Abstract
Background
Respiratory cryptosporidiosis is recognized as a late-stage complication in persons with HIV/AIDS. However, respiratory signs and symptoms are common in otherwise healthy children with intestinal cryptosporidiosis, suggesting that respiratory infection may occur in immunocompetent hosts.
Methods
We recruited children aged 9–36 months who presented with diarrhea to Mulago Hospital in Kampala, Uganda. Children with Cryptosporidium-positive and -negative stools were selected for further evaluation, including sputum induction in those with cough or unexplained respiratory signs, and collection of saliva and blood. Sputum samples were subjected to comprehensive bacteriological testing, and both sputum and saliva were tested for Cryptosporidium by nested-PCR.
Results
Of 926 fecal samples screened, 116 (12.5%) were Cryptosporidium positive. Seventeen of 48 (35.4%) sputum samples tested from stool-positive children were positive for Cryptosporidium. Sixteen of the 17 children with confirmed respiratory cryptosporidiosis were HIV-seronegative and 10/17 (58.8%) children were normally nourished. None of the 12 sputum specimens tested from stool-negative children were Cryptosporidium positive (p=0.013 compared to stool-positive children). Parasite DNA was only detected in 2/103 (1.9%) saliva samples (p<0.0001 compared to sputum).
Conclusions
Respiratory cryptosporidiosis was documented in one third of HIV-seronegative children who were tested. These novel findings suggest the potential for respiratory transmission. This study is registered with ClinicalTrials.gov, number NCT00507871.
Keywords: cryptosporidiosis, respiratory, HIV, transmission
Cryptosporidium spp. are well-recognized, enteric parasites. Transmission occurs following ingestion of oocysts that are passed in the feces of infected hosts. Cryptosporidiosis is characterized by diarrhea, dehydration, and wasting, which can be severe in persons with HIV/AIDS or malnutrition. Cryptosporidium has been rarely documented to infect the respiratory tract of immunocompromised persons, overwhelmingly those with HIV/AIDS [1]. The diagnosis of respiratory cryptosporidiosis is achieved by demonstration of parasites or parasite DNA in biopsy, bronchoalveolar lavage, or sputum specimens [2–7]. Curiously, several studies have reported that 40–50% of healthy children experience respiratory symptoms during intestinal cryptosporidiosis [8–10]. In none of these reports was the etiology of the respiratory symptoms determined. Systematic studies to evaluate the possibility of respiratory cryptosporidiosis in HIV-seronegative hosts have not been reported. This is an important lacunae in the epidemiology of cryptosporidiosis since respiratory infection may indicate that transmission can occur via this route. Accordingly, we conducted a study to determine if, and to what extent, respiratory cryptosporidiosis occurs in HIV-seronegative children. Our primary objective was to confirm or refute the hypothesis that respiratory cryptosporidiosis is common in both HIV-seropositive and -seronegative hosts.
METHODS
Study design, setting, and participants
Children presenting with diarrhea to the Acute Care Unit at Mulago Hospital in Kampala, Uganda were enrolled between November, 2007 and January, 2009. The primary outcome of interest was the presence of Cryptosporidium parasites in sputum. Children were eligible to participate if they were 9–36 months of age and had acute or persistent diarrhea on presentation (defined as 3 or more loose stools per day; ≥14 days duration for persistent diarrhea). Children with pre-existing medical conditions, who were moribund, or who had a recent history of choking or suspected foreign body inhalation were excluded. Caretakers provided written informed consent in English or Luganda (the local language); illiterate caretakers provided their consent with a thumbprint following verbal discussion of the consent document. The study was approved by the research ethics committees of Makerere University Medical School, the Uganda National Council for Science and Technology, Tufts Medical Center/Tufts University Health Sciences Campus, and the study sponsor (NIAID). This study is registered with ClinicalTrials.gov, number NCT00507871.
Clinical procedures
Following a detailed physical examination, including chest auscultation and pulse oximetry, a stool sample was collected from each child using a rectal swab. Stool was screened for Cryptosporidium using a modified acid-fast staining procedure. All stool-positive children were eligible for further clinical evaluation, which included saliva and blood collection, and sputum induction, if indicated (see criteria below). For every 4 stool-positive children who underwent sputum induction, one stool-negative child who was eligible for sputum induction was selected for further clinical evaluation. Saliva was collected from all children using Oracol collection devices (Malvern Medical Developments Ltd., Worcester, UK). Blood tests included a complete blood count in all children, and electrolyte assessment if the child was eligible for sputum induction. HIV testing was performed according to an established serial testing algorithm at Mulago Hospital, after caretaker consent. CD4 lymphocyte counts were measured using microcapillary cytometry (Guava Technologies Inc., Hayward, CA) in HIV-seropositive children.
Children with cough, unexplained tachypnea or unexplained hypoxia were eligible for sputum induction. Exclusion criteria for this procedure included asthma, chronic lung disease, hypersensitivity to salbutamol, hypoxia (oxygen saturation <92%) refractory to 30 minutes oxygen therapy, thrombocytopenia (platelets <75 × 106/ml) and hypokalemia (potassium <3.5mEq/L; salbutamol may exacerbate hypokalemia). The sputum induction procedure is described in detail elsewhere [11]. Briefly, sputum was obtained via nasopharyngeal suctioning after inhaled salbutamol treatment and processed for routine bacteriology. Children received treatment for anemia and any pathogens identified in the stool or sputum. Children testing positive for HIV were referred to the Pediatric Infectious Disease Clinic at Mulago Hospital for clinical care.
Laboratory procedures
Stool screening, routine bacteriology and blood tests were conducted in the clinical laboratories of Mulago Hospital. Sputum and saliva were spotted onto FTA cards (Whatman, Inc., Clifton, New Jersey) and shipped to Tufts University in North Grafton, MA, for molecular analysis. FTA cards were washed, and DNA eluted under alkaline conditions, according to the manufacturers recommendations. Sputum and saliva eluates were tested for Cryptosporidium using an established nested-polymerase chain reaction (PCR) assay, which amplifies a fragment of the Cryptosporidium 18S rRNA gene [12, 13]. This was followed by restriction fragment length polymorphism (RFLP) analysis to distinguish between species. Sputum eluates were also tested for Mycobacterium tuberculosis complex and Pneumocystis jiroveci by PCR, using established protocols [14, 15]. All samples were screened for potential inhibition using primers specific for a human house-keeping gene (β-globin), prior to PCR screening for pathogens.
Statistical analysis
Categorical variables were compared using Pearson’s χ2 test or Fishers exact test. Continuous variables were compared using t-tests, or non-parametric Mann-Whitney U tests where data were non-normally distributed. Statistical analysis was performed using SPSS software v16.0 (SPSS Inc., Chicago, IL).
RESULTS
Participants
Between November, 2007 and January, 2009, 1156 children aged 9–36 months presented to the Acute Care Unit with diarrhea. Of these, 926 were eligible to participate and were enrolled into the study. Study population characteristics are shown in Table 1. Stool assessment identified 116 children (12.5%) who were Cryptosporidium positive and 810 children (87.5%) who were parasite negative. Consistent with previous findings in the same population [16], stool-positive children were more likely to be younger, have persistent diarrhea and/or malnutrition. Of 116 children with intestinal cryptosporidiosis, 53 underwent sputum induction. Reasons for exclusion from this procedure included no respiratory signs (n=31), hypokalemia and/or thrombocytopenia (n=21), and caretaker refusal of phlebotomy and/or further testing (n=4). Seven eligible children did not undergo sputum induction because of caretaker withdrawal before completion of study procedures (n=2), physician withdrawal due to severe illness (n=3), or death due to unrelated causes (n=2). Thirteen stool-negative children with unexplained cough also underwent sputum induction. Sputum induction was successfully completed without adverse event in all 66 children who underwent this procedure.
Table 1.
Characteristics of the study population.
| Stool-positive for Cryptosporidium (n=116) | Stool-negative for Cryptosporidium (n=810) | P | |
|---|---|---|---|
| Demographic characteristics | |||
| Age category | 0.002 | ||
| 9–12 months | 80 (69.0) | 434 (53.5) | |
| 13–36 months | 36 (31.0) | 376 (46.4) | |
| Sex | 0.369 | ||
| Male | 58 (50.0) | 441 (54.4) | |
| Female | 58 (50.0) | 369 (45.6) | |
| Medical history | |||
| Diarrhea duration | 0.002 | ||
| <14 days (acute) | 85 (73.3) | 687 (84.8) | |
| ≥14 days (persistent) | 31 (26.7) | 123 (15.2) | |
| Recent history of vomiting | 82 (70.7) | 593 (73.2) | 0.568 |
| Clinical findings | |||
| Hydration statusa | 0.577 | ||
| No dehydration | 90 (77.6) | 593 (73.2) | |
| Some dehydration | 20 (17.2) | 173 (21.4) | |
| Severe dehydration | 6 (5.2) | 44 (5.4) | |
| Cough present | 85 (73.3) | 539 (66.5) | 0.148 |
| Difficulty breathing | 13 (11.2) | 71 (8.8) | 0.392 |
| Initial O2 saturation | 0.282 | ||
| Median | 97% | 98% | |
| Range | 92%–100% | 84%–100% | |
| Hypoxiab | 0 (0) | 5 (0.6) | 1.00 |
| Initial respiratory rate (breaths/min) | 0.459 | ||
| Median | 38 | 36 | |
| Range | 28–68 | 20–96 | |
| Tachypneac | 27 (23.3) | 178 (22.0) | 0.752 |
| Nutritional statusd | |||
| Stunted (≤-2 HAZ) | 28 (24.1) | 224 (27.7) | 0.426 |
| Wasted (≤-2 WHZ) | 43 (37.1) | 212 (26.2) | 0.014 |
| Underweight (≤-2 WAZ) | 58 (50.0) | 348 (43.0) | 0.153 |
| Suspected AIDSe | 13 (11.2) | 72 (8.9) | 0.419 |
NOTE: Data are presented as no. (%) of patients, unless otherwise indicated.
Assessed using UNICEF and WHO guidelines;
Defined as initial pulse-oximetry O2 saturation <92%;
Defined as respiratory rate ≥50 breaths/minute in children aged <12 months or ≥40 breaths/minute in children aged ≥12 months;
HAZ = height-for-age z-score, WHZ = weight-for-height z-score, WAZ = weight-for-age z-score;
Based on WHO clinical criteria for suspected AIDS.
Respiratory signs and symptoms
Cough was highly prevalent in the study population, occurring in 624/926 (67.4%) children with diarrhea. Cough was more common in children with an history of vomiting than in those without vomiting (474/675, 70.2% versus 150/251, 59.8%, p = 0.003). This relationship was true for both stool-positive and -negative children (p=0.024 and p=0.015, respectively). The frequency of respiratory signs and symptoms, including presence of cough, difficulty breathing, hypoxemia and tachypnea, were not significantly different between stool-positive and -negative children (Table 1). No differences in demographic and/or clinical characteristics were detected between stool-positive children with and without cough (not shown).
Sputum and saliva findings
Seventeen of the 48 (35.4%) sputum specimens tested from stool-positive children were positive for Cryptosporidium DNA, while none of the 12 sputum samples tested from stool-negative children contained parasite DNA (p=0.013). In five of the sputum samples, Cryptosporidium DNA was detected after the primary PCR and before the secondary PCR reaction, suggestive of high levels of parasite DNA. Sputum specimens from six children (five stool-positive and one stool-negative) were lost in transit. Assuming the study’s null hypothesis to be true, i.e. that the lost samples were parasite negative, the comparison between stool-positive and -negative children remained significant (p=0.015).
Parasite DNA was not detected in any of the saliva samples from children with confirmed respiratory cryptosporidiosis. Of 103 saliva samples tested, Cryptosporidium was only detected in two specimens (1.9%; p<0.001 compared to sputum), both collected from stool-positive children. The first child had a history of cough, tachypnea and vomiting, but was excluded from the sputum induction procedure due to hypokalemia. The second child had a history of cough without vomiting; Cryptosporidium DNA was not detected in this child’s sputum. Saliva specimens were not collected from 21 stool-positive children who did not undergo sputum induction, and 5 specimens were lost in transit; again, the comparison with sputum samples remained significant (p< 0.001) under the unlikely assumption that as many as half of the lost saliva samples would have been parasite positive.
The results of molecular characterization of the sputum and saliva isolates are shown in Figure 1. Four (23.5%) sputum isolates were C. parvum while 13 (76.5%) were C. hominis. Both saliva isolates were C. hominis.
Figure 1. Genotyping of Cryptosporidium parasites in sputum and saliva samples.
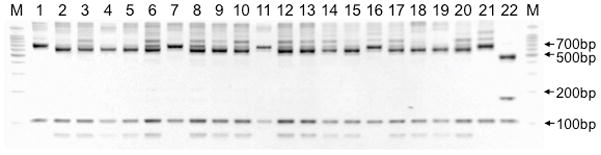
Restriction fragment length polymorphism (RFLP) analysis reveals the genotyping patterns of parasites in the sputum (lanes 1–17) and saliva (lanes 18,19). For sputum isolates, lane numbers correspond with the identification numbers in Table 2. Digestion of secondary PCR products with enzyme AseI (VspI) yields prominent bands at 70, 104 and 561bp for C. hominis, at 104 and 625 to 628bp for C. parvum and at 104, 171 and 456 for C. meleagridis [13]. Positive controls for the most common species in humans are indicated as follows: C. hominis (lane 20), C. parvum (lane 21), C. meleagridis (lane 22). M, 100bp marker.
Clinical characteristics of children with respiratory cryptosporidiosis
The clinical characteristics of children with respiratory cryptosporidiosis are shown in Table 2. Malnutrition was equally common in stool-positive children with and without confirmed respiratory involvement (7/17 vs. 19/31, p=0.181). Onset of cough was before diarrhea onset in 5/17 (29.4%) children with respiratory cryptosporidiosis compared to 7/31 (22.6%) of children without respiratory involvement (p=0.731). Neither duration of cough (median 7 days [range: 2–30] vs. 7 days [2–44], p=0.313) nor duration of diarrhea (median 7 days [range: 2–14] vs. 7 days [2–44], p=0.156) differed between these groups. Respiratory rates and oxygen saturation were likewise similar in stool-positive children with and without respiratory cryptosporidiosis (median 36 breaths/min [range: 28–58] vs. 36 breaths/min [28–60], p=0.786; median 96% [range: 92–100] vs. 98% [93–100], p=0.292). Children without a history of vomiting were uncommon in this study, thus limiting our ability to test the association between respiratory cryptosporidiosis and vomiting (16/40 children with history of vomiting had respiratory cryptosporidiosis vs. 1/8 children without such history; p=0.136).
Table 2.
Clinical characteristics of 17 children with respiratory cryptosporidiosis. All children were stool-positive for Cryptosporidium.
| ID | Age (months) | Sex | Nutritional statusa | Other respiratory pathogensb | Respiratory rate (breaths/min) | O2 saturation (%) | Duration of cough (days) | Duration of diarrhea (days) | Vomiting | HIV |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 9 | M | N | None detected | 52c | 100 | 2 | 4 | Y | N |
| 2 | 15 | M | N | Hi, Sp | 30 | 96 | 2 | 10 | Y | N |
| 3 | 11 | M | U,W | None detected | 32 | 100 | 14 | 14d | Y | N |
| 4 | 18 | F | N | None detected | 58c | 100 | 10 | 3 | Y | N |
| 5 | 13 | F | N | Sp | 40c | 96 | 3 | 7 | Y | N |
| 6 | 13 | M | U,W | None detected | 36 | 98 | 14 | 14d | Y | N |
| 7 | 11 | M | N | Hi, Sp | 34 | 97 | 2 | 7 | Y | N |
| 8 | 11 | F | S,U,W | Sp | 38 | 94 | 3 | 3 | Y | N |
| 9 | 12 | M | N | None detected | 32 | 96 | 30 | 7 | Y | N |
| 10 | 10 | F | N | None detected | 40 | 95 | 12 | 3 | Y | N |
| 11 | 12 | M | U,W | None detected | 34 | 95 | 4 | 7 | Y | N |
| 12 | 14 | F | N | None detected | 38 | 92 | 2 | 2 | Y | N |
| 13 | 12 | M | U,W | None detected | 40c | 92 | 14 | 3 | Y | N |
| 14 | 13 | F | N | Sa | 28 | 97 | 10 | 4 | Y | Y |
| 15 | 9 | F | U,W | None detected | 36 | 94 | 2 | 7 | Y | N |
| 16 | 11 | F | U.W | None detected | 32 | 100 | 7 | 14d | N | N |
| 17 | 9 | F | N | None detected | 38 | 97 | 7 | 7 | Y | N |
N = normally nourished (HAZ, WAZ and WHZ >-2.0), S = stunted (≤-2.0 HAZ), U = underweight (≤-2.0 WAZ), W = wasted (≤-2.0 WHZ);
Hi = Haemophilus influenzae, Sa = Staphylococcus aureus, Sp = Streptococcus pneumoniae;
Meets criteria for tachypnea (defined as respiratory rate ≥50 in children aged <12 months or ≥40 breaths/minute in children aged ≥12 months);
Meets criteria for persistent diarrhea (defined as 3 or more loose stool per day for ≥14 days).
DISCUSSION
Respiratory cryptosporidiosis is currently recognized as a rare, late-stage complication of chronic intestinal infection in persons with HIV/AIDS. We found that 17 of 48 (35.4%) children with intestinal cryptosporidiosis and cough had Cryptosporidium in their sputum. Sixteen of the 17 children with confirmed respiratory cryptosporidiosis were HIV-seronegative. The absence of parasite DNA in the saliva of these and indeed most children with intestinal cryptosporidiosis, indicates that these findings cannot be attributed to oral contamination during the sputum induction procedure.
It is well established that Cryptosporidium spp. are capable of infecting the respiratory tract. In humans, respiratory infection has been confirmed following direct observation of parasites in biopsy, bronchoalveolar lavage, and sputum specimens [1–5]. In the most recent review of respiratory cryptosporidiosis (1996), more than 50 cases had been reported in persons with AIDS, with fewer than 10 cases documented in persons with other immunodeficiencies [1]. Permissiveness of the respiratory tract to Cryptosporidium infection is not unique to humans. The avian species, C. baileyi, routinely causes respiratory disease in commercially important poultry [17]. Incidental respiratory infections have also been described in gnotobiotic piglets [18] and calves [19], and experimental rodent models using direct inoculation of parasites into the trachea have been developed [20]. These studies collectively demonstrate that Cryptosporidium is a genuine respiratory pathogen.
Respiratory cryptosporidiosis is believed to occur rarely, if at all, in immunologically normal humans. Only two case reports have documented respiratory infection in immunocompetent persons [21, 22]. Historically, studies have relied on staining methods to identify parasites in respiratory tissues and secretions. The low sensitivity of these methods may partly account for the limited number of reports in healthy persons. PCR detection of Cryptosporidium is diagnostically superior for detecting parasites in stool [23], and was recently applied to respiratory specimens [6, 7]. To our knowledge, this study is the first to use PCR detection of Cryptosporidium in sputum specimens within in a larger epidemiological setting.
We found that 13/17 (76.5%) of sputum isolates contained the human-adapted species, C. hominis, while only 4/17 (23.5%) contained C. parvum, which is adapted to many mammals, including humans. This genetic distribution is similar to our prior observations of intestinal cryptosporidiosis in the same population [16, 24]. Several of the sputum isolates had weakly-staining bands that are most likely non-specific background staining. We do no believe any mixed Cryptosporidium species respiratory infections were present. Mixed respiratory infection in immuncompromised persons has not been documented in the past; mixed intestinal infection is relatively rare in this population [16,24]; and it may be biologically unlikely due to competition between C. hominis and C. parvum [25].
Cough was highly prevalent among both stool-positive and -negative children. We detected a trend toward more compromised respiratory status, including presence of cough and/or difficulty breathing, in stool-positive children; however these differences were not significant, perhaps because of our relatively small sample size. Cryptosporidium was the only respiratory pathogen detected in the sputum of 12 of the 17 children with respiratory cryptosporidiosis and may well have been the cause of cough in some or all of these children. Previous studies have found that cough is present in as many as 40–50% of otherwise healthy children with intestinal cryptosporidiosis [8–10]. Furthermore, one European study reported that cough is more common in children with diarrhea due to cryptosporidiosis compared to other etiologies [9]. In persons with HIV/AIDS, respiratory infection is often accompanied by cough, dyspnea, and hypoxia, as well as infiltrates detectable on chest x-ray [2, 5]. However, co-infection with organisms such as cytomegalovirus and P. jiroveci can obscure the specific contribution of cryptosporidiosis to respiratory disease in persons with AIDS [5]. While the study population has a high incidence of pneumonia, malnutrition and other conditions that may influence respiratory function, the majority (10/17) of study children with respiratory cryptosporidiosis were well-nourished and had no other detected cause for their symptoms.
Our detection of parasites in sputum suggests that respiratory transmission of Cryptosporidium may occur. Transmission to others could arise if oocysts are aerosolized or ejected during coughing, as occurs with many other pathogens. Respiratory acquisition of infection could, in turn, occur through breathing aerosolized material or through contact with respiratory droplets on contaminated surfaces. Airborne transmission was first suggested in 1987 when a veterinary scientist became infected after exposure to aerosolized gastric contents from an infected calf [26]. Acquisition via the respiratory tract is also supported by documented cases where respiratory infection occurred before [2, 3], or even in the absence of [27], intestinal infection, and by studies that have documented the onset of respiratory symptoms before diarrhea onset. For example, an Australian study documented that 25% of immunocompetent persons experienced prodromal cough before the onset of diarrhea [28]. Respiratory transmission may be particularly relevant in enclosed spaces, or with close maternal-infant or other personal contact.
Alternative explanations for respiratory tract involvement include the aspiration of intestinal contents, or the hematogenous spread of the parasite from the gut to the lung. Vomiting is a common feature of intestinal cryptosporidiosis and the aspiration of intestinal contents could serve to seed the upper airways with infectious oocysts. While a history of vomiting was common in the study population, parasite DNA was infrequently recovered from the saliva of children with intestinal cryptosporidiosis, a finding that might have been expected if oocysts were present in vomitus. Respiratory infection has been documented in the absence of vomiting [1, 4], as was the case for one child in this study. Theoretically, hematogenous spread of the parasite from the gut to the lung could occur. Cryptosporidium parasites have been observed inside macrophages [5] and submucosal vessels of the colon [29] during infection. In laboratory animals, patent intestinal infections can be established when parasites are artificially administered via intraperitoneal [30] or intravascular [31] injection. The relevance of these observations to human infection is not known. Neither of these alternatives (aspiration or hematogenous spread) would explain the existence of prodromal respiratory symptoms or respiratory infection in the absence of intestinal involvement, nor do they preclude respiratory transmission as outlined above.
There are several limitations to this study. Stool specimens were screened for Cryptosporidium using modified acid-fast stain rather than more sensitive but laborious methods, such as PCR, to allow a rapid diagnosis. It is therefore likely that some children diagnosed as stool-negative by microscopy were, in fact, positive for the parasite. In our experience and in several published studies [32, 33], however, acid-fast smear positivity correlates well (≥ 85% sensitivity) with intestinal infection. Until recently, sputum induction was not routinely undertaken in young children because they do not expectorate but rather swallow their secretions. The safety and practicality of sputum induction for the diagnosis of tuberculosis and other infections in young children has now been demonstrated [11, 34]. However, for ethical reasons, we did not collect sputum from children who did not have cough, unexplained tachypnea or unexplained hypoxia. It is possible that some children with respiratory cryptosporidiosis do not have signs and symptoms of infection, in which case the prevalence of respiratory cryptosporidiosis may be underestimated in this study. Further, if respiratory infection precedes diarrhea onset or occurs in the absence of diarrhea, we may not have detected some children with respiratory cryptosporidiosis. We used PCR to detect Cryptosporidium in sputum because of its sensitivity and specificity for the parasite. For ethical reasons, we could not confirm our PCR results with the demonstration of parasites in biopsy specimens. The need to test the limited amount of sputum obtained from these children for other respiratory pathogens also precluded us from routinely testing the sputum with another diagnostic method such as immunofluorescence. Finally, we did not test for concurrent viral pathogens, which may have obscured a true association between cryptosporidiosis and cough.
This study demonstrates that respiratory involvement commonly occurs in HIV-seronegative children with intestinal cryptosporidiosis and cough. These findings indicate that respiratory infection is more universal than currently recognized. While the clinical importance of respiratory infection in normal hosts may be minor, the potential for respiratory transmission of cryptosporidiosis is of major concern, especially given the absence of effective treatment in populations most vulnerable to this infection. This study highlights the need for future research that elaborates on and refines the role of respiratory tract infection in the epidemiology of cryptosporidiosis.
Acknowledgments
Financial support for this study was provided by the National Institutes for Allergy and Infectious Diseases (R21AI068474 to J.K.G., S.T., and J.K.T.), and the University of Sydney (to S.M.M.).
The authors are indebted to the many study participants, doctors, nurses and laboratory staff at Mulago Hospital and Makerere University College of Health Sciences who contributed to this study. We thank the members of the safety monitoring committee and NIAID for their diligent reviews, and Dr. Melanie T. Cushion (University of Cincinnati College of Medicine) and Dr. Vera Naroditskaya (Massachusetts State Laboratory Institute, deceased) for kindly supplying the Pneumocystis and M. tuberculosis PCR controls, respectively. Finally, S.M.M. would like to extend sincere thanks to Dr. Elena N. Naumova (Tufts University School of Medicine) and Dr. Sam R. Telford III (Cummings School of Veterinary Medicine at Tufts University), as well as J.K.G. and S.T., for serving on her doctoral committee.
Footnotes
Potential conflicts of interest. All authors: no conflicts.
Summary: Cryptosporidium was detected in 17/48 (35.4%) sputum samples from children with intestinal cryptosporidiosis and cough; 16/17 of these children were HIV-seronegative. This finding challenges the current understanding of cryptosporidiosis, and raises the possibility of respiratory transmission.
References
- 1.Clavel A, Arnal AC, Sanchez EC, et al. Respiratory cryptosporidiosis: case series and review of the literature. Infection. 1996;24:341–6. doi: 10.1007/BF01716076. [DOI] [PubMed] [Google Scholar]
- 2.Dupont C, Bougnoux ME, Turner L, Rouveix E, Dorra M. Microbiological findings about pulmonary cryptosporidiosis in two AIDS patients. J Clin Microbiol. 1996;34:227–9. doi: 10.1128/jcm.34.1.227-229.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hojlyng N, Jensen BN. Respiratory cryptosporidiosis in HIV-positive patients. Lancet. 1988;1:590–1. doi: 10.1016/s0140-6736(88)91384-0. [DOI] [PubMed] [Google Scholar]
- 4.Lopez-Velez R, Tarazona R, Garcia Camacho A, et al. Intestinal and extraintestinal cryptosporidiosis in AIDS patients. Eur J Clin Microbiol Infect Dis. 1995;14:677–81. doi: 10.1007/BF01690873. [DOI] [PubMed] [Google Scholar]
- 5.Ma P, Villanueva TG, Kaufman D, Gillooley JF. Respiratory cryptosporidiosis in the Acquired Immune Deficiency Syndrome. Use of modified cold Kinyoun and Hemacolor stains for rapid diagnoses. JAMA. 1984;252:1298–301. [PubMed] [Google Scholar]
- 6.Mercado R, Buck GA, Manque PA, Ozaki LS. Cryptosporidium hominis infection of the human respiratory tract. Emerg Infect Dis. 2007;13:462–4. doi: 10.3201/eid1303.060394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meamar AR, Rezaian M, Rezaie S, et al. Cryptosporidium parvum bovine genotype oocysts in the respiratory samples of an AIDS patient: efficacy of treatment with a combination of azithromycin and paromomycin. Parasitol Res. 2006;98:593–5. doi: 10.1007/s00436-005-0097-4. [DOI] [PubMed] [Google Scholar]
- 8.Weikel CS, Johnston LI, De Sousa MA, Guerrant RL. Cryptosporidiosis in northeastern Brazil: association with sporadic diarrhea. J Infect Dis. 1985;151:963–5. doi: 10.1093/infdis/151.5.963. [DOI] [PubMed] [Google Scholar]
- 9.Egger M, Mausezahl D, Odermatt P, Marti HP, Tanner M. Symptoms and transmission of intestinal cryptosporidiosis. Arch Dis Child. 1990;65:445–7. doi: 10.1136/adc.65.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shahid NS, Rahman AS, Sanyal SC. Cryptosporidium as a pathogen for diarrhoea in Bangladesh. Trop Geogr Med. 1987;39:265–70. [PubMed] [Google Scholar]
- 11.Bakeera-Kitaka S, Musoke P, Downing R, Tumwine JK. Pneumocystis carinii in children with severe pneumonia at Mulago Hospital, Uganda. Ann Trop Paediatr. 2004;24:227–35. doi: 10.1179/027249304225019046. [DOI] [PubMed] [Google Scholar]
- 12.Xiao L, Bern C, Limor J, et al. Identification of 5 types of Cryptosporidium parasites in children in Lima, Peru. J Infect Dis. 2001;183:492–7. doi: 10.1086/318090. [DOI] [PubMed] [Google Scholar]
- 13.Xiao L, Morgan UM, Limor J, et al. Genetic diversity within Cryptosporidium parvum and related Cryptosporidium species. Appl Environ Microbiol. 1999;65:3386–91. doi: 10.1128/aem.65.8.3386-3391.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muhumuza J, Asiimwe BB, Kayes S, et al. Introduction of an in-house PCR for routine identification of M. tuberculosis in a low-income country. Int J Tuberc Lung Dis. 2006;10:1262–7. [PubMed] [Google Scholar]
- 15.Nuchprayoon S, Saksirisampant W, Jaijakul S, Nuchprayoon I. Flinders Technology Associates (FTA) filter paper-based DNA extraction with polymerase chain reaction (PCR) for detection of Pneumocystis jirovecii from respiratory specimens of immunocompromised patients. J Clin Lab Anal. 2007;21:382–6. doi: 10.1002/jcla.20200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tumwine JK, Kekitiinwa A, Nabukeera N, et al. Cryptosporidium parvum in children with diarrhea in Mulago Hospital, Kampala, Uganda. Am J Trop Med Hyg. 2003;68:710–5. [PubMed] [Google Scholar]
- 17.Sreter T, Varga I. Cryptosporidiosis in birds--a review. Vet Parasitol. 2000;87:261–79. doi: 10.1016/s0304-4017(99)00178-8. [DOI] [PubMed] [Google Scholar]
- 18.Tzipori S. Cryptosporidiosis in perspective. Adv Parasitol. 1988;27:63–129. doi: 10.1016/S0065-308X(08)60353-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mascaro C, Arnedo T, Rosales MJ. Respiratory cryptosporidiosis in a bovine. J Parasitol. 1994;80:334–6. [PubMed] [Google Scholar]
- 20.Meulbroek JA, Novilla MN, Current WL. An immunosuppressed rat model of respiratory cryptosporidiosis. J Protozool. 1991;38:113S–5S. [PubMed] [Google Scholar]
- 21.Harari MD, West B, Dwyer B. Cryptosporidium as cause of laryngotracheitis in an infant. Lancet. 1986;1:1207. doi: 10.1016/s0140-6736(86)91181-5. [DOI] [PubMed] [Google Scholar]
- 22.Campayo Ibanez A, Lacruz Rodrigo J, Valls Ferrer JM, Bonora Tamarit V. Med Clin. Vol. 103. Barc: 1994. Pulmonary cryptosporidiosis in an immunocompetent female patient; p. 237. [PubMed] [Google Scholar]
- 23.Balatbat AB, Jordan GW, Tang YJ, Silva J., Jr Detection of Cryptosporidium parvum DNA in human feces by nested PCR. J Clin Microbiol. 1996;34:1769–72. doi: 10.1128/jcm.34.7.1769-1772.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tumwine JK, Kekitiinwa A, Bakeera-Kitaka S, et al. Cryptosporidiosis and microsporidiosis in Ugandan children with persistent diarrhea with and without concurrent infection with the human immunodeficiency virus. Am J Trop Med Hyg. 2005;73:921–5. [PubMed] [Google Scholar]
- 25.Akiyoshi DE, Mor S, Tzipori S. Rapid displacement of Cryptosporidium parvum type 1 by type 2 in mixed infections in piglets. Infect Immun. 2003;71:5765–71. doi: 10.1128/IAI.71.10.5765-5771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hojlyng N, Holten-Andersen W, Jepsen S. Cryptosporidiosis: a case of airborne transmission. Lancet. 1987;2:271–2. doi: 10.1016/s0140-6736(87)90851-8. [DOI] [PubMed] [Google Scholar]
- 27.Palmieri F, Cicalini S, Froio N, et al. Pulmonary cryptosporidiosis in an AIDS patient: successful treatment with paromomycin plus azithromycin. Int J STD AIDS. 2005;16:515–7. doi: 10.1258/0956462054308332. [DOI] [PubMed] [Google Scholar]
- 28.Cruickshank R, Ashdown L, Croese J. Human cryptosporidiosis in North Queensland. Aust N Z J Med. 1988;18:582–6. doi: 10.1111/j.1445-5994.1988.tb00128.x. [DOI] [PubMed] [Google Scholar]
- 29.Gentile G, Baldassarri L, Caprioli A, et al. Colonic vascular invasion as a possible route of extraintestinal cryptosporidiosis. Am J Med. 1987;82:574–5. doi: 10.1016/0002-9343(87)90474-8. [DOI] [PubMed] [Google Scholar]
- 30.Yang S, Healey MC. Patent gut infections in immunosuppressed adult C57BL/6N mice following intraperitoneal injection of Cryptosporidium parvum oocysts. J Parasitol. 1994;80:338–42. [PubMed] [Google Scholar]
- 31.Yang S, Healey MC. Development of patent gut infections in immunosuppressed adult C57BL/6N mice following intravenous inoculations of Cryptosporidium parvum oocysts. J Eukaryot Microbiol. 1994;41:67S. [PubMed] [Google Scholar]
- 32.Morgan UM, Pallant L, Dwyer BW, Forbes DA, Rich G, Thompson RC. Comparison of PCR and microscopy for detection of Cryptosporidium parvum in human fecal specimens: clinical trial. J Clin Microbiol. 1998;36:995–8. doi: 10.1128/jcm.36.4.995-998.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stroup SE, Roy S, McHele J, et al. Real-time PCR detection and speciation of Cryptosporidium infection using Scorpion probes. J Med Microbiol. 2006;55:1217–22. doi: 10.1099/jmm.0.46678-0. [DOI] [PubMed] [Google Scholar]
- 34.Zar HJ, Tannenbaum E, Apolles P, Roux P, Hanslo D, Hussey G. Sputum induction for the diagnosis of pulmonary tuberculosis in infants and young children in an urban setting in South Africa. Arch Dis Child. 2000;82:305–8. doi: 10.1136/adc.82.4.305. [DOI] [PMC free article] [PubMed] [Google Scholar]


