Abstract
Background
Dysbindin (DTNBP1) is a widely-studied candidate gene for schizophrenia (SCZ); however, inconsistent results across studies triggered skepticism towards the validity of the findings. In this HapMap-based study, we reappraised the association between Dysbindin and SCZ in a large sample of German ethnicity.
Method
Six hundred thirty-four cases with DSM-IV SCZ, 776 controls, and 180 parent-offspring trios were genotyped for 38 Dysbindin SNPs. We also studied two phenotypically-defined subsamples: 147 patients with a positive family history of SCZ (FH-SCZ+) and SCZ patients characterized for cognitive performance with Trail-Making Tests A and B (TMT-A: n=219; TMT-B: n=247). Given previous evidence of gene-gene interactions in SCZ involving the COMT gene, we also assessed epistatic interactions between Dysbindin markers and 14 SNPs in COMT.
Results
No association was detected between Dysbindin markers and SCZ, or in the FH-SCZ+ subgroup. Only one marker (rs1047631, previously reported to be part of a risk haplotype), showed a nominally significant association with performance on TMT-A and TMT-B; these findings did not remain significant after correction for multiple comparisons. Similarly, no pairwise epistatic interactions between Dysbindin and COMT markers remained significant after correction for 504 pairwise comparisons.
Conclusions
Our results, based on one of the largest sample of European Caucasians and using narrowly-defined criteria for SCZ, do not support the etiological involvement of Dysbindin markers in SCZ. Larger samples may be needed in order to unravel Dysbindin's possible role in the genetic basis of proposed intermediate phenotypes of SCZ or to detect epistatic interactions.
Keywords: polymorphism, COMT, epistasis, endophenotype, cognitive function
1. Introduction
Since the original reports were published by Straub and colleagues (2002a, 2002b) noting an association between the Dystobrevin Binding Protein 1 (DTNBP1 or Dysbindin) gene on 6p22.3 and schizophrenia (SCZ), it has become one of the most widely studied candidate genes in SCZ (Bray et al., 2005; Bray et al., 2008; Funke et al., 2004; Kirov et al., 2004; Schwab et al., 2003; Sodhi et al., 2008; Van Den Bogaert et al., 2003; van den Oord et al., 2003; Williams et al., 2004). However, inconsistent findings have triggered skepticism towards their validity (Mutsuddi et al., 2006). Such inconsistencies could be due to differences in ascertainment and ethnic composition as well as phenotypic heterogeneity.
Previous studies from our group illustrate this inconsistent pattern; specifically, in Schwab et al. (2003), we reported an association between SCZ and Dysbindin in a sample of 78 sib-pair families as well as an independent sample of 125 parent-offspring trios. In that study, case definition included both SCZ and schizoaffective disorder (SAD) and the samples were enriched for familial cases. In a subsequent study (Van Den Bogaert et al, 2003) using case definitions of SCZ, SAD, and schizophreniform disorder, we studied case-control samples from Germany (418 cases, 285 controls), Poland (294 cases, 113 controls), and Sweden (142 cases, 272 controls). Of the three samples studied, only the Swedish sample showed an association between Dysbindin and SCZ, but this association became even stronger when only cases with a positive family history of SCZ were considered. This argued for a scenario in which the association between Dysbindin and SCZ or SCZ spectrum disorders could only be detected in samples with a high genetic load, as was the case for the initial studies by Straub and colleagues (2002b, 2003). However, questions remained. For instance, why was no association detected in the Polish sample or in the larger German sample, even when we analyzed a subsample of individuals with a positive family history of SCZ? Ethnic heterogeneity across the samples in question may be one explanation, given that the study by Schwab and colleagues (2003) included samples from Germany, Israel, and Hungary, and that the study by Van Den Bogaert and colleagues (2003) studied narrowly-defined groups with German, Polish, and Swedish ethnicity, respectively. Probably more importantly, due to the limited sample sizes, the subsamples of individuals with a positive family history in the study by Van Den Bogaert and colleagues (2003) had only limited statistical power (30%) to detect an association (power for the subsamples ranged from 19-35%). Thus, it is possible that an effect would have been seen if the sample of cases with a positive family history of SCZ had been larger. Finally, in these two previous studies by our group, a maximum of six Dysbindin markers were studied.
In order to more thoroughly investigate this issue, we conducted a systematic HapMap-based (http://www.hapmap.org/) study to reappraise any potential association between Dysbindin and SCZ in the German population by studying a large, combined case-control and family-based sample, totaling close to 2000 individuals. To decrease the level of diagnostic heterogeneity, case definition was confined to DSM-IV SCZ and only individuals of narrowly-defined German ethnicity were included. Thirty-six haplotype tagging single nucleotide polymorphisms (SNPs) and their three-marker haplotypes were tested for association with SCZ.
Several recent studies have implicated Dysbindin in cognitive performance in both healthy individuals and those suffering from SCZ (Burdick et al., 2006; Burdick et al., 2007; Donohoe et al., 2007; Fallgatter et al., 2006; Luciano et al., 2009; Zinkstok et al., 2007). One implication of these findings is that the association between Dysbindin and SCZ may be driven by the influence of Dysbindin on cognitive processes; thus, this issue may be better approached by investigating this biologically-based intermediate phenotype. We therefore also assessed the association between Dysbindin and cognitive performance in SCZ.
Finally, recent findings suggest that COMT, the gene that encodes Catechol-O-Methyl Transferase (COMT), may interact with other SCZ candidate genes to affect the pathophysiology of the disorder. Interactions between COMT and polymorphisms in RGS4, G72 (DAOA), DISC1, and GRM3 have all been found to influence SCZ risk, prefrontal function, or working memory (Buckholtz et al., 2007; Nicodemus et al., 2007; Tan et al., 2007). We therefore hypothesized that any association between Dysbindin and SCZ might be mediated via interaction with COMT. To test this hypothesis, we investigated any potential epistatic interaction between the 36 Dysbindin markers and 14 SNPs in COMT, including the widely studied val158met polymorphism.
2. Materials and Methods
2.1 Samples and Measures
The sample comprised 634 cases (351 males, 283 females, mean age = 38.5 ± 11.7) drawn from consecutive admissions to inpatient units, and 776 controls (446 males, 330 females, mean age = 45.5 ± 13.5). Three hundred and twenty of these cases originated from the Van Den Bogaert et al. (2003) sample. We studied an additional family sample of 177 parent-offspring trios and three dyads (one parent and one offspring) (119 males, 61 female, mean age 29.4 = ± 7.4). Thirty-six of these families were included in the Schwab et al. (2003) sample. All cases had a diagnosis of SCZ. Diagnostic assessment based on a best estimate approach was performed using IPGS, a comprehensive inventory for phenotype characterization (Fangerau et al., 2004). This included the Structured Clinical Interview for DSM IV (Diagnostic and Statistical Manual, 4th edition) Disorders (SCID-I; First et al., 1994) and the Operational Criteria Checklist for Psychotic Illness program (OPCRIT; McGuffin et al., 1991), a review of medical records, and a family history. One hundred and forty-seven cases (112 in the case-control sample, 35 in the trio sample) had a positive family history of SCZ (FH-SCZ+), as defined as having either a first- or second-degree relative with SCZ. Controls were randomly recruited from the list of registered inhabitants with the support of the local Census Bureau of the city of Bonn (North Rhine-Westphalia, Germany; for details on ascertainment, see Hoefgen et al., 2005). All subjects were of German descent. Written informed consent was obtained prior to study participation for all cases and controls.
The Trail-Making Test A and B (TMT-A and -B; Spreen and Strauss, 1998) was administered to a subsample of the SCZ cases in order to assess cognitive performance ((TMT-A: N=219; 125 males, 94 females; mean age 38.3 ± 10.5), (TMT-B: N=247; 140 males, 107 females; mean age 38.7 ± 10.6). TMT-A and -B measure speed of cognitive processing (visual search and motor speed skills) as well as cognitive flexibility and motor control within the executive functioning domain (Bowie and Harvey, 2006).
2.2 SNP Genotyping
Miller and colleagues' (1988) conventional salting-out protocol was applied to extract genomic DNA from EDTA whole blood samples. Samples were genotyped using the GoldenGate assay (Illumina, San Diego, CA). According to Gabriel and colleagues' (2002) haplotype block definition, the Dysbindin gene is covered by six haplotype blocks. We selected 38 haplotype tagging (h) SNPs from HapMap phase I+II data (HapMap release 20, dbSNP 125), which capture all haplotypes at a minimum frequency of 1% in the Utah population (CEU) of the Centre d'Etude du Polymorphisme Humain (CEPH) Project (for a detailed description of the marker selection procedure, see Treutlein et al. 2009 and Le Hellard et al. 2008). These markers also comprised SNPs in inter-block regions, SNPs that were associated with SCZ in the previous studies by Mutsuddi and colleagues (2006), and SNPs associated with cognitive performance in SCZ and healthy controls (Table 1). Two markers (rs9464797 and rs3213207 deviated from Hardy-Weinberg equilibrium (by standard 1df chi-square test, p<0.05) and were excluded from the analysis. The final list of 36 markers is given in Table 2. Figure 1 depicts the linkage disequilibrium (LD) structure, generated with the HAPLOVIEW software 4.0 (Barrett et al., 2005). The LD structure is based on the controls and on Gabriel and colleagues' (2002) definition.
Table 2.
Association analysis of the 36 Dysbindin SNPs
| Marker rs # | position (bp) | alleles | MAF Cases | MAF Controlls | MAF Indices | MAF Parents | p | p (FH)* |
|---|---|---|---|---|---|---|---|---|
| rs9396591 | 15624264 | A/G | 0.24 (A) | 0.25 (A) | 0.27 (A) | 0.25 (A) | 0.8415 | 0.4755 |
| rs909626 | 15625663 | A/G | 0.15 (A) | 0.14 (A) | 0.16 (A) | 0.12 (A) | 0.0721 | 0.7512 |
| rs3778651 | 15626511 | A/G | 0.07 (A) | 0.07 (A) | 0.07 (A) | 0.06 (A) | 0.7983 | 0.5392 |
| rs13195001 | 15627382 | C/T | 0.25 (T) | 0.26 (T) | 0.25 (T) | 0.29 (T) | 0.1474 | 0.1394 |
| rs13198512 | 15627864 | C/T | 0.49 (C) | 0.49 (C) | 0.49 (C) | 0.48 (C) | 0.9189 | 0.1154 |
| rs1047631 | 15631080 | A/G | 0.16 (G) | 0.15 (G) | 0.16 (G) | 0.17 (G) | 0.9389 | 0.3351 |
| rs17470454 | 15631427 | A/G | 0.06 (A) | 0.07 (A) | 0.05 (A) | 0.07 (A) | 0.2926 | 0.1682 |
| rs742106 | 15632459 | C/T | 0.37 (T) | 0.35 (T) | 0.35 (T) | 0.36 (T) | 0.39 | 0.5033 |
| rs2056943 | 15632542 | A/G | 0.04 (G) | 0.04 (G) | 0.03 (G) | 0.04 (G) | 1 | 1 |
| rs16876575 | 15633136 | C/T | 0.15 (T) | 0.14 (T) | 0.13 (T) | 0.14 (T) | 0.427 | 0.4015 |
| rs9296976 | 15633432 | A/G | 0.08 (G) | 0.09 (G) | 0.07 (G) | 0.09 (G) | 0.4492 | 0.4678 |
| rs6937379 | 15633976 | A/G | 0.24 (A) | 0.26 (A) | 0.24 (A) | 0.25 (A) | 0.2189 | 0.305 |
| rs4712253 | 15634396 | C/T | 0.44 (T) | 0.43 (T) | 0.41 (T) | 0.43 (T) | 0.5594 | 0.7935 |
| rs4236167 | 15641930 | C/T | 0.49 (T) | 0.47 (T) | 0.49 (C) | 0.47 (T) | 0.2539 | 0.4537 |
| rs9396592 | 15646989 | A/G | 0.36 (A) | 0.36 (A) | 0.38 (A) | 0.35 (A) | 0.5467 | 0.3468 |
| rs12527121 | 15654192 | C/T | 0.08 (T) | 0.07 (T) | 0.09 (T) | 0.07 (T) | 0.3644 | 0.5023 |
| rs12203173 | 15705548 | A/G | 0.14 (G) | 0.14 (G) | 0.12 (G) | 0.14 (G) | 0.7536 | 0.132 |
| rs7752070 | 15712898 | A/G | 0.08 (G) | 0.09 (G) | 0.07 (G) | 0.08 (G) | 0.4885 | 0.8357 |
| rs1011313 | 15741411 | A/G | 0.11 (A) | 0.11 (A) | 0.08 (A) | 0.09 (A) | 0.8857 | 0.5616 |
| rs7768128 | 15756679 | A/G | 0.25 (G) | 0.27 (G) | 0.23 (G) | 0.27 (G) | 0.2886 | 0.3592 |
| rs2619528 | 15757808 | A/G | 0.2 (A) | 0.2 (A) | 0.19 (A) | 0.2 (A) | 0.8384 | 0.6198 |
| rs2619522 | 15761628 | G/T | 0.2 (G) | 0.2 (G) | 0.19 (G) | 0.2 (G) | 0.8211 | 0.6722 |
| rs2743854 | 15763248 | C/T | 0.08 (T) | 0.08 (T) | 0.07 (T) | 0.08 (T) | 0.5838 | 0.7152 |
| rs2619519 | 15764181 | A/G | 0.08 (A) | 0.08 (A) | 0.07 (A) | 0.08 (A) | 0.614 | 0.823 |
| rs1018381 | 15765049 | C/T | 0.08 (T) | 0.08 (T) | 0.06 (T) | 0.08 (T) | 0.6036 | 0.7546 |
| rs1474605 | 15766191 | A/G | 0.2 (G) | 0.2 (G) | 0.2 (G) | 0.21 (G) | 0.971 | 0.5535 |
| rs12196958 | 15766584 | A/T | 0.01 (A) | 0.01 (A) | 0.01 (A) | 0.01 (A) | 1 | 1 |
| rs1997679 | 15766884 | C/T | 0.31 (T) | 0.33 (T) | 0.29 (T) | 0.32 (T) | 0.3344 | 0.7447 |
| rs13192791 | 15767518 | C/T | 0.24 (T) | 0.25 (T) | 0.23 (T) | 0.25 (T) | 0.3595 | 0.6981 |
| rs9476886 | 15769440 | C/T | 0.26 (T) | 0.27 (T) | 0.24 (T) | 0.26 (T) | 0.616 | 0.111 |
| rs2619536 | 15771826 | C/T | 0.07 (C) | 0.09 (C) | 0.08 (C) | 0.09 (C) | 0.1775 | 0.8787 |
| rs2619538 | 15773188 | A/T | 0.47 (A) | 0.44 (A) | 0.47 (A) | 0.43 (A) | 0.0933 | 0.2158 |
| rs742207 | 15776846 | C/T | 0.08 (T) | 0.1 (T) | 0.09 (T) | 0.09 (T) | 0.1494 | 0.8267 |
| rs760760 | 15782155 | A/G | 0.17 (G) | 0.16 (G) | 0.2 (G) | 0.16 (G) | 0.6703 | 0.6062 |
| rs7755766 | 15783620 | C/T | 0.15 (C) | 0.15 (C) | 0.17 (C) | 0.16 (C) | 0.728 | 0.8622 |
| rs17407828 | 15784456 | C/G | 0.32 (C) | 0.33 (C) | 0.28 (C) | 0.35 (C) | 0.1885 | 0.1083 |
Positive family history of schizophrenia
Figure 1.
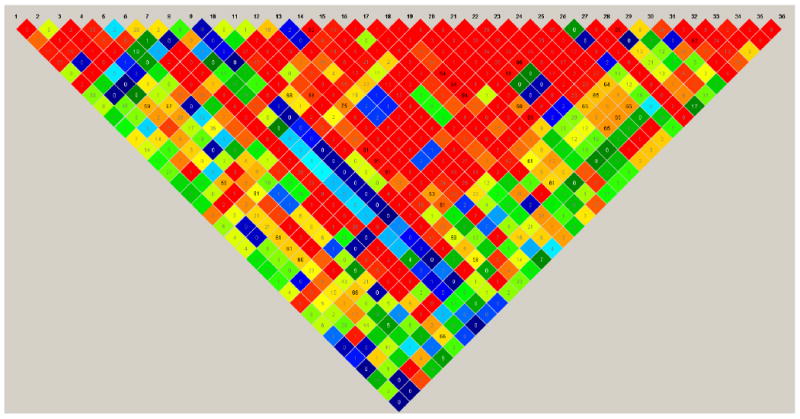
Heat map for the 36 Dysbindin SNPs based on the control sample
To test for a potential interaction between Dysbindin and COMT markers, we also genotyped the following 14 SNPs in COMT, using the same genotyping protocol described in this section: rs6518592, rs2020917, rs737865, rs5993883, rs740603, rs4633, rs2239393, rs4680 (i.e. the val158met), rs4646316, rs165774, rs174697, rs174699, rs9332377, and rs165599.
2.3 Statistical Analyses
Single marker analyses were performed for the whole sample and after stratification for FH-SCZ+. In the subsample tested for cognitive performance, we analyzed single marker and haplotypes that had previously been found to be associated with cognitive performance in SCZ as well as in healthy individuals. TMT-A and B scores (in seconds) were standardized by applying a z-transformation. Analyses were carried out using version 3.013 of UNPHASED (Dudbridge, 2003). Ten thousand permutations were performed. Power calculations were performed using the Genetic Power Calculator (http://pngu.mgh.harvard.edu/∼purcell/gpc/) (Purcell et al., 2003). We assumed a multiplicative model, a disease prevalence of 0.01, and various disease allele frequencies ranging from 0.1 to 0.9.
To test for a potential interaction between Dysbindin and COMT, we used the --epistasis option in PLINK (Purcell et al., 2007). We tested all possible two-pair combinations between the 36 Dysbindin and the 14 COMT SNPs, yielding a total of 504 comparisons.
3. Results
Results from the power analysis including Bonferroni correction for the number of SNPs assessed are presented in Table 3. For genotype relative risks above 1.3 (Aa) and 1.66 (AA), respectively, power estimates above > 0.8 were observed for an increasing number of the 36 Dysbindin SNPs studied.
Table 3.
Power calculation for the 36 Dysbindin SNPs studied (power estimates equal to or larger than 0.8 are bolded)
| Genotype relative risk (Aa/AA) | ||||
|---|---|---|---|---|
| Marker | 1.2/1.44 | 1.3/1.66 | 1.4/1.96 | 1.5/2.25 |
| rs9396591 | 0.51 | 0.82 | 0.96 | 0.99 |
| rs909626 | 0.35 | 0.64 | 0.85 | 0.96 |
| rs3778651 | 0.22 | 0.40 | 0.61 | 0.79 |
| rs13195001 | 0.52 | 0.83 | 0.97 | 1.00 |
| rs13198512 | 0.60 | 0.89 | 0.98 | 1.00 |
| rs1047631 | 0.38 | 0.68 | 0.89 | 0.97 |
| rs17470454 | 0.22 | 0.41 | 0.62 | 0.80 |
| rs742106 | 0.58 | 0.87 | 0.98 | 1.00 |
| rs2056943 | 0.15 | 0.27 | 0.42 | 0.58 |
| rs16876575 | 0.36 | 0.65 | 0.86 | 0.96 |
| rs9296976 | 0.26 | 0.50 | 0.72 | 0.88 |
| rs6937379 | 0.51 | 0.82 | 0.96 | 1.00 |
| rs4712253 | 0.60 | 0.89 | 0.98 | 1.00 |
| rs4236167 | 0.60 | 0.89 | 0.98 | 1.00 |
| rs9396592 | 0.58 | 0.88 | 0.98 | 1.00 |
| rs12527121 | 0.22 | 0.41 | 0.62 | 0.80 |
| rs12203173 | 0.36 | 0.65 | 0.86 | 0.96 |
| rs7752070 | 0.26 | 0.49 | 0.71 | 0.87 |
| rs1011313 | 0.30 | 0.55 | 0.78 | 0.91 |
| rs7768128 | 0.52 | 0.83 | 0.97 | 1.00 |
| rs2619528 | 0.45 | 0.76 | 0.93 | 0.99 |
| rs2619522 | 0.45 | 0.76 | 0.93 | 0.99 |
| rs2743854 | 0.24 | 0.46 | 0.68 | 0.84 |
| rs2619519 | 0.24 | 0.46 | 0.68 | 0.84 |
| rs1018381 | 0.24 | 0.46 | 0.68 | 0.84 |
| rs1474605 | 0.45 | 0.77 | 0.94 | 0.99 |
| rs12196958 | 0.08 | 0.11 | 0.15 | 0.20 |
| rs1997679 | 0.56 | 0.87 | 0.98 | 1.00 |
| rs13192791 | 0.51 | 0.82 | 0.96 | 0.99 |
| rs9476886 | 0.52 | 0.83 | 0.97 | 1.00 |
| rs2619536 | 0.26 | 0.50 | 0.72 | 0.88 |
| rs2619538 | 0.60 | 0.89 | 0.98 | 1.00 |
| rs742207 | 0.28 | 0.52 | 0.75 | 0.90 |
| rs760760 | 0.39 | 0.69 | 0.89 | 0.97 |
| rs7755766 | 0.38 | 0.68 | 0.88 | 0.97 |
| rs17407828 | 0.57 | 0.87 | 0.98 | 1.00 |
No significant associations were found using either the single marker or haplotype analyses with a 3-marker sliding window, either in the overall SCZ sample or in the FH-SCZ+ subsample (Table 2; haplotype data not shown).
In the subsample screened for cognitive performance, none of the previously identified Dysbindin risk markers or haplotypes for cognitive dysfunction in SCZ yielded any association with performance on either the TMT-A or TMT-B. We subsequently performed exploratory single marker and haplotype analyses with a 3-marker sliding window for all 36 SNPs. The only findings of note were single marker associations between TMT-A (p=0.0074) and TMT-B (p=0.021) and rs1047631, which was part of a risk haplotype studied by Bray and colleagues (2005). However, results did not remain significant after correction for multiple testing.
In our analysis of a potential epistatic interaction between Dysbindin and COMT markers, 64 out of the 504 pair-wise comparisons were of nominal significance, with the smallest p-value observed for the marker pair comprising rs909626 in Dysbindin and rs5748489 in COMT (p=0.0002; odds ratio for the interaction=0.58). However, none of these nominally significant findings remained present after correction for multiple testing (see Supplemental Table 1). In our sample, the COMT markers showed no association with SCZ (manuscript in preparation).
4. Discussion
Dysbindin has long been considered a major susceptibility gene for SCZ; however, the inconsistent results associated with Dysbindin have put these findings into question for three main reasons (Mutsuddi et al., 2006). First, the Dysbindin haplotypes associated with SCZ differed between studies, suggesting a possible allelic heterogeneity or population differences in LD structure across the gene. Second, the same SNPs have not been genotyped in every association study. Third, causal variants that may contribute to SCZ have not yet been identified, and a demonstrated function associated with any of the risk haplotypes is still absent. Mutsuddi and colleagues (2006) genotyped all associated SNPs reported in six studies (Bray et al., 2005; Funke et al., 2004; Kirov et al., 2004; Schwab et al., 2003; Van Den Bogaert et al., 2003; van den Oord et al., 2003; Williams et al., 2004) in a HapMap (CEU) trio European sample of the Centre d'Étude du Polymorphisme Humain (CEPH). Their results revealed that SNPs or haplotypes were of a similar frequency in the CEU-sample and in the association samples, suggesting that each sample was genetically similar and differences in published results could not be explained by population stratification. The authors did not exclude the possibility that differences in sample size, statistical power, diagnosis, variety of samples, and environmental factors yielded different results.
In this study, we attempted to investigate any possible association between SCZ and Dysbindin in one of the largest combined case-control and family-samples, and one of the largest samples overall; all participants were of German descent. The 36 SNPs analyzed in the current study yielded enough polymorphic markers to cover the whole genetic sequence, and sufficient power to detect an effect. In accordance with our previous findings on a German and Polish sample (Van Den Bogaert et al., 2003), as well as previous findings in Anglo-Irish, European-Ancestry American, Finnish, and Korean populations (Joo et al., 2006; Morris et al., 2003; Peters et al., 2008; Sanders et al., 2008; Turunen et al., 2007), we found no association between Dysbindin and SCZ: neither single marker nor haplotype analyses yielded any significant results for the overall SCZ or the FH-SCZ+ sample. We would like to point out, however, that we only tested common variants of Dysbindin, possible associations between SCZ and rare variants or very common variants were not examined.
As noted previously, recent findings suggest an association between Dysbindin and cognitive performance in individuals with SCZ as well as healthy individuals (Burdick et al., 2006; Burdick et al., 2007; Donohoe et al., 2007; Fallgatter et al., 2006; Hallmayer et al., 2005; Luciano et al., 2009; Posthuma et al., 2005; Zinkstok et al., 2007). These results suggest that the study of intermediate phenotypes in SCZ—such as cognitive dysfunction—may clarify inconsistent genetic findings and increase our understanding of the mechanisms leading from a genetic variation to a complex phenotype. Cognitive deficits are a common finding in patients with SCZ, are considered a core feature of the clinical phenotype (Green, 1996; Heinrichs, 2005), and strongly predict functional outcome (Gold, 2004; Brekke et al., 2007). Furthermore, Dysbindin is believed to be involved in both glutamatergic and dopaminergic signalling pathways (Harrison and Weinberger, 2005; Iizuka et al., 2007; Sodhi et al., 2008; Talbot et al., 2004; Weikert et al., 2004), which play a major role in cognitive processes usually impaired in SCZ (e.g., working memory) (Castner and Williams, 2007; Gray and Roth, 2007; Meisenzahl et al., 2007).
The present study therefore investigated the putative association between Dysbindin markers and cognitive performance using the TMT-A and TMT-B in a subsample of individuals with SCZ. Only one SNP (rs1047631) was associated with performance on the TMT-A and -B at a nominal level of significance, but this finding did not survive correction for multiple testing. However, the methodological design of our study may have prevented us from detecting robust associations between Dysbindin and cognitive function in SCZ; specifically, we used a very broad measure of cognitive performance in our study. While the TMT-A and TMT-B measure speed of cognitive processing and cognitive flexibility within the executive functioning domain (Bowie and Harvey, 2006), previous positive findings have been obtained by studies that administered very comprehensive measures of cognitive performance that allow for the identification of deficits in specific areas of cognitive function. For instance, Zinkstok and colleagues (2007) administered the Wechsler Adult Intelligence Test – third version (WAIS III) and Burdick and colleagues (2006; 2007) used a wide range of cognitive performance tests. Thus, it is possible that specific Dysbindin markers or haplotypes may be associated with deficits in very specific areas of cognitive function. Luciano and colleagues (2009) found associations between single Dysbindin markers and measures of executive functioning; one marker was associated with verbal ability and yet another marker with memory, speed, and executive function. A comprehensive and detailed characterization of intermediate phenotypes may therefore be essential to clarify if, and which, specific cognitive deficits are associated with Dysbindin.
Recent findings also suggest that COMT, which encodes the enzyme COMT, may interact with other SCZ candidate genes in the pathophysiology of the disorder (Buckholtz et al., 2007; Nicodemus et al., 2007; Tan et al., 2007). The val158met polymorphism of COMT has been widely studied and long been considered an important factor involved in the pathophysiology of SCZ and cognition (Honea et al., 2009). COMT is an enzyme that metabolizes released dopamine. More specifically, it metabolizes cortical dopamine (Tunbridge et al., 2006; Weinshilboum et al., 1990), which may be particularly important in the pathophysiology of SCZ (Winterer and Weinberger, 2004). Given the prior evidence for interaction between SCZ candidate genes, we hypothesized that an association between Dysbindin and SCZ might be mediated through an interaction with COMT. We thus performed a formal analysis of epistasis between markers in both genes. Testing all possible 504 two-marker combinations, which included the val158met polymorphism of COMT, we detected no epistatic effects that were significant after correction for multiple testing.
In sum, we could not replicate previous association findings between Dysbindin and SCZ in one of the largest European samples to date. We also found no support for the notion that an association between Dysbindin and SCZ would more typically be seen in samples with a high genetic load for SCZ. Finally, our analyses investigating the potential influence of Dysbindin on cognitive function in SCZ and on a potential epistatic interaction with COMT yielded no results that withstood correction for multiple testing. We conclude that definitively elucidating Dysbindin's role in proposed intermediate phenotypes of SCZ and its potential epistatic effects may require sample sizes well beyond the ones studied here.
Supplementary Material
Table 1.
Dysbindin SNPs and their association with cognitive function (CF)
Gray shading indicates positive association in respective study.
| Dysbindin SNPs | rs1047631 | rs3213207 | rs1011313 | rs2619528 | rs2005976 | rs760761 | rs2619522 | rs1018381 | rs1474605 | rs909706 | rs2619538 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Association with SZ (Mutsuddi et al, 2006) |
|||||||||||
| Association with CF (Burdick et al., 2006) |
|||||||||||
| Association with CF (Burdick et al., 2007) |
|||||||||||
| Association with CF (Donohoe et al., 2006) |
|||||||||||
| Association with CF (Zinkstok et al., 2007) |
|||||||||||
| Association with CF (Luciano et al., 2009) |
|||||||||||
| SNPs genotyped in present sample | yes | yes | yes | yes | yes | yes | yes | yes |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21(2):263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Bowie CR, Harvey PD. Administration and interpretation of the Trail Making Test. Nat Protoc. 2006;1(5):2277–2281. doi: 10.1038/nprot.2006.390. [DOI] [PubMed] [Google Scholar]
- Bray NJ, Holmans PA, van den Bree MB, Jones L, Elliston LA, Hughes G, Richards AL, Williams NM, Craddock N, Owen MJ, O'Donovan MC. Cis- and trans- loci influence expression of the schizophrenia susceptibility gene DTNBP1. Hum Mol Genet. 2008;17(8):1169–1174. doi: 10.1093/hmg/ddn006. [DOI] [PubMed] [Google Scholar]
- Bray NJ, Preece A, Williams NM, Moskvina V, Buckland PR, Owen MJ, O'Donovan C. Haplotypes at the destrobrevin binding protein 1 (DTNBP1) gene locus mediate risk for schizophrenia through reduced DTNBP1 expression. Hum Mol Genet. 2005;14(14):1947–1954. doi: 10.1093/hmg/ddi199. [DOI] [PubMed] [Google Scholar]
- Brekke JS, Hoe M, Long J, Green MF. How neurocognition and social cognition influence functional change during community-based psychosocial rehabilitation for individuals with schizophrenia. Schizophr Bull. 2007;33(5):1247–1256. doi: 10.1093/schbul/sbl072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholtz JW, Sust S, Tan HY, Mattay VS, Straub RE, Meyer-Lindenberg A, Weinberger DR, Callicott JH. FMRI evidence for functional epistasis between COMT and RGS4. Mol Psychiatry. 2007;12(10):893–895. doi: 10.1038/sj.mp.4002008. [DOI] [PubMed] [Google Scholar]
- Burdick KE, Goldberg TE, Funke B, Bates JA, Lencz T, Kucherlapati R, Malhotra AK. DTNBP1 genotype influences cognitive decline in schizophrenia. Schizophr Res. 2007;89(1-3):169–172. doi: 10.1016/j.schres.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick KE, Lencz T, Funke B, Finn CT, Szeszko PR, Kane JM, Kucherlapati R, Malhotra AK. Genetic variation in DTNBP1 influences general cognitive ability. Hum Mol Genet. 2006;15(10):1563–1568. doi: 10.1093/hmg/ddi481. [DOI] [PubMed] [Google Scholar]
- Castner SA, Williams GV. Tuning the engine of cognition: a focus on NMDA/D1 receptor interactions in prefrontal cortex. Brain Cogn. 2007;63(2):94–122. doi: 10.1016/j.bandc.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Donohoe G, Morris DW, Clarke S, McGhee KA, Schwaiger S, Nangle JM, Garavan H, Robertson IH, Gill M, Corvin A. Variance in neurocognitive performance is associated with dysbindin-1 in schizophrenia: A preliminary study. Neuropsychologia. 2007;45(2):454–458. doi: 10.1016/j.neuropsychologia.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Dudbridge F. Pedigree disequilibrium tests for multilocus haplotypes. Genet Epidemiol. 2003;25(2):115–21. doi: 10.1002/gepi.10252. [DOI] [PubMed] [Google Scholar]
- Fallgatter AJ, Herrmann MJ, Hohoff C, Ehlis AC, Jarczok TA, Freitag CM, Deckert J. DTNBP1 (Dysbindin) gene variants modulate prefrontal brain function in healthy individuals. Neuropsychopharmacology. 2006;31(9):2002–2010. doi: 10.1038/sj.npp.1301003. [DOI] [PubMed] [Google Scholar]
- Fangerau H, Ohlraun S, Granath RO, Nöthen MM, Rietschel M, Schulze TG. Computer-assisted phenotype characterisation for genetic research in psychiatry. Hum Hered. 2004;58(3-4):122–130. doi: 10.1159/000083538. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams J. Structured Clinical Interview for Axis I DSM-IV Disorders. Biometrics Research; New York: 1994. [Google Scholar]
- Funke B, Finn CT, Plocik AM, Lake S, DeRosse P, Kane JM, Kucherlapati R, Malhotra AK. Association of the DTNBP1 locus with schizophrenia in a US population. Am J Hum Genet. 2004;75(5):891–898. doi: 10.1086/425279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, Higgins J, DeFelice M, Lochner A, Faggart M, Liu-Cordero SN, Rotimi C, Adeyemo A, Cooper R, Ward R, Lander ES, Daly MJ, Altshuler D. The structure of haplotype blocks in the human genome. Science. 2002;296(5576):2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- Gold JM. Cognitive deficits are treatment targets in schizophrenia. Schizophr Res. 2004;72(1):21–28. doi: 10.1016/j.schres.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153(3):321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Hallmayer JF, Kalaydjieva l, Badcock J, Dragovic M, Howell S, Michie PT, Rock D, Vile D, Williams R, Corder EH, Hollingsworth K, Jablensky A. Genetic evidence for a distinct subtype of schizophrenia characterized by pervasive cognitive deficits. Am J Hum Genet. 2005;77(3):468–476. doi: 10.1086/432816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10(1):40–68. doi: 10.1038/sj.mp.4001558. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW. The primacy of cognition in schizophrenia. Am Psychol. 2005;60(3):229–242. doi: 10.1037/0003-066X.60.3.229. [DOI] [PubMed] [Google Scholar]
- Hoefgen B, Schulze TG, Ohlraun S, von Widdern O, Höfels S, Gross M, Heidmann V, Kovalenko S, Eckermann A, Kölsch H, Metten M, Zobel A, Becker T, Nöthen MM, Propping P, Heun R, Maier W, Rietschel M. The power of sample size and homogenous sampling: association between the 5-HTTLPR serotonin transporter polymorphism and major depressive disorder. Biol Psychiatry. 2005;57(3):247–251. doi: 10.1016/j.biopsych.2004.11.027. [DOI] [PubMed] [Google Scholar]
- Honea R, Verchinski BA, Pezawas L, Kolachana BS, Callicott JH, Mattay VS, Weinberger DR, Meyer-Lindenberg A. Impact of interacting functional variants in COMT on regional gray matter volume in human brain. Neuroimage. 2008;45(1):44–51. doi: 10.1016/j.neuroimage.2008.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka Y, Sei Y, Weinberger DR, Straub RE. Evidence that the BLOC-1 protein Dysbindin modulates dopamine D2 receptor internalization and signaling but not D1 internalization. J Neurosci. 2007;27(45):12390–12395. doi: 10.1523/JNEUROSCI.1689-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo EJ, Lee KY, Jeong SH, Ahn YM, Koo YJ, Kim YS. The Dysbindin gene (DTNBP1) and schizophrenia: No support for an association in the Korean population. Neurosci Lett. 2006;407(2):101–106. doi: 10.1016/j.neulet.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Kirov G, Ivanov D, Williams NM, Preece A, Nikolov I, Milev R, Koleva S, Dimitrova A, Tonchva D, O'Donovan MC, Owen MJ. Strong evidence for association between Dystrobrevin Binding Protein 1 Gene (DTNBP1) and schizophrenia in 488 parent-offspring trios from. Bulgaria Biol Psychiatry. 2004;55(10):971–975. doi: 10.1016/j.biopsych.2004.01.025. [DOI] [PubMed] [Google Scholar]
- Le Hellard S, Mühleisen TW, Djurovic S, Fernø J, Ouriaghi Z, Mattheisen M, Vasilescu C, Raeder MB, Hansen T, Strohmaier J, Georgi A, Brockschmidt FF, Melle I, Nenadic I, Sauer H, Rietschel M, Nöthen MM, Werge T, Andreassen OA, Cichon S, Steen VM. Polymorphisms in SREBF1 and SREBF2, two antipsychotic-activated transcription factors controlling cellular lipogenesis, are associated with schizophrenia in German and Scandinavian samples. Mol Psychiatry. 2008 Oct 21; doi: 10.1038/mp.2008.110. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Luciano M, Miyajima F, Lind PA, Bates TC, Horan M, Harris SE, Wright MJ, Ollier WE, Hayward C, Pendleton N, Gow AJ, Visscher PM, Starr JM, Deary IJ, Martin NG, Payton A. Variation in the Dysbindin gene and normal cognitive function in three independent population samples. Genes Brain Behav. 2009;8(2):218–227. doi: 10.1111/j.1601-183X.2008.00462.x. [DOI] [PubMed] [Google Scholar]
- McGuffin P, Farmer AE, Harvey I. A polydiagnostic application of the operational criteria in studies of psychotic illness. Arch Gen Psychiatry. 1991;48(8):764–770. doi: 10.1001/archpsyc.1991.01810320088015. [DOI] [PubMed] [Google Scholar]
- Meisenzahl EM, Schmitt GJ, Scheuerecker J, Möller HJ. The role of dopamine for the pathophysiology of schizophrenia. Int Rev Psychiatry. 2007;19(4):337–345. doi: 10.1080/09540260701502468. [DOI] [PubMed] [Google Scholar]
- Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16(3):1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris DW, McGhee KA, Schwaiger S, Scully P, Quinn J, Meagher D, Waddington JL, Gill M, Corvin AP. No evidence for association of the Dysbindin gene (DTNBP1) with schizophrenia in an Irish population-based study. Schizophr Res. 2003;60(2-3):167–172. doi: 10.1016/s0920-9964(02)00527-3. [DOI] [PubMed] [Google Scholar]
- Mutsuddi M, Morris DW, Waggoner SG, Daly MJ, Scolnick EM, Sklar P. Analysis of high-resolution HapMap of DTNBP1 (Dysbindin) suggests no consistency between reported common variant associations and schizophrenia. Am J Hum Genet. 2006;79(5):903–909. doi: 10.1086/508942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicodemus KK, Kolachana BS, Vakkalanka R, Straub RE, Giegling I, Egan MF, Rujescu D, Weinberger DR. Evidence for statistical epistasis between catechol-O-methyltransferase (COMT) and polymorphisms in RGS4, G72 (DAOA), GRM3, and DISC1: influence on risk of schizophrenia. Hum Genet. 2007;120(6):889–906. doi: 10.1007/s00439-006-0257-3. [DOI] [PubMed] [Google Scholar]
- Peters K, Wiltshire S, Henders AK, Dragovicl M, Badcock JC, Chandler D, Howell S, Ellis C, Bouwer S, Montgomery GW, Palmer LJ, Kalaydjieva L, Jablensky A. Comprehensive analysis of tagging sequence variants in DTNBP1 shows no association with schizophrenia or with its composite neurocognitive endophenotypes. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(7):1159–1166. doi: 10.1002/ajmg.b.30741. [DOI] [PubMed] [Google Scholar]
- Posthuma D, Luciano M, de Geus EJC, Wright MJ, Slagboom PE, Montgomery GW, Boomsma DI, Martin NG. A genomwide scan for intelligence identifies quantitative trait loci on 2q and 6p. Am J Hum Genet. 2005;77(2):318–326. doi: 10.1086/432647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Cherny SS, Sham PC. Genetic Power Calculator: design of linkage and association genetic mapping studies of complex traits. Bioinformatics. 2003;19(1):149–150. doi: 10.1093/bioinformatics/19.1.149. [DOI] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders AR, Duan J, Levinson DF, Shi J, He D, Hou C, Burrell GJ, Rice JP, Nertney DA, Olincy A, Rozic P, Vinogradov S, Buccola NG, Mowry BJ, Freedman R, Amin F, Black DW, Silverman JM, Byerley WF, Crowe RR, Cloninger CR, Martinez M, Gejman PV. No significant association of 14 candidate genes with schizophrenia in a large European ancestry sample: implications for psychiatric genetics. Am J Psychiatry. 2008;165(4):497–506. doi: 10.1176/appi.ajp.2007.07101573. [DOI] [PubMed] [Google Scholar]
- Schwab SG, Knapp M, Mondabon S, Hallmayer J, Borrmann-Hassenbach M, Albus M, Lerer B, Rietschel M, Trixler M, Maier W, Wildenauer DB. Support for association of schizophrenia with genetic variation in the 6p22.3 gene, Dysbindin, in sib-pair families with linkage and in an additional sample of triad families. Am J Hum Genet. 2003;72(1):185–190. doi: 10.1086/345463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodhi M, Wood KH, Meador-Woodruff J. Role of glutamate in schizophrenia: integrating excitatory avenues of research. Expert Rev Neurother. 2008;8(9):1389–1406. doi: 10.1586/14737175.8.9.1389. [DOI] [PubMed] [Google Scholar]
- Spreen O, Strauss E. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. second. Oxford Univerity Press; New York: 1998. [Google Scholar]
- Straub RE, Jiang X, MacLean CJ, Ma Y, Webb BT, Myakishev MV, Harris-Kerr C, Wormley B, Sadek H, Kadambi B, Cesare AJ, Gibberman A, Wang X, O'Neill FA, Walsh D, Kendler KS. Genetic variation in the 6p22.3 Gene DTNBP1, the human ortholog of the mouse Dysbindin gene, is associated with schizophrenia. Am J Hum Genet. 2002a;71(2):337–348. doi: 10.1086/341750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub RE, MacLean CJ, Ma Y, Webb BT, Myakishev MV, Harris-Kerr C, Wormley B, Sadek H, Kadambi B, O'Neill FA, Walsh D, Kendler KS. Genome-wide scans of three independent sets of 90 Irish multiplex schizophrenia families and follow-up of selected regions in all families provides evidence for multiple susceptibility genes. Mol Psychiatry. 2002b;7(6):542–559. doi: 10.1038/sj.mp.4001051. [DOI] [PubMed] [Google Scholar]
- Tan HY, Chen Q, Sust S, Buckholtz JW, Meyers JD, Egan MF, Mattay VS, Meyer-Lindenberg A, Weinberger DR, Callicott JH. Epistasis between catechol-O-methyltransferase and type II metabotropic glutamate receptor 3 genes on working memory brain function. Proc Natl Acad Sci USA. 2007;104(30):12536–12541. doi: 10.1073/pnas.0610125104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot K, Eidem WL, Tinsley CL, Benson MA, Thompson EW, Smith RJ, et al. Dysbindin is reduced in intrinsic, glutamatergic terminals of the hippocampal formation in schizophrenia. J Clin Invest. 2004;113(9):1353–1363. doi: 10.1172/JCI20425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treutlein J, Mühleisen TW, Frank J, Mattheisen M, Herms S, Ludwig KU, Treutlein T, Schmael C, Strohmaier J, Bösshenz KV, Breuer R, Paul T, Witt SH, Schulze TG, Schlösser RG, Nenadic I, Sauer H, Becker T, Maier W, Cichon S, Nöthen MM, Rietschel M. Dissection of phenotype reveals possible association between schizophrenia and Glutamate Receptor Delta 1 (GRID1) gene promoter. Schizophr Res. 2009;111(1-3):123–130. doi: 10.1016/j.schres.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Harrison PJ, Weinberger DR. Catechol-omethyltransferase, cognition and psychosis: Val158Met and beyond. Biol Psychiatry. 2006;60(2):141–151. doi: 10.1016/j.biopsych.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Turunen JA, Peltonen JO, Pietiläinen OPH, Hennah W, Loukola A, Paunio T, Silander K, Ekelund J, Varlio T, Partonen T, Lönnqvist J, Peltonen L. The role of DTNBP1, NRG1, and AKT1 in the genetics of schizophrenia in Finnland. Schizophr Res. 2007;91(1-3):27–26. doi: 10.1016/j.schres.2006.11.028. [DOI] [PubMed] [Google Scholar]
- Van Den Bogaert A, Schumacher J, Schulze TG, Otte AC, Ohlraun S, Kovalenko S, Becker T, Freudenberg J, Jönsson EG, Mattila-Evenden M, Sedvall GC, Czerski PM, Kapelski P, Hauser J, Maier W, Rietschel M, Propping P, Nöthen MM, Cichon S. The DTNBP1 (Dysbindin) gene contributes to schizophrenia, depending on family history of the disease. Am J Hum Genet. 2003;73(6):1438–1443. doi: 10.1086/379928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Oord EJCG, Sullivan PF, Jiang Y, Walsh D, O'Neill FA, Kendler KS, Riley BP. Identification of a risk-haplotype for the dystrobrevin binding protein 1 (DTNBP1) gene in the Irish study of high-density schizophrenia families. Mol Psychiatry. 2003;8(5):499–510. doi: 10.1038/sj.mp.4001263. [DOI] [PubMed] [Google Scholar]
- Weikert CS, Straub RE, McClintock BW, Matsumoto M, Hashimoto R, Hyde TM, Herman MM, Weinberger DR, Kleinman JE. Human Dysbindin (DTNBP1) gene expression in normal brain and in schizophrenic prefrontal cortex and midbrain. Arch Gen Psychiatry. 2004;61(6):544–555. doi: 10.1001/archpsyc.61.6.544. [DOI] [PubMed] [Google Scholar]
- Weinshilboum RM, Otterness DM, Szumlanski CL. Methylation pharmacogenetics: catechol O-methyltransferase, thiopurine methyltransferase, and histamine N-methyltransferase. Annu Rev Pharmacol Toxicol. 1990;39:19–52. doi: 10.1146/annurev.pharmtox.39.1.19. [DOI] [PubMed] [Google Scholar]
- Williams NM, Preece A, Morris DW, Spurlock G, Bray NJ, Stephens M, Norton N, Williams H, Clement M, Dwyer S, Curran C, Wilkinson J, Moskvina V, Waddington JL, Gill M, Corvin AP, Zammit S, Kirov G, Owen MJ, O'Donovan MC. Identification in 2 independent samples of a novel schizophrenia risk haplotype of the dystrobrevin binding protein gene (DTNBP1) Arch Gen Psychiatry. 2004;61(4):336–344. doi: 10.1001/archpsyc.61.4.336. [DOI] [PubMed] [Google Scholar]
- Winterer G, Weinberger DR. Genes, dopamine and cortical signal-to-noise ratio in schizophrenia. Trends Neurosci. 2004;27(11):683–690. doi: 10.1016/j.tins.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Zinkstok JR, de Wilde O, van Amelsvoort TAMJ, Tanck MW, Baas F, Linszen DH. Association between the DTNBP1 gene and intelligence: a case-control study in young patients with schizophrenia and related disorders and unaffected siblings. Behav Brain Funct. 2007;3:19–28. doi: 10.1186/1744-9081-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


