Abstract
Background
We previously reported reduced prefrontal cortex (PFC) grey matter volume among first-episode, antipsychotic-naïve schizophrenia subjects (SZ) exposed to HSV1 but not among healthy subjects (HS) (Prasad et al. 2007). Independently, rs1051788, an exonic polymorphism of the MHC Class I polypeptide-related sequence B (MICB) gene was associated with HSV1 seropositivity, as well as SZ risk. In this study, we examined whether PFC grey matter changes associated with HSV1 exposure varied against the background of MICB genotypes.
Methods
We examined Caucasian individuals from the sample we studied in our previous report (Prasad et al. 2007) (SZ, n=21 and HS, n=19). Whole-brain voxel-wise analysis of structural MRI scans was conducted using Statistical Parametric Mapping, ver 5 (SPM5). The impact of rs1051788 variation and HSV1 seropositivity on grey matter volumes was examined using regression models on the combined sample of cases and controls, and then within each diagnostic group.
Results
In the combined sample of cases and controls, we observed the main effects of HSV1 seropositivity and genotypes, and a significant joint effect of HSV1 seropositivity and genotype mainly in the PFC. The joint effect was more prominent among cases than among controls.
Discussion
Our observations suggest that rs1051788 and HSV1 seropositivity are associated individually and jointly with reduced PFC grey matter volume. The patterns of these associations differ by diagnostic status, and these factors explain only a “small” portion of the variance in the grey matter volume reductions.
1. INTRODUCTION
Up to 90% of persons in the US depending on age, socioeconomic status and ethnicity are exposed to the Herpes Simplex Virus type 1 (HSV1) - a neurotropic virus (NHANES II 1987, revised 2007; Schillinger et al. 2004; Xu et al. 2006). The majority of such individuals do not develop encephalitis. Recent replicable evidence indicates that the serological evidence of exposure to HSV1 (HSV1 seropositivity) is associated with cognitive impairments in such individuals (Dickerson et al. 2003; Shirts et al. 2008a). The effect size of HSV1 exposure on cognitive impairment was greater among subjects with schizophrenia compared to subjects without psychiatric disorders (Dickerson et al. 2008; Dickerson et al. 2003; Shirts et al. 2008a). Recently, we observed reduced prefrontal cortex (PFC) grey matter volumes among SZ subjects with serological evidence of exposure to HSV1 compared to those without but not among healthy subjects (HS) suggesting a mechanistic association that requires confirmation (Prasad et al. 2007). We had hypothesized that HSV1 exposure may interact with other illness related variables such as genetic variation.
We have separately analyzed host genetic variations in association with HSV1 seropositivity in our association studies of SZ. We focused on the Human Leukocyte Antigen (HLA) region because it is frequently associated with susceptibility to infectious diseases. Following a series of genetic association studies (Kim et al. 2007; Shirts et al. 2007), we focused on the Major Histocompatibility Complex (MHC) Class I related polypeptide-related sequence B (MICB) gene. Our analyses of rs1051788, an exonic polymorphism of MICB suggested novel associations. Allele A of this SNP was associated with increased risk for SZ, whereas allele G was associated with elevated HSV1 antibody titers among healthy comparison subjects (HS), suggesting a pleiotropic effect (Shirts et al. 2007). This polymorphism leads to substitution of aspartic acid with asparagine at amino acid position 136 on one of the α-helical folds of MICB protein, suggesting that the substitution may be functionally relevant.
In view of the novel associations in our prior imaging and genetic association studies, we examined grey matter variations related to HSV1 exposure among first episode antipsychotic naïve SZ subjects and HS against the background of rs1051788 genotypes at MICB. Our goal in this study was to examine whether our previously observed differences in grey matter variations in the PFC among HSV1-exposed SZ subjects and HS is related to variation at a specific MICB polymorphism.
2. METHODS
This sample was derived from our prior study, in which we examined associations between HSV1 exposure and grey matter volumes in first episode antipsychotic naïve SZ subjects and HS (Prasad et al. 2007). From this sample, we selected individuals with Caucasian ancestry in order to enable genetic analyses in a more ethnically homogenous sample (n=40; 21 first episode, antipsychotic-naïve SZ or schizoaffective disorder subjects, and 19 HS). The subjects were recruited from inpatient and outpatient services of the Western Psychiatric Institute and Clinic. DSM IV (American Psychiatric Association 1994) diagnoses were derived by reviewing data from the Structured Clinical Interview for DSM IV diagnosis (SCID) (First 1997) and medical records in a consensus diagnostic conference of senior clinicians. All diagnoses were reviewed after following the subjects for a minimum of 6 months. We also obtained demographic information, including the socioeconomic status (SES) using the Hollingshead scale (Hollingshead 1975). HS were recruited through local advertisements from the same geographic region as the patients. None of the subjects had mental retardation, substance dependence in the last 6 months or abuse in the month before participation per DSM IV criteria. Subjects with medical or neurological disorders including head injury, encephalitis and epilepsy were not recruited. After fully explaining the experimental procedures, informed consents were obtained from all subjects. The Institutional Review Board of the University of Pittsburgh approved the study.
2.1 Imaging Methods
MRI scans were obtained with a GE 1.5T whole body scanner (GE Medical Systems, Milwaukee, Wisconsin). The detailed scanning protocol has been described in our earlier publication (Gilbert et al. 2001). Briefly, the scans were three-dimension spoiled gradient recalled (SPGR), acquired in a steady-state pulse sequence (124 coronal slices, 1.5 mm thickness, TE=5 msec, TR=25 msec, acquisition matrix=256×192, FOV=24 cm, flip angle 40°). None of the scans had motion artifacts.
Images were analyzed using the Statistical Parametric Mapping (SPM 5) software (http://www.fil.ion.ucl.ac.uk/spm/software/spm5/). The voxel-based morphometry (VBM) analysis was implemented on a Mac Pro workstation using the SPM5 package on Matlab 7 platform (Mathworks Inc 2002). SPM5 offers several advantages over the previous version such as updated segmentation procedure and correction for misclassified non-brain tissue. It reduces the reliance on template brain to perform these steps. The MR-images were normalized to the standard T1 template and segmented into grey matter, white matter and cerebrospinal fluid (CSF) depending on the intensity and the spatial probability of that voxel belonging to any of these tissue categories. The images were smoothed by convolving with a Gaussian smoothing kernel (12×12×12 fwhm) in order to reduce noise. The normalization procedure was reapplied to the original images to obtain optimized segmentation of normalized images. In order to correct for volume changes, segmented images were modulated by the Jacobian determinants from spatial normalization. All images were then smoothed with a 12×12×12 FWHM Gaussian kernel.
We first examined the grey matter density setting a significance threshold of uncorrected p<0.001 and an extent threshold of 20 voxels. We report only those clusters that remained significant after correction using the false discovery rate (FDR) method in the whole brain analyses. From these clusters of grey matter densities, we extracted the volumes from the native space using MarsBar (Brett et al. 2002) from all images used in that particular contrast. For example, in a contrast that compared HSV1 seropositive SZ subjects with HSV1 seronegative SZ subjects (HSV1+<HSV1−), the significant cluster that showed reduction in a given Brodmann area among HSV1 seropositive SZ subjects area was masked and the volume estimated from the native space of the images from HSV1 seropositive group; the corresponding volume from the native space of images from seronegative SZ subjects was also estimated using the same mask. The extracted volumes were compared using appropriate statistics on SPSS version 16 (SPSS 2005). When a cluster consisted of multiple peaks of maximum intensity projections of grey matter density corresponding to a single Brodmann area, we added up the extracted volumes from these clusters and used this composite volume in the SPSS package for additional statistical tests as stated earlier. In order to locate the clusters to the Brodmann areas they correspond to, we converted the MNI coordinates to Talairach coordinates before using the Talairach database. We preferred to use the voxel-based morphometric approach because this method would offer a whole brain analysis as opposed to region-of-interest approach. Other studies (Gitelman et al. 2001; Vartiainen et al. 2009) including our previous study on SZ subjects and HS (Prasad et al. 2007) have successfully demonstrated the usefulness of voxel based morphometry.
2.2 Immunological assays
The IgG antibodies to HSV1 were tested with solid-phase enzyme immunoassay directed at the HSV-1 specific gG1 glycoprotein (Ashley 1998; Dickerson et al. 2003). Assays using different microtiter plates were standardized by using reference samples run on each assay plate. Antibody titers were quantified as signal/cutoff (S/C) ratios, which were calculated for each sample by dividing the optical density measurements generated in the assay by the optical density of a standard cutoff serum. Individuals were considered seropositive using criteria previously described (Prasad et al. 2007). In this study, subjects with antibody ratios greater than 1.3 were considered to have serological evidence of HSV1 exposure (HSV1 seropositive) and those with less than 1.3 were considered HSV1 seronegative.
2.3 Genotype assays
A detailed description of the selection of SNPs and genetic assays are described in our previous publications (Shirts et al. 2007; Shirts et al. 2008b). Briefly, a ligation-based SNPlex assay (ABI Inc) was used to genotype the MICB SNP. Positive controls and negative controls were included in the assay plates for quality control. Genotype clusters were examined on GeneMapper v 4.0. Allele calls were independently checked and confirmed by two investigators (MB and KMP). In addition, we genotyped 7 randomly selected subjects by sequencing; no mismatches were found.
2.4 Statistical Plan
We first examined the association of grey matter volume and individual independent variables (HSV1 serological status, MICB genotypes, gender and diagnostic status) in the entire sample of cases and controls. Our goal was to analyze the contribution of diagnosis (SZ/HS), serological status (HSV1+/HSV1−), and genotype (GG/AG) to the grey matter volumes individually and jointly, covarying for key demographic variables (age, sex and SES). Specifically, we fit the grey matter volume to the covariates in the entire case-control sample and obtained residuals. Next, we fit the residuals to genotypes and serological status separately first and then jointly in the entire sample of cases and controls using t-tests. We then repeated the same design within each diagnostic group (SZ/HS) to obtain residuals and then fit the residuals to genotypes and serological status for each diagnostic group. The regions that remained significant after FDR corrected whole brain analyses were extracted and examined using ANOVA for between subjects effects using SPSS version 16 (SPSS 2005). Since these volumes were adjusted for covariates, we did not include covariates again when we examined the volumetric differences. We report both FDR-corrected whole-brain grey matter density differences and the volumetric data from these clusters that met the statistical threshold. We did not correct the volumetric data for multiple tests because these data were obtained after a stringent voxelwise correction for the entire brain and, thus attempted to avoid committing type II error.
3. RESULTS
The key characteristics of the sample are provided in table 1. There were no significant case-control differences with regard to age, SES, HSV1 seropositivity or genotype distribution. However, there were proportionately more women among the HS (χ2=9.95, p=0.003). Similarly, age, SES and genotypes distribution were not significantly different with regard to serological status within SZ and HS. However, none of the females within HS group showed serological evidence of exposure to HSV1. Hence, we examined the association of sex with variations in grey matter volumes by including age and SES as covariates. There was no significant difference in the serological status or genotype distribution among SZ and HS. In this sample, there were no individuals homozygous for the allele A.
Table 1.
Sample Characteristics
| Schizophrenia (SZ) | Healthy Control Subjects (HS) | ||||||
|---|---|---|---|---|---|---|---|
| Total (n=21) | HSV1− (n=11) |
HSV1+ (n=10) |
Total (n=19) | HSV1− (n=13) | HSV1+ (n=6) | ||
| Age (in years)1 Mean ± SD |
24.16±8.63 | 21.34±6.21 | 27.26±10.11 | 24.39±6.29 | 23.89±6.01 | 25.48±7.32 | |
| Gender2 | Male | 17 | 10 | 7 | 6 | 6 | 0 |
| Female | 4 | 1 | 3 | 13 | 7 | 6 | |
| Av SES3 | 41.38±14.66 | 40.36±12.93 | 42.50±17.01 | 46.55±11.16 | 46.46 (11.78) | 46.75 (10.71) | |
|
MICB Genotypes |
GG | 9 | 3 | 6 | 7 | 1 | 6 |
| AG | 12 | 7 | 5 | 12 | 5 | 7 | |
| AA | 0 | 0 | 0 | 0 | 0 | 0 | |
HSV1: Herpes Simplex Virus, Subtype 1; SES: Socio-economic Status
t-tests:
SZ and HS did not differ in age. Similarly, HSV1+ SZ and HSV1− SZ subjects (t=1.63 p=0.12), and HSV1+ Controls and HSV1− Controls (t=0.50 p=0.62) did not differ in age
There were more females among HS (χ2=9.95, p=0.003)
SES did not differ between SZ and HS (t=1.26, p=0.22). In addition, HSV1+ SZ and HSV1− SZ subjects (t=0.33 p=0.75), and HSV1+ Controls and HSV1− SZ Controls did not differ in SES (t=0.05, p=0.96)
3.1. Association between grey matter volume and individual independent variables
In the combined sample of cases and controls, we observed that the serological status, and genotype, but not the diagnostic status or gender were associated with grey matter volumes.
HSV1 Status: HSV1 seropositive subjects in comparison to HSV1 seronegative subjects showed grey matter volume reductions in the following regions when the entire sample of cases and controls were analyzed: right middle and superior frontal gyrus (BA 9), and the right superior temporal gyrus (BA 22). In addition, the grey matter densities in the right dorsal anterior cingulate (BA 32), and the left anterior cingulate gyrus showed trends toward significance but the extracted volumes from these clusters did show significant differences (Table 2). Prefrontal volume (summated BA9 and BA 10) was reduced by 9.24% among seropositive individuals (4.62±0.57 cc) compared to seronegative (5.09±0.56 cc)(F(1, 40)=6.64, p=0.014)(Fig 1). MICB genotypes: Heterozygous individuals (cases + controls) (AG) compared to homozygous individuals (GG) had smaller volumes at the left inferior frontal gyrus (BA 45), left inferior parietal lobule (BA 40), and the left precuneus (BA 7) (Table 2). Gender: Due to the difference in gender distribution among cases and controls, the association with gender was also analyzed. Females subjects showed increased grey matter density in the left caudate, left inferior temporal gyrus (BA 37), left cuneus (BA 19), left thalamus, right middle temporal gyrus (BA 21) and right inferior frontal gyrus (BA 47). However, extracted volumes from these regions did not show significant differences between the genders after covarying for age and SES (Table 2). Diagnostic status: We did not observe a main effect of diagnostic status on grey matter density or volumes.
Table 2.
Associations of the pooled sample with independent variables
| Grey matter density | Grey matter Volumes | |||||||
|---|---|---|---|---|---|---|---|---|
| Association of grey matter volumes with HSV1 exposure status | ||||||||
| MNI Coordinate | Region | Brodmann Area |
T | FDR corrected p | HSV1 Neg (cc) | HSV1 Pos (cc) | F (1, 39) |
p |
| 54 8 39 26 44 36 |
R Middle Frontal G R Superior Frontal G |
BA 9 | 5.60 4.74 |
0.026 0.036 |
4.79±0.52 | 4.34±0.54 | 6.96 | 0.012 |
| 62 −2 −2 | R Superior Temporal G | BA 22 | 4.37 | 0.048 | 0.14±0.02 | 0.12±0.03 | 4.97 | 0.032 |
| −6 66 4 | L Superior Frontal G | BA 10 | 4.34 | 0.048 | 0.30±0.05 | 0.28±0.05 | 1.31 | 0.26 |
| 8 6 50 | R Dorsal Ant Cingulate G | BA 32 | 4.17 | 0.056* | 0.14±0.02 | 0.13±0.02 | 4.55 | 0.039 |
| −2 10 34 | Left Ant Cingulate G | BA 24 | 4.03 | 0.060* | 0.21±0.02 | 0.19±0.03 | 7.79 | 0.008 |
| Association of grey matter volumes with MICB Genotypes | ||||||||
| MNI Coordinate | Region | Brodmann Area |
T | FDR corrected p | GG (cc) | AG (cc) | F (1, 39) |
p |
| −20 −38 58 | L Postcentral G | BA 3 | 5.73 | 0.031 | 0.97±0.14 | 0.91±0.16 | 1.59 | 0.22 |
| −6 16 54 | L Superior Frontal G | BA 8 | 5.30 | 0.031 | 0.57±0.11 | 0.56±0.11 | 0.05 | 0.83 |
| −18 −26 −8 | L Parahippocampal G | BA 28 | 5.16 | 0.031 | 0.43±0.06 | 0.41±0.05 | 1.81 | 0.19 |
| 4 −34 36 | L Dorsal Ant Cingulate G | BA 31 | 5.07 | 0.031 | 1.42±0.18 | 1.36±0.18 | 1.35 | 0.25 |
| −14 −64 34 | L Precuneus | BA 7 | 4.59 | 0.038 | 0.30±0.06 | 0.23±0.08 | 10.63 | 0.002 |
| −48 −30 34 | L Inferior Parietal Lobule | BA 40 | 4.59 | 0.038 | 0.21±0.06 | 0.16±0.05 | 6.28 | 0.017 |
| −4 −46 14 | L Ant Cerebellum Culmen | 4.52 | 0.038 | 0.79±0.12 | 0.74±0.13 | 1.22 | 0.28 | |
| −8 48 30 | L Medial Frontal G | BA 9 | 4.23 | 0.046 | 0.22±0.03 | 0.23±0.04 | 0.89 | 0.35 |
| −40 24 18 | L Inferior Frontal G | BA 45 | 4.19 | 0.048 | 0.13±0.04 | 0.09±0.05 | 6.12 | 0.018 |
| 24 −32 60 | R Postcentral G | BA 3 | 4.14 | 0.049 | 0.26±0.07 | 0.24±0.04 | 0.74 | 0.40 |
| Association of grey matter volumes with Gender | ||||||||
| MNI Coordinate | Region | Brodmann Area |
T | FDR corrected p | Female (cc) | Male (cc) | F (1, 39) |
p |
| −12 12 4 | L Caudate | 5.49 | 0.023 | 2.54±0.24 | 2.67±0.26 | 2.74 | 0.11 | |
| 62 −56 0 | R Middle Temporal G | BA 21 | 5.34 | 0.023 | 1.67±0.22 | 1.75±0.18 | 1.79 | 0.19 |
| −52 −74 −2 | L Inferior Temporal G | BA 37 | 4.74 | 0.023 | 0.84±0.10 | 0.87±0.09 | 0.83 | 0.37 |
| −26 −88 20 | L Cuneus | BA 19 | 4.51 | 0.026 | 1.14±0.19 | 1.17±0.14 | 0.21 | 0.65 |
| −2 −24 2 | L Thalamus | 4.04 | 0.040 | 0.79±0.12 | 0.76±0.08 | 1.25 | 0.27 | |
| 34 32 −10 | R Inferior Frontal G | BA 47 | 4.14 | 0.036 | 0.10±0.02 | 0.10±0.04 | 0.04 | 0.85 |
These regions are included here because we observed changes in cingulate gyrus in our previous publication (Prasad et al., 2007)
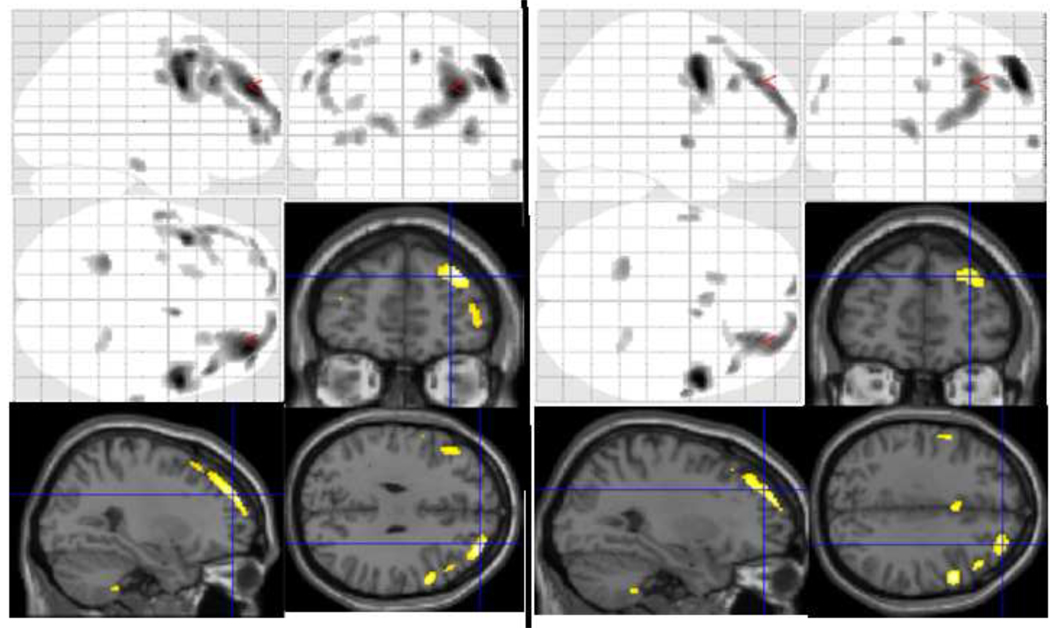
Fig 1.
Glass brain pictures and rendering of significant voxel clusters on to T1 image of grey matter density and volume across HSV1 serological status in the entire sample of cases and controls in a regression model. Left: Regression model included age, sex, SES and diagnostic status with MICB genotypes; Right: Regression model without MICB genotypes as covariates.
See table 3 and table 2, respectively for volumetric differences
3.2 Multivariate analysis of grey matter volumes including all key variables in the combined sample of cases and controls
Using regression models, we examined grey matter volume as the outcome variable in relation to the HSV1 exposure status by including MICB genotypes in addition to age, sex, diagnosis and SES as covariates in the entire sample. HSV1 seropositive subjects showed reduced grey matter volumes compared to seronegative subjects in the following regions: right BA 9, left BA 10, right inferior temporal gyrus (BA 20), and the right posterior lobe of cerebellum (Table 3) (Fig 1). This analysis also showed that the summated volume of prefrontal cortex was reduced by 8.5% among HSV1 seropositive subjects compared to seronegative subjects (t=2.33, p=0.025).
Table 3.
Grey matter density and volume across HSV1 serological status in the entire sample of cases and controls in a regression model that included MICB genotypes, age, sex, SES and diagnostic status as covariates
| Grey matter density | Grey matter Volumes | |||||||
|---|---|---|---|---|---|---|---|---|
| MNI Coordinate | Region | Brodmann Area |
T | FDR corrected p | HSV1 Neg (cc) | HSV1 Pos (cc) | F (1, 39) |
p |
| 54 8 34 | R Inferior Frontal G | BA 9 | 5.91 | 0.015 | 1.99±0.23 | 1.82±0.21 | 6.73 | 0.013 |
| −26 60 0 −26 56 12 38 54 2 −12 68 10 |
L Superior and Middle Frontal G |
BA 10 | 4.21 3.83 4.88 4.39 |
0.032 0.046 0.017 0.028 |
1.39±0.17 | 1.28±0.15 | 4.53 | 0.04 |
| 32 50 30 | R Superior Frontal G | BA 10 | 5.73 | 0.015 | 3.97±0.50 | 3.63±0.50 | 4.50 | 0.04 |
| 66 −20 −20 | R Inferior Temporal Gyrus |
BA 20 | 4.14 | 0.034 | 0.17±0.02 | 0.14±0.04 | 7.68 | 0.009 |
| 24 −38 −46 | R Cerebellar Tonsil | ---- | 3.87 | 0.044 | 0.27±0.03 | 0.24±0.03 | 6.80 | 0.013 |
| −56 −4 38 −16 18 62 −28 −4 60 −40 −12 52 |
L Precentral and Mid Frontal G |
BA 6 | 4.31 3.72 4.58 5.45 |
0.030 0.051* 0.023 0.015 |
2.50±0.32 | 2.31±0.31 | 3.42 | 0.072 |
| 8 4 52 | R Medial Frontal G | BA 6 | 4.58 | 0.023 | 0.19±0.03 | 0.18±0.03 | 3.29 | 0.078 |
This region was included because we observed changes in middle frontal gyrus in our previous publication (Prasad et al., 2007)
3.2.1. Within diagnostic group differences
In order to investigate which diagnostic group contributed to the grey matter changes in the above regression model, we conducted analyses within each diagnostic group using the same design as above.
SZ cases
Consistent with the association of HSV1 exposure as an independent variable with grey matter reductions in the entire sample of cases and controls, HSV1 seropositive SZ subjects showed reduced grey matter volumes compared to seronegative subjects in the following regions: right BA 9, left middle frontal gyrus (BA 47), left precentral gyrus (BA 6), left medial frontal gyrus (BA 10), and right dorsal anterior cingulate gyrus (BA 32) when MICB genotypes were included along with age, sex and SES as covariates (Table 4). The summated PFC volume showed significant reduction among HSV1 seropositive SZ compared to seronegative SZ subjects (t=3.03, p=0.007). When MICB genotypes were removed from the analysis while retaining age, sex and SES as covariates, we observed reductions in left BA 6, right superior temporal gyrus (BA 22), right BA 9, left precuneus and (BA 47) (Table 4). The total PFC volume reduction showed a trend toward significance (t=1.91, p=0.072). When MICB genotypes were covaried, we observed a greater reduction in grey matter volumes within SZ subjects. Mean reduction in the summated prefrontal volumes in seropositive SZ subjects when MICB genotypes were covaried was 1.22 cc (14.9% reduction) compared to 0.44 cc (6.4% reduction) when genotypes were not covaried.
Table 4.
Grey matter density and volume across HSV1 serological status with and without MICB genotypes as an additional covariate within SZ group in a regression model with age, sex, and SES as covariates
| Grey matter density | Grey matter Volumes | |||||||
|---|---|---|---|---|---|---|---|---|
| MNI Coordinate | Region | Brodmann Area |
T | FDR corrected p | HSV1 Neg (cc) | HSV1 Pos (cc) | F (1, 19) |
p |
| Regression model for grey matter volumes including MICB genotypes, Age, Sex and SES | ||||||||
| 54 8 34 | R Inf Frontal G | BA 9 | 6.72 | 0.003 | 7.51±0.65 | 6.38±0.20 | 11.38 | 0.003 |
| −54 −6 40 | L Precentral G | BA 6 | 5.39 | 0.007 | 3.76±0.43 | 3.32±0.48 | 5.11 | 0.036 |
| −12 68 10 | L Medial Frontal G | BA 10 | 4.85 | 0.011 | 0.68±0.08 | 0.59±0.06 | 8.74 | 0.008 |
| 8 6 50 | R Dorsal Ant Cingulate G | BA 32 | 4.45 | 0.018 | 0.14±0.02 | 0.12±0.02 | 5.51 | 0.03 |
| −2 8 34 | L Ant Cingulate G | BA 24 | 3.92 | 0.032 | 0.12±0.01 | 0.11±0.01 | 3.76 | 0.067 |
| −48 44 −6 | L Mid Frontal G | BA 47 | 3.79 | 0.037 | 0.18±0.02 | 0.14±0.02 | 20.71 | 0.0002 |
| −46 −66 46 | L Inf Par Lobule | BA 39 | 3.70 | 0.041 | 0.17±0.03 | 0.15±0.03 | 3.82 | 0.066 |
| −56 −34 48 | L Inf Par Lobule | BA 40 | 3.84 | 0.037 | 0.15±0.01 | 0.14±0.02 | 2.74 | 0.11 |
| Regression model for grey matter volumes including Age, Sex and SES but not MICB genotypes | ||||||||
| −6 28 42 −56 −4 36 −38 10 52 |
L Med/Mid Frontal & Precentral G |
BA 6 | 5.50 5.87 7.06 |
0.024 0.016 0.005 |
1.05±0.12 | 1.14±0.13 | 5.19 | 0.028 |
| −8 −48 −42 | L Posterior Cingulate G | BA 31 | 4.43 | 0.054* | 0.16±0.02 | 0.14±0.02 | 5.08 | 0.036 |
| 60 −2 0 | R Superior Temporal G | BA 22 | 5.42 | 0.026 | 0.25±0.03 | 0.21±0.05 | 4.54 | 0.046 |
| 24 42 40 54 6 40 |
R Superior Frontal G R Middle Frontal G |
BA 9 | 5.51 10.92 |
0.024 0.001 |
6.52±0.76 | 5.84±0.89 | 3.55 | 0.075 |
| −58 −26 38 | L Postcentral G | BA 2 | 5.17 | 0.033 | 0.36±0.05 | 0.33±0.04 | 3.59 | 0.073 |
| 26 −8 −38 | R Uncus | BA 28 | 5.37 | 0.027 | 0.17±0.03 | 0.15±0.03 | 3.14 | 0.092 |
| −48 12 28 | L Inf Frontal G | BA 9 | 5.13 | 0.033 | 0.34±0.05 | 0.31±0.04 | 2.11 | 0.163 |
| 22 −56 58 | R Precuneus | BA 7 | 5.26 | 0.030 | 0.11±0.03 | 0.09±0.03 | 1.84 | 0.190 |
| 28 −22 58 | R Precentral G | BA 4 | 4.75 | 0.044 | 0.08±0.02 | 0.07±0.02 | 0.12 | 0.730 |
| 8 2 50 | R Anterior Cingulate G | BA 24 | 5.91 | 0.015 | 3.96±0.48 | 3.58±0.57 | 0.06 | 0.809 |
This is included here because we observed changes in the cingulate gyrus in our previous publication (Prasad et al., 2007)
Healthy Control Subjects
Similar set of analyses among HS (with and without covarying for MICB genotypes) did not show significant differences in any regions that met our statistical threshold.
3.2.2 Leave 1-out analyses
As the prefrontal grey matter reductions were associated with the joint effect of HSV1 exposure and MICB genotypes, we examined whether these observations were due to outliers within this sample. Using the jackknife (leave 1-out) approach, we reanalyzed the BA9 cluster, retaining the earlier analytical design for the entire sample of cases and controls. The p-values were plotted after negative log conversion. We observed that the significance for the full-sample study was well below the mean p value of the leave-1 out analyses suggesting that our observations may not be entirely due to the outliers or large effect of a subgroup of SZ subjects (Supplemental data).
4. DISCUSSION
We report novel associations between a non-synonymous exonic polymorphism of an immune-related gene, MICB, and exposure to HSV1 on grey matter volumes in the same individuals. Our staged analyses suggest that these observations may not be due to illness chronicity, SES, antipsychotic medications, or outliers within this cohort. Consistent with prior studies of HSV1 related brain pathology, the observed grey matter changes are in the regions that are known to undergo neuronal dysfunction or death following HSV1 reactivation (Cleator and Klapper 2004a, b). It is important to note that none of our subjects had history of encephalitis, in which temporal lobes in addition to other regions may be affected (Cleator and Klapper 2004a). The main effect of HSV1 exposure on PFC grey matter reduction in SZ subjects observed in this report is somewhat weaker compared to our earlier study, presumably due to the smaller sample size. The reduction in the sample size was due to the inclusion of Caucasian subjects only and was necessitated by the addition of genetic analysis. Reductions in the grey matter volumes were observed mainly in the prefrontal cortex; abnormalities in this region have been implicated in SZ (Minzenberg et al. 2009; Weinberger et al. 1986). Here, we find that reduced grey matter volumes occurred more prominently among subjects with SZ in the background of MICB genotypes than when genotypes were not included. Thus, the joint effect was mainly quantitative in nature on the prefrontal region among SZ subjects. Illness-related variables other than the MICB variation and HSV1 exposure may additionally explain the observed differences in the patterns of associations between the cases and controls. For example, there may also be case-control differences in the distribution of other immune-related genes. The relatively small sample precludes additional genetic analyses.
Our selection of the genetic polymorphism and the environmental factor merits discussion. Regardless of the sample size, genetic studies conducted without considering the environmental background or vice versa may be limited in their implications. In addition, concurrent examination of the neurobiological impact of genetic variations and the environmental factors adds further information. Studies that examine either the joint effect or interactions between genetic and environmental factors are faced with the challenge of making an appropriate choice of risk factors. An additional consideration is the multiple comparisons issue. Hence we focused on only one polymorphism, namely rs1051788. Allele A on rs1051788 which was associated with risk for SZ (Shirts et al. 2007), did not show a main effect on PFC grey matter volumes; whereas there was a significant joint effect when examined against the background of exposure to HSV1. Furthermore, rs1051788 is a nonsynonymous exonic polymorphism that substitutes aspartic acid with asparagine at amino acid position 136 on one of the α-helical folds of MICB protein. The variation could have a functional impact in vivo. Normally, following HSV1 infection, MICB is presented to an activating receptor NKG2D on the natural killer (NK) cells and CD8αβT cells that modulates the immune response through cytokine production. Cytokines could increase cytotoxicity and also alter neuronal dendrite growth. Further studies are necessary to evaluate the impact of variation at rs1051788 on these interactions.
These observations raise several questions. First, the timing of exposure to HSV1 is unclear in our sample. While some subjects might have been exposed in utero, many others might have been exposed postnatally. Further, within the postnatal period some may have been exposed closer to the onset of illness while others well before the onset. Therefore, the impact of exposure to HSV1 on grey matter volumes may be variable. Second, whether volumetric changes observed in this study may be due to the direct effects of repeated cycles of HSV1 latency followed by reactivation, due to immune mediators modulated by exposure to HSV1 or both is unclear. Third, the modest sample size included in this study did not allow us to examine less frequent genotype, i.e., individuals homozygous for the A allele. Fourth, the functional impact of grey matter reductions needs further studies in adequately powered samples. This is especially important since the Brodmann areas within the prefrontal cortex were somewhat different across comparisons. However, it is intriguing that most of the effects were observed in Brodmann areas 9 and 10 that are proposed to be the key regions in the regulation of often impaired working memory and executive functions in SZ. Impaired working memory and executive functions have been associated with exposure to HSV1 in SZ subjects (Dickerson et al. 2003; Shirts et al. 2008a).
Our morphometric analytical approach to examine joint effects is relatively stringent from a statistical viewpoint. In this study, we estimated volumes of voxel clusters that showed significant grey matter density differences compared to another group in a given contrast; the differences grey matter density in these clusters were corrected for the entire brain using the FDR approach. These estimations were covaried for common confounders, namely age, sex and SES. We used a conservative threshold by correcting the statistical significance using FDR approach for the entire brain at an extent threshold of 20 voxels. This threshold greatly minimizes the false positive results as random field theory suggests that the effects are less likely to be false positive when they cluster together. Volumes were extracted from the native space of images within the groups and corresponding voxels in the comparison group. These volumes were included as dependent variables to examine overall effect and then individual effects. We used this method instead of extracting regional volumes either from the corresponding gyri or from the Brodmann areas because the gyral extent and volumes are large, and are likely to be functionally heterogeneous. For example, frontal gyri include several Brodmann areas with distinct functional representations, and estimating volumes from such functionally diverse regions may not inform the putative functional significance of such morphometric alterations. Extracting volumes of Brodmann areas, although methodologically feasible, is based on rule-based boundaries assigned by the Talairach database and do not precisely represent the absolute anatomy of the Brodmann areas (Maldjian, WFU Pickatlas manual) (Maldjian et al. 2003). In addition, many clusters that reached stringent statistical threshold defined in this study extended beyond one Brodmann area. Therefore, comparing extracted volumes of Brodmann areas across the groups would not have represented the impact of predictor variables accurately. For these reasons, we extracted the voxel clusters that showed differences in grey matter density at a relatively stringent statistical threshold. Since the analyses were conducted within the MNI space, we converted the MNI coordinates to Talairach coordinates using the mni2tal scripts to identify the regions. Although this method produces discrepancies in the inferior, superior frontal and occipital regions, the observations in this study do not change drastically even when MNI coordinates are used.
Some of the strengths of this study include the selection of study sample, genetic polymorphism and our approach. Our study sample represents ethnically homogeneous (Caucasians only), first episode antipsychotic naïve subjects who met criteria for schizophrenia or schizoaffective disorder after a minimum of 6 months follow up. Healthy control subjects were recruited from the same geographic region. The selected polymorphism is supported by a series of studies conducted in our group. Our statistical plan included common confounders such as age, gender and socioeconomic status as covariates. Further, genotype quality control was achieved by allele calls made by two investigators and by sequencing a few randomly selected samples. However, the study is limited by a relatively small sample.
In summary, our results suggest that the joint effect of exposure to HSV1 and a non-synonymous MICB SNP on the grey matter volumes in the prefrontal region is more than the individual main effects among SZ subjects. These observations are consistent with our prior imaging studies (Prasad et al. 2007) and provide in vivo support for our previous genetic association studies (Shirts et al. 2007). These results provide initial clues to the importance of examining the impact of genetic variations or environmental factors in the background of the other.
Supplementary Material
Plot of p values in leave 1-out analyses where one subject’s scan was excluded systematically and the design was estimated by including age, sex, SES, diagnostic status and MICB genotypes in the entire sample of cases and controls. The p value of the entire sample is plotted at the right most extreme (red filled circle).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- American Psychiatric Association. Diagnostic & Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Ashley RL. Genital herpes. Type-specific antibodies for diagnosis and management. Dermatol Clin. 1998;16(4):789, xiii–793. doi: 10.1016/s0733-8635(05)70048-6. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J, Valabregue R, Poline J. Region of interest analysis using an SPM toolbox. 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan.2002. [Google Scholar]
- Cleator GM, Klapper PE. Herpes Simplex. In: Zuckerman AJ, Banatvala JE, Pattison JR, editors. Principles and Practice of Clinical Virology. New York: John Wiley and Sons, Ltd; 2004a. [Google Scholar]
- Cleator GM, Klapper PE. The Herpesviridae. In: Zuckerman AJ, Banatvala JE, Pattison JR, editors. Principles and Practice of Clinical Virology. New York: John Wiley & Sons, Ltd; 2004b. pp. 23–26. [Google Scholar]
- Dickerson F, Stallings C, Sullens A, Origoni A, Leister F, Krivogorsky B, Yolken R. Association between cognitive functioning, exposure to Herpes Simplex Virus type 1, and the COMT Val158Met genetic polymorphism in adults without a psychiatric disorder. Brain, behavior, and immunity. 2008 doi: 10.1016/j.bbi.2008.04.156. [DOI] [PubMed] [Google Scholar]
- Dickerson FB, Boronow JJ, Stallings C, Origoni AE, Ruslanova I, Yolken RH. Association of serum antibodies to herpes simplex virus 1 with cognitive deficits in individuals with schizophrenia. Arch Gen Psychiatry. 2003;60(5):466–472. doi: 10.1001/archpsyc.60.5.466. [DOI] [PubMed] [Google Scholar]
- First MB. The Structured Clinical Interview for DSM-IV for Axis I disorders: Clinical Version, Administration Booklet. Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- Gilbert AR, Rosenberg DR, Harenski K, Spencer S, Sweeney JA, Keshavan MS. Thalamic volumes in patients with first-episode schizophrenia. Am J Psychiatry. 2001;158(4):618–624. doi: 10.1176/appi.ajp.158.4.618. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, Ashburner J, Friston KJ, Tyler LK, Price CJ. Voxel-Based Morphometry of Herpes Simplex Encephalitis. NeuroImage. 2001;13(4):623–631. doi: 10.1006/nimg.2000.0734. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four-factor index of social status. New Haven, CT: Yale University; 1975. [Google Scholar]
- Kim JJ, Shirts BH, Dayal M, Bacanu SA, Wood J, Xie W, Zhang X, Chowdari KV, Yolken R, Devlin B, Nimgaonkar VL. Are exposure to cytomegalovirus and genetic variation on chromosome 6p joint risk factors for schizophrenia? Ann Med. 2007;39(2):145–153. doi: 10.1080/07853890601083808. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Mathworks Inc. Matlab. Natick, MA: Mathworks Inc.; 2002. [Google Scholar]
- Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66(8):811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NHANES II. Second National Health and Nutrition Examination Survey 1976–1980. CDC, Center for Disease Control and Prevention; 1987. revised 2007. [Google Scholar]
- Prasad KM, Shirts BH, Yolken RH, Keshavan MS, Nimgaonkar VL. Brain morphological changes associated with exposure to HSV1 in first-episode schizophrenia. Mol Psychiatry. 2007;12(1):105–113. doi: 10.1038/sj.mp.4001915. 1. [DOI] [PubMed] [Google Scholar]
- Schillinger JA, Xu F, Sternberg MR, Armstrong GL, Lee FK, Nahmias AJ, McQuillan GM, Louis ME, Markowitz LE. National seroprevalence and trends in herpes simplex virus type 1 in the United States, 1976–1994. Sex Transm Dis. 2004;31(12):753–760. doi: 10.1097/01.olq.0000145852.43262.c3. [DOI] [PubMed] [Google Scholar]
- Shirts BH, Kim JJ, Reich S, Dickerson FB, Yolken RH, Devlin B, Nimgaonkar VL. Polymorphisms in MICB are associated with human herpes virus seropositivity and schizophrenia risk. Schizophr Res. 2007;94(1–3):342–353. doi: 10.1016/j.schres.2007.04.021. [DOI] [PubMed] [Google Scholar]
- Shirts BH, Prasad KM, Pogue-Geile MF, Dickerson F, Yolken RH, Nimgaonkar VL. Antibodies to cytomegalovirus and Herpes Simplex Virus 1 associated with cognitive function in schizophrenia. Schizophr Res. 2008a;106(2–3):268–274. doi: 10.1016/j.schres.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirts BH, Wood J, Yolken RH, Nimgaonkar VL. Comprehensive evaluation of positional candidates in the IL-18 pathway reveals suggestive associations with schizophrenia and herpes virus seropositivity. Am J Med Genet B Neuropsychiatr Genet. 2008b;147(3):343–350. doi: 10.1002/ajmg.b.30603. [DOI] [PubMed] [Google Scholar]
- SPSS. Statistical Package for Social Sciences, Ver 16. Chicago, Illinois: 2005. [Google Scholar]
- Vartiainen N, Kallio-Laine K, Hlushchuk Y, Kirveskari E, Seppanen M, Autti H, Jousmaki V, Forss N, Kalso E, Hari R. Changes in brain function and morphology in patients with recurring herpes simplex virus infections and chronic pain. Pain. 2009;144(1–2):200–208. doi: 10.1016/j.pain.2009.04.015. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch Gen Psychiatry. 1986;43(2):114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- Xu F, Sternberg MR, Kottiri BJ, McQuillan GM, Lee FK, Nahmias AJ, Berman SM, Markowitz LE. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA. 2006;296(8):964–973. doi: 10.1001/jama.296.8.964. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Plot of p values in leave 1-out analyses where one subject’s scan was excluded systematically and the design was estimated by including age, sex, SES, diagnostic status and MICB genotypes in the entire sample of cases and controls. The p value of the entire sample is plotted at the right most extreme (red filled circle).