Abstract
Objective
This study examined the impact of a 6-month, empowerment-based diabetes self-management support (DSMS) intervention on clinical outcomes, self-care behaviors, and quality of life (QOL) compared to a 6-month control period.
Methods
This control-intervention cohort study recruited 77 African-American adults with type 2 diabetes. Baseline, 6-month, and 12-month assessments measured A1C, weight, body mass index (BMI), blood pressure, lipids, self-care behaviors, and QOL. During the control period, participants received weekly educational newsletters. During the intervention period, participants attended weekly DSMS groups as frequently as they needed. Sessions were guided by participants’ self-management questions and concerns, and also emphasized experiential learning, coping, problem-solving, and goal-setting.
Results
The control period found significant improvements for diastolic BP (p<0.05), serum cholesterol (p<0.001), following a healthy diet (p<0.01), and monitoring blood glucose (p<0.01). The intervention period found significant additional improvements for A1C (p<0.001), weight (p<0.05), BMI (p<0.05), and LDL (p<0.001). Compared to the control period, participation in the intervention led to a significant reduction in A1C (p< 0.01).
Conclusion
Findings suggest that an empowerment-based, DSMS intervention is promising for improving and/or maintaining diabetes-related health, particularly A1C.
Practical implications
Incorporating empowerment principles in DSMS interventions may be useful for supporting patients’ self-management efforts in “real-world” settings.
1. Introduction
Patient empowerment has become widely recognized as a compelling paradigm for self-management education and behavior change in diabetes care [1–3]. As conceptualized by Anderson and Funnell [4]. The empowerment approach encompasses three guiding principles: First, diabetes is a patient-managed disease. Patients, not providers, make the majority of daily decisions (e.g., dietary choices, physical activity, blood glucose monitoring) regarding their diabetes care. Second, diabetes care should emphasize a collaborative patient-provider relationship in which the provider functions as an educator and/or consultant to the patient who ultimately makes informed self-care decisions. Third, patients are in the best position to identify self-management priorities that have the greatest impact on their lives. When patients self-select behavior changes that are personally meaningful, they will be more motivated to initiate and sustain the behavior change.
As patient-centered care has become a central tenet of health care delivery, patient empowerment has received greater attention. In fact, a growing number of self-management interventions have been designed based on the empowerment approach or have incorporated empowerment principles [5–19]. Participation in these interventions have been associated with improvements in metabolic and cardiovascular outcomes, including A1C [7–9], [11–12], [14], [16], [19], serum cholesterol [7–8], [12], [18–19], LDL [7], [12], [18], HDL [11], [18], systolic blood pressure (SBP) [7], and diastolic blood pressure (DBP) [7], [12]. These interventions have also led to reductions in weight [8], [16], [19], body mass index (BMI) [8], [18], and waist circumference [8]. Post-intervention data have also documented greater frequency of performing self-care practices, including making healthy nutritional choices [18], consuming fruits and vegetables [8], participating in physical activity [8], [18], monitoring blood glucose [18], and inspecting feet [8], [18]. Studies measuring psychological and emotional functioning have found positive changes in quality of life [14], [17–18], self-empowerment [8], [11], [19], psychosocial functioning [17], perceived health status [9], and satisfaction with care [8], [12].
A hallmark of patient empowerment is the focus on patient-directed versus curriculum-directed interventions. For instance, Anderson and colleagues [19] developed an empowerment-based self-management education program driven entirely by patients’ diabetes-related questions, concerns, and priorities rather than a predetermined curriculum with discrete self-management topics delivered in a fixed sequence. Given that patients live with diabetes in “real-world” settings that present unpredictable life challenges under ever-changing conditions, it would appear that a patient-driven self-management support program would be more suitable and responsive than a curriculum-driven program.
When a description of the empowerment-based program was presented to African Americans and Latinos with type 2 diabetes in a focus group setting, an overwhelming majority expressed great interest in participating [20]. In addition, they strongly agreed that this type of program would help patients improve emotional coping, develop effective self-management strategies, and enhance self-care practices. However, transportation and competing life demands were the most frequently cited barriers to participation. Therefore, in addition to patient-directed discussion, the intervention, itself, needs to be flexible and accommodating to “real-world” deterrents.
Considering these findings, Tang et al. [18] piloted the feasibility and acceptability of a weekly, empowerment-based, self-management support intervention for African Americans with type 2 diabetes. Conducted in a central location in the community, patients were encouraged to attend sessions as frequently as they needed based on their individual support needs. The study successfully recruited the target number of participants and met the pre-established weekly attendance criteria. Moreover, significant improvements were found for BMI, lipids, self-care practices, quality of life, and other measures of psychosocial functioning. However, a significant limitation to the pilot study was the lack of a control group.
This study addresses this limitation by utilizing an attention-control condition. The purpose of an attention-control condition is to determine if any significant changes observed in the experimental condition are a result of the intervention itself rather than the additional attention participants would receive by simply enrolling into a research study [21]. Unlike a “usual care” control condition that receives no treatment during the course of a research study, an “attention” control condition receives a placebo treatment that provides the same time and attention as the experimental treatment condition.
In this study, we elected to use a mailed intervention as our attention-control condition. Specifically, we provided clinical feedback to participants and their physicians immediately following baseline assessment, and also mailed participants weekly educational newsletters. We considered this mailed intervention as an appropriate attention-control condition because it emulates the “usual care” any patient with diabetes should receive (clinical feedback to patient and their provider and patient education material). In addition, this control condition provides the same time and attention (weekly educational newsletter) as the experimental treatment (weekly DSMS groups). It should be noted that we define same time and attention as the same number of encounters with the study whether these encounters are face-to-face or mailings.
The objectives of this study are two-fold:
To examine the impact of a 6-month empowerment-based self-management intervention (Lifelong Management) on metabolic and cardiovascular outcomes, self-care practices, and diabetes-specific quality of life compared to a 6-month attention-control period.
To determine the acceptability of an ongoing empowerment-based program using attendance rate and patterns.
2. Methods
2.1. Participants and recruitment
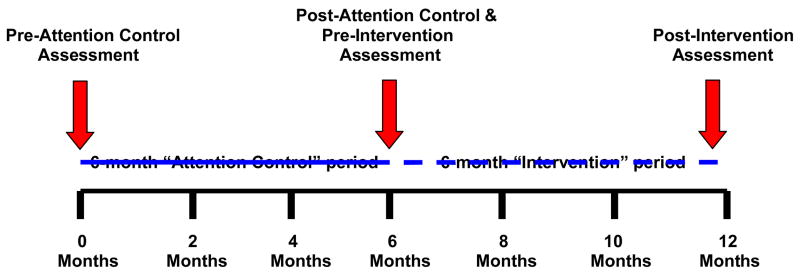
This study was approved by the University of Michigan Institutional Review Board. In contrast to Tang et al’s [18] pilot study which did not have a control group, the present study follows a control-intervention time-series design with subjects serving as their own controls. This study design allows us to compare the intervention condition with a control condition. In the first six months of the study (months 0 to 6), subjects participate in the “attention-control” period (See Figure 1). In the second six months of the study (months 7 to 12), subjects participate in the Lifelong Management intervention. We recruited African-American adults with type 2 diabetes living in the greater Ypsilanti, Michigan area via newspapers advertisement, flyers in local community centers, health centers, and organizations; and invited presentations at churches with a large African-American membership. An initial telephone screening assessed the following eligibility criteria: being 40 years or older, being diagnosed with type 2 diabetes for at least one year, having received some form of diabetes education in the past three years, and being under the care of a health care provider. Eligible participants attended an enrollment orientation where the researcher (T.S.T) discussed the purpose of the study and what participation involved. Those interested gave informed consent, provided a blood sample, completed a survey, and were reimbursed $50 for their time and effort.
Figure 1.
Control-Intervention Time-Series Study Design
2.2. Measures
Outcome variables
Metabolic and cardiovascular measures included A1C, lipids, blood pressure (SBP and DBP), weight, and BMI and were collected in person at assessment sessions. A1C and lipids (serum cholesterol, HDL, LDL) were obtained via venous puncture.
Diabetes-specific quality of life was measured by the Diabetes Distress Scale (DDS), a 17-item instrument that assesses emotional distress and functioning specific to living with diabetes [22]. Responses are scored on a 6-point Likert scale from “1” = no problem to “6” = serious problem. Scores can range from 17–102, with higher scores indicating poorer diabetes-specific quality of life.
Self-care behavior was assessed using items from the Summary of Diabetes Self-Care Activities Measure-revised [23]. Selected items assessed the frequency (over the past 7 days) of following a healthy diet, spacing out carbohydrates evenly across the day, eating high fat foods, participating in physical activity, monitoring blood glucose, inspecting feet, and taking medication and/or insulin. Responses, which are based on a 7-day week, ranged from 0 days to 7 days, with greater number of days reflecting better self-management.
Diabetes empowerment was measured by the Diabetes Empowerment Scale-Short Form, an 8-item scale assessing perceived ability to manage the psychosocial demands and challenges associated with diabetes [24]. The DES-SF is scored on a 5-point Likert scale with a higher mean score indicating greater diabetes empowerment.
Demographic items included age, race, marital status, education, income, insurance coverage, years since diagnosis, diabetes treatment, and perceived health status.
Immediately following each assessment, results of the A1C, weight, BMI, blood pressure, and lipids were sent to participants and their diabetes care provider. During the 6-month attention-control, we mailed participants a weekly newsletter (24 in total) focusing on different diabetes self-management topics. Updated newsletters from a previous study [25] addressed topics such as blood sugar testing, healthy eating, exercise, long-term complications, psychosocial and behavioral issues, etc. Following the 6-month control period, participants completed a follow-up assessment. Data collected post-attention-control period also served as pre-intervention period data (See Figure 1).
2.3. The Lifelong Management (LM) intervention
A detailed description of the LM intervention has been published in a previous paper by Tang and colleagues [18]. Briefly, the LM intervention is based on Anderson and Funnell’s [1–4] empowerment approach and consists of weekly sessions over a period of 24-months. This study uses data collected in the first 6-months of the intervention. Sessions were co-facilitated by a certified diabetes educator (M.M.F) and a clinical psychologist (T.S.T). Discussion was driven entirely by patients’ questions, concerns, and priorities. Although the content of sessions were patient-directed, the intervention involved 5 core processes: (1) reflecting on relevant self-management experiences, (2) discussing emotions and feelings, (3) engaging in problem-solving, (4) addressing questions about diabetes and its care, and (5) behavioral goal-setting. For instance, if a patient raised a concern about having a low blood sugar upon waking that morning, facilitators would encourage the patient to talk about how he/she felt when this situation occurred, initiate a group dialogue about possible causes of a low fasting blood sugar, discuss strategies to prevent and/or treat low blood sugars, and assist the patient in a process of problem-solving. To accommodate participants’ differing schedules, sessions were held in the morning and afternoon. Participants were invited to attend sessions as frequently as they felt they needed.
2.4. Statistical Analyses
Descriptive statistics were used to assess the demographic and diabetes care-related characteristics of the sample. Bivariate correlations were conducted to examine the relationship between frequency of attendance and demographic variables. For the purposes of examining the relationship between frequency of attendance and demographic variables, we recoded martial status to 1 = “currently married” and 2 = “not currently married”; education was recoded to 1 = “high school graduate/GED or less” and 2 = “some college for more”; income was recoded to 1 = “$0 – $19,999” and 2 = “$20,000 – $59,999”, 3 = “$60,000 or more”; employment status was recoded to 1 = “currently employed” and 2 = “not currently employed.”
Paired t-tests were performed to examine pre- post- changes associated with the control period and with the intervention period. To examine differences between the changes observed in the control period and the changes observed in the intervention period, we applied a paired t-test to the observed changes within each period (i.e., to the post-pre differences).
3. Results
3.1. Characteristics of the sample
Table 1 presents the demographics of the sample. At post-LM intervention, 12 participants had dropped out of the study yielding an attrition rate of 13% (below the expected rate of 20% per year). Participants were between the ages of 40 and 84 years with a mean of 61 years (SD = 10.4). Thirty-one percent (n=24) were men; 69% (n=53) were women. Forty-three percent were currently married (n=33). Thirty-one percent (n=24) had a high school degree or less; 18% (n=14) were currently employed; 56% had Medicare (n=43).
Table 1.
Characteristics of Sample (N=77)
| Variable | Mean ± SD | N | % |
|---|---|---|---|
| Age | 61.0 ± 10.4 | ||
| Years since diagnosis | 12.1 ±10.8 | ||
| Gender | |||
| Female | 53 | 69 | |
| Male | 24 | 31 | |
| Martial Status | |||
| Currently married | 33 | 43 | |
| Separated/Divorced | 21 | 27 | |
| Widowed | 11 | 14 | |
| Other | 12 | 16 | |
| Race | |||
| African American | 77 | 100 | |
| Education | |||
| 8 grades or less | 2 | 3 | |
| Some high school | 7 | 9 | |
| High school graduate or GED | 15 | 19 | |
| Some college or technical school | 39 | 51 | |
| College graduate or higher | 14 | 18 | |
| Household income | |||
| $0 – $9,999 | 16 | 21 | |
| $10,000 – $19,999 | 14 | 19 | |
| $20,000 – $29,999 | 14 | 19 | |
| $30,000 – $59,999 | 17 | 22 | |
| $60,000 or more | 14 | 19 | |
| Employment Status | |||
| Currently working | 14 | 18 | |
| Not currently working | 63 | 82 | |
| Insurance Coverage | |||
| Individual Plan | 5 | 7 | |
| Employee Plan | 38 | 49 | |
| U.S. Governmental Health Plan | 6 | 8 | |
| Medicare | 43 | 56 | |
| Medicaid | 14 | 18 | |
| No health insurance | 0 | 0 | |
| Diabetes treatment | |||
| Using insulin | 22 | 29 | |
| Taking pills | 58 | 76 | |
| Using Byetta | 3 | 4 | |
| Using Symlin | 0 | 0 | |
| Other medication | |||
| Taking cholesterol pills | 51 | 67 | |
| Taking blood pressure pills | 63 | 84 | |
| Perceived Health Status | |||
| Poor | 4 | 5 | |
| Fair | 29 | 38 | |
| Good | 36 | 47 | |
| Very good | 7 | 9 | |
| Excellent | 1 | 1 | |
3.2. Attendance patterns
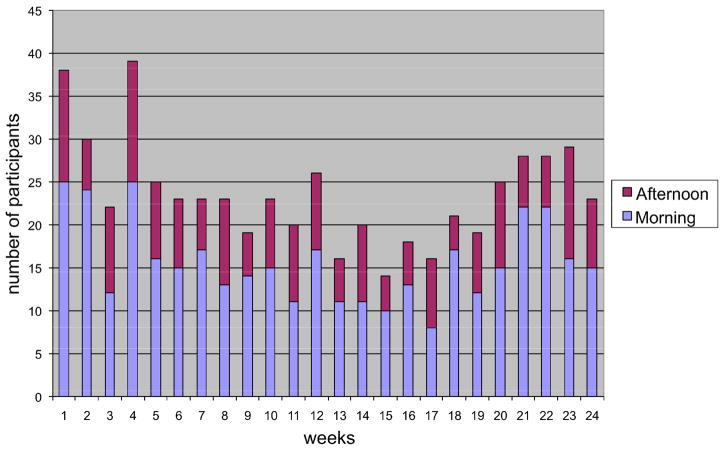
Figure 2 presents the weekly attendance rate during the 6-month intervention period. Two sessions (morning or afternoon) were offered each week over the 24-week period. The frequencies with which the participant chose to attend the morning or afternoon session are also presented in Figure 1. Over the 24 weeks, 1% (n=1) attended no weeks, 61% (n=47) attended 1 to 8 weeks, 23% (n=18) attended 9–16 weeks, and 15% (n=11) attended 17–24 weeks. Total attendance for any single week (2 sessions per week) ranged from 14–39 (m=22). Morning sessions attracted more participants with a mean attendance of 16 (SD = 4.8; range = 8 to 25); mean attendance for the afternoon sessions was 8 (SD= 2.8; range=4 to14). On average, 31% (n=24) of the total sample attended each week.
Figure 2.
Lifelong Management Attendance
There was a positive correlation between frequency of attendance and age (r=0.48, p<0.001). There was no relationship between frequency of attendance and other demographic variables, including gender, marital status, income, education, employment status, years since diagnosis, insurance status, or perceived health status.
3.3. Pre- and post- control period changes
Table 2 presents participants’ clinical, self-care, and psychosocial measures prior to and immediately following the 6-month control period. Significant improvements were found for serum cholesterol (167.3 vs. 151.1 mg/dL; p<0.001) and DBP (80.2 vs. 76.0 mm/Hg; p<0.05). Following the control period, participants had lower serum cholesterol levels and lower diastolic blood pressure readings. No significant changes were found for A1C, HDL, LDL, SBP, weight, or BMI.
Table 2.
Within- and Between- period changes in diabetes-related health outcomes
| Variable | Control Period | Intervention Period | Difference between Δ1 and Δ2 | p-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Pre | Posta | Post-Pre Δ1 | n | Pre | Posta | Post-Pre Δ2 | |||
| Clinical indices | ||||||||||
| A1C | 77 | 7.9±2.1 | 8.2±2.2 | 0.31±2.0 | 77 | 8.2±2.2 | 7.6±2.0 | −0.68±1.7† | −0.98±3.2 | 0.008 |
| Weight | 75 | 209.3±48.5 | 209.0±48.4 | −0.30±10.6 | 76 | 209.5±48.3 | 206.3±44.8 | −3.3±11.9* | −2.97±15.4 | 0.100 |
| BMI | 75 | 34.7±8.2 | 34.7±8.2 | −0.03±1.7 | 76 | 34.7±8.2 | 34.2±7.6 | −0.54±2.0* | −0.51±2.5 | 0.089 |
| BP-systolic | 77 | 137.2±17.7 | 137.6±17.3 | 0.39±16.7 | 76 | 137.2±17.0 | 133.6±17.5 | −3.63±19.2 | −3.83±29.8 | 0.265 |
| BP-diastolic | 77 | 80.2±10.6 | 76.0±11.1 | 4.27±15.1* | 77 | 76.0±11.1 | 76.9±10.8 | 0.97±11.8 | 5.25±23.1 | 0.054 |
| Serum cholesterol | 77 | 167.3±41.2 | 151.1±35.5 | 16.2±31.1‡ | 77 | 151.1±35.5 | 147.6±36.0 | −3.5±36.1 | 12.68±56.2 | 0.051 |
| HDL | 77 | 53.0±13.8 | 52.4±13.5 | −0.58±8.1 | 77 | 52.4±13.5 | 48.0±14.1 | −4.5.±9.6‡ | −3.90.±14.7 | 0.023 |
| LDL | 77 | 99.1±32.9 | 93.8±31.4 | −5.3±28.2 | 77 | 93.8±31.4 | 80.0±26.6 | −13.7±29.9‡ | −8.47±49.8 | 0.140 |
| Self-care behaviorsb | ||||||||||
| Following a healthy diet | 76 | 3.6±2.2 | 4.3±2.1 | 0.72±2.1† | 77 | 4.3±2.1 | 4.6±1.9 | 0.26±1.8 | −0.50±3.4 | 0.201 |
| Spacing carbohydrates | 73 | 3.5 ±2.7 | 3.4±2.6 | −0.08 ±2.3 | 76 | 3.5 ±2.5 | 3.9±2.4 | 0.47±2.7 | 0.59±4.2 | 0.234 |
| Exercising | 77 | 2.6±2.5 | 2.9±2.4 | 0.26±2.7 | 77 | 2.9±2.4 | 3.4±2.8 | 0.48±3.0 | 0.22±5.1 | 0.705 |
| Monitoring BG | 77 | 5.0±2.6 | 5.7±2.2 | 0.69±2.1† | 76 | 5.7±2.2 | 5.7±2.3 | 0.01±1.9 | −0.61±3.3 | 0.112 |
| Inspecting feet | 77 | 5.0±2.7 | 5.5±2.3 | 0.51±2.3 | 76 | 5.5±2.3 | 5.4±2.4 | −0.07±1.8 | −0.49±3.0 | 0.163 |
| Taking medication | 65 | 5.8±2.5 | 5.7±2.6 | −0.06±2.4 | 40 | 5.7±2.6 | 5.5±2.7 | −0.20±1.9 | −0.15±3.6 | 0.733 |
| Using insulin | 35 | 4.3±3.3 | 4.0±3.3 | −0.35±2.1 | 69 | 4.2±3.3 | 3.9±3.3 | −0.23±1.7 | 0.10±3.5 | 0.874 |
| Psychosocial indices | ||||||||||
| Quality of lifec | 69 | 32.7±16.7 | 30.8±15.8 | −1.97±10.2 | 72 | 30.8±15.7 | 30.5±15.6 | −0.38±13.5 | 1.48±19.2 | 0.531 |
| Empowermentd | 77 | 3.9±0.9 | 3.9±0.9 | 0.003±0.81 | 77 | 3.9±0.9 | 4.0±0.9 | 0.10±0.92 | 0.10±1.4 | 0.527 |
p < 0.05,
p < 0.01,
p < 0.001
Post-control and pre-intervention means may be slightly different as Post-Pre differences are based on complete pairs
Responses range from 0 to 7 days;
Responses range from 17 to 102 with higher scores indicating poorer diabetes-specific qualiy of life
Responses scored on a 5-point likert scale with “1” = strongly disagree to “5” = strongly agree. Due to a technical error, 1 of the original 8-item scale was dropped. A recalculation of the chronbach’s alpha indicate the reliability for the 7-items was excellent ranging from .85 to .90.
For self-care behaviors, significant improvements were found for healthy diet (3.6 vs. 4.3 days/wk; p<0.01) and blood glucose testing (5.0 vs 5.7 days/wk; p<0.01). Following the control period, participants reported a greater frequency of making healthy dietary choices and testing blood glucose. No changes were found for physical activity, medication and/or insulin use.
No changes were found for diabetes-specific quality of life and diabetes empowerment.
3.4. Pre- and post- intervention period changes
Table 2 presents participants’ clinical, self-care, and psychosocial measures prior to and immediately following the 6-month LM intervention. Modest improvements were found for glycemic control (8.2% vs. 7.6%; p<0.001), weight (209.5 vs. 206.3 lbs; p<0.05), BMI (34.7 vs. 34.2 kg/m2; p<0.05), and LDL (93.8 vs. 80 mg/dL; p<0.001). For HDL, a change in the undesired direction (52.4 vs. 48 mg/dL; p<0.001) was found.
No changes were found for self-care behaviors, diabetes-specific quality of life and diabetes empowerment.
3.5. Differences between the control period changes and the intervention period changes
Table 2 presents the differences between the control period and the intervention period. Compared to the control period, participation in the LM intervention was associated with a significant improvement in glycemic control (Δ0.31 vs. Δ−0.68; p<0.01). Compared to the LM intervention, participation in the control period was associated with significant improvement in HDL (Δ−0.58 vs. Δ−4.5; p<0.05).
4. Discussion and conclusion
4.1. Discussion
The Lifelong Management (LM) intervention was designed to support patients’ efforts in achieving and sustaining self-management goals in a “real-world” setting. Specifically, the LM intervention followed an empowerment-based approach that was patient-driven and flexible to individual needs, priorities, and life circumstances. This study examined the diabetes-related health impact of the first 6-months of the LM intervention on clinical, self-care, and psychosocial outcomes compared to a 6-month attention-control period with participants serving as their own controls.
While the attention-control condition (i.e., the mailed intervention) was not originally designed as an active intervention, it appeared to serve as a low-intensity intervention that led to modest improvements in diabetes-related health outcomes that were sustained and or further improved during the LM intervention. Following the first 6-months of the attention-control period, participants made significant improvements in DBP and serum cholesterol. They also reported a higher frequency of following a healthy diet, and monitoring blood glucose.
In addition to classic study effects such as volunteer bias and greater motivation at the start of a behavioral intervention, another possible explanation for the health gains associated with the attention-control period is the quality and quantity of “attention” participants received. Immediately following the baseline assessment, clinical results (e.g., A1C, blood pressure, lipid panel) were sent to participants and their diabetes care providers. The feedback given to patients included an explanation of the measure, their results, desired target range, and specific behavioral strategies that could influence the results (e.g., eat less saturated fats). Although we consider this type of feedback to be a standard of care that all patients with diabetes should receive, it is possible that some participants were not receiving this minimal level of care prior to participation in the study. For this reason, the clinical feedback could have prompted providers to make treatment adjustments and/or participants to initiate health-promoting practices. Research has shown that feedback can function as a cue that stimulates behavior change [26–27].
For instance, reductions in DBP and serum cholesterol could be attributed to treatment intensification on the part of providers. In fact, a greater percentage of patients reported taking blood pressure (pre-control 85% vs. post-control 89%) and cholesterol medication (pre-control 66% vs. post-control 72%) at the end of the control period compared to baseline. Consistent with this explanation, Hiss and colleagues [28] found that, among patients whose clinical values were above normal range, clinical feedback to physicians was associated with improvements in glycemic control, blood pressure and cholesterol at 1-year follow-up.
In addition to clinical feedback, we also mailed weekly newsletters on core self-management topics. These newsletters provided diabetes information, addressed psychosocial concerns, and included strategies to improve self-management practices to an already motivated group. Six out of the 24 newsletters addressed healthy eating and/or blood sugar testing. Consequently, weekly reminders of self-care behaviors coupled with clinical feedback may have contributed to the positive changes patients reported in dietary patterns and blood sugar monitoring. In fact, a study by Anderson and colleagues found that providing diabetes education newsletters on a weekly basis activated behavior change in diet, exercise, glucose monitoring, and weight loss [25].
Similar to the attention-control period, participation in the LM intervention was also associated with modest improvements in health outcomes including glycemic control, weight, BMI, and LDL. Compared to the control period, the LM intervention led to a significant reduction in A1C.
In terms of psychosocial outcomes, there were no changes in diabetes-specific quality of life associated with the control or intervention period. It is important to note that, at baseline, the group, on average reported a highly positive diabetes-specific quality of life (mean=32.7, sd=16.7). On a 6-point likert scale with scores ranging from 17 – 102 (lower scores indicate higher quality of life), a baseline mean under 33 leaves a very narrow range for improvement. Therefore, the lack of change in this variable could be partially due to a floor effect.
We also found no within- or between- period differences in diabetes empowerment. This finding is inconsistent with Anderson and colleague’s [6] study that indicated improvements in diabetes empowerment for the control group and empowerment-based intervention group. While this study and Anderson et al.’s [6] study used a similar empowerment-based approach to self-management, there were some distinct differences. Anderson et al.’s [6] intervention consisted of 6 self-management education sessions conducted over a 6-week period with the expectation that participants would attend all 6 sessions. In contrast, the LM intervention consisted of 24 weekly self-management support sessions conducted over a period of 6-months in which participants were encouraged to attend sessions as frequently as needed to obtain support and/or education. It is possible that improvements in diabetes empowerment are most prominent in the short-term, particularly with concentrated and frequent exposure to empowerment-based support. It is equally plausible that this intervention had no impact on diabetes empowerment.
Contrary to other self-management programs, the LM intervention was specifically designed to accommodate individuals with type 2 diabetes living under “real-life” conditions. In other words, given the variability in participants’ support needs, priorities, responsibilities, coping styles, etc., we did not expect the attendance rate to be 100%. In fact, patients were informed that while the sessions would be available weekly, they were invited to attend only as often as they felt it would be helpful for them. Based on our goal to attract at least 15% to 20% (n=12 to 15 participants) of the sample each week, we met our expectation for morning sessions (m=21%; n=16 participants) and fell below our expectation for afternoon sessions (m=10%; n=8 participants). Overall, attendance remained relatively stable over the course of 24 sessions.
It should be noted that 5 out of 48 sessions (10% of total morning and afternoon sessions) attracted a group size of 20–25 participants. While we recognize that a group of this size may not be ideal, our experience was that all participants who came with specific questions or issues were able to discuss them either in the session or afterwards with the facilitators. Our primary goal was to develop and evaluate an innovative model for providing ongoing self-management support that is flexible and can accommodate a large number of participants with different support needs and attendance patterns. Indeed, a disadvantage of this type of intervention is the small chance that sessions may fill over the optimal capacity. Therefore, when implementing this intervention in the future, it would be beneficial to consider adding an additional weekly session contingent on total group size.
Similar to our previous study [18], the frequency and patterns of attendance varied across individuals. Some participants attended weekly, some attended monthly, and others attended less consistently. Given that frequency of attendance was not related to diabetes-related health outcomes, a possible interpretation is that people, indeed, have the ability to accurately assess the level of support they need and seek this support accordingly.
Several limitations to this study need to be acknowledged. Due to funding constraints, we were unable to use a randomized controlled trial (RCT) design for this study. Instead, we employed a control-intervention time-series design with participants serving as their own controls. Findings suggest that our attention-control condition served as an active intervention that produced improvements, thereby diluting potential control-intervention differences. Moreover, study effects such as greater enthusiasm at the start of a behavioral intervention may have further enhanced the improvements made in the first 6 months of the study (i.e., attention-control condition). These methodological issues underscore the importance of designing the optimal control group particularly for studies where an RCT design is not financially feasible.
As mentioned previously, treatment intensification triggered by clinical feedback may have contributed to improvements associated with the control condition. However, we did not collect detailed documentation of treatment changes (e.g., type of medication or dosage) during the 6-month attention-control period. Therefore, the unique influence of provider behavior cannot be examined. Future investigations should conduct medical chart reviews to monitor and track provider-initiated treatment changes particularly in response to clinical feedback. It is possible that clinical feedback can serve as an effective intervention on its own. In summary, this program complemented and enhanced the treatment these patients were receiving and resulted in modest metabolic improvements.
Alternatively, this study could be conceived as testing an intervention consisting of two phases with phase one: a low-intensity, mailed education and clinical feedback intervention followed by phase two: a high-intensity empowerment-based, weekly self-management support intervention conducted in a group setting. With this perspective, the findings would suggest that participation in a low-intensity education and feedback intervention is associated with improvements in some diabetes-related clinical (e.g., DBP, serum cholesterol) and behavioral (e.g., following a healthy diet, monitoring blood sugar) outcomes with additional clinical improvements (e.g., A1C, weight, BMI, LDL) when followed by a high-intensity, empowerment-based self-management support intervention.
4.2. Conclusion
Findings from this study suggest that an empowerment-based approach to diabetes self-management support is promising for improving and/or sustaining diabetes-related health outcomes, particularly glycemic control. Using a control-intervention time-series design may have diminished the actual impact of the LM intervention. For this reason, future investigations should employ a randomized controlled trial (RCT) design comparing the LM intervention to a “usual care” control group. With an RCT, the presence of any study effects would have an equal impact on both the control and intervention groups. When working with low-resource and disadvantaged patient populations, alternative designs including a wait-list RCT and crossover study should also be considered.
4.3. Practical implications
Given that diabetes disproportionately affects certain ethnic groups who are often medically underserved, neither health professionals nor their patients have the luxury of working and living in an “optimal” environment for sustaining self-management over the long-term. The empowerment approach to self-management support embodies basic principles that accommodate the unique differences every person brings to the experience of living with diabetes. Ultimately, the goal is to design interventions that are intended to be ongoing, patient-driven, and flexible to the dynamic and evolving conditions of patients’ “real-world” environment and life circumstances.
Acknowledgments
This study was supported by a K23 patient-oriented career development award from National Institutes of Health, K23 DK068375, National Institutes of Diabetes and Digestive and Kidney Diseases, and by a grant NIH P60 DK20572 from the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anderson RM, Funnell MM. Patient empowerment: reflections on the challenge of fostering the adoption of a new paradigm. Patient Educ Couns. 2005;57:153–7. doi: 10.1016/j.pec.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Funnell MM, Anderson RM. Empowerment and self-management of diabetes. Clin Diabetes. 2004;22:123–127. [Google Scholar]
- 3.Funnell MM, Anderson RM. Patient empowerment: A look back, a look ahead. Diabetes Educ. 2003;29:454–464. doi: 10.1177/014572170302900310. [DOI] [PubMed] [Google Scholar]
- 4.Anderson RM, Funnell MM. Using the empowerment approach to help patients change behavior. In: Anderson Barbara J, Rubin Richard R., editors. Practical Psychology for Diabetes Educators. 2. American Diabetes Association; 2002. [Google Scholar]
- 5.Funnell MM, Nwankwo, Gillard ML, Anderson RM, Tang TS. Implementing an empowerment-based diabetes self-management education program. Diabetes Educ. 2005;31:53–61. doi: 10.1177/0145721704273166. [DOI] [PubMed] [Google Scholar]
- 6.Greene C, McClellan L, Gardner T, Larson CO. Diabetes management among low-income African Americans. A description of a pilot strategy for empowerment. J Ambul Care Manage. 2006;29:162–66. doi: 10.1097/00004479-200604000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Glimer TP, Philis-Tsimikas A, Walker C. Outcomes of Project Dulce: A culturally specific diabetes management program. Ann Pharmacother. 2005;39:817–22. doi: 10.1345/aph.1E583. [DOI] [PubMed] [Google Scholar]
- 8.Deakin TA, Cade E, Williams R, Greenwood C. Structured patient education: The Diabetes X-pert Programme makes a difference. Diabet Med. 2006;23:944–954. doi: 10.1111/j.1464-5491.2006.01906.x. [DOI] [PubMed] [Google Scholar]
- 9.Keers JC, Blaauwwiekel EE, Hania M, Bouma J, Scholten-Jaegers SMHJ, Sanderman R, Links TP. Diabetes rehabilitation: Development and first results of a Multidisciplinary Intensive Education Program for patients with prolonged self-management difficulties. Patient Educ Couns. 2004;52:151–7. doi: 10.1016/s0738-3991(03)00019-3. [DOI] [PubMed] [Google Scholar]
- 10.Keers JC, Bouma J, Links TP, Maaten JC, Gans ROB, Wolffenbuttel BHR, Sanderman R. One-year follow-up effects of diabetes rehabilitation for patients with prolonged self-management difficulties. Patient Educ Couns. 2006;60:16–23. doi: 10.1016/j.pec.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 11.Siminerio LM, Piatt G, Zgibor JC. Implementing the chronic care model for improvements in diabetes care and education in a rural primary care practice. Diabetes Educ. 2005;31:225–234. doi: 10.1177/0145721705275325. [DOI] [PubMed] [Google Scholar]
- 12.Phili-Tsimikas A, Walker C, Rivard L, Talavera G, Reimann JOF, Salmon M, Araugo R. Improvement in diabetes care of underinsured patients enrolled in Project Dulce: A community-based, culturally appropriate, nurse case management and peer education diabetes care model. Diabetes Care. 2004;27:110–11. doi: 10.2337/diacare.27.1.110. [DOI] [PubMed] [Google Scholar]
- 13.Anderson RM, Funnell MM, Butler PM, Arnold MS, Fitzgerald JT, Feste CC. Patient empowerment: Results of a randomized controlled trial. Diabetes Care. 1995;18:943–9. doi: 10.2337/diacare.18.7.943. [DOI] [PubMed] [Google Scholar]
- 14.Pibernik-Okanovic M, Prasek M, Poljicanin-Filipovic T, Pavlic-Renar I, Metelko Z. Effects of an empowerment-based psychosocial intervention on quality of life and metabolic control in type 2 diabetic patients. Patient Educ Couns. 2004;52:193–199. doi: 10.1016/s0738-3991(03)00038-7. [DOI] [PubMed] [Google Scholar]
- 15.Adolfsson ET, Walker-Engstrom ML, Smide B, Wikblad Patient education in type 2 diabetes A randomized controlled 1-year follow-up study. Diabetes Res Clin Prac. 2007;76:341–350. doi: 10.1016/j.diabres.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 16.Mayer-Davis EI, D’Antonio AM, Smith SM, Kirkner G, Martin SL, Parra-Medina D, Schultz R. Pounds off with empowerment (POWER): A clinical trial of weight management strategies for Black and White adults with diabetes who live in medically underserved rural communities. Am J Public Health. 2004;94:1736–1742. doi: 10.2105/ajph.94.10.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forlani G, Zannoni C, Tarrini G, Melchionda N, Marchesini G. An empowerment-based educational program improves psychological well-being and health-related quality of life in Type 1 diabetes. J Endocrinol Invest. 2006;29:405–412. doi: 10.1007/BF03344123. [DOI] [PubMed] [Google Scholar]
- 18.Tang TS, Gillard ML, Funnell MM, Nwankwo R, Parker E, Spurlock D, Anderson RM. Developing a new generation of ongoing diabetes self-management support interventions: A preliminary report. Diabetes Educ. 2005;31:91–97. doi: 10.1177/0145721704273231. [DOI] [PubMed] [Google Scholar]
- 19.Anderson RM, Funnell MM, Nwankwo R, Gillard ML, Oh M, Fitzgerald JT. Evaluating a problem-based empowerment program for African Americans with diabetes: Results of a randomized controlled trial. Ethn & Dis. 2005;15:671–678. [PubMed] [Google Scholar]
- 20.Sarkisian C, Brusuelas RJ, Steers WN, Davidson MB, Brown AF, Norris KC, Anderson RM, Mangione CM. Using focus groups of older African Americans and Latinos with diabetes to modify a self-care empowerment intervention. Ethn & Dis. 2005;15:283–291. [PubMed] [Google Scholar]
- 21.Bootzin RR. The role of expectancy in behavior change. In: White L, Turskey B, Schwartz G, editors. Placebo: Theory, research, and mechanisms. New York: Guilford Press; 1982. pp. 196–210. [Google Scholar]
- 22.Polonsky WH, Fisher L, Earles J, Dudl RJ, Lees J, Mullan J, Jackson RA. Assessing psychosocial distress in diabetes: Development of the Diabetes Distress Scale. Diabetes Care. 2005;28:626–631. doi: 10.2337/diacare.28.3.626. [DOI] [PubMed] [Google Scholar]
- 23.Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: Results from 7 studies and a revised scale. Diabetes Care. 2000;23:943–50. doi: 10.2337/diacare.23.7.943. [DOI] [PubMed] [Google Scholar]
- 24.Anderson RM, Fitzgerald JT, Gruppen LD, Funnell MM, Oh MS. The Diabetes Empowerment Scale-Short Form (DES-SF) (Letter) Diabetes Care. 2003;26:1641–1642. doi: 10.2337/diacare.26.5.1641-a. [DOI] [PubMed] [Google Scholar]
- 25.Anderson RM, Fitzgerald JT, Funnell MM, Barr PA, Stepien CJ, Hiss RG, Armbruster BA. Evaluation of an activated patient diabetes education newsletter. Diabetes Educ. 1994;20:29–34. doi: 10.1177/014572179402000106. [DOI] [PubMed] [Google Scholar]
- 26.DiClemente CC, Marinilli AS, Singh M, Bellino LE. The role of feedback in the process of health behavior change. Amer J Health Behav. 2001;25:217–227. doi: 10.5993/ajhb.25.3.8. [DOI] [PubMed] [Google Scholar]
- 27.Becker H, Roberts G, Voelmeck W. Explanations for improvement in both experimental and control groups. Western J Nursing Res. 2003;25:746–755. doi: 10.1177/0193945903253002. [DOI] [PubMed] [Google Scholar]
- 28.Hiss RG, Gillard ML, Armbruster BA, McClure LA. Comprehensive evaluation of community-based diabetic patients. Effect of feedback to patients and their physicians: a randomized controlled trial. Diabetes Care. 2001;24:690–694. doi: 10.2337/diacare.24.4.690. [DOI] [PubMed] [Google Scholar]