Abstract
Telomerase activity plays an essential role in cel0l survival, by lengthening telomeres and promoting cell growth and longevity. It is now possible to quantify the low levels of telomerase activity in human leukocytes. Low basal telomerase activity has been related to chronic stress in people and to chronic glucocorticoid exposure in vitro. Here we test whether leukocyte telomerase activity changes under acute psychological stress. We exposed 44 elderly women, including 22 high stress dementia caregivers and 22 matched low stress controls, to a brief laboratory psychological stressor, while examining changes in telomerase activity of peripheral blood mononuclear cells (PBMC). At baseline, caregivers had lower telomerase activity levels than controls, but during stress telomerase activity increased similarly in both groups. Across the entire sample, subsequent telomerase activity increased by 18% one hour after the end of the stressor (p<0.01). The increase in telomerase activity was independent of changes in numbers or percentages of monocytes, lymphocytes, and specific T cell types, although we cannot fully rule out some potential contribution from immune cell redistribution in the change in telomerase activity. Telomerase activity increases were associated with greater cortisol increases in response to the stressor. Lastly, psychological response to the tasks (greater threat perception) was also related to greater telomerase activity increases in controls. These findings uncover novel relationships of dynamic telomerase activity with exposure to an acute stressor, and with two classic aspects of the stress response -- perceived psychological stress and neuroendocrine (cortisol) responses to the stressor.
Keywords: stress, telomerase activity, cortisol, caregiving, immune cell trafficking
INTRODUCTION
The successful maintenance of telomeres, the protective caps at the ends of chromosomes, is critical to human health. Normal telomere maintenance requires the cellular enzyme telomerase. Telomerase is a cellular ribonucleoprotein reverse transcriptase enzyme that adds telomeric DNA to shortened telomeres, thus extending telomere length and protecting the chromosomes. Shortened telomeres and lower telomerase are linked to age-related risk factors and disease (Aviv et al., 2006; Benetos et al., 2001; Brouilette, Singh, Thompson, Goodall, & Samani, 2003; Gardner et al., 2005; Jeanclos et al., 2000; Nawrot, Staessen, Gardner, & Aviv, 2004; Samani, Boultby, Butler, Thompson, & Goodall, 2001; Sampson, Winterbone, Hughes, Dozio, & Hughes, 2006; Valdes et al., 2005) and several studies report that short telomeres predict early mortality (Bakaysa et al., 2007; Cawthon, Smith, O'Brien, Sivatchenko, & Kerber, 2003; Honig, Schupf, Lee, Tang, & Mayeux, 2006; Kimura et al., 2008; Lin et al., 2009; Martin-Ruiz et al., 2006). These findings highlight the importance of understanding how telomerase regulates telomere length in vivo.
Several studies have now shown that chronic life stress is linked to shorter telomeres (Damjanovic et al., 2007; Epel et al., 2004; Parks et al., 2009), and has been related to both dampened telomerase activity (Epel et al., 2004) and, paradoxically, elevated telomerase activity (Damjanovic et al., 2007). The cause of these different findings on telomerase activity under stress is unclear. While telomeres are thought to change slowly over time, telomerase is a dynamic enzyme that can increase quickly, and it is thus possible to examine how acute stress may impact telomerase activity in an experimental setting. In addition to protecting telomeres, telomerase has telomere-independent roles, important to cell survival in the face of physiological stress, and it could potentially be affected by acute psychological stress as well. In this study, we addressed whether acute psychological stress impacts telomerase activity, and whether this differs in individuals with high and low levels of chronic stress.
Telomerase activity is under multiple modes of dynamic control. For example, upon mitogenic stimulation of resting immune T lymphocytes, telomerase activity is upregulated at least 18 fold three days later (Hathcock, Chiang, & Hodes, 2005). One study assessed the telomerase activity increase after antigen stimulation of B lymphocytes, and found it was elevated as early as 12 hours later (Igarashi & Sakaguchi, 1997). After phytohemagglutinin stimulation, telomerase in T cells becomes elevated within 2 days, potentially in the G1 phase (when cells are preparing for DNA replication) of even the first cell cycle the ensuing cell cycles associated with clonal proliferation (Buchkovich & Greider, 1996). It is unclear if telomerase changed earlier than these time periods, since immediate changes were not studied.
Acute psychological stress can promote increases in cortisol, catecholamines, and oxidative stress (Gidron, Russ, Tissarchondou, & Warner, 2006), factors that may regulate telomerase activity. For example, exposure to cortisol in vitro dampens telomerase activity three days later (Choi, Fauce, & Effros, 2008). UV irradiation of lens epithelial cells in vitro increases stress proteins as well as telomerase activity in a dose dependent fashion (Wu & Zhang, 2005), indicating that telomerase can change acutely. The greater the level of oxidative stress in cells in vitro, the greater the level of telomerase activity (Nishikawa et al., 2009). Under high oxidative stress (H2O2 exposure), telomerase translocates from the nucleus to the mitochondria and telomerase activity increases within 3 hours (Saretzki, 2009). Although little is known about the physiological significance of this observation, it has been suggested that the telomerase response to oxidative stress may be cell-protective, rather than causing cell proliferation, at least in lens epithelial cells (Colitz, Whittington, Carter, & Warren, 2004).Little is known about the kinetics of telomerase regulation in vivo, and whether telomerase activity might upregulate in response to acute stress. While changes over days may be due in part to reduction in transcription of hTERT (Choi et al., 2008) it is possible that through rapid post-translational changes, telomerase activity can upregulate within minutes.
Acute psychological stress affects the immune system in complex ways (Segerstrom & Miller, 2004), including changing the distribution of cell types in the circulation vs. in tissue (F. Dhabhar & McEwen, 1997). During acute stress, immune cells mobilize into and then traffic out of the bloodstream (F. Dhabhar & McEwen, 1997; F. Dhabhar, Miller, McEwen, & Spencer, 1996; F. S. Dhabhar, 1998; Schedlowski et al., 1993). In contrast, chronic stress impairs such acute stress-induced immune cell redistribution, and suppresses and/or dysregulates immune function (Dhabhar & McEwen, 1997; Dhabhar, 2009; Dhabhar & McEwen, 2001). Given the known changes in immune cell composition in the blood during acute stress, such as the increases in lymphocytes and monocytes, and that telomerase activity levels differ depending on immune cell type (Lin et al., 2009), with B cells having the highest and CD8 cells having the lowest telomerase activity, it is possible that cell trafficking patterns might contribute to any observed changes in telomerase activity in circulating PBMCs during stress. Therefore, to investigate the sources of possible changes in telomerase activity during acute stress, it is important to concurrently measure changes in cell distribution during acute stress.
Our goal here was to test whether acute psychological stress affects PBMC telomerase activity levels, and whether this differs as a function of chronic stress exposure. We exposed healthy women who had high versus low levels of chronic stress to a standardized short-term laboratory stressor. We measured changes in their PBMC telomerase activity throughout the stressor, as well as two classic aspects of the human stress response: cortisol reactivity and psychological threat responses during the stressor. As described above, stress-induced redistributions of immune cells might cause changes in cell subsets present in peripheral blood during stress, which could impact the levels of telomerase activity as measured in the total PBMC population. Therefore, at each of the four blood draw points we also measured immune cell distributions using flow cytometry in a subset of 27 participants.
METHODS
Recruitment
The sample was recruited in the San Francisco Bay Area, from the community and service centers for caregivers, such as support groups, neurology clinics, and adult daycare centers, as well as from notices in public places and electronic bulletin boards. The study requested healthy nonsmoking women. The caregivers were the primary caregiver for a husband or partner with dementia (typically Alzheimer’s disease or frontal-temporo-lobe dementia). They had to identify as the primary caregiver, providing at least 4 hours of care a day.
The study was approved by the UCSF Human Subjects Committee. Each participant provided written informed consent. The sample is part of a larger sample of caregivers and controls that were enrolled at baseline, and completed health and psychological assessments. The sample that performed the lab stressor and had cells collected for telomerase activity includes 47 subjects in two groups: 24 caregivers and 23 controls. However, three participants had only baseline telomerase activity measured for the Trier Social Stress Test (one control and one caregiver declined to continue with the Stress Test tasks, and one caregiver reported getting a headache and so the session was ended). The other participants from the parent study did not have cells collected during the stress test, or in a few cases, did not sign up for the Stress Test for various reasons, including participant preference, as well as various health history reasons (poorly controlled hypertension, history of detached retina, and history of trauma exposure).
Eligibility criteria
Women had to be within the ages of 50 to 80, postmenopausal, without major or unstable medical conditions, endocrine disorders, or confounding medications (corticosteroid-containing). Antidepressant use was allowed in the caregiving group. Participants were screened to be healthy by self report and then by a physical exam by the study physician (L.K.), as well pass a chemistry screening for normal glucose, electrolytes, thyroid function and liver and kidney function. To recruit low stress controls, potential controls were excluded if they scored above the national mean score (12) on the Perceived Stress Scale (Cohen, Kamarck, & Mermelstein, 1983). Conversely, caregivers were included only if they scored above the mean on perceived stress.
Procedures
Women who called or emailed their interest were then screened for eligibility criteria by telephone. They had a physical exam, fasting blood draw, and provided written consent at the UCSF CCRC (CTSI Clinical Research Center). They were scheduled to return on a separate afternoon a week later, for the stress test. On this day, they ate a standardized lunch provided by the CCRC metabolic kitchen, and had an intravenous forearm catheter inserted around 1:00 pm. Participants had a one hour resting baseline period, while listening to relaxing music using headphones, after catheter insertion. At the end of this baseline period (Time 0), the baseline blood draw was performed. A modified form of the Trier Social Stress Test (TSST) (Kirschbaum, Pirke, & Hellhammer, 1993) was administered. Women were asked to perform a speech and math task. The phases of the stressor included four five-minute stressful periods (20 minutes total), including introduction to two trained stony-faced (expressionless) evaluators for description of the tasks, a quiet preparatory period for the speech, a speech (about strengths and weaknesses, rather than job interview, to fit the age group which includes many retirees), and lastly, a math task (subtraction of consecutive numbers).
Saliva and blood sample collections
Blood draws for leukocytes were performed through the indwelling catheter at baseline, 20 minutes, 50 minutes, and 90 minutes after the onset of the stressor (60 minutes after the cessation of the stressor). After each blood draw, PBMCs were immediately separated using density gradient centrifugation (Ficoll) and cryopreserved in a liquid nitrogen tank until they were assayed. A separate blood sample for each blood draw was processed immediately, so that no sample was left sitting longer than others. Lastly, one tube of fresh whole blood was same-day couriered to Stanford University (Dr. Dhabhar’s laboratory) for flow cytometric assessment of circulating leukocytes.
Saliva samples were collected via passive drool method, using IBL polypropylene saliva tubes at six time points throughout, for assessment of cortisol. Samples were collected at the following times in minutes: 0 (baseline), 15 (after the speech task), 20 (after the stressor ended), 30 (to capture cortisol peak), 50 (short term recovery) and 90 minutes (long term recovery, which is 70 minutes after the stressor ended, when cortisol is typically back to baseline levels) (Kirschbaum et al., 1993).
Saliva samples were kept on ice and frozen at the end of each session, and sent for batch assay to Dresden, Germany (lab of Clemens Kirschbaum). Salivary cortisol was assayed using a chemiluminescence immunoassay (CLIA). Intra-assay CV was 2.9% for high levels and 7.7 % for low levels. The inter-assay CV was 5.7% % for high levels and 9.1% for low levels. The sensitivity lower limit was 0.16 ng/ml.
Physiological measures
Telomerase activity
Telomerase activity was assayed by the Telomerase Repeat Amplification Protocol (TRAP) using a commercial kit (TRAPeze, Telomerase Detection Kit, Upstate/CHEMICON, Temecula, CA). This assay measures telomerase enzymatic activity, not amount of telomerase (hTERT) protein. Cryopreserved PBMCs were thawed by incubating the tubes at 37°C for 2 minutes and washed twice in 10 ml of cold DPBS (PBS without Mg++ and Ca++; Invitrogen, Carlsbad, CA, USA). Cells were pelleted by centrifugation in a Sorvall Legend RT tabletop centrifuge (Thermo Fisher Scientific, Waltham, MA, USA) and resuspended in 1 ml of DPBS. Live cells were counted with a hemocytometer (Bright-Line hemocytometer, Reichert, Buffalo, NY, USA) by use of Trypan blue (Invitrogen) exclusion criteria. One million live cells were pelleted and lysed with 1×CHAPS [3-(3-cholamidopropyl) dimethylammonio-1-propanesulfonate] buffer as directed by the manual for the TRAPeze kit. For each PBMC sample, an extract corresponding to 5000 cells/µL was made and two concentrations, corresponding to 5000 and 10,000 cells, were assayed for each sample to ensure the assay was in the linear range. The reaction was done according to the manual for TRAPeze kit and radioactive products fractionated by 10% polyacrylamide-8M urea sequencing gel electrophoresis (National Diagnostics, Atlanta, GA, USA). The gel was exposed to a phosphorimager plate overnight and scanned on STORM 860 (GE Healthcare). As positive control standards, 293T human cancer cells were used and telomerase activity was expressed as the equivalent of number of 293T cells. Telomerase activity was quantified by use of ImageQuant 5.2 software (GE Healthcare) as published (Ornish et al., 2008).
Flow cytometry
Flow cytometry was used to quantify major leukocyte subpopulations in order to detect cell trafficking patterns, via direct staining of whole fresh blood. Whole blood from the same blood draw collection that was used for telomerase activity at each point, was collected into sodium heparin tubes, and maintained at room temperature until it was stained. White blood cell counts were obtained on a cell counter (Beckman Coulter, Miami, FL). Specific leukocyte subtypes were identified by staining with directly conjugated monoclonal antibodies followed by flow cytometric analyses (FACSCalibur, Becton Dickinson, San Jose, CA). Briefly, whole blood was stained using antibodies conjugated with fluorescein isothiocyanate (FITC), phycoerythrin (PE), phycoerythrin-cyanine-5 (Pe-Cy5), or allophycocyanin (APC) (BD Pharmingen, San Diego, CA). The specific leukocyte subtypes were identified using the following antibody clones: CD3 (clone UCHT1), CD4 (clone RPA-T4), CD8 (clone RPA-T8),CD19 (clone HIB19), CD16 (Clone 3G8) and CD56 (clone B159). Cell suspensions were incubated with antibody for 20 min at room temperature, lysed with FACS Brand Lysing Solution (Becton Dickinson, San Jose, CA), resulting in simultaneous lysis of red blood cells and fixation of leukocytes. Cells were washed with PBS after lysis and read on the FACSCalibur with approximately 5,000 events being acquired from each preparation. Forward versus side scatter characteristics were used to identify lymphocyte, monocyte and neutrophil subpopulations. Matched antibody isotype controls were used to set negative staining criteria. Data were analyzed using Cell Quest-pro software (Becton Dickinson, San Jose, CA).
Psychological Measures
Threat and challenge responses to the stressor
Psychological responses (threat and challenge appraisals) to the stressor were measured immediately before onset of the stressor and after the stressor ended.
We assessed threat appraisals, which measure the perception that demands of the situation outweigh personal resources, and challenge appraisals, which reflect perceptions that one can cope successfully). These appraisals are derived from coping theory (Folkman & Lazarus, 1985) based measures that predict cardiovascular reactivity (Tomaka, 1997). Ratings for cognitive threat measures ranged from – 4 (strongly disagree) to + 4 (strongly agree). The items for threat asked how much the upcoming tasks would be “very demanding,” and “very stressful,” and how much “the upcoming situation is very uncertain,” (Cronbach’s α = .83). Emotions reflecting threat included state ratings of how much the subject felt “anxious,” “fearful,” and “worried” (Cronbach’s α = .83). The cognitive and emotional threat subscales were converted into z scores and the mean was taken as a measure of both cognitive and emotional components of a threat response. The same questions were asked immediately post stressor, in past tense, to assess retrospective evaluations of level of threat experienced.
Psychological profiles of challenge were measured in a similar way as threat. Cognitive challenge was measured with the items that assessed how much one feels they will be ‘able to complete the tasks successfully,” and “have control over how I do”. The subjects also rated how much they felt “I’m the kind of person who does well in these types of situations,” and “The evaluators will rate my performance well.” (Cronbach’s α = .83). Emotional challenge was assessed by ratings of how much they felt hopeful, confident, eager, and excited (Cronbach’s α = .81). Thus, the measures included one scale for challenge (cognitive and emotional), and one scale for threat (cognitive and emotional), and both were assessed pre- and post-stressor. We analyzed pre- and post- challenge and threat appraisals separately. Since people often feel both aspects of challenge and threat, we created a measure of threat relative to challenge, by subtracting the z score of challenge from the z score of threat. This measures the relative weighting of threat to challenge.
Perceived Stress over the past month was measured using the 10-item Cohen Perceived Stress Scale, which includes ratings of feeling overwhelmed, out of control, and stressed, over the last month, and has been extensively validated (S. Cohen & Williamson, 1988)
Depressive Symptoms were assessed using the Inventory for Depressive Symptomatology–Self Rated (IDS-SR). The self-reported IDS rates the severity of depressive symptoms and is highly correlated with the Hamilton Rating Scale for Depression (Rush, 1996).
Statistical analyses
Data preparation
Distributions of telomerase activity and cortisol were generally right skewed, so we computed the natural log for telomerase and cortisol. All values in statistical analyses are logged unless otherwise noted.
Computation of cortisol and telomerase changes
Examination of group means showed that as expected cortisol reached peak levels 30 minutes after onset of the stressor. Cortisol reactivity was defined as the change from baseline to peak response, and secondarily we examined early change (15 minutes after onset of stress For telomerase activity, there was a linear increase across the 90 minute period, so telomerase responsivity was assessed as both change from baseline to 90 minutes, as well as indices of cumulative telomerase secreted using AUC. We computed total Area Under the Curve (AUC), and reactive AUC (rAUC), the latter using the sum of trapezoidal areas-- multiplying change in time and the mean value for each variable across the corresponding time points, and subtracting the trapezoidal area for baseline. We note that while AUC is a cumulative measure and not a reactivity measure per se, it can be important to include in cases when baseline (pre-stressor) levels of a parameter might be affected by anticipatory psychological stress. This is relevant in this case since for controls high threat at baseline was related to high baseline telomerase activity, described below (Table 3). We also examined magnitude of telomerase at 90 minutes, the distal post stress response, as a secondary measure of responsivity.
Table 3.
Spearman correlations (rho) between appraisals and telomerase responsivity in controls
| Baseline Challenge |
Baseline Threat |
Baseline Threat - Challenge |
|
|---|---|---|---|
| Baseline telomerase | −.28 | .47* | .45* |
|
Post-stressor Challenge |
Post-Stressor Threat |
Post-Stressor Threat - Challenge |
|
| Telomerase Post Stress (90 min) |
−.34 | .51* | .49* |
| Change in telomerase (90 minus baseline) |
−.04 | .58** | .33 |
| Telomerase Reactivity (AUC minus baseline) |
.01 | .36+ | .18 |
| Telomerase Total (AUC) |
−.45* | .36+ | .48* |
p < .01,
p < .05,
p = .10, 2-tailed (Caregivers showed no significant correlations).
Groups were similar on age and BMI. To test group differences on the variables of interest (psychological factors, telomerase, cortisol, immune cell distributions) between caregivers vs. controls, we used t-tests. To test relationships between telomerase with psychological factors and cortisol we used Spearman rank order correlations, both across the sample and stratified by caregiving status.
Immune cell trafficking
Increases in telomerase activity may be per cell (increases within a cell) or due to increases in relative numbers of cells with high telomerase activity. Ideally, we would have measured telomerase activity in each blood cell type at each time point. However, as that would require very large amounts of blood for cell sorting at each point, we examined changes in cell redistribution as an indirect measure of whether telomerase changes occurred per cell or were due to changes in distributions of cells. Thus, the goal of measuring cell redistribution was to assess whether changes in telomerase activity vary with changes in redistribution of certain subpopulations of immune cells. We examined leukocyte distributions at each of the four time points (0, 20, 50, and 90 minutes after the onset of the stressor). Although neutrophils are not included in the measures of telomerase activity (which used purified PBMCs), it is still feasible that the pattern of change might have been associated with changes in telomerase activity in cell types other than PBMCs. Therefore, we also examined neutrophil changes. To examine whether acute increases in telomerase are due to immune cell redistribution (i.e., a shift in certain types of cells in the blood under acute stress), we used a mixed linear regression (with PASW 17.0), to evaluate telomerase over time using cell distribution as time varying correlate, as described in detail below.
RESULTS
Sample description
One participant, a control, had telomerase activity values that were more than 4 SD’s above the mean for the majority of samples (3 of the 4 samples), and was thus excluded from telomerase analyses. The final sample with complete telomerase measures, and excluding this outlier as well as the 3 women with incomplete telomerase values, included 43 postmenopausal women, 22 who were caregivers and 21 controls. The women had a mean age of 62, ranging from 51 to 75 years. They were 84% Caucasian, 5% African American, and 11% Asian. Their mean body mass index (BMI) in kg/m2 was 26.3 (SD 5.4), mean years of education was 15.7 (SD 1.9), and mean household income was $89K (SD $54K). There were no significant differences between caregivers and controls in BMI or the above demographics.
Psychological distress and psychological response to the lab stressor
As expected, caregivers had significantly higher levels of perceived life stress than controls (M =19.1, SE =1.1 vs. M = 9.9, SE = 1.3, t=−5.6, p<0.0001) as well as higher levels of depressive symptoms (M=17.5, SE=1.8 vs. M=9.0, SE=1.3, t=3.8, p<0.0001.
We compared threat and challenge response profiles, both anticipatory (before task) and evaluative (post-task) responses to the stressor. Caregivers reported significantly higher perceived threat in anticipation of the stressful tasks, compared to controls (caregivers: M = .20, SE = .13, vs. controls: M = −.52, SE = .18, p = .002), slightly lower challenge appraisals before the tasks (M = .02, SE = .19 vs. M = .34, SD = .18), and greater threat to challenge difference score (M = .18, SE = .27 vs. M = −.90, SE = .31, p = .01). Groups were similar in threat and challenge appraisals after the tasks
Telomerase responsivity during the laboratory stressor
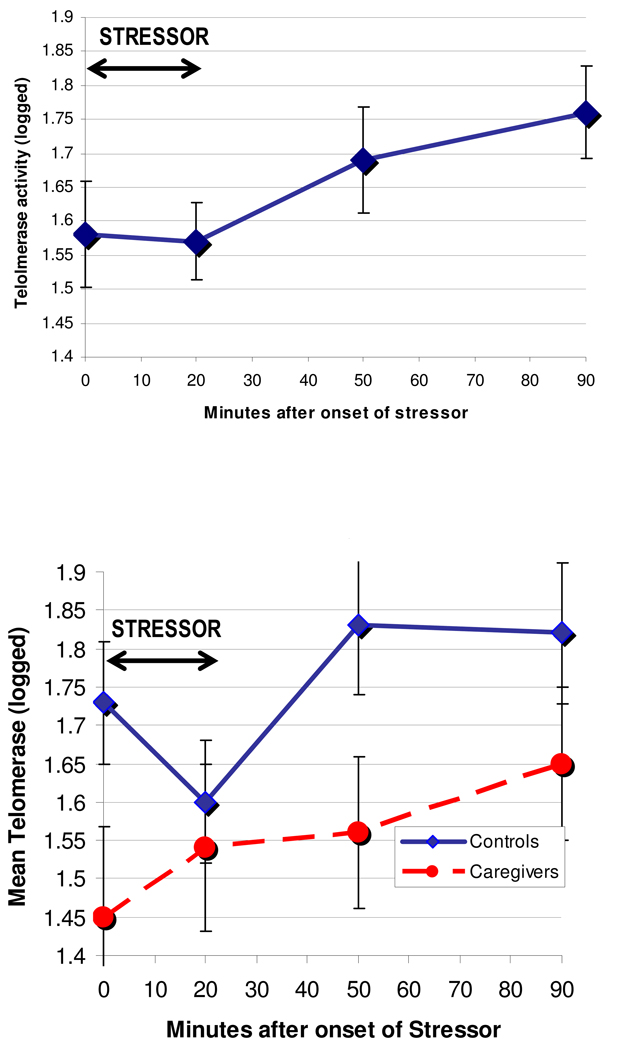
In the entire sample, there was a significant linear trend of increasing telomerase activity over time, F=9.9, p=0.003 (Figure 1, logged values), with a significant increase from baseline to 50 and 90 minutes after stress, p values < .02 (paired t-tests). We examined the trajectory of telomerase in response to the stressor by employing mixed modeling statistical designs. We fitted a restricted maximum likelihood to our mixed models and an unstructured covariance structure. To start, we modeled an unconditional model for telomerase to partition its variation. Results indicated statistically significant variation (p<.01) at both the within person and between person levels. Within and between person variation estimates were .14 (SE=.02) and .16 (SE=.04), respectively, indicating an intraclass correlation coefficient equal to .53. Thus, 53% of the variation in telomerase is a function of between person effects. We next modeled time, and found that residual variation decreased to .13, a drop in approximately 8% in unexplained variation from the unconditional model. Across the sample, telomerase increased significantly over time (b= .18 for changes over one hour, SE = .05, p < .01). Because we used natural logged values, this estimate is directly translatable into percent change (18% change). As described below after taking into account either total percentage or numbers of each cell subtype, this estimate appeared stable, varying narrowly from .17 to .21.
Figure 1.
Telomerase activity (logged) during the lab stressor
Figure 1b: Telomerase activity (logged) by caregivers and controls during lab stressor
Caregivers, compared to controls, had marginally lower baseline telomerase activity (p = .06) and significantly lower mean telomerase activity across time points (b = 1.56 mean logged telomerase for caregivers, SE=.05, and b=1.76 f for controls, SE=.08, p<.01). However, telomerase activity change over time was not a function of group. Although groups had different values on average, their slope over time was not significantly different, caregiver × time b = −.01, SE = .10, p = .94. Therefore, group was not included in further analyses examining telomerase covarying immune cell redistribution.
Telomerase responsivity and Immune Cell Redistribution
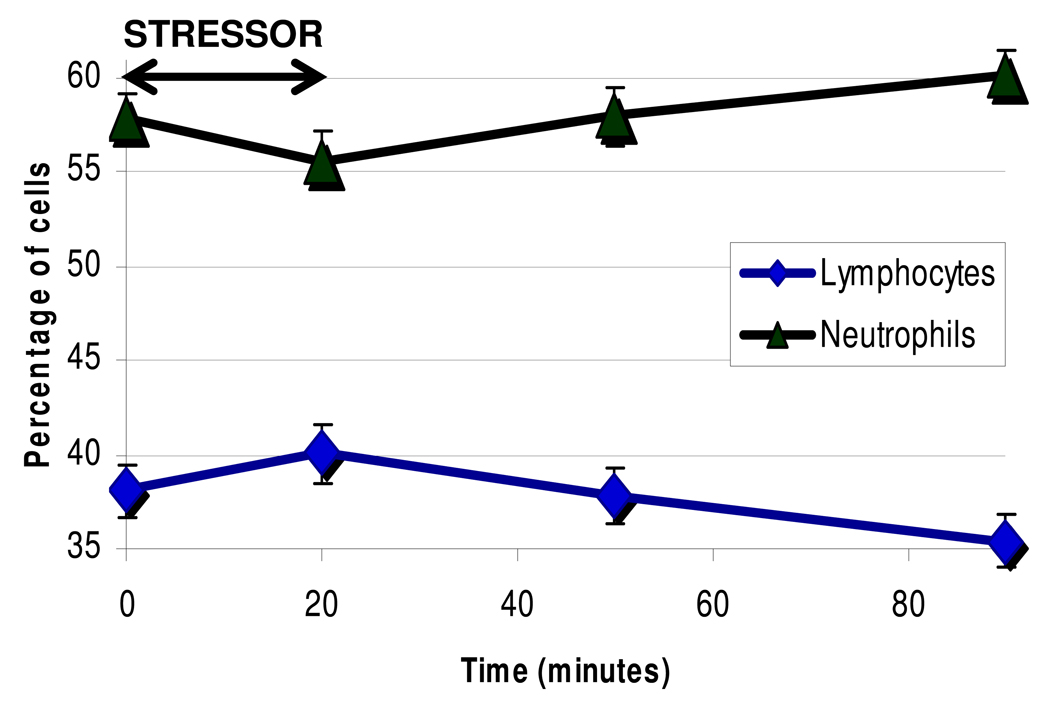
Cell frequencies were obtained in a subset of 27 participants (11 caregivers, 16 controls) to assess cell redistribution during stress. Redistribution patterns occurred as expected. For example, as shown in Figure 3, lymphocytes increase immediately post-stress, and traffic out of the blood, whereas neutrophils show the opposite pattern, continuously increasing during recovery from stress. Next, we performed a series of models predicting telomerase activity at each time as a function of each immune cell type percent (see Table 1 Model A). This model simply assesses whether total PMBC telomerase activity is in part a function of certain cell subpopulation frequencies, as would be expected given that different cell types have different levels of telomerase activity (Lin et al., 2009) . It assesses associations between telomerase and cell % at each time point, controlling for the dependency occurring between data points within each person. In Model A, we present intercepts (mean level of telomerase) and then the percent that telomerase is altered as a function of cell type %. As can be seen in Table 1, Model A, 1% more lymphocytes was related to a 2% lower level of telomerase activity. Conversely, 1% higher monocytes and neutrophils were related to a 7% and 2% higher telomerase activity, respectively. There were no relationships between CD4+, CD8+, or B cells with telomerase at each point. We note that neutrophils were not represented in the measured PBMC telomerase activity, so this relationship must be indirect.
Figure 3.
Lymphocytes and Neutrophils (%) during the lab stressor
Table 1.
Hierarchical model relationships between telomerase and cell subtype percentages.
| Log transformed telomerase | ||||
|---|---|---|---|---|
| Model A | Model B | |||
| Intercept B (SE) Baseline telomerase |
Factor B (SE) Mean telomerase |
Intercept B (SE) Baseline telomerase |
Factor B (SE) (telomerase over time) |
|
| CD4 | 1.80 (.10)* | −.00 (.00) | 1.69 (.11)* | −.00 (.00) |
| Time | - | - | - | .21 (.06)* |
| CD8 | 1.88 (.16)* | −.00 (.01) | 1.78 (.16)* | −.01 (.01) |
| Time | - | - | - | .21 (.06)* |
| B cells | 1.60 (.12)* | .02 (.01) | 1.54 (.12)* | .01 (.01) |
| Time | - | - | - | .19 (.06)* |
| Lymphocytes (L) | 2.40 (.20)* | −.02 (.00)* | 2.21 (.21)* | −.01 (.00)* |
| Time | - | - | - | .17 (.07)* |
| Monocytes | 1.49 (.15)* | .07 (.03)* | 1.36 (.15)* | .07 (.03)* |
| Time | - | - | - | .21 (.06)* |
| Neutrophils | .85 (.30)* | .02 (.01)* | .92 (.29)* | .01 (.00)* |
| Time | - | - | - | .18 (.07)* |
Next, in Model B (Table 1), we examined telomerase reactivity over the 90 minutes, covarying each cell type % at each point, in order to rule out the possibility that telomerase changes over time were simply a function of immune cell type percentages (which can change due to mobilizing into and trafficking out of the blood system). As can be seen in the rows for “Time”, after covarying each cell type, telomerase activity still increased approximately 17% to 21% post stressor.
Table 2 presents similar models; however, we used raw numbers of cell types instead of percent cell types. In the following models, one unit (which is 1000 cells per microliter) higher CD8+ cells is related to a 51% higher telomerase activity, which means that one standard deviation higher total CD8+ cells (220 cells/microliter) is related to 11.2% higher telomerase. In contrast, one unit higher lymphocytes was related to a 17% lower telomerase, which means one standard deviation higher number of lymphocytes (694 cells per microliter) is related to 11.8% higher telomerase.
Table 2.
Hierarchical model relationships between telomerase and cell subtype frequencies (per 1000 cells per microliter).
| NUMBER CELL TYPE |
Log transformed telomerase | |||
|---|---|---|---|---|
| Model A | Model B | |||
| Intercept B (SE) Baseline telomerase |
Factor B (SE) Mean telomerase |
Intercept B (SE) Baseline telomerase |
Factor B (SE) telomerase over time) |
|
| CD4 | 1.86 (.08)* | −.19 (.11) | 1.75 (.08)* | −.19 (.06) |
| Time | .21 (.06)* | |||
| CD8 | 2.03 (.10)* | −.51 (.14)* | 1.91 (.10)* | −.49 (.14)* |
| Time | .20 (.07)* | |||
| B cells | 1.87 (.11)* | −.35 (.42) | 1.74 (.11)* | −.36 (.06) |
| Time | .21 (.06)* | |||
| Lymphocytes (L) | 2.16 (.13)* | −.17 (.05)* | 1.95 (.16)* | −.13 (.06)* |
| Time | .20 (.07)* | |||
| Monocytes | 1.74 (.11)* | .18 (.40) | 1.64 (.12)* | .10 (.39) |
| Time | .21 (.06)* | |||
| Neutrophils | 1.76 (.14)* | .01 (.04) | 1.70 (.14) | −.01 (.04) |
| Time | .21 (.06)* | |||
denotes p < .05;
Finally, in Model B, we examined telomerase activity level as a function of time covarying numbers of cells in order to rule out the possibility that telomerase changes over time were simply a function of immune cell type numbers. As can be seen in the row “Time”, after covarying each cell type, telomerase activity still increased approximately 20% to 21% post stressor. Lastly, to directly test the concern that groups with and without trafficking data may be different, we added the group factor of whether subjects have cell type data or not. The interaction was not at significant (b = .02, SE = .09, p = .83).
Confounds
In the final mixed model testing telomerase over time, we examined telomerase activity over time in response to stress, while covarying potential confounds, including age, BMI, physical activity level, alcohol use, education and beta-blocker use (4 subjects vs. all others). In all analyses, telomerase activity significantly increased with time, even after covarying each confound individually. With each confound, telomerase activity significantly increased between 16% and 19% over time. A preferred model would consider all potential confounds together; however, due to our sample size, we covaried each individually.
Relations between Appraisals of the Stressor and Telomerase Responses
Across the sample, greater telomerase AUC was only marginally associated with perceptions after the stressor - lower challenge appraisals (r = −.27, p = .06), and greater threat-to-challenge appraisal difference scores (r = .29, p = .06), and not related to threat appraisals (r = .23, ns). These relationships appeared to be due to the controls, who showed a consistent pattern between appraisals and indices of telomerase output and reactivity; most notably, greater threat after the stressor with higher telomerase increase at 90 minutes (see Table 1). In caregivers alone, no relationships of appraisals (either pre- or post-stressor) with telomerase reached statistical significance.
As shown in Table 1, for controls, there was a pattern of relationships across the measures of appraisal and telomerase. Feeling greater relative threat-to-challenge pre-stressor (at baseline) was significantly associated with higher baseline telomerase activity. After the stressor, greater threat-to-challenge appraisals were related to higher telomerase at 90 minutes, and also to telomerase AUC. Feeling threatened post-stressor was also significantly related to indices of telomerase responsivity (higher telomerase at 90 minutes and higher telomerase responsivity increase from baseline to 90 minutes).
Relations between cortisol responses and telomerase responses to the stressor
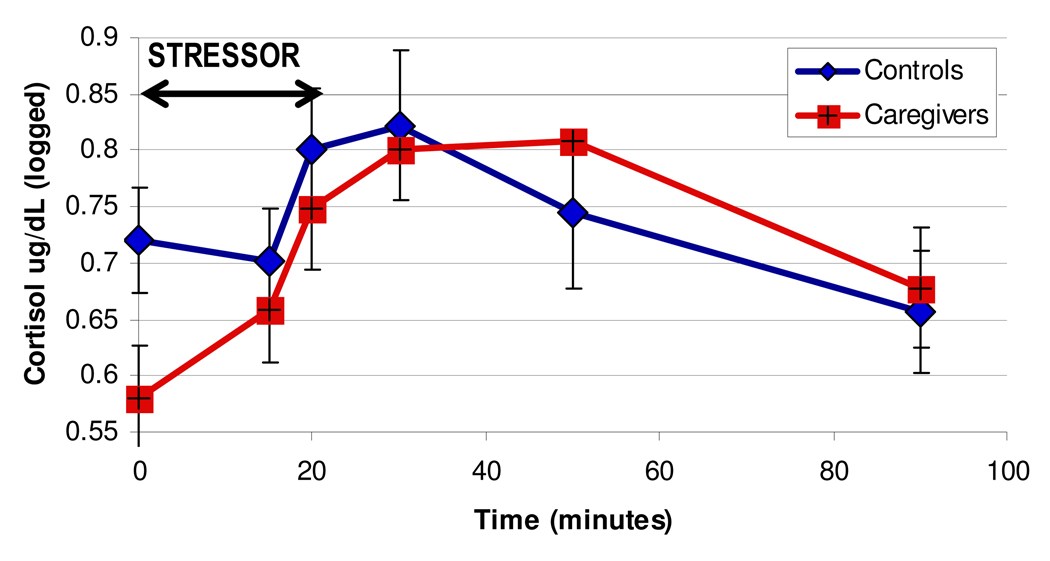
Across the sample, salivary cortisol increased by 16% from baseline to ten minutes into the stressor (early reactivity), and by 73% 30 minutes after onset of stressor, the time of peak cortisol response. Caregivers and controls had similar baseline cortisol. The caregiving group had early cortisol reactivity, with a mean percent change of 33.4%, compared to no increase (−0.55%) in controls (T = 2.4, p = .03). There were no significant group differences in peak (30 minute) cortisol reactivity. The change scores of cortisol from baseline are shown in Figure 2, by group.
Figure 2.
Cortisol change during laboratory stressor by caregivers and controls
We then examined correlations between cortisol reactivity both early (15 minutes) and at peak (30 minutes) with telomerase measures. Across the sample, cortisol at baseline was not related to telomerase at baseline (r = −.07). Telomerase activity at 90 minutes (but not change in telomerase at 90 minutes) was significantly associated with both early cortisol reactivity (r = .40, p = .02) and peak cortisol reactivity (r = .33, p = .05) across the sample. Total telomerase (AUC) but not rAUC was also related to early cortisol reactivity (r = .25, p < .05) and marginally related to peak cortisol reactivity (r = .31, p = .07)..
DISCUSSION
In this study, we examined whether leukocyte telomerase activity changes in response to acute psychological stress, and whether it is related to being a caregiver, or to responding in the moment to the stressor with greater perceived threat or cortisol reactivity. We found that in response to the Trier Social Stress Test telomerase activity increased by around 18%. Further, telomerase activity increased more those reacting with greater cortisol, and among controls, for those feeling more threatened. This is the first demonstration that telomerase activity levels can change acutely in vivo within short periods. We term this phenomenon ‘telomerase responsivity.’ Given that telomerase appears to decrease in some in vitro conditions with cortisol exposure (Choi et al., 2008), and there appeared to be a decrease in the early phase of the stress response in controls in the present study, we term this dynamic behavior of telomerase activity levels “responsivity,” indicating changeability in either direction, rather than “reactivity” (which implies a unidirectional increase).
The function of telomerase responsivity is unknown. However, as with most stress responses, it is likely a protective functional response. It may protect cells or even telomeric DNA from stress-induced acute increases in stress mediators such as cortisol or oxidative stress. It is also possible it acts to prepare the immune cells for proliferation, in the event of antigen exposure which evolutionarily occurs during times of acute stress (e.g., running from a predator before wounding). In mice, after stimulation with antigen or virus while lymphocytes undergo proliferative clonal expansion, their telomerase activity levels increase dramatically, and telomerase activity remains high in memory cells (Hathcock et al., 2005).
Telomerase offers cell protection especially in the context of short telomeres (Zhu, Wang, Bishop, & Blackburn, 1999). The telomerase core protein, hTERT, in human fibroblasts protects DNA (by telomere lengthening) from radiation and oxidative stress, but only in cells with short telomeres (Rubio, Davalos, & Campisi, 2004). Basic research on telomerase has shown it also has other roles in determining cellular programs and properties besides elongating telomeres, (Hsu et al., 2007; Park et al., 2009). These roles may also be relevant to the responses to stress we report here.
In an in vitro study, by exposing immune cells to higher-than-physiological levels of glucocorticoid, Choi et al (Choi et al., 2008) found that such continuous cortisol exposure of PBMCs was associated with decreased PBMC telomerase activity. On the surface, these findings appear to be in opposition with our in vivo findings, but it is difficult to compare in vivo findings with these in vitro findings. Supraphysiological levels of glucocorticoids tend to have a suppressive rather than stimulatory effect on immune function (Dhabhar, 2009). Indeed, our results suggest that the lower concentrations of cortisol relevant to mild stress in humans (typically very short term, often only two-fold increases) and the ensuing stress responsive mediators may have a stimulatory effect rather than a suppressive effect on telomerase activity. Further, there are often curvilinear relationships between cortisol exposure and defense mechanisms and regulatory systems. As described in detail elsewhere, low levels of cortisol have facilitative or permissive effects whereas high levels of cortisol can have suppressive effects on various systems including aspects of immune function (Sapolsky, Romero, & Munck, 2000)
Choi et al reported that physiological levels (around .1 µM) of cortisol when applied in vitro did not affect PBMC telomerase. However, their study is not comparable to the current study in several important ways. They used supraphysiological levels, up to 30 µM, which are much higher than expected during mild stress in humans. Further, they did not examine acute effects one hour after cortisol exposure as we did for our in vivo mild acute stress session, as they studied only a much longer time frame of 3 days. They also found that such high levels of cortisol had different effects depending on cell type (more suppressive in CD8+ cells than in CD4+ cells). Hence telomerase responsivity may depend on duration and dose of cortisol as well as type of cells, at least in vitro. Their longer term effect appeared to be mediated by reduced transcription of hTERT.
The mechanism for the acute effect we observed is unknown. Telomerase activity can be modulated via transcriptional regulation of the protein subunit hTERT, and by other mechanisms including alternative splicing (Anderson, Hoare, Ashcroft, Bilsland, & Keith, 2006; Jalink et al., 2007). However, such gene expression changes would likely manifest over hours, rather than minutes. Therefore, any acute changes are likely to occur through post-translational modifications of telomerase, such as phosphorylation, and/or changes in subcellular localization. Both mechanisms have been reported to regulate telomerase activity (Kang, Kwon, Kwon, & Do, 1999; Li, Zhao, Funder, & Liu, 1997; Liu, Hodes, & Weng, 2001; Wong, Kusdra, & Collins, 2002). In particular, post-translational regulation of telomerase activity in immune cells likely involves signaling via kinase family members PI3K/AKT, through NFKB and protein kinase C (Li, Zhao, Yang, Funder, & Liu, 1998; Plunkett et al., 2007; Sheng, Chen, & Wang, 2006). The impact of acute psychological stress on these regulatory modalities in vivo warrants further investigation.
Lastly, we note that cortisol levels in blood do not necessarily reflect cortisol signaling at the cellular and genomic levels (Miller et al., 2008). Hence, it is unclear whether the greater cortisol reactivity is mechanistically involved in the increases in telomerase activity we observed, or rather a proxy measure for history of greater exposure to chronic stress and related changes at the cellular level.
Telomerase Responsivity and Psychological Stress
We also examined whether women under chronic stress (being a dementia patient caregiver who reported high perceived stress) had a different response compared to low stress controls. Indeed, the caregivers had lower telomerase activity at baseline, but their slope of increase following the stressor was not significantly different than that of caregivers. For controls, who were lower in life stress, feeling threatened (e.g., anxious, stressed), and feeling greater threat relative to feeling positively challenged (e.g., eager, enthusiastic), were related to stress induced increases in telomerase activity. It is unclear why, in caregivers, acute appraisals were not similarly related to telomerase responsivity.
Baseline telomerase and threat appraisals
It was particularly notable that appraisals at baseline, before the stressor onset, were associated with telomerase activity at that time, during the baseline blood draw. This suggests that the higher telomerase activity at the baseline time point may be attributable at least in part to anticipation of completing the stressful tasks. This finding was limited to controls, who also showed relations between threat during the stressor and changes in telomerase activity. Hence, in the controls the baseline telomerase measure may already be reflecting stress-induced elevations. It is striking that, even despite this possibility, greater feelings of threat (and threat-to-challenge) after the stressor were still related to higher telomerase increase from baseline to 90 minutes. Together, these findings that telomerase activity increases in association with perceived threat and increases in cortisol suggest that telomerase activity may be stress-reactive. These results imply that research that aims to measure basal telomerase should have a resting period before measures are taken, as with any stress hormone.
Telomerase Reactivity and Immune Trafficking
This study examined the dynamics of telomerase levels using total PBMCs. Therefore, we tested whether shifts in the distributions of cell subtypes that differ in their telomerase activity levels might underlie or at least help explain the observed responsivity response. As one might expect, given the varying levels of telomerase per cell type (Lin et al., 2009) the percentages of cell subtypes present can influence the overall measure of telomerase activity in PBMCs. We found greater percentages of monocytes were related to greater telomerase, whereas greater percentages of lymphocytes were related to lower telomerase. Greater numbers of CD8+ cells were related to lower telomerase, a finding which might reflect greater CD8+ cell senescence. Thus, composition of immune cell types clearly influences overall PBMC telomerase activity, which is important to consider whenever interpreting findings across cell types. Despite these relationships, the responsivity of PBMC telomerase activity during and following acute stress existed even after covarying blood cell composition in that time period, leaving open the possibility that telomerase responsivity is caused by dynamic changes in the regulation of telomerase enzymatic activity levels in response to psychological stress.
Although our results suggest that total PBMC telomerase activity increases independently of cell distribution, it is not possible to determine from this study whether telomerase activity increases on a per cell basis, or whether the increase is mediated by stress-induced changes in immune cell distribution. Future studies examining telomerase activity within individual cell types rather than in pooled PBMCs should address this possibility. It is also possible that cells lost during purification process of PBMCs for telomerase measurement may weaken any correlation between telomerase enzyme activity and whole blood flow cytometric enumeration of cell types. Overall, the changes in telomerase activity that were observed are likely to be mediated by a combination of stress-induced changes in the distribution of immune cells, as well as changes in telomerase activity on a per cell basis.
Limitations and Conclusions
Strengths of this study are careful measurement of telomerase activity over time with a highly controlled standardized protocol and study sample, concurrently with psychological, hormonal, and immune cell responses during acute stress. This has revealed novel relationships. However, the study is limited by its sample size and by the fact that immune cell redistribution was measured on only a subsample of 27. It is also a limitation that we did not have a control group with no stress exposure, to test for catheter insertion, time of day, and other non-specific factors. It will be informative to have such a control group in future studies, as well as test mediation by they sympathetic nervous system. Given that stress parameters (threat appraisals and changes in cortisol) were correlated with changes in telomerase activity, it is unlikely that the changes in telomerase activity were due to time of day, especially since our data suggest little change across the day (unpublished results).
Despite the above limitations, the study advances the understanding of the relationship between acute stress and cell aging in vivo, in terms of the telomerase-mediated telomere length maintenance system. Although telomerase is an intracellular ribonucleoprotein enzyme, and does not communicate from cell to cell, nevertheless, like stress-reactive hormones, telomerase appears dynamic and responsive to stress, which could have major implications for both research and clinical applications.
ACKNOWLEDGMENTS
This study was supported in part by an NIMH K08, NIA R56, and UCSF Academic Senate Grant and REAC grant (ESE), Bernard and Barbro Foundation (EHB), the Carl & Elizabeth Naumann Fund Startup Grant (FSD), the UCSF Sandler Integrative Research Award (EHB) and the UCSF CCRC NIH grant UL1 RR024131. Our sincere acknowledgments to Jean Tillie for flow cytometry, Alanie Lazaro for laboratory management, Wendy Wolfson, Hana Tylova, and Jessica Chan for study coordination, and Aoife O’Donovan for comments. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: All authors declare that there are no conflicts of interest.
REFERENCES
- Anderson CJ, Hoare SF, Ashcroft M, Bilsland AE, Keith WN. Hypoxic regulation of telomerase gene expression by transcriptional and post-transcriptional mechanisms. Oncogene. 2006;25(1):61–69. doi: 10.1038/sj.onc.1209011. [DOI] [PubMed] [Google Scholar]
- Aviv A, Valdes A, Gardner JP, Swaminathan R, Kimura M, Spector TD. Menopause modifies the association of leukocyte telomere length with insulin resistance and inflammation. J Clin Endocrinol Metab. 2006;91(2):635–640. doi: 10.1210/jc.2005-1814. [DOI] [PubMed] [Google Scholar]
- Bakaysa SL, Mucci LA, Slagboom PE, Boomsma DI, McClearn GE, Johansson B, et al. Telomere length predicts survival independent of genetic influences. Aging Cell. 2007;6(6):769–774. doi: 10.1111/j.1474-9726.2007.00340.x. [DOI] [PubMed] [Google Scholar]
- Benetos A, Okuda K, Lajemi M, Kimura M, Thomas F, Skurnick J, et al. Telomere length as an indicator of biological aging: the gender effect and relation with pulse pressure and pulse wave velocity. Hypertension. 2001;37(2 Part 2):381–385. doi: 10.1161/01.hyp.37.2.381. [DOI] [PubMed] [Google Scholar]
- Brouilette S, Singh RK, Thompson JR, Goodall AH, Samani NJ. White cell telomere length and risk of premature myocardial infarction. Arterioscler Thromb Vasc Biol. 2003;23(5):842–846. doi: 10.1161/01.ATV.0000067426.96344.32. [DOI] [PubMed] [Google Scholar]
- Buchkovich KJ, Greider CW. Telomerase regulation during entry into the cell cycle in normal human T cells. Mol Biol Cell. 1996;7(9):1443–1454. doi: 10.1091/mbc.7.9.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon R, Smith K, O'Brien E, Sivatchenko A, Kerber R. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- Choi J, Fauce SR, Effros RB. Reduced telomerase activity in human T lymphocytes exposed to cortisol. Brain Behav Immun. 2008;22(4):600–605. doi: 10.1016/j.bbi.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- Cohen S, Williamson G. Perceived stress in a probability sample of the United States. In: Oskamp SSS, editor. The social psychology of health: Claremont Symposium on applied social psychology. Newbury Park, CA: Sage; 1988. [Google Scholar]
- Colitz CM, Whittington A, Carter R, Warren J. The effects of oxidative stress on telomerase activity and other stress-related proteins in lens epithelial cells. Exp Eye Res. 2004;78(2):235–242. doi: 10.1016/j.exer.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Damjanovic AK, Yang Y, Glaser R, Kiecolt-Glaser JK, Nguyen H, Laskowski B, et al. Accelerated telomere erosion is associated with a declining immune function of caregivers of Alzheimer's disease patients. J Immunol. 2007;179(6):4249–4254. doi: 10.4049/jimmunol.179.6.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar F, McEwen B. Acute stress enhances while chronic stress suppresses, immune function in vivo: A role for luekocyte trafficking. Brain, Behavior, and Immunity. 1997;11:286–306. doi: 10.1006/brbi.1997.0508. [DOI] [PubMed] [Google Scholar]
- Dhabhar F, Miller A, McEwen B, Spencer R. Stress-induced changes in blood leukocyte distribution: Role of adrenal steroid hormones. Journal of Immunology. 1996;157:1638–1644. [PubMed] [Google Scholar]
- Dhabhar FS. Stress-induced enhancement of cell-mediated immunity. Ann N Y Acad Sci. 1998;840:359–372. doi: 10.1111/j.1749-6632.1998.tb09575.x. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS. Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection and immunopathology. Neuroimmunomodulation. 2009;16(5):300–317. doi: 10.1159/000216188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS. Bidirectional Effects Of Stress & Glucocorticoid Hormones On Immune Function: Possible Explanations For Paradoxical Observations. In Psychoneuroimmunology. In: Ader DR, Felten L, Cohen N, editors. Psychoneuroimmunology. 3rd edition. Vol. San Diego: Academic Press; 2001. pp. 301–338. [Google Scholar]
- Epel E, Blackburn E, Lin J, Dhabhar F, Adler N, Morrow JD, et al. Accelerated telomere shortening in response to exposure to life stress. PNAS. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman S, Lazarus R. If it changes it must be a process: Study of emotion nad coping during three stages of a college examination. Journal of Personality and Social Psychology. 1985;48:150–170. doi: 10.1037//0022-3514.48.1.150. [DOI] [PubMed] [Google Scholar]
- Gardner JP, Li S, Srinivasan SR, Chen W, Kimura M, Lu X, et al. Rise in insulin resistance is associated with escalated telomere attrition. Circulation. 2005;111(17):2171–2177. doi: 10.1161/01.CIR.0000163550.70487.0B. [DOI] [PubMed] [Google Scholar]
- Gidron Y, Russ K, Tissarchondou H, Warner J. The relation between psychological factors and DNA-damage: a critical review. Biol Psychol. 2006;72(3):291–304. doi: 10.1016/j.biopsycho.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Hathcock KS, Chiang Y, Hodes RJ. In vivo regulation of telomerase activity and telomere length. Immunol Rev. 2005;205:104–113. doi: 10.1111/j.0105-2896.2005.00267.x. [DOI] [PubMed] [Google Scholar]
- Honig LS, Schupf N, Lee JH, Tang MX, Mayeux R. Shorter telomeres are associated with mortality in those with APOE epsilon4 and dementia. Ann Neurol. 2006;60(2):181–187. doi: 10.1002/ana.20894. [DOI] [PubMed] [Google Scholar]
- Hsu M, McEachern M, Dandjinou A, Tzfati Y, Orr E, Blackburn E, et al. Telomerase core components protect Candida telomeres from aberrant overhang accumulation. Proc Natl Acad Sci U S A. 2007;104:11682–11687. doi: 10.1073/pnas.0700327104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi H, Sakaguchi N. Telomerase activity is induced in human peripheral B lymphocytes by the stimulation to antigen receptor. Blood. 1997;89(4):1299–1307. [PubMed] [Google Scholar]
- Jalink M, Ge Z, Liu C, Bjorkholm M, Gruber A, Xu D. Human normal T lymphocytes and lymphoid cell lines do express alternative splicing variants of human telomerase reverse transcriptase (hTERT) mRNA. Biochem Biophys Res Commun. 2007;353(4):999–1003. doi: 10.1016/j.bbrc.2006.12.149. [DOI] [PubMed] [Google Scholar]
- Jeanclos E, Schork NJ, Kyvik KO, Kimura M, Skurnick JH, Aviv A. Telomere length inversely correlates with pulse pressure and is highly familial. Hypertension. 2000;36(2):195–200. doi: 10.1161/01.hyp.36.2.195. [DOI] [PubMed] [Google Scholar]
- Kang SS, Kwon T, Kwon DY, Do SI. Akt protein kinase enhances human telomerase activity through phosphorylation of telomerase reverse transcriptase subunit. J Biol Chem. 1999;274(19):13085–13090. doi: 10.1074/jbc.274.19.13085. [DOI] [PubMed] [Google Scholar]
- Kimura M, Hjelmborg JV, Gardner JP, Bathum L, Brimacombe M, Lu X, et al. Telomere length and mortality: a study of leukocytes in elderly Danish twins. Am J Epidemiol. 2008;167(7):799–806. doi: 10.1093/aje/kwm380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke K, Hellhammer D. The "Trier Social Stress Test"--A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Li H, Zhao L, Yang Z, Funder JW, Liu JP. Telomerase is controlled by protein kinase Calpha in human breast cancer cells. J Biol Chem. 1998;273(50):33436–33442. doi: 10.1074/jbc.273.50.33436. [DOI] [PubMed] [Google Scholar]
- Li H, Zhao LL, Funder JW, Liu JP. Protein phosphatase 2A inhibits nuclear telomerase activity in human breast cancer cells. J Biol Chem. 1997;272(27):16729–16732. doi: 10.1074/jbc.272.27.16729. [DOI] [PubMed] [Google Scholar]
- Lin J, Epel E, Cheon J, Kroenke C, Wolkowitz O, Mellon S, et al. Analyses and Comparisons of Telomerase Activity and Telomere Length in Human T and B cells: Insights for Epidemiology of Telomere maintenance. Journal of Immunological Methods. 2009 doi: 10.1016/j.jim.2009.09.012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Hodes RJ, Weng N. Cutting edge: telomerase activation in human T lymphocytes does not require increase in telomerase reverse transcriptase (hTERT) protein but is associated with hTERT phosphorylation and nuclear translocation. J Immunol. 2001;166(8):4826–4830. doi: 10.4049/jimmunol.166.8.4826. [DOI] [PubMed] [Google Scholar]
- Martin-Ruiz C, Dickinson HO, Keys B, Rowan E, Kenny RA, Von Zglinicki T. Telomere length predicts poststroke mortality, dementia, and cognitive decline. Ann Neurol. 2006;60(2):174–180. doi: 10.1002/ana.20869. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Sze J, Marin T, Arevalo JM, Doll R, et al. A functional genomic fingerprint of chronic stress in humans: blunted glucocorticoid and increased NF-kappaB signaling. Biol Psychiatry. 2008;64(4):266–272. doi: 10.1016/j.biopsych.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrot TS, Staessen JA, Gardner JP, Aviv A. Telomere length and possible link to X chromosome. Lancet. 2004;363(9408):507–510. doi: 10.1016/S0140-6736(04)15535-9. [DOI] [PubMed] [Google Scholar]
- Nishikawa T, Nakajima T, Katagishi T, Okada Y, Jo M, Kagawa K, et al. Oxidative stress may enhance the malignant potential of human hepatocellular carcinoma by telomerase activation. Liver Int. 2009;29(6):846–856. doi: 10.1111/j.1478-3231.2008.01963.x. [DOI] [PubMed] [Google Scholar]
- Ornish D, Lin J, Daubenmier J, Weidner G, Epel ES, Kemp C, et al. Increased Telomerase Activity in a Pilot Study of Comprehensive Lifestyle Changes. The Lancet Oncology. 2008 doi: 10.1016/S1470-2045(08)70234-1. in press. [DOI] [PubMed] [Google Scholar]
- Park J, Venteicher A, JY H, Choi J, Jun S, Shkreli M, et al. Telomerase modulates Wnt signalling by association with target gene chromatin. Nature. 2009;460:66–72. doi: 10.1038/nature08137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks CG, Miller DB, McCanlies EC, Cawthon RM, Andrew ME, DeRoo LA, et al. Telomere length, current perceived stress, and urinary stress hormones in women. Cancer Epidemiol Biomarkers Prev. 2009;18(2):551–560. doi: 10.1158/1055-9965.EPI-08-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plunkett FJ, Franzese O, Finney HM, Fletcher JM, Belaramani LL, Salmon M, et al. The loss of telomerase activity in highly differentiated CD8+CD28-CD27- T cells is associated with decreased Akt (Ser473) phosphorylation. J Immunol. 2007;178(12):7710–7719. doi: 10.4049/jimmunol.178.12.7710. [DOI] [PubMed] [Google Scholar]
- Rubio MA, Davalos AR, Campisi J. Telomere length mediates the effects of telomerase on the cellular response to genotoxic stress. Exp Cell Res. 2004;298(1):17–27. doi: 10.1016/j.yexcr.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Rush J, Gullion C, Basco M, Jarrett R, Trivedi M. The Inventory of Depressive Symptomatology (IDS): Psychometric properties. Psychological Medicine. 1996;26:477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- Samani NJ, Boultby R, Butler R, Thompson JR, Goodall AH. Telomere shortening in atherosclerosis. Lancet. 2001;358(9280):472–473. doi: 10.1016/S0140-6736(01)05633-1. [DOI] [PubMed] [Google Scholar]
- Sampson MJ, Winterbone MS, Hughes JC, Dozio N, Hughes DA. Monocyte telomere shortening and oxidative DNA damage in type 2 diabetes. Diabetes Care. 2006;29(2):283–289. doi: 10.2337/diacare.29.02.06.dc05-1715. [DOI] [PubMed] [Google Scholar]
- Sapolsky R, Romero M, Munck A. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Saretzki G. Telomerase, mitochondria and oxidative stress. Exp Gerontol. 2009;44(8):485–492. doi: 10.1016/j.exger.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Schedlowski M, Jacobs G, Stratman S, Richter A, Hädike U, Tewes T, et al. Changes of natural killer cells during acute psychological stress. J. Clin. Immunol. 1993;13:119–126. doi: 10.1007/BF00919268. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull. 2004;130(4):601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng WY, Chen YR, Wang TC. A major role of PKC theta and NFkappaB in the regulation of hTERT in human T lymphocytes. FEBS Lett. 2006;580(30):6819–6824. doi: 10.1016/j.febslet.2006.11.044. [DOI] [PubMed] [Google Scholar]
- Tomaka J, Blaskovich J, Kibler J, Ernst JM. Cognitive and Physiological Antecedents of Threat and Challenge Appraisal. Journal of Personality & Social Psychology. 1997;73(1):63–72. doi: 10.1037//0022-3514.73.1.63. [DOI] [PubMed] [Google Scholar]
- Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, et al. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366(9486):662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- Wong JM, Kusdra L, Collins K. Subnuclear shuttling of human telomerase induced by transformation and DNA damage. Nat Cell Biol. 2002;4(9):731–736. doi: 10.1038/ncb846. [DOI] [PubMed] [Google Scholar]
- Wu ZH, Zhang JS. The effect of UV-irradiation on telomerase activity and other stress-related proteins in human lens epithelial cells. Zhonghua Yan Ke Za Zhi. 2005;41(5):459–463. [PubMed] [Google Scholar]
- Zhu J, Wang H, Bishop J, Blackburn E. Telomerase extends the lifespan of virus-transformed human cells without net telomere lengthening. Proc Natl Acad Sci U S A. 1999;96:3723–3728. doi: 10.1073/pnas.96.7.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]