+benfotiamine; L, LPS; LB, LPS+benfotiamine.
Effect of benfotiamine on LPS-induced activation of caspase-3 and mitochondrial membrane potential
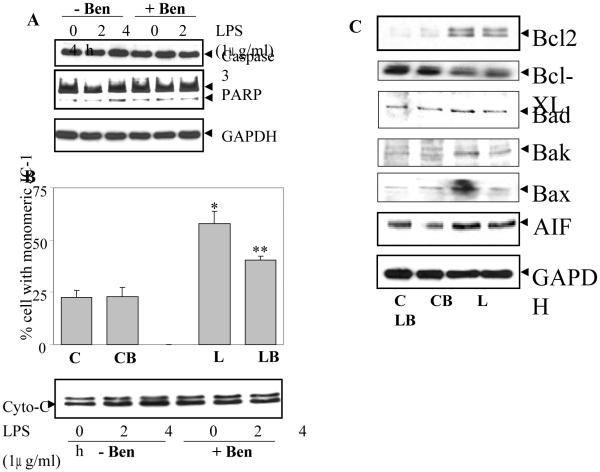
To further examine the effect of benfotiamine on LPS-induced apoptosis caspase-3 activation and PARP cleavage were determined. As shown in Fig. 3A LPS caused increased caspase-3 in the cytosol after 4h of treatment which was prevented by benfotiamine. Further, PARP cleavage was also observed during the same time period which was consistent with the caspase-3 increase in cytosol.
Fig. 3. Benfotiamine prevents LPS-induced activation of caspase-3, mitochondrial membrane potential (MMP) and Bcl-2 family proteins in RAW cells.
A. Growth-arrested RAW cells without or with Benfotiamine were incubated with 1 μg/ml of LPS for 24 h and Caspase-3 activation and PARP cleavage were determined in the cell lysate by western blot analysis using specific antibodies. A representative blot is shown (n=4). B. For assay of MMP, the growth-arrested RAW cells were treated with LPS (2.5 μg/mL) for 4h with or without benfotiamine. The cells were harvested and washed with PBS and MMP was evaluated by staining with JC-1 dye and analyzed with flow cytometry. Twenty thousand events were acquired for each sample. The data are means ± SD; (n=3). *p<0.0007 Vs Control; **p< 0.006 Vs LPS. Cytochrome-C release in the cytosol was measured after 2 and 4 h of LPS treatment by western blot using specific antibodies. GAPDH was used as loading control. C. The expression of Bcl-2 family proteins and AIF in the cell lysate from (A) was determined by western blot analysis using specific antibodies. A representative blot is shown (n=4). C, control; CB, control+benfotiamine; L, LPS; LB, LPS+benfotiamine.
Since apoptosis involves changes in mitochondria which release various apoptotic intermediates, the effects of benfotiamine on the changes in mitochondrial membrane potential (MMP) were investigated. As shown in Fig. 3B, LPS treatments caused increased percentage of cells with the monomeric form of JC-1 dye which indicates that in those cells there was decreased in electrochemical gradient across the mitochondrial membrane, an early event during apoptosis. In the cells treated with benfotiamine, there was significantly reduced percentage of cells with monomeric JC-1, suggesting that benfotiamine prevented the alteration in MMP in response to LPS. Since mitochondria releases cytochrome-c during apoptosis as an early intermediate, the level of cytochrome-c in the cytosol was determined, which increased significantly after 4 h of LPS treatment and benfotiamine prevented it (Fig. 3B).
Effect of benfotiamine on the regulation of LPS-induced Bcl-2 family of proteins
Since, LPS-induced oxidative stress resulting in apoptosis is caused by altered expression of anti- and pro-apoptotic proteins and benfotiamine prevented apoptosis in RAW cells, the effect of benfotiamine on the status of Bcl-2 family proteins under LPS challenge was examined. As shown in Fig. 3C, treatment with LPS decreased the expression of Bcl-xl protein while at the same time enhanced the expression of Bcl-2, Bax, Bad and Bak. Benfotiamine significantly reversed the changes caused by LPS but did not affect the basal levels of these proteins when given alone. Similarly, increased expression of apoptosis inducing factor (AIF) was also observed after 24 h incubation with LPS which was significantly prevented by benfotiamine treatment.
Effect of benfotiamine on LPS-induced reactive oxygen species (ROS) in RAW264.7 cells
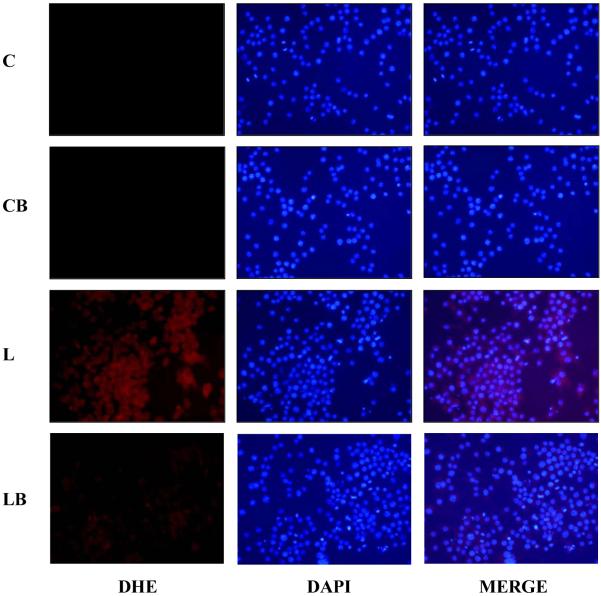
Since LPS-induced ROS is known to induce cell death [33], the level of ROS in RAW cells was examined by DHE staining. LPS treatment for 1h caused marked increase in the ROS formation in RAW cells as observed by the increased red fluorescence associated with DHE conversion in ethidium (Fig. 4). Pre-incubation of cells with benfotiamine prevented the increase in ROS levels in LPS-treated cells. These observations suggest that benfotiamine could quench the oxidative stress-induced by ROS in cells.
Fig. 4. Benfotiamine prevents LPS-induced ROS generation in RAW cells.
The RAW cells were growth-arrested by incubating in 0.1% FBS medium with Benfotiamine (100 μM) or carrier for overnight followed by stimulation with LPS (1 μg/ml) or carrier (PBS) for 16 h. The cells were washed with cold PBS and stained with ROS-sensitive dye dihydroethidium (DHE; 2.5 μM) for 15 min at 37° C. The cells were washed and mounted with floursave mounting medium with DAPI. Photomicrographs were acquired using a fluorescence microscope (Nikon). A representative picture is shown (n=3); Magnification 200X. C, control; CB, control+benfotiamine; L, LPS; LB, LPS+benfotiamine.
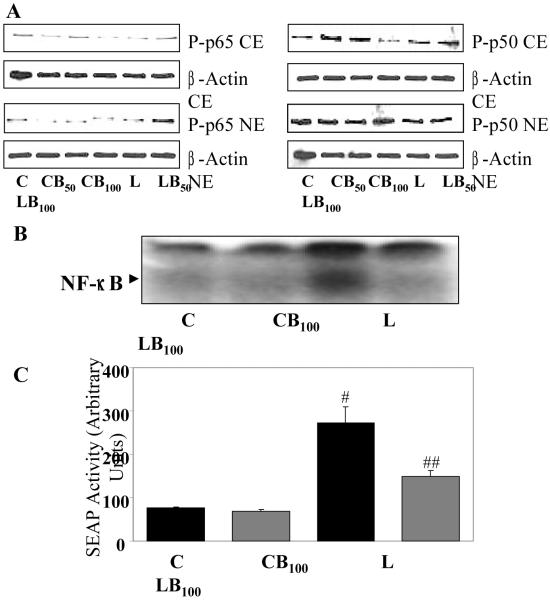
Effect of benfotiamine on LPS-induced redox sensitive transcription factors
LPS-induced activation of redox-sensitive transcription factor NF-κB is critical for the induction of cytokines, chemokines and other inflammatory markers such as iNOS and COX-2 [34, 35], hence, effect of benfotiamine treatment on LPS-induced phosphorylation, nuclear translocation and DNA binding activity of NF-κB in macrophages was studied. As shown in Fig. 5A, LPS caused increased phosphorylation and nuclear translocation of p65 as well as p50 subunits of NF-κB which was dose dependently inhibited by benfotiamine. Next, DNA binding activity of NF-κB was determined by electrophoretic mobility shift assay (EMSA), which showed approximately 4-fold increased in DNA binding in response to LPS and benfotiamine inhibited it significantly (Fig. 5B). Further, to confirm the preventive role of benfotiamine on the NF-κB activation, a more sensitive NF-κB- dependent SEAP reporter assay was used which also showed that NF-κB-dependent SEAP activity in response to LPS was significantly decreased by benfotiamine treatment (Fig. 5C). These results suggest that benfotiamine’s protective role against oxidative stress-induced cell toxicity could be due to inhibition of transcription factor NF-κB.
Fig. 5. Benfotiamine prevents LPS-induce activation of NF-κB in RAW cells.
The RAW cells were growth-arrested by incubating in 0.1% FBS medium with Benfotiamine (50 and 100 μM) or carrier for overnight and stimulated with LPS (1 μg/mL) for 1h with or without benfotiamine. Nuclear extract was prepared and equal amounts of nuclear proteins were subjected to either A. western blot or B. electrophoretic mobility gel-shift assays using specific NF-κB antibodies or oligonucleotide probes, respectively. A representative blot is shown (n=3). C. The RAW cells were transiently transfected with pNF-κB-SEAP reporter vector. The cells pre-treated without or with benfotiamine (100 μM) for 24 h and incubated with 1 μg/ml of LPS for additional 24 h. The cell culture supernatants were assayed for SEAP activity using chemiluminescence kit according to supplier’s instructions. Data represents mean ± SD (n = 3). #p<0.001 Vs Control; ##p<0.01 Vs LPS. C, Control; CB50, Control+benfotiamine (50 μM); CB100, Control+benfotiamine (100 μM); L, LPS; LB50, LPS+benfotiamine (50 μM); LB100, LPS+benfotiamine (100 μM).
Effect of benfotiamine on LPS-induced NF-κB upstream signals
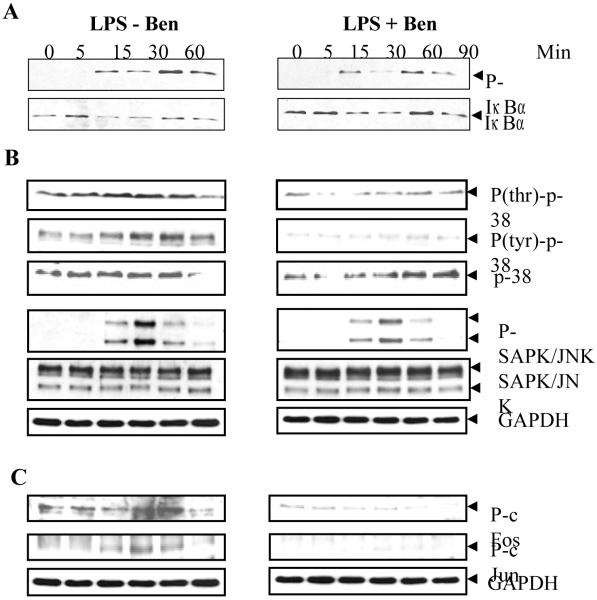
Since NF-κB activation by LPS is preceded by the phosphorylation and subsequent degradation of Iκ-B [35], further investigations were carried out to examine if benfotiamine treatment could inhibit the phosphorylation of Iκ-B. As shown in Fig. 6A, LPS stimulation induced phosphorylation and subsequent degradation of Iκ-B which were prevented by benfotiamine. Corresponding to the phosphorylation of Iκ-B there was reduced amount of un-phosphorylated protein present in the cytosol after 30 min, suggesting degradation of phosphorylated protein which partially recovered by 60 min. In benfotiamine-treated cells, reduced phosphorylation corresponded with increased un-phosphorylated protein in the cytosol which indicates inhibition of degradation. These results indicate that inhibition of NF-κB activation by benfotiamine could be via reduced phosphorylation and resultant decrease in the degradation of Iκ-B.
Fig. 6. Benfotiamine prevents LPS-induce activation of NF-κB upstream signaling kinases in RAW cells.
The RAW cells were growth-arrested by incubating in 0.1% FBS medium with Benfotiamine (100 μM) or carrier for overnight and stimulated with LPS (1 μg/mL) for different time intervals with or without benfotiamine. Cytosolic extract was prepared and equal amounts of cytosolic proteins were subjected to western blot analysis using antibodies against A. phospho-IκB and unphosphorylated IκB antibodies. B. phospho-p38 (phospho-thr and phospho-tyr), phospho-SAPK/JNK, unphosphorylated p38, SAPK/JNK, and C. phospho-cFos and -cJun. GAPDH immunoblotting of the stripped membrane was done as loading control. A representative blot is shown (n=3). Ben, benfotiamine.
Since p38-MAPK and JNK/SAPK have been shown to be involved in the LPS-induced release of inflammatory markers [36, 37], further studies were performed to investigate whether benfotiamine would prevent the activation of these signaling kinases. As shown in Fig 6B, LPS caused increase in the phosphorylation of (thr180 and tyr182) p38-MAPK within 5 min which returned to basal level by 90 min, whereas increased phosphorylation of JNK was observed within 15 min which also returned to basal levels by 90 min. Treatment with benfotiamine significantly reduced the phosphorylation of p38-MAPK but had very little or no effect on the phosphorylation of JNK/SAPK. There was no change in the levels of un-phosphorylated p38-MAPK and JNK/SAPK. Because MAPK are known to activate ligand-dependent transcription factors, such as c-fos and c-jun, which constitute the transcription factor AP-1 [38], the effects of benfotiamine on the activation of c-fos and c-jun was investigated. As shown in Fig 6C, LPS caused time-dependent increase in phosphorylation of c-fos and c-jun proteins which was inhibited by benfotiamine.
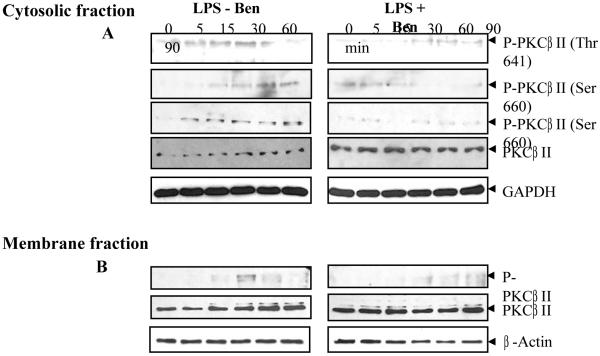
LPS-induced activation of PKC-βII subunit has been implicated in the cytotoxicity and inflammation [26]. As they phosphorylate in response to LPS stimulus, PKC translocate to the membrane which indicates the activation of the kinase. As shown in Fig. 7A, when Raw cells were stimulated with LPS, there was increased phosphorylation at Thr641 and Ser660 residues of PKC-βII within 5 min of LPS-stimulation and increased time-dependently in the cytosolic fraction, whereas in the membrane fraction phosphorylated PKC was observed after 15 min of LPS stimulus which increased to maximum at 30 min and decreased by 90 min after LPS stimulus (Fig. 7B). Also, total protein gradually decreased in the cytosol and correspondingly the protein level increased in the membrane fraction which suggests membrane translocation of the phosphorylated PKC-βII. In benfotiamine-treated cells, there was minimal increase in the phosphorylation at Thr641 residue while almost none at Ser660 residue and the total protein remained constant and did not change with time in the cytosol as well as in the membrane fraction in response to LPS (Fig. 6A and B). These results indicate that benfotiamine prevented the phosphorylation of PKC-βII in response to LPS challenge in RAW cells. Similar results were also observed for the phosphorylation and membrane translocation of other PKC isozymes such as PKCα, PKCδ, and PKCμ in response to LPS challenge (data not shown).
Fig. 7. Benfotiamine prevents LPS-induce activation of PKC in RAW cells.
The RAW cells were growth-arrested by incubating in 0.1% FBS medium with Benfotiamine (100 μM) or carrier for overnight and stimulated with LPS (1 μg/mL) for 0-90 min. The cytosolic and membrane fractions were prepared and equal amounts of A. cytosolic proteins (30 μg) and B. membrane proteins (10 μg) were used for western blot analysis using antibodies against phospho-PKCβII (phospho-thr and phospho-ser) and unphosphorylated PKC. The stripped membrane was probed with GAPDH and β-actin antibodies to depict equal loading. A representative blot is shown (n=3).
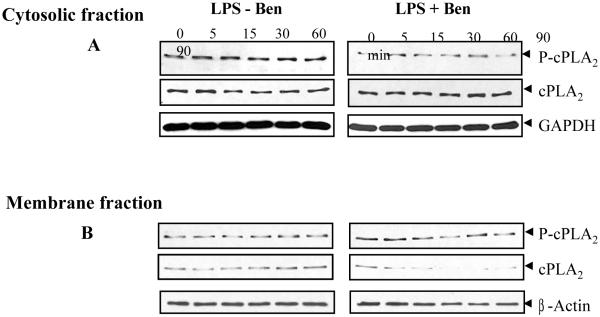
Since cPLA2 has been shown to be induced by many factors and is involved in oxidant-induced cytotoxicity and is known to be phosphorylated by MAPK [39], activation of cPLA2 in RAW cells was determined by immunoblott analysis. There was phosphorylation of cPLA2 within 5 min of LPS-stimulation and increased with time in the cytosolic fraction (Fig. 8A), whereas in the membrane fraction phosphorylated cPLA2 was observed not before 15 min of LPS stimulus which decreased by 90 min after LPS stimulus (Fig. 8B). A gradual decrease in the cPLA2 protein level in cytosol and corresponding increase in the protein level in the membrane fraction were observed. In benfotiamine-treated cells, there was minimal increase in the phosphorylation of cPLA2 and the total protein remained constant in cytosol and decreased with time in the membrane fraction in response to LPS. Because cPLA2 is involved in the arachidonic acid metabolim, these data suggest that benfotiamine could modulate PGE2 synthesis and COX-2 activation via cPLA2 in additional to molecular signaling cascade.
Fig. 8. Benfotiamine prevents LPS-induce activation of cPLA2 in RAW cells.
The RAW cells were growth-arrested and pretreated with or without benfotiamine (100 uM) in 0.1% serum medium for 24 h and stimulated with LPS (1 μg/mL) for 0-90 min. The equal amounts of A. cytosolic proteins (30 μg) and B. membrane proteins (10 μg) were subjected to western blot analysis using antibodies against phospho-cPLA2 (phospho-thr and phospho-ser) and unphosphorylated cPLA2. The stripped membrane was probed with GAPDH and β-actin antibodies to depict equal loading. A representative blot is shown (n=3).
Effect of benfotiamine on LPS-induced NF-κB downstream signals
Since LPS is known to stimulate an inflammatory response in macrophages by inducing the synthesis of inflammatory mediators such as cytokines and chemokines which cause cytotoxicty [38], next, the effect of benfotiamine on LPS-induced synthesis of various cytokines and chemokines in RAW cells was examined using a mouse specific cytokine array system. LPS caused significantly increased synthesis of cytokines such as IL-1α, IL-4, IL-5, IL-6, IL-12, TNF-α, TNFRII, and chemokines such as MCP-5, LIX, P-selectine, C TACK, MIP3α, CXCL 16, MIG, GM CSF, G CSF, lymphotactine, and fractalkaline (Table 1). These inflammatory markers play important role in the macrophage activation during pathogen invasion and assist in recruitment, activation and phagocytosis. The pre-treatment with benfotiamine significantly prevented the release of these cytokines and chemokines (Table 1).
Table 1. Benfotiamine prevents LPS-induced inflammatory cytokines and chemokines in RAW cell culture medium.
| Inflammatory Markers |
RAW cells (no treatment) |
RAW cells + benfotiamine |
RAW cells + LPS |
RAW cells + LPS and benfotiamine |
|---|---|---|---|---|
| IL-1α | 2240±730 | 990±200 | 7140±670* | 0## |
| IL-4 | 4920±970 | 4900±520 | 14540±620* | 6890±1310# |
| IL-5 | 850±210 | 890±160 | 18610±520** | 5320±1050## |
| IL-6 | 0 | 0 | 158220±4720** | 87910±2330# |
| IL-12 | 4540±1220 | 3500±710 | 8920±2870* | 3080±520# |
| Lymphotactine | 7820±1110 | 7710±690 | 68950±2740** | 31200±3680# |
| Fractalkaline | 0 | 0 | 4410±1290** | 1890±150# |
| GCSF | 2260±400 | 0 | 94800±7130** | 68670±3570# |
| GM-CSF | 0 | 0 | 20580±1610** | 14330±1570# |
| MIG | 0 | 0 | 9410±10** | 3780±20# |
| TARC | 2100±70 | 0 | 13100±6760** | 4450±2670# |
| TIMP-1 | 670±0 | 0 | 12350±2990** | 5040±1310# |
| TNF-α | 3200±0 | 0 | 82540±11030** | 44870±2490# |
| α-TNFRII | 20510±3180 | 22620±1000 | 91670±11540* | 60420±2940# |
| CXCL 16 | 61870±5280 | 62960±320 | 76600±3660* | 42530±2950# |
| MCP5 | 15430±1240 | 14500±720 | 50280±11450* | 18040±2140# |
| LIX | 8270±530 | 10620±170 | 30910±2370** | 19390±960# |
| P-Selectin | 18580±1490 | 19010±260 | 37170±450* | 25360±180# |
| C-TACK | 7570±1510 | 9360±2350 | 19530±2350* | 7910±1680# |
| MIP-3α | 0 | 0 | 7500±510** | 0## |
The RAW cells were growth-arrested and pretreated with or without benfotiamine (100 uM) in 0.1% serum medium for 24 h and stimulated with LPS (1 μg/mL). The culture medium was collected 24 h after LPS-challenge and secreted cytokines and chemokines were determined by antibody array system as described in the methods. The values represent arbitrary units of pixel density for cytokine-specific spots on the array determined by densitometry (n=4).
p<0.01 Vs Control
p<0.001 Vs Control
p<0.01 Vs LPS
p<0.001 Vs LPS. 0 = equals not detected.
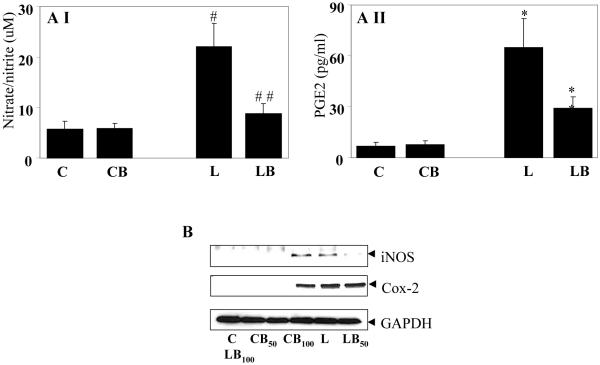
Further, macrophages are known to produce other inflammatory markers such as PGE2 and NO when stimulated with LPS. Thus, the effect of benfotiamine on LPS-induced production of these inflammatory markers by RAW cells was determined. There was more than three-fold increased production of NO and approximately 9-fold increased synthesis of PGE2 in LPS-stimulated cells and benfotiamine treatment significantly (p<0.01 and p<0.008, respectively) prevented these changes (Fig. 9A). Since NO and PGE2 are synthesized by inducible enzymes, iNOS and COX-2 respectively, in response to oxidative stress, expression of these proteins was examined next. LPS treatment caused profound expression of iNOS and COX-2 in RAW cells as compared to control cells where these proteins could barely be noticed by immunoblotting. Benfotiamine, dose-dependently inhibited the expression of these proteins (Fig. 9B). These results suggest that benfotiamine could regulate the expression and synthesis of inflammatory markers which could be responsible for its protective role against oxidative stress.
Fig. 9. Befotiamine prevents LPS-induced NO and PGE2 production in RAW cells. (AI and II).
NO and PGE2 levels in the culture medium collected 24 h after LPS challenge were measured by ELISA kits as described in Methods. Each value represents the mean ± SD (n=4), #p<0.005 Vs Control; ##p<0.01 Vs LPS. *p<0.0005 Vs Control; **p<0.008 Vs LPS. C, Control; CB, Control+benfotiamine; L, LPS; LB, LPS+benfotiamine. B. Growth-arrested RAW cells without or with Benfotiamine were incubated with 1 μg/ml of LPS for 24 h. The expression of COX-2 and iNOS proteins was determined by western blot analysis using specific antibodies. A representative blot is shown (n=4). C, Control; CB50, Control+benfotiamine (50 μM); CB100, Control+benfotiamine (100 μM)L, LPS; LB50, LPS+benfotiamine (50 μM); LB100, LPS+benfotiamine (100 μM).
Discussion
The present study for the first time shows that benfotiamine, vitamin B1 analogue, prevents LPS-induced inflammatory signals leading to apoptotic cell death of murine macrophages. Alteration of mitochondrial membrane potential and subsequent release of apoptotic intermediates including cytochrome-c, and activation of caspase-3 are early events during apoptosis [40]. Further, the release of inflammatory markers promotes oxidative stress which results in programmed cell-death mediated by Bcl-2 family of proteins including both pro-apoptotic such as Bax, Bad, and Bak and anti-apoptotic proteins such as Bcl-2 and BclXL [41-43]. Inhibition of inflammatory signals by benfotiamine could regulate the apoptotic events such as activation of Bcl-2 family proteins and caspase-3 which can cause macrophage cell death.
LPS is well known to cause oxidative stress in immune cells by binding to its receptor TLR-4 which consequently starts a myriad of signaling reactions regulated by phosphorylation and dephosphorylation of various kinases which eventually lead to the activation of redox-sensitive transcription factors such as NF-κB and AP-1 [38, 44, 45]. The activation of NF-κB results in the transcription of various inflammatory markers such as cytokines, chemokines and inflammatory enzymes including COX-2 and iNOS [35, 46, 47]. These inflammatory reactions are critical in the immune response against pathogens and foreign antigens, which are then phagocytozed and killed and removed from the body. However, excessive and persistent exposure to pathogens leads to hyper-response resulting in the release of enormous amount of inflammatory markers, which cause cytotoxicity and tissue injury, requiring therapeutic intervention to contain the inflammation. Treatment with antibiotics generally kills the bacterial pathogen, but the bacterial toxins released in the body keep the inflammation recurring, causing damage to the tissues. In this scenario anti-inflammatory and/or antioxidant therapeutic approach would block the inflammatory circuitry and give the body enough time and capacity to convalesce from the inflammation [48].
Many studies have suggested the use of different anti-inflammatory agents, which inhibit a particular kinase such as MAPK, PI3K, IKK, PKC or transcription factor NF-κB [49-54]. However, inhibition of one signaling kinase does not block the inflammation because an alternative signaling kinase would get activated to recompense for the inhibition of previous kinase molecule. Therefore, a drug or agent that reduces the overall hyper-responsiveness against the pathogen and inhibits most of the inflammatory circuitry would be a better therapeutic alternative. This could be possible only by regulating the cellular metabolism in such as way that there is reduced ROS generation in response to an insult, which not only reduces the inflammation by overall inhibition of ROS-dependent signaling cascade, as its effect would be upstream of ROS formation, but also provide antioxidant milieu for the tissue to dispose off ROS efficiently. Various antioxidants such as N-acetylcysteine, Vit C and Vit E have been shown to inhibit ROS-induced inflammatory response [55-59]. In best of our knowledge this is one of the first reports that a lipophilic analogue of Vit B1, benfotiamine, could prevent bacterial LPS-induced inflammatory signals and cytotoxicity in RAW cells. Previously, benfotiamine has been shown to prevent oxidative stress-induced complications through its anti-oxidant properties in vitro and in vivo animal models. Nishikawa et al, have shown that benfotiamine blocks three of the major molecular pathways leading to diabetic complications [60]. It prevents the hyperglycemia-induced increase in UDP-N-acetylglucosamine leading to enhanced hexosamine pathway activity and advanced glycation end products (AGE) formation [61, 62]. Benfotiamine also shown to inhibit hyperglycemia –induced NF-κB pathway via PKC [26, 62]. Since PKC activation has been shown to activate NADPH oxidase which is responsible for generation of ROS, the ability of benfotiamine to inhibit PKC activation may contribute to its anti-oxidative effect [63, 64]. Further, benfotiamine also shown to inhibits aldose reductase activity that regulates glucose flux in the polyol pathway of glucose metabolism [65]. Through its anti-oxidant mechanism benfotiamine has been shown to prevent high glucose-induced increase in DNA fragmentation and caspase-3 activity and consequently endothelial cell damage and apoptosis in endothelial cells and pericytes [66]. Further, benfotiamine has been shown to reduce the oxidative stress and genomic damage caused by the mutagenic model compound NQO, the uremic toxin indoxyl sulfate and the peptide hormone angiotensin II in renal cells indicating its direct antioxidant capacity [67]. during hyperglycemia benfotiamine has been shown to regulate the activity of glycolytic enzyme transketolase and divert glucose utilization via pentose phosphate pathway [20, 59]. This could prevent the excessive ROS production due to glucose over-utilization by mitochondria which depends on glycolysis for reducing intermediates, NAD(P)H. Benfotiamine has been shown to be useful in the diabetic complications such as diabetic neuropathy, micro-angiopathy and nephropathy [27-29; 60].
In consistence with previous studies that show benfotiamine inhibits high glucose-induced activation of PKC and NF-κB [27], our current observations indicate that benfotiamine also prevents the LPS-induced activation of p-38 MAPK, PKCβII, PKCδ, PKCμ, and transcription factors NF-κB and c-fos and c-jun. These signaling molecules are key components of oxidative stress-induced inflammatory circuitry and potential therapeutic targets. Inhibition of these signaling molecules by benfotiamine could be responsible for reduction in the LPS-induced inflammatory markers such as cytokines including IL-1α, IL-4, IL-5, IL-6, IL-12, TNF-α, TNFRII, and chemokines such as MCP-5, LIX, P-selectine, C TACK, MIP3α, CXCL 16, MIG, GM-CSF, G-CSF, lymphotactine, fractalkaline, as observed in RAW cells in this study. Also, activation of cPLA2 by LPS has been implicated in arachidonic acid metabolism, which regulates COX-2 activity and PGE2 synthesis [68, 69]. Inhibition of COX-2 expression and reduced PGE2 formation in benfotiamine-treated cells could be secondary to the inhibition of cPLA2 phosphorylation/activation, though transcriptional regulation by NF-κB could be another route to regulate the COX-2 expression.
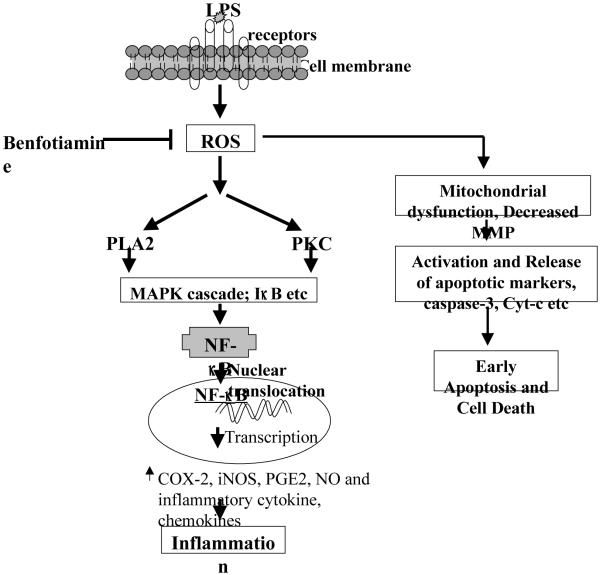
In conclusion, our results suggest that fat soluble form of vitamin B1, benfotiamine, could modulate the macrophage inflammatory response against the bacterial toxin-induced inflammation by regulating signaling intermediates such as MAPK/PKC/IκB leading to the expression of NF-κB-dependent inflammatory markers which by autocrine and paracrine manner causes cytotoxicity (Fig 10). Therefore, vitamin B1 supplementation could be beneficial in preventing inflammation-induced pathological conditions.
Fig. 10.
The mechanism of benfotiamine regulated inflammatory signals. Benfotiamine prevents ROS formation and thereby blocks the activation of subsequent adverse effects including mitochondrial dysfunction and activation of apoptotic markers that sets an early apoptosis. Benfotiamine could also prevent the activation of protein kinases leading to activation of NF-κB which transcribes inflammatory genes and causes inflammation.
Acknowledgements
This study was supported in part by NIH grants GM71036 and EY018591 (to KVR), and DK36118 (to SKS).
Abbreviation list
- PARP
Poly (ADP-ribose) polymerase
- AIF
apoptosis inducing factor
- p38
mitogen-activated protein kinase p38
- JNK/SAPK
c-Jun NH2-terminal kinase/Stress-activated protein kinase
- cPLA2
cytoplasmic phospholipase A2
- IκBα
inhibitory κB alpha
- PKC
Protein kinase C
- NF-κB
nuclear factor-κB
- AP-1
activator protein 1
- iNOS
inducible nitric oxidase
- COX-2
cycloxygenase-2
- NO
nitric oxides
- PGE2
prostaglandin E2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Ramana KV, Fadl AA, Tammali R, Reddy AB, Chopra AK, Srivastava SK. Aldose reductase mediates the lipopolysaccharide-induced release of inflammatory mediators in RAW264.7 murine macrophages. J Biol Chem. 2006;281:33019–33029. doi: 10.1074/jbc.M603819200. [DOI] [PubMed] [Google Scholar]
- [2].Ginsburg I. The role of bacteriolysis in the pathophysiology of inflammation, infection and post-infectious sequelae. APMIS. 2002;110:753–770. doi: 10.1034/j.1600-0463.2002.1101101.x. [DOI] [PubMed] [Google Scholar]
- [3].Jean-Baptiste E. Cellular mechanisms in sepsis. J Intensive Care Med. 2007;22:63–72. doi: 10.1177/0885066606297123. [DOI] [PubMed] [Google Scholar]
- [4].Liu SF, Malik AB. NF-kappa B activation as a pathological mechanism of septic shock and inflammation. Am J Physiol Lung Cell Mol Physiol. 2006;290:L622–L645. doi: 10.1152/ajplung.00477.2005. [DOI] [PubMed] [Google Scholar]
- [5].Woo CH, Lim JH, Kim JH. Lipopolysaccharide induces matrix metalloproteinase-9 expression via a mitochondrial reactive oxygen species-p38 kinase-activator protein-1 pathway in RAW 264.7 cells. J Immunol. 2004;173:6973–6980. doi: 10.4049/jimmunol.173.11.6973. [DOI] [PubMed] [Google Scholar]
- [6].Asehnoune K, Strassheim D, Mitra S, Kim JY, Abraham E. Involvement of reactive oxygen species in Toll-like receptor 4-dependent activation of NF-kappa B. J Immunol. 2004;172:2522–2529. doi: 10.4049/jimmunol.172.4.2522. [DOI] [PubMed] [Google Scholar]
- [7].Müller JM, Ziegler-Heitbrock HW, Baeuerle PA. Nuclear factor kappa B, a mediator of lipopolysaccharide effects. Immunobiology. 1993;187:233–256. doi: 10.1016/S0171-2985(11)80342-6. [DOI] [PubMed] [Google Scholar]
- [8].Blackwell TS, Christman JW. The role of nuclear factor-kappa B in cytokine gene regulation. Am J Respir Cell Mol Biol. 1997;17:3–9. doi: 10.1165/ajrcmb.17.1.f132. [DOI] [PubMed] [Google Scholar]
- [9].Murphy K, Haudek SB, Thompson M, Giroir BP. Molecular biology of septic shock. New Horiz. 1998;6:181–193. [PubMed] [Google Scholar]
- [10].Sebai H, Ben-Attia M, Sani M, Aouani E, Ghanem-Boughanmi N. Protective effect of resveratrol in endotoxemia-induced acute phase response in rats. Arch Toxicol. 2009;83:335–340. doi: 10.1007/s00204-008-0348-0. [DOI] [PubMed] [Google Scholar]
- [11].Toklu HZ, Tunali-Akbay T, Velioglu-Ogunc A, Ercan F, Gedik N, Keyer-Uysal M, Sener G. Silymarin, the antioxidant component of Silybum marianum, prevents sepsis-induced acute lung and brain injury. J Surg Res. 2008;145:214–222. doi: 10.1016/j.jss.2007.03.072. [DOI] [PubMed] [Google Scholar]
- [12].Victor VM, Rocha M, De la Fuente M. N-acetylcysteine protects mice from lethal endotoxemia by regulating the redox state of immune cells. Free Radic Res. 2003;37:919–929. doi: 10.1080/1071576031000148727. [DOI] [PubMed] [Google Scholar]
- [13].De la Fuente M, Victor VM. Ascorbic acid and N-acetylcysteine improve in vitro the function of lymphocytes from mice with endotoxin-induced oxidative stress. Free Radic Res. 2001;35:73–84. doi: 10.1080/10715760100300611. [DOI] [PubMed] [Google Scholar]
- [14].Ortolani O, Conti A, De Gaudio AR, Moraldi E, Cantini Q, Novelli G. The effect of glutathione and N-acetylcysteine on lipoperoxidative damage in patients with early septic shock. Am J Respir Crit Care Med. 2000;161:1907–1911. doi: 10.1164/ajrccm.161.6.9903043. [DOI] [PubMed] [Google Scholar]
- [15].Zhang H, Spapen H, Nguyen DN, Benlabed M, Buurman WA, Vincent JL. Protective effects of N-acetyl-L-cysteine in endotoxemia. Am J Physiol. 1994;266:H1746–H1754. doi: 10.1152/ajpheart.1994.266.5.H1746. [DOI] [PubMed] [Google Scholar]
- [16].Sompamit K, Kukongviriyapan U, Nakmareong S, Pannangpetch P, Kukongviriyapan V. Curcumin improves vascular function and alleviates oxidative stress in non-lethal lipopolysaccharide-induced endotoxaemia in mice. Eur J Pharmacol. 2009;616:192–199. doi: 10.1016/j.ejphar.2009.06.014. [DOI] [PubMed] [Google Scholar]
- [17].Wilson JX. Mechanism of action of vitamin C in sepsis: ascorbate modulates redox signaling in endothelium. Biofactors. 2009;35:5–13. doi: 10.1002/biof.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Uchiyama K, Takano H, Yanagisawa R, Inoue K, Naito Y, Yoshida N, Yoshino S, Murase H, Ichinose T, Yoshikawa T. A novel water-soluble vitamin E derivative prevents acute lung injury by bacterial endotoxin. Clin Exp Pharmacol Physiol. 2004;31:226–230. doi: 10.1111/j.1440-1681.2004.03981.x. [DOI] [PubMed] [Google Scholar]
- [19].Reiter E, Jiang Q, Christen S. Anti-inflammatory properties of alpha- and gamma-tocopherol. Mol Aspects Med. 2007;28:668–691. doi: 10.1016/j.mam.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Thornalley PJ. The potential role of thiamine (vitamin B1) in diabetic complications. Curr Diabetes Rev. 2005;1:287–298. doi: 10.2174/157339905774574383. [DOI] [PubMed] [Google Scholar]
- [21].Malecka SA, PopRAWski K, Bilski B. Prophylactic and therapeutic application of thiamine (vitamin B1)--a new point of view. Wiad Lek. 2006;59:383–387. [PubMed] [Google Scholar]
- [22].Hinze-Selch D, Weber MM, Zimmermann U, Pollmächer T. Thiamine treatment in psychiatry and neurology. Fortschr Neurol Psychiatr. 2000;68:113–120. doi: 10.1055/s-2000-11622. [DOI] [PubMed] [Google Scholar]
- [23].Wooley JA. Characteristics of thiamin and its relevance to the management of heart failure. Nutr Clin Pract. 2008;23:487–493. doi: 10.1177/0884533608323430. [DOI] [PubMed] [Google Scholar]
- [24].Benfotiamine. Monograph. Altern Med Rev. 2006;11:238–242. [PubMed] [Google Scholar]
- [25].Loew D. Pharmacokinetics of thiamine derivatives especially of benfotiamine. Int J Clin Pharmacol Ther. 1996;34:47–50. [PubMed] [Google Scholar]
- [26].Hammes HP, Du X, Edelstein D, Taguchi T, Matsumura T, Ju Q, Lin J, Bierhaus A, Nawroth P, Hannak D, Neumaier M, Bergfeld R, Giardino I, Brownlee M. Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat Med. 2003;9:294–299. doi: 10.1038/nm834. [DOI] [PubMed] [Google Scholar]
- [27].Stracke H, Hammes HP, Werkmann D, Mavrakis K, Bitsch I, Netzel M, Geyer J, Köpcke W, Sauerland C, Bretzel RG, Federlin KF. Efficacy of benfotiamine versus thiamine on function and glycation products of peripheral nerves in diabetic rats. Exp Clin Endocrinol Diabetes. 2001;109:330–336. doi: 10.1055/s-2001-17399. [DOI] [PubMed] [Google Scholar]
- [28].Stirban A, Negrean M, Stratmann B, Gawlowski T, Horstmann T, Götting C, Kleesiek K, Mueller-Roesel M, Koschinsky T, Uribarri J, Vlassara H, Tschoepe D. Benfotiamine prevents macro- and microvascular endothelial dysfunction and oxidative stress following a meal rich in advanced glycation end products in individuals with type 2 diabetes. Diabetes Care. 2006;29:2064–2071. doi: 10.2337/dc06-0531. [DOI] [PubMed] [Google Scholar]
- [29].Yadav UC, Subramanyam S, Ramana KV. Prevention of endotoxin-induced uveitis in rats by benfotiamine, a lipophilic analogue of vitamin B1. Invest Ophthalmol Vis Sci. 2009;50:2276–2282. doi: 10.1167/iovs.08-2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yadav UC, Naura AS, Aguilera-Aguirre L, Ramana KV, Boldogh I, Sur S, Boulares HA, Srivastava SK. Aldose reductase inhibition suppresses the expression of Th2 cytokines and airway inflammation in ovalbumin-induced asthma in mice. J Immunol. 2009;183:4723–32. doi: 10.4049/jimmunol.0901177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kalariya NM, Ramana KV, Srivastava SK, van Kuijk FJ. Carotenoid derived aldehydes-induced oxidative stress causes apoptotic cell death in human retinal pigment epithelial cells. Exp Eye Res. 2008;86:70–80. doi: 10.1016/j.exer.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Pladzyk A, Reddy AB, Yadav UC, Tammali R, Ramana KV, Srivastava SK. Inhibition of aldose reductase prevents lipopolysaccharide-induced inflammatory response in human lens epithelial cells. Invest Ophthalmol Vis Sci. 2006;47:5395–5403. doi: 10.1167/iovs.06-0469. [DOI] [PubMed] [Google Scholar]
- [33].Simon F, Fernández R. Early lipopolysaccharide-induced reactive oxygen species production evokes necrotic cell death in human umbilical vein endothelial cells. J Hypertens. 2009;27:1202–1216. doi: 10.1097/HJH.0b013e328329e31c. [DOI] [PubMed] [Google Scholar]
- [34].Sharif O, Bolshakov VN, Raines S, Newham P, Perkins ND. Transcriptional profiling of the LPS induced NF-kappaB response in macrophages. BMC Immunol. 2007;8:1. doi: 10.1186/1471-2172-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ramana KV, Srivastava SK. Mediation of aldose reductase in lipopolysaccharide-induced inflammatory signals in mouse peritoneal macrophages. Cytokine. 2006;36:115–122. doi: 10.1016/j.cyto.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hambleton J, Weinstein SL, Lem L, DeFranco AL. Activation of c-Jun N-terminal kinase in bacterial lipopolysaccharide-stimulated macrophages. Proc Natl Acad Sci U S A. 1996;93:2774–2778. doi: 10.1073/pnas.93.7.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–69. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- [38].Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001;13:85–94. doi: 10.1016/s0898-6568(00)00149-2. [DOI] [PubMed] [Google Scholar]
- [39].Coulon L, Calzada C, Moulin P, Véricel E, Lagarde M. Activation of p38 mitogen-activated protein kinase/cytosolic phospholipase A2 cascade in hydroperoxide-stressed platelets. Free Radic Biol Med. 2003;35:616–625. doi: 10.1016/s0891-5849(03)00386-1. [DOI] [PubMed] [Google Scholar]
- [40].Cho HJ, Kwon GT, Park JH. trans-10, cis-12 Conjugated linoleic acid induces depolarization of mitochondrial membranes in HT-29 human colon cancer cells: a possible mechanism for induction of apoptosis. J Med Food. 2009;12:952–958. doi: 10.1089/jmf.2009.0056. [DOI] [PubMed] [Google Scholar]
- [41].Adams JM, Cory S. Life-or-death decisions by the Bcl-2 protein family. Trends Biochem Sci. 2001;26:61–66. doi: 10.1016/s0968-0004(00)01740-0. [DOI] [PubMed] [Google Scholar]
- [42].Tsujimoto Y. Role of Bcl-2 family proteins in apoptosis: apoptosomes or mitochondria? Genes Cells. 1998;3:697–707. doi: 10.1046/j.1365-2443.1998.00223.x. [DOI] [PubMed] [Google Scholar]
- [43].Kutuk O, Basaga H. Bcl-2 protein family: implications in vascular apoptosis and atherosclerosis. Apoptosis. 2006;11:1661–1675. doi: 10.1007/s10495-006-9402-7. [DOI] [PubMed] [Google Scholar]
- [44].Asehnoune K, Strassheim D, Mitra S, Kim JY, Abraham E. Involvement of reactive oxygen species in Toll-like receptor 4-dependent activation of NF-kappa B. J Immunol. 2004;172:2522–2529. doi: 10.4049/jimmunol.172.4.2522. [DOI] [PubMed] [Google Scholar]
- [45].Kim HJ, Lee HS, Chong YH, Kang JL. p38 Mitogen-activated protein kinase up-regulates LPS-induced NF-kappaB activation in the development of lung injury and RAW 264.7 macrophages. Toxicology. 2006;225:36–47. doi: 10.1016/j.tox.2006.04.053. [DOI] [PubMed] [Google Scholar]
- [46].Srivastava SK, Ramana KV. Focus on molecules: nuclear factor-kappaB. Exp Eye Res. 2009;88:2–3. doi: 10.1016/j.exer.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Gloire G, Legrand-Poels S, Piette J. NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol. 2006;72:1493–505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- [48].Berger MM, Chioléro RL. Antioxidant supplementation in sepsis and systemic inflammatory response syndrome. Crit Care Med. 2007;35:S584–S590. doi: 10.1097/01.CCM.0000279189.81529.C4. [DOI] [PubMed] [Google Scholar]
- [49].Reddy DB, Reddanna P. Chebulagic acid (CA) attenuates LPS-induced inflammation by suppressing NF-kappaB and MAPK activation in RAW 264.7 macrophages. Biochem Biophys Res Commun. 2009;381:112–117. doi: 10.1016/j.bbrc.2009.02.022. [DOI] [PubMed] [Google Scholar]
- [50].Zhou HY, Shin EM, Guo LY, Youn UJ, Bae K, Kang SS, Zou LB, Kim YS. Anti-inflammatory activity of 4-methoxyhonokiol is a function of the inhibition of iNOS and COX-2 expression in RAW 264.7 macrophages via NF-kappaB, JNK and p38 MAPK inactivation. Eur J Pharmacol. 2008;586:340–349. doi: 10.1016/j.ejphar.2008.02.044. [DOI] [PubMed] [Google Scholar]
- [51].Yun KJ, Kim JY, Kim JB, Lee KW, Jeong SY, Park HJ, Jung HJ, Cho YW, Yun K, Lee KT. Inhibition of LPS-induced NO and PGE2 production by asiatic acid via NF-kappa B inactivation in RAW 264.7 macrophages: possible involvement of the IKK and MAPK pathways. Int Immunopharmacol. 2008;8(3):431–441. doi: 10.1016/j.intimp.2007.11.003. [DOI] [PubMed] [Google Scholar]
- [52].Zhang WJ, Wei H, Hagen T, Frei B. Alpha-lipoic acid attenuates LPS-induced inflammatory responses by activating the phosphoinositide 3-kinase/Akt signaling pathway. Proc Natl Acad Sci U S A. 2007;104:4077–4082. doi: 10.1073/pnas.0700305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Jang SI, Kim HJ, Kim YJ, Jeong SI, You YO. Tanshinone IIA inhibits LPS-induced NF-kappaB activation in RAW 264.7 cells: possible involvement of the NIK-IKK, ERK1/2, p38 and JNK pathways. Eur J Pharmacol. 2006;542:1–7. doi: 10.1016/j.ejphar.2006.04.044. [DOI] [PubMed] [Google Scholar]
- [54].Lee TY, Lee KC, Chen SY, Chang HH. 6-Gingerol inhibits ROS and iNOS through the suppression of PKC-alpha and NF-kappaB pathways in lipopolysaccharide-stimulated mouse macrophages. Biochem Biophys Res Commun. 2009;382(1):134–139. doi: 10.1016/j.bbrc.2009.02.160. [DOI] [PubMed] [Google Scholar]
- [55].Victor VV, Guayerbas N, Puerto M, Medina S, De la Fuente M. Ascorbic acid modulates in vitro the function of macrophages from mice with endotoxic shock. Immunopharmacology. 2000;46:89–101. doi: 10.1016/s0162-3109(99)00162-9. [DOI] [PubMed] [Google Scholar]
- [56].De la Fuente M, Victor VM. Ascorbic acid and N-acetylcysteine improve in vitro the function of lymphocytes from mice with endotoxin-induced oxidative stress. Free Radic Res. 2001;35:73–84. doi: 10.1080/10715760100300611. [DOI] [PubMed] [Google Scholar]
- [57].Abe S, Tanaka Y, Fujise N, Nakamura T, Masunaga H, Nagasawa T, Yagi M. An antioxidative nutrient-rich enteral diet attenuates lethal activity and oxidative stress induced by lipopolysaccharide in mice. JPEN J Parenter Enteral Nutr. 2007;31:181–187. doi: 10.1177/0148607107031003181. [DOI] [PubMed] [Google Scholar]
- [58].Reiter E, Jiang Q, Christen S. Anti-inflammatory properties of alpha- and gamma-tocopherol. Mol Aspects Med. 2007;28:668–691. doi: 10.1016/j.mam.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Beltramo E, Berrone E, Tarallo S, Porta M. Effects of thiamine and benfotiamine on intracellular glucose metabolism and relevance in the prevention of diabetic complications. Acta Diabetol. 2008;45:131–141. doi: 10.1007/s00592-008-0042-y. [DOI] [PubMed] [Google Scholar]
- [60].Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- [61].Pomero F, Molinar MA, La Selva M, Allione A, Molinatti GM, Porta M. Benfotiamine is similar to thiamine in correcting endothelial cell defects induced by high glucose. Acta. Diabetol. 2001;38:135–138. doi: 10.1007/s005920170010. [DOI] [PubMed] [Google Scholar]
- [62].Babaei-Jadidi R, Karachalias N, Ahmed N, Battah S, Thornalley PJ. Prevention of incipient diabetic nephropathy by high-dose thiamine and benfotiamine. Diabetes. 2003;52:2110–2120. doi: 10.2337/diabetes.52.8.2110. [DOI] [PubMed] [Google Scholar]
- [63].Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, Sano H, Utsumi H, Nawata H. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C--dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000;49:1939–1945. doi: 10.2337/diabetes.49.11.1939. [DOI] [PubMed] [Google Scholar]
- [64].Inoguchi T, Sonta T, Tsubouchi H, Etoh T, Kakimoto M, Sonoda N, Sato N, Sekiguchi N, Kobayashi K, Sumimoto H, Utsumi H, Nawata H. Protein kinase C-dependent increase in reactive oxygen species (ROS) production in vascular tissues of diabetes: role of vascular NAD(P)H oxidase. J Am Soc Nephrol. 2003;14:S227–S232. doi: 10.1097/01.asn.0000077407.90309.65. [DOI] [PubMed] [Google Scholar]
- [65].Berrone E, Beltramo E, Solimine C, Ape AU, Porta M. Regulation of intracellular glucose and polyol pathway by thiamine and benfotiamine in vascular cells cultured in high glucose. J Biol Chem. 2006;281:9307–9313. doi: 10.1074/jbc.M600418200. [DOI] [PubMed] [Google Scholar]
- [66].Beltramo E, Berrone E, Buttiglieri S, Porta M. Thiamine and benfotiamine prevent increased apoptosis in endothelial cells and pericytes cultured in high glucose. Diabetes Metab Res Rev. 2004;20:330–336. doi: 10.1002/dmrr.470. [DOI] [PubMed] [Google Scholar]
- [67].Schmid U, Stopper H, Heidland A, Schupp N. Benfotiamine exhibits direct antioxidative capacity and prevents induction of DNA damage in vitro. Diabetes Metab Res Rev. 2008;24:371–377. doi: 10.1002/dmrr.860. [DOI] [PubMed] [Google Scholar]
- [68].Qi HY, Shelhamer JH. Toll-like receptor 4 signaling regulates cytosolic phospholipase A2 activation and lipid generation in lipopolysaccharide-stimulated macrophages. J Biol Chem. 2005;280:38969–38975. doi: 10.1074/jbc.M509352200. [DOI] [PubMed] [Google Scholar]
- [69].Dieter P, Scheibe R, Kamionka S, Kolada A. LPS-induced synthesis and release of PGE2 in liver macrophages: regulation by CPLA2, COX-1, COX-2, and PGE2 synthase. Adv Exp Med Biol. 2002;507:457–462. doi: 10.1007/978-1-4615-0193-0_71. [DOI] [PubMed] [Google Scholar]